Abstract
Insufficient perfusion of the trophoblast by maternal blood is associated with an increased generation of reactive oxygen species and complications of the placenta. In this study, we first examined whether rosiglitazone, an agonist of the peroxisome proliferator-activated receptor-γ (PPARγ), protects the human trophoblast from oxidative injury by regulating key antioxidant proteins, catalase (CAT) and the superoxide dismutases (SOD1 and SOD2). In first trimester placental explants, localization of CAT was limited to cytotrophoblasts, whereas SOD1 was expressed in both the cyto- and syncytiotrophoblasts. In first trimester placental explants, hypoxia decreased the expression of both SOD1 and SOD2, and increased apoptosis. Treatment with rosiglitazone dose-dependently upregulated anti-oxidative CAT and SOD2, and rescued hypoxic injury in first trimester villous explants and JEG-3 cells, strongly suggesting the involvement of the PPARγ in regulating their expressions. Rosiglitazone facilitated transcription activity of PPARγ, and enhanced promotor binding, increased transcriptional activity at the CAT promoter, and elevated protein expression/activity. Treatment of hypoxic JEG-3 cells with rosiglitazone resulted in mitochondrial membrane potential increase and a reduction of caspase 9 and caspase 3 activity which is consistent with improved cell survival. To complement PPARγ activation data, we also utilized the antagonist (SR-202) and siRNA to suppress PPARγ expression and demonstrate the specific role of PPARγ in reducing ROS and oxidative stress. Ex vivo examination of term human placenta revealed lower expression of antioxidant proteins in pathologic compared to healthy placental tissues, which could be rescued by rosiglitazone, indicating that rosiglitazone can improve survival of the trophoblast under pathological conditions. These findings provide evidence that the PPARγ pathway directly influences cellular antioxidants production and the pathophysiology of placental oxidative stress.
Keywords: PPARγ, human placenta, oxidative stress, hypoxia, preeclampsia
Rosiglitazone (a PPARγ agonist) improves survival of the trophoblast under pathological conditions by regulation of antioxidants.
Introduction
Oxidative stress is a hallmark of placental insufficiency in hypertensive disorders of pregnancy such as preeclampsia (PE) [1, 2]. Shallow uterine trophoblast invasion can cause placental hypoperfusion and a prolonged hypoxic state which predisposes the placenta to potential injury by generating free radicals and reactive oxygen species (ROS) [3]. Elevated ROS and a lack of scavenger molecules in this hypoxic state can cause trophoblast dysfunction and apoptosis, which are thought to play central roles in the pathophysiology of PE [4]. The elevation of lipid peroxidation in both maternal blood and placentas of preeclamptic patients provided the first direct evidence that oxidative stress, ROS overproduction, mitochondrial damage, and endothelial dysfunction are linked [5]. Prolonged oxidative stress can induce apoptosis and could explain, in part, the high rate of apoptosis in preeclamptic placentas [6–9]. Considering the substantial contribution of oxidative stress in pathophysiology of placental insufficiency, any effective therapeutic strategies that directly target ROS scavengers could potentially improve maternal and fetal health by reducing placental and endothelial damage.
The generation of the superoxide radical anion, O2−, by the activation of NADPH oxidase or xanthine oxidase initiates the ROS reaction cascade. In healthy cells, ROS are continuously produced at low levels [10], and cells are naturally equipped with antioxidant defense systems to scavenge O2−. Superoxide radicals can be converted to a less toxic H2O2 by superoxide dismutases (SODs), either in the mitochondrial matrix (SOD2) or in the intermembrane space and cytosol (SOD1). H2O2 can be further neutralized by conversion to H2O through the catalase (CAT) and glutathione peroxidase (GPx) pathways. Excessive H2O2 enters the cytosol, binds ferrous ions, and generates a highly reactive hydroxyl radical that can cause oxidative damage [11]. It is known that pathological elevation of O2− can also cause oxidation of lipids and proteins, which negatively affects cell function [10]. Accumulation of intracellular ROS damages the mitochondria and permeabilizes its outer membrane, which subsequently releases cytochrome c into the cytosol to initiate the intrinsic apoptosis pathway through activation of caspases [12].
Increased placental ROS production has been reported in PE pregnancies [13]. It has been proposed that impaired ROS scavenging by SOD [14] and CAT [15] in maternal blood could contribute to the pathophysiology of placenta-related complications, which could serve as a useful risk assessment tool in early pregnancy [5, 16, 17]. Superoxide dismutase-1 (SOD1) is significantly downregulated in preeclamptic placentas [18] suggesting that ROS processing plays a key role in PE. However, the expression patterns and subcellular localization of anti-oxidative proteins in healthy and pathological conditions are still poorly understood.
Rosiglitazone belongs to the thiazolidinediones group of drugs, and is a selective agonist of the nuclear transcription factor peroxisome proliferator-activated receptor-γ (PPARγ). PPARγ is a ligand-dependent nuclear receptor that regulates gene expression through transactivation or transrepression mechanisms [19, 20]. Rosiglitazone reduces insulin resistance in the liver, fat, and skeletal muscle. Additionally, several studies have reported cytoprotective properties of rosiglitazone against oxidative stress in neural [21], myocardial [22, 23], and endothelial [24] cells. Rosiglitazone directly affects cellular function by inducing nuclear translocation of PPARγ, which regulates transcriptional activity and the expression of downstream target genes involved in angiogenesis [25], trophoblast differentiation [26], cell migration [27], and apoptosis [28]. In one report, rosiglitazone improved vascular endothelial function via heme oxygenase 1 (HO1) in a rat model of PE, linking PPARγ activity and PE [29]. Furthermore, a new role was recently proposed for this PPARγ agonist in prevention of preterm birth by suppressing the macrophage-mediated inflammatory response [30]. The cytoprotective effects of rosiglitazone against oxidative damage in the placenta are thought to be carried out through nitric oxide (NO)-dependent regulation of lipid homeostasis. Capobianco et al. have reported that placentas of type 2 diabetic patients treated with rosiglitazone show significantly suppressed NO overproduction and reduced lipoperoxidation [31]. Beyond this evidence, little is known about the role of PPARγ-dependent regulation of antioxidants in the human placenta under healthy or pathological conditions.
In order to address this, we (a) investigate the effect of hypoxia on trophoblast apoptosis and ROS scavenger expression in first trimester placental explants, (b) examine the cytoprotective properties of rosiglitazone in hypoxic human trophoblasts (using first trimester placental explants and the JEG-3 choriocarcinoma cell line), (c) identify the ROS scavenging proteins that mediate the antioxidant properties of rosiglitazone, and (d) profile the expression of intercellular antioxidants in pathologic placentas (PE/IUGR) to test whether rosiglitazone could alter their intercellular levels ex vivo.
Materials and methods
Patient selection and placental tissue
First trimester (10–12 weeks of gestation) placental tissues (n = 6) were obtained with written informed consent from healthy pregnant women undergoing elective termination of pregnancy. The Institutional Review Board (IRB) of Wayne State University approved all consent forms and protocols used in this study, which abide by the NIH research guidelines. Term placental samples were obtained either by the Research Centre for Women's and Infants’ Health (RCWIH) BioBank program of Mount Sinai Hospital in Toronto, Canada, in accordance with the policies of the Mount Sinai Hospital Research Ethics Board or from Hutzel Women's Hospital in Detroit, MI, approved by the IRB with waiver of parental consent. Specimens were collected from age-matched preterm pregnancies not complicated by PE (PTC) (n = 6; gestational age = 31.5 ± 1.8 weeks), and pregnancies complicated by severe early-onset PE (n = 9; gestational age = 30.8 ± 1.0 weeks) that were delivered by caesarean section. In this study, we used “idiopathic” preterm control samples. Inclusion criteria for PE was in accordance with the guidelines of The American Congress of Obstetricians and Gynecologists, presence of hypertension > 160/110 mm Hg on two occasions longer than 6 h apart, proteinuria > 5 g/day and oliguria > 500 mL/day, with or without fetal growth restriction. Placental tissues were obtained within 1 h after delivery. A standardized random sampling protocol was applied dissecting random five 1 cm × 1 cm × 1 cm cuboidal sections to avoid sampling bias. The collected tissues were washed and transported to the laboratory in ice cold HBSS (Hank's Balanced Salt Solution), and processed within a maximum of 2 h after collection. On arrival, tissues were snap-frozen in liquid nitrogen for further analysis. For ex vivo modeling, individual clusters of villous trees were dissected under a stereomicroscope, and cultured in 1 ml of DMEM/F12 media containing 10% fetal bovine serum (FBS) and 1% antibiotic-antimycotic (Gibco). Frozen 900 μM stock solutions of rosiglitazone maleate were dissolved in warm water, as recommended by the manufacturer (Selleckchem). Culture medium was supplemented with 10, 50, or 100 μM of rosiglitazone. The explants were maintained overnight at either 8% or 1.5% O2 with 5% CO2 at 37°C.
Cell culture
The human choriocarcinoma cell lines JEG-3 and BeWo were purchased from the American Type Culture Collection (ATCC). Both cell lines were cultured in Dulbecco Modified Eagle Medium (DMEM) and Ham F12 (1:1 DMEM/F12) medium (Invitrogen) containing 10% FBS and 1% Antibiotic-Antimycotic (Gibco) in a humidified incubator at 5% CO2. The H/R (hypoxia/reoxygenation) protocol was conducted as previously described [32]. Briefly, cells (from passage 8–10) were exposed to hypoxia (2% O2) for 2 h, followed by replacement with fresh medium equilibrated at 20% O2 and incubation for 6 h. Medium was supplemented with a selective PPARγ antagonist 400 μM SR-202 (TOCRIS Bioscience) and/or various concentrations of rosiglitazone during the reoxygenation step.
