Abstract
BACKGROUND
Previous studies addressing the influence of surgery on the outcome of patients with glioblastomas (GBM) have not addressed molecular markers. The value of surgery versus the tumor's major biological markers remains unclear.
OBJECTIVE
We investigate the extent of resection as a prognosticator for patients with newly diagnosed primary GBM with the incorporation of molecular diagnostics as per the updated WHO 2016 diagnostic criteria for GBM.
METHODS
Patients with newly diagnosed GBM who underwent resection were prospectively included within a database. We analyzed patients with newly diagnosed GBM and excluded patients who presented with IDH1 R132H mutations. Gross total resection (GTR) was defined as complete removal of enhancing disease.
RESULTS
One hundred seventy-five patients were included within the analysis. One hundred four patients (59.4%) had GTR, 71 patients (40.6%) had subtotal or partial resection. Eighty patients (45.7%) displayed O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation, 95 patients (54.3%) showed no MGMT promoter methylation. In Cox regression analysis, MGMT promoter methylation (hazard ratio [HR] 1.55; 95% confidence interval [CI], 1.01-2.19; P = .0133) and GTR (HR 1.48; 95% CI, 1.06-2.07; P = .0206) were significantly associated with favorable progression-free survival. MGMT promoter methylation (HR 2.13; 95% CI, 1.45-3.12; P = .0001) and GTR (HR 1.81; 95% CI, 1.24-2.63; P = .002) were associated with favorable overall survival (OS). Of other risk factors analyzed, age (>60 vs ≤ 60 yr) was significantly associated with progression-free survival (HR 1.60; 95% CI, 1.14-2.24; P = .006) and OS (HR 2.19; 95% CI, 1.51-3.19; P < .0001).
CONCLUSION
GTR and MGMT promoter methylation are independent prognosticators for improved overall and progression-free survival in a homogeneous cohort of newly diagnosed patients with IDH wild-type glioblastoma.
Keywords: Primary glioblastoma, Extent of resection, MGMT promoter methylation, IDH1 mutation, Concordance probability estimate
ABBREVIATIONS
- CPE
concordance probability estimate
- EOR
extent of resection
- GBM
glioblastomas
- GTR
gross total resection
- HR
hazard ratio
- IDH
isocitrate dehydrogenase
- KPS
Karnofsky Performance Score
- MGMT
O6-methylguanine-DNA methyltransferase
- MRI
magnetic resonance imaging
- MSP
methylation-specific polymerase
- OS
overall survival
- PFS
progression-free survival
- SE
standard error
- TMZ
temozolomide.
Glioblastoma (GBM) is the most common and malignant brain tumor in adults.1 Following surgery, standard adjuvant treatment includes combined radio- and chemotherapy.2 On a molecular level, patients who present with tumors that are hyper-methylated within the promoter region of the gene encoding O6-methylguanine-DNA
methyltransferase (MGMT) display an improved prognosis as compared to those patients whose tumors lack such MGMT promoter methylation.3
Beyond MGMT promoter methylation status an increased extent of resection (EOR) has been reported to be favorable for patients suffering from GBM, with gross total resection (GTR) having been put forward as the best surgical outcome.4-7 However, some studies have demonstrated only a moderate favorable prognostic effect with regard to GTR in the era of combined radio- and chemotherapy.2,8 Unfortunately, none of the aforementioned studies claiming a beneficial effect of GTR, with the exception of the report from Kreth et al,8 have included data on the MGMT promoter methylation status of the patients examined.
Isocitrate dehydrogenase (IDH)1 mutation analysis allows one to differentiate between primary and secondary GBM, thereby creating homogenous and pathologically distinct subgroups.9 Critically, none of the previous studies dealing with the EOR have considered IDH1 mutational status in their patient populations and have therefore compared biologically10 and clinically11 distinct tumor patient populations.
In contrast to previous studies, this monocentric observational study was designed to identify prognostic factors in primary GBM patients with regard to EOR and MGMT promoter methylation status. Therefore, we chose to exclude those patients in whom immunohistochemistry indicated secondary glioblastoma because of positive immunohistochemical staining with a mutation-specific IDH1 antibody.
METHODS
Patients
All patients with histologically confirmed newly diagnosed GBM undergoing surgery at the corresponding author's institution between 2007 and 2012 were prospectively included in an SPSS-database (version 20, SPSS, IBM Inc, Armonk, New York). The database was closed for analysis in December 2014. The University ethics committee gave approval to this study (SNO_09-13). All patients gave informed consent.
