Abstract
The macrocyclic lactone anthelmintics are the only class of drug currently used to prevent heartworm disease. Their extremely high potency in vivo is not mirrored by their activity against Dirofilaria immitis larvae in vitro, leading to suggestions that they may require host immune functions to kill the parasites. We have previously shown that ivermectin stimulates the binding of canine peripheral blood mononuclear cells (PBMCs) and polymorphonuclear leukocytes (PMNs) to D. immitis microfilariae (Mf). We have now extended these studies to moxidectin and examined the ability of both drugs to stimulate canine PBMC and PMN attachment to Mf from multiple strains of D. immitis, including two that are proven to be resistant to ivermectin in vivo. Both ivermectin and moxidectin significantly increased the percentage of drug-susceptible parasites with cells attached at very low concentrations (<10 nM), but much higher concentrations of ivermectin (>100 nM) were required to increase the percentage of the two resistant strains, Yazoo-2013 and Metairie-2014, with cells attached. Moxidectin increased the percentage of the two resistant strains with cells attached at lower concentrations (<10 nM) than did ivermectin. The attachment of the PBMCs and PMNs did not result in any parasite killing in vitro. These data support the biological relevance of the drug-stimulated attachment of canine leukocytes to D. immitis Mf and suggest that this phenomenon is related to the drug resistance status of the parasites.
Keywords: Dirofilaria immitis, Ivermectin, Moxidectin, Polymorphonuclear leukocytes, Peripheral blood mononuclear cells, Drug resistance
Graphical abstract
Highlights
-
•
Ivermectin promotes attachment of PMN and PBMC to D. immitis microfilariae in vitro.
-
•
Moxidectin has a similar effect.
-
•
Higher ivermectin concentrations are needed if Mf of ML-resistant strains are used.
-
•
Moxidectin is more effective at promoting cell attachment to resistant Mf.
-
•
Neither PMN nor PBMC attachment does not result in parasite death in vitro.
1. Introduction
The macrocyclic lactone anthelmintics, which include ivermectin and moxidectin, are the only class of drug currently commercially available for the prevention of heartworm disease in dogs and cats caused by Dirofilaria immitis. These drugs are labelled to be 100% effective at preventing the development of third stage larvae (L3) from susceptible parasites to adulthood when used at very low doses (Hampshire, 2005). At higher doses, they are also effective at removing microfilariae (Mf) from the circulation of infected animals (Bowman and Mannella, 2011). However, in vitro incubation of Mf or L3 with concentrations of ivermectin equivalent to those present in infected animals has little effect on the parasites motility or ability to migrate (Bourguinat et al., 2011; Evans et al., 2013; Storey et al., 2014). This has led to speculation that host factors may be required for their full anthelmintic activity in vivo and attention has focused on the immune system (Wolstenholme et al., 2016; Carithers, 2017). We have previously shown that ivermectin (an avermectin) stimulates the attachment of polymorphonuclear leukocytes (PMNs) and peripheral blood mononuclear cells (PBMCs) from naïve dogs to D. immitis Mf (Vatta et al., 2014) in vitro, but did not examine the ability of milbemycins to do this. In addition, the drug-susceptible Missouri strain used in this study had been maintained in the laboratory by FR3 for many years (Michalski et al., 2011) prior to the experiments being carried out, and we were concerned that this might have affected its performance in this assay. We have therefore extended our experiments to include a more recently isolated susceptible strain, Georgia-2, and to study the ability of moxidectin to stimulate leukocyte attachment to the Mf of both strains.
In recent years, it has been confirmed that D. immitis parasites resistant to the macrocyclic lactones are circulating in the United States (Pulaski et al., 2014; Bourguinat et al., 2015; Blagburn et al., 2016; Maclean et al., 2017). In our hands, in vitro motility and migration assays have proved to be ineffective at differentiating resistant from susceptible parasites, either at the Mf or L3 stages (Evans et al., 2017; Maclean et al., 2017) and only the in vivo Mf suppression test seems to provide a reliable way of identifying drug-resistant D. immitis (Geary et al., 2011) without requiring the euthanasia of infected animals. If our observation that ivermectin stimulated the in vitro binding of leukocytes from uninfected dogs to D. immitis Mf is relevant to the in vivo anthelmintic efficacy of the drug, then we would predict that this effect would be reduced when Mf of resistant isolates were tested. There are conflicting accounts of the efficacy of moxidectin as a preventative against these resistant worms, which may reflect the dose of drug the parasite is exposed to following different routes of administration. One resistant isolate, JYD-34, was reported to remain susceptible to topically applied moxidectin (Blagburn et al., 2016), in that no adult worms were present at necropsy following experimental infection with infective L3 larvae and treatment, but to be resistant to either one or three oral doses (3 μg/kg) of the drug (McTier et al., 2017). Another isolate, Jd2009-2, was fully resistant to a long-acting injectable formulation of the drug given 6 months after infection (Bourguinat et al., 2015). We therefore compared the ability of ivermectin and moxidectin to increase the in vitro attachment of canine PMNs and PBMCs to Mf of JYD-27, which was isolated by the FR3 from a blood sample from the same donor dog that gave rise to the JYD-34 strain, and of two other resistant strains of D. immitis, Yazoo-2013 and Metairie-2014 (Maclean et al., 2017), in addition to two susceptible strains, Missouri (Michalski et al., 2011; Bowman et al., 2016) and Georgia-2.
