ABSTRACT
Background
Both excessive sodium intake and obesity are risk factors for hypertension and cardiovascular disease. The association between sodium intake and obesity is unclear, with few studies assessing sodium intake using 24-h urine collection.
Objectives
Our objective was to assess the association between usual 24-h sodium excretion and measures of adiposity among US adults.
Methods
Cross-sectional data were analyzed from a sample of 730 nonpregnant participants aged 20–69 y who provided up to 2 complete 24-h urine specimens in the NHANES 2014 and had data on overweight or obesity [body mass index (kg/m2) ≥25] and central adiposity [waist circumference (WC): >88 cm for women, >102 cm for men]. Measurement error models were used to estimate usual sodium excretion, and multiple linear and logistic regression models were used to assess its associations with measures of adiposity, adjusting for sociodemographic, health, and dietary variables [i.e., energy intake or sugar-sweetened beverage (SSB) intake]. All analyses accounted for the complex survey sample design.
Results
Unadjusted mean ± SE usual sodium excretion was 3727 ± 43.5 mg/d and 3145 ± 55.0 mg/d among participants with and without overweight/obesity and 3653 ± 58.1 mg/d and 3443 ± 35.3 mg/d among participants with or without central adiposity, respectively. A 1000-mg/d higher sodium excretion was significantly associated with 3.8-units higher BMI (95% CI: 2.8, 4.8) and a 9.2-cm greater WC (95% CI: 6.9, 11.5 cm) adjusted for covariates. Compared with participants in the lowest quartile of sodium excretion, the adjusted prevalence ratios in the highest quartile were 1.93 (95% CI: 1.69, 2.20) for overweight/obesity and 2.07 (95% CI: 1.74, 2.46) for central adiposity. The associations also were significant when adjusting for SSBs, instead of energy, in models.
Conclusions
Higher usual sodium excretion is associated with overweight/obesity and central adiposity among US adults.
Keywords: 24-h urinary sodium, sodium intake, obesity, overweight, overweight/obesity, adiposity, central adiposity, waist circumference
Introduction
Both excessive sodium intake and obesity are risk factors for hypertension, and hypertension increases cardiovascular disease risk (1–3). Several studies in developed countries suggest that sodium intake and obesity are positively associated (4–11). Previous studies in children and adolescents suggested an indirect association between sodium intake and obesity through sugar-sweetened beverage (SSB) intake, a major source of energy intake in children. This may be via a mechanism of more sodium intake triggering greater thirst (4, 12, 13). In addition, sodium intake is highly correlated with energy intake (3). However, results from several recent cross-sectional studies in both children and adults indicated a direct association between sodium intake and obesity that was independent of total energy or SSB intake (5–7, 9, 10, 14). One postulated mechanism for this association may be an effect on body fat mass. High sodium intake has been reported to increase white adipocyte mass and plasma leptin concentration in rats (15) and has been associated with several adiposity measures including fat mass and percentage body fat in humans (6, 8).
Among the studies in adults, some used self-reported dietary measures to assess both sodium and energy intake (6, 7, 14, 16), which may be problematic due to recall bias and greater underreporting of intakes among overweight and obese adults (17). Few studies have used 24-h sodium excretion, the recommended gold standard, for assessment of sodium intake (7–10). Among them, a longitudinal study in 215 Danish individuals reported a significant association of 24-h sodium excretion with body fat and fat-free mass, but not with body weight or waist circumference (WC). The authors proposed that they may have overlooked some association due to lack of power (8). Studies from Japan and the United Kingdom indicated a positive association of 24-h sodium excretion with overweight/obesity and central adiposity, independent of total energy intake (7, 10). Among adults living in New York City, 24-h sodium excretion was positively associated with obesity, BMI, body weight, and WC using diet quality as a proxy indicator to adjust for energy intake (9). All of these studies used 1 d of 24-h sodium excretion without accounting for within-person day-to-day variation, which can attenuate the true association. Because up to 2 d of 24-h urine samples were collected in the NHANES for the first time in 2014, we were able to use measurement error models to estimate usual urinary sodium excretion, which should provide a better estimate of the true association with measures of adiposity. Hence, the aim of this study was to examine the relation between measures of adiposity and usual sodium excretion, independent of energy or SSB intake, in a nationally representative sample of US adults.
Methods
Data source and participants
The NHANES includes a series of cross-sectional, nationally representative surveys, which use a complex, stratified, multistage, probability-sampling procedure to collect health and nutritional data from a representative sample of the civilian, noninstitutionalized US population. For the purpose of this study, data from the 24-h urine collection component in NHANES 2014 were used, which are available through the National Center for Health Statistics Research Data Center (http://www.cdc.gov/rdc) and described in detail elsewhere (18). In 2014, 66% of selected NHANES participants aged 20–69 y were examined in a mobile examination center (MEC). Among those, 1103 nonpregnant participants were randomly selected to collect a 24-h urine specimen and 827 provided a complete specimen for the initial collection (completeness criteria defined below). This resulted in an overall component response rate of 50% [75% (24-h urine response rate) × 66% (examination response rate for adults aged 20–69 y)] for the initial 24-h urine collection (19). Of the 585 selected to complete a second 24-h urine collection, 436 participants (75%) provided a second complete specimen. The 24-h urine collection protocol was approved by the National Center for Health Statistics Research Ethics Review Board. All participants provided informed consent. For this analysis, participants with unreliable initial dietary recalls or whose recalls did not meet minimum criteria (n = 48) (20), those with missing anthropometric measures (n = 18), or missing data for covariates (n = 31) were sequentially excluded. This yielded a final sample of 730 participants for the analyses (Supplemental Figure 1).
Measures
Outcomes.
All anthropometric data were collected in the MEC using standard procedures and equipment by trained health technicians, as described in detail elsewhere (21). BMI was calculated as body weight in kilograms divided by height in meters squared (kg/m2). Obesity was defined as BMI ≥30, overweight as 25 ≤ BMI < 30; overweight/obesity was defined as BMI ≥25 (22). We used WC as a measure of central adiposity (defined as WC >88 cm for women and >102 cm for men) (23).
Usual 24-h sodium excretion.
