ABSTRACT
The governments of the United States and Canada have jointly undertaken the development of the Dietary Reference Intakes (DRIs) since the mid-1990s. The Federal DRI committees from each country work collaboratively to identify DRI needs, prioritize nutrient reviews, advance work to resolve methodological issues that is necessary for new reviews, and sponsor DRI-related committees through the National Academies of Sciences, Engineering and Medicine. In recent years, the Joint Canada-US DRI Working Group, consisting of members from both Federal DRI committees, developed an open and transparent nomination process for prioritizing nutrients for DRI review, by which sodium, the omega-3 (n–3) fatty acids, vitamin E, and magnesium were identified. In addition, discussions during the nutrient nomination process prompted the Federal DRI committees to address previously identified issues related to the use of chronic disease endpoints when setting DRIs. The development of guiding principles for setting DRIs based on chronic disease risk reduction will be applied for the first time during the DRI review of sodium and potassium. In summary, the US and Canadian governments have worked collaboratively to adapt our approach to prioritizing nutrients for DRI review and to broaden the scope of the DRIs to better incorporate the concept of chronic disease risk reduction in order to improve public health.
Keywords: Dietary Reference Intakes, reference values, nutrient requirements, nutrition policy
Brief History of the Dietary Reference Intakes Process
Prior to 1994, the key nutrient reference values were the Recommended Dietary Allowances (RDAs) in the United States and the Dietary Standards/Recommended Nutrient Intakes in Canada. In 1994, the Food and Nutrition Board of the Institute of Medicine (IOM) report “How Should the Recommended Dietary Allowances Be Revised?” proposed: 1) a new approach for the review of current reference intake values, where sufficient new knowledge is available; 2) the inclusion of the concept of chronic disease risk reduction when setting future reference intake values, where sufficient data for efficacy and safety exist; and 3) consideration of a new format for future reference intakes (1). The new format included 3 values: the Estimated Average Requirement (EAR), the RDA, and the Tolerable Upper Intake Level (UL) (Table 1) (2).
TABLE 1.
| DRIs | Definition |
|---|---|
| EAR | The average daily nutrient intake level that is estimated to meet the requirements of half of the healthy individuals in a particular life stage and gender group. |
| RDA | The average daily dietary nutrient intake level that is sufficient to meet the nutrient requirements of nearly all (97–98%) healthy individuals in a particular life stage and gender group. |
| UL | The highest average daily nutrient intake level that is likely to pose no risk of adverse health effects for almost all individuals in the general population. As intake increases above the UL, the potential risk of adverse effects may increase. |
| AI | The recommended average daily intake level based on observed or experimentally determined approximations or estimates of nutrient intake by a group (or groups) of apparently healthy people that are assumed to be adequate; used when an RDA cannot be determined. |
| AMDR | The range of intakes of an energy source that is associated with a reduced risk of chronic disease yet can provide adequate amounts of essential nutrients; expressed as a percentage of total energy intake. |
| EER | The average dietary energy intake that is predicted to maintain energy balance in a healthy individual of a defined age, gender, weight, height, and level of physical activity consistent with good health. In children and pregnant and lactating women, the EER also accounts for the needs associated with growth, the deposition of tissues, or the secretion of milk at rates consistent with good health. |
1AI, Adequate Intake; AMDR, Acceptable Macronutrient Distribution Range; DRI, Dietary Reference Intake; EAR, Estimated Average Requirement; EER, Estimated Energy Requirement; RDA, Recommended Dietary Allowance; UL, Tolerable Upper Intake Level.
Based on the 1994 recommendations, the United States and Canada cosponsored the development and publication of a harmonized set of nutrient-based Dietary Reference Intake (DRI) values. Six DRI reports were published between 1997 and 2005 by the IOM [now the Health and Medicine Division of the National Academies of Sciences, Engineering and Medicine (NASEM)] (3–8). The reports included DRIs for 29 vitamins and minerals, as well as for macronutrients and select components, such as specific fatty acids and amino acids, fiber, energy, and water. For some nutrients, the available evidence did not allow for an EAR and RDA to be set. As a result, additional reference values were devised to account for differences in available evidence: the Adequate Intake level, the Acceptable Macronutrient Distribution Range, and the Estimated Energy Requirement (Table 1) (2, 3, 8). During this period, the IOM also published a report on the development of the UL and 2 reports on the application of the DRIs (9–11).