2. Materials and methods
2.1. Parasites
All animal experiments were carried out in strict accordance with national and local guidelines and were approved by the University of Georgia IACUC (AUP# A2016 10-006, A2017 04-11, and A2017 05-002). The Missouri and JYD-27 strains of D. immitis were provided by the NIH/NIAID Filariasis Research Reagent Resource Center (www.filariasiscenter.org). The Georgia-2 strain was a kind gift from TRS Labs Inc., Athens, GA. The Yazoo-2013 and Metairie-2014 strains have been described previously (Maclean et al., 2017). Microfilariae isolation was based on the method of Franks and Stoll (1945). Blood from infected dogs was collected in heparinized tubes and centrifuged for 30 min at 1200×g at room temperature. The top layer of plasma was removed, and the mass of red blood cells and Mf was brought back to its original volume with 3.8% (w/v) saline-citrate (38 mg sodium citrate/100 mL physiological saline). 1 ml of 15% (w/v) saponin (Sigma-Aldrich, St Louis, United States) was added for every 15 mL of original volume and the tube was shaken for 30 s. The mixture was centrifuged for 30 min at 1200×g. The supernatant was discarded, and sodium-citrate was used to bring the volume back to 15 mL. The mixture was then centrifuged for 4 min at 1200×g. The worm mixture was transferred to a new conical tube and mixed with 1× PBS (10 mL). The PBS and Mf mixture was centrifuged for 5 min at 1200×g to pellet the Mf and the supernatant was removed. The pellet was resuspended in RPMI (Catalog # 11875-093, Sigma-Aldrich, St Louis, United States) prior to assessment of worm numbers.
2.2. Cell isolation
Blood from uninfected dogs was drawn by jugular punctures and put into heparinized tubes. Blood (10 mL) was transferred from the heparinized tube into a sterile, endotoxin free conical tube (50 mL) with 1:1 PBS. Two donor dogs were used, a female purpose-bred beagle and a neutered male purpose-bred mixed breed; neither dog had any history of exposure to heartworms nor of treatment with any macrocyclic lactone. The blood was then underlaid with Histopaque 1077 (5–10 mL) (Sigma-Aldrich, St Louis, United States) with an 18-gauge needle and a sterile syringe. The gradient was then centrifuged at 400×g at room temperature for 25 min with the brake off. The top layer of plasma was discarded and the middle layer, the PBMC layer, was placed in a separate sterile conical tube. Forty mls ACK buffer (155 mM ammonium chloride, 10 mM potassium hydrogen carbonate, 0.1 mM EDTA, pH 7.3) was added to the red blood cell/PMN mixture and gently mixed by inverting the tube. The blood mixture was set to rest for 5 min at room temperature in order for the red blood cells to lyse. The mixture was centrifuged for 5 min at 400×g to pellet PMNs. The PMN pellet was washed with PBS and re-suspended in 10 mls PBS-2% BSA then centrifuged again at 400×g for 5 min. After removing the supernatant, the pellet was re-suspended in 500 μl RPMI and 500 μl serum (Li et al., 2011).
2.3. Leukocyte attachment assays
Assays were set up essentially as described by Vatta et al. (2014). Each assay was performed in triplicate in a 96-well plate with a minimum of 4 biological replicates for each strain and drug combination. Each biological replicate is defined as independent Mf isolations in different weeks from the same dog. Each well contained 100 Mf of the strain under test, 20,000 cells (PBMCs or PMNs) and 10% uninfected dog serum in RPMI. The drug concentrations tested were 1, 3, 10, 30, 100, 300 and 1,000 nM, plus a vehicle (1% DMSO) control. The assays were incubated at 37 °C in a 5% CO2 atmosphere for 24 h (PMN) or 40 h (PBMC), before being visually scored. Attachment in this assay was defined by a motile Mf having at least one cell attached. Static worms were considered to be dead and were not counted. Since the background level of cell attachment did vary between Mf preparations, only those biological replicates where an average of less than 20% of Mf had any cells attached in the absence of any drug were analyzed further. In some cases this reduced the number of experiments analyzed, but at least three complete datasets were obtained for all strain/drug combinations.