Beside baseline demographics and neurological assessment, the patients’ status at admission was documented using Karnofsky Performance Score (KPS). Moreover, detailed information on patient- and GBM-specific characteristics, including MGMT promoter methylation status, expression of mutated IDH1-R132H protein, as well as patient gender and age, were recorded. EOR was determined by a radiologist blinded to intraoperative and histopathological findings in early (<72 h, 3T) postoperative magnetic resonance imaging (MRI).12 GTR was defined as complete removal of enhancing tissue (ie, lack of residual enhancing tumor tissue). In patients where GTR was not achieved, the EOR was calculated volumetrically via Brainlab software (Brainlab, Feldkirchen, Germany). Treatment decisions, including determination for surgery were rendered by the local interdisciplinary tumor board. Tumor progression was assessed according to the RANO criteria.13
MGMT Promoter Methylation Analysis
MGMT promoter methylation status was assessed by methylation-specific polymerase (MSP) chain reaction. Briefly, tumor specimens were histologically examined and subsequently those areas displaying the highest amount of vital tumor tissue (goal: >70% vital) were selected for MPS chain reaction. In specimens showing larger areas of necrosis or normal brain tissue, we performed macrodissection of the tumor to specifically select regions with the highest content of tumor tissue. One slide of 10μm thickness was cut from each paraffin block. Slides were deparaffinized using xylene and 2 × 96% alcohol. Cell lysis was performed using EpiTect Lyse All Lysis Kit (Quiagen, Hilden, Germany). Lysed cell solution was treated with sodium bisulfite, and then bisulfite-treated DNA was purified using the Epitect Fast DNA Bisulfite Kit (Quiagen). PCR runs were performed as previously described.14
Immunohistochemistry for IDH1-R132H
The tumor sections (3μm) were immunohistochemically stained using the following antibody: mouse IgG2a antihuman IDH1-R132H dilution 1:50 (clone H09; Dianova, Hamburg, Germany). Tissue labeling was performed with the DiscoveryXT immunohistochemistry system (Ventana, Strasbourg, France) as previously published.15 Patients with proven IDH1 mutation were excluded from analysis.
Statistical Analysis
Overall survival (OS) was defined as the time from surgery for GBM to the date of death; progression-free survival (PFS) was defined as the time from surgery for glioblastoma to the date of clinical or radiological progression. For both OS and PFS, subjects were censored at the time of their last clinical follow-up appointment. The association analysis was performed for the following 6 risk factor variables: age (≤60 vs >60), sex, KPS (≤70 and >70), GTR (incomplete vs complete), MGMT promoter status (unmethylated vs methylated), and adjuvant therapy (Stupp vs other). Other adjuvant therapy consisted mainly of experimental designs. OS was a primary outcome parameter. PFS was considered a secondary outcome parameter; P-values with P < 0.05 were considered statistically significant. The following statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina).
For each of the 6 risk factor variables, the Kaplan–Meier product-limit method was used to create survival curves and to estimate median survival time and 95% confidence intervals. The log-rank-test was applied to evaluate the difference in survival curves. For the 4 combinations of the 2 factors, MGMT and GTR, the P-values of log-rank test for the 6 pairwise multiple comparisons were adjusted using Tukey's method.
Stepwise Cox proportional regression with entry and stay significance level of 0.1 was performed for model selection. Graphical and statistical tests (with ASSESS statement16) were applied to examine the proportionality assumption for the 6 risk variables. For the variable with a violation of the proportionality assumption, a stratification technique was applied. Interactions between the risk variables were also examined. HR and 95% confidence intervals were estimated using the selected model. Concordance probability estimate (CPE) based on the Cox proportional hazards model was calculated to evaluate the global discrimination power and the predictive accuracy.17
RESULTS
All patients with newly diagnosed GBM were primarily considered for analysis. In total, 208 patients with newly diagnosed GBM were treated at our institution. In this study, we excluded 3 patients in whom MRI was contraindicated due to medical reasons, as EOR assessment was not achievable. Seventeen patients were excluded due to inconclusive MGMT promoter methylation status. Of the remaining, 13 patients were excluded because of IDH1 mutation, indicating secondary GBM thus leaving 175 patients for final analysis (Figure, Supplemental Digital Content 1).
Of these patients, 104 (59.4%) were of male and 71 (40.6%) of female sex. Ninety-six patients (55.8%) displayed a preoperative KPS > 70. GTR was achieved in 104 patients (59.4%) with the remainder having incomplete resections. A detailed overview of EOR may be found in Table 1. MGMT promoter methylation was found in 80 patients (45.7%) and nonmethylation in 95 patients (54.3%). For the entire group, median PFS was 275 d (95% CI, 256-326 d), and median OS was 541 days (95% CI, 472-616 d).
