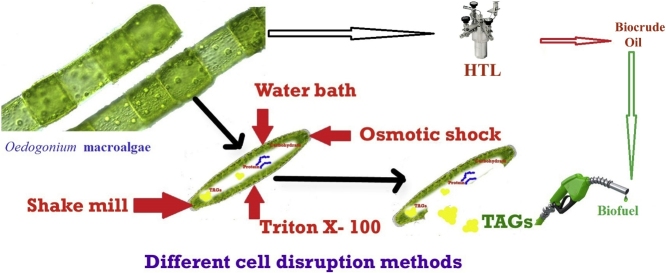
Graphical abstract
Keywords: Oedogonium, Macroalgae, Cell disruption, Lipid, Biodiesel, HTL
Highlights
-
•
Lipids yield increased by osmotic shock cell disruption method.
-
•
High percentage of hexadecanoic acid (52–68%) was obtained by soxhlet extraction.
-
•
Impurities of chlorophyll and protein were also detected in the extracted lipids.
-
•
Only one type of FAME, hexadecanoic acid methyl ester was obtained by Triton X-100.
-
•
23.3 wt% of crude oil was produced by HTL of algal biomass with TiO2 at 300 °C.
Abstract
Cell disruption and lipid extraction methods for macroalgae are not well reported. Therefore, we compared various lipid extraction methods and extraction efficiency of various solvents to improve lipid yields from Oedogonium fresh water macroalgae. Lipid extraction was done by 2 methods viz., modified Bligh and Dyer method and soxhlet extraction using either single solvents or mixtures. In soxhlet extraction method five solvents were used (1) Hexane commonly used solvent for lipid extractions, (2) chloroform: methanol (2:1), (3) Chloroform: hexane (1:1), (4) Chloroform: hexane (1:2), (5) Dichloromethane + methanol (2:1). To improve lipid extraction yields, various cell disruption methods were also compared during the present study. Impurities of chlorophyll and protein were also detected in the extracted lipids. Hydrothermal liquefaction of algal biomass with TiO2 was also conducted at 300 °C. HTL was more effective by which 23.3 wt% of bio-crude oil was obtained.
1. Introduction
Increase in population is linked to the rate of exploration of energy conventional fuels. A serious attempt is needed to search for viable alternatives of conventional fuels in the form of renewable energy sources. Algal biomass depends on species rich in useful compounds like protein, carbohydrate and lipid. This algal biomass can be used as sustainable bioenergy resource to meet the futuristic demands of fuel [1].
Algal cell walls are diverse in nature in terms of molecular component, linkages and overall structure [2] The algal cell wall comprises of two important components, (i) an organized microfibrillar structure which serves as framework of the cell wall and (ii) the gel like protein matrix within which the fibrillar component is fixed firmly, thus providing the structural integrity [3]. In addition to the cell wall, some microalgae have an external inorganic covering composed of silica frustules and calcium carbonate [4] making it more resistant towards cell disruption. Interestingly, the cell wall of microalgae alters significantly under different environmental conditions such as nutrient depletion, light fluctuations, salt and heavy metal stress, hampering the recovery of intracellular lipids [5,3]. To date, various potential microalgal species have been reported to accumulate high lipid content intracellularly, but only a few commercially important species (Chlamydomonas sp., Chlorella sp., Haematococcus sp., and Nannocloropsis sp.) are the most extensively explored microalgae because of their prominent relevance in field of biotechnology and bioenergy [6]. The basic composition of algae cells comprises of cellulosic; a polymer of β 1,4 linked D-glucose units in nature. However, the chlorophycean green algae have cell walls varying from cellulose pectin complexes to hydroxyproline rich glycoproteins respectively [7]. Polysaccharides of algal cell wall comprises of different polymers such as, hemicelluloses, chitin, pectins, fucans, alginates and carrageens which make them distinct from each other [8,9].
The cell wall of the unicellular microalgae Chlamydomonas reinhardtii encompasses a network of fibrils and glycoproteins, mainly comprising of hydroxyproline (Hyp)-rich glycoproteins (HRGPs) arranged in five distinct layers, with extended oligosaccharides side chain on them [10]. Structural analysis of cell walls of C. reinhardtii and C. gymnogama elucidated arabinose, glucose and galactose as the main sugar components bound to HRGPs [11]. These conserved Hyp- rich sequences in Chlamydomonas adds to the strength of the cell wall, as these sequences allow the protein molecules to acquire the polyprolines dominant conformations resulting in more stable form when glycosylated [12]. On the other hand, the cell wall of Chlorella consists of trilaminar layer having an outer covering of sporopollenin which is the main component leading to its toughness (REF). Beneath the outer layer, heterogeneous secondary wall is rich in mannose and glucosamine [13]. Interspecies variation has been reported in Chlorella depending upon the different growth conditions [6]. For example, an enhanced proportion of uronic acid and amino sugars associated with reduction in neutral sugars were reported in CO2 enriched conditions (2% CO2) [14].
Additionally, the polysaccharides in cell wall of marine alga such as Nannocloropsis sp, generally exist in sulphated form [15]. A bilayer structure composed of an outer layer made up of hydrophobic algaenan, covering the inner cellulosic layer contributes to the recalcitrant nature of the cell wall of Nannocloropsis [16]. Disrupting the cellular wall of algae allows for easier recovery of the intracellular lipids resulting in rapid and increased efficiencies in lipid extraction. The summary and comparison of cell disruption methods reported for lipid extraction from algae have been listed in Table 1. Lee et al. [17] reported that for microalgae of Botryococcus sp., Chlorella vulgaris and Scenedesmus sp the microwave treatment was best cell disruption method. Cell disruption method depends on the microorganism on which one is working, therefore, one cannot generalize the results obtained from one species to others [18].
Table 1.
