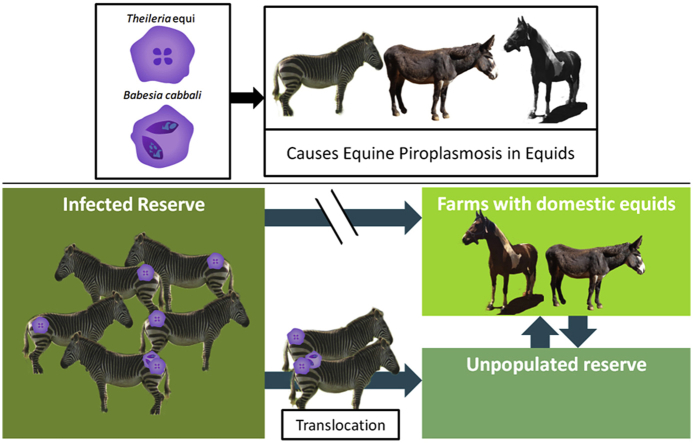
Abstract
Translocation of animals in fragmented habitats is an important means of dispersal and gene flow, however, the movement of animals has led to the spread of various diseases globally and wildlife are often the reservoirs of these diseases. Currently, Cape mountain zebra are translocated within South Africa as a management method for augmentation of isolated and fragmented populations. The movement of pathogens due to translocations in local regions have gone largely unchecked, particularly where there may still be isolated regions that can be negatively affected. Equine piroplasmosis is a tick-borne disease caused by Theilaria equi and/or Babesia caballi reported to occur in equids (Bhoora et al., 2010; Zweygarth et al., 2002). Here, the presence of T. equi and B. caballi was detected in 137 clinically healthy Cape mountain zebra from three South African reserves, Mountain Zebra National Park (MZNP), De Hoop Nature Reserve (DHNR) and Karoo National Park (KNP) using the multiplex EP real-time PCR (qPCR) assay. We observed 100% prevalence for T. equi and identified only one animal from MZNP with B. caballi. These results affirm that precautions should be taken prior to founding new populations of Cape mountain zebra and that potential farms and properties adjacent to prospective reserves should be screened for the presence of the organisms in order to mitigate risks of infection to domestic animals.
Keywords: Cape mountain zebra, Equus zebra zebra, Translocation, Piroplasm, Babesia caballi, Theilaria equi
Graphical abstract
Highlights
-
•
Piroplasms are prevalent in 3 Nature reserves containing Cape mountain zebra.
-
•
Translocation of Cape mountain zebra leads to risk of spread of equine piroplasms.
-
•
Equids in adjacent areas to new populations of Cape mountain zebra are at risk of infection.
1. Introduction
The Cape mountain zebra is endemic to South Africa and is a subspecies of mountain zebra (Equus zebra). Due to various threats including hunting and habitat loss, the population declined in the early twentieth century to less than 100 animals (Moehlman, 2002). From the 1960's, due to population growth in the Mountain Zebra National Park (MZNP), approximately 91% of Cape mountain zebra were translocated to 75 different localities, including 18 reserves that are formally protected (Hrabar et al., 2016; Hrabar and Kerley, 2015). Currently, it is estimated that there are more than 4000 Cape mountain zebra (Hrabar et al., 2016). In 2018, the Biodiversity Management Plan (BMP) for the Cape mountain zebra was published (Birss et al., 2018). As a management strategy it was recommended that in order to maintain the current population growth rates while preventing the loss of genetic diversity, animals need to be further translocated into different areas. Although translocation is considered an important management tool for dispersal in isolated species (IUCN, 1987), it is also known as a mode for the spread of diseases (Fèvre et al., 2006; Woodford and Rossiter, 1994). Humans and wildlife in the areas receiving translocated animals are particularly at risk of infection (Kock et al., 2010). For example, plains zebra (Equus quagga) translocated from Namibia to Spain, is suspected as the mode of transmission of African Horse Sickness to domestic horses (Mellor et al., 1990; Rodriguez et al., 1992).