2.4. Data analysis
Data were analyzed using Graphpad Prism®, v5 (GraphPad Software, Inc., San Diego, United States). Cell attachment within each strain was compared using 2-way ANOVA and Tukey's post-hoc test, using p < 0.05 as the level of significance.
3. Results
3.1. Ivermectin increases PMN and PBMC attachment to Mf from susceptible strains, but the effect is less marked on resistant parasites
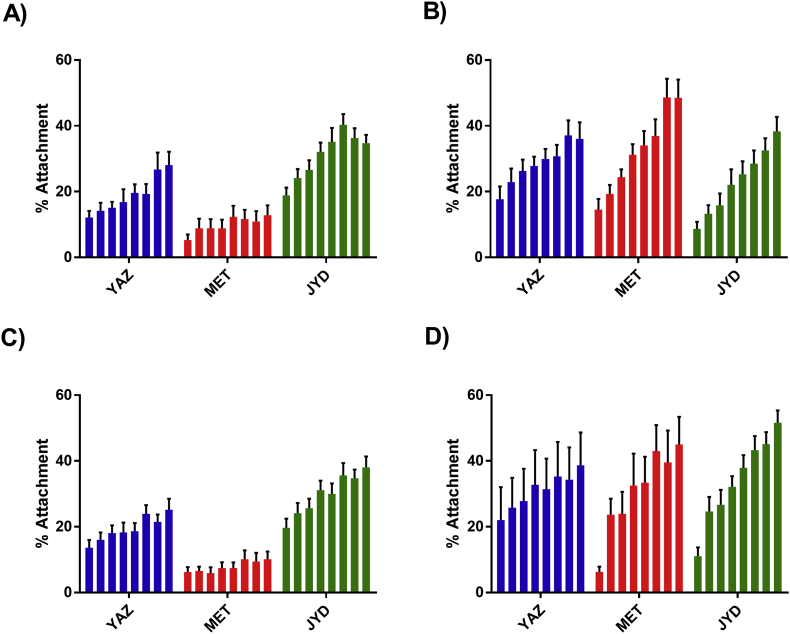
When purified canine PMNs and PBMCs isolated from uninfected dogs were cultured with D. immitis Mf we found that a low percentage of the parasites had cells attached to them after 16 h (PMNs) or 40 h (PBMCs), as previously reported (Fig. 1) (Vatta et al., 2014). This proportion did vary between Mf preparations, but for the purposes of this study we only considered experiments (biological replicates) where the number of Mf with cells attached in the control wells (absence of drug) was less than 20%. The addition of ivermectin to the cultures increased the percentage of the Mf with both PMNs and PBMCs attached in a concentration-dependent manner for nearly all the strain/cell type combinations tested, confirming the results previously reported for the Missouri strain (Vatta et al., 2014). The concentration at which a statistically significant increase in the percentage of Mf with cells attached over the no-drug controls was observed did vary between strains (Table 1). In general, the proportion of Mf with cells attached increased with the drug concentration, but attempts to fit conventional dose-response equations to the data were largely unsuccessful; in those cases where this was possible the slope of the curve generated was very low resulting in a very large degree of uncertainty in the estimates of EC50 values produced. For the Missouri and Georgia-2 strains, both of which are susceptible to current preventatives, 1–3 nM ivermectin was sufficient to cause a significant increase in the percentage of Mf with both PMNs and PBMCs attached. For the resistant Metairie-2014 and Yazoo-2013 strains, higher drug concentrations were required, 100 nM or greater for Yazoo-2013 and 1 μM or greater for Metairie-2014 (Fig. 2 & Table 1); there was no significant increase in the percentage of Metairie-2014 Mf with PBMCs attached at any concentration of ivermectin. In this assay, the Mf of the supposed resistant JYD-27 strain gave results that were more similar to those of the susceptible Missouri and Georgia-2 than the other resistant strains; ivermectin concentrations of 3–10 nM caused a statistically significant increase in the percentage of Mf with cell attachment. We also observed that the maximum percentage of the Mf with cells attached varied between the strains, even at the highest concentration tested (1 μM) (Table 2). When incubated with 1 μM ivermectin, 13% of the Metairie-2014 Mf had PMNs attached compared to 48% of the Missouri Mf; for the PBMCs the range was 10% of Metairie-2014 to 71% of the Georgia-2 Mf having cells attached.
Fig. 1.