TABLE 1.
Patient Characteristics
| Categorical variable | Categories | n (%) |
|---|---|---|
| Sex | ||
| male | 71 (40.6) | |
| female | 104 (59.4) | |
| Age | ||
| ≤60 yr | 85(48.6) | |
| >60 yr | 90(51.4) | |
| KPS | ||
| 100 | 13 (7.4) | |
| 90 | 32 (18.3) | |
| 80 | 51 (29.1) | |
| 70 | 45 (25.7) | |
| other | 34 (19.4) | |
| KPS > 70 | 96(55.8) | |
| KPS ≤ 70 | 76(44.2) | |
| MGMT promoter methylation status | ||
| Methylated | 80 (45.7) | |
| Unmethylated | 95 (54.3) | |
| Extent of resection | ||
| GTR (=100%) | 104 (59.4) | |
| STR | 71 (40.6) | |
| ≥95% | 49 | |
| ≥90% | 58 | |
| ≥80% | 66 | |
| ≥75% | 69 | |
| <75% | 2 | |
| Adjuvant therapy | ||
| Stupp | 94(53.7) | |
| Other | 81(46.3) | |
| Continuous variable | Median (range) | |
| Age (year) | 61 (23-84) | |
| Preoperative tumor volume (cc) | 32.1 (1.9-101.9) | |
| Postoperative tumor volume (cc) | 0 (0-30.7) | |
| Progression-free survival (day) | 266 (8-2225) | |
| Overall survival (day) | 466 (8-2225) | |
In total, 94 patients received standard radiochemotherapy including temozolomide (TMZ). No associations between postoperative adjuvant therapy were observed with both MGMT status (P = .216) and EOR (P = .1455). Further correlation analysis demonstrated that there is no association between EOR (binary) and MGMT status (P = 1.0). The Wilcoxon test also showed there is no association between EOR (continuous) and MGMT status (P = .4074).
Based on the Kaplan–Meier method (log-rank test), in our patient population, GTR was significantly associated with enhanced PFS (median complete 326 d; 95% CI, 262-396 d vs incomplete 265 d; 95% CI, 193-281 d; P = 0 .0244) and OS (median complete 604 d; 95% CI,510-748 d vs incomplete 459 d; 95% CI, 376-519 d; P = .0108; Table 2).
TABLE 2.
Univariable Analysis (Log-Rank Test) of Factors Associated With PFS or OS
| PFS (days) | OS (days) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Factors | N (%) | Median | 95% CI | P-value* | Median | 95% CI | P-value* | ||
| Age (years) | .0037 | .0002 | |||||||
| Age ≤60 | 85 (48.6) | 341 | 264 | 467 | 688 | 541 | 837 | ||
| Age >60 | 90 (51.4) | 265 | 229 | 294 | 464 | 361 | 527 | ||
| Sex | .9215 | .1606 | |||||||
| Female | 71 (40.6) | 314 | 256 | 388 | 485 | 386 | 543 | ||
| Male | 104 (59.4) | 266 | 232 | 323 | 573 | 527 | 748 | ||
| KPS | .2598 | .8456 | |||||||
| KPS >70 | 96 (55.8) | 294 | 268 | 361 | 559 | 510 | 686 | ||
| KPS ≤70 | 76 (44.2) | 250 | 209 | 361 | 472 | 370 | 645 | ||
| Extent of resection | .0244 | .0108 | |||||||
| Complete | 104 (59.4) | 326 | 262 | 396 | 604 | 510 | 748 | ||
| Incomplete | 71 (40.6) | 265 | 193 | 281 | 459 | 376 | 519 | ||
| MGMT promoter | .0376 | .0004 | |||||||
| Methylated | 80 (45.7) | 314 | 232 | 434 | 786 | 486 | 993 | ||
| Unmethylated | 95 (54.3) | 268 | 248 | 326 | 474 | 395 | 542 | ||
| Adjuvant therapy | .193 | .1342 | |||||||
| Stupp | 94 (53.7) | 294 | 256 | 386 | 544 | 474 | 724 | ||
| Other | 81 (46.3) | 266 | 198 | 323 | 488 | 386 | 641 | ||
*Log-rank test.
Likewise, MGMT promoter methylation was significantly associated with better PFS (median methylated 314 d; 95% CI, 232-434 d vs unmethylated 268 d; 95% CI, 248-326 d; P = .0376) and OS (median methylated 786 d; 95% CI, 486-993 d vs unmethylated 474 d; 95% CI, 395-542 d; P = .0004; Table 2).
