Abstract
Plasma xanthine oxidase (XO) activity was defined as a source
of enhanced vascular superoxide (O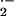 ) and
hydrogen peroxide (H2O2) production in both
sickle cell disease (SCD) patients and knockout-transgenic SCD mice.
There was a significant increase in the plasma XO activity of SCD
patients that was similarly reflected in the SCD mouse model. Western
blot and enzymatic analysis of liver tissue from SCD mice revealed
decreased XO content. Hematoxylin and eosin staining of liver tissue of
knockout-transgenic SCD mice indicated extensive hepatocellular injury
that was accompanied by increased plasma content of the liver enzyme
alanine aminotransferase. Immunocytochemical and enzymatic analysis of
XO in thoracic aorta and liver tissue of SCD mice showed increased
vessel wall and decreased liver XO, with XO concentrated on and in
vascular luminal cells. Steady-state rates of vascular
O
) and
hydrogen peroxide (H2O2) production in both
sickle cell disease (SCD) patients and knockout-transgenic SCD mice.
There was a significant increase in the plasma XO activity of SCD
patients that was similarly reflected in the SCD mouse model. Western
blot and enzymatic analysis of liver tissue from SCD mice revealed
decreased XO content. Hematoxylin and eosin staining of liver tissue of
knockout-transgenic SCD mice indicated extensive hepatocellular injury
that was accompanied by increased plasma content of the liver enzyme
alanine aminotransferase. Immunocytochemical and enzymatic analysis of
XO in thoracic aorta and liver tissue of SCD mice showed increased
vessel wall and decreased liver XO, with XO concentrated on and in
vascular luminal cells. Steady-state rates of vascular
O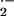 production, as indicated by coelenterazine
chemiluminescence, were significantly increased, and nitric oxide
(⋅NO)-dependent vasorelaxation of aortic ring segments was
severely impaired in SCD mice, implying oxidative inactivation of
⋅NO. Pretreatment of aortic vessels with the superoxide
dismutase mimetic manganese
5,10,15,20-tetrakis(N-ethylpyridinium-2-yl)porphyrin
markedly decreased O
production, as indicated by coelenterazine
chemiluminescence, were significantly increased, and nitric oxide
(⋅NO)-dependent vasorelaxation of aortic ring segments was
severely impaired in SCD mice, implying oxidative inactivation of
⋅NO. Pretreatment of aortic vessels with the superoxide
dismutase mimetic manganese
5,10,15,20-tetrakis(N-ethylpyridinium-2-yl)porphyrin
markedly decreased O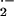 levels and significantly
restored acetylcholine-dependent relaxation, whereas catalase had no
effect. These data reveal that episodes of intrahepatic
hypoxia-reoxygenation associated with SCD can induce the release of XO
into the circulation from the liver. This circulating XO can then bind
avidly to vessel luminal cells and impair vascular function by creating
an oxidative milieu and catalytically consuming ⋅NO via
O
levels and significantly
restored acetylcholine-dependent relaxation, whereas catalase had no
effect. These data reveal that episodes of intrahepatic
hypoxia-reoxygenation associated with SCD can induce the release of XO
into the circulation from the liver. This circulating XO can then bind
avidly to vessel luminal cells and impair vascular function by creating
an oxidative milieu and catalytically consuming ⋅NO via
O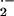 -dependent mechanisms.
-dependent mechanisms.
The β-globin mutation in sickle cell disease (SCD) is manifested by a glutamic acid to valine substitution and, ultimately, vascular dysfunction. Upon deoxygenation, intracellular polymerization of HbS occurs, and sickle erythrocytes acquire altered rheological properties (1). Even though the capillary transit time of red cells is brief in comparison to the kinetics of HbS polymerization, increased blood cell interactions with vascular endothelium will occur as a consequence of altered red cell membrane properties and increased vessel wall adhesiveness. Incompletely described signaling mechanisms also induce an inflammatory-like activation state in vascular endothelium indicated by elevated endothelial expression of Fc receptor and the integrins ICAM-1, VCAM-1, and P-selectin (2–5). There are also increased plasma levels of leukocytes (6), “activated” circulating endothelial cells, proinflammatory cytokines, platelet-activating factor, C-reactive protein, and angiogenic stimuli (7, 8).
The mechanisms underlying regional blood flow deprivation during sickle cell crises, as well as the associated pain with consequent tissue injury, remain poorly understood. If tissue ischemia in SCD patients resulted solely from Hb polymerization and red cell deformation, occlusion of small blood vessels such as terminal arterioles would predominate. Whereas this phenomenon certainly contributes to tissue injury in the liver, lungs, kidney, and spleen, it is not sufficient to explain large vessel vasculopathies. For example, stroke in SCD patients occurs in large and medium-sized arteries (internal carotid and middle cerebral arteries; refs. 9 and 10). Importantly, the quantity or proportion of sickled or dense red cells in the circulation does not correlate with the incidence of painful episodes or other manifestations of vascular occlusion (11, 12). This implies that much of the morbidity and mortality of SCD is caused by alterations in vascular function that occur secondary to red cell sickling, rather than as a consequence of direct vaso-occlusive actions of sickled red cells.
Multiple features of SCD strongly infer a pathogenic role for impaired
⋅NO-dependent vascular regulation. For example, vascular
production of ⋅NO seems to be chronically activated to maintain
vasodilation, as indicated by low baseline blood pressure (13) and
decreased plasma arginine levels (14). Also, decreased pressor
responses to angiotensin II (15), renal hyperfiltration (16), a
tendency for priapism (17), and elevated plasma nitrite and nitrate
(NO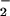 + NO
+ NO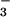 ) levels occur in SCD
(18). During vaso-occlusive crisis, an increased metabolic demand for
arginine and an inverse relationship between subjective pain scores and
plasma NO
) levels occur in SCD
(18). During vaso-occlusive crisis, an increased metabolic demand for
arginine and an inverse relationship between subjective pain scores and
plasma NO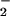 + NO
+ NO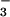 levels has been
reported (18, 19). Finally, therapeutic benefit has been observed in
SCD patients receiving inhaled ⋅NO and hydroxyurea, a drug
frequently used to treat SCD that not only induces fetal Hb levels in
SCD patients but also is metabolized to ⋅NO (20, 21).
levels has been
reported (18, 19). Finally, therapeutic benefit has been observed in
SCD patients receiving inhaled ⋅NO and hydroxyurea, a drug
frequently used to treat SCD that not only induces fetal Hb levels in
SCD patients but also is metabolized to ⋅NO (20, 21).
Another hallmark of SCD, increased tissue rates of production of
reactive oxygen species, may also contribute to impaired NO signaling.
Compared with HbA red cells, HbS red cells have been reported to
generate ≈2-fold greater extents of O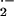 ,
H2O2, hydroxyl radical
(⋅OH), and lipid oxidation products (LOOH, LOO⋅) (22,
23). Also, decompartmentalization of redox-active transition metals
such as iron has been observed in HbS red cells (23). Finally, mice
expressing human βS-hemoglobin displayed
indices of increased lipid oxidation and aromatic hydroxylation
reactions and, upon exposure to hypoxia, had ≈10% increase in the
conversion of liver and kidney xanthine oxidoreductase to the
O
,
H2O2, hydroxyl radical
(⋅OH), and lipid oxidation products (LOOH, LOO⋅) (22,
23). Also, decompartmentalization of redox-active transition metals
such as iron has been observed in HbS red cells (23). Finally, mice
expressing human βS-hemoglobin displayed
indices of increased lipid oxidation and aromatic hydroxylation
reactions and, upon exposure to hypoxia, had ≈10% increase in the
conversion of liver and kidney xanthine oxidoreductase to the
O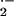 and
H2O2-producing oxidase form
(24). Appreciating that ⋅NO reacts at diffusion-limited rates
with O
and
H2O2-producing oxidase form
(24). Appreciating that ⋅NO reacts at diffusion-limited rates
with O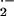 and lipid peroxyl radicals
(LOO⋅) to produce secondary products such as peroxynitrite
(ONOO−) and nitrated lipids
[LNO2, L(O)NO2] (25–27),
it is proposed that the impaired vascular function and inflammatory
activation of SCD vessels could be a consequence of oxygen
radical-dependent consumption of ⋅NO and production of secondary
reactive species (e.g.,
H2O2 or
ONOO−) that can also impair vascular function.