Detailed urine data collection procedures have been described elsewhere (18, 24, 25). Briefly, a random half sample of nonpregnant participants aged 20–69 y, examined in the MEC, were selected to collect their urine for a 24-h period. Initially, half of those who completed the initial 24-h urine collection were randomly selected to collect a second 24-h urine specimen 3–10 d later, but later in the study, all those with a complete initial 24-h urine collection were invited to participate. The 24-h urine collection started and ended at a urine study MEC. The completeness of 24-h urine collection was defined on the basis of the following criteria: 1) the collection start and end times were recorded; 2) the length of collection was ≥22 h; 3) the total urine volume was ≥400 mL; 4) the participant reported no more than a few drops of urine missed during collection; and 5) if the participant was a female, she reported not menstruating during the urine collection. Data from ≤2 specimens/participant were used in measurement error models (described below in the “Statistical analyses” section) to adjust for within-person day-to-day variability in sodium excretion.
Covariates.
Age, sex, race–Hispanic origin, other demographic characteristics, and health behaviors were self-reported (26). Race–Hispanic origin was categorized as non-Hispanic white, non-Hispanic black, non-Hispanic Asian, Hispanic, and other (including multiracial). Poverty-income ratio (PIR) was calculated as family income relative to the 2014 Department of Health and Human Services poverty guidelines and was categorized as ≤130% or >130% on the basis of commonly used thresholds for federal assistance programs. Educational attainment was categorized as ≤12 y of education or GED (general equivalency diploma) compared with >12 y of education. Self-reported physical activity was based on responses to a questionnaire about work, transportation, and leisure-time activities in a typical week, which is based on the Global Physical Activity Questionnaire. Physical activity was defined as active (≥75 min/wk of vigorous intensity or ≥150 minutes/wk of moderate or an equivalent combination of moderate and vigorous intensity activity), intermediate (5–74 min/wk of vigorous intensity or 10–149 min/wk of moderate or an equivalent combination of moderate and vigorous intensity activity), or inactive (27). Smoking status was categorized as current smoker, former smoker, or never smoked. Heavy alcohol use was defined as consumption of ≥8 drinks/wk for women or ≥15 drinks/wk for men.
Usual dietary energy and SSB intake were examined as potential confounding/mediating factors of the sodium excretion–adiposity associations. Participants provided at least one 24-h dietary recall. The first dietary recall was administered in person at the MEC, followed by a second recall via telephone 3–10 d later. Nutrient intakes from foods and beverages were estimated by using the USDA's Food and Nutrient Database for Dietary Studies 2013–2014 (28). The definition of SSBs is consistent with other reports and includes regular soda, fruit drinks (including sweetened bottled waters and fruit juices and nectars with added sugars), sports and energy drinks, sweetened coffees and teas, and other SSBs (including horchata and sugar cane beverages) (29). SSBs do not include diet drinks; 100% fruit juice; beverages sweetened by the participant, including coffee and teas; alcohol; or flavored milks. The USDA food codes for the beverages above were used to aggregate data to calculate participants’ total SSB intake (28). The amount of food or beverage consumed is listed in grams by NHANES; therefore, SSB intake was calculated and reported as grams per day.
Statistical analyses
Estimating usual sodium excretion and nutrient and food intake.
In order to estimate participants’ usual sodium excretion or usual dietary intakes of total energy and SSBs, we used a method developed by the National Cancer Institute (NCI) to account for within- and between-person variations in excretion/intake. The NCI method for estimating usual excretion/intake uses 2 steps. The first step is a 2-part model (MIXTRAN macro) for repeated measures of nutrient data with correlated random effects. The first part of the model estimates the probability of the consumption of an episodically consumed dietary constituent (i.e., SSB). Because nearly every participant consumed sodium and energy daily, for these variables we used only the second part of the 2-part model to specify the consumption-day amount, whereas for SSB intake we used both parts of the model. The data on amount consumed were transformed to approximate normality using Box-Cox transformation. The second step in the NCI method calculates the individual's usual excretion/intake using estimated model parameters from the first step (30). The NCI method requires that at least some of the respondents have multiple days of nutrient values to estimate the within- and between-individual variations (30, 31). In our study, 390 (54.1%) participants provided two 24-h urine collections and 672 participants (92.4%) had 2 reliable dietary recalls.
For the baseline characteristics, the models included the following covariates: an indicator of sequence number (first compared with second day of urine collection or dietary recall), day of the week when the 24-h urine or dietary recall was collected [weekday compared with weekends (Friday–Sunday)], age, sex, and race–Hispanic origin. For the association analyses, along with the aforementioned covariates, we further included physical activity, educational attainment, smoking status, alcohol consumption, and either usual total energy or SSB intake based on the adjusted covariates in each linear or logistic model (described below).
Statistical tests and models.
To assess mean differences of continuous variables between groups, t tests were used. Rao-Scott F-adjusted chi-square tests were used for categorical variables. Multiple linear regression was used to examine the association of the estimated usual sodium excretion (independent variable) with continuous measures of BMI and WC (dependent variables). We used multiple logistic regression with usual sodium excretion as a continuous variable to assess its associations with overweight/obesity and central adiposity. To present prevalence ratios (PRs) in a quartile fashion, we calculated the estimated usual sodium excretion at the middle value of each quartile: at the 87.5th percentile for the highest quartile (quartile 4), the 62.5th percentile for quartile 3, the 37.5th percentile for quartile 2, and the 12.5th percentile for the lowest quartile (quartile 1). We used the parameters from the logistic regression models to estimate the adjusted PRs by comparing the prevalences for the 87.5th, 62.5th, and 37.5th percentiles of usual sodium excretion as a continuous variable with that for the 12.5th percentile (quartiles 4, 3, and 2 compared with quartile 1).
For both linear and logistic regression analyses, we adjusted for demographic variables including age, sex, and race–Hispanic origin (model 1). Next, we further adjusted for physical activity, educational attainment, smoking status, and alcohol consumption (model 2). To examine potential mediation by usual total energy intake or SSB intake, model 3 included all variables in model 2 and added usual energy intake, whereas model 4 included all variables in model 3 but replaced energy intake with SSB intake. Using the parameters from the multiple linear regression (model 3), we also calculated the adjusted mean BMI and WC across the middle value of the quartiles (12.5th, 37.5th, 62.5th, and 87.5th percentiles) of estimated usual sodium excretion. We examined the interaction between usual sodium excretion and other covariates by including the interaction terms in the multiple regression models. Bonferroni correction was used to correct P values for multiple comparisons when examining potential interactions.