Cell death assay
A total of six placentas were used for histological evaluation and quantification of apoptosis in paraffin-embedded section. A similar number of villi were dissected from all five regions mixed and then randomly picked from the pool for each experiment to avoid sampling bias. Each dissected explant contained approximately 8–10 mg tissue, four to five villi, or cultured cells. All treatments were performed in triplicates. DNA-strand breaks were detected by TUNEL (terminal deoxynucleotidyl transferase [TdT] dUTP nick-end labeling), using a fluorescein-based in situ cell death detection kit (Roche Applied Science, Indianapolis, IN), according to the manufacturer's instructions. Nuclei were counterstained with DAPI (EMD Biosciences, Billerica, MA). Sections were imaged with a Nikon Eclipse 90i epifluorescence microscope (Nikon Inc., Melville). The apoptotic cells (TUNEL-positive nuclei) were counted at ×200 from four random fields on each section from three samples for each treatment, along with the total number of nuclei (DAPI-labeled) to calculate the percentage of TUNEL/DAPI-labeled nuclei (TUNEL index). Additional sections subjected to treatment without TdT were assessed as negative controls.
Fluorometric and quantitative evaluation of ROS generation
To observe the basic changes of intracellular ROS, a Cellular Reactive Oxygen Species Detection Assay Kit (Abcam, Cambridge, MA) was utilized as instructed by the manufacturer. The mitochondrial content of the cells was simultaneously assessed using MitoTracker Green FM fluorescent mitochondrial stain (Molecular Probes, Life Technologies). Initially, JEG-3 and BeWo cells were seeded in an eight-chamber slide at 10,000 cells/chamber. After a 48-h incubation, cells were washed with PBS and preloaded with 1X ROS probes and 100 nM MitoTracker Green FM for 45 min at 37°C. Cells were then washed with PBS. Cells were examined under a Nikon Eclipse 90i epifluorescence microscope (Nikon Inc.) with appropriate filters. The intracellular ROS generation was evaluated quantitatively, using the oxidant-sensing probe 2΄,7΄-dichlorofluorescin diacetate DCFDA (Sigma). Briefly, JEG-3 cells were plated at 5000 cells/well in a 96-well plate. After treatment, cells were washed twice with PBS and loaded with 25 μM DCFDA for 30 min at 37°C. The fluorescence intensity was quantified using a Synergy H1 microplate reader (BioTek) with an excitation wavelength of 429 nm and an emission wavelength of 517 nm.
Mitochondrial membrane potential (ΔΨm)
The alteration of the mitochondrial membrane potential (ΔΨm) was determined using the fluorescent dye JC-10. JEG-3 cells were seeded at a density of 5000 cells/well in a 96-well plate and incubated overnight for attachment. After experimental treatment, cells were washed with PBS and incubated with 1 μM JC-10 (Enzo Life Sciences) for 15 min, washed again with PBS, and fluorescence was quantified by microplate reader. Microplate reader-based measurement for J-monomers and J-aggregates were carried out at 485 nm (Ex)/516 nm (Em) and 528 nm (Ex)/590 nm (Em), respectively.
Caspase activity assay
The activation of caspase 3 and caspase 9 was determined using the corresponding fluorometric substrates, Ac-DEVD-AMC and Ac-LEHD-AMC (Enzo Life Sciences), respectively. JEG-3 cells were seeded at a density of 70,000 cells/well in a 6-well plate. After treatment, a nondenaturing lysis buffer was added to extract cellular protein, as described previously [33]. Total protein (35 μg) and 40 μl of substrate were added to 50 μl of reaction buffer (1% NP-40, 10% glycerol in TBS). The mixture was incubated at 37°C for 3 h, and the fluorescence intensity was quantified using a microplate reader.
Immunohistochemistry/immunofluorescence
Placental villi were labeled for immunohistochemistry as described previously [34]. Briefly, the placental villi were fixed in 4% paraformaldehyde in PBS, embedded in paraffin, sectioned at 5 μm thickness, and mounted onto glass slides. Following deparaffinization and rehydration, sections were incubated overnight at 4°C with anti-CAT (ab16731; Abcam) or anti-SOD2 (ab68155; Abcam). One slide was incubated with 10 μg/ml nonimmune rabbit IgG in parallel to serve as control. Slides were washed with PBS, labeled with 10 μg/ml biotin-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch), and stained using Envision System peroxidase kits (DAKO). After dehydration, slides were coverslipped and examined by light microscopy. JEG-3 cells cultured on an eight-chambered slide were fixed in 4% paraformaldehyde in PBS for 10 min, permeabilized for 7 min with 0.01% Triton X-100 in PBS, blocked in 3% BSA in PBS for 1 h at room temperature, and incubated overnight at 4°C with anti-cytochrome C (ab133504; Abcam). Rhodamine (TRITC)-conjugated donkey anti-rabbit secondary antibody (Jackson ImmunoResearch) was applied for 2 h at room temperature. Hoechst 33342 was used to stain nuclei. Slides were analyzed in an Eclipse 90i (Nikon Inc.) epifluorescence microscope.
siRNA-mediated PPARγ suppression
JEG-3 cells were transfected with PPARγ siRNA or scrambled siRNA using GeneMute transfection reagent (SignaGen laboratories), according to the manufacturer's instructions. Briefly, 50% confluent cells in a 6-well culture plate were treated with 50 pM of four MISSION® predesigned siRNAs (Sigma) targeting different sites of PPARγ mRNA, or scramble siRNA, diluted in 100 μl of transfection buffer. After addition of 4 μl of transfection reagent, the mixture was incubated for 20 min at room temperature. The mixture was added dropwise to 900 μl OPTI-MEM medium (Gibco) per well. Cells were incubated with siRNA transfection mixture for 5 h, followed by replacement with 10% FBS-supplemented DMEM/F12. After 48 h, cells were either harvested or treated for another 18 h prior to harvesting.
Chromatin immunoprecipitation
Following experimental treatments, the medium was aspirated from JEG-3 cells cultured in 6-well plates and cells were gently washed with 1 ml cold PBS (Life Technologies). The washed cells were cross-linked by adding 4% formaldehyde in PBS (Thermo Scientific, Rockford, IL) and shaking for 6 min at room temperature. Crosslinking was stopped by adding 125 mM glycine (Fischer Scientific), and cells were washed with 1 ml cold PBS for 5 min to remove the residual formaldehyde. The cells were incubated in cell lysis buffer (10 mM HEPES, 60 mM KCl, 1 mM EDTA, 1 mM 1,4-dithiothreitol, 1 mM PMSF, 0.075% NP-40, pH 7.6) for 3 min and spun at 1500 rpm for 4 min. The pellet was resuspended in nuclear lysis buffer (10 mM EDTA, 50 mM Tris HCl, 1% SDS, pH 8.1) and incubated on ice for 30 min to obtain chromatin. The chromatin lysate was then sonicated on a focused ultrasonicator (S220, Covaris) to obtain DNA fragments of 200–500 bp. Post sonication, the amount of DNA was quantified using a spectrophotometer (Nanodrop). For each treatment, immunoprecipitation was conducted using anti-POL2 CTD (MA1-46093; Thermo Scientific) and anti-PPARγ antibodies (PA3-821A; Thermo Scientific), saving an aliquot representing the input fraction. Simultaneous immunoprecipitations were also performed using anti-rabbit IgG (CA-011-000-003 Jackson Immunoresearch) and anti-mouse IgG (CAS 12-371, Millipore) to serve as negative controls for PPARγ and POL2 CTD antibodies. Each immunoprecipitation reaction contained approximately 5 μg chromatin, 4 μg respective antibody, and 9 μl Dynabeads Protein G magnetic beads (Invitrogen), and was rotated overnight at 4°C. The beads were then washed by rotating for 5 min at 4°C. The bound DNA was eluted by adding 40 μl Chelex beads (Biorad) and heating at 100°C for 10 min, then adding 2 μl proteinase K and incubating at 55°C for 60 min, followed by RNase treatment for 30 min at 37°C. Eluted DNA (3 μl) was used for qPCR analysis, using a primer pair (forward: 5΄—CTC GGG TGG TTG CTT CAG AAT—3΄ and reverse: 5΄—GAC CCC ACA ACT ATG AAA GCG—3΄) spanning the PPARγ-binding site in the CAT promoter (Figure 7B; upper panel). The online MEME SUITE (http://meme-suite.org/tools/tomtom) was used to confirm the presence of PPARγ-binding site in the upstream promoter region of our target gene (CAT) [35]. The fold enrichment of CAT promoter over respective IgG controls was calculated to determine antibody specificity. For comparing treatment effects, enrichment of the CAT promoter in the N/T and rosiglitazone treatment groups was calculated individually using the percent input method. The change in CAT promoter enrichment due to rosiglitazone treatment was calculated by taking a ratio over the enrichment in the corresponding N/T-treated group. Higher enrichment of the CAT promoter in the PPARγ and POL2 CTD precipitations over their respective IgG controls validated the specificity of the antibodies used (data not shown).
