Abstract
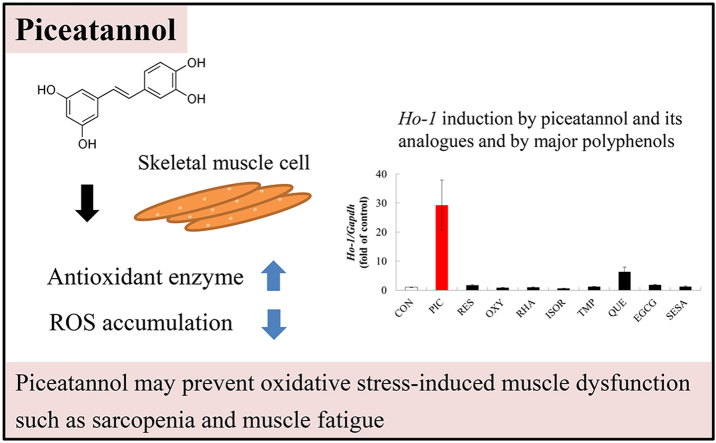
Piceatannol (PIC), a phytochemical, is abundant in passion fruit (Passiflora edulis) seeds. In this study, we investigated the effects of PIC on the expression levels of antioxidant enzymes in C2C12 skeletal muscle cells and compared its effects with those of PIC analogues and polyphenols. We also evaluated its effects on hydrogen peroxide–induced accumulation of reactive oxygen species in C2C12 myotubes. Treatment with PIC led to dose-dependent upregulation of heme oxygenase-1 (Ho-1) and superoxide dismutase 1 (Sod1) mRNA expression in C2C12 myotubes. PIC was the most potent inducer of Ho-1 among the PIC analogues and major polyphenols tested. In addition, treatment with PIC suppressed the hydrogen peroxide–induced increase in intracellular reactive oxygen species levels. Our results suggest that PIC protects skeletal muscles from oxidative stress by activating antioxidant enzymes such as HO-1 and SOD1 and can therefore help prevent oxidative stress–induced muscle dysfunction such as muscle fatigue and sarcopenia.
Keywords: Piceatannol, Skeletal muscle cell, HO-1, Oxidative stress, Antioxidant enzymes
Graphical abstract
Highlights
-
•
PIC induced antioxidant enzymes in C2C12 skeletal muscle cell line.
-
•
PIC was the most potent inducer of Ho-1 among other polyphenols tested.
-
•
Induction potency of PIC for Sod1 was similar level with those of other polyphenols.
-
•
PIC suppressed the hydrogen peroxide-induced increase in intracellular ROS levels.
1. Introduction
Reactive oxygen species (ROS) are generated by physical exercise and muscle contraction. Physiological levels of ROS, which are generated because of moderate exercise, are important for muscle function [1,2]; however, continuous high-intensity exercises generate high levels of ROS, which promotes skeletal muscle contractile dysfunction resulting in muscle fatigue [3]. In addition, excessive ROS accumulation during aging is suggested to trigger sarcopenia [4].
The Kelch-like ECH-associated protein 1 (Keap1)–nuclear factor erythroid 2–related factor 2 (Nrf2) pathway regulates the gene expression of antioxidant enzymes such as heme oxygenase-1 (Ho-1) and superoxide dismutase 1 (Sod1); this signaling pathway preserves intracellular redox homeostasis [5]. ROS stimulates redox-sensitive signaling pathways, and activation of antioxidant enzymes prevents oxidative damage to tissues. Imbalances in normal redox states cause oxidative damage, and attenuation of antioxidant activity during aging is reported to contribute to the age-related loss of muscle [6]. Therefore, increasing the antioxidant capacity of skeletal muscles is one of the most valuable therapeutic approaches against for muscle dysfunction [7,8].