Rationale for Development of the Nomination and Prioritization Process
The US DRI Sub-committee of the Interagency Committee on Human Nutrition Research and the Canadian Interdepartmental/Interagency Steering Committee on the Dietary Reference Intakes [for the purpose of this report, the 2 committees are referred to as the Federal DRI Sub-/Steering Committees (SCs)] work collaboratively to identify their DRI needs, funding, and to coordinate government sponsorship of DRI reviews and related activities. It should be noted that the DRI reviews are costly, requiring systematic reviews and the sponsoring of a NASEM DRI committee, and that there is no dedicated stable, long-term funding for the DRI-related work. Because of this, ongoing cyclical reviews of nutrients have not been performed. Since the initial DRI reviews, only 2 nutrients, calcium and vitamin D, have undergone a second review (12). The vitamin D and calcium review was initiated because of the availability of significant, new, and relevant evidence relating nutrient status to bone health, risk of falls, and potential adverse effects, and new evidence demonstrating dose–response relations between nutrient intakes, indicators of adequacy (e.g., circulating 25-hydroxyvitamin D, a biomarker of vitamin D adequacy), and health outcomes. Also factors were identified that affected the interpretation of these relations (e.g., baseline status, race-ethnicity, BMI), which were not, or not fully, accounted for in the original report (13).
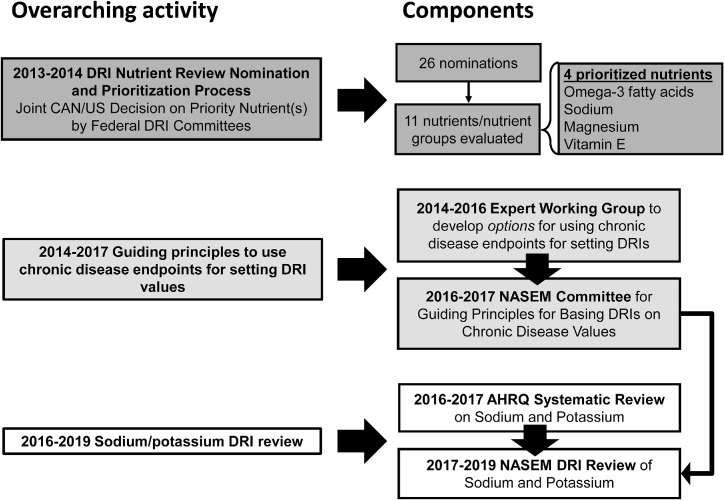
Although an ad hoc procedure can be used to prioritize nutrients for DRI review, a more systematic approach could facilitate the identification of nutrients for review and justification for funding. This report outlines the development of the approach taken by the Federal DRI SCs to prioritize nutrients for DRI review and subsequent DRI-related activities, including the development of guiding principles for basing DRIs on chronic disease endpoints and the review of the DRIs for sodium and potassium (Figure 1). The Federal DRI SCs agreed to establish a more transparent framework for reviewing DRIs. Owing to the broad range of uses of the DRIs, SC members recognized the importance of input from individuals and organizations from both within and outside government in informing future DRI prioritization decisions. As such, the Federal DRI SCs created a web-based nomination process (https://health.gov/dietaryguidelines/dri/nutrient-assessment.asp) that was open to the public to help identify candidate nutrients for DRI review and improve openness and transparency. The nomination process was implemented in mid-2013 after broad promotion on the US Health and Human Services website (https://health.gov/dietaryguidelines/dri/nutrient-assessment.asp), the Health Canada website (https://www.canada.ca/en/health-canada/services/food-nutrition/healthy-eating/dietary-reference-intakes/status-dietary-reference-intakes-nomination-process-may-13-2014.html), and in electronic newsletters from several professional organizations. In addition, nominations were solicited through an opportunity announcement published in the US Federal Register on 30 April, 2013 (https://www.gpo.gov/fdsys/pkg/FR-2013-04-30/pdf/2013-10054.pdf).
FIGURE 1.
Overarching activities related to the DRIs, their specific components, and timelines. Three main activities were undertaken: the DRI nutrient review nomination and prioritization; development of guiding principles to use chronic disease endpoints for setting DRIs; and a sodium and potassium DRI review. AHRQ, Agency for Healthcare Research and Quality; CAN, Canada; DRI, Dietary Reference Intake; NASEM, National Academies of Sciences, Engineering and Medicine.
Nutrient nominations were limited to nutrients that had undergone a previous review by an IOM DRI Committee. Several criteria were established a priori to prioritize eligible nominated nutrients, building on the criteria established for the calcium and vitamin D review (13). The criteria included evidence of significant, new, and relevant research since the last DRI review, relevance to current public health concerns, and availability of funds for the initiation of the DRI reviews. “Significant” data refers to the overall scientific quality of the evidence (e.g., type of study and quality rating scores), the number of new studies, consistency of results, and whether the new study results appear to expand the DRI-related information available to the original DRI expert panel. Examples of DRI-related information could include indicators of adequacy or hazard, health outcomes, or life stage groups for which evidence was previously unavailable or limited. “New” data refers to research that was unlikely to have been available to the previous DRI expert panel. “Relevant” means that new available study results are generalizable to the context of DRI development including applicability to the general North American population, and primary prevention or risk reduction. The evidence should also include information on causal relations between intake and outcomes of interest as well as data on quantitative dose–response relations. Furthermore, the evidence should be available for the DRI life stage groups.