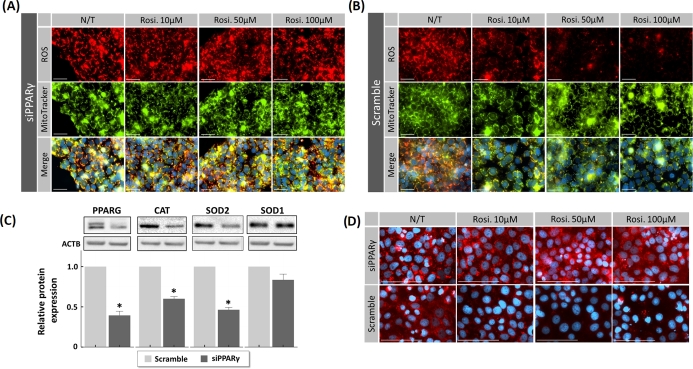
Figure 7.
Transfection of JEG-3 cells with siRNA for PPARγ and rosiglitazone anti-ROS activity during H/R exposure. JEG-3 cells were transfected with either siRNA against PPARγ (siPPARγ) or nontargeted control siRNA (scrambled) for 48 h, followed by H/R challenge in the absence (N/T) or presence of rosiglitazone (10, 50, and 100 μM). (A and B) Fluorescence imaging of ROS probes (red) and MitoTracker green probes in JEG-3 cells transfected with siPPARγ (A) or scrambled siRNA (B). (C) Western blot analysis for the expression of PPARγ, as well as antioxidant proteins after transfection with scrambled siRNA or siRNA for PPARγ in JEG-3 cells. (D) Immunostaining of cytochrome c shows an increase in the cytoplasmic presence in PPARγ-silenced JEG-3 cells compared to scramble control (N/T). The addition of rosiglitazone reduced cytochrome c presence in dose-dependent manner only in nonsilenced JEG-3 cells. N = 3; * P < 0.05 vs N/T control, N/T = no-treatment, Rosi. = Rosiglitazone, scale bar = 100 μm, bars present mean ± SEM.
Proximity ligation assay
Proximity ligation assay (PLA) was performed in situ using a Duolink In Situ Red Starter Kit for Mouse/Rabbit (Sigma, St. Louis), according to the manufacturer's instructions. Briefly, JEG-3 cells were fixed, permeabilized, and incubated overnight at 4 °C with primary anti-Cytochrome C and anti-APAF1 antibodies (Abcam) for one assay and anti-POL2 CTD and anti-PPARγ antibodies (Thermo Scientific) for another in pre-blocking buffer (0.05% Triton X-100 in PBS, pH 7.4). A negative control was prepared by incubating cells in blocking solution without primary antibodies. Cells were washed and incubated with rabbit plus and mouse minus PLA probes for 60 min at 37°C. After washing, the ligation-ligase mixture was added and cells were incubated for 30 min at 37°C, followed by an amplification step that generates a rolling circle DNA. Hoechst 33342 was used to stain nuclei. The fluorescently labeled oligonucleotides were visualized by a Nikon Eclipse 90i epifluorescence microscope (Nikon Inc.).
Mitochondria isolation
The isolation of mitochondrial and cytosolic fractions from JEG-3 cells was performed using the Mitochondria Isolation Kit for Cultured Cells (Thermo Scientific) according to the manufacturer's instructions. Briefly, following experimental incubations, cells were harvested and pelleted in ice cold conditions (all steps of the mitochondrial isolation were performed on ice or in refrigerated centrifuges). Cells were resuspended in cold buffer A with added protease inhibitors, vortexed, and incubated for 2 min. Next, buffer B was added, vortexed, and incubated for 5 min followed by adding buffer C and centrifugation at 700 × g for 10 min. The supernatant was further centrifuged at 12,000 × g for 15 min to pellet the mitochondria. The supernatant from this spin was removed and saved as the cytosolic portion. The crude mitochondrial fraction was resuspended for washing and centrifuged at 12,000 × g for 15 min. The pellet was collected as the mitochondrial fraction and cells were lysed using 2% CHAPS in Tris-buffered saline (TBS; 25 mM Tris, 0.15M NaCl; pH 7.2). Soluble protein from both cytosolic and mitochondrial fractions was analyzed by BCA protein assay reagent (Thermo Scientific), according to the manufacturer's instructions.
Protein extraction and immunoblotting
JEG-3 cells were lysed in 130 μl of RIPA lysis buffer (Sigma) supplemented with complete protease and phosphatase inhibitor cocktail (Roche Diagnostics GmbH, Mannheim, Germany). Protein extraction from tissues (20–30 mg) was performed as previously described [36]. Protein concentration was determined with BCA™ protein assay (Thermo Scientific), according to the manufacturer's instructions. Equal amounts of protein (35 μg) were denatured (8 min, 95°C) in Laemmli sample buffer (BioRad) and separated using SDS–PAGE, following by semi-dry (Trans-Blot, BioRad) transfer to a PVDF membrane. The membranes were blocked with 5% non-fat dry milk in 1X TBS containing 0.05% Tween-20, and incubated overnight at 4°C with primary antibodies used for immunostaining. Subsequently, membranes were incubated with HRP-conjugated secondary antibodies for 1 h at room temperature, and developed with Western Lightning ECL Pro (PerkinElmer). Signals were visualized using a ChemiDoc Imaging System (BioRad) and Image Lab V.5.1 software (BioRad). Densities of immunoreactive bands were measured as arbitrary units by ImageJ software (NIH). Protein levels were normalized to a housekeeping protein (β-actin, 1:20,000; Abcam).
Statistical analysis
All experiments were performed at least three times. A one-way ANOVA followed by Tukey post hoc test was performed to analyze differences between cohorts. An effect was considered significant when P < 0.05. For arbitrary units, results were calculated relative to no treatment (N/T) controls (set as 1), and presented as mean ± standard error (SEM).
Results
Rosiglitazone decreases hypoxia-induced apoptosis in first trimester placental explants
To evaluate the anti-oxidative/anti-apoptotic effects of rosiglitazone on first trimester human placental tissues, placental explants (n = 6) were incubated overnight under normoxic (8% O2, control) or hypoxic (1.5% O2) conditions. Hypoxic tissues were treated with rosiglitazone (10, 50, and 100 μM) and compared to no treatment (N/T) hypoxic control. Overnight exposure to hypoxia increased apoptosis to 15.2% ± 0.7 (P < 0.001), compared to no-treatment normoxic control (5.3% ± 0.5) (Figure 1A and B). Rosiglitazone treatment of hypoxic cultures at 10, 50, and 100 μM reduced TUNEL to 8.6% ± 0.8 (P < 0.001), 6.0% ± 0.7 (P < 0.001), and 4.9% ± 1.1 (P < 0.001), respectively.
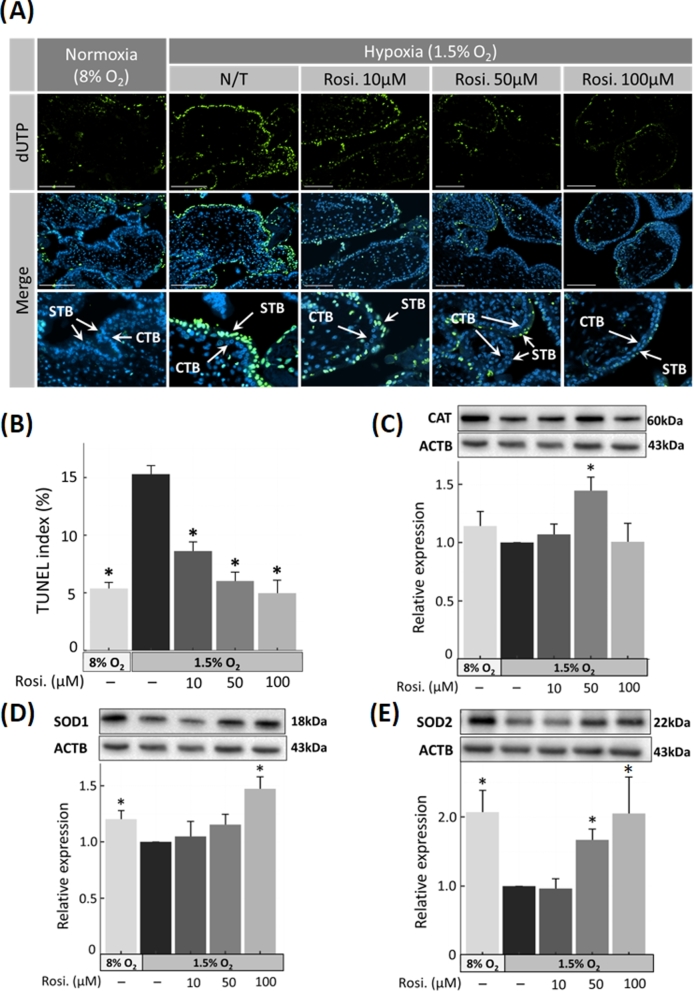
Figure 1.
