ABSTRACT
Background
Saturated fatty acids (SFAs) of different chain lengths have unique metabolic and biological effects, and a small number of recent studies suggest that higher circulating concentrations of the very-long-chain SFAs (VLSFAs) arachidic acid (20:0), behenic acid (22:0), and lignoceric acid (24:0) are associated with a lower risk of diabetes. Confirmation of these findings in a large and diverse population is needed.
Objective
We investigated the associations of circulating VLSFAs 20:0, 22:0, and 24:0 with incident type 2 diabetes in prospective studies.
Methods
Twelve studies that are part of the Fatty Acids and Outcomes Research Consortium participated in the analysis. Using Cox or logistic regression within studies and an inverse-variance-weighted meta-analysis across studies, we examined the associations of VLSFAs 20:0, 22:0, and 24:0 with incident diabetes among 51,431 participants.
Results
There were 14,276 cases of incident diabetes across participating studies. Higher circulating concentrations of 20:0, 22:0, and 24:0 were each associated with a lower risk of incident diabetes. Pooling across cohorts, the RR (95% CI) for incident diabetes comparing the 90th percentile to the 10th percentile was 0.78 (0.70, 0.87) for 20:0, 0.84 (0.77, 0.91) for 22:0, and 0.75 (0.69, 0.83) for 24:0 after adjustment for demographic, lifestyle, adiposity, and other health factors. Results were fully attenuated in exploratory models that adjusted for circulating 16:0 and triglycerides.
Conclusions
Results from this pooled analysis indicate that higher concentrations of circulating VLSFAs 20:0, 22:0, and 24:0 are each associated with a lower risk of diabetes.
Keywords: saturated fatty acids, very-long-chain saturated fatty acids, diabetes, meta-analysis, Fatty Acids and Outcomes Research Consortium, Cohorts for Heart and Aging Research in Genomic Epidemiology
Introduction
Type 2 diabetes is a major cause of morbidity and mortality, and the global burden of the disease has reached epidemic proportions. In 2014, the WHO estimated that 8.5% of adults 18 y of age or older have type 2 diabetes worldwide (1), and this estimate is expected to rise with an increase in life expectancy, global urbanization, and the adoption of Western lifestyles (2, 3). Identification of risk factors associated with the development of diabetes is therefore of considerable public health interest.
Circulating SFAs, which can be derived from both endogenous metabolic processes as well as diet, provide biomarkers of specific SFAs of different chain lengths. The very-long-chain SFAs (VLSFAs) arachidic acid (20:0), behenic acid (22:0), and lignoceric acid (24:0) are found in foods, such as peanuts, peanut butter, and macadamia nuts, and are also produced endogenously from the elongation of shorter-chain SFAs [e.g., palmitic acid (16:0) to stearic acid (18:0) and 20:0, and then of 20:0 to 22:0 and 24:0] (4). SFAs 16:0 and 18:0 may originate from dietary and metabolic sources; dietary sources of SFAs 16:0 and 18:0 include red meats, hard cheeses, and tropical oil (5, 6), whereas de novo lipogenesis in the presence of low-fat and high-carbohydrate diets (7–10) is a major metabolic pathway for synthesis of SFAs 16:0 and 18:0.
Recent studies suggest that associations of circulating SFAs with diabetes risk may vary by SFA chain length, likely due to the unique metabolic and biological effects of different SFAs (11–13). Particularly, these studies suggest that higher concentrations of circulating VLSFAs 20:0, 22:0, and 24:0 are associated with a lower risk of diabetes than lower concentrations of circulating VLSFAs 20:0, 22:0, and 24:0. Although these findings are important because circulating concentrations of VLSFAs are at least in part modifiable through diet (e.g., intake of peanut butter), the study designs, covariates of interest, measurement of VLSFAs, and ascertainment of incident diabetes differed across studies, and the generalizability of the study results is unknown because the studies were performed among primarily Caucasian adults in Europe (12, 13) and the United States (11). To address this gap, we investigated the associations of circulating VLSFAs 20:0, 22:0, and 24:0 with incident type 2 diabetes among 12 prospective cohort studies as part of the Fatty Acids and Outcomes Research Consortium (FORCE) (14). We hypothesized that higher concentrations of VLSFAs 20:0, 22:0, and 24:0 are associated with a lower risk of type 2 diabetes.
Methods
Study sample
The study sample comprised participants from 12 prospective cohort studies that are part of the FORCE: a consortium derived from the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) to examine the associations of circulating fatty acids of interest with nongenetic outcomes. Details on FORCE have been described previously (15, 16). For the present analysis, we included all cohorts that are part of FORCE who were interested in the project and who had available data on VLSFAs, and whose cohorts included participants 18 y of age or older who were free of prevalent type 2 diabetes (as defined by self-reported diabetes, fasting glucose ≥126 mg/dL, or use of diabetes drugs) at the time of fatty acid measurement (Supplemental Figure 1). Standardized analysis plans were developed and provided to each of the 12 participating cohorts, including inclusion and exclusion criteria; definitions for exposures, outcomes, and covariates of interest; and a detailed statistical analysis protocol. Contributing studies included: the Age, Gene, Environmental Susceptibility-Reykjavik Study (17); the Chin-Shan Community Cardiovascular Cohort Study (CCCC) (18); the Cardiovascular Health Study (CHS) (19); the Framingham Heart Study (20); the European Prospective Investigation into Cancer-InterAct (EPIC-Interact) (21); the Insulin Resistance Atherosclerosis Study (IRAS) (22); the Kuopio Ischaemic Heart Disease Risk Factor Study (KIHD) (23); the Melbourne Collaborative Cohort Study (MCCS) (24); the Multi-Ethnic Study of Atherosclerosis (MESA) (25); the Metabolic Syndrome in Men Study (METSIM) (26); the Women's Health Initiative Memory Study (WHIMS) (27); and the Three City Study (3C-Study) (28). Of the 12 participating studies, 2 (CHS and EPIC-Interact) have previously assessed associations of VLSFAs and incident diabetes (11, 12). All procedures followed were in accordance with the Helsinki Declaration of 1975 as revised in 1983. Each participating study had local institutional review board approval and written informed consent was obtained from all participants.