Overview of the Nutrient Nomination Process
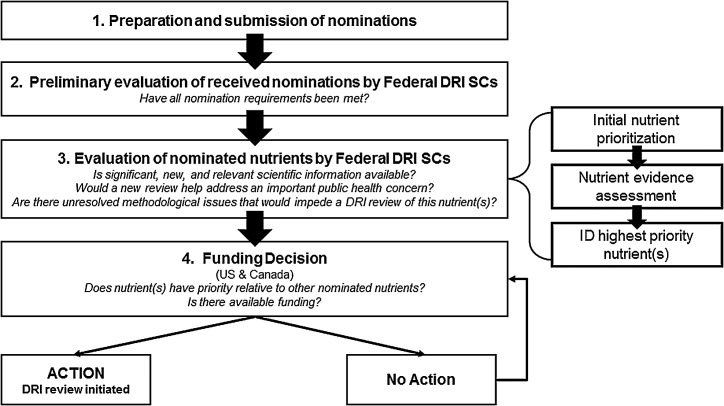
The assessment of nutrient nominations was completed in 4 steps: nutrient nomination submission; preliminary evaluation of nutrient nomination submissions to ensure completeness; evaluation and prioritization of the nutrients; and decision to fund the activity (Figure 2). Nutrient nominations were accepted by email by both governments between 30 April and 31 July, 2013. A nutrient nomination submission consisted of 2 components: a cover letter and a literature search. The cover letter could be no more than 2 pages, and the nominators had to provide a rationale and description of why a review of a nutrient, or a small group of interrelated nutrients, was warranted and how it would address a current public health concern. If relevant, the cover letter could identify any methodological issues that impeded the last DRI review of the nutrient(s), or issues that were generally relevant to a new review of the nutrient(s) that had since been resolved. Such issues were identified in previous DRI reviews of individual nutrients (https://www.nal.usda.gov/fnic/dietary-reference-intakes) and in subsequent reviews of the overall DRI process (14, 15).
FIGURE 2.
DRI nutrient nomination and prioritization process. DRI, Dietary Reference Intake; SC, steering committee/subcommittee.
The literature search was required to be comprehensive and objective without nominator review or interpretation. The nominator was to provide a list of relevant human studies that evaluated the linkage of the nutrient(s) to a health outcome of public health interest, encompassing the period since the last nutrient DRI review. A description of the search strategy, including all search terms, databases used, languages, years, and inclusion or exclusion criteria, had to be provided. A list of included studies (with abstracts) and relevant systematic reviews (with abstracts) also had to be included. Furthermore, a list of excluded studies, with a rationale for exclusion, had to be provided.
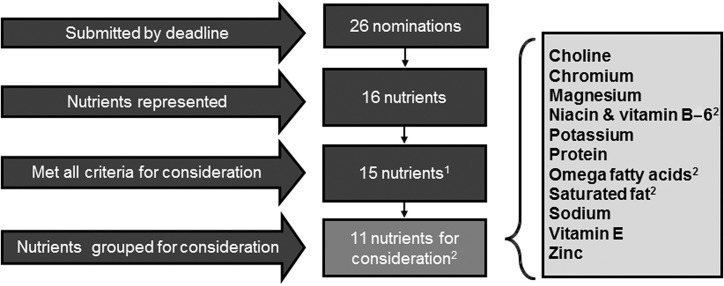
In total, 26 nominations representing 16 nutrients were received and reviewed for completeness to ensure all requirements were met (Figure 3). In the case of incomplete submissions, nominators were given an opportunity to revise and resubmit their nomination by the end of the nomination period. Two nominations were excluded because they did not nominate a specific nutrient within scope, and 5 were excluded because of incomplete or missing literature searches. Ultimately, 19 nominations representing 15 nutrients met all requirements (Figure 3). To avoid bias during prioritization, Federal staff who would not be involved in the nutrient prioritization process removed all identifying information of the nominator before nominations were forwarded to the Federal DRI SCs for consideration.
FIGURE 3.
Nutrient nomination screening before consideration for prioritization. 1Viscous and fermentable fiber nomination excluded because it did not meet the basic criteria for acceptance. 2Closely related nutrients were grouped. These included: niacin and vitamin B-6; EPA, DHA, and arachidonic acid; saturated fat and stearic acid.