We performed several sensitivity analyses, as follows:
Because there was a relatively large number of participants with missing PIR (n = 41), we assessed the effect of PIR on the associations of usual sodium excretion with adiposity outcomes separately.
We categorized BMI as obesity (≥30.0) compared with nonobesity (<30) and analyzed the association between usual 24-h sodium excretion with obesity.
We categorized BMI as obesity (≥30.0), overweight (25.0–29.9), and normal weight (<25) and conducted multinomial logistic regression to investigate the association of usual 24-h sodium excretion with obesity and overweight, respectively.
We stratified analyses by sex, given that higher sodium and energy intakes have been observed in men than in women.
We replaced estimated usual sodium excretion with the first 24-h sodium excretion to examine if the associations still held when not accounting for within-person day-to-day variability in excretion.
Among the 730 participants who provided a complete initial 24-h urine collection, we further excluded participants with potentially incomplete 24-h urine collection based on both Joossens and Geboers’ (32) and Mage's creatinine criteria to determine whether incomplete urine collection could explain our associations (33). No creatine criteria are universally accepted. Although Joossens and Geboers’ criteria are more commonly used than Mage's, Joossens and Geboers’ criteria were developed and validated in a mainly white population. Mage's criteria adjust for differences by race-ethnicity (34). Participants with an observed-to-expected 24-h creatinine excretion ratio <0.7 were identified as having potentially incomplete 24-h urine collection (34, 35) and were excluded.
All of the statistical analyses were conducted in SAS (version 9.3; SAS Institute, Inc.) or SAS-callable SUDAAN (RTI International) with 1-y 24-h urine sample weights to account for nonresponse and the complex sampling design of NHANES. All tests were 2-sided, and P values <0.05 were considered significant.
Results
The weighted prevalences ± SEs of overweight/obesity (n = 523) and central adiposity (n = 417) among US adults aged 20–69 y were 72.5% ± 2.8% and 58.8% ± 3.1%, respectively (Table 1, Supplemental Table 1). Participants who were overweight/obese or who had central adiposity differed from their counterparts in age, race–Hispanic origin, physical activity, and educational levels (Table 1). Mean ± SE BMI and WC for the study population were 30 ± 0.5 and 101 ± 1.2 cm, respectively. The mean usual sodium excretion estimated from up to two 24-h urine sodium excretion was 3567 ± 40.0 mg/d. Persons with overweight/obesity or central adiposity had a significantly higher estimated usual sodium excretion compared with other adults. On the other hand, no difference in estimated usual energy or SSB intakes was observed by overweight/obesity or central adiposity status (Table 1).
TABLE 1.
Sociodemographic and other characteristics by overweight/obesity and central adiposity among US adults aged 20–69 y, NHANES 20141
| Overweight/obesity | Central adiposity | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | All participants | Yes | No | P | Yes | No | P |
| All,2 % | 72.5± 2.8 | 27.5 ± 2.8 | 58.8 ± 3.1 | 41.2 ± 3.1 | |||
| Age, y | 43 ± 1.0 | 44 ± 1.0 | 41 ± 1.6 | 0.03 | 46 ± 1.0 | 40 ± 1.2 | <0.0001 |
| Male, % | 49.5 ± 2.2 | 51.6 ± 2.1 | 43.8 ± 4.8 | 0.15 | 42.1 ± 3.1 | 59.9 ± 4.0 | 0.004 |
| Race–Hispanic origin,3 % | 0.01 | 0.01 | |||||
| Non-Hispanic white | 65.1 ± 5.7 | 63.9 ± 5.8 | 68.2 ± 7.0 | 65.9 ± 6.0 | 64.0 ± 5.9 | ||
| Non-Hispanic black | 11.2 ± 3.2 | 12.0 ± 3.3 | 9.2 ± 3.0 | 11.7 ± 3.6 | 10.5 ± 2.8 | ||
| Non-Hispanic Asian | 4.9 ± 1.5 | 3.0 ± 0.9 | 10.1 ± 3.2 | 2.3 ± 0.9 | 8.7 ± 2.5 | ||
| Hispanic | 15.8 ± 2.9 | 18.6 ± 3.2 | 8.6 ± 3.0 | 17.8 ± 3.5 | 13.1 ± 2.5 | ||
| Family poverty-income ratio,4 % | 0.32 | 0.17 | |||||
| ≤130% | 26.9 ± 3.1 | 27.9 ± 3.0 | 24.3 ± 4.3 | 29.0 ± 2.9 | 24.0 ± 4.1 | ||
| >130% | 73.1 ± 3.1 | 72.1 ± 3.0 | 75.7 ± 4.3 | 71.0 ± 2.9 | 76.0 ± 4.1 | ||
| Physical activity,5 % | 0.03 | 0.003 | |||||
| Active | 62.4 ± 3.2 | 59.6 ± 3.4 | 69.7 ± 4.7 | 57.3 ± 3.2 | 69.6 ± 4.4 | ||
| Intermediate | 15.8 ± 2.6 | 15.4 ± 2.0 | 16.8 ± 5.3 | 16.7 ± 1.9 | 14.6 ± 4.0 | ||
| Inactive | 21.8 ± 2.3 | 25.0 ± 2.5 | 13.5 ± 2.5 | 26.1 ± 2.7 | 15.8 ± 2.4 | ||
| Education, % | <0.001 | 0.004 | |||||
| ≤12 y or GED | 39.8 ± 3.2 | 44.8 ± 3.4 | 26.6 ± 2.7 | 45.0 ± 3.4 | 32.4 ± 3.4 | ||
| >12 y | 60.2 ± 3.2 | 55.2 ± 3.4 | 73.4 ± 2.7 | 55.0 ± 3.4 | 67.6 ± 3.4 | ||
| Smoking status, % | 0.50 | 0.13 | |||||
| Current smoker | 22.4 ± 1.7 | 21.8 ± 1.6 | 24.0 ± 3.8 | 22.6 ± 1.9 | 22.2 ± 2.8 | ||
| Former smoker | 19.8 ± 2.5 | 21.1 ± 2.7 | 16.5 ± 3.6 | 22.4 ± 3.3 | 16.1 ± 2.5 | ||