VLSFA assessment
Details on the measurement of circulating fatty acid biomarkers for each participating cohort are described in Supplemental Table 1. In brief, gas chromatography was used to assess individual fatty acid concentrations in each cohort in ≥1 lipid compartment including plasma phospholipids (the Age, Gene, Environmental Susceptibility-Reykjavik Study, CHS, EPIC-Interact, MCCS, MESA, METSIM), total plasma (CCCC, IRAS, KIHD, 3C Study), or red blood cells (Framingham Heart Study, WHIMS, 3C Study, METSIM). Fatty acid levels in each cohort were expressed as a percentage of total measured fatty acids. A list of the VLSFAs of interest available in each study is given in Table 1; all studies had each of VLSFA 20:0, 22:0, and 24:0 available, except KIHD did not measure VLSFA 20:0, and MESA and 3C Study did not measure VLSFA 24:0.
TABLE 1.
Description of 12 studies that participated in analyses of circulating very-long-chain SFAs and incident diabetes1
| Study | Country | Study design2 | Biomarker compartment | Year of blood sampling | Fatty acids assessed | Year follow-up ended |
|---|---|---|---|---|---|---|
| AGES-Reykjavik | Iceland | PC | Plasma phospholipid | 2002–2006 | 20:0, 22:0, 24:0 | 2007–2011 |
| CCCC | Taiwan | PC | Total plasma | 1992 | 20:0, 22:0, 24:0 | 2000 |
| CHS | United States | PC | Plasma phospholipid | 1992–1993 | 20:0, 22:0, 24:0 | 2011 |
| EPIC-InterAct | Europe | PCC | Plasma phospholipid | 1993–1997 | 20:0, 22:0, 24:0 | 2007 |
| FHS | United States | PC | Red blood cells | 2005–2008 | 20:0, 22:0, 24:0 | 2015 |
| IRAS | United States | PCC | Total plasma | 1992–1994 | 20:0, 22:0, 24:0 | 1999 |
| KIHD | Finland | PC | Total plasma | 1998–2001 | 22:0, 24:0 | 2010 |
| MCCS | Australia | PC | Plasma phospholipid | 1992 | 20:0, 22:0, 24:0 | 2002 |
| MESA | United States | PC | Plasma phospholipid | 2000–2002 | 20:0, 22:0 | 2010–2012 |
| METSIM | Finland | PC | Plasma phospholipid | 2006–2010 | 20:0, 22:0, 24:0 | 2014 |
| WHIMS | United States | PC | Red blood cells | 1995 | 20:0, 22:0, 24:0 | 2009 |
| 3C Study | France | PC | Red blood cells, total plasma | 1999–2000 | 20:0, 22:0 | 2011–2012 |
1AGES-Reykjavik, Age, Gene, Environmental Susceptibility-Reykjavik Study (17); CCCC, Chin-Shan Community Cardiovascular Cohort Study (18); CHS, Cardiovascular Health Study (19); EPIC-Interact, European Prospective Investigation into Cancer-InterAct (21); FHS, Framingham Heart Study (20); IRAS, Insulin Resistance Atherosclerosis Study (22); KIHD, Kuopio Ischaemic Heart Disease Risk Factor Study (23); MCCS, Melbourne Collaborative Cohort Study (24); MESA, Multi-Ethnic Study of Atherosclerosis (25); METSIM, Metabolic Syndrome in Men Study (26); PC, prospective cohort; PCC, prospective nested case-control; WHIMS, Women's Health Initiative Memory Study (27); 3C Study, Three City Study (28).
2Details on the design of each study are described in Supplemental Table 1.
Ascertainment of incident diabetes
Cohort-specific methods for assessing development of diabetes are described in detail in Supplemental Table 1. Briefly, for most participating cohorts, incident diabetes was defined based on ≥1 criterion: fasting glucose concentrations ≥126 mg/dL, nonfasting or 2-h postchallenge glucose concentrations ≥200 mg/dL, glycated hemoglobin ≥6.5%, use of insulin or oral hypoglycemic medications, or self-report. For 3 European studies (EPIC-InterAct, KIHD, and METSIM), diabetes was ascertained by linkage to registries of primary care, secondary care, medication use, hospital admissions, or mortality.
Measurement of covariates
The standardized analysis plan included detailed definitions and categorizations for the major risk factors of interest, including physical activity, smoking, alcohol use, prevalent hypertension, prevalent dyslipidemia, prevalent coronary artery disease, and self-reported health status. The standardized definitions of risk factors were adopted to minimize heterogeneity across cohorts (29). Details on data collection methods for covariates for each cohort are described in Supplemental Table 1.
Cohort statistical analyses
Each cohort performed new individual-level analyses and provided results to the lead author (AMF) using a standardized electronic form. In 10 of the participating cohorts, Cox regression models were used to examine the associations of each VLSFA of interest with incident type 2 diabetes. For these study participants, follow-up time was assessed from baseline (i.e., time of fatty acid measurement) to date of development of incident diabetes, death from any cause, or loss to follow-up. The MCCS and the IRAS did not have detailed time-to-event data available for participants, and therefore used logistic regression. For each study, each VLSFA was incorporated in models as a continuous linear variable in units of the study-specific interquintile range (i.e., the difference between the 90th and 10th percentiles) and, in separate models, as quintiles in indicator (i.e., dummy) categorical variables with the referent group as the lowest quintile of each circulating VLSFA: 20:0, 22:0, or 24:0. Each cohort estimated coefficients and SEs for the associations from 3 prespecified multivariable models. The first model adjusted for major potential confounders including age, sex, clinic, race, education, physical activity, smoking, alcohol use, prevalent hypertension, prevalent dyslipidemia, prevalent coronary artery disease, and self-reported health status. A second model also adjusted for BMI and waist circumference to better understand if these factors influence the associations of each VLSFA with incident diabetes (primary model). A third model (exploratory model) further adjusted for circulating SFA 16:0 and triglycerides (TGs) (model 3), biomarkers of hepatic de novo lipogenesis in the presence of low-fat and high-carbohydrate diets (30, 31). In the CHS, SFA 16:0 and TGs were shown to potentially mediate or confound the association of VLSFAs and incident diabetes (11).
We examined the potential interactions of age, sex, and BMI with each VLSFA of interest (modeled linearly) on risk of incident diabetes. Participating cohorts provided coefficients and SEs for multiplicative interaction terms for each factor of interest with each VLSFA of interest, after adjustment for the covariates included in the primary model described above.