In support of this precept, a combination of clinical and
knockout-transgenic SCD mouse studies are reported herein that show
increased rates of xanthine oxidase (XO)-dependent vessel wall
production of ⋅NO-inactivating O
and lipid peroxyl radicals
(LOO⋅) to produce secondary products such as peroxynitrite
(ONOO−) and nitrated lipids
[LNO2, L(O)NO2] (25–27),
it is proposed that the impaired vascular function and inflammatory
activation of SCD vessels could be a consequence of oxygen
radical-dependent consumption of ⋅NO and production of secondary
reactive species (e.g.,
H2O2 or
ONOO−) that can also impair vascular function.
In support of this precept, a combination of clinical and
knockout-transgenic SCD mouse studies are reported herein that show
increased rates of xanthine oxidase (XO)-dependent vessel wall
production of ⋅NO-inactivating O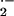 in
SCD. The increased rates of vessel wall oxidant production caused
impairment of ⋅NO-dependent vascular relaxation in SCD mouse
vessels that were corrected by a catalytic metalloporphyrin
superoxide dismutase (SOD) mimetic. Finally, multiple lines of evidence
showed that the vessel wall, not red cells, was the primary source of
⋅NO-consuming free radical species in SCD.
in
SCD. The increased rates of vessel wall oxidant production caused
impairment of ⋅NO-dependent vascular relaxation in SCD mouse
vessels that were corrected by a catalytic metalloporphyrin
superoxide dismutase (SOD) mimetic. Finally, multiple lines of evidence
showed that the vessel wall, not red cells, was the primary source of
⋅NO-consuming free radical species in SCD.
Materials and Methods
Erythrocyte Superoxide, Hydrogen Peroxide, and Lipid Hydroperoxide Production.
Blood was collected from healthy HbA adult volunteers and
homozygous HbS patients in anticoagulated (EDTA) vacutainers as
approved by the Institutional Review Board for Human Use at the
University of Alabama at Birmingham. All individuals were evaluated for
cytochrome b5 reductase and
glucose-6-phosphate dehydrogenase activity, and none were reported
deficient. After centrifugation, plasma and buffy coat was discarded,
and cells were washed and filtered through a cellulose column (Sigma,
type 50 and α cellulose) to remove neutrophils and platelets. Packed
RBCs were diluted to a hematocrit of 2.5% (vol/vol) hemoglobin
concentration determined with Drabkin's reagent at 540 nm (28), and
rates of O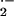 release over 2 h were
quantified spectrophotometrically by CuZn SOD-inhibitable (100 units
ml−1, equivalent to ≈33 μg
ml−1 SOD) reduction of cytochrome c
(50 μM) at 550 nm (ɛM = 21
mM−1⋅cm−1). In some
experiments, O
release over 2 h were
quantified spectrophotometrically by CuZn SOD-inhibitable (100 units
ml−1, equivalent to ≈33 μg
ml−1 SOD) reduction of cytochrome c
(50 μM) at 550 nm (ɛM = 21
mM−1⋅cm−1). In some
experiments, O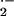 release was measured in cells
pretreated with 2,3-dimethoxy-1-napthoquinone (DMNQ; Oxis, 100 μM),
3hydroxy-1,2-dimethyl-4-pyridone (Aldrich, 0.5 mM), and
4,4-diisothiocyano-2,2 disulfonic acid stilbene (Sigma, 200 μM).
Possible Hb interference in determination of rates of cytochrome
c reduction was evaluated by performing a singular value
decomposition analysis (Matlab, Mathworks, Natick, MA). Intracellular
H2O2 concentrations were
calculated from aminotriazole (AT)-mediated inactivation of catalase
activity as described (29). Red cells were incubated with 10 mM AT at
37°C, and intracellular catalase activity was determined at 1-h
intervals for 4 h. Catalase activity was measured
spectrophotometrically based on the consumption of 10 mM
H2O2 at 240 nm
(ɛM = 43.6
M−1⋅cm−1). For
determining the extent of membrane lipid oxidation, packed RBCs were
lysed in hypotonic phosphate buffer (20 mosM, pH 7.4, 4°C),
centrifuged at 30,000 × g for 20 min, and the
supernatant was discarded. Membrane ghosts were washed eight times to
minimize Hb contamination and stored at −20°C. For use as a stimulus
of lipid oxidation, ONOO− was synthesized as
described (25), and its concentration was determined
spectrophotometrically at 302 nm (ɛM = 1670
M−1⋅cm−1). Lipid
hydroperoxide (LOOH) content was measured via N-benzoyl
leucomethylene blue oxidation and quantitated against a
t-butyl hydroperoxide standard. Protein concentrations were
measured at 595 nm by a modified Bradford assay by using
Coomassie Plus reagent with BSA as a standard.
release was measured in cells
pretreated with 2,3-dimethoxy-1-napthoquinone (DMNQ; Oxis, 100 μM),
3hydroxy-1,2-dimethyl-4-pyridone (Aldrich, 0.5 mM), and
4,4-diisothiocyano-2,2 disulfonic acid stilbene (Sigma, 200 μM).
Possible Hb interference in determination of rates of cytochrome
c reduction was evaluated by performing a singular value
decomposition analysis (Matlab, Mathworks, Natick, MA). Intracellular
H2O2 concentrations were
calculated from aminotriazole (AT)-mediated inactivation of catalase
activity as described (29). Red cells were incubated with 10 mM AT at
37°C, and intracellular catalase activity was determined at 1-h
intervals for 4 h. Catalase activity was measured
spectrophotometrically based on the consumption of 10 mM
H2O2 at 240 nm
(ɛM = 43.6
M−1⋅cm−1). For
determining the extent of membrane lipid oxidation, packed RBCs were
lysed in hypotonic phosphate buffer (20 mosM, pH 7.4, 4°C),
centrifuged at 30,000 × g for 20 min, and the
supernatant was discarded. Membrane ghosts were washed eight times to
minimize Hb contamination and stored at −20°C. For use as a stimulus
of lipid oxidation, ONOO− was synthesized as
described (25), and its concentration was determined
spectrophotometrically at 302 nm (ɛM = 1670
M−1⋅cm−1). Lipid
hydroperoxide (LOOH) content was measured via N-benzoyl
leucomethylene blue oxidation and quantitated against a
t-butyl hydroperoxide standard. Protein concentrations were
measured at 595 nm by a modified Bradford assay by using
Coomassie Plus reagent with BSA as a standard.
NO Consumption.
Anaerobic solutions of 1.9 mM ⋅NO were prepared by equilibrating ⋅NO gas (Matheson) in argon-saturated deionized water. Contaminating nitrogen dioxide (NO2) or nitrous oxide (N2O) was removed by first passing the NO gas through 5 M NaOH. NO (2.5–15 μM) was added to diluted RBCs (Hb = 3 × 10−5 g/ml−1; 0.5 μM), and rates of ⋅NO consumption were measured electrochemically (Iso-NO, WPI Instruments, Waltham, MA) under normoxic and hypoxic (20–40 mmHg O2 tension) conditions in a closed, thermally regulated (37°C) and stirred polarographic cell. Hypoxic conditions were established in the reaction chamber by partially equilibrating buffers with nitrogen gas and quantified by using a Clark model YSI 5300 oxygen electrode (Yellow Springs Instruments).
XO and Alanine Aminotransferase Analysis.
All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham. Plasma and tissue XO activity was measured by reversed-phase HPLC with electrochemical detection of uric acid as described (30). For biochemical analysis of liver tissue, isolated livers were weighed and homogenized in ice-cold homogenizing buffer (50 mM K2HPO4/80 μM leupeptin/2.1 mM Pefabloc SC/1 mM PMSF/1 μg ml−1 aprotinin, pH 7.4). Homogenates were centrifuged (40,000 × g, 30 min, 4°C), and supernatants were stored at −80°C. Plasma alanine aminotransferase activity was measured on an automated spectrofluorometer (Cobas-Fara II, Roche Diagnostic Systems). A rabbit polyclonal antibody against recombinant human xanthine oxidoreductase (XOR) fragment was used (1:1,000 dilution) for immunoblot analysis (32). Horseradish peroxidase-conjugated goat anti-rabbit IgG (1:10,000) was used as a secondary antibody, and immunoreactive proteins were visualized by enhanced chemiluminescence (SuperSignal West Pico Substrate, Pierce).