Piceatannol (PIC), a natural polyphenolic compound, is present in large amounts in passion fruit (Passiflora edulis) seeds [9]. It also presents in red grape and other plants [10]. PIC is an analogue of resveratrol (RES), displays a wide spectrum of biological activities like RES. Our previous studies showed that PIC causes improvement of vascular function [11,12], protects the skin from UV irradiation [13], and promotes Sirtuin 1 expression [14]. Moreover, PIC has many beneficial effects such as anti-cancer [15] and anti-inflammatory effects [16], and also helps prevent type 2 diabetes [[17], [18], [19]].
PIC has been recently found to suppress aging via activation of Nrf2 and its downstream antioxidant enzyme in the cranial nerve [20]. PIC also activates antioxidant enzymes in tissues other than brain nerves [[21], [22], [23]]. As well as PIC, plant-derived polyphenols are known to upregulate antioxidant enzymes. RES and quercetin (QUE) upregulate Ho-1 mRNA expression in astrocytes and microglia [24], and epigallocatechin gallate (EGCG) upregulates Ho-1 in human aortic endothelial cells [25]. However, the effects of PIC on skeletal muscles have not yet been elucidated. In the present study, we investigated whether PIC induces antioxidant enzymes in a cultured skeletal muscle cell line and compared the effects of PIC with those of PIC analogues and major polyphenols on the induction of antioxidant enzymes. In addition, the effects of treatment with PIC on hydrogen peroxide (H2O2)-induced ROS accumulation in C2C12 myotubes were evaluated.
2. Materials and methods
2.1. Materials
PIC, RES, oxyresveratrol (OXY), rhapontigenin (RHA), isorhapontigenin (ISOR), 3,3′,4,5′-tetramethoxypiceatannol (TMP), and sesamin (SESA) were obtained from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Dimethyl sulfoxide (DMSO), EGCG, H2O2, and N-acetylcysteine (NAC) were obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). QUE was obtained from Tocric Co., Ltd. (Bristol, UK). Dulbecco's modified Eagle's medium (DMEM), heat-inactivated horse serum (HS), Hanks' Balanced Salt solution (HBSS), and penicillin and streptomycin solutions were obtained from Gibco (MD, USA). Fetal bovine serum (FBS) was obtained from HyClone (UT, USA). The 5-(and-6) chloromethyl-2′,7′ dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) was obtained from Invitrogen (CA, USA).
2.2. Cell culture and treatment
C2C12 skeletal muscle cells were obtained from the European Collection of Authenticated Cell Cultures (Salisbury, UK). The cells were grown in DMEM medium containing 10% FBS and 1% penicillin and streptomycin solution in a humidified incubator at 37 °C with 5% CO2. When C2C12 myoblast cultures reached confluence, the cells were cultured in DMEM containing 2% HS (differentiation medium), and the mediums were changed every three days. After 6 days, the cells differentiated into myotubes. PIC or the other test compounds were dissolved in DMSO, diluted in differentiation medium to the desired concentration before use, and added to the cell culture. The DMSO concentration in the medium was 0.1% for all conditions. The cytotoxicity was not observed in this treatment.
2.3. Real-time PCR analysis of Ho-1 and Sod1 mRNA expression
C2C12 myotubes were cultured in 12-well plates and incubated for 6 h in differentiation medium containing different concentrations of PIC or other test compounds. Total RNA was extracted from cells by using the QIAshredder and the RNeasy Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions. RNA (1 μg) was reverse transcribed into cDNA by using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, CA, USA). Real-time PCR were performed using a LightCycler 480 Real-Time PCR system II (Roche Molecular Diagnostics, Basel, Switzerland) with the LightCycler 480 Probes Master and Universal Probe Library Probes (Roche Molecular Diagnostics). The assay was performed with probe No. 17 for Ho-1, probe No. 49 for Sod1, and probe No. 29 for glyceraldehyde 3-phosphate dehydrogenase (Gapdh). The PCR primers used were as follows: for Ho-1: forward, 5′-aggctaagaccgccttcct-3′ and reverse, 5′-tgtgttcctctgtcagcatca-3′; for Sod1: forward, 5′-caggacctcattttaatcctcac-3′ and reverse, 5′-tgcccaggtctccaacat-3′; and for Gapdh: forward, 5′-gccaaaagggtcatcatctc-3′ and reverse, 5′-cacacccatcacaaacatgg-3′. The amplification conditions were as follows: 50 °C for 2 min; 95 °C for 10 min; and 45 cycles each of 95 °C for 10 s and 60 °C for 25 s. Ho-1 and Sod1 mRNA expression were normalized to Gapdh mRNA expression levels, and relative Ho-1 and Sod1 mRNA expression were determined in comparison with the corresponding levels in control cells.