Meta-analyses
Results from each cohort were compiled and combined using inverse-variance-weighted meta-analysis in STATA version 13.1 (Stata Corporation). Inverse-variance-weighted fixed-effects meta-analysis approximates results that would be obtained if the data from all studies could be analyzed together with adjustment for study (32). Heterogeneity between studies was assessed using the I2 index derived from the Cochran Q statistic (33). In preliminary meta-analyses, IRAS contributed 65–71% of the sample weight in each model despite a small total sample size (n = 719) and few cases of diabetes (n = 146) owing to influential outliers in concentrations of VLSFAs for some participants. As the cohort was unable to provide updated results excluding influential outliers, it was subsequently excluded from primary analyses. In sensitivity analyses, we repeated each meta-analysis omitting 1 cohort at a time to confirm that individual cohorts were not overly influencing the observed levels of association. We also performed additional exploratory meta-regression according to lipid compartment (i.e., plasma phospholipid, total plasma, or red blood cell measures) and region (i.e., cohorts based in the United States, Europe, Asia, or Australia).
Results
Descriptions and baseline characteristics for each of the 12 participating cohorts are given in Tables1 and 2. The mean cohort age ranged from 52.3 y to 76.0 y, and BMI (in kg/m2) from 23.2 to 28.1. Mean cohort fasting glucose ranged from 86.4 to 104.8 mg/dL. Most cohorts included primarily participants of European descent, although several included significant proportions of other races/ethnicities including the CCCC study (100% Chinese), the Cardiovascular Health Study (11% African American), IRAS (33.2% Hispanic, 24.5% African American), MESA (23.9% Hispanic, 22.2% African American, 25.5% Asian), and WHIMS (6.0% African American, 2.1% Hispanic, 1.7% Asian). Mean cohort levels of each VLSFA ranged from 0.13% to 0.62% for VLSFA 20:0, 0.23% to 1.67% for VLSFA 22:0, and 0.20% to 4.0% for VLSFA 24:0. For most cohorts, VLSFA 20:0, 22:0, and 24:0 were moderately-to-highly correlated, and each VLSFA was negatively correlated with circulating 16:0 (Supplemental Table 2). Circulating concentrations of fatty acids were generally similar across region (i.e., cohorts based in the United States, Europe, Asia, or Australia) or year of blood sampling (data not shown).
TABLE 2.
Characteristics of participating cohorts at time of fatty acid biomarker measurement1
| Study | n (incident cases of diabetes) | Age, y | Sex (% female) | BMI (kg/m2) | 20:02 | 22:02 | 24:02 | Baseline fasting glucose, mg/dL |
|---|---|---|---|---|---|---|---|---|
| AGES-Reykjavik | 753 (28) | 75.5 ± 5.2 | 59.5 | 27.0 ± 4.0 | 0.62 ± 0.10 | 1.67 ± 0.27 | 1.33 ± 0.22 | 99.0 ± 8.9 |
| CCCC | 616 (128) | 58.7 ± 9.7 | 40.0 | 23.2 ± 2.9 | 0.48 ± 0.29 | 0.18 ± 0.31 | 0.80 ± 0.33 | 104.8 ± 13.8 |
| CHS | 3107 (282) | 75.1 ± 5.3 | 61.5 | 26.4 ± 4.5 | 0.50 ± 0.08 | 1.70 ± 0.32 | 1.40 ± 0.28 | 97.8 ± 9.8 |
| EPIC-InterAct | 27,296 (12,132) | 52.3 ± 9.2 | 62.3 | 26.0 ± 4.2 | 0.13 ± 0.04 | 0.24 ± 0.08 | 0.23 ± 0.07 | 89.3 ± 23.2 |
| FHS | 1870 (95) | 64.4 ± 8.3 | 57.2 | 27.8 ± 5.0 | NA | NA | 0.42 ± 0.16 | 100.1 ± 9.1 |
| IRAS | 719 (146) | 55.1 ± 8.5 | 55.8 | 28.4 ± 5.6 | 0.11 ± 0.03 | 0.23 ± 0.08 | 0.20 ± 0.08 | 98.1 ± 11.1 |
| KIHD | 1543 (205) | 62.7 ± 6.5 | 52.7 | 27.6 ± 4.4 | NA | 0.48 ± 0.09 | 0.48 ± 0.12 | 86.4 ± 8.1 |
| MCCS | 5617 (485) | 56.3 ± 8.6 | 53.9 | 27.0 ± 4.4 | 0.25 ± 0.07 | 0.71 ± 0.17 | 0.59 ± 0.15 | 99.6 ± 9.2 |
| MESA | 2252 (309) | 60.9 ± 9.7 | 53.9 | 27.6 ± 5.4 | 0.25 ± 0.09 | 0.56 ± 0.29 | NA | 89.7 ± 10.7 |
| METSIM | 1302 (71) | 55.0 ± 7.1 | 0 | 26.4 ± 3.5 | 0.38 ± 0.07 | 0.74 ± 0.16 | 0.62 ± 0.14 | 102.8 ± 8.3 |
| WHIMS | 6510 (502) | 70.1 ± 3.8 | 100.0 | 28.1 ± 5.5 | 0.13 ± 0.06 | 0.16 ± 0.10 | 0.35 ± 0.24 | 94.7 ± 9.8 |
| 3C Study | 565 (39) | 76.0 ± 4.0 | 64.3 | 25.0 ± 4.0 | 0.46 ± 0.08 | 1.00 ± 0.30 | NA | 88.0 ± 10.0 |
1Values are means ± SDs unless otherwise indicated. AGES-Reykjavik, Age, Gene, Environmental Susceptibility-Reykjavik Study (17); CCCC, Chin-Shan Community Cardiovascular Cohort Study (18); CHS, Cardiovascular Health Study (19); EPIC-Interact, European Prospective Investigation into Cancer-InterAct (21); FHS, Framingham Heart Study (20); IRAS, Insulin Resistance Atherosclerosis Study (22); KIHD, Kuopio Ischaemic Heart Disease Risk Factor Study (23); MCCS, Melbourne Collaborative Cohort Study (24); MESA, Multi-Ethnic Study of Atherosclerosis (25); METSIM, Metabolic Syndrome in Men Study (26); WHIMS, Women's Health Initiative Memory Study (27); 3C Study, Three City Study (28).