Equine piroplasmosis (EP) is a tick-borne disease of equids (horses, donkeys, zebra and mules) (Equus caballus; E. asinus, Malekifard et al., 2014; Laus et al., 2015; Schein et al., 2018) that is caused by haemoparasites, Babesia caballi and Theilaria equi. Piroplasm infections were first identified in plains zebra in 1905 (Equus quagga burchelli; Koch, 1905). Inoculation of blood from this zebra into a susceptible horse caused fever and parasitaemia, thus providing support that zebra are carriers of the parasite (Koch, 1905). Subsequently, T. equi has been identified in Cape mountain zebra in South Africa (E. zebra zebra; Bhoora et al., 2010a; Zweygarth et al., 2002) and Babesia spp. Has been reported in plains and Grevy's zebra (Equus grevyi) from East Africa (Brocklesby and Vidler, 1965). Clinical signs vary from asymptomatic to severe and in acute cases with fever, hemolytic anaemia, jaundice, hemoglobinuria, and death. Mortality rates are reported to vary between 10% and 50% (De Waal, 1992). However, the disease may be associated with other non-specific clinical signs (De Waal, 1992). Nucleic acids of T. equi and B. caballi have been detected in several species including dromedary camels and domestic dog (Beck et al., 2009; Qablan et al., 2012). In a few cases, T. equi nucleic acids have been reported in sheep, goat, cow, South American tapir (Tapirus terrestris) and South American rodent (Thrichomys foster; Spickler, 2018i). In addition, organisms potentially related to T. equi have been observed in coati (Nasua nasua) (de Sousa et al., 2018), waterbuck (Kobus defassa; Githaka et al., 2014) and a Malayan tapir (Tapirus indicus; Spickler, 2018).
The occurrence of the disease is associated with the tick vector distribution (De Waal, 1992). Worldwide, ticks from the genera Dermecentor, Hyalomma, Rhipicephalus (De Waal, 1990; Mans et al., 2015; Scoles and Ueti, 2015) as well as experimentally infected Amblyomma (Scoles et al., 2011) have been identified as vectors for both parasites. In South Africa, Rhipicephalus evertsi evertsi and Hyalomma truncatum are the vectors of EP (Madder et al., 2013). The tick vectors are reported to be common within the Savannah and Grassland biomes of South Africa, which includes the natural distribution range of Cape mountain zebra (Spickett, 2013) and prefer domestic and wild equids, although sheep, goats, cattle as well as a range of antelope species will be fed upon (Spickett, 2013; Horak et al., 2017).
Various methods can be used to detect the parasites including the use of blood or organ smears stained with Romanowsky-type stains. Serological tests may be used to measure antibodies and therefore exposure to the parasite. The most commonly used tests are the indirect fluorescent antibody (IFA) assay, enzyme-linked immunosorbent assay (ELISA) as well as the competitive-inhibition ELISA (CI-ELISA). However, there are limitations with serological assays due to problems with non-specificity, cross-reactivity and antibody detection limits (Bruning et al., 1997). Lastly, in cases where it needs to be determined if treatment has eliminated the parasites from a carrier, more labour intensive diagnostic methods may be employed such as in vitro culture (Spickler, 2018). Advances in molecular biological techniques have resulted in the improved detection, identification and genetic characterization of many haemoparasites (Caccio et al., 2000 and Nagore et al., 2004). Various PCR tests are available for the identification of T. equi and B. caballi, and are more sensitive than direct observation. (Bhoora et al., 2010b; Malekifard et al., 2014; Zweygarth et al., 2002). The real-time PCR (qPCR) test has been described as being more sensitive than standard PCR analysis (Bhoora et al., 2010b; Laus et al., 2015).
In the present study, we investigated the prevalence of equine piroplasms in Cape mountain zebra from three South African nature reserves in order to increase our knowledge of the epidemiology of equine piroplasmosis in this sub-species. This work will inform conservation management strategies as directed by the BMP of Cape mountain zebra with regards to mitigating and managing the impact of current and emerging diseases.