Effect of ivermectin and moxidectin on PMN and PBMC attachment to microfilariae of the Missouri and Georgia-2 strains. A) Effect of ivermectin on attachment of PMNs. B) Effect of moxidectin on attachment of PMNs. C) Effect of ivermectin on attachment of PBMCs. D) Effect of moxidectin on attachment of PBMCs. For each panel the bars represent the average percentage of Mf with at least one cell attached at varying concentrations of the drug; from left to right these were 0, 1, 3, 10, 30, 100, 300 and 1000 nM. MO = Missouri, GA-2 = Georgia-2. In each case N = 4, each experiment carried out in triplicate. Error bars indicate the standard error of the mean.
Table 1.
The lowest drug concentration [ivermectin (IVM) or moxidectin (MOX)] at which a statistically significant increase (p=<0.05), judged by 2-way ANOVA, in cell attachment was observed compared to the no-drug control.
| Strain | Missouri | Georgia-2 | JYD-27 | Yazoo-2013 | Metairie-2014 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Drug | IVM | MOX | IVM | MOX | IVM | MOX | IVM | MOX | IVM | MOX |
| PMN | 3 nM | 3 nM | 1 nM | 3 nM | 3 nM | 10 nM | 100 nM | 3 nM | 1 μMa | 10 nM |
| PBMC | 3 nM | 1 nM | 1 nM | 1 nM | 10 nM | 1 nM | 100 nM | 10 nM | >1 μMa | 1 nM |
1 μM was the highest concentration of either drug tested.
Fig. 2.
Effect of ivermectin and moxidectin on PMN and PBMC attachment to microfilariae of D. immitis strains with suspected resistance against at least one macrocyclic lactone. A) Effect of ivermectin on attachment of PMNs. B) Effect of moxidectin on attachment of PMNs. C) Effect of ivermectin on attachment of PBMCs. D) Effect of moxidectin on attachment of PBMCs. For each panel the bars represent the average percentage of Mf with at least one cell attached at varying concentrations of the drug; from left to right these were 0, 1, 3, 10, 30, 100, 300 and 1000 nM. YAZ = Yazoo-2013, MET = Metairie-2014, JYD = JYD-27. In each case N = 4-5, each experiment carried out in triplicate. Error bars indicate the standard error of the mean.
Table 2.
Percentage (±SEM) of Mf with cells attached after incubation with 1 μM ivermectin (IVM) or moxidectin (MOX), rounded to the nearest whole number.
| Strain | Missouri | Georgia-2 | JYD-27 | Yazoo-2013 | Metairie-2014 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Drug | IVM | MOX | IVM | MOX | IVM | MOX | IVM | MOX | IVM | MOX |
| PMN | 48 ± 6% | 65 ± 5% | 41 ± 6% | 54 ± 6% | 33 ± 4% | 35 ± 4% | 25 ± 5% | 28 ± 5% | 13 ± 3% | 42 ± 6% |
| PBMC | 60 ± 3% | 63 ± 5% | 71 ± 5% | 63 ± 5% | 33 ± 3% | 52 ± 4% | 18 ± 4% | 19 ± 3% | 10 ± 2% | 49 ± 8% |
3.2. Moxidectin has similar effects to ivermectin, but was more effective at promoting attachment to Mf from Metairie-2014 and Yazoo-2013
In general, when we repeated the attachment experiments using moxidectin instead of ivermectin, the results were very similar (Fig. 1, Fig. 2), with a concentration dependent increase in the percentage of Missouri, Georgia-2 and JYD-27 Mf with both PMNs and PBMCs attached. However, a concentration-dependent increased percentage of Metairie-2014 Mf with cells attached was also observed with moxidectin, in contrast to ivermectin. The concentration of moxidectin at which a significant increase in the percentage of Mf with attachment was observable with this strain was lower than with ivermectin (Table 1) and the maximum percentage of Mf with cells attached was higher (Table 2). Both the concentration of moxidectin at which a significant increase in the percentage of Mf with attachment was observable and the maximum percentage of cells attached by concentration were similar to the susceptible strains. The results with Yazoo-2013 were more equivocal; the concentration of moxidectin at which increased attachment was observed was similar to all the other strains at 3–10 nM (Table 1), but the proportion of the Mf with cells attached tended to be lower (<30% at 1 μM).