Fluorescence Microscopy.
Frozen aortic sections and paraffin-embedded liver sections from C57BL/6J or knockout-transgenic SCD mouse were processed for immunofluorescence. Primary antibody incubations were carried out for 60 min at 25°C by using a rabbit polyclonal antibody against XO (1:50). The secondary antibody was Alexa-594 conjugated goat anti-rabbit (1:100, Molecular Probes). For control studies, the primary anti-XO was preabsorbed with excess (1 unit ml−1) bovine XO. Nuclei were counterstained with Hoechst 33258 (20 μg ml−1, Sigma). Images were acquired through a Leitz Orthoplan microscope and analyzed with ip lab spectrum software (Scanalytics, Billerica, MA).
Vessel Relaxation and Superoxide Production.
Isometric tension was measured in aortic segments from mice as
described (33). Aortic segments were exposed in some cases to various
mediators and inhibitors for 1 h, submaximally contracted with
phenylephrine (10−8–10−7
M), and acetylcholine (ACh; 1 × 10−9 to
3 × 10−6 M) was added in a cumulative
manner to induce relaxation. Vessel O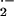 production was monitored in aortic segments by coelenterazine-enhanced
chemiluminescence (34) by using an automated microplate luminescence
reader (Lumistar Galaxy, BMG Lab Technologies). Previous studies have
shown coelenterazine to reflect both tissue O
production was monitored in aortic segments by coelenterazine-enhanced
chemiluminescence (34) by using an automated microplate luminescence
reader (Lumistar Galaxy, BMG Lab Technologies). Previous studies have
shown coelenterazine to reflect both tissue O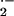 and ONOO− production, differentiable by
selective use of SOD and NO synthase inhibitors (34). Isolated vessels
were equilibrated in Hanks' balanced salt solution (HBSS) for 30 min
at 37°C, and coelenterazine (10 μM, Calbiochem) was added. In some
experiments, O
and ONOO− production, differentiable by
selective use of SOD and NO synthase inhibitors (34). Isolated vessels
were equilibrated in Hanks' balanced salt solution (HBSS) for 30 min
at 37°C, and coelenterazine (10 μM, Calbiochem) was added. In some
experiments, O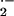 was measured in vessels that
were pretreated with xanthine (50 μM, Sigma), allopurinol (100 μM,
Sigma), CuZn SOD (30 units ml−1, Oxis),
manganese
5,10,15,20-tetrakis(N-ethylpyridinium-2-yl)-porphyrin
(MnTE-2-PyP; 50 μM), catalase (20 units ml−1,
Worthington), BOF-4272
[sodium-8-(3-methoxy-4-phenylsulfinylphenyl)pyrazolol(1,5α)-1,3,5-triazine-4-olate-monohydrate]
a gift from Otsuka Pharmaceutical, Tokushima, Japan (25 μM),
and DMNQ (100 μM). The assay system was calibrated by using 0.1–0.5
mU XO plus 50 μM xanthine, generating known rates of
O
was measured in vessels that
were pretreated with xanthine (50 μM, Sigma), allopurinol (100 μM,
Sigma), CuZn SOD (30 units ml−1, Oxis),
manganese
5,10,15,20-tetrakis(N-ethylpyridinium-2-yl)-porphyrin
(MnTE-2-PyP; 50 μM), catalase (20 units ml−1,
Worthington), BOF-4272
[sodium-8-(3-methoxy-4-phenylsulfinylphenyl)pyrazolol(1,5α)-1,3,5-triazine-4-olate-monohydrate]
a gift from Otsuka Pharmaceutical, Tokushima, Japan (25 μM),
and DMNQ (100 μM). The assay system was calibrated by using 0.1–0.5
mU XO plus 50 μM xanthine, generating known rates of
O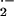 -dependent cytochrome c reduction.
-dependent cytochrome c reduction.
Results
Red Cell Production of Reactive Oxygen Species.
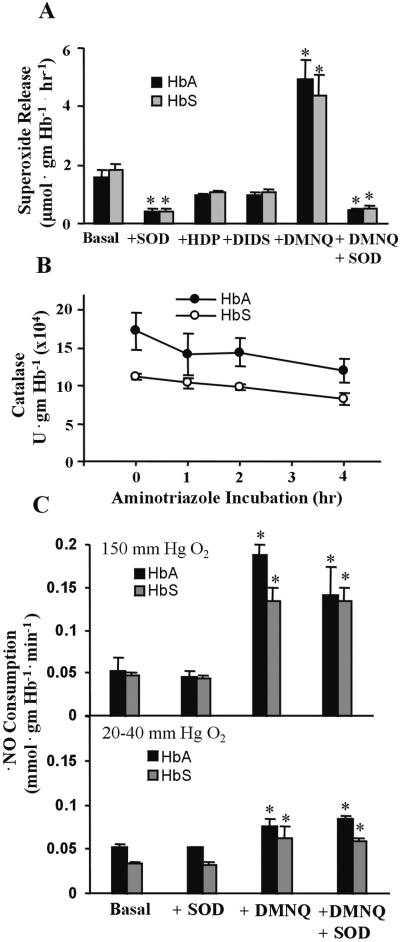
Endogenous rates of O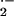 release under
normoxic (150 mmHg O2, pH 7.4) conditions were
not significantly different in human HbA (1.60 ± 0.25 μmol
gmHb−1⋅h−1) vs.
homozygous HbS (1.84 ± 0.2 μmol
gmHb−1⋅h−1) red
cells (Fig. 1A). The mean Hb
content of HbA and HbS red cell preparations was 0.36 and 0.39 gmHb
ml−1 packed cells, respectively. Potential
interference of lysed cell-derived Hb in the analysis of cytochrome
c reduction was ruled out by singular value decomposition
analysis, which separated the spectral components of the reaction
system and identified the elements contributing to the recorded
absorbance. The singular value decomposition analysis of red
cell-dependent cytochrome c reduction showed a
time-dependent increase at 550 nm in the absence of SOD, with only one
significant spectral component, reduced cytochrome c (not
shown). To elucidate the nature of O
release under
normoxic (150 mmHg O2, pH 7.4) conditions were
not significantly different in human HbA (1.60 ± 0.25 μmol
gmHb−1⋅h−1) vs.
homozygous HbS (1.84 ± 0.2 μmol
gmHb−1⋅h−1) red
cells (Fig. 1A). The mean Hb
content of HbA and HbS red cell preparations was 0.36 and 0.39 gmHb
ml−1 packed cells, respectively. Potential
interference of lysed cell-derived Hb in the analysis of cytochrome
c reduction was ruled out by singular value decomposition
analysis, which separated the spectral components of the reaction
system and identified the elements contributing to the recorded
absorbance. The singular value decomposition analysis of red
cell-dependent cytochrome c reduction showed a
time-dependent increase at 550 nm in the absence of SOD, with only one
significant spectral component, reduced cytochrome c (not
shown). To elucidate the nature of O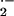 release
and verify the specificity of the assay system, DMNQ (100 μM) was
added to stimulate red cell O
release
and verify the specificity of the assay system, DMNQ (100 μM) was
added to stimulate red cell O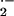 production.
Preincubation of cells with the metal chelator
3-hydroxy-1,2-dimethyl-4-pyridone (0.5 mM) induced ≈36% decrease in
O
production.
Preincubation of cells with the metal chelator
3-hydroxy-1,2-dimethyl-4-pyridone (0.5 mM) induced ≈36% decrease in
O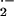 , suggesting that cellular Fe-dependent
reactions partially contributed to cell O
, suggesting that cellular Fe-dependent
reactions partially contributed to cell O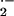 production. When cells were pretreated with a stilbene sulfonate
chloride-bicarbonate exchange protein inhibitor (4,4-diisothiocyano-2,2
disulfonic acid stilbene, 200 μM), both HbA and HbS red cells showed
≈35% decrease in rates of cytochrome c reduction,
indicating that some extracellular O
production. When cells were pretreated with a stilbene sulfonate
chloride-bicarbonate exchange protein inhibitor (4,4-diisothiocyano-2,2
disulfonic acid stilbene, 200 μM), both HbA and HbS red cells showed
≈35% decrease in rates of cytochrome c reduction,
indicating that some extracellular O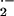 was
released through anion channels.
was
released through anion channels.