2Measured as percentage of total fatty acids.
Higher circulating concentrations of 20:0, 22:0, and 24:0 were each associated with a lower risk of incident diabetes. Across the 11 studies, there were 14,276 cases of incident diabetes. Pooling across cohorts, comparing the 90th percentile to the 10th percentile, the RR of incident diabetes was 0.78 (95% CI: 0.70, 0.87) for 20:0, 0.84 (95% CI: 0.77, 0.91) for 22:0, and 0.75 (95% CI: 0.69, 0.83) for 24:0 after adjustment for age, sex, site, race, education, occupation, physical activity, smoking, alcohol use, hypertension, dyslipidemia, coronary heart disease, self-reported health status, BMI, and waist circumference (Figure 1). The model without BMI and waist circumference did not produce materially different results (Supplemental Figure 2). In contrast, further adjustment for TGs and circulating SFA 16:0 fully attenuated observed associations with RRs across the interquintile range of 0.93 (95% CI: 0.83, 1.04) for 20:0, 1.04 (95% CI: 0.94, 1.14) for 22:0, and 0.97 (95% CI: 0.88, 1.08) for 24:0 (Supplemental Figure 3). Omitting 1 cohort at a time did not materially alter RR estimates (data not shown). Results were similar in: 1) analyses that included IRAS (Supplemental Figures 4–6) and 2) pooled analyses that assessed each VLSFA in quintiles as indicator categories (Supplemental Figures 7–9).
FIGURE 1.
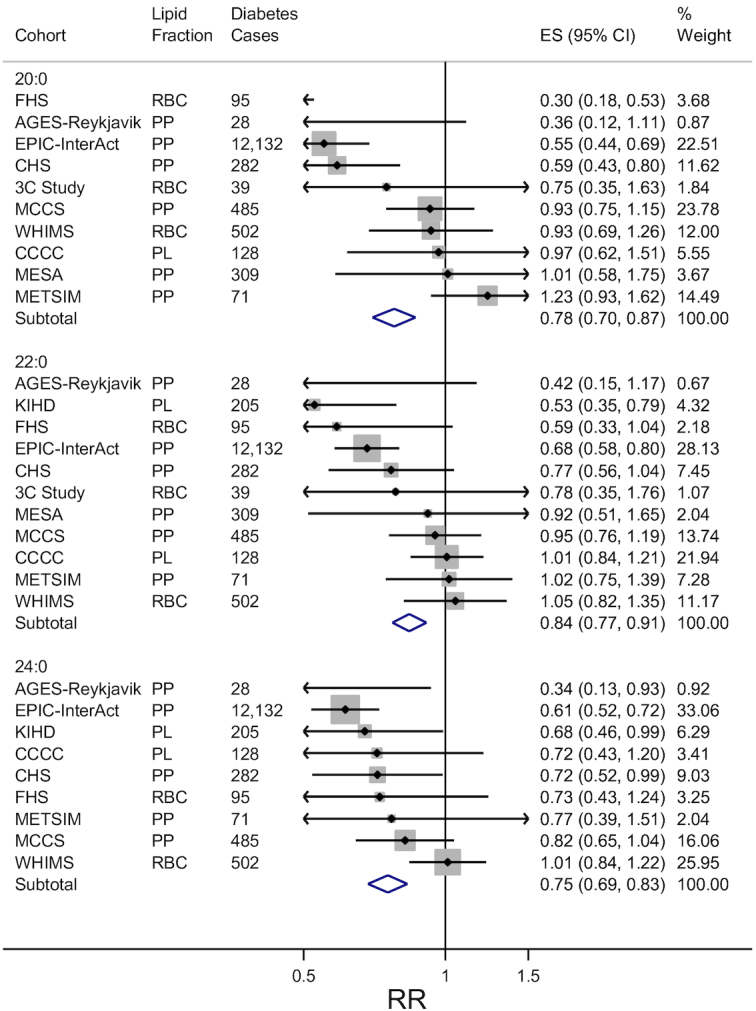
Forest plots of prospective associations of circulating very-long-chain SFAs with incident diabetes in 11 studies. RR and 95% CI per interquintile range (medians of the first and fifth quintile in each cohort) are represented by a filled circle and horizontal line for each cohort, and by a diamond for the overall pooled results. Cohort-specific associations were assessed in multivariable models adjusted for age, sex, clinic, race, education, physical activity, smoking, alcohol use, prevalent hypertension, prevalent dyslipidemia, prevalent coronary heart disease, self-reported health status, BMI, and waist circumference. The size of the shaded square is a marker of study weight in the inverse-variance-weighted meta-analysis. AGES-Reykjavik, Age, Gene, Environmental Susceptibility-Reykjavik Study (17); CCCC, Chin-Shan Community Cardiovascular Cohort Study (18); CHS, Cardiovascular Health Study (19); EPIC-Interact, European Prospective Investigation into Cancer-InterAct (21); ES, effect size; FHS, Framingham Heart Study (20); IRAS, Insulin Resistance Atherosclerosis Study (22); KIHD, Kuopio Ischaemic Heart Disease Risk Factor Study (23); MCCS, Melbourne Collaborative Cohort Study (24); MESA, Multi-Ethnic Study of Atherosclerosis (25); METSIM, Metabolic Syndrome in Men Study (26); PC, prospective cohort; PCC, prospective nested case-control; PL, total plasma; PP, plasma phospholipid; RBC, red blood cell; WHIMS, Women's Health Initiative Memory Study (27); 3C Study, Three City Study (28).
We observed little evidence of effect modification for each VLSFA with age, sex, or BMI on risk of incident diabetes (Supplemental Table 3). Although we observed notable heterogeneity between studies (i.e., the I2 index for each primary analysis of VLSFA 20:0, 22:0, and 24:0 was 78.3%, 60.5%, and 57.2%, respectively), the heterogeneity was not explained by region (i.e., cohorts in Europe, the United States, Australia, or Asia) or fatty acid compartment (i.e., plasma phospholipids, total plasma, or red blood cells) in post hoc meta-regression analyses (data not shown). Although fixed-effects meta-analyses have been shown to produce valid estimates of risk across heterogeneous studies (34), in sensitivity analyses, we reran all analyses using a random-effects meta-analysis, and results were similar (data not shown).