Comparison of different cell disruption methods.
| Cell Disruption method | Most Efficient method | Algae | % lipid Extracted | Reference |
|---|---|---|---|---|
| Autoclaving Bead beating Microwaves Sonication Osmotic shock |
Microwaves | Microalgae Chlorella vulgaris |
11 | [17] |
| Sonication Osmotic shock Microwave Autoclave Bead beating |
Sonication | Microalgae Nostoc sp. |
18.2 | [52] |
| Grinding Sonication Bead milling Enzymatic lysis Microwaves |
Grinding | Microalgae Chlorella vulgaris |
29 | [18] |
| Grinding Bead vortexing Osmotic shock Water bath Sonication Shake mill |
Osmotic shock | Microalgae Schizochytrium sp. S31 |
48.7 | [35] |
| Sonication French press homogenisation |
Sonication | Microalgae Schizochytrium sp. S31 |
34.5 | [69] |
| Grinding Ultrasonication Microwave |
Ultrasonication | Microalgae Scenedesmus sp. |
90.8 | [70] |
| Osmotic Shock Water Bath Shake Mill Triton X- 100 |
Osmotic Shock | Macroalgae Oedogonium |
16.3 | Current Study |
For algal biomass there is a new promising alternative processes for biocrude oil production called as Hydrothermal liquefaction (HTL). HTL is an oxygen-free thermochemical process which directly converts the wet biomass into biofuels, carried out at temperatures (200–400 °C), and high pressure (6–15 MPa) respectively [19]. In this process cellulose, hemicellulose and lignin is converted into four phases namely biocrude oil, aqueous phase, solid residue and gaseous products [20].
Previous studies have reported the maximum yield of bio-oil using HTL process at 300 °C [21,22]. Catalyst plays a crucial role to maximize the crude oil production and increases the conversion rate of feedstock in biocrude oil [23,24]. Titanium Oxide (TiO2) is mainly used as catalyst due to its high thermostability [25]. Different concentrations of catalyst have been reported by different researchers in their studies, but using 10% catalyst ratio to feed stock has been reported to give the maximum conversion rate of feedstock to crude oil by HTL [26,27].
Small numbers of studies have reported the potential of macroalgae for biofuels production and majority of algae research is concentrated on microalgae [28]. Macroalgae form dense floating mats on the water surface which make cost-efficient harvesting as compared to microalgae [29]. In our previous study we have extracted and blended macroalgae biodiesel with butanol-diesel fuel which resulted in good efficiency and exhaust emissions characteristics [30].
Genus Oedogonium is a filamentous macroalgae that are one cell thick. The cells are cylindrical and reproduce sexually and asexually. They are either free-floating or attached to other microorganisms like bacteria, fungi, protozoa, and sessile animals, altogether called ‘periphyton. It has a biochemical composition suitable for a range of biomass applications [[31], [32]]. The choice of efficient lipid extraction method is an important step towards commercial fuel production from macro algal species. Our key target species for this study was genus Oedogonium, a filamentous macroalgae that is an appropriate biofuels feedstock [33].
The present study focuses on two main objectives, which are as listed as follows: First, different cell disruptions and solvent extraction methods have been investigated and second was hydrothermal liquefaction of algal biomass with TiO2. However, to the best of our knowledge, it has not been reported the most efficient lipid extraction method from Oedogonium macroalgae.
2. Materials and methods
2.1. Materials
Oedogonium algae was collected from the fresh water rivers in Dehradun, India. All solvents and reagents used in this study were HPLC grade.
2.2. Identification and sample preparation
Oedogonium was identified based on the morphological characteristics. The algae was examined under compound light microscope to study their morphological characteristics and each sample was identified to species level using taxonomic keys. Sample was prepared by drying the wet algal biomass at 40 °C.
2.3. Isolation of lipid
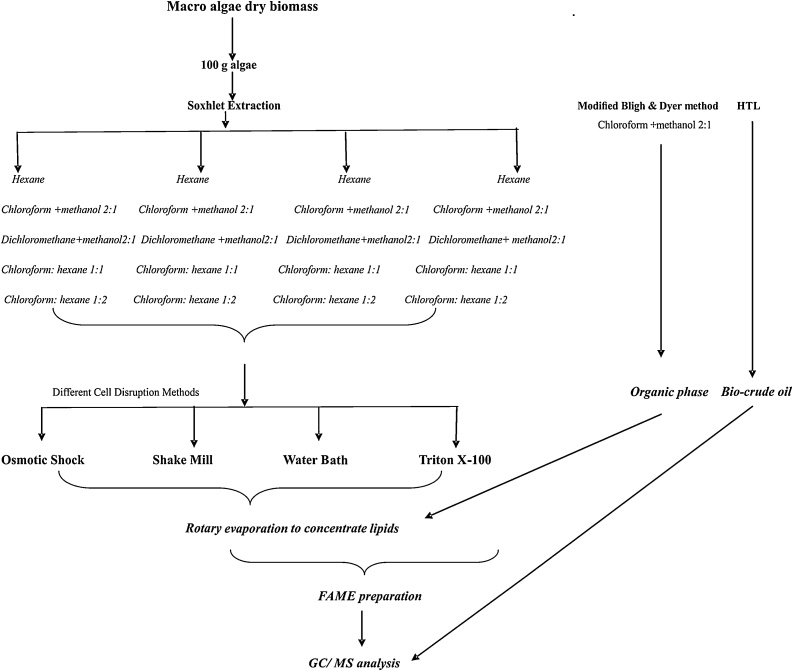
In order to improve lipid productivity from macroalgae, lipid was extracted by two different methods. First soxhlet extraction was performed with different solvents and the lipids were estimated by gravimetric method. The second method used was the modified E.G. Bligh and W.J. Dyer method [34] (Fig. 1).
Fig. 1.
Brief overview of lipid extraction methods used.
2.3.1. Modified Bligh and Dyer method
Total lipids were extracted by modified E.G. Bligh and W.J. Dyer method [34]. 100 g of fine powder of algal biomass was treated with 250 ml of Chloroform: methanol (2:1 Ratio) in conical flask. Conical flask was shaked vigorously (200 rpm) on a rotary shaker for 30 min followed by a 10 min stop and then shaked vigorously for 20 min. The conical flask was kept at room temperature overnight where the supernatant (lipid) would separate from the remaining residue of the algae.
2.3.2. Soxhlet extraction
The soxhlet extraction was implemented with 100 g biomass (small pieces 2–3 cm) on a soxhlet system for 6 h of extraction process with 250 ml of solvents.
2.3.2.1. Solvents used
Lipid extraction was done using five different solvents (1) Hexane commonly used solvent for lipid extractions, less toxic than other solvents (2) Chloroform: methanol (2:1) (Standard solvent, mainly used in lipid extraction) (3) Chloroform: hexane (1:1) (Extract total lipids), (4) Chloroform: hexane (1:2), (5) Dichloromethane: methanol (2:1) as used by R. Byreddy et al., [35].
2.4. Different Cell Disruption methods used for lipid extraction
Small pieces (2–3 cm) of algae biomass was disrupted by four different cell disruption methods (Fig. 1). Lipid content (%w/w) was measured using the following equation:
| LC = TLC/ CDW |
Where LC was the lipid content (%, w/w), and TLC and CDW were the total lipid concentration (g/L) and the cell dry weight concentration (g/L), respectively.