2.4. Measurement of intracellular ROS levels
C2C12 myotubes were cultured in 96-well plates. After the myotubes were treated with the test compounds for 24 h, they were loaded with CM-H2DCFDA (3 μM) at 37 °C under dark condition for 30 min. Subsequently, the cells were washed with HBSS containing 0.2% HS and treated with H2O2 at 37 °C for 30 min. The fluorescence (excitation/emission at 495 nm/525 nm), reflecting the ROS concentration, was analyzed using a fluorescence plate reader (BMG Labtech, Ortenberg, Germany).
2.5. Statistical analysis
All results have been presented in terms of mean and standard deviation (S.D.). Statistical analyses were performed with one-way analysis of variance (ANOVA), with the Tukey (if data were homoscedastic) or Games-Howell (if data were non-homoscedastic) post-hoc test being used for multiple comparisons with the SPSS software (SPSS Inc., Tokyo, Japan.). A p value of < 0.05 was considered to indicate significant differences between groups.
3. Results
3.1. Effects of PIC on Ho-1 and Sod1 mRNA expression
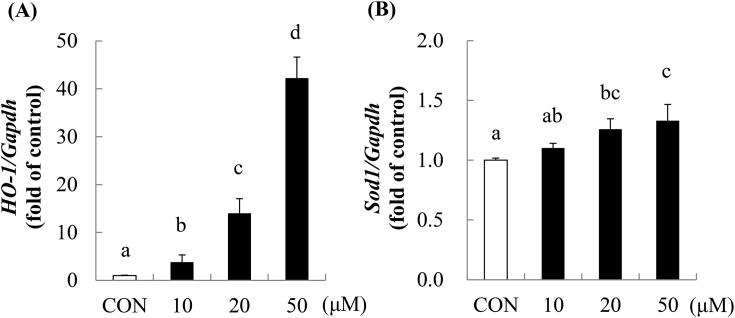
To examine whether PIC affects the gene expression of antioxidant enzymes in skeletal muscle cells, Ho-1 and Sod1 mRNA expression were analyzed by real-time PCR analysis. When C2C12 myotubes were treated with 10–50 μM PIC for 6 h, Ho-1 and Sod1 mRNA expression was upregulated in a dose-dependent manner (Fig. 1). In particular, PIC induced marked Ho-1 mRNA expression: treatment with 10, 20, and 50 μM PIC upregulated Ho-1 mRNA expression by 3.8-, 14.0-, and 42.2-fold, respectively. Treatment with 10, 20, and 50 μM PIC upregulated Sod1 mRNA expression by 1.1-, 1.3-, and 1.3-fold, respectively.
Fig. 1.
Effects of PIC on Ho-1 and Sod1 mRNA expression. C2C12 myotubes were incubated with the DMSO control (CON; white bar) or 10–50 μM piceatannol (black bars) for 6 h, and Ho-1 (A) and Sod1 (B) mRNA expression was analyzed by real-time PCR. Values have been expressed in terms of the fold change compared with the control, which was arbitrarily set to 1. Results have been provided as mean + S.D. values from at least three separate experiments. Different alphabets represent significant difference at p < 0.05; the analysis involved ANOVA with the Games–Howell post-hoc test (A) or Tukey post-hoc test (B).