Figure 1.
Red cell production of reactive oxygen species. (A) Rates of superoxide release by HbA and HbS red cells. Values are mean ± SEM (n = 3–9). Statistical analysis was by two-way ANOVA with the Tukey post hoc test. *, P < 0.05. (B) Aminotriazole-mediated catalase inactivation by HbA and HbS red cells. Values at each time point represent mean ± SD with n = 5. (C) Rates of HbA and HbS red cell NO consumption. Values represent mean ± SEM (n = 4–14). Statistical analysis was by two-way ANOVA with the Tukey post hoc test. *, P < 0.05 compared with basal.
The similar slopes of the time course of aminotriazole-dependent red
cell catalase inactivation in HbA and HbS red cells revealed that
steady-state H2O2 levels
were not significantly different, with calculated
H2O2 concentrations of
3.11 ± 2.61 pM and 4.9 ± 2.25 pM, respectively, at 150 mmHg
O2, pH 7.4 (Fig. 1B). This occurred
despite the 36% lower catalase specific activity in HbS red cells,
suggesting other compensatory mechanisms for intracellular
H2O2 scavenging, e.g.,
glutathione peroxidase. Accepting that ⋅NO reacts at almost
diffusion-limited rates with O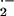 (25), the
relative rates of red cell ⋅NO consumption was determined as
probe for differences in intracellular and extracellular
O
(25), the
relative rates of red cell ⋅NO consumption was determined as
probe for differences in intracellular and extracellular
O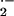 production by HbA and HbS red cells. NO
consumption, measured during both normoxic (150 mmHg
O2) and sickling-inducing hypoxic (20–40 mmHg
O2) conditions, was not significantly different
in HbA vs. HbS red cells, with normoxic values of 0.053 ± 0.016
and 0.047 ± 0.003 mmol
gmHb−1⋅min−1,
respectively (Fig. 1C). Addition of extracellular CuZn SOD
(100 units ml−1) did not impact the rate of
⋅NO consumption, whereas stimulation of cell
O
production by HbA and HbS red cells. NO
consumption, measured during both normoxic (150 mmHg
O2) and sickling-inducing hypoxic (20–40 mmHg
O2) conditions, was not significantly different
in HbA vs. HbS red cells, with normoxic values of 0.053 ± 0.016
and 0.047 ± 0.003 mmol
gmHb−1⋅min−1,
respectively (Fig. 1C). Addition of extracellular CuZn SOD
(100 units ml−1) did not impact the rate of
⋅NO consumption, whereas stimulation of cell
O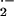 generation by DMNQ (100 μM) added as a
positive control significantly increased rates of red cell ⋅NO
consumption. The presence of endogenous LOOH was
undetectable in both HbA and HbS RBC membranes. When membrane oxidation
was stimulated by the addition of OONO−, HbS red
cell membranes showed greater tendency to undergo lipid peroxidation
(not shown).
generation by DMNQ (100 μM) added as a
positive control significantly increased rates of red cell ⋅NO
consumption. The presence of endogenous LOOH was
undetectable in both HbA and HbS RBC membranes. When membrane oxidation
was stimulated by the addition of OONO−, HbS red
cell membranes showed greater tendency to undergo lipid peroxidation
(not shown).
Plasma XO and Alanine Aminotransferase Activities.
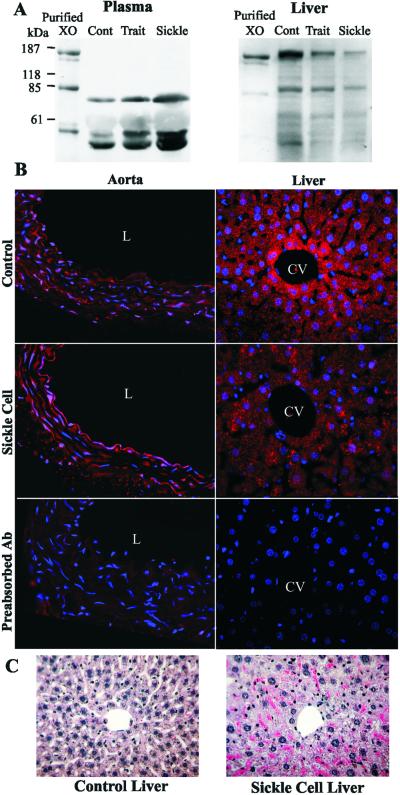
The catalytic activity of XO was significantly increased in the plasma of SCD patients vs. controls (Table 1). This also occurred in the plasma of a knockout-transgenic mouse model of SCD that synthesizes exclusively human Hb in the murine RBCs (31). The observed increase in plasma XO activity in the knockout-transgenic SCD mouse was accompanied by a decrease in liver XO activity and an increase in plasma alanine aminotransferase activity (Table 1). Western blot analysis of plasma and liver XOR revealed increased plasma and decreased liver XOR protein content in knockout-transgenic SCD mice compared with control or knockout-transgenic sickle cell trait mice, which synthesize both human βS and βA (Fig. 2A). XOR, which is rapidly converted to the oxidase form (XO) in plasma, revealed immunoreactive 20-kDa, 40-kDa, and 85-kDa proteolytic fragments upon Western blot analysis. Knockout-transgenic sickle cell trait (heterozygous for HbS) mice were also not significantly different from control C57BL/6J mice in plasma and liver XO and plasma alanine aminotransferase activities (not shown). Immunocytochemical localization of XO in aorta and liver (Fig. 2B) of SCD mice showed increased vessel wall and decreased liver XO immunoreactivity, with XO concentrated on and in vascular luminal cells. Hematoxylin and eosin staining of liver of knockout-transgenic SCD mice revealed extensive hepatocellular injury associated with pericentral necrosis. Sickled erythrocytes were also observed in intrahepatic sinusoids (Fig. 2C) (31).
Table 1.
Activity of XO and ALT in sickle cell disease
| Measurement | Enzyme
activity
|
|
|---|---|---|
| Control | SCD* | |
| Human | ||
| Plasma XO (μU/ml) | 0.89 ± 0.3 (15) | 3.30 ± 0.9 (18) |
| Mouse | ||
| Plasma XO (mU/ml) | 2.2 ± 0.26 (13) | 5.6 ± 1.5 (15) |
| ALT (mU/ml) | 24.2 ± 2.3 (10) | 270.5 ± 24.5 (12) |
| Liver XO (mU/g tissue) | 53.9 ± 7.8 (6) | 18.6 ± 4.4 (6) |
| Aortic XO (mU/mg protein) | 0.29 ± 0.01 (3) | 0.46 ± 0.03 (4) |
, P < 0.05 from control. n for each measurement is italicized in parentheses.
Figure 2.
Immunocytochemical analysis of XOR in C57BL/6J control and sickle cell mouse tissues. (A) Western blot analysis of plasma and liver XO in SCD mice. (B) Descending thoracic aortic segments from knockout-transgenic SC mice display intense immunofluorescent staining for XO (red) that is associated with the endothelium and, to a lesser extent, smooth muscle cells (L, lumen). Liver sections from SC mice show decreased XOR staining in the pericentral hepatocytes when compared with controls (CV, central vein). Nuclei were counterstained with Hoechst in all experiments. (C) Hematoxylin and eosin staining of liver sections from control and sickle cell mouse tissues.
Superoxide Production and Endothelial-Dependent Relaxation of Vessels.