Expression of ROS scavengers and apoptosis in first trimester placenta. (A) Detection of apoptotic cells by TUNEL in sectioned chorionic villous explants. The nuclei of TUNEL-positive cells are indicated by green fluorescence, with DAPI (blue) as a nuclear counterstain. The population of apoptotic cells increased with hypoxia (in no-treatment N/T group), compared to untreated control cells cultured at 8% O2, whereas exposure to increasing concentrations of rosiglitazone reduced apoptosis. Arrows indicate apoptotic cells. (B) Percentage of TUNEL-positive nuclei was calculated for cells treated as in A. (C–E) The protein expression of ROS regulators in first trimester placental explants was semiquantified by western blotting, compared to β-actin (ACTB). (C) Catalase (CAT), (D) superoxide dismutase 1 (SOD1), and 2 (E) SOD2. N = 6; * P < 0.05 vs hypoxia no-treatment (N/T) control, Rosi. = Rosiglitazone, scale bar = 100 μm, bars present mean ± SEM.
Rosiglitazone increases the expression of ROS scavengers in hypoxic first trimester placental explants
Expression levels of CAT, SOD1 (cytosolic Cu-ZnSOD), and SOD2 (mitochondrial MnSOD) were measured by western blot, and represented as fold change compared to no treatment (N/T) controls. The expression of both SOD1 and SOD2 was higher in normoxia (1.20-fold ± 0.08; P = 0.03) and (2.05-fold ± 0.31; P = 0.009), respectively (Figure 1C–E; Supplementary Figure S1). Under hypoxic conditions and in response to rosiglitazone treatment (50 μM), CAT was significantly elevated (1.44-fold ± 0.11; P = 0.04) (Figure 1C). SOD1 expression increased in response to rosiglitazone and reached (1.47-fold ± 0.10; P = 0.02) at 100 μM treatment concentration (Figure 1D). SOD2 similarly increased up to 1.66-fold (±0.16; P = 0.02) and 2.05-fold (±0.5; P = 0.02) at 50 and 100 μM, respectively (Figure 1E). Immunohistochemical analysis confirmed the quantitative data and revealed a change in SOD1 expression in both cyto- (CTB) and syncytiotrophoblasts (STB), while the expression of CAT was mostly limited to cytotrophoblasts (Figure 2).
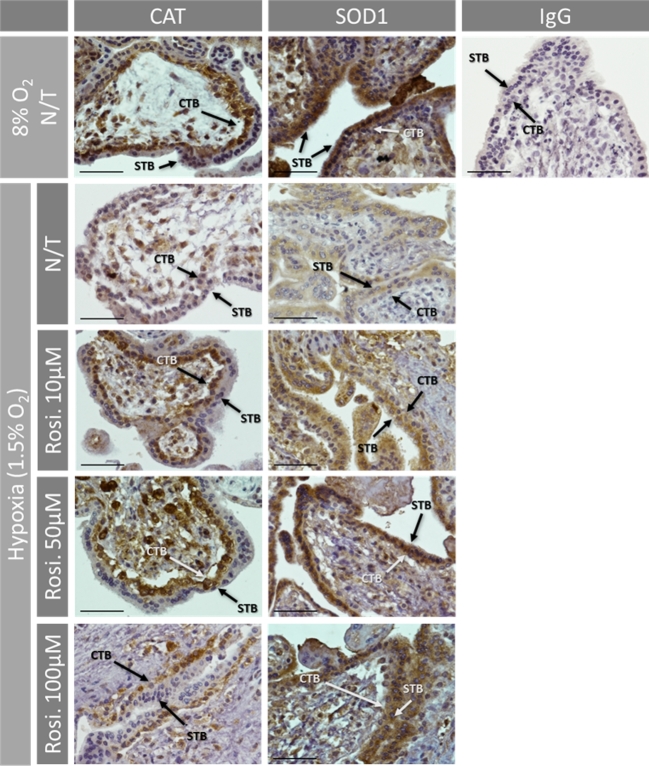
Figure 2.
Immunohistochemistry for CAT and SOD1 in first trimester placenta tissue cultured at normoxia (8% O2) and hypoxia (1.5% O2) with or without (N/T) rosiglitazone. SOD1 was widely distributed in both cytotrophoblasts (CTB) and syncytiotrophoblasts (STB), whereas CAT was mainly limited to the CTB. The expression of both proteins was reduced by hypoxia compared to normoxia, and restored by rosiglitazone treatment, compared to no-treatment (N/T) controls. No staining was observed in IgG control. Arrows indicate the localization of proteins in trophoblast cells. Scale bar = 100 μm.
Rosiglitazone-treated JEG-3 cells show decreased apoptosis and increased SOD2 and CAT expression
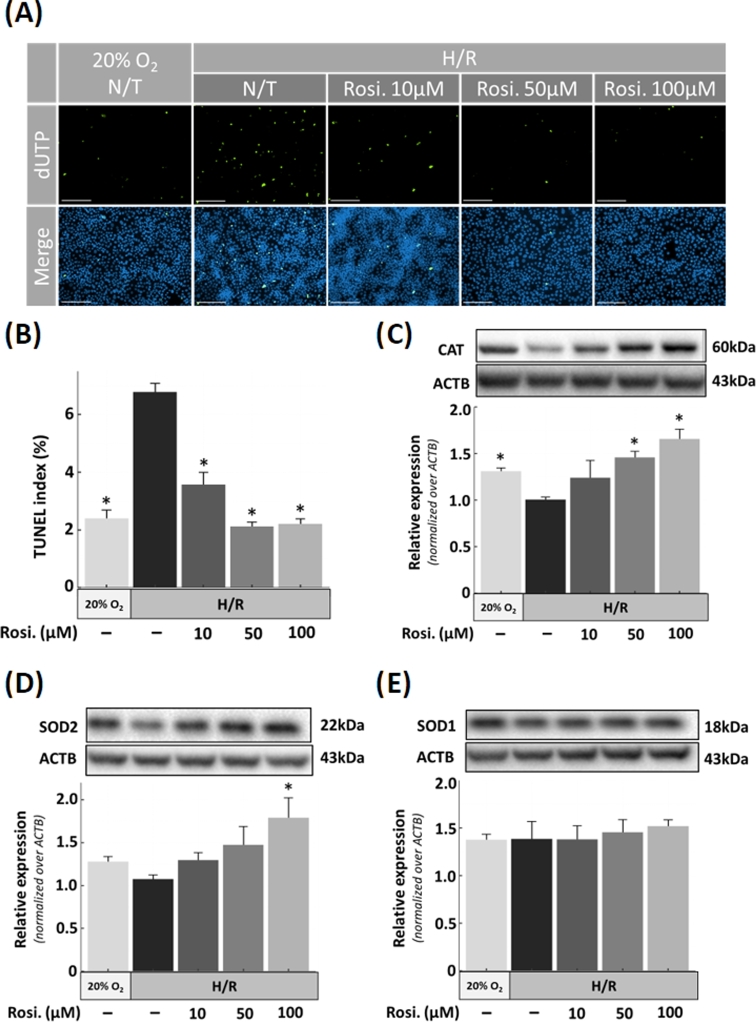
To examine the effect of rosiglitazone on apoptosis in trophoblast cells, human choriocarcinoma-derived JEG-3 cells were exposed to H/R in the presence of increasing concentrations of rosiglitazone. Based on preliminary results, we determined that JEG-3 cells required H/R injury in order to consistently induce apoptosis whereas placental explants were more vulnerable to low oxygen conditions. As such, apoptosis was increased in H/R condition (6.7% ± 0.3; P < 0.001), compared to normoxia (2.3% ± 0.3). However, a significant decrease in apoptosis was revealed by TUNEL following rosiglitazone (3.5% ± 0.4; P < 0.001, 2.1% ± 0.1; P < 0.001 and 2.2% ± 0.1; P < 0.001 for 10, 50, and 100 μM, respectively) treatment in cells exposed to H/R (Figure 3A and B). Next, we investigated whether the reduction in apoptosis by rosiglitazone treatment was linked to expression of CAT, SOD1, and SOD2 levels (Figure 3C–E; Supplementary Figure S2). Western blotting showed that the downregulation of CAT by exposure to H/R was reversed by rosiglitazone 1.41-fold ± 0.10 (P = 0.04) at 50 μM and 1.59-fold ± 0.08 (P = 0.006) at 100 μM, compared to no-treatment controls. SOD2 expression was also augmented by rosiglitazone 1.70-fold ± 0.20 (P = 0.04) at 100 μM. In contrast, SOD1 levels were not affected by H/R or treatment with rosiglitazone.
Figure 3.
Apoptosis and antioxidant protein expression in JEG-3 cells subjected to H/R and treatment with rosiglitazone. (B) Density of apoptotic cells was quantified by TUNEL in cells exposed to H/R and rosiglitazone and compared to the normoxia (20% O2) condition, as indicated, with representative images shown in A. (C–E) ROS regulating proteins were analyzed by western blotting in extracts of JEG-3 cells exposed to H/R. (C) Catalse (CAT), (D) superoxide dismutase-1 (SOD1), and 2 (E) SOD2. N = 3; * P < 0.05 vs N/T control, N/T = no-treatment, Rosi. = Rosiglitazone, scale bar = 100 μm, bars present mean ± SEM.