2. Materials and methods
2.1. Ethical considerations
The samples used in this study were obtained through CapeNature and South African National Parks (SanParks) and the National Zoological Garden, South African National Biodiversity Institute (NZG SANBI). Ethical clearance was obtained through the University of the Free State's Animal Ethics Committee (Ref: UFS-AED2017/0011), The NZG Research Ethics and Scientific Committee (Ref: NZG/RES/P17/19) and approval was also granted under the Department of Agriculture Forestry and Fisheries Section 20 of the Animal Disease Act 1984 (Ref: 12/11/1/1/8).
2.2. Sample collection and DNA extraction
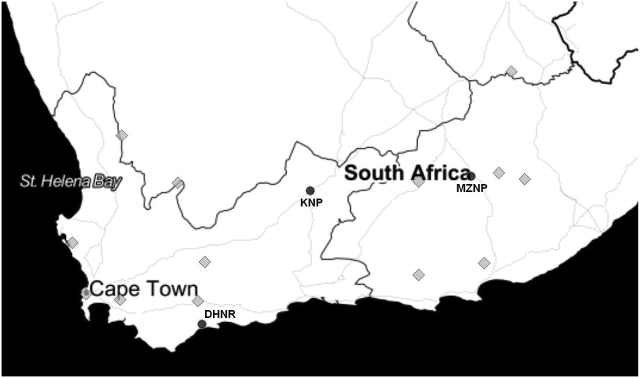
Whole blood was collected in ethylene diamine tetractic acid (EDTA) vacuum tubes from a total of 137 Cape mountain zebra at the two points, 2016 and 2018 (Supplementary Table 1). These Samples were collected from individuals from MZNP (n = 100) prior to translocation to a holding farm in the Eastern Cape for resale, and a private game reserve in the Western Cape respectively. In addition, samples collected from Karoo National Park (KNP, n = 10) (Fig. 1) were obtained from the NZG Biobank. The blood was stored at the NZG Biobank at −20 °C until use. DNA was extracted from 500 μl of whole blood using the Quick-DNA™ Universal Kit (Zymo Research) as per the manufacturer's instructions. The extracted DNA was stored at −20 °C until analysis.
Fig. 1.
A map of south-western South Africa, showing the localities of the 3 reserves from which samples were obtained (black circles) namely Mountain Zebra National Park (MZNP), De Hoop Nature Reserve (DHNR) and Karoo National Park (KNP). The grey diamonds show other known municipal nature reserves and national parks which contain Cape Mountain zebra sourced from Cradock, Eastern Cape, South Africa.
2.3. Statistical analysis
Confidence intervals (CI) of the sample proportion were determined for a representative sample at a 95% confidence level for the populations based on Czaplewski et al. (1983). The Formula is as follows:
Where: CI is the confidence interval, Z is the Z-index (Set to 1.96 for 95% Confidence), n is the sample size, p is the assumed proportion of population infected (Set at 0.5, for a conservative estimate), q is (1-p) and N is the total population size.
2.4. Quantitative PCR assay
The presence of T. equi and B. caballi was detected using the multiplex EP real-time PCR (qPCR) assay as described by Bhoora et al. (2018). The samples were run using Luna® Universal qPCR Master Mix (New England Biolabs) as per manufacturer's instructions. Briefly, reactions prepared in a final volume of 20 μl contained 10 μl Luna universal probe qPCR master mix (New England Biolabs), 2 μl of 10 x primer/probe mix and 5 μl of template DNA. Quantitative PCR assays were performed using the Quantstudio™ 5 Real-time PCR system (Thermo Fisher Scientific). Cycling conditions included enzyme activation at 95 °C for 30 s, followed by 45 cycles of 10 s at 95 °C, 45 s at 60 °C. Data was analysed using QuantStudio™ Design analysis software.