Discussion
The results from this pooled analysis of new, harmonized, individual-level analyses in 12 prospective cohort studies globally indicate that higher concentrations of circulating VLSFAs 20:0, 22:0, and 24:0 are each associated with a lower risk of diabetes. Results were robust to adjustment for major diabetes risk factors, including measures of adiposity. In comparison, results were fully attenuated after adjustment for circulating 16:0 and TGs (30).
The relative contributions of metabolism and diet on circulating concentrations of VLSFAs are unknown, but studies provide evidence that VLSFAs, as well as other SFAs, are derived from both endogenous and dietary sources. For example, these fatty acids can be synthesized from the elongation of 18:0 to 20:0, 22:0, and then 24:0 (4, 35, 36). In the diet, VLSFAs 20:0, 22:0, and 24:0 are contained in meaningful amounts only in selected foods, including peanuts, peanut butter, and Macadamia nuts (37, 38). A previous study indicated that consumption of peanuts and peanut butter is inversely associated with diabetes risk (39). Although the authors attributed these findings to the high amounts of monounsaturated fat, polyunsaturated fat, fiber, and magnesium found in peanuts and peanut butter (39), the findings reported herein suggest that VLSFAs 20:0, 22:0, and 24:0 contained in these foods may also partly explain these associations. In other words, circulating VLSFAs may be a marker of peanut, peanut butter, or Macadamia nut consumption—which is associated with diabetes risk.
Compared to other long-chain SFAs, VLSFAs possess properties that appear to have distinct effects on specific biological processes, although these processes are complex and not completely understood (40–44). For example, circulating VLSFAs are major components of ceramides and sphingomyelins, and it is possible that the inverse associations of VLSFAs and incident diabetes reported herein may be explained at least in part by the impact of ceramides and sphingomyelins on diabetes-related pathways. Both animal and in vitro studies suggest that 1) ceramides play a role in insulin resistance and glucose homeostasis (40, 41) and 2) effects of ceramides and sphingomyelins on cardiometabolic outcomes may be dependent on the chain length of the incorporated fatty acids. For instance, ceramides of different chain lengths differentially permeabilize mitochondria (43), and studies in animal and in vitro models have indicated that ceramides containing SFA 16:0 induce apoptosis in β-cells (42, 44), whereas ceramides containing fatty acids 20:0 and 22:0 inhibit apoptosis in β-cells (42, 45–47). Apoptosis may influence type 2 diabetes by means of β-cell death and reduced insulin secretion (48–51).
The findings in the present investigation were fully attenuated after adjustment for SFA 16:0 and TGs in exploratory models. This attenuation may be due to potential mediation by SFA 16:0 and TGs of observed associations of VLSFAs with diabetes risk (11). Alternatively, as SFA 16:0 is inversely associated with VLSFAs 20:0, 22:0, and 24:0, it has been proposed that this attenuation may be a result of residual confounding due to an unhealthy lifestyle. This theory is based on the premise that SFA 16:0 may be a marker of poor diet quality (i.e., diet high in red meats, processed meats), and thus low concentrations of circulating VLSFAs may reflect metabolic dysfunction associated with poor diet quality (31). In addition, high TGs are a major risk factor for insulin resistance [and a marker of an unhealthy diet high in simple carbohydrates and processed meats, and low levels of physical activity (52)], and VLSFAs have been shown to be associated with lower concentrations of fasting TGs in previous studies (11, 53). These theories are hypothesis-generating, and more studies are needed to better understand the interplay of VLSFAs, 16:0, TGs, and diabetes risk.
To date, only a handful of studies have assessed the associations of VLSFAs 20:0, 22:0, and 24:0 with diabetes risk (11–13). In the CHS and EPIC-Interact, both included in the present study, 20:0, 22:0, and 24:0 were each associated with a lower risk of diabetes (11, 12). Our analysis builds upon and greatly extends these prior findings by pooling data from 12 prospective studies from 13 countries and 4 continents—and incorporates data on an additional 22,000 participants not previously included in previous reports (11, 12).
Our study has several strengths. To our knowledge, this is the largest and most complete analysis to date to examine the associations of VLSFAs with incident diabetes. Owing to the richness of the data available for each participating study, we were able to employ a standard analysis plan to perform de novo individual-level analyses and adjust for major potential confounders and mediators. The 12 participating studies also represent a broad range of ages, geographical regions, and background diets, increasing generalizability. Compared to reports of individual studies, for which positive results are much more likely to be published, our methods for identifying and including studies reduce the possibility of publication bias.
This study also has potential limitations. Circulating fatty acids were only measured at a single time, and we were unable to adjust for changes in VLSFA concentrations over time in this meta-analysis. Given the prospective design, changes in VLSFA concentrations over time would likely attenuate results toward the null. Our study sample comprised primarily participants of European descent, although several of the cohorts included significant numbers of other races/ethnicities. Although we adjusted for several factors that may be associated with SFAs and diabetes, residual confounding by imprecisely measured or unknown factors is possible. In addition, the intercorrelations of VLSFAs make it challenging to interpret the independent associations of each individual VLSFA with risk of diabetes. Finally, because our primary interest was in circulating concentrations of VLSFAs, analyses of the relations of foods that contain VLSFAs with incident diabetes were beyond the scope of this project.
In conclusion, the results of this study suggest that higher concentrations of circulating VLSFAs 20:0, 22:0, and 24:0 are each associated with a lower risk of diabetes, perhaps because of their association with lower de novo lipogenesis. This study adds to the growing body of evidence that supports positive health outcomes with higher concentrations of VLSFAs (11, 12, 37, 54) and highlights the need for additional research studies to identify relevant biological mechanisms and pathways that may contribute to observed associations.
Supplementary Material
ACKNOWLEDGEMENTS
Age, Gene/Environment Susceptibility Study Reykjavik thanks Pho Diep for technical assistance with fatty acid analyses. The Insulin Resistance Atherosclerosis Study acknowledges Lipomics Inc. (Metabolon) for performing the fatty acid analyses. The Interact study acknowledges the laboratory teams at the Medical Research Council Epidemiology Unit for sample management and at Medical Research Council Human Nutrition Research for biochemical analysis; and Nicola Kerrison for data management. The Melbourne Collaborative Cohort Study acknowledge Mark Neumann for technical assistance in the analysis of the plasma phospholipid fatty acids.