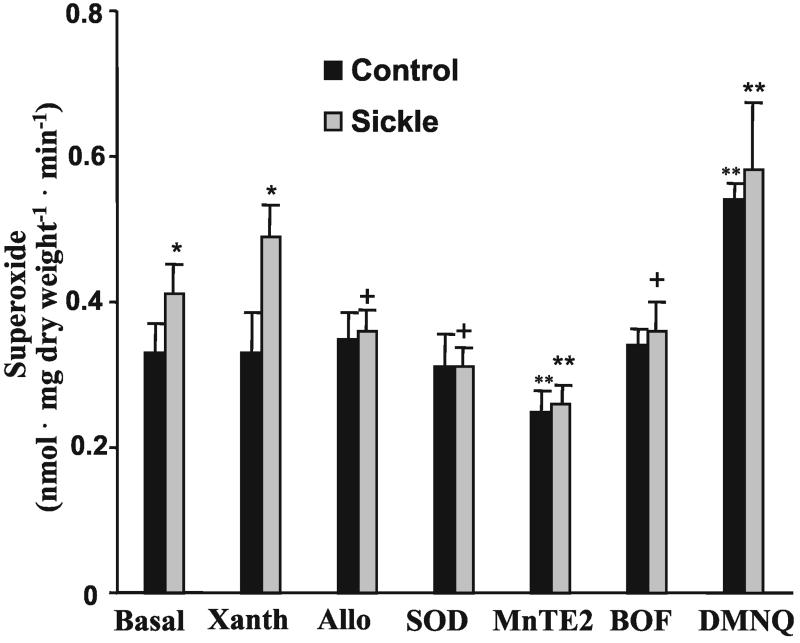
The catalytic activity of XO was significantly increased in the aorta
of SCD mice (Table 1) with a parallel increase in XOR protein observed
by Western blot analysis as well (not shown). Basal rates of
O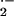 production were significantly increased in
the aorta of SCD mice vs. controls, with rates of 0.41 ± 0.04 and
0.33 ± 0.04 nmol⋅mg dry
weight−1⋅min−1,
respectively (Fig. 3). In SCD, but not
wild-type mouse vessels, rates of O
production were significantly increased in
the aorta of SCD mice vs. controls, with rates of 0.41 ± 0.04 and
0.33 ± 0.04 nmol⋅mg dry
weight−1⋅min−1,
respectively (Fig. 3). In SCD, but not
wild-type mouse vessels, rates of O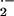 production
were enhanced by addition of xanthine and returned to basal rates when
vessels were pretreated with CuZn SOD (30 units
ml−1), allopurinol (100 μM), or the XO
inhibitor BOF-4272 (25 μM). Pretreatment of aorta with the SOD
mimetic MnTE-2-PyP (50 μM) significantly decreased rates of
detectable O
production
were enhanced by addition of xanthine and returned to basal rates when
vessels were pretreated with CuZn SOD (30 units
ml−1), allopurinol (100 μM), or the XO
inhibitor BOF-4272 (25 μM). Pretreatment of aorta with the SOD
mimetic MnTE-2-PyP (50 μM) significantly decreased rates of
detectable O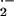 production, whereas DMNQ (100
μM) addition significantly enhanced rates of
O
production, whereas DMNQ (100
μM) addition significantly enhanced rates of
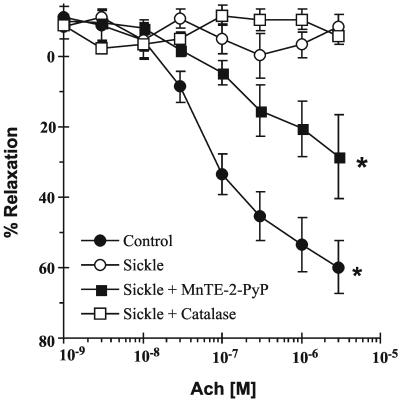
O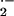 production by both control and SCD mouse
vessels. NO-dependent vessel relaxation, elicited by ACh,
was severely impaired in SCD mice (Fig.
4). The ⋅NO-dependent stimulus of
smooth muscle cell guanylate cyclase activity, sodium nitroprusside
(100 nM), induced maximal vessel relaxation in SCD mouse vessels,
whereas addition of arginine (3 mM) had no effect (not shown).
Treatment of SCD mouse vessels with the SOD mimetic MnTE-2-PyP (10
μM) significantly restored ACh-dependent relaxation, whereas addition
of catalase had no effect (Fig. 4).
production by both control and SCD mouse
vessels. NO-dependent vessel relaxation, elicited by ACh,
was severely impaired in SCD mice (Fig.
4). The ⋅NO-dependent stimulus of
smooth muscle cell guanylate cyclase activity, sodium nitroprusside
(100 nM), induced maximal vessel relaxation in SCD mouse vessels,
whereas addition of arginine (3 mM) had no effect (not shown).
Treatment of SCD mouse vessels with the SOD mimetic MnTE-2-PyP (10
μM) significantly restored ACh-dependent relaxation, whereas addition
of catalase had no effect (Fig. 4).
Figure 3.
Superoxide production by C57BL/6J control and sickle cell mouse vessels. Values represent mean ± SD (n = 4). Statistical analysis was by two-way ANOVA with the Duncan's post hoc test. *, P < 0.05 compared with control. +, P < 0.05 compared with xanthine-treated sickle cell vessels. **, P < 0.05 compared with control and all treated vessel groups.
Figure 4.
ACh-dependent vascular relaxation in C57BL/6J control and sickle cell mouse vessels. Values represent mean ± SEM (n = 4–8). All statistical analyses were by one-way ANOVA with Student–Newman Keuls pairwise multiple comparison. *, P < 0.05 compared with sickle cell mouse vessel response.
Discussion
Insight into the mechanisms underlying impaired vascular
function in SCD is important for guiding the design of more effective
therapies to limit vaso-occlusive crisis, acute chest syndrome, stroke,
and other circulatory manifestations of this hemoglobinopathy. In
contrast to previous reports (22, 23), there is not a significant
difference in rates of O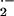 and
H2O2 production and basal
levels of membrane LOOH content in HbA vs. HbS red cells (Fig. 1
A and B). Also, HbA vs. HbS red cells display
similar rates of ⋅NO consumption under both normoxic and
sickling-inducing hypoxic conditions (Fig. 1C). This rules
out the possibility that unique oxidative or free radical metabolic
properties of HbS red cells contribute to enhanced rates of vascular
⋅NO scavenging and in turn initiate pathogenic signal
transduction events in SCD vessels, due to suppression of the
anti-inflammatory properties of ⋅NO. Although the inherent
instability of sickle Hb and the possible impairment of tissue free
radical defense mechanisms in SCD may render red cells more susceptible
to oxidative damage (23, 35), a well-integrated network of oxidant
defense mechanisms evidently prevents HbS red cells from becoming
significant loci of reactive species production and oxygen
radical-dependent inactivation of vascular ⋅NO signaling.
and
H2O2 production and basal
levels of membrane LOOH content in HbA vs. HbS red cells (Fig. 1
A and B). Also, HbA vs. HbS red cells display
similar rates of ⋅NO consumption under both normoxic and
sickling-inducing hypoxic conditions (Fig. 1C). This rules
out the possibility that unique oxidative or free radical metabolic
properties of HbS red cells contribute to enhanced rates of vascular
⋅NO scavenging and in turn initiate pathogenic signal
transduction events in SCD vessels, due to suppression of the
anti-inflammatory properties of ⋅NO. Although the inherent
instability of sickle Hb and the possible impairment of tissue free
radical defense mechanisms in SCD may render red cells more susceptible
to oxidative damage (23, 35), a well-integrated network of oxidant
defense mechanisms evidently prevents HbS red cells from becoming
significant loci of reactive species production and oxygen
radical-dependent inactivation of vascular ⋅NO signaling.
The observation that XO activity is elevated in the plasma of SCD patients is recapitulated in the SCD mouse (Table 1), suggesting that this enzyme may serve as a significant source of reactive oxygen species production in SCD. There is only trace XO activity in the plasma and vascular endothelium of healthy humans (36, 37). During pathological conditions such as hepatocellular damage (38, 39), ischemia-reperfusion (40), atherosclerosis (41), adult respiratory distress syndrome (36), alcoholic liver disease (42), and now SCD, in which organs replete in XOR can release this proinflammatory enzyme into the circulation, plasma XO levels increase. This can lead to an enhancement of vascular oxidant production, often in tissue compartments having minimal defense against oxidative injury.