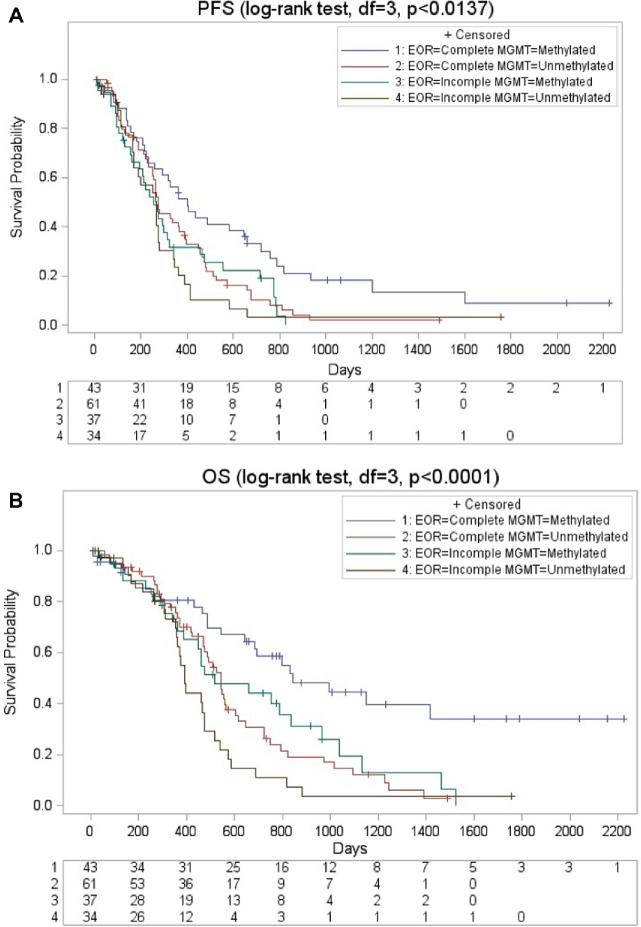
Patients were then stratified according to GTR and MGMT promoter methylation status resulting in 4 different groups (group 1: GTR, MGMT promoter methylated; group 2: GTR, MGMT promoter unmethylated; group 3: Incomplete resection, MGMT promoter methylated; group 4: Incomplete resection, MGMT promoter unmethylated). Kaplan–Meier analysis revealed significant differences between the 4 groups (Table 3) regarding PFS (group 1 median 399 days; 95% CI, 271-527 d; group 2 median 275 days; 95% CI, 207-343 d; group 3 median 256 d; 95% CI, 182-330 d; group 4265 d; 95% CI, 173-357; P < 0 .001; Figure A) and OS (group 1 median 842 d; 95% CI, 533-1151 d; group 2 median 541 d; 95% CI, 483-599 d; group 3 median 519 d; 95% CI, 304-734 d; group 4 median 392 d; 95% CI, 359-425; P < .001; Figure B).
TABLE 3.
Multivariate Analysis of Parameters Associated With PFS or OS
| Strata | Median | Test of equality over strata | Multiple comparisona | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EOR | MGMT | code | Estimate | 95% CI | Chi-Sq | DF | P-value | P-value | |||
| PFS | |||||||||||
| Complete | Unmethylated | 1 | 275 | 248 | 386 | 10.6544 | 3 | .0137 | |||
| Complete | Methylated | 2 | 399 | 232 | 657 | 2 vs 3 | .0073 | ||||
| Incomplete | Unmethylated | 3 | 266 | 167 | 281 | ||||||
| Incomplete | Methylated | 4 | 256 | 161 | 323 | ||||||
| OS | |||||||||||
| Complete | Unmethylated | 1 | 541 | 470 | 604 | 21.291 | 3 | <.0001 | 1 vs 2 | .0109 | |
| Complete | Methylated | 2 | 842 | 543 | |||||||
| Incomplete | Unmethylated | 3 | 392 | 353 | 474 | 2 vs 3 | <.0001 | ||||
| Incomplete | Methylated | 4 | 519 | 360 | 837 | 2 vs 4 | .0324 | ||||
aTukey method was used for adjusting multiple comparisons.
FIGURE.
Kaplan–Meier curves for survival. A, Kaplan–Meier curves illustrating progression-free survival stratified between MGMT promoter methylation status and EOR. B, Kaplan–Meier curves illustrating overall survival stratified between MGMT promoter methylation status and EOR. EOR = Extent of resection; MGMT = O6-methylguanine-DNA methyltransferase.