The authors’ contributions were as follows—AMF, FI, BM, DM, and RNL: designed the research (project conception, development of the overall research plan, and study oversight); NGF, KR, TBH, LD, NJW, LMS, A. Koulman, DS, IAB, VG, SV, BH, M Lankinen, JV, CS, BTS, CH, NS, AK, MU, MYT, LW, CB, and K-LC: conducted the research (hands-on conduct of the experiments and data collection); NGF, AK, DS, IAB, VG, SV, BH, ML, MU, MYT, LW, CB, and K-LC: provided essential materials; AMF, FI, CIY, RAM, NT, JKV, ML, KW, ACW, and WTQ: analyzed the data or performed statistical analysis; AMF, FI, MM, RM, JHYW, DM, and RNL: wrote the paper; AMF: had primary responsibility for final content.All authors read and approved the final manuscript. JHYW and RM report research support from Unilever for other projects on fatty acid biomarkers. CH reports receiving fees for a conference from Novartis. IAB reports involvement in a research project partly funded by Unilever. DM reports receiving ad hoc honoraria from Bunge, Pollock Institute, and Quaker Oats; ad hoc consulting for Foodminds, Life Sciences Research Organization, Nutrition Impact, Amarin, AstraZeneca, Winston, and Strawn LLP; membership in Unilever North America Scientific Advisory Board; and chapter royalties from UpToDate. FI, NGF, and NJW report research support from the United Kingdom Medical Research Council Epidemiology Unit core grants MC_UU_12015/1 and MC_UU_12015/5. None of the other authors reported a conflict of interest related to the study.
Notes
Supported by NIH grant 5KL2TR000421 (to AMF), a University of New South Wales Scientia Fellowship (to JHYW), MRC Epidemiology Unit grants MC_UU_12015/1 and MC_UU_12015/5 (to NJW, NGF, and FI), National Institute for Health Research Biomedical Research Centre Cambridge grant IS-BRC-1215-20014 (to NGF, AK, and NJW), MRC Elsie Widdowson Laboratory grant MC_UD99999906 (to A Koulman), and Cambridge Lipidomics Biomarker Research Initiative grant G0800783 (to A Koulman). Age, Gene/Environment Susceptibility Study Reykjavik was funded by Office of Dietary Supplements, NIH contract N01-AG012100, the National Institute of Aging (NIA) Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament), with additional contribution from Canadian Cancer Society grant #704735. Chin-Shan Community Cardiovascular Study was funded by Ministry of Science and Technology and National Taiwan University, Taiwan grants MOST 103-2314-B-002-135–MY3 (to K-LC), NSC 100-2314-B-002-113–MY3, NTUH 105-S3120, and NTUH 106-S3453. The Cardiovascular Health Study (CHS) was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS); additional support was provided by National Institute on Aging (NIA) grant R01AG023629. The Framingham Heart Study was supported by the NHLBI in collaboration with Boston University (contract no. N01-HC-25195). The Insulin Resistance Atherosclerosis Study was funded by NHLBI grants U01-HL-47892, U01-HL-47902, DK-29867, R01-58329, and DK-079888 and NIH grant M01-RR-43. The EPIC-Interact project was funded by the EU FP6 programme, grant no. LSHM_CT_2006_037197. The Kuopio Ischaemic Heart Disease Risk Factor Study (KIHD) was supported mainly by funding from the Academy of Finland to Jukka T Salonen. The Melbourne Collaborative Cohort Study was funded by VicHealth, The Cancer Council Victoria, and the National Health and Medical Research Council, grants 124317, 126402, 126403, 180705, 180706, 194327, 209057, 251533. The Multi-Ethnic Study of Atherosclerosis (MESA) was funded by NHLBI contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169, and by National Center for Advancing Translational Sciences grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420. The Metabolic Syndrome in Men Study was funded by grants from the European Union, the Academy of Finland, and the Juselius Foundation. The Three City Study was conducted under a partnership agreement between the Institut National de la Santé et de la Recherche Médicale, the University Bordeaux 2 Victor Segalen, and Sanofi. The Fondation pour la Recherche Médicale funded the preparation and initiation of the study. The Three City Study was also supported by the Caisse Nationale Maladie des Travailleurs Salaries, Direction Générale de la Santé, MGEN, Institut de la Longévité, Conseils Régionaux d'Aquitaine et Bourgogne, Fondation de France, Ministry of Research—Institut National de la Santé and de la Recherche Médicale Programme Cohortes, Agence Nationale de la Recherche grant COGINUT ANR-06-PNRA-005, Fondation Plan Alzheimer grant FCS 2009–2012, and the Caisse Nationale de Solidarité pour l'Autonomie. The Women's Health Initiative (WHI) program was funded by the NHLBI, NIH, US Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C. A full listing of the CHS, MESA, and WHI investigators can be found at http://www.CHS-NHLBI.org, http://www.mesa-nhlbi.org, and http://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf, respectively.
The funders had no role in study design, data collection, analysis or interpretation of data, the writing of the report, or the decision to publish.
Supplemental Tables 1–3 and Supplemental Figures 1–9 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: CCCC, Chin-Shan Community Cardiovascular Cohort Study; CHS, Cardiovascular Health Study; EPIC-Interact, European Prospective Investigation into Cancer-InterAct; FORCE, Fatty Acids and Outcomes Research Consortium; IRAS, Insulin Resistance Atherosclerosis Study; KIHD, Kuopio Ischaemic Heart Disease Risk Factor Study; MCCS, Melbourne Collaborative Cohort Study; MESA, Multi-Ethnic Study of Atherosclerosis; METSIM, Metabolic Syndrome in Men Study; SFA, saturated fatty acids; TG, triglyceride; VLSFA, very-long-chain SFA; WHIMS, Women's Health Initiative Memory Study; 3C Study, Three City Study.