3.2. Effects of PIC analogues and major polyphenols on the mRNA expression of Ho-1 and Sod1
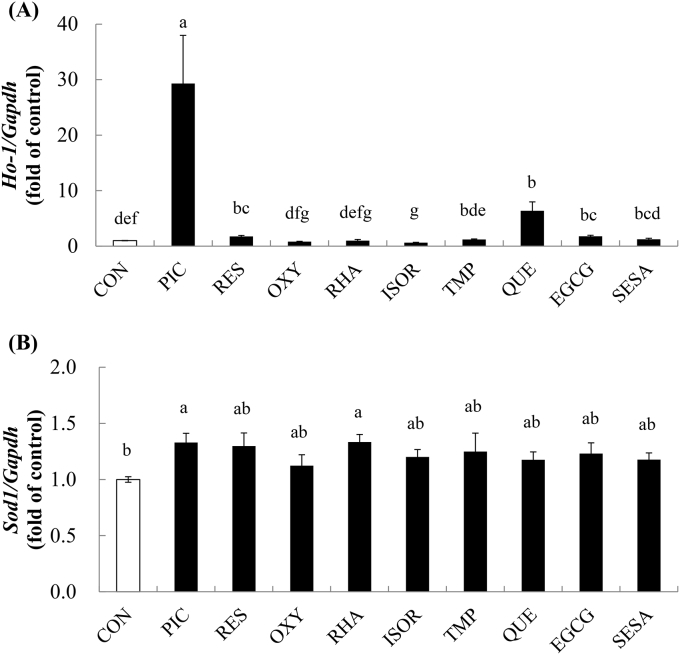
To investigate the potency of PIC against antioxidant enzyme induction, the effects of PIC on antioxidant enzyme expression were compared to those of PIC analogues and major polyphenols. Treatment with PIC, RES, QUE, and EGCG significantly upregulated Ho-1 mRNA expression (Fig. 2A) by 29.3-, 1.8-, 6.4-, and 1.8-fold, respectively. PIC dramatically upregulated Ho-1 mRNA expression, and was the most potent Ho-1 inducer among those tested. PIC and RHA significantly upregulated Sod1 mRNA expression by 1.3- and 1.3- fold, respectively (Fig. 2B). The Sod1-inducing effect of PIC was similar to that of the other compounds.
Fig. 2.
Effects of PIC and other compounds on Ho-1 and Sod1 mRNA expression. C2C12 myotubes were stimulated with the DMSO control (CON; white bar) or stimulants (50 μM; black bars) for 6 h, and Ho-1 (A) and Sod1 (B) mRNA expression was analyzed by real-time PCR. The stimulants were piceatannol (PIC), resveratrol (RES), oxyresveratrol (OXY), rhapontigenin (RHA), isorhapontigenin (ISOR), 3,3',4,5'-tetramethoxypiceatannol (TMP), quercetin (QUE), epigallocatechin gallate (EGCG), and sesamin (SESA). Values have been expressed in terms of the fold change compared with the control, which was arbitrarily set to 1. Results have been provided as mean + S.D. values from four separate experiments. Different alphabets represent significant difference at p < 0.05; the analysis involved ANOVA with the Games–Howell post-hoc test.