Outcomes of the Nomination Process
Prioritization of 4 top nutrients or nutrient groups
Each country's DRI SC independently identified and selected ≤3 higher-priority nutrients based on public health importance and the policy needs for each country. The combined prioritized nutrients from both countries were sodium, omega-3 fatty acids, vitamin E, and magnesium (Figure 1 and Table 2). Four nutrient evidence assessment working groups consisting of US and Canadian Federal scientists and experienced reviewers were established to perform an internal nutrient evidence assessment. The working groups were tasked to evaluate whether there was any significant and new scientific evidence relevant to the development of a new DRI. Using a uniform process independent of that used by the nominators, evidence scans were conducted and new evidence was reviewed. Each working group produced a summary report using a template, which included an evidence map table that described for each study the endpoints used, study design, sample size, life stage, sex, study population, and relevance to DRI development. The evidence assessment process was launched at the end of November, 2013 and the 4 nutrient evidence assessment working groups completed their evaluation by 24 January, 2014 for joint consideration by the 2 Federal DRI SCs. The Federal DRI SCs focused their joint discussion around the public health importance; policy need addressed; the availability of significant, new, relevant scientific data; and the likelihood of securing funding for a DRI review for each prioritized nutrient.
TABLE 2.
Nutrients considered for prioritization for DRI review1
| Nutrients | Date of review | Type of DRI values | Limitations of previous DRI | New available evidence |
|---|---|---|---|---|
| Sodium | 2005 | AI, UL (>1 y only) | • Lacked evidence for an indicator of adequacy evaluated at multiple levels of intake, precluding the setting of an EAR• Use of an AI in population assessment is difficult; AIs are often misinterpreted | • New evidence using indicators of chronic disease risk reduction (e.g., blood pressure) |
| Omega fatty acids | 2005 | AI for linoleic and linolenic acids only | • AI based on the median intake in the United States where a deficiency for either fatty acid is nonexistent in healthy individuals• Use of an AI in population assessment is difficult; AIs are often misinterpreted | • New RCT evidence using indicators of chronic disease risk reduction (e.g., visual function in children, CVD and blood pressure in adults) |
| Vitamin E | 2000 | AI (<1 y), EAR (>1 y), RDA (>1 y), UL (>1 y) | • Derived based on serum concentrations of vitamin E that provide adequate protection in a test measuring the survival of erythrocytes when exposed to hydrogen peroxide in experimentally vitamin E–depleted men• Reference values only apply to intake of RRR-α-tocopherol from food and the 2R-stereoisomeric forms of α-tocopherol from fortified foods, and supplements | • Some additional evidence examining the relation between vitamin E intake and erythrocyte hemolysis• Data from an ongoing trial examining vitamin E kinetics will aid in future estimation of vitamin E requirements |
| Magnesium | 1997 | AI (<1 y), EAR (>1 y), RDA (>1 y), UL (>1 y) | • Based on balance studies with most using a single intake level• Evidence for women aged >30 y based on a single study• Intake from water not considered• Sweat and dermal losses not usually considered | • New evidence based on additional balance studies in multiple life stage groups, using Western-type diets, with larger sample sizes• May be new endpoints relevant to setting DRIs (e.g., Ca:Mg intake ratio; chronic disease endpoints) |
1Sodium received 2 complete nominations. Magnesium received 5 complete nominations. AI, Adequate Intake; CVD, cardiovascular disease; DRI, Dietary Reference Intake; EAR, Estimated Average Requirement; RCT, randomized controlled trial; RDA, Recommended Daily Allowance; UL, Upper Tolerable Intake Level.
Sodium.
Assessment
Data were deemed inadequate to determine an EAR and RDA in the previous sodium DRI review (7). Adequate intake values were developed based on sodium intake that ensures that the overall diet provides an adequate intake of other important essential nutrients and covers sodium sweat losses in unacclimated individuals exposed to high temperatures or who become physically active (2, 7). In the absence of a previously identified indicator of adequacy for sodium, the sodium evidence assessment working group performed a literature search based on indicators evaluated, but not necessarily used, in the previous DRI review. These included studies on sodium balance or homeostasis, blood pressure, growth and development in infants and children, insulin resistance, or plasma renin activity. In the absence of a framework for using chronic disease endpoints for setting DRIs, the working group did not include chronic disease outcomes or endpoints [e.g., cardiovascular disease (CVD), cancer, all-cause mortality, hypertension] in the literature search. However, relevant randomized controlled trials (RCTs) on the relation between sodium and blood pressure were included. Blood pressure, a well-accepted CVD risk factor for which dose–response effects of sodium intake have been documented, was used to set the current UL (7). The working group identified new, relevant evidence on blood pressure that addressed the previously documented reductions in blood pressure with decreases in dietary sodium intake.
The availability of new sodium balance studies for the North American population was limited. The sodium evidence assessment working group evaluated literature related to plasma renin activity to identify whether new, relevant scientific evidence existed that may support its use as an indicator of status, following recommendations for its consideration as an indicator laid out in the previous DRI report (7). However, the evidence for the impact of sodium on plasma renin activity or the renin–angiotensin–aldosterone system was considered unlikely to be relevant for developing an EAR.
Special considerations
Although sodium and potassium were nominated separately, the Federal DRI SCs agreed that if sodium was to be selected for review, then potassium should be included. There is a strong rationale for grouping these nutrients for review considering their biological interdependence. Reference values for each nutrient would ideally take the other into account. In addition, given that the primary endpoint considered by the 2005 DRI committee for sodium (and potassium) was blood pressure, the development of a framework for the use of chronic disease outcomes in setting DRIs would facilitate a DRI review.