| Never smoked | 57.8 ± 2.7 | 57.1 ± 2.9 | 59.5 ± 5.3 | 55.1 ± 2.9 | 61.6 ± 3.5 | ||
| Heavy user of alcohol,6 % | 9.6 ± 1.5 | 8.4 ± 1.6 | 12.6 ± 3.5 | 0.31 | 8.7 ± 1.7 | 10.8 ± 2.8 | 0.55 |
| Twenty-four-hour sodium excretion, mg/d | 3567 ± 40.0 | 3727 ± 43.5 | 3145 ± 55.0 | <0.0001 | 3653 ± 58.1 | 3443 ± 35.3 | 0.01 |
| BMI, kg/m2 | 30 ± 0.5 | 33 ± 0.4 | 22 ± 0.1 | <0.0001 | 34 ± 0.5 | 24 ± 0.2 | <0.0001 |
| Waist circumference, cm | 101 ± 1.2 | 108 ± 1.2 | 81.9 ± 0.6 | <0.0001 | 111 ± 1.1 | 86 ± 0.6 | <0.0001 |
| Energy intake, kcal/d | 2157 ± 33.4 | 2182 ± 38.0 | 2091 ± 62.0 | 0.22 | 2102 ± 38.9 | 2236 ± 61.2 | 0.08 |
| SSB intake,7 g/d | 418 ± 17.8 | 435 ± 18.2 | 369 ± 39.9 | 0.15 | 408 ± 23.3 | 434 ± 30.1 | 0.52 |
Values are weighted means ± SEs for continuous variables or weighted percentages ± SEs for categorical variables. Overweight/obesity was defined as BMI (kg/m2) ≥25; central adiposity was defined as waist circumference >102 cm for men and >88 cm for women. For categorical variables, we used Rao-Scott F-adjusted chi-square test; t test was used to examine whether the mean of continuous variables varied by groups. All tests were 2-sided. GED, general equivalency diploma; SSB, sugar-sweetened beverage.
Row percentages are presented in this line; column percentages are presented in all other lines. Unweighted sample sizes are provided in Supplemental Table 1.
Estimates from participants who reported other non–Hispanic races including non–Hispanic multiracial are not presented separately.
Family poverty-income ratio was defined as total family income divided by 2014 Department of Health and Human Services poverty guidelines multiplied by 100. Participants with missing income (n = 46) were not included.
Active: ≥150 min/wk at moderate intensity or ≥75 min/wk at vigorous intensity or ≥150 min/wk at moderate + vigorous intensity. Intermediate: 10–149 min/wk at moderate intensity or 5–74 min/wk at vigorous intensity or 10–149 min/wk at moderate + vigorous intensity.
Heavy user of alcohol was defined as self-reported consumption of ≥8 drinks/wk for women or ≥15 drinks/wk for men.
Analysis was conducted in a subsample of participants who consumed SSBs on either dietary recall day because 38.9% (n = 284) of participants did not report consuming any SSBs on the day of survey, which resulted in left-skewed distribution of SSBs.
Estimated usual sodium excretion was positively associated with BMI and WC after adjustment for potential confounding variables. As shown in Table 2, each 1000-mg/d higher usual sodium excretion was significantly associated with a BMI that was 3.8 units higher (95% CI: 2.8, 4.8 units; P < 0.001) and a WC that was 9.2 cm greater (95% CI: 6.9, 11.5 cm; P < 0.001), after adjusting for age, sex, race–Hispanic origin, physical activity, alcohol consumption, smoking, and education (model 2). The associations were almost the same when we further adjusted for either usual total energy intake (model 3) or usual SSB intake (model 4).
TABLE 2.
Association of usual 24-h sodium excretion (independent variable) with BMI and WC (dependent variable) among US adults aged 20–69 y, NHANES 20141
| BMI | WC | |||||
|---|---|---|---|---|---|---|
| Usual sodium excretion | β-Coefficient | (95% CI) | P | β-Coefficient | (95% CI) | P |
| Model 1 | 3.8 | (2.8, 4.8) | <0.001 | 9.1 | (6.6, 11.6) | <0.001 |
| Model 2 | 3.8 | (2.8, 4.8) | <0.001 | 9.2 | (6.9, 11.5) | <0.001 |
| Model 3 | 3.8 | (2.8, 4.8) | <0.001 | 9.2 | (6.9, 11.5) | <0.001 |
| Model 4 | 3.8 | (2.8, 4.7) | <0.001 | 9.2 | (7.0, 11.4) | <0.001 |
The unweighted sample size in each model is 730. β-Coefficients for usual sodium excretion represent change in BMI or WC (centimeters) associated with per 1000-mg/d higher usual sodium excretion. Model 1 adjusted for age, sex, and race–Hispanic origin; model 2 adjusted for age, sex, race-Hispanic origin, physical activity, alcohol consumption, smoking status, and educational attainment; model 3 adjusted for covariates of model 2 plus usual total energy intake; model 4 adjusted for covariates of model 2 plus usual sugar-sweetened beverage intake. All tests were 2-sided and based on Satterthwaite adjusted F test. WC, waist circumference.
The adjusted mean BMI was significantly higher with greater usual sodium excretion—that is, from 25.7 (95% CI: 25.0, 26.4) at the 12.5th percentile of usual sodium excretion to 33.8 (95% CI: 31.9, 35.7) at the 87.5th percentile (P-trend < 0.001) as shown in Figure 1. A similar trend was observed for WC; adjusted mean WC was greater with higher usual urinary sodium excretion (P-trend < 0.001) from 91.0 cm (95% CI: 89.0, 93.1 cm) at the 12.5th percentile to 110.6 cm (95% CI: 106.3, 114.8 cm) at the 87.5th percentile.
FIGURE 1.