XOR is found mainly in the splanchnic system, where it exists
predominantly as a dehydrogenase (XDH/XO; EC 1.1.1.204/1.1.3.22)
(43). The hepatic localization of XOR is zonally distributed, with
higher activity in pericentral, compared with periportal, hepatocytes
(44). The zonal distribution of XOR corresponds to regions where
ischemia and hypoxia would be most severe, as oxygen is extracted as
blood flows from the portal triad to the central vein branches. During
ischemia, XOR can be converted to XO by either proteolytic cleavage of
the amino terminus or more rapidly by intramolecular and mixed
disulfide formation (45, 46). Significant irreversible proteolytic
conversion of cellular XOR to XO is slow, requiring 4–6 h of ischemia
(47). However, XOR can be released from ischemic hepatocytes into the
circulation before detectable intracellular XOR to XO conversion (48),
whereupon either proteolytic cleavage or thiol oxidation may occur
rapidly. Repeated interruption of blood flow and the resultant
transient ischemia associated with SCD can thus provide the basis for
ischemic liver injury and elevated plasma XO levels. During
experimental hypoxia, XOR to XO conversion has been shown to be
significantly greater in the liver and kidneys of a sickle cell mice
model that had a mixture of human HbS and murine Hb (24). In the
knockout-transgenic mouse model of SCD used herein, extensive
hepatocellular injury (Fig. 2C), accompanied by increased
plasma alanine aminotransferase levels (Table 1) and decreased liver XO
immunoreactivity (Fig. 2B) and catalytic activity
(Table 1), was observed even under normoxic conditions. Even though
rodents have much greater XOR-specific activities in plasma than humans
(49), release of this enzyme from XOR-replete human splanchnic tissues
can still have potent pathophysiologic effects. Present evidence
supports that not only the extent of XO-dependent tissue
O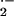 production, but also its anatomic location,
will determine whether or not vascular ⋅NO-dependent signaling
is adversely affected (50).
production, but also its anatomic location,
will determine whether or not vascular ⋅NO-dependent signaling
is adversely affected (50).
The immunohistochemical localization of increased XO in the vessel wall of SCD mice could be a manifestation of either the binding and uptake of circulating XO by vascular endothelial cells (33, 50, 51) or the increased expression of XOR by activated vascular endothelium (52). Considering the high association constant of XO for binding to vascular endothelium (Kd = 6 nM; ref. 53), the presence of XO in the systemic circulation of both SCD mice and humans with SCD implies that a significant deposition of XO can occur in the vessel wall, even when considering the typically low expression of vascular cell XOR in humans (53).
The ability of endothelial-bound XO to remain catalytically active and
generate O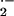 and
H2O2 at anatomic sites
remote from the locus of XO release (50) is consistent with the
increased basal rates of O
and
H2O2 at anatomic sites
remote from the locus of XO release (50) is consistent with the
increased basal rates of O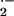 production
quantified in SCD mouse aorta (Fig. 3). Addition of xanthine enhanced
rates of O
production
quantified in SCD mouse aorta (Fig. 3). Addition of xanthine enhanced
rates of O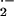 release that were normalized with
the XO inhibitors allopurinol and BOF-4272, confirming the XO-mediated
increase of vascular O
release that were normalized with
the XO inhibitors allopurinol and BOF-4272, confirming the XO-mediated
increase of vascular O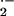 and
H2O2 production in sickle
vessels (Fig. 3). The preincubation of control and SCD mouse vessels
with CuZn SOD caused a 10% and 32% reduction in rates of detectable
O
and
H2O2 production in sickle
vessels (Fig. 3). The preincubation of control and SCD mouse vessels
with CuZn SOD caused a 10% and 32% reduction in rates of detectable
O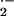 , respectively. The modest inhibition of
detectable rates of O
, respectively. The modest inhibition of
detectable rates of O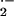 generation by CuZn SOD
supports the concept that size exclusion and charge repulsion of this
enzyme from the intracellular milieu impedes its ability to scavenge
O
generation by CuZn SOD
supports the concept that size exclusion and charge repulsion of this
enzyme from the intracellular milieu impedes its ability to scavenge
O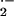 generated by cell-associated XO (50).
Pretreatment of aortic vessels with the more cell-avid and
membrane-permeable SOD mimetic MnTE-2-PyP (54, 55) had a greater impact
on scavenging O
generated by cell-associated XO (50).
Pretreatment of aortic vessels with the more cell-avid and
membrane-permeable SOD mimetic MnTE-2-PyP (54, 55) had a greater impact
on scavenging O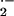 , giving a 33% and 50%
reduction in rates of O
, giving a 33% and 50%
reduction in rates of O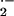 release in control and
sickle vessels, respectively.
release in control and
sickle vessels, respectively.
XO-derived O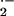 will react with ⋅NO
at diffusion-limited rates to impair ⋅NO signaling and
concomitantly yield secondary oxidizing species, such as
ONOO−, that can further propagate tissue injury
(25, 56, 57). A challenge for the future is the delineation of acute
versus chronic actions of XO on vascular function in SCD. Although no
restorative effects of catalase on vessel relaxation were observed
(Fig. 4), XO-derived H2O2
can serve as a peroxidase substrate (e.g., for neutrophil
myeloperoxidase) that in turn stimulates the catalytic consumption of
⋅NO and the formation of secondary oxidizing and nitrating
species, possibly leading to more chronic forms of vessel injury (58).
A precedent for these phenomena exists in animal models and clinical
studies of atherosclerosis. In a hypercholesterolemic rabbit model of
atherosclerosis, a 2.5-fold increase in plasma XO severely impairs
ACh-mediated relaxation of the thoracic aorta (33, 59). Heparin-induced
dissociation of XO from vessel wall binding sites and
allopurinol-mediated XO inhibition partially restores ACh-dependent
relaxation and decreases vessel O
will react with ⋅NO
at diffusion-limited rates to impair ⋅NO signaling and
concomitantly yield secondary oxidizing species, such as
ONOO−, that can further propagate tissue injury
(25, 56, 57). A challenge for the future is the delineation of acute
versus chronic actions of XO on vascular function in SCD. Although no
restorative effects of catalase on vessel relaxation were observed
(Fig. 4), XO-derived H2O2
can serve as a peroxidase substrate (e.g., for neutrophil
myeloperoxidase) that in turn stimulates the catalytic consumption of
⋅NO and the formation of secondary oxidizing and nitrating
species, possibly leading to more chronic forms of vessel injury (58).
A precedent for these phenomena exists in animal models and clinical
studies of atherosclerosis. In a hypercholesterolemic rabbit model of
atherosclerosis, a 2.5-fold increase in plasma XO severely impairs
ACh-mediated relaxation of the thoracic aorta (33, 59). Heparin-induced
dissociation of XO from vessel wall binding sites and
allopurinol-mediated XO inhibition partially restores ACh-dependent
relaxation and decreases vessel O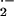 production
(33). In atherosclerotic and diabetic humans, the XO inhibitors
allopurinol and oxypurinol increase forearm blood flow and decrease
blood pressure (60, 61). Thus, increased circulating and vessel wall XO
in SCD can account for hemodynamic instability and contribute to the
pathogenesis of a broader systemic vascular dysfunction. This is
exemplified by the observation of impaired ⋅NO-dependent
vasorelaxation in SCD mice (Fig. 4) and affirms the blunted vascular
relaxation that occurs in SCD mouse vessels in response to a calcium
ionophore and the ⋅NO donor DEA-NONOate (62). This impaired
vessel relaxation was restored by denudation of endothelium (62),
supporting the presently observed generation of ⋅NO-inactivating
species by the endothelium of SCD vessels. Importantly, the inhibition
of vessel relaxation observed herein was completely restored by sodium
nitroprusside (not shown). Because sodium nitroprusside is metabolized
by smooth muscle cells (63), this excludes the existence of an
end-organ defect in ⋅NO signaling and further confirms that
⋅NO is being consumed by endothelial-derived free radical
reactions in SCD vessels. This impairment of ⋅NO-dependent
vascular relaxation by increased rates of reactive oxygen species
generation was underscored by the observation that the SOD mimetic
MnTE-2-PyP restored ACh-dependent relaxation of SCD mouse vessels.