2.4.1. Osmotic shock
100 g of algae biomass was treated with 250 ml of 10% NaCl solution and vortexed for 2 min. The contents were further incubated for 48 h at room temperature, followed by soxhlet extraction.
2.4.2. Water bath
100 g of algal biomass was added to 250 ml of water in a beaker and placed in preheated water bath. Sample was kept in water bath for 30 min at 90 °C, followed by soxhlet extraction.
2.4.3. Shake mill
100 g of algal biomass was mixed with 250 ml solvent in a conical flask. Further glass beads (0.4–0.6 mm) were added to the beaker in the ratio of 3:1 (Biomass: Glass Bead) and kept in a shake mill (1060 cycle/min) for 30 min, followed by soxhlet extraction.
2.4.4. Triton X- 100
100 g of algal biomass was suspended in 250 ml of 2% triton X-100 for 12 h, followed by soxhlet extraction.
2.5. Detection of triacylglycerols (TAGs)
For triacylglycerols (TAGs) detection, 5 μl of isolated lipid sample was spotted on 0.25-mm-thick silica gel and visualized with methanolic MnCl2 solution [36].
2.6. Biochemical analysis
Pigments were also present in crude oil extracted with different extraction methods. For the photosynthetic pigments (chlorophyll a, chlorophyll b, and carotenoids) estimation absorbance were taken at 665.2, 652.4, 470 and 750 nm. Amount of pigments were determined using the following equations given by H.K. Lichtenthaler [37]:
| Chlorophyll a (Chl a; μg/mL) = 16.72 A665.2 − 9.16 A652.4 |
| Chlorophyll b (Chl b; μg/mL) = 34.09 A652.4 − 15.28 A665.2 |
| Carotenoids (μg/mL) = (1000 A470 − (1.63 Chl a − 104.9 Chl b))/221 |
Total protein extracted with crude algae oil was estimated by Lowery method [38]. The carbohydrate content of lipid extracted algal biomass was isolated by 5% H2SO4 [39] and estimated by phenol sulphuric acid method [40].
2.7. Analysis of fatty acid profile and biodiesel quality by GC
Transesterification of extracted lipid with different methods was done according to Hossain and Salleh [41] method, in briefly 0.25 g NaOH was mixed with 24 ml methanol as a catalyst. Then solution of catalyst and methanol was mixed with algal oil in a Teflon-coated screw-cap tube. The mixture was kept in a water bath at 60 °C for 2 h with gentle shaking. The mixture was followed by addition of n-hexane (2 mL) and water (1 mL). The FAME (fatty acid methyl ester) was collected in the n-hexane. FAMEs analyzed by gas chromatography–mass spectroscopy (GC–MS; Agilent technologies,USA) under operating conditions that have previously been reported by V. Kumar et al. method [36].
2.8. Analysis of biodiesel physical properties
Important parameters of biodiesel were determined by different empirical formulas given below [[42], [43], [44], [45]]:
| Acid value = (Volume of KOH x Normality of KOH × Eq. wt × 1000) / Weight of Oil sample | (1) |
| Saponification value = = Σ 560 (% FA) / Mi | (2) |
| Iodine value: (Titer value of blank-titer value of oil samples) ml × 0.01269 × 100/ Weight of sample (g) | (3) |
| Specific gravity: Density of object/ Density of pure water | (4) |
| Long-chain saturation factor = (0.1 * C16) + (0.5 * C18) | (5) |
| Cold filter plugging point = (3.417 * LCSF) – 16.477 | (6) |
| Cetane number (CN) = (46.3 + 5458) / SV – (0.255 * IV) | (7) |
| High heating value (HHV; MJ/Kg) = 49.43 – 0.041 (SV) – 0.015 (IV) | (8) |
Pensky-Martens closed cup tester was used for the analysis of two important factors which were fire and flash point.
2.9. Hydrothermal liquefaction (HTL) process
For Hydrothermal liquefaction (HTL) of algal biomass, 100 ml reactor was used which operated in a batch mode. 20 g of algal biomass was added in 50 ml of distilled water. To the above added 2 g of TiO2 as catalyst. The reaction conditions were selected based on modified method given by S. Karagoz et al. [46]. The reactor was heated up to 300 °C and pressure 4 MPa with heating rate 5 °C/min for 30 min. After heating the reactor was cooled at room temperature and water phase and solid mixture was separated from each other. Bio-crude oil from solid mixture was extracted using soxhlet extraction apparatus (6 h) with acetone (150 ml) as solvent. After extraction acetone was recovered at 60 °C. The extracted oil phase was weighed and marked as bio-crude oil 1. Water phase was treated with treated with 50 ml of diethyl ether. Diethyl ether was evaporated in a rotary evaporator and remaining fraction was weighed and marked as bio-crude oil 2. Bio-crude oil 1 was mixed with bio-crude oil 2 for calculation of total bio-crude oil (wt%).
| Bio-crude oil (wt%) = bio-oil/ algal biomass ×100% |
To determine the composition of biocrude oil GC–MS (Clarus 500, Perkin Elmer) analysis was done. In GC–MS varian DB-5 column was used with helium as the carrier gas (1 ml/min). The temperature was ramped to 250 °C and 320 °C. The mass transfer line and ion source were set at 250 and 320 °C, respectively. The components of crude oil were determined with electron ionization (70 eV) in scan mode (20–650 m/z) [46].
2.10. Statistical analysis
In this study, all experiments were conducted in triplicates. The data were expressed as mean ± standard deviation and were analyzed with one-way analysis of variance (ANOVA) using Microsoft Office Excel 2016, with p-values of <0.05 being regarded as significant.
3. Results and discussion
In the present study the wet algal biomass was dried at 40°C. It is significant correct drying temperature, which eliminates its negative impact on biomass quality [47]. E.N. Frankel [48] has reported that the drying temperature had a significant effect on lipid content of biomass. In another study conducted by A. Piasecka et al. [49] have reported that a rapid fall in the lipid contents with increasing drying temperature.