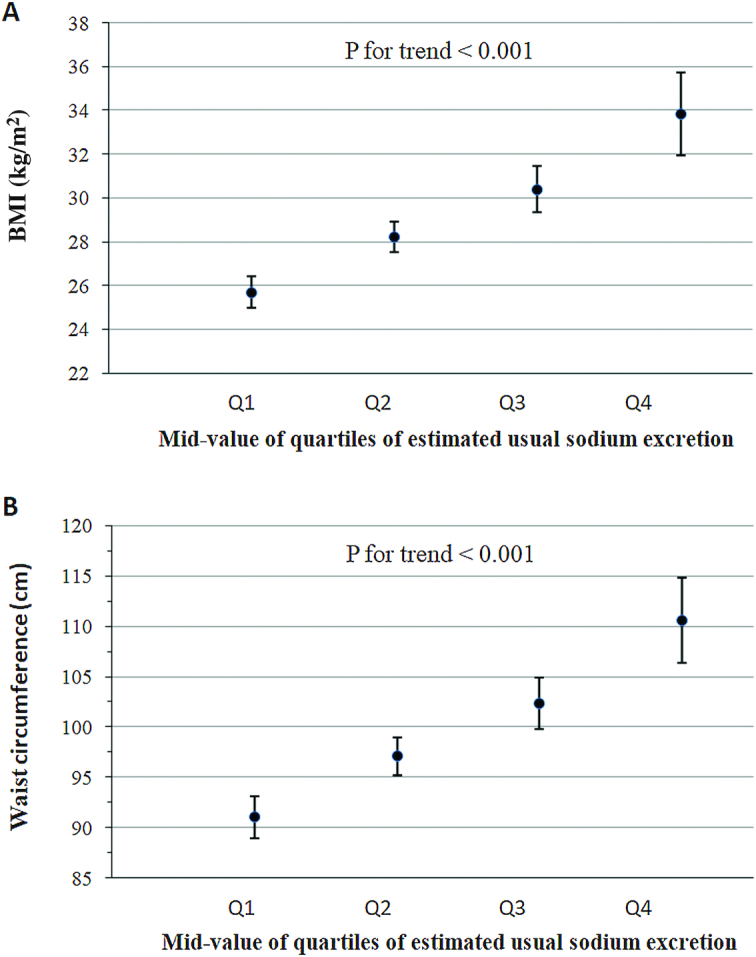
Adjusted mean BMI (A) and waist circumference (B) by midvalue of the quartile of the estimated usual 24-h sodium excretion: NHANES 2014. Adjusted variables were age, sex, race–Hispanic origin, physical activity, alcohol consumption, smoking status, educational attainment, and usual total energy intake. The midpoint values for usual sodium excretion in each quartile are as follows: quartile 1, 2554 mg/d; quartile 2, 3192 mg/d; quartile 3, 3775 mg/d; quartile 4, 4770 mg/d. P values indicate tests for trend from the survey-adjusted regression models. Q, quartile.
Positive associations of estimated usual sodium excretion with overweight/obesity and central adiposity were observed and are summarized in Table 3. The adjusted PR comparing the prevalence of overweight/obesity among adults in the highest quartile of sodium excretion with those in the lowest quartile was 1.93 (95% CI: 1.69, 2.20), after adjusting for age, sex, race–Hispanic origin, physical activity, alcohol consumption, smoking, education, and usual total energy intake (Table 3, model 3). When usual SSB intake was substituted for usual total energy intake, the positive association with overweight/obesity was not meaningfully altered (adjusted PR: 1.86; 95% CI: 1.64, 2.11) (Table 3, model 4). Similarly, the predicted prevalence of central adiposity was ∼2 times higher in the highest quartile of sodium excretion compared with the lowest quartile after adjusting for potential confounding/mediating variables including either usual total energy intake in model 3 (adjusted PR: 2.07; 95% CI: 1.74, 2.46) or usual SSB intake in model 4 (adjusted PR: 1.99; 95% CI: 1.68, 2.36).
TABLE 3.
Association between usual sodium excretion (independent variable) and overweight/obesity status (dependent variable) among US adults aged 20–69 y, NHANES 20141
| Midvalue of quartiles of estimated usual sodium excretion, PR (95% CI) | |||||
|---|---|---|---|---|---|
| Q1: 12.5th percentile | Q2: 37.5th percentile | Q3: 62.5th percentile | Q4: 87.5th percentile | P-trend | |
| Usual sodium excretion,2 mg | 2505 | 3176 | 3753 | 4662 | |
| Overweight/obesity | |||||
| Model 1 | Ref | 1.33 (1.24, 1.43) | 1.61(1.44, 1.79) | 1.86 (1.64, 2.12) | <0.001 |
| Model 2 | Ref | 1.36 (1.26, 1.46) | 1.60 (1.44, 1.79) | 1.87 (1.65, 2.13) | <0.001 |
| Model 3 | Ref | 1.38 (1.28, 1.49) | 1.65 (1.48, 1.85) | 1.93 (1.69, 2.20) | <0.001 |
| Model 4 | Ref | 1.35 (1.26, 1.45) | 1.60 (1.44, 1.77) | 1.86 (1.64, 2.11) | <0.001 |
| Central adiposity | |||||
| Model 1 | Ref | 1.30 (1.20, 1.40) | 1.59 (1.40, 1.81) | 1.97 (1.66, 2.34 | <0.001 |
| Model 2 | Ref | 1.33 (1.23, 1.43) | 1.60 (1.41, 1.81) | 2.00 (1.69, 2.37) | <0.001 |
| Model 3 | Ref | 1.35 (1.25, 1.46) | 1.65 (1.45, 1.88) | 2.07 (1.74, 2.46) | <0.001 |
| Model 4 | Ref | 1.32 (1.22, 1.43) | 1.60 (1.41, 1.81) | 1.99 (1.68, 2.36) | <0.001 |
The unweighted sample size in each model is 730. P values for trend across percentiles of estimated usual sodium intake based on Satterthwaite adjusted F test; all tests were 2-sided. Model 1 adjusted for age, sex, and race–Hispanic origin; model 2 adjusted for age, sex, race-Hispanic origin, physical activity, alcohol consumption, smoking status, and educational attainment; model 3 adjusted for covariates of model 2 plus usual total energy intake; model 4 adjusted for covariates of model 2 plus usual sugar-sweetened beverage intake. PR, prevalence ratio; Q, quartile; Ref, reference.
This row shows midvalues of quartiles of estimated usual sodium excretion.