3.3. PBMC/PML binding has no effect on Mf survival
The hypothesis underlying these experiments is that the macrocyclic lactones may interact with the host immune system against filarial parasites (Wolstenholme et al., 2016; Carithers, 2017). Previous studies on PBMC and PML attachment to Brugia malayi Mf in vitro have shown that this results in the death of the parasites after five days in culture, and that death was increased when the Mf were incubated with a mixture of both PBMCs and PMLs (McCoy et al., 2017). We postulated that the drug-induced increase in PBMC and PML attachment to D. immitis Mf that we have observed here and in previous studies (Vatta et al., 2014) would result in the death of the parasites. We therefore tested the survival of the Mf of the Missouri strain when cultured with a mixed population of PBMCs and PMLs in the presence of varying concentrations of ivermectin (Fig. 3). Although increased attachment of the cells was observed in the presence of the drug, no difference in the survival of the Mf was observed at any concentration of the drug after 5 days incubation.
Fig. 3.

Survival of Missouri Mf in the presence of canine PBMCs and varying concentrations of ivermectin. Mf were cultured together with PBMCs for 5 days in different concentrations of ivermectin, and the number of motile larvae counted as a proportion of those originally added. N = 3. Error bars indicate the standard error of the mean.
4. Discussion
There have been reports over many years that ivermectin increases attachment to or killing of microfilariae of various species by granulocytes or peripheral blood mononuclear cells in vitro (Rao et al., 1987; Zahner et al., 1997) and we extended these observations to D. immitis and canine PBMC and PMNs (Vatta et al., 2014). However, the relevance of these in vitro observations to the possible in vivo anthelmintic efficacy has been difficult to assess. We have approached this problem in two ways; if the in vitro attachment is relevant to the in vivo efficacy of the drugs then other members of the avermectin/milbemycin anthelmintic class should also promote it, and at pharmacologically relevant concentrations. The recent confirmation that resistance to the macrocyclic lactone anthelmintics has emerged in D. immitis (Pulaski et al., 2014; Bourguinat et al., 2015; Wolstenholme et al., 2015) allowed us to test two strains with confirmed ivermectin resistance (Maclean et al., 2017). If the leukocyte attachment is relevant to the drug's in vivo anthelmintic efficacy, then we would predict that attachment would be reduced in the presence of the drug to Mf of resistant strains as opposed to those of susceptible ones.
In general those predictions were supported by the data we obtained. In the absence of the drugs, cell attachment to the Mf was usually very low, with less than 20% of the parasites having any cells attached. In a few experiments the background attachment was higher; this may have reflected the Mf used in these assays, as work with B. malayi showed that variations in the attachment of human cells resulted from variability in the parasites used, not the human donors (Reaves et al., 2018). Presumably PBMCs and PMLs are better able to recognize damaged or unhealthy Mf, and the parasites could have been damaged during their isolation from blood. With B. malayi and human cells, PBMC and PML attachment varied between experiments and it was originally postulated (McCoy et al., 2017) that this reflected donor to donor variation, though this was later shown not to be the case (Reaves et al., 2018). Resource limitations meant that we could only test cells and serum from two uninfected donor dogs; no difference was observed in the results obtained between them.
Ivermectin and moxidectin both increased cellular attachment to the Missouri and Georgia-2 Mf at very low concentrations (<10 nM) which correspond to those reported to be present in the plasma of treated dogs (Gokbulut et al., 2006; McKellar and Gokbulut, 2012). The effects of ivermectin on attachment to the resistant Metairie-2014 and Yazoo-2013 strains were much less marked (Fig. 2). These results suggest that the in vitro drug-promoted leukocyte attachment is indeed relevant to the full in vivo potential of the macrocyclic lactone anthelmintics and support the hypothesis that the host immune response is required for the heartworm prevention provided by these drugs (Wolstenholme et al., 2016; Carithers, 2017). They also suggest that the Mf are a suitable surrogate life-stage for studying resistance in D. immitis, supporting the utility of the Mf suppression test as a diagnostic tool for resistance in infected dogs (Geary et al., 2011).
Perhaps surprisingly, our data suggest that the JYD-27 strain, isolated from the same dog as the JYD-34 strain (Blagburn et al., 2016; Bourguinat et al., 2017), may not be drug-resistant. The Mf we used in this study were supplied by FR3 and the infected dog that was the source of the Mf had never been treated with any heartworm preventative; when JYD-27 infective L3 larvae were used in an attempt to infect an animal that did receive preventative no patent infection was established (A. Moorhead, personal communication). This suggests that the JYD source animal was either co-infected with both resistant and susceptible parasites, or that the isolate evolved towards drug susceptibility over time; perhaps both processes took place. Resource limitations meant that only a limited number of experimental infections could be performed, making it difficult to draw too many conclusions from these parasites.