Omega-3 Fatty acids.
Assessment
The omega fatty acid evidence assessment working group examined the relations of DHA and EPA with infant/child eye function, CVD, and blood pressure. Arachidonic acid was nominated with DHA in 1 of the 3 nominations received related to the omega fatty acids. Owing to the large size of the nomination package encompassing information from all 3 nominations, the evidence assessment working group chose to limit the scope of their review to the highest-quality information, including only RCTs and systematic reviews of DHA and EPA. The omega fatty acid evidence assessment working group identified numerous new RCTs and systematic reviews covering several life stage groups for each of the endpoints that had been published since the last DRI review (8). Many of the studies included populations and dosing conditions that could be relevant to setting DRIs. Limitations of the studies included different types of exposure (e.g., fetal compared with breast milk compared with formula in children), variable effect size and direction, use of clinical populations, and lack of clarity as to whether the intervention resulted in prevention or treatment of the disease endpoint. An important limitation was that studies were secondary prevention studies, which could make them difficult to generalize to the primary prevention context and increase uncertainties when deriving DRI values.
Special considerations
The Federal SCs felt that a new DRI review for DHA and EPA would require prior resolution of the question of appropriate models for using chronic disease outcomes when deriving DRIs. In addition, if omega-3 fatty acids were selected for review, the Federal DRI SCs considered whether it would make sense to evaluate the other fatty acids that were nominated, which included arachidonic acid, stearic acid, and other SFAs. If fatty acids were evaluated, then total fat might also be considered, which in turn might make it sensible to include all fatty acids and macronutrients simultaneously. The current macronutrient DRI report includes energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (8). Any, or all, of these could be evaluated simultaneously. Given the number of new studies on diets of different macronutrient distributions and their importance to dietary recommendations, a simultaneous evaluation of the macronutrients could be useful for the development of dietary guidelines for both countries and would fit multiple agency priorities. There may also be reasons to re-evaluate estimates for the Estimated Energy Requirement and Acceptable Macronutrient Distribution Range for the 3 macronutrients (e.g., energy intake too high for weight maintenance in a sedentary population, restriction on total fat not supported by strong data, need to address ketogenic diets, carbohydrate requirement without consideration of hepatic gluconeogenesis).
Vitamin E.
Assessment
The EAR for vitamin E was based on the prevention of hydrogen peroxide–induced hemolysis and applies only to intake of the 2R-steroisomeric forms of α-tocopherol (5). A few studies were identified that measured erythrocyte hemolysis directly since the last DRI report was published. Although oxidative stress markers have been used as indicators for estimating vitamin E requirements, there are concerns that they are not specific to vitamin E status. There is no clear consensus in the scientific community on which vitamin E indicators are valid and sensitive, and thus suitable for setting a DRI. Vitamin E kinetics was identified to likely be a more suitable indicator of vitamin E requirement by the IOM panel that set the current vitamin E DRIs (5).
The vitamin E evidence assessment working group also noted that there is an ongoing clinical trial (clinicaltrials.gov identifier NCT00862433: Vitamin E pharmacokinetics and biomarkers in normal and obese women), funded by the National Institute of Diabetes and Digestive and Kidney Diseases. This study proposes turnover kinetics as a new means to estimate vitamin E requirements. This trial has 3 arms to examine vitamin E requirements in women aged 18–40 y. The objectives are to 1) determine the amount of fat required to get the best vitamin E absorption from a meal; 2) determine the amount (i.e., best dose) of vitamin E that must be consumed before it can be measured in the blood; and 3) examine how vitamin E and vitamin C work together in the body, in conjunction with diet and vitamin supplements. The results of this study may not be available for some time. Some of the studies reviewed by the working group administered large amounts of supplemental vitamin E. More work needs to be done to determine the bioavailability of vitamin E from supplements compared with that from food sources, such as nuts, seeds, and vegetable oils (e.g., olive, sunflower, or safflower oils). Although the evidence in support of bioavailability or absorption studies of vitamin E in various forms, food matrices, or dietary patterns is relevant for determining vitamin E reference intakes, these studies were beyond the scope of the evidence review.
Special considerations
The vitamin E evidence assessment working group suggested the possibility of holding a workshop that could help identify the best endpoints and oxidative biomarkers specific to vitamin E, and how to measure them. The working group also recommended waiting for the results of the ongoing clinical trial to determine whether this information, in conjunction with the findings from Novotny et al. (16), would be relevant for setting a new DRI for vitamin E. If not, then a workshop, as suggested above, may help to identify potential endpoints for consideration by a DRI committee.
Magnesium.