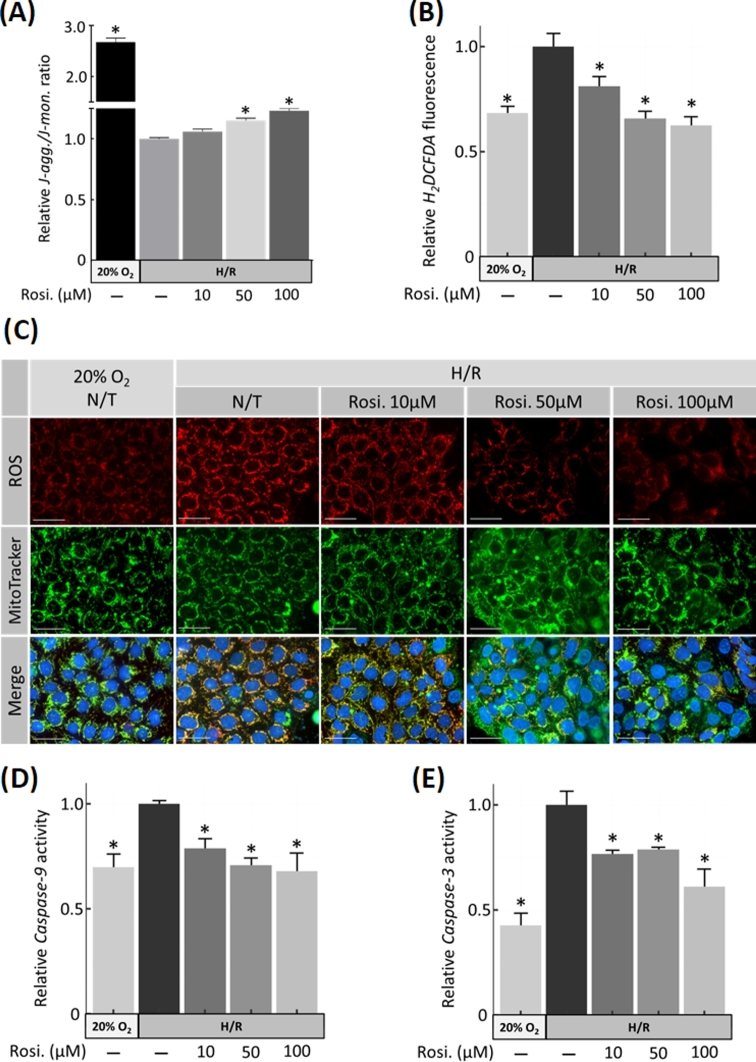
Rosiglitazone prevents mitochondrial depolarization and blocks caspase 9/3 activity
To determine the mitochondrial contribution to the cytoprotective effect of rosiglitazone, the mitochondrial potential (ΔΨm) in JEG-3 cells exposed to H/R was semiquantified using JC-10 immunofluorescence microscopy. Under physiological conditions, JC-10 forms red fluorescent aggregates in the mitochondrial matrix, but in damaged mitochondria, it converts to its monomeric form and changes to green. The JC-10 aggregate/monomer ratio significantly decreased by H/R (2.68-fold ± 0.08 (P = 0.001)) and increased after exposure to rosiglitazone 1.15-fold ± 0.02 (P = 0.001) and 1.23-fold ± 0.03 (P < 0.001) at 50 and 100 μM, respectively compared to N/T control (Figure 4A), consistent with lower mitochondrial damage. To further investigate the cytoprotective mechanism and determine if rosiglitazone regulates antioxidant expression and intracellular ROS generation, human choriocarcinoma-derived JEG-3 cells were exposed to H/R and increasing concentrations of rosiglitazone. The increased ROS production due to H/R declined in a dose-dependent manner in response to rosiglitazone treatment (Figure 4C). This finding was further supported by a significant reduction in the levels of ROS-dependent H2DCFDA oxidation in response to rosiglitazone treatment to 80% ± 3.8 (P = 0.01) at 10 μM, 65% ± 2.3 (P < 0.001) at 50 μM, and 62% ± 2.6 (P < 0.001) at 100 μM (Figure 4B). The effect of rosiglitazone treatment was also confirmed on another trophoblast-derived choriocarcinoma cell line (BeWo) under same conditions as JEG-3 cells (Supplementary Figure S3). Caspase-9 and caspase-3 enzymatic activities were also assessed using LEHD and DEVD cleavage rates, respectively. LEHD cleavage increased by H/R (1.68-fold ± 0.05; P = 0.001). Under H/R, LEHD activity decreased significantly by 0.78-fold ± 0.04 (P = 0.04), 0.70-fold ± 0.03 (P = 0.001), and 0.67-fold ± 0.08 (P < 0.001) (n = 4) when exposed to 10, 50, and 100 μM rosiglitazone, respectively (Figure 4D). DEVD cleavage was similarly increased by H/R (2.2-fold ± 0.06; P = 0.001) and reduced 0.76-fold ± 0.01 (P = 0.02), 0.78-fold ± 0.01 (P = 0.03), and 0.61-fold ± 0.08 (P < 0.001) at 10, 50, and 100 μM rosiglitazone in H/R condition, respectively (Figure 4E).
Figure 4.
Mitochondrial apoptotic pathway in H/R-injured JEG-3 cells and treatment with rosiglitazone. (A) Mitochondrial membrane potential (ΔΨm) was assessed as the J-aggregate/J-monomer ratio using JC-10 assay. (B) Quantification of ROS in JEG-3 cells using 2’,7’–dichlorofluorescin diacetate (DCFDA) showed that ROS production due to H/R decreased in response to rosiglitazone, as indicated by fluorescent dye intensity changes in C. Mitotracker green was used as mitochondrial marker. (D) Caspase 9 and (E) caspase 3 activities were assessed using fluorogenic substrates. N = 3; * P < 0.05 vs N/T control, N/T = no-treatment, Rosi. = Rosiglitazone, scale bar = 100 μm, bars present mean ± SEM.
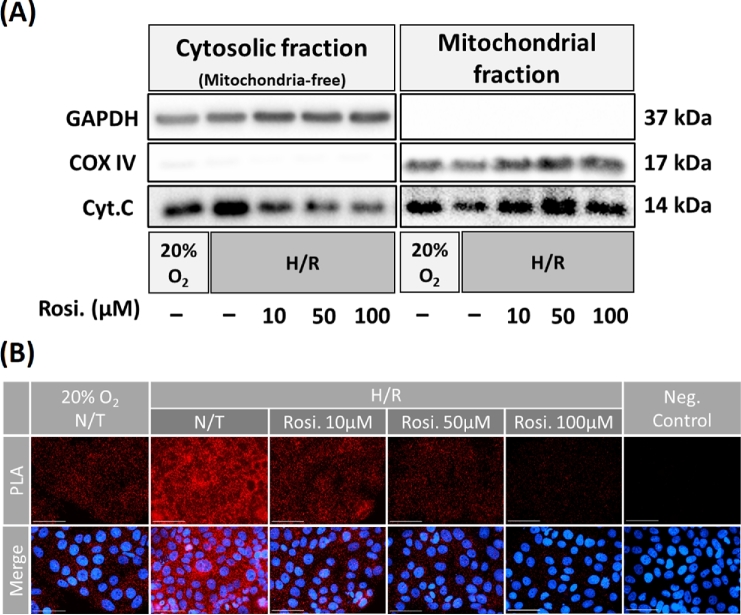
Rosiglitazone prevents the cytosolic release of cytochrome C and decreases its interaction with APAF1 under H/R conditions
The cytosolic release of cytochrome C was investigated next in order to further evaluate how rosiglitazone affects mitochondrial integrity in JEG-3 cells. After JEG-3 cells exposure to H/R with or without rosiglitazone treatment, mitochondrial and cytosolic fractions were isolated. JEG-3 cells were incubated under H/R condition and cytochrome c levels were quantified in both cytosol and mitochondria by western blot. H/R triggered the release of cytochrome c from mitochondria into the cytosol, which was inhibited by rosiglitazone exposure (Figure 5A; Supplementary Figure S4). To visualize the functional role of cytochrome C (apoptosis initiation), PLA was performed to show the interaction of cytochrome C and apoptotic protease-activating factor 1 (APAF1). The results confirmed that the elevated interaction of cytochrome C with APAF1 in H/R was reversed in presence of rosiglitazone (Figure 5B).
Figure 5.
Rosiglitazone prevents the cytosolic release of cytochrome C and decreases its interaction with APAF1 under H/R condition. (A) The expression of cytochrome C in the cytosol and mitochondrion fractions isolated from JEG-3 cells exposed to H/R and rosiglitazone in comparison to normoxia (20% O2) condition. COX IV, an inner membrane protein is used for control of mitochondrial protein loading and GAPDH used for total cell lysate loading. (B) Proximity ligation assay (PLA) showing the interaction between cytochrome C and APAF1 (red dots). Negative control (negative) imaged cells processed after treatment with rosiglitazone (Rosi.) without primary antibodies. N = 3; scale bar = 100 μm.