3.1. Lipid extraction by different solvents
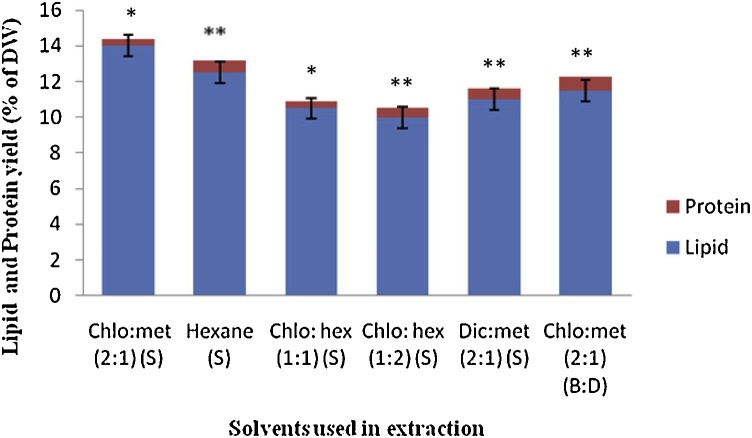
Lipid extraction was done by different organic solvents for identification of a suitable solvent for maximum lipid extraction. The lipid extracted by soxhlet extraction method displayed a difference in extraction efficiency of hexane and other solvents as given in the Fig. 2. Chloroform: methanol (2:1) showed maximum lipid extraction 14% (DW), followed by 12.5% in hexane (2:1) and dichloromethane: methanol (2:1) by soxhlet extraction. In terms of lipid extraction, the order of extraction efficiency could be ranked as Chl:Met (2:1) > Hexane > Dic:Met (2:1)>Chl:Hex(1:1)>Chl:Hex (1:2). Modified Bligh and Dyer extraction with solvent Chloroform: methanol (2:1) showed 11.5% (% DW) lipid. The present findings also supported the results obtained by Y. Shen et al., [50] who have reported that hexane extract more lipids as compared to combination of solvents from algal biomass. In another study R. Byreddy et al. [35], have reported that among the single organic solvents, hexane extracted more lipids from biomass. Their findings using the combination of solvents contradicts our conclusion, that chloroform:methanol (2:1) give the maximum yield of lipid from Schizochytrium sp. F. Shahidi and P.K. Wanasundara [51] have reported that hexane has low compatibility towards contamination and is more suitable for neutral lipid extraction.
Fig. 2.
Comparison of lipid recovery by Soxhlet extraction utilizing different solvents.
Chlo:Met (2:1) (S)- Chloroform: methanol (2:1) in Soxhlet extraction, Hexane (S)-Hexane used in Soxhlet extraction, Chl:hex (2:1) (S)-Chloroform: hexane (1:1) in Soxhlet extraction, Chlo:hex (1:1) (S)- Chloroform: hexane (1:2) in Soxhlet extraction, Dic:met (2:1) (S)- Dichloromethane + methanol (2:1) in Soxhlet extraction, Chl:meth (2:1) (B:D)-Chloroform: methanol (1:2) in Bligh and Dyer extraction with modification. Data are mean ± S.D. for three independent replicates (*p < 0.05; **p < 0.01).
3.2. Comparison of cell disruption methods
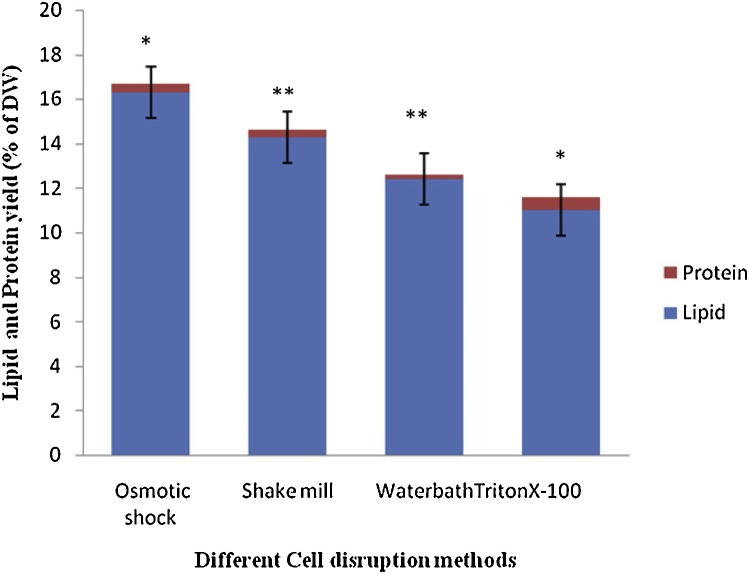
In the present study four different methods of cell disruption were evaluated in order to increase the lipid productivity in Oedogonium macroalgae. The efficiency of each cell disruption method was determined using percentages of lipid contents (Fig. 3). Different cell disruption methods used in the study were able to disrupt macroalgae cells, although lipid yields varied with methods. A maximum lipid yield of 16.3% was recorded by disrupting the macroalgae cells using Osmotic Shock and hexane as a solvent. Shake mill, water bath, and triton X-100 treatments extracted 14.3%, 12.4%, and 11% of lipids, respectively. The advantages and disadvantages of different cell disruption methods used in this study are summarized in Table 2. R. Byreddy et al. [35] have reported osmotic shock as an effective method for extracting lipids from microalgae. In another study carried on Chlorella sp. also osmotic shock has been reported as an effective method for extracting lipids [52]. Available literature suggests that the efficiency of different cell disruption methods in improving lipid extraction varies for different algae species.
Fig. 3.
Effect of different cell disruption methods on lipid extraction from Oedogonium (hexane as solvent). Data are mean ± S.D. for three independent replicates (*p < 0.05; **p < 0.01).
Table 2.
Advantages and disadvantages of selected cell disruption methods.
| Cell disruption methods | Advantages | Disadvantages |
|---|---|---|
| Shake mill |
|
|
| Osmotic shock |
|
|
| Water bath |
|
|
| Triton X-100 |
|
|
3.3. Effects of different solvents and cell disruption methods on biochemical composition of isolated lipids
During the present study protein content as impurity of isolated algae oil was high (0.8%) with chloroform: methanol (2:1) as solvent using modified E.G. Bligh and W.J. Dyer method [34]. Effect of different solvents and cell disruption on protein impurity is given in Figs. 2 & 3 . Using soxhlet extraction maximum impurity of protein (0.7%) was recorded with chloroform: methanol (2:1) as solvent. Protein impurities of 0.6% were recorded with Triton X-100 and exane as solvent. A. J. Cole et al., [29] have reported high protein content (18%) from the macroalgae Oedogonium. Other researchers have also reported species of macroalgae displaying 20–40% protein content [53,54,55].