Assessment
The magnesium evidence assessment working group identified new balance studies published since the last DRI review that could be relevant to setting DRIs (3). The new data provide information for multiple life stage groups and were derived from studies that used Western-type diets. Many new studies include larger sample sizes than those available for the last DRI review for magnesium. Evidence in support of re-examining the EARs for magnesium based on other criteria (i.e., Ca:Mg intake ratio and chronic disease endpoints) was not evaluated by the working group. The working group did not include endpoints associated with chronic disease risk given the lack of a chronic disease framework, but suggested that evidence based on these endpoints could be considered as part of any future DRI review.
Special considerations
The Federal DRI SCs considered the possibility of grouping magnesium with sodium and potassium because they all play a role in regulating blood pressure. However, the Federal DRI SCs decided that the rationale for a combined review was not strong enough because magnesium has other roles in the body (e.g., bone health) and because of the likelihood that sodium and potassium intake would not factor into the determination of magnesium reference values.
Guiding principles for using chronic disease endpoints for setting DRIs
During the nomination review and assessment process, it became clear that most of the new scientific evidence was related to nutrient–chronic disease relations. Given that chronic disease endpoints have historically been inconsistently applied in previous DRI nutrient reviews (14, 15), the Federal DRI SCs proposed to assemble an expert working group, whose work would include a workshop on the potential use of chronic disease endpoints in setting DRI values before a nutrient DRI review would be undertaken. If appropriate, the results of the expert working group could be used as a foundation for the development of guiding principles for setting DRI values based on chronic disease endpoints.
Expert working group for identifying options for basing DRIs on chronic disease endpoints.
A multidisciplinary expert working group led by Federal coordinators from Health Canada and the US NIH was assembled and met from November, 2014 to April, 2016 to identify key scientific challenges that past DRI committees encountered in using chronic disease endpoints to establish reference values (17).
The working group focused its discussions on 3 key questions:
What are the important evidentiary challenges for selecting and using chronic disease endpoints in future DRI reviews?
What intake–response models can future DRI committees consider when using chronic disease endpoints?
What are the arguments for and against continuing to include chronic disease endpoints in future DRI reviews?
The Federal DRI SCs asked the working group to critically evaluate the scientific issues related to the 3 key questions, to identify a range of options for addressing the scientific issues, and to elucidate the strengths and weaknesses of each option, while keeping in mind current and future DRI contexts and uses. The SCs did not ask the group to reach a consensus on which options had the highest priority. Final decisions about the feasibility and appropriate options for specific approaches for deriving DRIs based on chronic disease outcomes were to rest with a future NASEM committee. The “Options Report” was published in January, 2017 (17).
NASEM report on guiding principles for basing DRIs on chronic disease endpoints.
Using the “Options Report” as a foundation, a NASEM committee was sponsored in July, 2016 by the Canadian and US governments to develop guiding principles that will be used by future NASEM committees to develop DRIs based on chronic disease endpoints (18). The committee was asked to provide a rationale for the choice of each option and, in the case of an option that was not included in the “Options Report,” a rationale for its inclusion in the guiding principles. The NASEM committee was charged with determining the acceptable level of confidence that the relation between a food substance (nutrient or other naturally occurring substance in food) and a chronic disease is causal, the acceptable level of confidence in the intake–response relation, the approach for identifying and characterizing the intake–response relation, and the organizational process by which a NASEM committee could recommend the setting of a DRI based on chronic disease. The committee recommended 11 guiding principles for basing DRIs on chronic disease endpoints, which were published in August, 2017 (18). With the guiding principles in hand, the Federal DRI SCs decided that a prioritized nutrient could go forward for a DRI review. Based on the priorities of both governments, sodium and potassium were selected to undergo a DRI review.
Sodium and potassium DRI review
Agency for Healthcare Research and Quality systematic review for sodium and potassium.
The commission of an independent systematic review is an integral part of the process to update a DRI. The Federal DRI SCs collaborated to support an Agency for Healthcare Research and Quality (AHRQ) systematic review, “Effects of dietary sodium and potassium intake on chronic disease outcomes and related risk factors” (19). The AHRQ Effective Health Care Program supports Evidence-based Practice Centers (EPCs) to perform in-depth reviews of existing evidence. The goal was to provide the NASEM Committee to Review the Dietary Reference Intakes for Sodium and Potassium with a systematic review of evidence on the effects of dietary sodium and potassium intake on blood pressure and risk of CVDs, renal disease outcomes and related risk factors, and risk of all-cause mortality. The systematic review included the general body of evidence reviewed by the 2005 DRI panel (7) and updated evidence through 2017. It addressed 8 key questions on sodium and potassium posed by the Federal sponsors (Table 3).