3.3. Effects of PIC on intracellular levels of H2O2-induced ROS
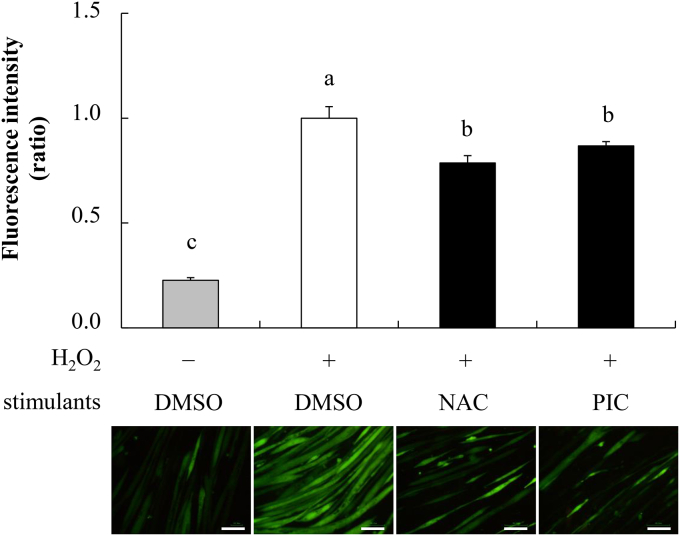
To examine whether PIC affects oxidative stress-induced ROS accumulation, the levels of ROS induced by H2O2 were measured. Addition of 50 μM H2O2 induced 4.5-fold increase in intracellular ROS levels. Pretreatment with 1 mM NAC (powerful antioxidant) and 20 μM PIC for 24 h suppressed H2O2-induced ROS production (Fig. 3), leading to a significant decrease in the ROS levels by 21% and 13%, respectively.
Fig. 3.
Effects of PIC on H2O2-induced intracellular ROS levels. The cells were incubated with the DMSO control, 1mM N-acetylcysteine (NAC), or 20 μM piceatannol (PIC) and loaded with 3 μM CM-H2DCFDA for 30 min. ROS accumulation was determined on treatment with or without 50 μM H2O2. The graph shows the fluorescence intensity produced by ROS. Values have been expressed in terms of the fold change compared with the DMSO control with H2O2, which was arbitrarily set to 1. Results have been provided as mean + S.D. values from at least four separate experiments. Different alphabets represent significant difference at p < 0.05; the analysis involved ANOVA with the Tukey post-hoc test. The bottom panel depicts representative fluorescence microscopy images showing the fluorescence intensity produced by ROS. The scale bar is 100 μm.
4. Discussion
In the present study, we found that (a) PIC induced antioxidant enzymes in a cultured skeletal muscle cell line, (b) PIC had superior effect on Ho-1 mRNA expression compared to those of PIC analogues and major polyphenols, and (c) the induction potency of PIC for Sod1 was on the similar level with those of PIC analogues and major polyphenols. An inducer of HO-1 suppress proinflammatory cytokine and ROS production, which results in ameliorating muscle mass loss [26], therefore PIC may be an optimal polyphenol that improve muscle dysfunctions.
The induction potency for Ho-1 differed between PIC, RES, and other PIC analogues. The mechanism underlying Ho-1 upregulation by polyphenols has been investigated previously. Genes encoding antioxidant enzymes such as Ho-1 exist downstream of the antioxidant response element (ARE), and their expression is mainly controlled by the Keap-Nrf2 pathway [27,28]. Some polyphenols, including PIC, activate Nrf2 signaling [20,22,29]. In particular, PIC and QUE activate Nrf2 through structural changes in Keap1. PIC and QUE have a catechol moiety; it is proposed that an electrophilic quinone is formed because of oxidation of PIC or QUE and interacts with the critical cysteine thiol of Keap1, thereby facilitating the dissociation of Nrf2 [21,30]. RES, which has no catechol moiety, did not show a strong Ho-1 inducing effect in this study. ISOR and RHA, a structure in which one of the hydroxyl groups of the catechol group in PIC was methylated, showed no Ho-1 inducing effects as seen when PIC was treated. No Ho-1 induction was observed either for TMP in which all the hydroxyl groups of PIC were methylated. Therefore, these results suggest that the presence of catechol moiety strongly affects Ho-1 expression. OXY, which has the same number of hydroxyl groups as PIC, did not show Ho-1 induction like PIC. Therefore it is considered that the position of the hydroxyl group, not the number of hydroxyl groups, is important for Ho-1 induction. EGCG and SESA, which is known to have high antioxidant ability, did not have strong Ho-1 inducing effects as seen in PIC.