M. Bahmaei et al [56] have reported that high levels of chlorophyll like compounds are mainly found in lipid extracts from plants. Effect of cell disruption on chlorophyll yield is shown in Table 3. Highest yield was recorded using water bath (Chl a + Chl b 14.02 μg/ml) while lowest yield was recorded was with osmotic shock (Chl a + Chl b 1.39 μg/ml). Cell disruption methods play important role in diffusion of algae proteins and pigments in the aqueous phase [57]. For carbohydrate extraction, lipid extracted algae biomass was treated with 5% H2SO4. During the present study 35.4% of carbohydrate content was recorded from Oedogonium. Green macroalgae have high content of carbohydrates in the form of cellulose and starch [58].
Table 3.
Effects of cell disruption methods on Oedogonium algae pigments (hexane as solvent).
| Cell Disruption method | Chl a* (μg/ml) |
Chl b** (μg/ml) |
Car*** (μg/ml) |
Chl a + Chl b | Chl a/Chl b | Car/ Chl a + Chl b |
|---|---|---|---|---|---|---|
| Shake mill | 3.77 ± 0.02 | 1.59 ± 0.04 | 5.18 ± 0.02 | 5.36 ± 0.04 | 2.37 ± 0.01 | 0.96 ± 0.01 |
| Water bath | 11.19 ± 0.05 | 2.837 ± 0.01 | 6.55 ± 0.03 | 14.02 ± 0.05 | 3.94 ± 0.03 | 0.46 ± 0.03 |
| Osmotic shock | 0.94 ± 0.02 | 0.45 ± 0.01 | 1.258 ± 0.02 | 1.39 ± 0.03 | 0.208 ± 0.01 | 0.89 ± 0.02 |
| Triton x 100 | 2.41 ± 0.03 | 7.70 ± 0.03 | 2.01 ± 0.01 | 10.11 ± 0.02 | 0.312 ± 0.02 | 0.19 ± 0.01 |
| Bligh and Dyer | 1.01 ± 0.02 | 2.69 ± 0.01 | 0.65 ± 0.02 | 3.70 ± 0.01 | 0.375 ± 0.03 | 0.175 ± 0.01 |
Note: Data values are average of two independent experiments with p < 0.05.
3.4. Fatty acid profile and characterization of biodiesel
Algal cells synthesize different types of lipids which are neutral lipids, glycolipids and phospholipids to perform different metabolic functions. Triacylglycerol (neutral lipids) is the main lipid stored in algal cells used to produce biodiesel. TLC is the cost effective and best method to detect all the class of lipids extracted by different methods. Results showed that by using different extraction methods variation in lipid productivity and composition is obtained (Fig. 3). GC–MS analysis of FAMEs by different cell disruption methods showed hexadecanoic acid (C-16:0) methyl ester as major fatty acids (52–62%) obtained in the present study. Other fatty acids i.e., myristic acid (C14:0), methyl ester (C16:0), stearic acid (C18:0) ando leic acid (18:1) were also detected in lower amount using shake mill and osmotic shock methods. With cell disruption using Triton X- 100, only Hexadecanoic acid (C16:0) and methyl ester (58%) were obtained. S.G. Musharraf et al., [59] have reported hexadecanoic acid (C-16:0) and methyl ester, ranging from 29 to 61% from S. quadricauda, S. acuminatus, Nannochloropsis sp., Anabaena sp., Chlorella sp. and Oscillatroria sp.
Bligh and Dyer extraction yielded tridecanoic Acid (C13:0), dodecanoate (C12:0), tetradecanoate (C14:0), palmitic acid (C16:0), stearic acid (C18:0), oleic acid (18:1), linoleic acid (C18:2), heptacosanoic Acid (C27:1) and triacontanoic Acid (30:0) as major fatty acids. Using soxhlet extraction lower yields of unsaturated fatty acids were obtained. This may be due to thermo-degradation of long chain polyunsaturated fatty acids during soxhlet extraction [60]. Fatty acids C16, C18:1 and C18:2 are normally treated as the major components microalgal biodiesel [61]. E. Ryckebosch et al. [62], has reported that main properties of biodiesel depend on the length and unsaturation of FAMEs [62]. The extraction of long chain unsaturated fatty acids were significantly dependent on the extraction method [63]. Important parameters of biodiesel production are summarized in Table 4. High fire point was reported in diesel obtained after water bath pretreatment method. While high cetane number was reported in Triton X-100.
Table 4.
Comparison of physical properties of different FAMEs obtained from Oedogonium with plant oil methyl esters (JPE, PME) and commercial biodiesel.
| Physical properties |
Plant oil methyl esters |
Different cell disruption methods |
Bligh and Dyer | Commercial biodiesel |
||||
|---|---|---|---|---|---|---|---|---|
| JME | PME | Shake mill | Water bath | Osmotic shock | Triton x 100 | |||
| Saponification Value (mg KOH) |
187 | 49.56 | 144.63 | 100.16 | 106.82 | 83.86 | 170.24 | |
| Iodine value (g I2/100 g) |
54 | 61 | 40.21 | 36 | 38 | 20.47 | 42.72 | 130 |
| Specific gravity (kg−1) | – | – | 0.766 | 0.784 | 0.770 | .824 | 0.873 | – |
| Acid Value mg KOH g−1 | – | – | 2.8 | 2.8 | 2.8 | 11.22 | 2.8 | 0.50 |
| Flash point | 180 | – | 45 | 39 | 46 | 35 | 45 | 35 |
| Fire point | 256 | – | 40 | 53 | 52 | 42 | 50 | – |
| Cetane Value | 40 | 40.94 | 60.50 | 56.66 | 69.99 | 37.44 | 47 | |
| High Heating Value | – | 42.00 | 44.17 | 43.84 | 45.22 | 42.11 | ||
| Long chain saturation factor (% wt) |
– | – | 6.7 | 5.9 | 5.6 | 6.2 | 3.2 | – |
| Cold flow plugging property (° C) |
−2 | 13 | 6.4 | 3.68 | 2.65 | 4.70 | −5.54 | −5 |
-No standard limit designated by biodiesel standards, JPE = Jatropha methyl ester, PME = Palm oil methyl ester.
3.5. Hydrothermal liquefaction (HTL)
After HTL of algal biomass, 23.3% bio-crude oil at 300 °C with TiO2 was obtained. Z. Zhu et al., [64] reported the maximum yield of bio-crude oil 34.9 wt% at 300 °C from barley straw. N. Neveux et al. [33], reported highest yield of bio-crude oil 26.2 wt% from Oedogonium macroalgae at temperature 350 °C. Presence of catalyst during HTL leads to increase in biocrude oil yield and decrease in the biochar formation [65]. W.Wang et al. [26], reported that by using TiO2 in HTL of microalgae at 300 °C lead to the highest bio-oil yield and the maximum liquefaction conversion.