Moxidectin was more effective at promoting attachment to the Metairie-2014 and Yazoo-2013 Mf than was ivermectin (Table 1). With other parasitic nematodes, moxidectin has been shown to be effective against ivermectin-resistant isolates (Kaplan et al., 2007; Prichard et al., 2012) and a moxidectin-containing heartworm preventative was more effective against a resistant isolate (JYD-34) than an ivermectin-containing one (Blagburn et al., 2016), though another isolate, Jd2009-1, was resistant to moxidectin (Bourguinat et al., 2015). This implies that at least some of the resistant heartworm isolates currently in circulation may retain some susceptibility to moxidectin, though this may vary depending on the effective drug concentration the parasites are exposed to. This in turn will be influenced by the route of administration; different preparations, one topical, the other a long-lasting injectable were used by Blagburn et al. (2016) and Bourguinat et al. (2015) respectively. McTier et al. (2017) reported that JYD-34 was resistant to oral administration of moxidectin. In small ruminants the use of moxidectin to control ivermectin-resistant nematodes resulted in the rapid development of moxidectin resistance (Kaplan et al., 2007) which suggests that the use of moxidectin-based preventatives will provide only a short-term solution, if any.
The attachment of human leukocytes to Brugia malayi Mf results in the death of the parasites (McCoy et al., 2017), and this is mediated by both PBMC and PMNs though the formation of DNA-based extracellular traps is not involved. In contrast, we never observed any significant death of the Mf of any of the D. immitis strains we studied here, even after 5 days of incubation in the presence of high concentrations of ivermectin (Fig. 3). D. immitis Mf, like those of B. malayi, are able to induce the formation of DNA-based extracellular traps (Munoz-Caro et al., 2018) but the lack of any killing observed in our assays suggests that, as with B. malayi, these are not sufficient to cause parasite death. In vivo, it is possible that the attachment of PBMCs and/or PMLs to the D. immitis Mf will lead to their removal from the circulation, even if they are not directly killed by the cells. Ivermectin and moxidectin are both active at nematode glutamate-gated chloride channels (Wolstenholme and Rogers, 2005; Prichard et al., 2012), and these are expressed in the excretory/secretory pore of B. malayi Mf (Moreno et al., 2010). Ivermectin inhibits secretion from B. malayi (Moreno et al., 2010; Harischandra et al., 2018); if the macrocyclic lactones have the same effect on D. immitis Mf, this would be consistent with a model in which the macrocyclic lactone anthelmintic drugs inhibit the parasites’ ability to manipulate and evade host immunity. This effect would be reduced in drug-resistant strains, whatever the mechanism of resistance.
At present, we do not have a convenient in vitro test for drug resistance in D. immitis, and this is limiting our ability to survey its current prevalence. Though aspects of our current protocol, such as the need for serum and freshly isolated cells from an uninfected dog, make it inconvenient for development as a diagnostic or survey tool at present it may be possible to refine it to be more useful. This would require extensive testing on Mf taken from field infections, which may consist of infections with parasites of a varying resistance status. Regardless of whether or not that proves to be possible, we believe that the results presented here clearly show that drug-promoted attachment of PBMCs and PMNs to D. immitis Mf is relevant to the mode of action of the macrocyclic lactones, providing additional evidence for a relevant contribution of the host immune system in the prevention of a fatal filarioid disease.
Acknowledgements
This work was supported in part by a grant from Bayer Animal Health GmbH, Leverkusen, Germany and by award R01AI103140 from the National Institutes of Health. We should like to thank TRS Labs, Inc. for the Georgia-2 strain of D. immitis and the NIH/NIAID Filariasis Research Reagent Resource Center (www.filariasiscenter.org) for the Missouri and JYD-27 parasites. We should also like to thank Dr. Andrew Moorhead for useful discussions and for sharing unpublished data on the JYD-27 strain.
Contributor Information
Tessa Berrafato, Email: tess.berrafato@gmail.com.
Ruby Coates, Email: rc715@cam.ac.uk.
Barbara J. Reaves, Email: bjreaves@uga.edu.
Daniel Kulke, Email: daniel.kulke@bayer.com.
Adrian J. Wolstenholme, Email: adrianw@uga.edu.