3. Results
3.1. Quantitative PCR assays
All Cape mountain zebra included in this study tested positive for T. equi and two specimens were identified to have a co-infection of T. equi and B. caballi. A summary of these results can be found in Table 1 and Supplementary Table 1. Quantification cycle (Cq) values of each of the animals are provided in Table 1. The multiplex EP qPCR assay detected T. equi parasite DNA in 100% of samples tested; with Cq values between 19 and 31 indicating high levels of parasitaemia. In contrast, B. caballi was detected in only 2 of the 137 samples (∼1.5%) and as a mixed infection with T. equi. One of these samples was detected at a Cq value below the reported 95% sensitivity of the assay. At higher Cq values, the sensitivity of the assay is highly compromised and therefore results below the reportable range of the assay are regarded as false-positives. The samples for MZNP and DHNR were shown to be statistically representative samples of the population determined through the confidence interval of the sample proportion. Based on these results we show that 100% of the populations MZNP (±9.38 CI) and DHNR (±16.57 CI) are infected with T. equi, and that only 2% (±9.38 CI) of MZNP is infected with B. caballi.
Table 1.
Summary of the real time PCR results obtained when screening the Cape Mountain zebra. Nd = nothing detected, CI = confidence interval at 95%.
| Reserve | Population | Samples |
T. equi |
B. caballi |
CI (95%) | ||
|---|---|---|---|---|---|---|---|
| No of (+) | Prevalence | No of (+) | Prevalence | ||||
| MZNP | 1191 | 100 | 100 | 100% | 2 | 2% | ±9.38 |
| DHNR | 115 | 27 | 27 | 100% | nd | 0% | ±16.57 |
| KNP | 842 | 10 | 10 | 100% | nd | 0% | ±30.82 |
4. Discussion
In this study, we report the prevalence of equine piroplasmosis in Cape mountain zebra (Equus zebra zebra) from MZNP, KNP and DHNR. Molecular screening using qPCR detected T. equi parasite DNA in 100% of samples, while B. caballi infections could only be detected as a mixed infection in 1 of the samples (animal from MZNP). The results reported here are similar to previous studies where T. equi was detected in ten Cape mountain zebra in the Bontebok National Park (100% tested positive; Bhoora et al., 2010a) and six Cape mountain zebra from the KNP (100% tested positive, Zweygarth et al., 2002). Equine piroplasmosis is endemic in South Africa and the associated tick vectors have an overlapping distribution, thus it is not surprising that Cape Mountain zebra were found to be positive for T. equi. The zebra tested in our study were positive for T. equi with Cq values that range between 19 and 35. In a tick transmission study conducted by Ueti et al. (2005), the authors used a real-time PCR assay targeting the equi merozoite (ema-1) gene of T. equi to quantify the parasite load required for ticks to successfully transmit the parasite from chronically infected horses. The authors report that ticks infected with a minimum dose of 105.5 parasites/ml were able to transmit the parasites. Determining the minimum parasite dose required by the vectors in South Africa to transmit these parasites does not fall within the scope of this study and therefore a tick transmission study was not conducted. However, we could infer that based on the 95% sensitivity of the T. equi qPCR assay (Bhoora et al., 2018), at a cut-off Cq value of 37, the multiplex EP assay could detect as little at 105.1 parasites/ml. Given that the observed Cq values of T. equi detected in zebra, fall below the reported cut-off Cq of 37, we can conclude that the observed parasitaemia is zebra could allow for successful transmission by the tick vectors.
Previous studies also reported lower prevalence of B. caballi infections in horses (Bhoora et al., 2010b; Zweygarth et al., 2002). Lower prevalence of B. caballi infection has also been reported in zebra. A study conducted by Bhoora et al. (2010a) that included 70 plains zebra and 10 Cape mountain zebra samples, detected T. equi parasite DNA in 90% of the samples tested, while B. caballi could only be detected in 27% of the samples at high Cq values, indicative of low parasite parasitaemia. Zweygarth et al. (2002) reported two out of six Cape mountain zebra obtained from KNP tested positive for B. caballi (Zweygarth et al., 2002). In addition, three animals tested positive for B. caballi from the Bontebok National Park (Zweygarth et al., 2002). It has been previously noted that B. caballi is typically present in their respective hosts at very low parasitaemia at 1%–0.01% (De Waal, 1992). In addition, T. equi has been found to remain as a lifelong infection, whereas B. caballi occurs in its host for approximately 1.5 years (Rüegg et al., 2008).