Six main compounds 3-Penten-2-one, 4-methyl, 2-Pentadecanone, 6,10,14-trimethyl, n-Hexadecanoic acid, Pentadecanoic acid and Phytol were analyzed by GC–MS. The compounds such as amides, fatty acids acid, phenols, alkanes, ketones and alkenes were considered as main components of biocrude oil obtained by HTL of algal biomass [66]. Amines and amides were produced due to the conversion of algal protein [67]. Ketones and phenols were produced during HTL process from algal carbohydrates [68].
4. Conclusion
The findings of the present study displayed variation in percentage of saturated fatty acid yield with different cell disruption methods. Using modified Bligh and Dyer extraction varying amounts of different saturated fatty acids, monounsaturated fatty acids and polyunsaturated fatty acids were obtained in the extracted oil. Whereas with soxhlet extraction only saturated fatty acids and monounsaturated fatty acids were obtained. However, the total fatty acid yield was recorded more using soxhlet extraction than modified Bligh and Dyer extraction. Impurities of chlorophyll and protein were also detected in extracted lipids by different extraction methods. Highest yield (23.3%) of bio-crude oil was obtained by HTL method.
Conflict of interest
The author(s) declare no conflict interests.
References
- 1.Brennan L., Owende P. Biofuels from microalgae-a review of technologies for production, processing, and extraction of biofuels and co-products. Renew. Sustain. Energy Rev. 2010;14:557–577. [Google Scholar]
- 2.Work V.H., Bentley F.K., Scholz M.J., D’Adamo S., Gu H., Vogler B.W., Franks D.T., Stanish L.F., Jinkerson R.E., Posewitz M.C. Biocommodities from photosynthetic microorganisms. Environ. Prog. Sustain. 2013;32:989–1001. [Google Scholar]
- 3.Yap B.H.J., Crawford S.A., Dumsday G.J., Scales P.J., Martin G.J.O. A mechanistic study of algal cell disruption and its effect on lipid recovery by solvent extraction. Algal Res. 2014;5:112–120. [Google Scholar]
- 4.Bolton C.T., Hernández-Sánchez M.T., Fuertes M.A., González-Lemos S., Abrevaya L., Mendez-Vicente A., José-Abel F., Ian P., Liviu G., Joel J., Stoll H.M. Decrease in coccolithophore calcification and CO2 since the middle Miocene. Nat. Commun. 2016;7:10284. doi: 10.1038/ncomms10284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Praveen Kumar R., Lee K., Lee J., Oh Y.-K. Breaking dormancy: an energy-efficient means of recovering astaxanthin from microalgae. Green Chem. 2015;17:1226–1234. [Google Scholar]
- 6.Lee S.Y., Cho J.M., Chang Y.K., Oh Y.K. Cell disruption and lipid extraction for microalgal biorefineries: a review. Bioresour. Technol. 2017;244:1317–1328. doi: 10.1016/j.biortech.2017.06.038. [DOI] [PubMed] [Google Scholar]
- 7.Domozych D.S., Ciancia M., Fangel J.U., Mikkelsen M.D., Ulvskov P., Willats W.G. The cell walls of green algae: a journey through evolution and diversity. Front. Plant Sci. 2012;3(82):82. doi: 10.3389/fpls.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domozych D.S., Serfis A., Kiemle S.N., Gretz The structure and biochemistry of charophycean cell walls: I. Pectins of Penium margaritaceum. Protoplasma. 2017;230:99–115. doi: 10.1007/s00709-006-0197-8. [DOI] [PubMed] [Google Scholar]
- 9.Michel G., Helbert W., Kahn R., Dideberg O., Kloareg B. The structural bases of the processive degradation of ι-carrageenan, a main cell wall polysaccharide of red algae. J. Mol. Biol. 2003;334:421–433. doi: 10.1016/j.jmb.2003.09.056. [DOI] [PubMed] [Google Scholar]
- 10.Arnold A.A., Genard B., Zito F., Tremblay R., Warschawski D.E., Marcotte I. Identification of lipid and saccharide constituents of whole microalgal cells by 13C solid-state NMR. Biochim. Biophys. Acta Biomembr. 2015;1848:369–377. doi: 10.1016/j.bbamem.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Bollig K., Lamshöft M., Schweimer K., Marner F.J., Budzikiewicz H., Waffenschmidt S. Structural analysis of linear hydroxyproline-bound O-glycans of Chlamydomonas reinhardtii-conservation of the inner core in Chlamydomonas and land plants. Carbohydr. Res. 2007;342:2557–2566. doi: 10.1016/j.carres.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Ferris P.J., Waffenschmidt S., Umen J.G., Lin H., Lee J.H., Ishida K. Plus and minus sexual agglutinins from Chlamydomonas reinhardtiip. Plant Cell. 2005;17:597–615. doi: 10.1105/tpc.104.028035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim D.Y., Vijayan D., Praveenkumar R. Cell-wall disruption and lipid/astaxanthin extraction from microalgae: Chlorella and Haematococcus. Bioresour. Technol. 2016;199:300–310. doi: 10.1016/j.biortech.2015.08.107. [DOI] [PubMed] [Google Scholar]
- 14.Cheng Y.S., Labavitch J.M., Vandergheynst J.S. Elevated CO2 concentration impacts cell wall polysaccharide composition of green microalgae of the genus Chlorella. Lett. Appl. Microbiol. 2015;60:1–7. doi: 10.1111/lam.12320. [DOI] [PubMed] [Google Scholar]
- 15.Kloareg B., Quatrano R.S. Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Oceanogr. Mar. Biol. Annu. Rev. 1998;26:259–315. [Google Scholar]
- 16.Scholz M.J., Weiss T.L., Jinkerson R.E. Ultrastructure and composition of the Nannochloropsis gaditana cell wall. Eukaryot. Cell. 2014;13:1450–1464. doi: 10.1128/EC.00183-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J.Y., Yoo C., Jun S., Ahn C.Y., Oh H.M. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010;101:S75–S77. doi: 10.1016/j.biortech.2009.03.058. [DOI] [PubMed] [Google Scholar]
- 18.Zheng H., Yin J., Gao Z., Huang H., Ji X., Dou C. Disruption of Chlorella vulgaris cells for the release of biodiesel-producing lipids: a comparison of grinding, ultrasonication, bead milling, enzymatic lysis, and microwaves. Appl. Biochem. Biotechnol. 2011;164:1215–1224. doi: 10.1007/s12010-011-9207-1. [DOI] [PubMed] [Google Scholar]