PIC dramatically upregulated Ho-1 expression, though the change in Sod1 expression was moderate. A study involving mouse embryonic fibroblasts derived from Nrf2-knockout mice revealed suppressed Ho-1 expression and elevated Sod1 expression [31]. Another study showed that Nrf2 knockdown in C2C12 downregulated many antioxidant enzymes such as Ho-1 and Catalase, whereas Sod1 expression did not differ from that of the wild type [32]. These results suggest that Sod1 is not mainly dependent on the Nrf2-ARE pathway. PIC has been reported to activate the Nrf2 pathway; therefore, the difference in the contribution of the Nrf2 pathway against Ho-1 and Sod1 may involve the difference in induction potency of PIC for Ho-1 and Sod1.
In C2C12 myoblast, inhibition of HO-1 activity dramatically abolishes the suppression of ROS accumulation [33,34]. In human endothelial cells, 12 h PIC-treatment suppresses intracellular ROS accumulation, and this effect is canceled by inhibition of HO-1 activity [23]. Therefore, upregulation of Ho-1 expression is related to suppression of ROS accumulation in various cells. PIC induced marked Ho-1 mRNA expression, though the suppression of intracellular ROS by PIC was not so dramatic in this study. The highest Ho-1 induction was observed at 6 h PIC-treatment, and ROS accumulation was examined after 24 h PIC-treatment, therefore, inhibition efficacy may be changed by the treatment time of PIC.
In summary, we showed that PIC upregulates Ho-1 and Sod1 gene expression and decreases H2O2-induced ROS accumulation in C2C12 myotubes. These results suggested that PIC protects skeletal muscles from oxidative stress by activating antioxidant enzymes. The findings would also be helpful in future research on prevention of oxidative stress–induced muscle dysfunction such as sarcopenia and muscle fatigue by using PIC.
Footnotes
Transparency document related to this article can be found online at https://doi.org/10.1016/j.bbrep.2019.100643
Transparency document
References
- 1.Jackson M.J. Redox regulation of skeletal muscle. IUBMB Life. 2008;60:497–501. doi: 10.1002/iub.72. [DOI] [PubMed] [Google Scholar]
- 2.Merry T.L., McConell G.K. Skeletal muscle glucose uptake during exercise: a focus on reactive oxygen species and nitric oxide signaling. IUBMB Life. 2009;61:479–484. doi: 10.1002/iub.179. [DOI] [PubMed] [Google Scholar]
- 3.Powers S.K., Jackson M.J. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fulle S., Protasi F., Di Tano G., Pietrangelo T., Beltramin A., Boncompagni S., Vecchiet L., Fano G. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp. Gerontol. 2004;39:17–24. doi: 10.1016/j.exger.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Kensler T.W., Wakabayashi N., Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 6.Jackson M.J. Redox regulation of muscle adaptations to contractile activity and aging. J. Appl. Physiol. 1985;119:163–171. doi: 10.1152/japplphysiol.00760.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh S., Komine S., Warabi E., Akiyama K., Ishii A., Ishige K., Mizokami Y., Kuga K., Horie M., Miwa Y., Iwawaki T., Yamamoto M., Shoda J. Nuclear factor (erythroid derived 2)-like 2 activation increases exercise endurance capacity via redox modulation in skeletal muscles. Sci. Rep. 2017;7:12902. doi: 10.1038/s41598-017-12926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira L.F., Reid M.B. Muscle-derived ROS and thiol regulation in muscle fatigue. J. Appl. Physiol. 1985;104:853–860. doi: 10.1152/japplphysiol.00953.2007. [DOI] [PubMed] [Google Scholar]