Step-wise Cox regression analysis identified age >60 yr (hazard ratio (HR) 1.60; 95% CI, 1.14-2.24; P = .006), incomplete resection (HR 1.48; 95% CI, 1.06-2.07; P = .0206), and nonmethylation of the MGMT promoter (HR 1.55; 95% CI, 1.10-2.19; P = .0133) as being associated with shorter PFS (Table 4).
TABLE 4.
Cox Regression Analysis of Overall Survival and Progression-Free Survival
| PFS | OS* | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age (>60 vs ≤60) | 1.61 | 1.15-2.26 | .0052 | 2.12 | 1.46-3.1 | .0028 |
| Sex (male vs female) | – | |||||
| KPS(≤70 vs >70) | 1.43 | 1.01-2.01 | .0442 | – | ||
| EOR (incomplete vs complete) | 1.57 | 1.12-2.21 | .0092 | 1.75 | 1.21-2.53 | <.0001 |
| MGMT promoter (methylated vs non-methylated) | 1.53 | 1.08-2.16 | .0165 | 2.22 | 1.52-3.25 | <.0001 |
| Adjuvant therapy | 1.37 | 0.97-1.92 | .0715 | – | ||
–the variable was not selected in the stepwise Cox regression (using P = .1 as entry and stay).
*stratified (by sex) Cox-regression model was used since proportional hazard (PH) assumption for sex was violated.
Step-wise Cox regression analysis identified age >60 yr (HR 2.19; 95% CI, 1.51-3.19; P < .0001), incomplete resection (HR 1.78; 95% CI, 1.23-2.57; P = .0023), and MGMT promoter-nonmethylation (HR 2.13; 95% CI, 1.47-3.11; P < .0001) as being associated with shorter OS (Table 4).
We further examined the potential impact of postoperative residual tumor volume and hence performed additional statistical analyses. Thus, we employed a statistical model focusing on EOR as a continuous variable: since both continuous EOR and residual tumor volume were very skewed, inverse transformation was applied. A Cox-regression analysis was performed using the following 3 models: Model 1 for binary EOR, model 2 for continuous EOR, and model 3 for residual tumor volume. Continuous EOR and residual tumor volume were strongly correlated (Pearson r = −0.976, and Pearson r = −0.961) based on transformed data. Tables, Supplemental Digital Content 2 and 3 indicate that the 3 variables (binary EOR, continuous EOR, and residual tumor volume) had similar effect on the 2 outcomes as P-values remain significant for both MGMT promoter methylation and EOR in all 3 models (binary EOR/continuous EOR/postoperative volume).
CPEs based on Cox regression model indicated that GTR and MGMT had similar predictive accuracy: for PFS, with age and KPS as covariates, CPEs (standard error [SE]) were 0.589 (0.023) and 0.590 (0.023) for MGMT and GTR respectively; for OS, with age and sex as covariates, CPE (SE) were 0.640 (0.023) and 0.632 (0.024). Including both biomarkers in the model slightly improves predictive accuracy: CPEs were 0.604 (0.022) and 0.659 (0.022) for PFS and OS, respectively (Table, Supplemental Digital Content 4).
DISCUSSION
With this study we aimed to determine the impact of EOR and MGMT promoter methylation status in newly diagnosed primary GBM patients. Here, we studied patient data derived from a prospective database including all patients undergoing resection of GBM between 2007 and 2012.
The literature initially identified patient age and clinical condition as significant parameters with regard to patient prognosis in GBM patients.18 With the establishment of radiotherapy and concomitant TMZ followed by intermittent TMZ in clinical practice2 (hereafter referred to as Stupp-scheme) several studies have gone on to identify MGMT promoter methylation status as one of the most important predictive and prognostic factors for outcomes in GBM patients.19-22
With regard to EOR, studies have emerged to suggest that achieving GTR is in fact linked to better outcomes in GBM patients.6,12,23,24 As a result of such findings, techniques with the aim of increasing the EOR via a minimization of residual tumor have evolved. Of note, a large retrospective analysis has indicated favorable outcome if an EOR of ≥78% can be achieved.7 Therefore, the notion of “the more, the better” has gained increasing interest within the neurosurgical community.