TABLE 3.
| Nutrient | Key question |
|---|---|
| Sodium | 1. Among adults and children of all age groups (including both sexes and pregnant and lactating women), what is the effect (benefits and harms) of interventions to reduce dietary sodium intake on blood pressure at the time of the study and in later life? |
| 2. Among adults and children, what is the association between dietary sodium intake and blood pressure? | |
| 3. Among adults, what is the effect (benefits and harms) of interventions to reduce dietary sodium intake on CVD and kidney disease morbidity and mortality and on total mortality? | |
| 4. Among adults, what is the association between dietary sodium intake and CVD, CHD, stroke, and kidney disease morbidity and mortality and between dietary sodium intake and total mortality? | |
| Potassium | 1. Among children and adults, what is the effect of interventions to increase potassium intake on blood pressure and kidney stone formation? |
| 2. Among children and adults, what is the association between potassium intake and blood pressure and kidney stone formation? | |
| 3. Among adults, what is the effect of interventions aimed at increasing potassium intake on CVD and kidney disease morbidity and mortality, and total mortality? | |
| 4. Among adults, what is the association between dietary potassium intake and CVD, CHD, stroke, and kidney disease morbidity and mortality, and between dietary potassium and total mortality? |
1AHRQ, Agency for Healthcare Research and Quality; CHD, coronary heart disease; CVD, cardiovascular disease.
The AHRQ EPC worked with a Technical Expert Panel to seek guidance on the draft review protocol including key questions, inclusion/exclusion criteria, and key question PICOTSS (population, intervention/exposure, comparison group, outcome, time, setting, and study design). The protocol was published on the AHRQ Effective Healthcare Program website in February, 2017 (20). The draft report was published online in December, 2017 for a 4-wk public comment period. Simultaneously, the report was peer reviewed by the Technical Expert Panel and additional peer reviewers. The EPC reviewed and updated the report, considering the review comments and the final report was published in March, 2018 (19). The report was presented to the NASEM Committee to Review the Dietary Reference Intakes for Sodium and Potassium at a NASEM public workshop (21). The AHRQ systematic review, along with the “Guiding principles for developing dietary reference intakes based on chronic disease,” is serving as a key reference for the ongoing sodium and potassium DRI review.
NASEM committee for the DRI review of sodium and potassium.
The Federal DRI SCs worked jointly to write a statement of task and sponsored the NASEM to undertake an independent 18-mo consensus study to assess current relevant data and update, as appropriate, the DRIs for sodium and potassium (7). As indicated on the NASEM website (21):
“The review will include consideration of indicators of deficiency, inadequacy, and toxicities, as well as relevant chronic disease endpoints. The study will incorporate the AHRQ systematic evidence review of sodium and potassium on chronic disease endpoints, as appropriate, and the NASEM report on guiding principles for inclusion of chronic disease endpoints along with the DRI organizing framework. Indicators for adequacy and excess will be selected based on the strength and quality of the evidence and the demonstrated public health significance, taking into consideration sources of uncertainty in the evidence. Estimates of dietary intake of sodium and potassium will be compatible with optimal health throughout the lifespan and may decrease risk of chronic disease where data indicate they play a role.”
As for previous DRIs, the NASEM convened an expert study committee with a range of expertise integral to the task (http://nationalacademies.org/hmd/activities/nutrition/reviewdriforsodiumandpotassium.aspx). To date, the committee has conducted 3 sessions open to the public and sponsors, including 2 webinars and a public workshop, to understand both the need for the DRIs and the related science (21). All other sessions were closed to allow open deliberations among committee members. This is the second DRI review for which an independent systematic evidence review will be used to inform the DRIs. The DRI guiding principles for inclusion of chronic disease endpoints will be used for the first time in DRI development, thus serving as a model for future DRIs.
Summary
In order to facilitate the identification, prioritization, and funding of nutrient DRI reviews, the Federal DRI SCs worked collaboratively to develop a nutrient nomination process open to all stakeholders. This process not only identified candidate nutrients for DRI review, but also prompted the 2 governments to address the long-standing issue of using chronic disease endpoints in the setting of DRI values. With these new guiding principles in hand, the Federal DRI SCs moved forward with sodium and potassium to undergo a DRI review. We anticipate that the processes outlined within this report will simplify future prioritization of nutrients for DRI review and improve public health through the consistent use of chronic disease endpoints when setting DRIs.
Acknowledgements
We acknowledge the past and present members of the Joint Canada-US Dietary Reference Intakes Working Group who were involved in the reported DRI-related activities, the US DRI Sub-committee of the Interagency Committee on Human Nutrition Research, the Canadian Interdepartmental/Interagency Steering Committee on the Dietary Reference Intakes, the Federal nutrient evidence assessment working groups, and the expert working group for identifying options for basing DRIs on chronic disease endpoints. The authors’ responsibilities were as follows—AJM, MEC, JMdJ, LSG-F, DMK, KR, and SY: wrote individual sections of the manuscript; AJM: coordinated manuscript writing and was responsible for the final content; and all authors: contributed to the concept for the manuscript, and reviewed, read, and approved the final manuscript. The authors had no conflicts of interest to declare.
Notes
Supported by Health Canada, which paid publication costs.