- 19.Behrendt F., Neubauer Y., Oevermann M., Wilmes B., Zobel N. Direct liquefaction of biomass. Chem. Eng. Technol. 2008;31:667–677. [Google Scholar]
- 20.Kruse A., Dinjus E. Hot compressed water as reaction medium and reactant: properties and synthesis reactions. J. Supercrit. Fluids. 2007;39:362–380. [Google Scholar]
- 21.Zhu Z., Rosendahl L., Toor S.S., Yu D., Chen G. Hydrothermal liquefaction of barley straw to bio-crude oil: effects of reaction temperature and aqueous phase recirculation. Appl. Energy. 2015;137:183–192. [Google Scholar]
- 22.Arun J., Shreekanth S.J., Sahana R., Raghavi M.S., Gopinath K.P., Gnanaprakash D. Studies on influence of process parameters on hydrothermal catalytic liquefaction of microalgae (Chlorella vulgaris) biomass grown in wastewater. Bioresour. Technol. 2017;244:963–968. doi: 10.1016/j.biortech.2017.08.048. [DOI] [PubMed] [Google Scholar]
- 23.Chen W.T., Qian W., Zhang Y., Mazur Z., Kuo C.-T., Scheppe K. Effect of ash on hydrothermal liquefaction of high-ash content algal biomass. Algal Res. 2017;25:297–306. [Google Scholar]
- 24.Zhang C., Duan P., Xu Y., Wang B., Wang F., Zhang L. Catalytic upgrading of duckweed biocrude in subcritical water. Bioresour. Technol. 2014;166:37–44. doi: 10.1016/j.biortech.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 25.Aranda-Perez N., Ruiz M.P., Echave J., Faria J. Enhanced activity and stability of Ru-TiO2 rutile for liquid phase ketonization. Appl. Catal. A Gen. 2017;531:106–118. [Google Scholar]
- 26.Wang W., Yu Qi, Meng Han, Han Wei, Li Jie, Zhang Jinglai. Catalytic liquefaction of municipal sewage sludge over transition metal catalysts in ethanol-water co-solvent. Bioresour. Technol. 2018;249:361–367. doi: 10.1016/j.biortech.2017.09.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Z., Rosendahl L., Toor S.S., Yu D., Chen G. Hydrothermal liquefaction of barley straw to bio-crude oil: effects of reaction temperature and aqueous phase recirculation. Appl. Energy. 2015;137:183–192. [Google Scholar]
- 28.Yun J.H., Smith V.H., de Noyelles F.J., Roberts G.W., Stagg-Williams S.M. Freshwater macroalgae as a biofuels feedstock: mini-review and assessment of their bioenergy potential. Ind. Biotechnol. 2014;10 [Google Scholar]
- 29.Cole A.J., Mata L., Paul N.A., de Nys R. Using CO2 to enhance carbon capture and biomass applications of freshwater macroalgae. Glob. Change Biol. Bioenergy. 2013 [Google Scholar]
- 30.Kumar V., Nanda M., Joshi H.C., Singh A., Sharma S., Verma M. Production f biodiesel and bioethanol using algal biomass harvested from fresh water river. Renew. Energy. 2018;116:606–612. [Google Scholar]
- 31.Cole A.J., de Nys R., Paul N.A. Removing constraints on the biomass production of freshwater macroalgae by manipulating water exchange to manage nutrient flux. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0101284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naselli-Flores L., Barone R. Green algae. In: Likens Gene E., editor. Vol. 1. Elsevier; Oxford: 2009. pp. 166–173. (Encyclopedia of Inland Waters). [Google Scholar]
- 33.Neveux N., Yuen A.K.L., Jazrawi C., Magnusson M., Haynes B.S., Masters A.F., Montoya A., Paul N.A., de Nys T., Maschmeyer R. Biocrude yield and productivity from the hydrothermal liquefaction of marine and freshwater green macroalgae. Bioresour. Technol. 2014;155:334–341. doi: 10.1016/j.biortech.2013.12.083. [DOI] [PubMed] [Google Scholar]
- 34.Bligh E.G., Dyer W.J. A rapid method for total lipid extraction and purification. Can. J. Biochem. Phys. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 35.Byreddy Avinesh R., Gupta Adarsha, Barrow Colin J., Puri Munish. Comparison of cell disruption methods for improving lipid extraction from thraustochytrid strains. Mar. Drugs. 2015;13:5111–5127. doi: 10.3390/md13085111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar V., Nanda M., Verma M. Application of agar liquidgel transition in cultivation and harvesting of microalgae for biodiesel production. Bioresour. Technol. 2017;243:163–168. doi: 10.1016/j.biortech.2017.06.080. [DOI] [PubMed] [Google Scholar]
- 37.Lichtenthaler H.K. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. [Google Scholar]
- 38.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 39.Kumar V., Nanda M., Verma M., Singh A. An integrated approach for extracting fuel, chemicals, and residual carbon using pine needles. Biomass Conv. Bioref. 2018;8:447–454. [Google Scholar]
- 40.Sturgeon R.J. Monosaccharides. In: Dey P.M., editor. Methods in Plant Biochemistry. Vol. 2. Carbohydrates. Academic Press; London: 1990. pp. 1–37. [Google Scholar]
- 41.Hossain A.B., Salleh Aishah M.S. Biodiesel fuel production 300 from algae as renewable energy. Am. J. Biochem. Biotechnol. 2008;4:250–254. [Google Scholar]
- 42.Caballero-Co´rdoba G.M., Sgarbieri V.C. Nutritional and toxicological evaluation of yeast (Saccharomyces cereVisiae) biomass and a yeast protein concentrate. J. Sci. Food Agric. 2000;80:341–351. [Google Scholar]
- 43.Guarnieri M.T., Nag A., Yang S., Pienkos P.T. Proteomic analysis of Chlorella vulgaris: potential targets for enhanced lipid accumulation. J. Proteome. 2013;93:245–253. doi: 10.1016/j.jprot.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 44.Bhola V., Desikan R., Santosh S.K., Subburamu K., Sanniyasi E., Bux F. Effects of parameters affecting biomass yield and thermal behaviour of Chlorella vulgaris. J. Biosci. Bioeng. 2011;111:377–382. doi: 10.1016/j.jbiosc.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Kumar V., Kumar R., Rawat D., Nanda M. Synergistic dynamics of light, photoperiod and chemical stimulants influences biomass and lipid productivity in Chlorella singularis (UUIND5) for biodiesel production. Appl. Biol. Chem. 2018;61:7–13. [Google Scholar]