- 9.Matsui Y., Sugiyama K., Kamei M., Takahashi T., Suzuki T., Katagata Y., Ito T. Extract of passion fruit (Passiflora edulis) seed containing high amounts of piceatannol inhibits melanogenesis and promotes collagen synthesis. J. Agric. Food Chem. 2010;58:11112–11118. doi: 10.1021/jf102650d. [DOI] [PubMed] [Google Scholar]
- 10.Piotrowska H., Kucinska M., Murias M. Biological activity of piceatannol: leaving the shadow of resveratrol. Mutat. Res. 2012;750:60–82. doi: 10.1016/j.mrrev.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Kinoshita Y., Kawakami S., Yanae K., Sano S., Uchida H., Inagaki H., Ito T. Effect of long-term piceatannol treatment on eNOS levels in cultured endothelial cells. Biochem. Biophys. Res. Commun. 2013;430:1164–1168. doi: 10.1016/j.bbrc.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 12.Sano S., Sugiyama K., Ito T., Katano Y., Ishihata A. Identification of the strong vasorelaxing substance scirpusin B, a dimer of piceatannol, from passion fruit (Passiflora edulis) seeds. J. Agric. Food Chem. 2011;59:6209–6213. doi: 10.1021/jf104959t. [DOI] [PubMed] [Google Scholar]
- 13.Maruki-Uchida H., Kurita I., Sugiyama K., Sai M., Maeda K., Ito T. The protective effects of piceatannol from passion fruit (Passiflora edulis) seeds in UVB-irradiated keratinocytes. Biol. Pharm. Bull. 2013;36:845–849. doi: 10.1248/bpb.b12-00708. [DOI] [PubMed] [Google Scholar]
- 14.Kawakami S., Kinoshita Y., Maruki-Uchida H., Yanae K., Sai M., Ito T. Piceatannol and its metabolite, isorhapontigenin, induce SIRT1 expression in THP-1 human monocytic cell line. Nutrients. 2014;6:4794–4804. doi: 10.3390/nu6114794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madreiter-Sokolowski C.T., Gottschalk B., Parichatikanond W., Eroglu E., Klec C., Waldeck-Weiermair M., Malli R., Graier W.F. Resveratrol specifically kills cancer cells by a devastating increase in the Ca2+ coupling between the greatly tethered endoplasmic reticulum and mitochondria. Cell. Physiol. Biochem. 2016;39:1404–1420. doi: 10.1159/000447844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Youn J., Lee J.S., Na H.K., Kundu J.K., Surh Y.J. Resveratrol and piceatannol inhibit iNOS expression and NF-kappaB activation in dextran sulfate sodium-induced mouse colitis. Nutr. Canc. 2009;61:847–854. doi: 10.1080/01635580903285072. [DOI] [PubMed] [Google Scholar]
- 17.Minakawa M., Miura Y., Yagasaki K. Piceatannol, a resveratrol derivative, promotes glucose uptake through glucose transporter 4 translocation to plasma membrane in L6 myocytes and suppresses blood glucose levels in type 2 diabetic model db/db mice. Biochem. Biophys. Res. Commun. 2012;422:469–475. doi: 10.1016/j.bbrc.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Uchida-Maruki H., Inagaki H., Ito R., Kurita I., Sai M., Ito T. Piceatannol lowers the blood glucose level in diabetic mice. Biol. Pharm. Bull. 2015;38:629–633. doi: 10.1248/bpb.b15-00009. [DOI] [PubMed] [Google Scholar]
- 19.Oritani Y., Okitsu T., Nishimura E., Sai M., Ito T., Takeuchi S. Enhanced glucose tolerance by intravascularly administered piceatannol in freely moving healthy rats. Biochem. Biophys. Res. Commun. 2016;470:753–758. doi: 10.1016/j.bbrc.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y., Zhang L.H., Chen X., Zhang N., Li G. Piceatannol attenuates behavioral disorder and neurological deficits in aging mice via activating the Nrf2 pathway. Food Funct. 2018;9:371–378. doi: 10.1039/c7fo01511a. [DOI] [PubMed] [Google Scholar]