References
- 1. World Health Organization. Global Report on Diabetes. [Internet] 2016; [cited 24 Oct, 2017]. Available from: http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf. [Google Scholar]
- 2. Lam DW, LeRoith D. The worldwide diabetes epidemic. Curr Opin Endocrinol Diabetes Obes. 2012;19(2):93–6. [DOI] [PubMed] [Google Scholar]
- 3. Mattei J, Malik V, Wedick NM, Campos H, Spiegelman D, Willett W, Hu FB. A symposium and workshop report from the Global Nutrition and Epidemiologic Transition Initiative: nutrition transition and the global burden of type 2 diabetes. Br J Nutr. 2012;108(7):1325–35. [DOI] [PubMed] [Google Scholar]
- 4. Kihara A. Very long-chain fatty acids: elongation, physiology and related disorders. J Biochem. 2012;152(5):387–95. [DOI] [PubMed] [Google Scholar]
- 5. Schwab US, Maliranta HM, Sarkkinen ES, Savolainen MJ, Kesaniemi YA, Uusitupa MI. Different effects of palmitic and stearic acid-enriched diets on serum lipids and lipoproteins and plasma cholesteryl ester transfer protein activity in healthy young women. Metabolism. 1996;45(2):143–9. [DOI] [PubMed] [Google Scholar]
- 6. Iggman D, Riserus U. Role of different dietary saturated fatty acids for cardiometabolic risk. Clin Lipidol. 2011;6(2):209–23. [Google Scholar]
- 7. Chong MF, Hodson L, Bickerton AS, Roberts R, Neville M, Karpe F, Frayn KN, Fielding BA. Parallel activation of de novo lipogenesis and stearoyl-CoA desaturase activity after 3 d of high-carbohydrate feeding. Am J Clin Nutr. 2008;87(4):817–23. [DOI] [PubMed] [Google Scholar]
- 8. Knopp RH, Retzlaff B, Walden C, Fish B, Buck B, McCann B. One-year effects of increasingly fat-restricted, carbohydrate-enriched diets on lipoprotein levels in free-living subjects. Proc Soc Exp Biol Med. 2000;225(3):191–9. [DOI] [PubMed] [Google Scholar]
- 9. Hudgins LC, Hellerstein M, Seidman C, Neese R, Diakun J, Hirsch J. Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J Clin Invest. 1996;97(9):2081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. King IB, Lemaitre RN, Kestin M. Effect of a low-fat diet on fatty acid composition in red cells, plasma phospholipids, and cholesterol esters: investigation of a biomarker of total fat intake. Am J Clin Nutr. 2006;83(2):227–36. [DOI] [PubMed] [Google Scholar]
- 11. Lemaitre RN, Fretts AM, Sitlani CM, Biggs ML, Mukamal K, King IB, Song X, Djousse L, Siscovick DS, McKnight B et al.. Plasma phospholipid very-long-chain SFAs and incident diabetes in older adults: the Cardiovascular Health Study. Am J Clin Nutr. 2015;101(5):1047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kroger J, Schulze MB, Crowe FL, Huerta JM, Guevara M, Beulens JW et al.. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. 2014;2(10):810–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kroger J, Zietemann V, Enzenbach C, Weikert C, Jansen EH, Doring F, Joost HG, Boeing H, Schulze MB. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr. 2011;93(1):127–42. [DOI] [PubMed] [Google Scholar]
- 14. Tufts University Friedman School of Nutrition Science and Policy. FORCE - Fatty Acids and Outcomes Research Consortium. [Internet]. [cited 1 Aug, 2018]. Available from: https://nutrition.tufts.edu/research/projects-initiatives/force-fatty-acids-and-outcomes-research-consortium. [Google Scholar]
- 15. Wu JHY, Marklund M, Imamura F, Tintle N, Ardisson Korat AV, de Goede J, Zhou X, Yang WS, de Oliveira Otto MC, Kroger J et al.. Omega-6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol. 2017;5(12):965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Del Gobbo LC, Imamura F, Aslibekyan S, Marklund M, Virtanen JK, Wennberg M, Yakoob MY, Chiuve SE, Dela Cruz L, Frazier-Wood AC et al.. ω-3 Polyunsaturated fatty acid biomarkers and coronary heart disease: pooling project of 19 cohort studies. JAMA Intern Med. 2016;176(8):1155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, Thorgeirsson G, Aspelund T, Garcia ME, Cotch MF et al.. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165(9):1076–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chien KL. Mini-review of the Chin-Shan Community Cardiovascular Cohort Study in population health research in Taiwan. Acta Cardiol Sin. 2017;33(3):226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A et al.. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–76. [DOI] [PubMed] [Google Scholar]
- 20. Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4(4):518–25. [DOI] [PubMed] [Google Scholar]
- 21. Langenberg C, Sharp S, Forouhi NG, Franks PW, Schulze MB, Kerrison N, Ekelund U, Barroso I, Panico S, Tormo MJ et al.. Design and cohort description of the InterAct Project: an examination of the interaction of genetic and lifestyle factors on the incidence of type 2 diabetes in the EPIC Study. Diabetologia. 2011;54(9):2272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wagenknecht LE, Mayer EJ, Rewers M, Haffner S, Selby J, Borok GM, Henkin L, Howard G, Savage PJ, Saad MF et al.. The insulin Resistance Atherosclerosis Study (IRAS): objectives, design, and recruitment results. Ann Epidemiol. 1995;5(6):464–72. [DOI] [PubMed] [Google Scholar]
- 23. Salonen JT. Is there a continuing need for longitudinal epidemiologic research? The Kuopio Ischaemic Heart Disease Risk Factor Study. Ann Clin Res. 1988;20(1–2):46–50. [PubMed] [Google Scholar]
- 24. Hodge AM, Simpson JA, Gibson RA, Sinclair AJ, Makrides M, O'Dea K, English DR, Giles GG. Plasma phospholipid fatty acid composition as a biomarker of habitual dietary fat intake in an ethnically diverse cohort. Nutr Metab Cardiovasc Dis. 2007;17(6):415–26. [DOI] [PubMed] [Google Scholar]
- 25. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K et al.. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–81. [DOI] [PubMed] [Google Scholar]
- 26. Lankinen MA, Stančáková A, Uusitupa M, Ågren J, Pihlajamäki J, Kuusisto J, Schwab U, Laakso M. Plasma fatty acids as predictors of glycaemia and type 2 diabetes. Diabetologia. 2015;58(11):2533–44. [DOI] [PubMed] [Google Scholar]