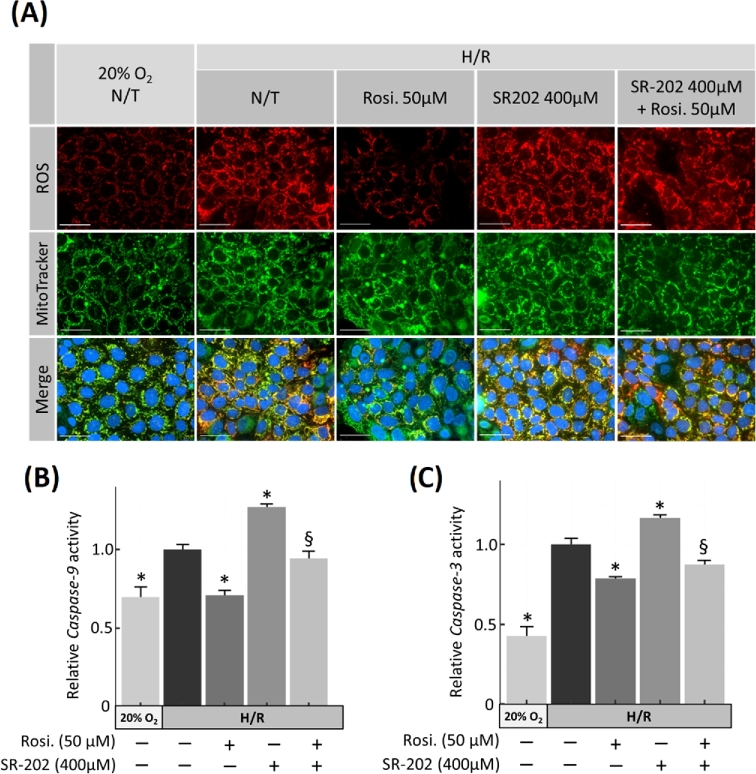
A PPARγ antagonist generates ROS and increases caspase activity in JEG-3 cells under H/R condition
To demonstrate the direct involvement of PPARγ in the regulation of cell survival, JEG-3 cells were incubated under H/R condition and treated with a specific PPARγ antagonist SR-202 [37] (400 μM), rosiglitazone (50 μM), and both. SR-202 treatment significantly increased intracellular generation of ROS but rosiglitazone co-treatment partially attenuated SR-202-induced ROS (Figure 6A). Elevation of ROS by SR-202 correlated with an increase of both caspase 9 (1.26-fold ± 0.02; P < 0.001, n = 3) and caspase 3 (1.16-fold ± 0.02; P = 0.002, n = 3) activities, compared to no treatment (N/T) controls. These effects of SR-202 were reversed by rosiglitazone co-treatment (Figure 6B and C).
Figure 6.
Effect of PPARγ antagonist (SR-202) and rosiglitazone on ROS production and apoptosis in JEG-3 cells exposed to H/R. (A) Production of ROS (red) in H/R injured JEG-3 cells treated with or without rosiglitazone and the PPARγ antagonist SR-202, as indicated. Caspase 9 (B) and Caspase 3 (C) were measured in cells treated similarly. N = 3; * P < 0.05 vs N/T control, N/T = no-treatment, Rosi. = Rosiglitazone, scale bar = 100 μm, bars present mean ± SEM.
PPARγ knockdown by siRNA reverses rosiglitazone effects on ROS generation
To validate the involvement of rosiglitazone and PPARγ in ROS neutralization, siRNA knockdown was performed to reduce PPARγ expression. JEG-3 cells were transfected with either PPARγ-specific siRNA or scrambled siRNA for 48 h prior to H/R exposure. PPARγ siRNA reversed rosiglitazone-dependent attenuation of intracellular ROS production (Figure 7A and B; Supplementary Figure S5) compared to scrambled control siRNA. PPARγ-specific siRNA reduced PPARγ protein levels (0.39-fold ± 0.05; P < 0.001, n = 4) compared to scrambled control (Figure 7C). Reduction of PPARγ significantly reduced the expressions of catalase (0.59-fold ± 0.02; P < 0.001) and SOD2 (0.46-fold ± 0.03; P < 0.001 n = 4) but had no effect on SOD1 expression (Figure 7C). RNA interference also abrogated the inhibitory effect of rosiglitazone on cytochrome C release (Figure 7D).
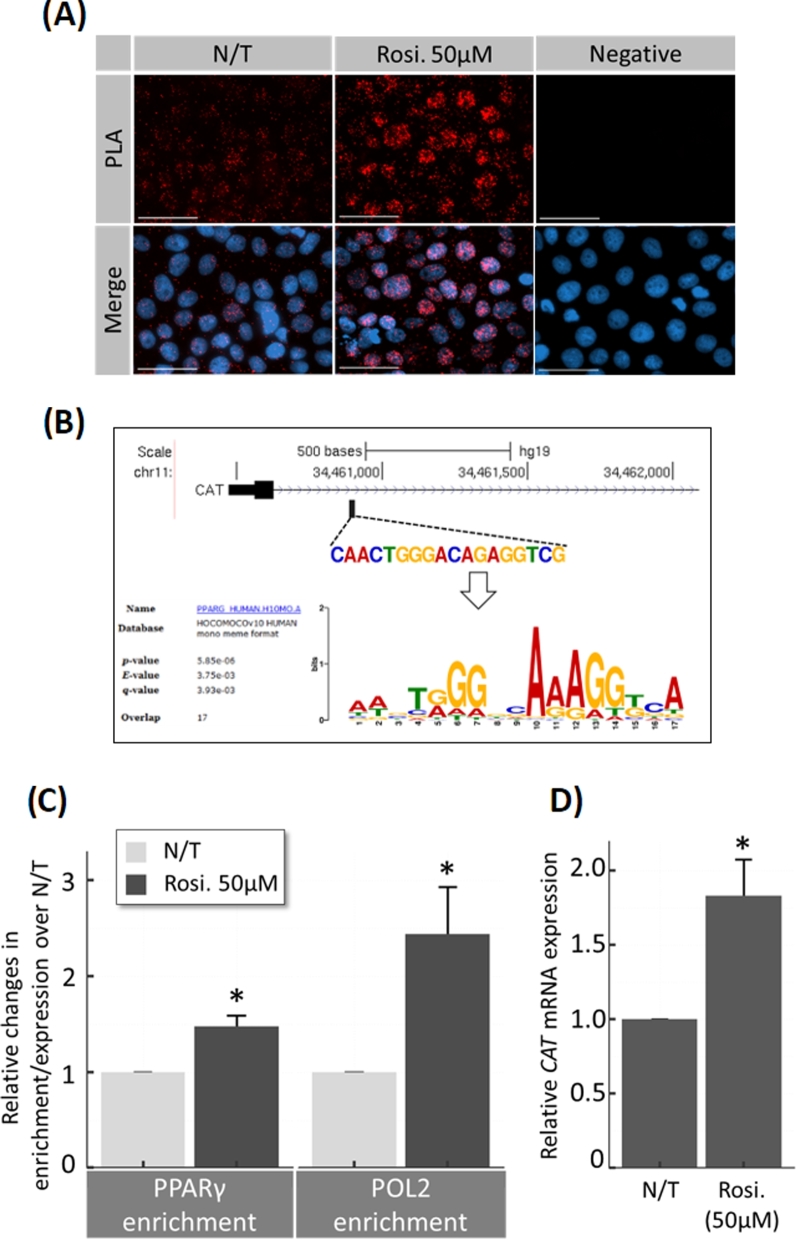
Rosiglitazone induces transcription of catalase by nuclear translocation of PPARγ and promoter binding
The transcriptional activity of PPARγ was analyzed using in situ PLA to evaluate the physical association of PPARγ with RNA-polymerase II (POL2) at transcriptional start sites. Nuclear PPARγ-POL2 association was increased by 50 μM rosiglitazone (Figure 8A). The CAT gene has one putative PPARγ-binding site (Figure 8B). The enrichment of PPARγ and POL2 at the CAT promoter was demonstrated by chromatin immunoprecipitation (ChIP) assay with primers specific for the region flanking the PPARγ-binding site in the catalase promoter. Recruitment of both PPARγ and POL2 at the CAT promoter increased in presence of 50 μM rosiglitazone 1.47-fold ± 0.11 (P = 0.003 n = 3) and 2.44-fold ± 0.48 (P = 0.02 n = 3), respectively. This enrichment correlated with an elevation of CAT mRNA expression (1.37-fold ± 0.01; P < 0.001, n = 3; Figure 8C and D).
Figure 8.
Rosiglitazone effect on nuclear translocation of PPARγ and CAT transcription in JEG-3 cells. (A) Immunofluorescence proximity ligation assay (PLA) for global transcriptional activity of PPARγ in JEG-3 cells. Using primary antibodies against PPARγ and RNA polymerase-2 (POL2), the technique enables visualization of molecular proximity (potentially at gene promoters) at single molecule resolution. Negative control (negative) imaged cells processed after treatment with rosiglitazone (Rosi.) without primary antibodies. (B) ChIP assays demonstrating the occupancy of PPARγ and RNA polymerase-2 (POL2) on the promoters of the CAT gene. The DNA fragments were IP using specific antibodies against PPARγ, POL2, or control IgG, and analyzed by qPCR. Input DNA served as an internal control. The target promoter region upstream of the transcription start site of the human CAT gene (verified by MEME SUITE; upper panel) was targeted. (C) Results were normalized to input DNA, and the enrichments were calculated relative to untreated (N/T) control. (D) RT-qPCR was performed in parallel to measure CAT mRNA expression. N = 3; * P < 0.05 vs N/T control, N/T = no-treatment, Rosi. = Rosiglitazone, scale bar = 100 μm, bars present mean ± SEM.