The Cape mountain zebra sampled in this study showed no indications of chronic infection, thus it is highly likely that sub-clinical infection is common in Cape mountain zebra. Previous reports have shown a prevalence of 26.4% of T. equi in clinically healthy horses from six geographical regions within Israel, thus providing support for sub-clinical infection (Steinman et al., 2012). In endemic areas (or enzootic) such as South Africa, constant infections with equine piroplasmosis induces stable immunity and therefore a state of endemic stability. This is similar to the observation in cattle, where super infections with babesiosis have been reported to induce stable immunity (De Vos, 1979). Fenton and Perkins (2010) suggested that in certain cases, it may be advantageous for the host to tolerate the parasitic infection/s rather than attempt to clear them in order to maintain a more stable, controlled dynamic for the entire parasite community.
Cape mountain zebra are currently regarded as a single meta-population where dispersal is exclusively through translocation activities. It has been suggested that in order for the population to continue to expand and maintain genetic diversity, animals should be translocated to new suitable localities (Hrabar and Kerley, 2015). A molecular epidemiology study of equine piroplasmosis in South Africa has identified as many as 25 distinct T. equi sequences belonging to three distinct genotypes (Bhoora et al., 2010a). In addition, eleven distinct T. equi sequences has been observed in horses from Sudan (Salim et al., 2010) and genetic variants within T. equi has been identified in Spanish horses based on 18 S sequence data (Criado-Fornelio et al., 2004; Nagore et al., 2004). In addition, Githaka et al. (2014) reported an organism potentially related to T. equi was transmissible to waterbuck in Kenya. A study in Kenya conducted by Hawkins et al. (2015) reported infection in zebra (Equus grevyi) and due to their proximity to domestic donkeys the authors suggested that transmission between the two species was likely. Thus, precautions pertaining to the movement of animals are recommended when new reserves are selected for founding Cape mountain zebra populations, to ensure these vectors and novel genotypes are not introduced into non-endemic areas. In addition, the movement of zebra that test positive for equine piroplasms may be a risk to other equids and possibly to other animal species in proximity to their release location. In equids, particularly horses, the control of piroplasmosis infections is desirable in order to avoid abortions, intrauterine transmission and clinical disease which restrict the international movement of horses for equestrian sporting events.
In conclusion, this study provides additional data with regards to the epidemiology of equine piroplasmosis in Cape mountain zebra. While Cape mountain zebra are largely asymptomatic carriers, translocation on to new reserves may prove to be challenging for adjacent farms where domestic equids may be present. It will thus be useful to screen the other reserves from which translocations are set to take place. In addition, it is recommended that when founding new populations in regions where there are no wild equids, surrounding farms first be screened for the presence of equine piroplasms as well as to genotype these piroplasms so as to mitigate risks of transmission to animals that are not currently affected.
Declarations of interest
None.