Likewise, several reports have indicated that extensive surgical resection carries survival benefits for patients with low-grade gliomas.25-27 However, more recent studies only observed a moderate favorable prognostic effect of GTR for glioblastoma in the era of radiotherapy plus chemotherapy.8 Moreover, no significant benefits were observed, when comparing incomplete resection with biopsy alone.8
Critically, the aforementioned studies did not incorporate MGMT promoter methylation status, and they did not differentiate between primary and secondary GBM; this differentiation might be facilitated by examining IDH1 mutational status that was first reported in 200828 and also can be performed by immunohistochemistry at least for mutational hot-spot IDH1-R132H.29 Recent literature spotlights IDH1 mutation to discriminate between primary and secondary GBM.9,30 There is a clear difference between the duration of clinical history when comparing primary and secondary GBM similar to the IDH1 mutation status.31 Moreover, clinical outcome is reported to be significantly better in patients with secondary GBM with IDH1 mutation. Such differentiation is of importance, as patients with an IDH1 mutation indicative of secondary GBM have been shown to a clinical course distinct from those patients with primary GBM.11,32 In previous studies addressing EOR and MGMT promoter methylation status, this difference was not taken into consideration, therefore possibly introducing bias.
In line with the literature, we considered IDH1 mutation as an indicator of secondary GBM and excluded patients with immunohistochemical staining for IDH1-R132H from our analysis, thereby creating a homogenous patient population. As a caveat, we may still have included a small number of patients with IDH2 mutations, or mutations of the IDH1 gene other than R132H (resulting from an IDH1 p.R132H mutation); however, IDH1-R132H mutations account for the vast majority of IDH mutations.29
In line with previously published data,8,23,24,33 our results confirm that GTR is able to prolong PFS and OS, when compared to incomplete resection. Further analysis of parameters associated with prolonged PFS and OS confirmed GTR as an independent parameter.
Analysis of MGMT methylation status demonstrated that patients with a methylated MGMT promoter displayed significantly longer PFS and OS. Analysis of independent variables associated with prolonged PFS or OS clearly highlighted MGMT promoter methylation status as a prognostic factor, which is in line with published data that did not discriminate between primary or secondary GBM patients.8
Other work assessing the effects of EOR and MGMT promoter methylation status on PFS and OS draw the conclusion that tumor biology far outweighs the prognostic impact of tumor resection.8 Although our data corroborate EOR and MGMT promotor methylation status as prognostic parameters, our analysis suggests that patient groups 2 (complete resection, MGMT promoter unmethylated) and 3 (incomplete resection, MGMT promoter methylated) have similar outcomes with regard to PFS and OS as reflected by a lack of significant difference between these groups. Furthermore, c-index-calculation does not support this conclusion: although minor, CPE of EOR is higher than that of MGMT promotor methylation status, indicating a better prediction of EOR based on the Cox regression analysis. Such observations may be due to the selection of a different patient populations (ie, including patients undergoing stereotactic biopsy and no respective surgery) as done by Kreth et al.8 Another explanation might be the exclusion of secondary GBM patients as done in our analysis.
Further assessment of pre- and postoperative tumor volume has recently resulted in a threshold of ≥78% for a linear increase of EOR and OS in a patient population not discriminating between MGMT promoter methylation status and IDH1 mutation.7 In another publication with demonstrated balance of MGMT promoter methylation status between GTR and non-GTR patients, no such threshold was observable.34 In our study, now taking into consideration both established molecular markers, thresholds at a very high EOR (≥99.5% EOR for PFS and ≥99.8% for OS) were necessary in order for surgery to elucidate a survival benefit. These data are contradictory to those previously published by Sanai et al.7 and it appears to be suggestive that a misdistribution of primary/secondary or MGMT promoter methylated/unmethylated patients might have been the underlying cause. Whether or not the threshold at this high rate of EOR is true or due to imprecise volumetric assessment,35 remains speculative. In line with our findings, Kreth et al8 found survival benefit only for patients undergoing GTR, but not subtotal resection. These data are further substantiated by the findings of Grabkowski et al. who identified a fairly high threshold for a beneficial EOR (98%) to display a survival benefit. While Grabkowski et al.34 identified a potential superiority of postoperative tumor volume over EOR as a predictor of survival in glioblastoma patients, we have not found such superiority but a high correlation of EOR and residual tumor volume (Pearson r = −0.976). While we acknowledge, that postoperative tumor volume might more meaningfully reflect the pathobiology of glioblastoma, our data do not support such an assumption as per the lack of a statistical difference.
Limitations
The nature of this retrospective study most certainly introduces a selection bias as not all patients admitted with newly diagnosed primary GBM underwent craniotomy and tumor removal. More specifically, patients with midline tumors/patients harboring a H3.3K27M mutation may have undergone only a diagnostic biopsy followed by a combinatory radio-/chemotherapy. However, the latter are now a distinct tumor entity as per the 2016 updated WHO classification and do not truly represent primary GBM. Likewise, GBM with IDH gene mutation are distinct from IDH-wild-type tumors. Although no statistical significance was noted, not all patients included for analysis underwent the same postoperative adjuvant treatment therefore introducing a possible bias; however, there was no statistically significant difference regarding outcome between patients receiving standard or experimental adjuvant therapy. As only patients treated in 1 surgical center were included in this analysis a sample bias cannot be excluded. Moreover, we only tested for mutated IDH1-R132H protein in patients with an IDH1 p.R132H mutation. Although being the most prominent IDH1 mutation, a minority of patients might have displayed other mutations of IDH1 or IDH2 36 and therefore remain undetected in regard to their mutation status.