In sensitivity analyses, we examined whether PIR modified the associations of usual sodium excretion with measures of adiposity in separate analyses (Supplemental Tables 2 and 3). The significant associations were not altered by adding PIR in the models; therefore, we did not include PIR in our main analyses. With BMI categorized as obesity (BMI ≥30) compared with nonobesity (BMI <30) (Supplemental Table 4) or obesity (BMI ≥30), overweight (25.0–29.9), and normal weight (BMI <25) (Supplemental Table 5), the positive associations between usual sodium excretion and obesity remained robust. There were no significant interactions between usual sodium excretion with the covariates in all multiple linear and logistic models after Bonferroni correction for multiple comparison (data not shown). Significant positive associations between usual sodium excretion and both overweight/obesity and central adiposity were observed among both male and female participants in stratified analyses (Supplemental Table 6).
When we used only the first 24-h urine sodium excretion (instead of estimated usual excretion based on measurement error models using up to two urine collections) to examine the association with adiposity outcomes, we observed weaker, but still significant, positive associations (Supplemental Tables 7 and 8). Last, in analyses excluding participants with potentially incomplete 24-h urine collection using an expected creatinine excretion ratio <0.7 based on both Joossens and Geboers’ (32) and Mage's (33) definitions, the associations did not substantially change (Supplemental Tables 9–12).
Discussion
In the present study with the use of 24-h urine collections in a nationally representative sample of US adults for the first time, we found that higher usual sodium excretion was associated with higher BMI, greater WC, and higher prevalences of overweight/obesity and central adiposity. These associations remained significant after further adjusting for usual total energy or SSB intake, which is consistent with previous studies.
A direct positive association between sodium intake and obesity has been reported in several studies (5–7, 9, 10, 14), although the magnitude of the association differs. The heterogeneity between studies may potentially be related to differences in methods used to assess sodium intake and study population and design. Among studies that used 24-h sodium excretion to assess sodium intake, a study in 1043 young Japanese women found that greater 24-h urinary sodium excretion was associated with a higher odds of overweight/obesity (adjusted OR comparing quartile 4 with quartile 1: 2.49; 95% CI: 1.15, 5.42) and central adiposity (adjusted OR comparing quartile 4 with quartile 1: 1.77; 95% CI, 1.00, 3.16) (7). A study from the United Kingdom showed that a 1-g/d higher intake of salt was associated with higher odds of overweight/obesity (OR: 1.26; 95% CI: 1.16, 1.37) and central adiposity (OR: 1.22; 95% CI: 1.14, 1.32) independent of energy intake (10). Similarly, in adults living in New York City, 24-h sodium excretion was positively associated with obesity (OR: 1.26; 95% CI, 1.11, 1.42), BMI, and WC; however, it was not certain if the association was independent of energy intake because energy intake data were not available and diet quality was used as a proxy for caloric intake (9).
Although our findings are consistent with these previous studies, the magnitude of the observed association was stronger in the present study. The stronger association in our study may be due to our use of measurement error models to estimate usual sodium excretion to account for day-to-day variability in sodium excretion. When we used only data from the first 24-h urine collection in sensitivity analyses, substantial attenuation occurred in the association between sodium excretion with all measures of adiposity (Supplemental Tables 7 and 8). Studies have shown that within-person day-to-day variation in 24-h sodium excretion was equal or greater than between-person variability (36). None of the aforementioned studies accounted for day-to-day variability in sodium excretion.
Several mechanisms may explain the association of sodium intake with obesity. One possibility could be that excessive sodium intake is the result of a higher intake of energy-dense food or higher intake of SSBs. It is well accepted that a high dietary salt intake induces thirst, which may lead to increased intake of SSBs and may thereby contribute to obesity (37). However, recent experimental studies in humans showed that increasing salt intake induced body osmolyte-free water accrual that actually reduced thirst and fluid intake (38). When we included total energy or SSB intake in our models, the associations were not altered, which raises the possibility that high sodium intake could directly be associated with obesity. However, biological mechanisms for a direct association are unclear. A recent human study indicated that a high-salt diet increased fasting ghrelin, which regulates appetite, glucose homeostasis, and fat deposition, and may contribute to the progression of obesity (39). In addition, salty food itself has been suggested as an obesogenic mechanism by which sodium intake is associated with obesity (40). In this hypothesis, salty foods stimulate the brain's reward and pleasure centers, thus producing an addiction to these foods, augmenting the incidence of overeating and obesity. In animal studies, a high-salt diet enhanced the adipocyte insulin sensitivity for glucose uptake and the insulin-induced glucose metabolism, promoting adipocyte hypertrophy and increasing the mass of white adipose tissue and leptin production in rats (15, 41). High salt intake induced leptin resistance and obesity in mice through endogenous fructose production (42). Several epidemiologic studies also showed that salt intake, independent of energy intake, was positively associated with abdominal adipose tissue, leptin concentrations, and percentage of body fat, indicating that higher sodium intake somehow alters body fat metabolism (8, 43, 44).
Our study has several strengths. To our knowledge, it is the first study to assess the association between 24-h sodium excretion and obesity among a nationally representative sample of US adults. We used 24-h urine collection, a recommended gold-standard method for assessing population sodium intake, which avoids the potential bias from underreporting that is often observed in dietary recalls (17) and captures sodium intake from all food sources including discretionary salt use (45, 46). Furthermore, we used a measurement error model to estimate usual sodium excretion from up to two 24-h urine collections to account for within-person day-to-day variation. Nevertheless, our study had several limitations. First, the collection of self-reported dietary data, which were used to derive usual energy and SSB intakes, was not concurrent with urine collection and may have been affected by recall bias, especially among overweight and obese persons (17). Second, although we adjusted for multiple confounding variables, the effects of residual or other unknown confounding factors cannot be excluded. Finally, due to the cross-sectional nature of the study, our results do not support causality. In order to determine whether this is a causal relation, longitudinal studies of high quality (i.e., large sample size), with repeated measures of 24-h sodium excretion, energy intake, and anthropometry measures, followed by interventional studies where sodium intake is reduced in adults are needed, along with further investigations of the biological mechanisms underlying the possible relation.
Our study, which used a nationally representative sample of adults aged 20–69 y in the US population, showed significant positive associations between usual sodium intake on the basis of 24-h sodium excretion and various measures of adiposity, independent of energy or SSB intake. Our findings, together with others, provide important evidence suggesting that high sodium intake may play a role in obesity. Further interventional studies might lead to a better understanding of whether reduction in sodium intake could help reduce weight or the risk of developing obesity.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—LZ: planned the data analyses and drafted the manuscript; MEC, QY, ZZ, SO, SLJ, T-CC, CML, C-YW, JDW, ALT, RM, and CLO: contributed to study planning, analysis, and manuscript development; LZ and T-CC: conducted data analysis; and all authors: read and approved the final manuscript. The authors reported no conflicts of interest or financial disclosures.