Acknowledgements
This research was co-funded through the National Zoological Garden, South African National Biodiversity Institute (SANBI-NZG) and the Agricultural Research Council Onderstepoort Veterinary Research (ARC-OVR), as well as partially funded through the South African National Research Foundation (NRF). We would like to thank CapeNature and SANParks for their cooperative efforts in collecting the samples. We would like to thank OVR for making use of their diagnostic assays. We would like to thank Dr. Mamohale Chaisi for referral to her contacts.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2019.04.010.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Beck R., Vojta L., Mrljak V., Marinculić A., Beck A., Živičnjak T., Cacciò S.M. Diversity of Babesia and Theileria species in symptomatic and asymptomatic dogs in Croatia. Int. J. Parasitol. 2009;39(7):843–848. doi: 10.1016/j.ijpara.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Bhoora R., Buss P., Guthrie A.J., Penzhorn B.L., Collins N.E. Genetic diversity of piroplasms in plains zebra (Equus quagga burchellii) and Cape mountain zebra (Equus zebra zebra) in South Africa. Vet. Parasitol. 2010;174:145–149. doi: 10.1016/j.vetpar.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Bhoora R., Quan M., Franssen L., Butler C.M., Van der Kolk J.H., Guthrie A.J., Zweygarth E., Jongejan F., Collins N.E. Development and evaluation of real-time PCR assays for the quantitative detection of Babesia caballi and Theileria equi infections in horses from South Africa. Vet. Parasitol. 2010;168:201–211. doi: 10.1016/j.vetpar.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Bhoora R.V., Pienaar R., Cornelius F., Josemans A., Matthee O., Marumo R., Troskie C., Mans B.J. Multiplex hydrolysis-probe assay for the simultaneous detection of Theileria equi and Babesia caballi infections in equids. Vet. Parasitol. 2018;255:61–68. doi: 10.1016/j.vetpar.2018.03.022. [DOI] [PubMed] [Google Scholar]
- Birss C., Cowell C., Hayward N., Peinke D., Hrabar H., Kotze A. vol. 2. 2018. pp. 1–30. (Biodiversity Management Plan for the Cape Mountain Zebra Equus Zebra Zebra in South Africa). [Google Scholar]
- Brocklesby D.W., Vidler B.O. Some parasites of East African wild animals. Afr. J. Ecol. 1965;3(1):120–122. [Google Scholar]
- Bruning A., Phipps P., Posnett E., Canning E.U. Monoclonal antibodies against Babesia caballi and Babesia equi and their application in serodiagnosis. Vet. Parasitol. 1997;68:11–26. doi: 10.1016/s0304-4017(96)01074-6. [DOI] [PubMed] [Google Scholar]
- Caccio S., Camma C., Onuma M., Severini C. The beta-tubulin gene of Babesia and Theileria parasites is an informative marker for species discrimination. Int. J. Parasitol. 2000;30:1181–1185. doi: 10.1016/s0020-7519(00)00105-3. [DOI] [PubMed] [Google Scholar]
- Criado-Fornelio A., Gonzalez-del-Rio M.A., Buling-Sarana A., Barba-Carretero J.C. The “expanding universe” of piroplasms. Vet. Parasitol. 2004;119:337–345. doi: 10.1016/j.vetpar.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Czaplewski R.L., Crowe D.M., McDonald L.L. Sample sizes and confidence intervals for wildlife population ratios. Wildl. Soc. Bull. 1983;11:121–128. [Google Scholar]
- de Sousa K.C.M., Fernandes M.P., Herrera H.M., Freschi C.R., Machado R.Z., André M.R. Diversity of piroplasmids among wild and domestic mammals and ectoparasites in Pantanal wetland, Brazil. Ticks and tick-borne diseases. 2018;9(2):245–253. doi: 10.1016/j.ttbdis.2017.09.010. [DOI] [PubMed] [Google Scholar]
- De Vos A. Epidemiology and control of bovine babesiosis in South Africa. J. S. Afr. Vet. Assoc. 1979;50(4):357–362. https://journals.co.za/content/savet/50/4/AJA00382809_3606 [Online], Available: [PubMed] [Google Scholar]