- 21.Lee H.H., Park S.A., Almazari I., Kim E.H., Na H.K., Surh Y.J. Piceatannol induces heme oxygenase-1 expression in human mammary epithelial cells through activation of ARE-driven Nrf2 signaling. Arch. Biochem. Biophys. 2010;501:142–150. doi: 10.1016/j.abb.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Wung B.S., Hsu M.C., Wu C.C., Hsieh C.W. Piceatannol upregulates endothelial heme oxygenase-1 expression via novel protein kinase C and tyrosine kinase pathways. Pharmacol. Res. 2006;53:113–122. doi: 10.1016/j.phrs.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Jeong S.O., Son Y., Lee J.H., Cheong Y.K., Park S.H., Chung H.T., Pae H.O. Resveratrol analog piceatannol restores the palmitic acid-induced impairment of insulin signaling and production of endothelial nitric oxide via activation of anti-inflammatory and antioxidative heme oxygenase-1 in human endothelial cells. Mol. Med. Rep. 2015;12:937–944. doi: 10.3892/mmr.2015.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Syapin P.J. Regulation of haeme oxygenase-1 for treatment of neuroinflammation and brain disorders. Br. J. Pharmacol. 2008;155:623–640. doi: 10.1038/bjp.2008.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pullikotil P., Chen H., Muniyappa R., Greenberg C.C., Yang S., Reiter C.E., Lee J.W., Chung J.H., Quon M.J. Epigallocatechin gallate induces expression of heme oxygenase-1 in endothelial cells via p38 MAPK and Nrf-2 that suppresses proinflammatory actions of TNF-alpha. J. Nutr. Biochem. 2012;23:1134–1145. doi: 10.1016/j.jnutbio.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu X., Han W., Wang C., Sui D., Bian J. 2018. Upregulation of Heme Oxygenase-1 by Hemin Alleviates Sepsis-Induced Muscle Wasting in Mice; p. 8927104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishii T., Itoh K., Yamamoto M. Roles of Nrf2 in activation of antioxidant enzyme genes via antioxidant responsive elements. Methods Enzymol. 2002;348:182–190. doi: 10.1016/s0076-6879(02)48637-5. [DOI] [PubMed] [Google Scholar]
- 28.Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I., Yamamoto M., Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 29.Ogborne R.M., Rushworth S.A., O'Connell M.A. Epigallocatechin activates haem oxygenase-1 expression via protein kinase Cdelta and Nrf2. Biochem. Biophys. Res. Commun. 2008;373:584–588. doi: 10.1016/j.bbrc.2008.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turpaev K.T. Keap1-Nrf2 signaling pathway: mechanisms of regulation and role in protection of cells against toxicity caused by xenobiotics and electrophiles. Biochemistry (Mosc.) 2013;78:111–126. doi: 10.1134/S0006297913020016. [DOI] [PubMed] [Google Scholar]
- 31.Wan Hasan W.N., Kwak M.K., Makpol S., Wan Ngah W.Z., Mohd Yusof Y.A. Piper betle induces phase I & II genes through Nrf2/ARE signaling pathway in mouse embryonic fibroblasts derived from wild type and Nrf2 knockout cells. BMC Complement Altern. Med. 2014;14:72. doi: 10.1186/1472-6882-14-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horie M., Warabi E., Komine S., Oh S., Shoda J. Cytoprotective role of Nrf2 in electrical pulse stimulated C2C12 myotube. PLoS One. 2015;10 doi: 10.1371/journal.pone.0144835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang J.S., Han M.H., Kim G.Y., Kim C.M., Kim B.W., Hwang H.J., Hyun Y. Nrf2-mediated HO-1 induction contributes to antioxidant capacity of a Schisandrae Fructus ethanol extract in C2C12 myoblasts. Nutrients. 2014;6:5667–5678. doi: 10.3390/nu6125667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang J.S., Choi I.W., Han M.H., Lee D.S., Kim G.Y., Hwang H.J., Kim B.W., Kim C.M., Yoo Y.H., Choi Y.H. The cytoprotective effect of petalonia binghamiae methanol extract against oxidative stress in C2C12 myoblasts: mediation by upregulation of heme oxygenase-1 and nuclear factor-erythroid 2 related factor 2. Mar. Drugs. 2015;13:2666–2679. doi: 10.3390/md13052666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.