Notes
Supported by the CDC and the NIH. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Research Data Center, the National Center for Health Statistics, or the official position of the CDC; the National Heart, Lung, and Blood Institute; the NIH; or the US Department of Health and Human Services.
Supplemental Figure 1 and Supplemental Tables 1–12 are available from the “Supplementary Data” link in the online posting of the article and from the same link in the online table of contents at: https://academic.oup.com/ajcn/.
Abbreviations: MEC, mobile examination center; NCI, National Cancer Institute; PIR, poverty-income ratio; PR, prevalence ratio; SSB, sugar-sweetened beverage; WC, waist circumference.
References
- 1. He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ. 2013;346:f1325. [DOI] [PubMed] [Google Scholar]
- 2. Landsberg L, Aronne LJ, Beilin LJ, Burke V, Igel LI, Lloyd-Jones D, Sowers J. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment: a position paper of The Obesity Society and the American Society of Hypertension. J Clin Hypertens (Greenwich). 2013;15(1):14–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whelton PK, Appel LJ, Sacco RL, Anderson CA, Antman EM, Campbell N, Dunbar SB, Frohlich ED, Hall JE, Jessup M et al.. Sodium, blood pressure, and cardiovascular disease: further evidence supporting the American Heart Association sodium reduction recommendations. Circulation. 2012;126(24):2880–9. [DOI] [PubMed] [Google Scholar]
- 4. He FJ, Marrero NM, MacGregor GA. Salt intake is related to soft drink consumption in children and adolescents: a link to obesity? Hypertension. 2008;51(3):629–34. [DOI] [PubMed] [Google Scholar]
- 5. Grimes CA, Riddell LJ, Campbell KJ, He FJ, Nowson CA. 24-h urinary sodium excretion is associated with obesity in a cross-sectional sample of Australian schoolchildren. Br J Nutr. 2016;115(6):1071–9. [DOI] [PubMed] [Google Scholar]
- 6. Yi SS, Firestone MJ, Beasley JM. Independent associations of sodium intake with measures of body size and predictive body fatness. Obesity (Silver Spring). 2015;23(1):20–3. [DOI] [PubMed] [Google Scholar]
- 7. Murakami K, Livingstone MB, Sasaki S, Uenishi K, Japan Dietetic Students' Study for Nutrition and Biomarkers Group . Ability of self-reported estimates of dietary sodium, potassium and protein to detect an association with general and abdominal obesity: comparison with the estimates derived from 24 h urinary excretion. Br J Nutr. 2015;113(8):1308–18. [DOI] [PubMed] [Google Scholar]
- 8. Larsen SC, Angquist L, Sorensen TI, Heitmann BL. 24h Urinary sodium excretion and subsequent change in weight, waist circumference and body composition. PLoS One. 2013;8(7):e69689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yi SS, Kansagra SM. Associations of sodium intake with obesity, body mass index, waist circumference, and weight. Am J Prev Med. 2014;46(6):e53–5. [DOI] [PubMed] [Google Scholar]
- 10. Ma Y, He FJ, MacGregor GA. High salt intake: independent risk factor for obesity?. Hypertension. 2015;66(4):843–9. [DOI] [PubMed] [Google Scholar]
- 11. Moosavian SP, Haghighatdoost F, Surkan PJ, Azadbakht L. Salt and obesity: a systematic review and meta-analysis of observational studies. Int J Food Sci Nutr. 2017;68(3):265–77. [DOI] [PubMed] [Google Scholar]
- 12. Grimes CA, Wright JD, Liu K, Nowson CA, Loria CM. Dietary sodium intake is associated with total fluid and sugar-sweetened beverage consumption in US children and adolescents aged 2–18 y: NHANES 2005–2008. Am J Clin Nutr. 2013;98(1):189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grimes CA, Riddell LJ, Campbell KJ, Nowson CA. Dietary salt intake, sugar-sweetened beverage consumption, and obesity risk. Pediatrics. 2013;131(1):14–21. [DOI] [PubMed] [Google Scholar]
- 14. Song HJ, Cho YG, Lee HJ. Dietary sodium intake and prevalence of overweight in adults. Metabolism. 2013;62(5):703–8. [DOI] [PubMed] [Google Scholar]
- 15. Fonseca-Alaniz MH, Brito LC, Borges-Silva CN, Takada J, Andreotti S, Lima FB. High dietary sodium intake increases white adipose tissue mass and plasma leptin in rats. Obesity (Silver Spring). 2007;15(9):2200–8. [DOI] [PubMed] [Google Scholar]
- 16. Yoon YS, Oh SW. Sodium density and obesity; the Korea National Health and Nutrition Examination Survey 2007–2010. Eur J Clin Nutr. 2013;67(2):141–6. [DOI] [PubMed] [Google Scholar]
- 17. Bailey RL, Mitchell DC, Miller C, Smiciklas-Wright H. Assessing the effect of underreporting energy intake on dietary patterns and weight status. J Am Diet Assoc. 2007;107(1):64–71. [DOI] [PubMed] [Google Scholar]
- 18. National Center for Health Statistics; CDC. National Health and Nutrition Examination Survey. 24-Hour urine study procedures manual. January 2014. [cited 2017 Aug 1]. Available from: https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/manuals/24_Hour_Urine_Study_Procedures_Manual.pdf. [Google Scholar]
- 19. Cogswell ME, Loria CM, Terry AL, Zhao L, Wang CY, Chen TC, Wright JD, Pfeiffer CM, Merritt R, Moy CS et al.. Estimated 24-hour urinary sodium and potassium excretion in US adults. JAMA. 2018;319(12):1209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Center for Health Statistics; CDC. National Health and Nutrition Examination Survey. 2013–2014 Data documentation, codebook, and frequencies. Dietary Interview—Individual Foods, first day. September 2016. [cited 2017 Aug 5]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/DR1IFF_H.htm. [Google Scholar]
- 21. National Center for Health Statistics; CDC. National Health and Nutrition Examination Survey (NHANES). MEC anthropometry procedures manual. [cited 2017 Aug 8]. Available from: https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/manuals/2013_Anthropometry.pdf. [Google Scholar]
- 22. WHO. Obesity and Overweight Fact Sheet. Updated June 2016. [cited 2017 Aug 18]. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/. [Google Scholar]