production
(33). In atherosclerotic and diabetic humans, the XO inhibitors
allopurinol and oxypurinol increase forearm blood flow and decrease
blood pressure (60, 61). Thus, increased circulating and vessel wall XO
in SCD can account for hemodynamic instability and contribute to the
pathogenesis of a broader systemic vascular dysfunction. This is
exemplified by the observation of impaired ⋅NO-dependent
vasorelaxation in SCD mice (Fig. 4) and affirms the blunted vascular
relaxation that occurs in SCD mouse vessels in response to a calcium
ionophore and the ⋅NO donor DEA-NONOate (62). This impaired
vessel relaxation was restored by denudation of endothelium (62),
supporting the presently observed generation of ⋅NO-inactivating
species by the endothelium of SCD vessels. Importantly, the inhibition
of vessel relaxation observed herein was completely restored by sodium
nitroprusside (not shown). Because sodium nitroprusside is metabolized
by smooth muscle cells (63), this excludes the existence of an
end-organ defect in ⋅NO signaling and further confirms that
⋅NO is being consumed by endothelial-derived free radical
reactions in SCD vessels. This impairment of ⋅NO-dependent
vascular relaxation by increased rates of reactive oxygen species
generation was underscored by the observation that the SOD mimetic
MnTE-2-PyP restored ACh-dependent relaxation of SCD mouse vessels.
Plasma uric acid and XO levels are strongly predictive of hypertension in normotensive subjects (64). Although hypertension is not associated with SCD, sickle patients with high blood pressure have an increased risk of stroke and death (65). Systolic blood pressure is not increased in an SCD mouse model compared with the background strain (62); however, a 5-fold greater increase in systolic blood pressure is observed in SCD mice when the NO synthase inhibitor l-NAME is administered, again affirming the concept of enhanced ⋅NO consumption and impaired vascular ⋅NO signaling in SCD.
In summary, episodes of hypoxia-reoxygenation associated with SCD lead
to the release of XO into the circulation from hepatic cells replete in
the activity of this source of O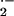 and
H2O2. Increased circulating
XO can then bind avidly to vessel luminal cells and impair vascular
function by creating an oxidative milieu and catalytically consuming
⋅NO via diffusion-limited reaction with
O
and
H2O2. Increased circulating
XO can then bind avidly to vessel luminal cells and impair vascular
function by creating an oxidative milieu and catalytically consuming
⋅NO via diffusion-limited reaction with
O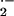 . Considering the critical role of
endothelial ⋅NO production in regulating endothelial adhesion
molecule expression, platelet aggregation, and both basal and
stress-mediated vasodilation, the O
. Considering the critical role of
endothelial ⋅NO production in regulating endothelial adhesion
molecule expression, platelet aggregation, and both basal and
stress-mediated vasodilation, the O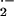 -mediated
reduction in ⋅NO bioavailability reported herein can
significantly contribute to the vascular disease that is the hallmark
of sickle cell anemia.
-mediated
reduction in ⋅NO bioavailability reported herein can
significantly contribute to the vascular disease that is the hallmark
of sickle cell anemia.
Acknowledgments
We appreciate insights and assistance provided by Denyse Thornley-Brown, MD, Elizabeth Lowenthal, MD, Phil Chumley, and Scott Sweeney. This work was supported by National Institutes of Health Grants RO1-HL64937, RO1-HL58115, and P6-HL58418 and by Aeolus, Inc.
Abbreviations
- ACh
acetylcholine
- DMNQ
2,3-dimethoxy-1-napthoquinone
- MnTE-2-PyP
manganese 5,10,15,20-tetrakis(N-ethylpyridinium-2-yl)-porphyrin
- SCD
sickle cell disease
- SOD
superoxide dismutase
- XO
xanthine oxidase
- XOR
xanthine oxidoreductase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Embury S H, Mohandas N, Paszty C, Cooper P, Cheung A T. J Clin Invest. 1999;103:915–920. doi: 10.1172/JCI5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belcher J D, Marker P H, Weber J P, Hebbel R P, Vercellotti G M. Blood. 2000;96:2451–2459. [PubMed] [Google Scholar]
- 3.Hebbel R P, Visser M R, Goodman J L, Jacob H S, Vercellotti G M. J Clin Invest. 1987;80:1503–1506. doi: 10.1172/JCI113233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiu Y T, Udden M M, McIntire L V. Blood. 2000;95:3232–3241. [PubMed] [Google Scholar]
- 5.Stuart M J, Setty B N. Blood. 1999;94:1555–1560. [PubMed] [Google Scholar]
- 6.Kaul D K, Hebbel R P. Clin Invest. 2000;106:411–420. doi: 10.1172/JCI9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solovey A A, Solovey A N, Harkness J, Hebbel R P. Blood. 2001;97:1937–1941. doi: 10.1182/blood.v97.7.1937. [DOI] [PubMed] [Google Scholar]
- 8.Solovey A, Gui L, Key N S, Hebbel R P. J Clin Invest. 1998;101:1899–1904. doi: 10.1172/JCI1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stockman J A, Nigro M A, Mishkin M M, Oski F A. N Engl J Med. 1972;287:846–849. doi: 10.1056/NEJM197210262871703. [DOI] [PubMed] [Google Scholar]
- 10.Merkel K H, Ginsberg P L, Parker J C, Jr, Post M J. Stroke. 1978;9:45–52. doi: 10.1161/01.str.9.1.45. [DOI] [PubMed] [Google Scholar]
- 11.Ballas S K, Larner J, Smith E D, Surrey S, Schwartz E, Rappaport E F. Blood. 1988;72:1216–1223. [PubMed] [Google Scholar]
- 12.Lande W M, Andrews D L, Clark M R, Braham N V, Black D M, Embury S H, Mentzer W C. Blood. 1988;72:2056–2059. [PubMed] [Google Scholar]
- 13.Johnson C S, Giorgio A J. Arch Intern Med. 1981;141:891–893. [PubMed] [Google Scholar]
- 14.Enwonwu C O. Med Sci Res. 1989;17:997–998. [Google Scholar]
- 15.Hatch F E, Crowe L R, Miles D E, Young J P, Portner M E. Am J Hypertens. 1989;2:2–8. doi: 10.1093/ajh/2.1.2. [DOI] [PubMed] [Google Scholar]
- 16.Allon M. Arch Intern Med. 1990;150:501–504. [PubMed] [Google Scholar]
- 17.Mantadakis E, Cavender J D, Rogers Z R, Ewalt D H, Buchanan G R. J Pediatr Hematol Oncol. 1999;21:518–522. [PubMed] [Google Scholar]
- 18.Rees D C, Cervi P, Grimwade D, O'Driscoll A, Hamilton M, Parker N E, Porter J B. Br J Haematol. 1995;91:834–837. doi: 10.1111/j.1365-2141.1995.tb05397.x. [DOI] [PubMed] [Google Scholar]
- 19.Morris C R, Kuypers F A, Larkin S, Sweeters N, Simon J, Vichinsky E P, Styles L A. Br J Haematol. 2000;111:498–500. doi: 10.1046/j.1365-2141.2000.02403.x. [DOI] [PubMed] [Google Scholar]
- 20.Atz A M, Wessel D L. Anesthesiology. 1997;87:988–990. doi: 10.1097/00000542-199710000-00037. [DOI] [PubMed] [Google Scholar]
- 21.Glover R E, Ivy E D, Orringer E P, Maeda H, Mason R P. Mol Pharmacol. 1999;55:1006–1010. doi: 10.1124/mol.55.6.1006. [DOI] [PubMed] [Google Scholar]