References
- Blagburn B.L., Arther R.G., Dillon A.R., Butler J.M., Bowles J.V., von Simson C., Zolynas R. Efficacy of four commercially available heartworm preventive products against the JYD-34 laboratory strain of Dirofilaria immitis. Parasites Vectors. 2016;9:191. doi: 10.1186/s13071-016-1476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguinat C., Keller K., Blagburn B., Schenker R., Geary T.G., Prichard R.K. Correlation between loss of efficacy of macrocyclic lactone heartworm anthelmintics and P-glycoprotein genotype. Vet. Parasitol. 2011;176:374–381. doi: 10.1016/j.vetpar.2011.01.024. [DOI] [PubMed] [Google Scholar]
- Bourguinat C., Lee A.C.Y., Lizundia R., Blagburn B.L., Liotta J.L., Kraus M.S., Keller K., Epe C., Letourneau L., Kleinman C.L., Paterson T., Gomez E.C., Montoya-Alonso J.A., Smith H., Bhan A., Peregrine A.S., Carmichael J., Drake J., Schenker R., Kaminsky R., Bowman D.D., Geary T.G., Prichard R.K. Macrocyclic lactone resistance in Dirofilaria immitis: failure of heartworm preventives and investigation of genetic markers for resistance. Vet. Parasitol. 2015;210:167–178. doi: 10.1016/j.vetpar.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Bourguinat C., Lefebvre F., Sandoval J., Bondesen B., Moreno Y., Prichard R.K. Dirofilaria immitis JYD-34 isolate: whole genome analysis. Parasites Vectors. 2017;10:494. doi: 10.1186/s13071-017-2437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman D.D., Grazette A.R., Basel C., Wang Y.Y., Hostetler J.A. Protection of dogs against canine heartworm infection 28 days after four monthly treatments with Advantage Multi (R) for Dogs. Parasites Vectors. 2016;9:12. doi: 10.1186/s13071-016-1293-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman D.D., Mannella C. Macrocyclic lactones and Dirofilaria immitis microfilariae. Top. Companion Anim. Med. 2011;26:160–172. doi: 10.1053/j.tcam.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Carithers D.S. Examining the role of macrolides and host immunity in combatting filarial parasites. Parasites Vectors. 2017;10:182. doi: 10.1186/s13071-017-2116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C.C., Moorhead A.R., Storey B.E., Blagburn B.L., Wolstenholme A.J., Kaplan R.M. Evaluation of the larval migration inhibition assay for detecting macrocyclic lactone resistance in Dirofilaria immitis. Vet. Parasitol. 2017;246:76–81. doi: 10.1016/j.vetpar.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Evans C.C., Moorhead A.R., Storey B.E., Wolstenholme A.J., Kaplan R.M. Development of an in vitro bioassay for measuring susceptibility to macrocyclic lactone anthelmintics in Dirofilaria immitis. Int. J. Parasitol. - Drugs Drug Res. 2013;3:102–108. doi: 10.1016/j.ijpddr.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks M.B., Stoll N.R. The isolation of microfilariae from blood for use as antigen. J. Parasitol. 1945;31:158–162. [Google Scholar]
- Geary T.G., Bourguinat C., Prichard R.K. Evidence for macrocyclic lactone anthelmintic resistance in Dirofilaria immitis. Top. Companion Anim. Med. 2011;26:186–192. doi: 10.1053/j.tcam.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Gokbulut C., Karademir U., Boyacioglu M., McKellar Q.A. Comparative plasma dispositions of ivermectin and doramectin following subcutaneous and oral administration in dogs. Vet. Parasitol. 2006;135:347–354. doi: 10.1016/j.vetpar.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Hampshire V.A. Evaluation of efficacy of heartworm preventive products at the FDA. Vet. Parasitol. 2005;133:191–195. doi: 10.1016/j.vetpar.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Harischandra H., Yuan W., Loghry H.J., Zamanian M., Kimber M.J. Profiling extracellular vesicle release by the filarial nematode Brugia malayi reveals sex-specific differences in cargo and a sensitivity to ivermectin. PLoS Neglected Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R.M., Vidyashankar A.N., Howell S.B., Neiss J.M., Williamson L.H., Terrill T.H. A novel approach for combining the use of in vitro and in vivo data to measure and detect emerging moxidectin resistance in gastrointestinal nematodes of goats. Int. J. Parasitol. 2007;37:795–804. doi: 10.1016/j.ijpara.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Li J., Birkenheuer A.J., Marr H.S., Levy M.G., Yoder J.A., Nordone S.K. Expression and function of triggering receptor expressed on myeloid cells-1 (TREM-1) on canine PMNs. Dev. Comp. Immunol. 2011;35:872–880. doi: 10.1016/j.dci.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Maclean M.J., Savadelis M.D., Coates R., Dzimianski M.T., Jones C., Benbow C., Storey B.E., Kaplan R.M., Moorhead A.R., Wolstenholme A.J. Does evaluation of in vitro microfilarial motility reflect the resistance status of Dirofilaria immitis isolates to macrocyclic lactones? Parasites Vectors. 2017;10:480. doi: 10.1186/s13071-017-2436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy C.J., Reaves B.J., Giguere S., Coates R., Rada B., Wolstenholme A.J. Human leukocytes kill Brugia malayi microfilariae independently of DNA-based extracellular trap release. PLoS Neglected Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKellar Q.A., Gokbulut C. Pharmacokinetic features of the antiparasitic macrocyclic lactones. Curr. Pharmaceut. Biotechnol. 2012;13:888–911. doi: 10.2174/138920112800399194. [DOI] [PubMed] [Google Scholar]