- 27. Harris WS, Luo J, Pottala JV, Margolis KL, Espeland MA, Robinson JG. Red blood cell fatty acids and incident diabetes mellitus in the Women's Health Initiative Memory Study. PloS One. 2016;11(2):e0147894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. 3C Study Group. Vascular factors and risk of dementia: design of the Three-City Study and baseline characteristics of the study population. Neuroepidemiology. 2003;22(6):316–25. [DOI] [PubMed] [Google Scholar]
- 29. Smith-Warner SA, Spiegelman D, Ritz J, Albanes D, Beeson WL, Bernstein L, Berrino F, van den Brandt PA, Buring JE, Cho E et al.. Methods for pooling results of epidemiologic studies: the Pooling Project of Prospective Studies of Diet and Cancer. Am J Epidemiol. 2006;163(11):1053–64. [DOI] [PubMed] [Google Scholar]
- 30. Chong MF, Fielding BA, Frayn KN. Metabolic interaction of dietary sugars and plasma lipids with a focus on mechanisms and de novo lipogenesis. Proc Nutr Soc. 2007;66(1):52–9. [DOI] [PubMed] [Google Scholar]
- 31. Lauritzen L, Hellgren LI. Plasma phospholipid very-long-chain saturated fatty acids: a sensitive marker of metabolic dysfunction or an indicator of specific healthy dietary components?. Am J Clin Nutr. 2015;101(5):901–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin DY, Zeng D. Meta-analysis of genome-wide association studies: no efficiency gain in using individual participant data. Genet Epidemiol. 2010;34(1):60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rice K, Higgins JPT, Lumley T. A re-evaluation of fixed effect(s) meta-analysis. J R Stat Soc Ser A Stat Soc. 2018;181(1):205–27. [Google Scholar]
- 35. Jakobsson A, Westerberg R, Jacobsson A. Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog Lipid Res. 2006;45(3):237–49. [DOI] [PubMed] [Google Scholar]
- 36. Guillou H, Zadravec D, Martin PG, Jacobsson A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: insights from transgenic mice. Prog Lipid Res. 2010;49(2):186–99. [DOI] [PubMed] [Google Scholar]
- 37. Fretts AM, Mozaffarian D, Siscovick DS, King IB, McKnight B, Psaty BM, Rimm EB, Sitlani C, Sacks FM, Song X et al.. Associations of plasma phospholipid SFAs with total and cause-specific mortality in older adults differ according to SFA chain length. J Nutr. 2016;146(2):298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. United States Department of Agriculture Agricultural Research Service, Nutrient Data Laboratory. USDA National Nutrient Database for Standard Reference. [Internet]. Available from: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/nutrient-data-laboratory/docs/usda-national-nutrient-database-for-standard-reference/.Published April 2018. Accessed: August 2018. [Google Scholar]
- 39. Jiang R, Manson JE, Stampfer MJ, Liu S, Willett WC, Hu FB. Nut and peanut butter consumption and risk of type 2 diabetes in women. JAMA. 2002;288(20):2554–60. [DOI] [PubMed] [Google Scholar]
- 40. Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem. 1999;274(34):24202–10. [DOI] [PubMed] [Google Scholar]
- 41. Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab. 2012;15(5):585–94. [DOI] [PubMed] [Google Scholar]
- 42. Grosch S, Schiffmann S, Geisslinger G. Chain length-specific properties of ceramides. Prog Lipid Res. 2012;51(1):50–62. [DOI] [PubMed] [Google Scholar]
- 43. Stiban J, Perera M. Very long chain ceramides interfere with C16-ceramide-induced channel formation: a plausible mechanism for regulating the initiation of intrinsic apoptosis. Biochim Biophys Acta. 2015;1848(2):561–7. [DOI] [PubMed] [Google Scholar]
- 44. Deng X, Yin X, Allan R, Lu DD, Maurer CW, Haimovitz-Friedman A, Fuks Z, Shaham S, Kolesnick R. Ceramide biogenesis is required for radiation-induced apoptosis in the germ line of C. elegans. Science (New York, NY). 2008;322(5898):110–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ussher JR, Koves TR, Cadete VJ, Zhang L, Jaswal JS, Swyrd SJ, Lopaschuk DG, Proctor SD, Keung W, Muoio DM et al.. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes. 2010;59(10):2453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Crowder CM. Ceramides–friend or foe in hypoxia?. Science (New York, NY). 2009;324(5925):343–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lee YG, Lee J, Cho JY. Cell-permeable ceramides act as novel regulators of U937 cell-cell adhesion mediated by CD29, CD98, and CD147. Immunobiology. 2010;215(4):294–303. [DOI] [PubMed] [Google Scholar]
- 48. Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced β cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci U S A. 1998;95(5):2498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Maedler K, Spinas GA, Dyntar D, Moritz W, Kaiser N, Donath MY. Distinct effects of saturated and monounsaturated fatty acids on β-cell turnover and function. Diabetes. 2001;50(1):69–76. [DOI] [PubMed] [Google Scholar]
- 50. Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic β-cell turnover and function. Diabetes. 2003;52(3):726–33. [DOI] [PubMed] [Google Scholar]
- 51. Favaloro B, Allocati N, Graziano V, Di Ilio C, De Laurenzi V. Role of apoptosis in disease. Aging (Albany NY). 2012;4(5):330–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. AlEssa HB, Malik VS, Yuan C, Willett WC, Huang T, Hu FB, Tobias DK. Dietary patterns and cardiometabolic and endocrine plasma biomarkers in US women. Am J Clin Nutr. 2017;105(2):432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zheng JS, Sharp SJ, Imamura F, Koulman A, Schulze MB, Ye Z, Griffin J, Guevara M, Huerta JM, Kroger J et al.. Association between plasma phospholipid saturated fatty acids and metabolic markers of lipid, hepatic, inflammation and glycaemic pathways in eight European countries: a cross-sectional analysis in the EPIC-InterAct study. BMC Med. 2017;15(1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fretts AM, Mozaffarian D, Siscovick DS, Djousse L, Heckbert SR, King IB, McKnight B, Sitlani C, Sacks FM, Song X et al.. Plasma phospholipid saturated fatty acids and incident atrial fibrillation: the Cardiovascular Health Study. J Am Heart Assoc. 2014;3(3):e000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