- 22.Hebbel R P, Eaton J W, Balasingam M, Steinberg M H. J Clin Invest. 1982;70:1253–1259. doi: 10.1172/JCI110724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuross S A, Hebbel R P. Blood. 1988;72:1278–1285. [PubMed] [Google Scholar]
- 24.Osarogiagbon U R, Choong S, Belcher J D, Vercellotti G M, Paller M S, Hebbel R P. Blood. 2000;1:314–320. [PubMed] [Google Scholar]
- 25.Beckman J S, Beckman T W, Chan J, Marshall P S, Freeman B A. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radi R, Beckman J S, Bush K M, Freeman B A. Arch Biochem Biophys. 1991;228:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 27.Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, Kirk M, Freeman B A. J Biol Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- 28.Beutler E R, editor. Cell Metabolism: A Manual of Biochemical Methods. 3rd Ed. Orlando, FL: Grune & Stratton; 1984. pp. 8–13. [Google Scholar]
- 29.Royall J A, Gwin P D, Parks D A, Freeman B A. Arch Biochem Biophys. 1992;294:686–694. doi: 10.1016/0003-9861(92)90742-f. [DOI] [PubMed] [Google Scholar]
- 30.Tan S, Radi R, Gaudier F, Evans R A, Rivera A, Kirk K A, Parks D A. Pediatr Res. 1993;34:303–307. doi: 10.1203/00006450-199309000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Ryan T M, Ciavatta D J, Townes T M. Science. 1997;278:873–876. doi: 10.1126/science.278.5339.873. [DOI] [PubMed] [Google Scholar]
- 32.Parks D. In: Reactive Oxygen Species in Biological Systems. Gilbert D L, Colton C, editors. New York: Plenum; 1998. pp. 397–420. [Google Scholar]
- 33.White R C, Darley-Usmar V, Berrington R W, McAdams M, Gore Z J, Thomson A J, Parks A D, Tarpey M M, Freeman B A. Proc Natl Acad Sci USA. 1996;93:8745–8749. doi: 10.1073/pnas.93.16.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarpey M M, White C R, Suarez E, Richardson G, Radi R, Freeman B A. Circ Res. 1999;84:1203–1211. doi: 10.1161/01.res.84.10.1203. [DOI] [PubMed] [Google Scholar]
- 35.Aslan M, Thornley-Brown D, Freeman B A. Ann NY Acad Sci. 2000;899:375–391. doi: 10.1111/j.1749-6632.2000.tb06201.x. [DOI] [PubMed] [Google Scholar]
- 36.Grum C M, Ragsdale R A, Ketai L H, Simon R H. J Crit Care. 1987;2:22–26. [Google Scholar]
- 37.Giler S, Sperling O, Brosh S, Urca I, De Vries A. Clin Chim Acta. 1975;63:37–40. doi: 10.1016/0009-8981(75)90375-7. [DOI] [PubMed] [Google Scholar]
- 38.Giler S, Sperling O, Brosh S, Urca I, De Vries A. Isr J Med Sci. 1975;11:1225. [PubMed] [Google Scholar]
- 39.Wolko K, Krawczynski J. Mater Med Pol. 1974;6:95–98. [PubMed] [Google Scholar]
- 40.Parks D A, Granger D N. Am J Physiol. 1983;245:G285–G289. doi: 10.1152/ajpgi.1983.245.2.G285. [DOI] [PubMed] [Google Scholar]
- 41.Mohacsi A, Kozlovszky B, Kiss I, Seres I, Fulop T., Jr Biochim Biophys Acta. 1996;1316:210–216. doi: 10.1016/0925-4439(96)00027-0. [DOI] [PubMed] [Google Scholar]
- 42.Parks D A, Skinner K A, Skinner H B, Tan S. Pathophysiology. 1998;5:49–66. [Google Scholar]
- 43.Kooij A, Schijns M, Frederiks W M, Van Noorden C J, James J. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;63:17–23. doi: 10.1007/BF02899240. [DOI] [PubMed] [Google Scholar]
- 44.Kooij A. Histochem J. 1994;26:889–915. [PubMed] [Google Scholar]
- 45.Enroth C, Eger B T, Okamoto K, Nishino T, Nishino T, Pai E F. Proc Natl Acad Sci USA. 2000;97:10723–10728. doi: 10.1073/pnas.97.20.10723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amaya Y, Yamazaki K, Sato M, Noda K, Nishino T. J Biol Chem. 1990;265:14170–14175. [PubMed] [Google Scholar]
- 47.Engerson T D, McKelvey T G, Rhyne D B, Boggio E B, Snyder S J, Jones H P. J Clin Invest. 1987;6:1564–1570. doi: 10.1172/JCI112990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yokoyama Y, Beckman J S, Beckman T K, Wheat J K, Cash T G, Freeman B A, Parks D A. Am J Physiol. 1990;258:G564–G570. doi: 10.1152/ajpgi.1990.258.4.G564. [DOI] [PubMed] [Google Scholar]
- 49.Al-Khalidi A S, Chaglassian T H. Biochem J. 1965;97:318–320. doi: 10.1042/bj0970318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Houston M, Estevez A, Chumley P, Aslan M, Marklund S, Parks D A, Freeman B. J Biol Chem. 1999;274:4985–4994. doi: 10.1074/jbc.274.8.4985. [DOI] [PubMed] [Google Scholar]
- 51.Radi R, Rubbo H, Bush K, Freeman B A. Arch Biochem Biophys. 1997;339:125–135. doi: 10.1006/abbi.1996.9844. [DOI] [PubMed] [Google Scholar]
- 52.Dupont G P, Huecksteadt T P, Marshall B C, Ryan U S, Michael J R, Hoidal J R. J Clin Invest. 1992;1:197–202. doi: 10.1172/JCI115563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paler-Martinez A, Panus P C, Chumley P H, Ryan U, Hardy M M, Freeman B A. Arch Biochem Biophys. 1994;311:79–85. doi: 10.1006/abbi.1994.1211. [DOI] [PubMed] [Google Scholar]
- 54.Patel M, Day B J. Trends Pharmacol Sci. 1999;9:359–364. doi: 10.1016/s0165-6147(99)01336-x. [DOI] [PubMed] [Google Scholar]
- 55.Spasojevic I, Batinic-Haberle I, Fridovich I. Nitric Oxide. 2000;5:526–533. doi: 10.1006/niox.2000.0303. [DOI] [PubMed] [Google Scholar]
- 56.Kissner R, Nauser T, Bugnon P, Lye P G, Koppenol W H. Chem Res Toxicol. 1997;10:1285–1292. doi: 10.1021/tx970160x. [DOI] [PubMed] [Google Scholar]
- 57.Villa L M, Salas E, Darley-Usmar V M, Radomski M W, Moncada S. Proc Natl Acad Sci USA. 1994;91:12383–12387. doi: 10.1073/pnas.91.26.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Vliet A, Eiserich J P, Halliwell B, Cross C E. J Biol Chem. 1997;272:7617–7625. doi: 10.1074/jbc.272.12.7617. [DOI] [PubMed] [Google Scholar]
- 59.White C R, Brock T A, Chang L Y, Crapo J, Briscoe P, Ku D, Bradley W A, Gianturco S H, Gore J, Freeman B A, Tarpey M M. Proc Natl Acad Sci USA. 1994;91:1044–1048. doi: 10.1073/pnas.91.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cardillo C, Kilcoyne C M, Cannon R O, III, Quyyumi A A, Panza J A. Hypertension. 1997;30:57–63. doi: 10.1161/01.hyp.30.1.57. [DOI] [PubMed] [Google Scholar]
- 61.Butler R, Morris A D, Belch J J, Hill A, Struthers A D. Hypertension. 2000;35:746–751. doi: 10.1161/01.hyp.35.3.746. [DOI] [PubMed] [Google Scholar]
- 62.Nath K A, Shah V, Haggard J J, Croatt A J, Smith L A, Hebbel R P, Katusic Z S. Am J Physiol. 2000;279:R1949–R1955. doi: 10.1152/ajpregu.2000.279.6.R1949. [DOI] [PubMed] [Google Scholar]
- 63.Kowaluk E A, Seth P, Fung H L. J Pharmacol Exp Ther. 1992;262:916–922. [PubMed] [Google Scholar]
- 64.Newaz M A, Adeeb N N, Muslim N, Razak T A, Htut N N. Clin Exp Hypertens. 1996;8:1035–1050. doi: 10.3109/10641969609081033. [DOI] [PubMed] [Google Scholar]
- 65.Pegelow C H, Colangelo L, Steinberg M, Wright E C, Smith J, Phillips G, Vichinsky E. Am J Med. 1997;2:171–177. doi: 10.1016/s0002-9343(96)00407-x. [DOI] [PubMed] [Google Scholar]