CONCLUSION
Herein we utilized the neuro-oncologic markers as per the 2016 WHO update on the classification of CNS tumors37 (identifying patients with an IDH1 p.R132H mutation) and in so doing compared the influence of a major biological marker (MGMT promoter methylation status) and a surgically achievable factor (EOR) in primary GBM while controlling for IDH1 mutational status.
In this population of newly diagnosed primary GBM patients, EOR and MGMT promoter methylation status are associated with PFS and OS. Surgical resection remains beneficial after excluding the likely confounding effect(s) of IDH1 mutations, thus underlining the importance of GTR, if achievable. Between both EOR and MGMT methylation status c-Index analysis demonstrated no significant difference, with EOR displaying a higher CPE value, indicating a better prediction based on Cox regression models.
By taking the molecular markers IDH1 mutation and MGMT promoter methylation into consideration, our data also demonstrate favorable outcome for incompletely resected GBM patients in regard to PFS (≥99.5% EOR) and to OS (≥98.8% EOR).
The results reported within our analysis substantiate recent efforts to increase EOR in GBM patients. Our data may therefore influence caregivers in the decision-making process in which achievable EOR needs to be balanced against potential harm by surgical procedures.
Disclosure
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Acknowledgments
The authors thank Marina Heibel and Anne Sicking for assistance with the preparation of figures and tables.
Notes
Parts of the manuscript were presented at the Congress of Neurological Surgeons Annual Meeting, in Boston, Massachusetts, October 7-11 2017.
Supplemental Digital Content 1. Figure. Flow chart illustrating data extraction from patients with newly diagnosed glioblastoma. GBM = glioblastoma; MGMT = O6-methylguanine-DNA methyltransferase; MRI = magnetic resonance imaging; IDH1 = isocitrate dehydrogenase.
Supplemental Digital Content 2. Table. CPE analysis. Concordance probability estimate (CPE) based on the Cox proportional hazards model was calculated to evaluate the global discrimination power and the predictive accuracy. MGMT = O6-methylguanine-DNA methyltransferase; GTR = Gross total resection, KPS = Karnofsky performance scale, PFS = progression-free survival, OS = overall survival, CPE = concordance probability estimate, SE = standard error.
Supplemental Digital Content 3. Table. Cox-regression analysis for PFS (n = 172); KPS = Karnofsky performance scale; EOR = Extent of Resection; MGMT = O6-methylguanine-DNA methyltransferase; HR = Hazard ratio; CI = Confidence interval.
–: the variable was not selected in the stepwise Cox regression (using P = .1 as entry and stay).
Supplemental Digital Content 4. Table. Cox-regression analysis for OS (n = 175)a; KPS = Karnofsky performance scale; EOR = Extent of Resection; MGMT = O6-methylguanine-DNA methyltransferase; HR = Hazard ratio; CI = Confidence interval.
–the variable was not selected in the stepwise Cox regression (using P = .1 as entry and stay).
astratified (by sex) Cox-regression model was used since proportional hazard(PH) assumption for sex was violated.