- 23. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr. et al.. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–52. [DOI] [PubMed] [Google Scholar]
- 24. Terry AL, Cogswell ME, Wang CY, Chen TC, Loria CM, Wright JD, Zhang X, Lacher DA, Merritt RK, Bowman BA. Feasibility of collecting 24-h urine to monitor sodium intake in the National Health and Nutrition Examination Survey. Am J Clin Nutr. 2016;104(2):480–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jackson SL, Cogswell ME, Zhao L, Terry AL, Wang CY, Wright J, Coleman King SM, Bowman B, Chen TC, Merritt RK et al.. Association between urinary sodium and potassium excretion and blood pressure among adults in the United States: National Health and Nutrition Examination Survey, 2014. Circulation. 2017;137(3):237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. CDC; National Center for Health Statistics. National Health and Nutrition Examination Survey. NHANES 2013–2014. [cited 2017 Aug 8]. Available from: https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Default.aspx?BeginYear=2013. [Google Scholar]
- 27. CDC; National Center for Health Statistics. National Health and Nutrition Examination Survey. 2013–2014 Data documentation, codebook, and frequencies. physical activity (PAQ_H). March 2017. [cited 2017 Aug 5]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/PAQ_H.htm. [Google Scholar]
- 28. USDA. Food and Nutrient Database for Dietary Studies. Beltsville (MD): Agricultural Research Service, Food Surveys Research Group; [cited 2017 Aug 8]. Available from: https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/fndds-download-databases/. [Google Scholar]
- 29. Rosinger A, Herrick K, Gahche J, Park S. Sugar-sweetened beverage consumption among U.S. adults, 2011–2014. NCHS Data Brief. 2017(270):1–8. [PubMed] [Google Scholar]
- 30. National Cancer Institute. Usual Dietary Intakes: the NCI method. [cited 2016 May]. Available from: http://appliedresearch.cancer.gov/diet/usualintakes/method.html. [Google Scholar]
- 31. Tooze JA, Kipnis V, Buckman DW, Carroll RJ, Freedman LS, Guenther PM, Krebs-Smith SM, Subar AF, Dodd KW. A mixed-effects model approach for estimating the distribution of usual intake of nutrients: the NCI method. Stat Med. 2010;29(27):2857–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Joossens JV, Geboers J. Monitoring salt intake of the population: methodological considerations. In: de Baker GG, Pedoe HT, Ducimetière P, editors. Euro-Nut Report 2: Surveillance of Dietary Habits of the Population with Regard to Cardiovascular Diseases. Wageningen (Netherlands): Department of Human Nutrition Agricultural University; 1984. p. 61–73. [Google Scholar]
- 33. Mage DT, Allen RH, Kodali A. Creatinine corrections for estimating children's and adult's pesticide intake doses in equilibrium with urinary pesticide and creatinine concentrations. J Expo Sci Environ Epidemiol. 2008;18(4):360–8. [DOI] [PubMed] [Google Scholar]
- 34. John KA, Cogswell ME, Campbell NR, Nowson CA, Legetic B, Hennis AJ, Patel SM. Accuracy and usefulness of select methods for assessing complete collection of 24-hour urine: a systematic review. J Clin Hypertens (Greenwich). 2016;18(5):456–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang CY, Cogswell ME, Loria CM, Chen TC, Pfeiffer CM, Swanson CA, Caldwell KL, Perrine CG, Carriquiry AL, Liu K et al.. Urinary excretion of sodium, potassium, and chloride, but not iodine, varies by timing of collection in a 24-hour calibration study. J Nutr. 2013;143(8):1276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu K, Cooper R, Soltero I, Stamler J. Variability in 24-hour urine sodium excretion in children. Hypertension. 1979;1(6):631–6. [DOI] [PubMed] [Google Scholar]
- 37. Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84(2):274–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rakova N, Kitada K, Lerchl K, Dahlmann A, Birukov A, Daub S, Kopp C, Pedchenko T, Zhang Y, Beck L et al.. Increased salt consumption induces body water conservation and decreases fluid intake. J Clin Invest. 2017;127(5):1932–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang Y, Li F, Liu FQ, Chu C, Wang Y, Wang D, Guo TS, Wang JK, Guan GC, Ren KY et al.. Elevation of fasting ghrelin in healthy human subjects consuming a high-salt diet: a novel mechanism of obesity? Nutrients. 2016;8(6):E323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cocores JA, Gold MS. The salted food addiction hypothesis may explain overeating and the obesity epidemic. Med Hypotheses. 2009;73(6):892–9. [DOI] [PubMed] [Google Scholar]
- 41. Fonseca-Alaniz MH, Takada J, Andreotti S, de Campos TB, Campana AB, Borges-Silva CN, Lima FB. High sodium intake enhances insulin-stimulated glucose uptake in rat epididymal adipose tissue. Obesity (Silver Spring). 2008;16(6):1186–92. [DOI] [PubMed] [Google Scholar]
- 42. Lanaspa MA, Kuwabara M, Andres-Hernando A, Li N, Cicerchi C, Jensen T, Orlicky DJ, Roncal-Jimenez CA, Ishimoto T, Nakagawa T et al.. High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc Natl Acad Sci USA. 2018;115(12):3138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhu H, Pollock NK, Kotak I, Gutin B, Wang X, Bhagatwala J, Parikh S, Harshfield GA, Dong Y. Dietary sodium, adiposity, and inflammation in healthy adolescents. Pediatrics. 2014;133(3):e635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Libuda L, Kersting M, Alexy U. Consumption of dietary salt measured by urinary sodium excretion and its association with body weight status in healthy children and adolescents. Public Health Nutr. 2012;15(3):433–41. [DOI] [PubMed] [Google Scholar]
- 45. McLean RM. Measuring population sodium intake: a review of methods. Nutrients. 2014;6(11):4651–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cook NR, Kumanyika SK, Cutler JA. Effect of change in sodium excretion on change in blood pressure corrected for measurement error: the Trials of Hypertension Prevention, phase I. Am J Epidemiol. 1998;148(5):431–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