- De Waal D.T. Transovarial transmission of Babesia caballi by Hyalomma truncatum. Onderstepoort J. Vet. Res. 1990;57:99–100. [PubMed] [Google Scholar]
- De Waal D.T. Equine piroplasmosis: a review. Br. Vet. J. 1992;148:6–14. doi: 10.1016/0007-1935(92)90061-5. [DOI] [PubMed] [Google Scholar]
- Fenton A., Perkins S.E. Applying predator-prey theory to modelling immune-mediated, within-host interspecific parasite interactions. Parasitology. 2010;137(6):1027–1038. doi: 10.1017/S0031182009991788. [DOI] [PubMed] [Google Scholar]
- Fèvre E.M., Bronsvoort B.M.D.C., Hamilton K.A., Cleaveland S. Animal movements and the spread of infectious diseases. Trends Microbiol. 2006;14:125–131. doi: 10.1016/j.tim.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Githaka N., Konnai S., Bishop R., Odongo D., Lekolool I., Kariuki E., Gakuya F., Kamau L., Isezaki M., Murata S., Ohashi K. Identification and sequence characterization of novel Theileria genotypes from the waterbuck (Kobus defassa) in a Theileria parva-endemic area in Kenya. Vet. Parasitol. 2014;202:180–193. doi: 10.1016/j.vetpar.2014.02.056. [DOI] [PubMed] [Google Scholar]
- Hawkins E., Kock R., McKeever D., Gakuya F., Musyoki C., Chege S.M., Mutinda M., Kariuki E., Davidson Z., Low B., Skilton R.A., Njahira M.N., Wamalwa M., Maina E. Prevalence of Theileria equi and Babesia cabballi as well as the identification of associated ticks in sympatric grevy's zebras (Equus grevyi) and donkeys (Equus africanus asinus) in Northern Kenya. J. Wildl. Dis. 2015;51:137–147. doi: 10.7589/2013-11-316. [DOI] [PubMed] [Google Scholar]
- Horak I.G., Heyne H., Halajian A., Booysen S., Smit W.J. Parasites of domestic and wild animals in South Africa. L. Ixodid ticks infesting horses and donkeys. Onderstepoort J. Vet. Res. 2017;84:1–6. doi: 10.4102/ojvr.v84i1.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabar H., Kerley G. 2015. Cape Mountain Zebra 2014/15 Status Report. [Google Scholar]
- Hrabar H., Birss C., Peinke D., King S., Kerley G., Child M.F. A conservation assessment of Equus zebra zebra. In: Child M.F., Roxburgh L., Do linh San E., Raimondo D., Davies-Mostert H., editors. The Red List of Mammals of South Africa, Lesotho and Swaziland. South African National Biodiversity Institute and Endangered WIldlife Trust; 2016. pp. 1–8. [Google Scholar]
- IUCN Translocaiton of living organisms, IUCN position statement. Int. Union Conserv. Nat. Species Surviv. Comm. 1987:1–13. [Google Scholar]
- Koch R. Vorlaufige Mitteilungen Ober die Ergebnisse einer Forschungsreise nach Ostafrika. Deutsche Medizin- ische Wochenschrift. 1905;31:1865–1869. Brocklesby and Vidler 1965. [Google Scholar]
- Kock R.A., Woodford M.H., Rossiter P.B. Disease risks associated with the translocation of wildlife. Rev. Sci. Tech. l’OIE. 2010;29:329–350. doi: 10.20506/rst.29.2.1980. [DOI] [PubMed] [Google Scholar]
- Laus F., Spaterna A., Faillace V., Veronesi F., Ravagnan S., Beribé F., Cerquetella M., Meligrana M., Tesei B. Clinical investigation on Theileria equi and Babesia caballi infections in Italian donkeys. BMC Vet. Res. 2015;11:1–7. doi: 10.1186/s12917-015-0411-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madder M., Horak I., Stoltsz H. Tick importance and disease transmission. 2013. http://www.afrivip.org/resources/tick-importance-and-disease-transmission
- Malekifard F., Tavassoli M., Yakhchali M., Darvishzadeh R. Detection of Theileria equi and Babesia caballi using microscopic and molecular methods in horses in suburb of Urmia, Iran. Vet. Res. Forum. 2014;5:129–133. [PMC free article] [PubMed] [Google Scholar]