The findings and conclusions expressed in this article are those of the authors and do not necessarily represent the official position of the CDC.
Abbreviations used: AHRQ, Agency for Healthcare Research and Quality; CVD, cardiovascular disease; DRI, Dietary Reference Intake; EAR, Estimated Average Requirement; EPC, Evidence-based Practice Center; IOM, Institute of Medicine; NASEM, National Academies of Sciences, Engineering and Medicine; RCT, randomized controlled trial; RDA, Recommended Dietary Allowance; SC, Sub-/Steering Committee; UL, Tolerable Upper Intake Level.
References
- 1. Institute of Medicine. How Should the Recommended Dietary Allowances be Revised?. Washington (DC): The National Academies Press; 1994. [PubMed] [Google Scholar]
- 2. Institute of Medicine. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington (DC): The National Academies Press; 2006. [Google Scholar]
- 3. Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington (DC): The National Academies Press; 1997. [PubMed] [Google Scholar]
- 4. Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington (DC): The National Academies Press; 1998. [PubMed] [Google Scholar]
- 5. Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington (DC): The National Academies Press; 2000. [PubMed] [Google Scholar]
- 6. Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington (DC): The National Academies Press; 2001. [PubMed] [Google Scholar]
- 7. Institute of Medicine. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington (DC): The National Academies Press; 2005. [Google Scholar]
- 8. Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington (DC): The National Academies Press; 2005. [Google Scholar]
- 9. Institute of Medicine. Dietary Reference Intakes: A Risk Assessment Model for Establishing Upper Intake Levels for Nutrients. Washington (DC): The National Academies Press; 1998. [PubMed] [Google Scholar]
- 10. Institute of Medicine. Dietary Reference Intakes: Applications in Dietary Assessment. Washington (DC): The National Academies Press; 2000. [PubMed] [Google Scholar]
- 11. Institute of Medicine. Dietary Reference Intakes: Applications in Dietary Planning. Washington (DC): The National Academies Press; 2003. [PubMed] [Google Scholar]
- 12. Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC): The National Academies Press; 2011. [PubMed] [Google Scholar]
- 13. Yetley EA, Brule D, Cheney MC, Davis CD, Esslinger KA, Fischer PW, Friedl KE, Greene-Finestone LS, Guenther PM, Klurfeld DM et al.. Dietary reference intakes for vitamin D: justification for a review of the 1997 values. Am J Clin Nutr. 2009;89:719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Institute of Medicine. The Development of DRIs 1994–2004: Lessons Learned and New Challenges: Workshop Summary. Washington (DC): The National Academies Press; 2008. [Google Scholar]
- 15. Taylor CL. Framework for DRI development: components “known” and components “to be explored” background paper [Internet]. 2008; [cited 11 Jul, 2018]. Available from: https://www.nal.usda.gov/sites/default/files/fnic_uploads//Framework_DRI_Development.pdf. [Google Scholar]
- 16. Novotny JA, Fadel JG, Holstege DM, Furr HC, Clifford AJ. This kinetic, bioavailability, and metabolism study of RRR-α-tocopherol in healthy adults suggests lower intake requirements than previous estimates. J Nutr. 2012;142:2105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yetley EA, MacFarlane AJ, Greene-Finestone LS, Garza C, Ard JD, Atkinson SA, Bier DM, Carriquiry AL, Harlan WR, Hattis D et al.. Options for basing dietary reference intakes (DRIs) on chronic disease endpoints: report from a joint US-/Canadian-sponsored working group. Am J Clin Nutr. 2017;105:249S–85S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Academies of Sciences, Engineering, and Medicine. Guiding Principles for Developing Dietary Reference Intakes Based on Chronic Disease. Washington (DC): The National Academies Press; 2017. [PubMed] [Google Scholar]
- 19. Newberry SJ, Chung M, Anderson C, Fu W, Chen C, Tang A, Zhao N, Booth M, Marks J, Hollands S et al.. Sodium and Potassium Intake: Effects on Chronic Disease Outcomes and Risks. Comparative Effectiveness Review No. 206. AHRQ Publication No. 18-EHC009-EF Rockville, MD: Agency for Healthcare Research and Quality; 2018. [PubMed] [Google Scholar]
- 20. Agency for Healthcare Research and Quality. Evidence-based practice center systematic review protocol: effects of dietary sodium and potassium intake on chronic disease outcomes and related risk factors [Internet]. 1 March, 2017; [cited 11 Jul, 2018]. Available from: https://effectivehealthcare.ahrq.gov/topics/sodium-potassium/research-protocol. [Google Scholar]
- 21. National Academies of Sciences, Engineering, and Medicine. Review of the dietary reference intakes for sodium and potassium [Internet]. 2018; [updated 8 Oct, 2018; cited 11 Jul, 2018]. Available from: http://www.nationalacademies.org/hmd/Activities/Nutrition/ReviewDRIforSodiumandPotassium.aspx. [Google Scholar]