Rosiglitazone induces ROS scavenger expression in preeclamptic placentas
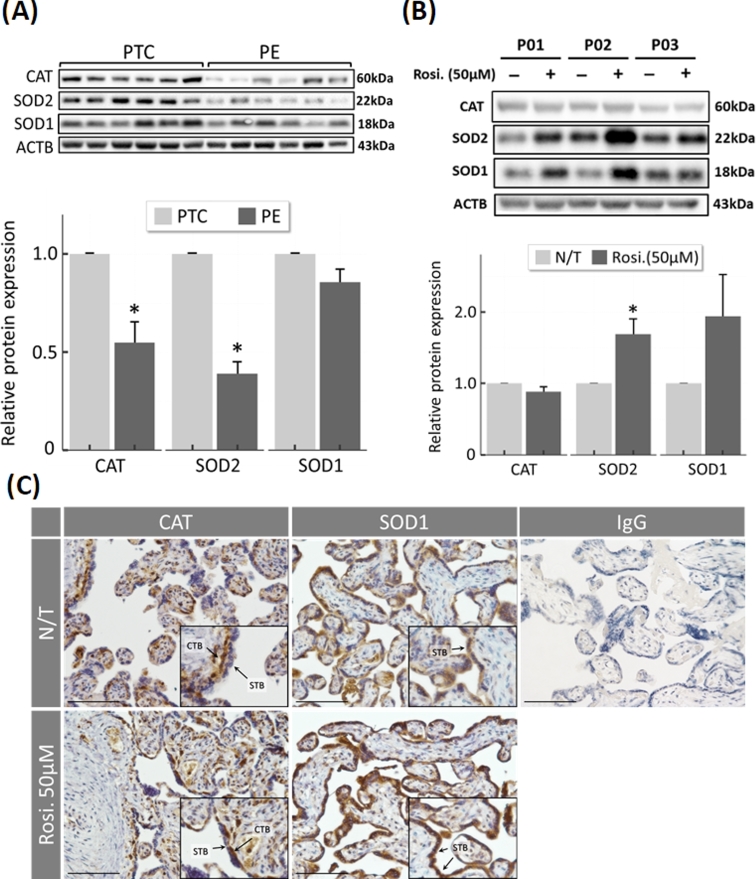
To determine whether the imbalance in ROS scavengers can be rescued in the preeclamptic placenta, the expression of CAT, SOD1, and SOD2 was analyzed semiquantitatively by western blotting in term placentas from preeclamptic (PE, n = 6) and preterm normotensive control (PTC; n = 6) patients. In preeclamptic tissues, CAT and SOD2 were significantly lower compared to controls (0.54-fold ± 0.10, P = 0.04, and 0.39-fold ± 0.06, P < 0.001) (Figure 9A; Supplementary Figure S6). To determine whether the protective properties of rosiglitazone observed in cell culture experiments can be translated to disease, term placental explants (n = 3) from PE/IUGR pregnancies were incubated overnight with rosiglitazone (50 μM) under normoxic (8% O2) conditions, and the expression of antioxidant proteins was semiquantified by immunoblotting. Treatment with rosiglitazone upregulated SOD2 expression (1.68-fold ± 0.21; P = 0.03) in all samples, while CAT expression remained unchanged (Figure 9B; Supplementary Figure S6). Immunocytochemistry confirmed an increase in SOD1 signal intensity in the presence of rosiglitazone (50 μM) within both CTB and STB, while CAT immunoreactivity was localized to the CTB (Figure 9C).
Figure 9.
Expression of ROS scavengers and effect of rosiglitazone treatment in preeclamptic placentas and normotensive preterm controls. )A) Western blot analysis of antioxidants in term placentas from preeclamptic (PE; N = 6) or preterm control (PTC; N = 6) labeled with antibodies to CAT and SOD2, showing individual bands and averages after densitometry. (B) Term placental explants from PE/IUGR patients (N = 3) were cultured overnight ex vivo in the absence or presence of 50 μM rosiglitazone. Tissue extracts were analyzed by immunoblotting, as indicated. (C) Representative images of CAT and SOD1 immunohistochemistry in human term placenta after ex vivo culture in absence (N/T) or presence of rosiglitazone (Rosi. 50 μM), showing the localization of antioxidant proteins in term placenta. Negative control using normal IgG is included. Arrows indicate the localization of trophoblast cells. * P < 0.05 vs N/T control, N/T = no-treatment, Rosi. = Rosiglitazone, scale bar = 100 μm, bars present mean ± SEM.
Discussion
The etiology and pathophysiology of the PE syndrome is not well understood. Placental oxidative stress caused by ROS appears to contribute to cellular damage in trophoblast cells of preeclamptic placentas [1–3]. In the current study, using ex vivo and in vitro models, we provide mechanistic evidence of rescue from ROS damage in hypoxic human placental tissue. The pharmacological PPARγ agonist rosiglitazone reduced ROS generation and damage by regulating detoxification enzymes (CAT, SOD1, and SOD2). Our results revealed that PPARγ transcriptional activity induced by rosiglitazone causes upregulation of key ROS scavenger proteins. In turn, this prevents mitochondrial damage by oxidative stress, cytochrome C release, and the activation of caspases controlling cell death.
Ischemic injury in the placenta occurs regularly in cases of placental insufficiencies such as PE where dysregulated placental perfusion results in oxidative stress and apoptosis [38]. In the current study, we observed higher levels of apoptosis in the syncytiotrophoblast compared to the underlying cytotrophoblasts of hypoxic first trimester explants. This is likely due to proximity of the insult. Rosiglitazone reduced this effect to normoxic control level.
The expression of both SOD1 and SOD2 was decreased under oxidative stress. Similar observations were reported previously [39, 40]. Wang et al. found that HIF1-α is key regulator of SOD1 and SOD2 [41] in a hypoxic pulmonary hypertensive rat model. Although we did not specifically study HIF1-α in our model, another study emphasized the importance of HIF1-α gene expression in the first trimester placenta and its contribution to promoting PE progression [42]. Another recent study found that transient activation of HIF-1α caused by hypoxia was attenuated by rosiglitazone [43] in pulmonary artery smooth muscle cells. We propose that a similar mechanism may occur in the human trophoblast.
Increased production of superoxide radical anion (O2−) and hydrogen peroxide (H2O2) in PE is potentially caused by reduced levels of detoxifying proteins in the placenta [44]. Our findings provide evidence that rosiglitazone exhibits cytoprotective effects through the upregulation of ROS scavenger proteins, including CAT, SOD1, and SOD2. Rosiglitazone activates the putative ROS regulator PPARγ. The cytoprotective and anti-apoptotic activity of PPARγ agonists has been linked to induction of CAT and SOD expression in neurons [45–47]. Morphological examination of CAT and SOD1 expression in first trimester placental explants has revealed different localization of both proteins. Watson and colleagues have reported that expressions of SODs gradually increase from 10 to 14 weeks of gestation [48]. However, such gestational effect was not observed in the current study, possibly due to limited samples size. The H2O2-degrading enzyme, CAT, was predominantly localized to cytotrophoblasts, whereas the cytosolic superoxide scavenger, SOD1, was expressed in both cyto- and syncytiotrophoblasts.
These findings are supported by previous reports on first trimester human placenta [49]. The low levels of the peroxide-clearing CAT in the syncytiotrophoblast might increase the vulnerability to ROS overproduction which could explain the high apoptosis rate observed in hypoxic syncytiotrophoblasts.
Rosiglitazone elevated catalase and SOD2 expression in H/R-injured JEG-3 cells, which was associated with a significant suppression of ROS generation. Placental SOD2 expression and activity are lower in pregnancy disorders like recurrent miscarriage [50] and robustly increase during labor [51]. Considering that trophoblast cells are enriched with mitochondria where SOD2 is constitutively expressed and metabolically active [52], we propose that the cytoprotective effect of rosiglitazone is at least in part caused by a disruption of mitochondrial damage, caspase activity, and intrinsic activation of the apoptotic network. In this process, mitochondrial membrane depolarization is considered the first sign of mitochondrial damage by ROS overproduction, followed by release of pro-apoptotic cytochrome C to activate the intrinsic apoptotic pathway [11]. We found that rosiglitazone decreased cytosolic cytochrome C in conjunction with a significant decrease in the cleavage of caspases 9 and 3 to prevent DNA fragmentation. Similar effects of rosiglitazone were previously reported in neural [47], retinal [53], and pancreatic islet [54] cells following oxidative stress.
siRNA-mediated reduction of PPARγ caused downregulation of catalase and SOD2, which in turn abolished the protective effect of rosiglitazone, providing direct evidence of the involvement of PPARγ signaling. Similar studies using liver [55] and neural stem cells [56] treated with rosiglitazone reported improved mitochondrial integrity, reduced ROS production, and decreased apoptosis.
Using PLAs we showed that rosiglitazone induces transcriptional activation via PPARγ–POL2 nuclear translocation. In silico analysis of the CAT gene using MEME SUITE [36] revealed the presence of a putative PPARγ response element (PPRE) and a ChIP assay demonstrated an increase in PPARγ and active POL2 in response to rosiglitazone, correlating with increased CAT expression. Similarly, PPARγ regulates the expression of CAT in mice [46] and humans [57] through a specific PPRE. In summary, we suggest that PPARγ regulates, at least in part, CAT expression transcriptionally but we cannot rule out other possible indirect mechanisms through which PPARγ might control CAT expression. Similarly, we speculate an indirect control of PPARγ on the expression of SODs since no PPRE was recognized in the promoter region. Our findings of PPARγ involvement in the regulation of the oxidative stress response are in line with previous reports where activation of PPARγ increases mitochondrial biogenesis, oxygen consumption, ΔΨm, and antioxidant defenses [58].