- 46.Karagoz S., Bhaskar T., Muto A., Sakata Y. Hydrothermal upgrading of biomass: effect of K2CO3 concentration and biomass/water ratio on products distribution. Bioresour. Technol. 2006;97:90–98. doi: 10.1016/j.biortech.2005.02.051. [DOI] [PubMed] [Google Scholar]
- 47.Houghton T.P., Stevens D.M., Pryfogle P.A., Wright C.T., Radtke C.W. The effect of drying temperature on the composition of biomass. Appl. Biochem. Biotechnol. 2009;153:4–10. doi: 10.1007/s12010-008-8406-x. [DOI] [PubMed] [Google Scholar]
- 48.Frankel E.N. In search of better methods to evaluate natural antioxidants and oxidative stability in food lipids. Trends Food Sci. Technol. 1993;4:220–225. [Google Scholar]
- 49.Piasecka A., Krzemiñska I., Tys J. Physical methods of microalgal biomass pretreatment. Int. Agrophys. 2014;28:341–348. [Google Scholar]
- 50.Shen Y., Pei Z.J., Yuan W.Q., Mao E.R. Effect of nitrogen and extraction method on algae lipid yield. Int. J. Agric. Biol. Eng. 2009;2:51–57. [Google Scholar]
- 51.Shahidi F., Wanasundara P.K., J.P.D . Extraction and analysis of lipids. In: Akoh C.C., Min D.B., editors. Food Lipids - Chemistry, Nutrition, and Biotechnology. Dekker Press; New York, USA: 2002. [Google Scholar]
- 52.Prabakaran P., Ravindran A.D. A comparative study on effective cell disruption methods for lipid extraction from microalgae. Lett. Appl. Microbiol. 2011;53:150–154. doi: 10.1111/j.1472-765X.2011.03082.x. [DOI] [PubMed] [Google Scholar]
- 53.Craggs R., Sutherland D., Campbell H. Hectare-scale demonstration of high rate algal ponds for enhanced wastewater treatment and biofuel production. J. Appl. Phycol. 2012;24:1–9. [Google Scholar]
- 54.Nielsen M., Bruhn A., Rasmussen M., Olesen B., Larsen M., Mølle Henrik B. Cultivation of Ulva lactuca with manure for simultaneous bioremediation and biomass production. J. Appl. Phycol. 2011:1–10. [Google Scholar]
- 55.Angell A.R., Mata L., Nys R., Paul N.A. Variation in amino acid content and its relationship to nitrogen content and growth rate in Ulva ohnoi (Chlorophyta) J. Phycol. 2014;50:216–226. doi: 10.1111/jpy.12154. [DOI] [PubMed] [Google Scholar]
- 56.Bahmaei M., Sabbaghian E.S., Farzadkishb E. Development of a method for chlorophyll removal from canola oil using mineral acids. J. Am. Oil Chem. Soc. 2005;82:679–684. [Google Scholar]
- 57.Safi C., Frances C., Ursu A.V., Laroche C., Pouzet C., Vaca-Garcia C., Pontalier P.-Y. Understanding the effect of cell disruption methods on the diffusion of Chlorella vulgaris proteins and pigments in the aqueous phase. Algal Res. 2015;8:61–68. [Google Scholar]
- 58.Lobban C.S., Harrison P.J. Cambridge University Press; Cambridge: 1996. Light and photosynthesis. In: Seaweed Ecology and Physiology; pp. 146–150. ISBN: 9780521408974. [Google Scholar]
- 59.Musharraf S.G., Ahmed M.A., Zehra N., Kabir N., Choudhary M.I., Rahman A. Biodiesel production from microalgal isolates of southern Pakistan and quantification of FAMEs by GC-MS/MS analysis. Chem. Cent. J. 2012;6:149. doi: 10.1186/1752-153X-6-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheung P.C.K., Leung A.Y.H., Ang P.O. Comparison of supercritical carbon dioxide and soxhlet extraction of lipids from a brown seaweed Sargassum hemiphyllum (Turn.). C Ag. J. Agric. Food Chem. 1998;46:4228–4232. [Google Scholar]
- 61.Halim R., Gladman B., Danquah M., Webley P. Oil extraction from microalgae for biodiesel production. Bioresour. Technol. 2011;102:178–185. doi: 10.1016/j.biortech.2010.06.136. [DOI] [PubMed] [Google Scholar]
- 62.Ryckebosch E., Myuylaert K., Foubert I. Optimisation of an analytical procedure for extraction of lipids from microalgae. J. Am. Oil Chem. Soc. 2012;89:189–198. [Google Scholar]
- 63.Li Y., Ghasemi Naghdi F., Garg S., Adarme-Vega T.C., Thurecht K.J., Ghafor W.A., Tannock S., Schenk P.M. A comparative study: the impact of different lipid extraction methods on current microalgal lipid research. Microb. Cell Fact. 2014;24:14. doi: 10.1186/1475-2859-13-14. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu Z., Rosendahl L., Toor S.S., Yu D., Chen G. Hydrothermal liquefaction of barley straw to bio-crude oil: effects of reaction temperature and aqueous phase recirculation. Appl. Energy. 2015;137:183–192. [Google Scholar]
- 65.Karagoz S., Bhaskar T., Muto A., Sakata Y., Uddin Md.A. Low-temperature hydrothermal treatment of biomass: effect of reaction parameters on products and boiling point distributions. Energy Fuels. 2014;18:234–241. [Google Scholar]
- 66.Jena U., Das K.C. Comparative evaluation of thermochemical liquefaction and pyrolysis for bio-oil production from microalgae. Energy Fuels. 2011;25:5472–5482. [Google Scholar]
- 67.Ross A.B., Biller P., Kubacki M.L., Li H., Lea-Langton A., Jones J.M. Hydrothermal processing of microalgae using alkali and organic acids. Fuel. 2010;89:2234–2243. [Google Scholar]
- 68.Zhou D., Zhang L., Zhang S., Fu H., Chen J. Hydrothermal liquefaction of macroalgae Enteromorpha prolifera to bio-oil. Energy Fuel. 2010;24:4054–4061. [Google Scholar]
- 69.Yel N.V., Yelboğa E., Tüter M., Karagüler N.G. Comparison of cell disruption and lipid extraction methods for improving lipid content of Schizochytrium sp. S31. J. Mol. Biol. Biotechnol. 2017;1:9–12. [Google Scholar]
- 70.Kim M.G., Hwang H.W., Nzioka A.M., Kim Y.J. Enhanced lipid extraction from microalgae in biodiesel production. Hem. Ind. 2017;71:167–174. [Google Scholar]