- McTier T.L., Six R.H., Pullins A., Chapin S., McCall J.W., Rugg D., Maeder S.J., Woods D.J. Efficacy of oral moxidectin against susceptible and resistant isolates of Dirofilaria immitis in dogs. Parasites Vectors. 2017;10:482. doi: 10.1186/s13071-017-2429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalski M.L., Griffiths K.G., Williams S.A., Kaplan R.M., Moorhead A.R. The NIH-NIAID Filariasis Research reagent Resource center. PLoS Neglected Trop. Dis. 2011;5 doi: 10.1371/journal.pntd.0001261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno Y., Nabhan J.F., Solomon J., Mackenzie C.D., Geary T.G. Ivermectin disrupts the function of the excretory-secretory apparatus in microfilariae of Brugia malayi. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20120–20125. doi: 10.1073/pnas.1011983107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Caro T., Conejeros I., Zhou E., Pikhovych A., Gartner U., Hermosilla C., Kulke D., Taubert A. Dirofilaria immitis microfilariae and third-stage larvae induce canine NETosis resulting in different types of PMN extracellular traps. Front. Immunol. 2018;9:968. doi: 10.3389/fimmu.2018.00968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard R., Menez C., Lespine A. Moxidectin and the avermectins: consanguinity but not identity. Int. J. Parasitol.-Drugs Drug Res. 2012;2:134–153. doi: 10.1016/j.ijpddr.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulaski C.N., Malone J.B., Bourguinat C., Prichard R., Geary T., Ward D., Klei T.R., Guidry T., Smith G.B., Delcambre B., Bova J., Pepping J., Carmichael J., Schenker R., Pariaut R. Establishment of macrocylic lactone resistant Dirofilaria immitis isolates in experimentally infected laboratory dogs. Parasites Vectors. 2014;7:494. doi: 10.1186/s13071-014-0494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao U.R., Chandrashekar R., Subrahmanyam D. Effect of ivermectin on serum dependent cellular interactions to Dipetalonema viteae microfilariae. Trop. Med. Parasitol. 1987;38:123–127. [PubMed] [Google Scholar]
- Reaves B.J., Wallis C., McCoy C.J., Lorenz W.W., Rada B., Wolstenholme A.J. Recognition and killing of Brugia malayi microfilariae by human immune cells is dependent on the parasite sample and is not altered by ivermectin treatment. Int. J. Parasitol.-Drugs Drug Resist. 2018;8:587–595. doi: 10.1016/j.ijpddr.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B., Marcellino C., Miller M., Maclean M., Mostafa E., Howell S., Sakanari J., Wolstenholme A., Kaplan R. Utilization of computer processed high definition video imaging for measuring motility of microscopic nematode stages on a quantitative scale: "The Worminator''. Int. J. Parasitol.-Drugs Drug Res. 2014;4:233–243. doi: 10.1016/j.ijpddr.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatta A.F., Dzimianski M., Storey B.E., Camus M.S., Moorhead A.R., Kaplan R.M., Wolstenholme A.J. Ivermectin-dependent attachment of PMNs and peripheral blood mononuclear cells to Dirofilaria immitis microfilariae in vitro. Vet. Parasitol. 2014;206:38–42. doi: 10.1016/j.vetpar.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Wolstenholme A.J., Evans C.C., Jimenez P.D., Moorhead A.R. The emergence of macrocyclic lactone resistance in the canine heartworm, Dirofilaria immitis. Parasitology. 2015;142:1249–1259. doi: 10.1017/S003118201500061X. [DOI] [PubMed] [Google Scholar]
- Wolstenholme A.J., Maclean M.J., Coates R., McCoy C.J., Reaves B.J. How do the macrocyclic lactones kill filarial nematode larvae? Invertebr. Neurosci. 2016;16:7. doi: 10.1007/s10158-016-0190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme A.J., Rogers A.T. Glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics. Parasitology. 2005;131:S85–S95. doi: 10.1017/S0031182005008218. [DOI] [PubMed] [Google Scholar]
- Zahner H., Schmidtchen D., Mutasa J.A. Ivermectin-induced killing of microfilariae in vitro by PMNs mediated by NO. Exp. Parasitol. 1997;86:110–117. doi: 10.1006/expr.1997.4160. [DOI] [PubMed] [Google Scholar]