REFERENCES
- 1. Louis DN, Ohgaki H, Wiestler OD et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3. Hegi ME, Diserens AC, Gorlia T et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 4. McGirt MJ, Chaichana KL, Gathinji M et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110(1):156–162. [DOI] [PubMed] [Google Scholar]
- 5. Stummer W, van den Bent MJ, Westphal M. Cytoreductive surgery of glioblastoma as the key to successful adjuvant therapies: new arguments in an old discussion. Acta Neurochir. 2011;153(6):1211–1218. [DOI] [PubMed] [Google Scholar]
- 6. Stummer W, Pichlmeier U, Meinel T et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401. [DOI] [PubMed] [Google Scholar]
- 7. Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3–8. [DOI] [PubMed] [Google Scholar]
- 8. Kreth FW, Thon N, Simon M et al. Gross total but not incomplete resection of glioblastoma prolongs survival in the era of radiochemotherapy. Ann Oncol. 2013;24(12):3117–3123. [DOI] [PubMed] [Google Scholar]
- 9. Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013;19(4):764–772. [DOI] [PubMed] [Google Scholar]
- 10. Lai A, Kharbanda S, Pope WB et al. Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. J Clin Oncol. 2011;29(34):4482–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nobusawa S, Watanabe T, Kleihues P, Ohgaki H. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res. 2009;15(19):6002–6007. [DOI] [PubMed] [Google Scholar]
- 12. Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol. 2011;12(11):997–1003. [DOI] [PubMed] [Google Scholar]
- 13. Macdonald DR, Cascino TL, Schold SC Jr., Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 14. Gessler F, Zappi J, Konczalla J et al. Secondary glioblastoma: molecular and clinical factors that affect outcome after malignant progression of a lower grade tumor. World Neurosurg. 2017;102:49–55. doi:10.1016/j.wneu.2017.02.104. [DOI] [PubMed] [Google Scholar]
- 15. Gessler F, Baumgarten P, Bernstock JD et al. Assessment of molecular markers demonstrates concordance between samples acquired via stereotactic biopsy and open craniotomy in both anaplastic astrocytomas and glioblastomas. J Neurooncol. 2017;133(2):399–407. [DOI] [PubMed] [Google Scholar]
- 16. Allison PD. , Institute SAS. Survival analysis using SAS: a practical guide 2010. [Google Scholar]
- 17. Gönen M, Heller G. Concordance probability and discriminatory power in proportional hazards regression. Biometrika. 2005;92(4):965–970. [Google Scholar]
- 18. Krex D, Klink B, Hartmann C et al. Long-term survival with glioblastoma multiforme. Brain. 2007;130(10):2596–2606. [DOI] [PubMed] [Google Scholar]
- 19. Martinez R, Schackert G, Yaya-Tur R, Rojas-Marcos I, Herman JG, Esteller M. Frequent hypermethylation of the DNA repair gene MGMT in long-term survivors of glioblastoma multiforme. J Neurooncol. 2007;83(1):91–93. [DOI] [PubMed] [Google Scholar]
- 20. Gorlia T, van den Bent MJ, Hegi ME et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. Lancet Oncol. 2008;9(1):29–38. [DOI] [PubMed] [Google Scholar]
- 21. Esteller M, Garcia-Foncillas J, Andion E et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343(19):1350–1354. [DOI] [PubMed] [Google Scholar]
- 22. Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res. 1999;59(4):793–797. [PubMed] [Google Scholar]
- 23. Lacroix M, Abi-Said D, Fourney DR et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–198. [DOI] [PubMed] [Google Scholar]
- 24. Stummer W, Reulen HJ, Meinel T et al. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62(3):564–576; discussion 564-576. [DOI] [PubMed] [Google Scholar]
- 25. Jakola AS, Myrmel KS, Kloster R et al. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA. 2012;308(18):1881–1888. [DOI] [PubMed] [Google Scholar]
- 26. Smith JS, Chang EF, Lamborn KR et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26(8):1338–1345. [DOI] [PubMed] [Google Scholar]
- 27. Roelz R, Strohmaier D, Jabbarli R et al. Residual tumor volume as best outcome predictor in low grade glioma—a nine-years near-randomized survey of surgery vs. biopsy. Sci Rep. 2016;6:32286, doi:10.1038/srep32286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parsons DW, Jones S, Zhang X et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Capper D, Weissert S, Balss J et al. Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol. 2010;20(1):245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170(5):1445–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64(6):479–489. [DOI] [PubMed] [Google Scholar]
- 32. Okamoto Y, Di Patre PL, Burkhard C et al. Population-based study on incidence, survival rates, and genetic alterations of low-grade diffuse astrocytomas and oligodendrogliomas. Acta Neuropathol. 2004;108(1):49–56. [DOI] [PubMed] [Google Scholar]
- 33. Laws ER, Parney IF, Huang W et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003;99(3):467–473. [DOI] [PubMed] [Google Scholar]
- 34. Grabowski MM, Recinos PF, Nowacki AS et al. Residual tumor volume versus extent of resection: predictors of survival after surgery for glioblastoma. J Neurosurg. 2014;121(5):1115–1123. [DOI] [PubMed] [Google Scholar]
- 35. Kubben PL, Postma AA, Kessels AG, van Overbeeke JJ, van Santbrink H. Intraobserver and interobserver agreement in volumetric assessment of glioblastoma multiforme resection. Neurosurgery. 2010;67(5):1329–1334. [DOI] [PubMed] [Google Scholar]
- 36. Beiko J, Suki D, Hess KR et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol. 2014;16(1):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Louis DN, Perry A, Reifenberger G et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.