- Mans B.J., Pienaar R., Latif A.A. A review of Theileria diagnostics and epidemiology. Int. J. Parasitol. Parasites Wildl. 2015;4:104–118. doi: 10.1016/j.ijppaw.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor P.S., Boned J., Hamblin C., Graham S. Isolations of African horse sickness virus from vector insects made during the 1988 epizootic in Spain. Epidemiol. Infect. 1990;105:447–454. doi: 10.1017/s0950268800048020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehlman P.D., editor. Status Survey and Conservation Action Plan: Equids: Zebras, Asses and Horses. IUCN; Gland: 2002. [Google Scholar]
- Nagore D., Garcia-Sanmartin J., Garcia-Perez A.L., Juste R.A., Hurtado A. Detection and identification of equine Theileria and Babesia species by reverse line blotting: epidemiological survey and phylogenetic analysis. Vet. Parasitol. 2004;123:41–54. doi: 10.1016/j.vetpar.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Qablan M.A., Sloboda M., Jirků M., Oborník M., Dwairi S., Amr Z.S., Hořín P., Lukeš J., Modrý D. Quest for the piroplasms in camels: identification of Theileria equi and Babesia caballi in Jordanian dromedaries by PCR. Vet. Parasitol. 2012;186(3–4):456–460. doi: 10.1016/j.vetpar.2011.11.070. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., Hooghuis H., Castaño M. African horse sickness in Spain. Vet. Microbiol. 1992;33:129–142. doi: 10.1016/0378-1135(92)90041-q. [DOI] [PubMed] [Google Scholar]
- Rüegg S.R., Heinzmann D., Barbour A.D., Torgerson P.R. Estimation of the transmission dynamics of Theileria equi and Babesia caballi in horses. Parasitology. 2008;135(5):555–565. doi: 10.1017/S0031182008004204. [DOI] [PubMed] [Google Scholar]
- Salim B., Bakheit M.A., Kamau J., Nakamura I., Sugimoto C. Nucleotide sequence heterogeneity in the small subunit ribosomal RNA gene within Theileria equi from horses in Sudan. Parasitol. Res. 2010;106(2):493. doi: 10.1007/s00436-009-1691-7. [DOI] [PubMed] [Google Scholar]
- Schein F.B., Maia M.O., Witter R., Marcili A., Nakazato L., Almeida E.M., De Castro A., Oliveira S. De, Pacheco R.D.C. Molecular survey and genetic diversity of piroplasmids in equids from. Midwestern Brazil. 2018;2961:464–472. doi: 10.1590/S1984-296120180048. [DOI] [PubMed] [Google Scholar]
- Scoles G.A., Ueti M.W. Vector ecology of equine piroplasmosis. Annu. Rev. Entomol. 2015;60:561–580. doi: 10.1146/annurev-ento-010814-021110. [DOI] [PubMed] [Google Scholar]
- Scoles G.A., Hutcheson H.J., Schlater J.L., Hennager S.G., Pelzel A.M., Knowles D.P. Equine piroplasmosis associated with Amblyomma cajennense ticks, Texas, USA. Emerg. Infect. Dis. 2011;17:1903–1905. doi: 10.3201/eid1710.101182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spickett A.M. 2013. Ixodid Ticks of Major Economic Importance and Their Distibution in South Africa. [Google Scholar]
- Spickler Anna Rovid. Equine piroplasmosis. 2018. http://www.cfsph.iastate.edu/DiseaseInfo/factsheets.php Retrieved from.
- Steinman A., Zimmerman T., Klement E., Lensky I.M., Berlin D., Gottlieb Y., Baneth G. Demographic and environmental risk factors for infection by Theileria equi in 590 horses in Israel. Vet. Parasitol. 2012;187(3–4):558–562. doi: 10.1016/j.vetpar.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Ueti M.W., Palmer G.H., Kappmeyer L.S., Statdfield M., Scoles G.A., Knowles D.P. Ability of the vector tick Boophilus microplus to acquire and transmit Babesia equi following feeding on chronically infected horses with low-level parasitemia. J. Clin. Microbiol. 2005;43:3755–3759. doi: 10.1128/JCM.43.8.3755-3759.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodford M.H., Rossiter P.B. Disease risks associated with wildlife translocation projects. Creat. Conserv. 1994;12:178–200. doi: 10.20506/rst.12.1.667. [DOI] [PubMed] [Google Scholar]
- Zweygarth E., Lopez-Rebollar L.M., Meyer P. In vitro isolation of equine piroplasms derived from Cape Mountain zebra (Equus zebra zebra) in South Africa. Onderstepoort J. Vet. Res. 2002;69:197–200. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.