In preeclamptic placentas, low levels of catalase and SODs have been reported [18, 59], which might contribute to the oxidative stress leading to endothelial dysfunction during pregnancy. We confirmed the reduced expression of CAT and SOD2 in preeclamptic placenta compared to controls. Our findings collectively suggest that stimulation of antioxidative enzymes protects the trophoblast during oxidative stress in vitro.
PE/IUGR placental tissues treated with rosiglitazone showed an increase in both SOD1 and SOD2 expression. In contrast to first trimester tissue, rosiglitazone did not significantly increase catalase expression in the term placenta. This observation could be explained by the differential expression of CAT, being mainly limited to cytotrophoblast cells which gradually decrease in number over the gestation period [60]. In the current study, a cell line model (JEG-3) was used in parallel to placental explants to confirm the general aspects of hypoxia detoxification pathways. However, employing other placental cell lines such as (BeWo) and comparing fused vs. nonfused cells may reveal insights into the mechanism of differential enzyme expression in the trophoblast cells and the syncytium.
Overall our data indicate that rosiglitazone regulates the expression of two key antioxidative enzymes SODs and catalase which may work in tandem or parallel to reduce detrimental ROS and by products. These results are clinically relevant since rosiglitazone is used in the treatment of diabetes [61].
The role of PPARG in placental dysfunction is still unknown and should be the subject of future studies. Current literature aimed to explore this process with conflicting results showing no changes [62], upregulation [63] or downregulation [64] of PPARγ protein expression in pathological placentas. This might be explained by the differences in sample size and disease definitions. All studies so far failed to critically assess PPARγ activity. Holdsworth-Carson used a DNA-binding assay to assess PPARγ activity in pathological placenta [63]. However, the provided data are not conclusive because PPARγ expression and DNA binding do not prove its molecular activity. PPARγ is able to induce [65, 66] and/or block [67, 68] transcriptional activity; therefore, a DNA-binding assay is indicative but not a proof. The current study aimed to evaluate the role of PPARγ activation in the human placenta under pathological conditions. The PLA was used to visualize the interaction of active RNA-POL2 and PPARγ to show the global PPARγ transcriptional activation.
In conclusion, this study provides a novel role for the cytoprotective properties of rosiglitazone and PPARγ in the human trophoblast, driven by the induction of ROS-detoxifying enzymes. Considering the substantial contribution of oxidative stress in pathophysiology of placental insufficiency, our findings suggest that rosiglitazone and its derivatives could provide a novel approach for therapeutic intervention to improve maternal and fetal health.
Supplementary data
Supplementary Figure S1. Full unedited blots of Figure 1.
Supplementary Figure S2. Full unedited blots of Figure 3.
Supplementary Figure S3. ROS production decreased in response to rosiglitazone in another trophoblast-derived choriocarcinoma cell line (BeWo) under H/R condition, as indicated by ROS fluorescent dye intensity changes. Mitotracker green was used as mitochondrial marker. N/T = no-treatment, Rosi. = Rosiglitazone, scale bar = 100 μm.
Supplementary Figure S4. Full unedited blots of Figure 5.
Supplementary Figure S5. Full unedited blots of Figure 7.
Supplementary Figure S6. Full unedited blots of Figure 9.
Acknowledgment
The authors thank the Northland Family Planning Centers of Michigan for participating in this research study.
Footnotes
Grant support: This research was supported by the NIH Grant HL128628 and the March of Dimes Foundation.
Conflict of Interest: The authors declare no conflict of interest.
References
- 1. Roberts JM, Hubel CA. Is oxidative stress the link in the two-stage model of pre-eclampsia? Lancet North Am Ed 1999; 354:788–789. [DOI] [PubMed] [Google Scholar]
- 2. Walsh SW. Maternal-placental interactions of oxidative stress and antioxidants in preeclampsia. Semin Reprod Med 1998; 16:93–104. [DOI] [PubMed] [Google Scholar]
- 3. Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet North Am Ed 2010; 376:631–644. [DOI] [PubMed] [Google Scholar]
- 4. Leung DN, Smith SC, To KF, Sahota DS, Baker PN. Increased placental apoptosis in pregnancies complicated by preeclampsia. Am J Obstet Gynecol 2001; 184:1249–1250. [DOI] [PubMed] [Google Scholar]
- 5. Wang Y, Walsh SW. Placental mitochondria as a source of oxidative stress in pre-eclampsia. Placenta 1998; 19:581–586. [DOI] [PubMed] [Google Scholar]
- 6. Allaire AD, Ballenger KA, Wells SR, McMahon MJ, Lessey BA. Placental apoptosis in preeclampsia. Obstet Gynecol 2000; 96:271–276. [DOI] [PubMed] [Google Scholar]
- 7. DiFederico E, Genbacev O, Fisher SJ. Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am J Pathol 1999; 155:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol 2002; 186:158–166. [DOI] [PubMed] [Google Scholar]
- 9. Longtine MS, Chen B, Odibo AO, Zhong Y, Nelson DM. Villous trophoblast apoptosis is elevated and restricted to cytotrophoblasts in pregnancies complicated by preeclampsia, IUGR, or preeclampsia with IUGR. Placenta 2012; 33:352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grivennikova VG, Vinogradov AD. Generation of superoxide by the mitochondrial Complex I. Biochim BiophysActa- Bioenergetics 2006; 1757:553–561. [DOI] [PubMed] [Google Scholar]
- 11. Finkel T. Signal transduction by reactive oxygen species. J Cell Biol 2011; 194:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 2010; 48:749–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sikkema JM, van Rijn BB, Franx A, Bruinse HW, de Roos R, Stroes ES, van Faassen EE. Placental superoxide is increased in pre-eclampsia. Placenta 2001; 22:304–308. [DOI] [PubMed] [Google Scholar]
- 14. Bakacak M, Kilinc M, Serin S, Ercan O, Kostu B, Avci F, Kiran H, Kiran G. Changes in copper, zinc, and malondialdehyde levels and superoxide dismutase activities in pre-eclamptic pregnancies. Med Sci Monit 2015; 21:2414–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dordevic NZ, Babic GM, Markovic SD, Ognjanovic BI, Stajn AS, Zikic RV, Saicic ZS. Oxidative stress and changes in antioxidative defense system in erythrocytes of preeclampsia in women. Reprod Toxicol 2008; 25:213–218. [DOI] [PubMed] [Google Scholar]
- 16. D'Souza V, Rani A, Patil V, Pisal H, Randhir K, Mehendale S, Wagh G, Gupte S, Joshi S. Increased oxidative stress from early pregnancy in women who develop preeclampsia. Clin Exp Hypertens 2016; 38:225–232. [DOI] [PubMed] [Google Scholar]
- 17. Genc H, Uzun H, Benian A, Simsek G, Gelisgen R, Madazli R, Guralp O. Evaluation of oxidative stress markers in first trimester for assessment of preeclampsia risk. Arch Gynecol Obstet 2011; 284:1367–1373. [DOI] [PubMed] [Google Scholar]
- 18. Wang Y, Walsh SW. Antioxidant activities and mRNA expression of superoxide dismutase, catalase, and glutathione peroxidase in normal and preeclamptic placentas. J Soc Gynecol Investig 1996; 3:179–184. [PubMed] [Google Scholar]
- 19. Li MD, Yang X. A retrospective on nuclear receptor regulation of inflammation: lessons from GR and PPARs. PPAR Res 2011; 2011:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moreno M, Lombardi A, Silvestri E, Senese R, Cioffi F, Goglia F, Lanni A, de Lange P. PPARs: nuclear receptors controlled by, and controlling, nutrient handling through nuclear and cytosolic signaling. PPAR Res 2010; 2010:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thouennon E, Cheng Y, Falahatian V, Cawley NX, Loh YP. Rosiglitazone-activated PPARgamma induces neurotrophic factor-alpha1 transcription contributing to neuroprotection. J Neurochem 2015; 134:463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pal Singh A, Kaur T, SinghDahiya R, Singh N, Singh Bedi PM. Ameliorative role of rosiglitazone in hyperhomocysteinemia-induced experimental cardiac hypertrophy. J Cardiovasc Pharmacol 2010; 56:53–59. [DOI] [PubMed] [Google Scholar]
- 23. Tao L, Wang Y, Gao E, Zhang H, Yuan Y, Lau WB, Chan L, Koch WJ, Ma XL. Adiponectin: an indispensable molecule in rosiglitazone cardioprotection following myocardial infarction. Circ Res 2010; 106:409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chou CA, Ng HY, Kuo WH, Chiou TY, Pei SN, Li LC, Lee YT, Lee CT. Rosiglitazone attenuates indoxyl sulphate-induced endothelial dysfunction. Clin Exp Pharmacol Physiol 2015; 42:287–292. [DOI] [PubMed] [Google Scholar]
- 25. Tong S, Kaitu’u-Lino TJ, Onda K, Beard S, Hastie R, Binder NK, Cluver C, Tuohey L, Whitehead C, Brownfoot F, De Silva M, Hannan NJ. Heme oxygenase-1 is not decreased in preeclamptic placenta and does not negatively regulate placental soluble fms-like tyrosine kinase-1 or soluble endoglin secretion novelty and significance. Hypertension 2015; 66:1073–1081. [DOI] [PubMed] [Google Scholar]
- 26. Levytska K, Drewlo S, Baczyk D, Kingdom J. PPAR-gamma regulates trophoblast differentiation in the BeWo cell model. PPAR Res 2014; 2014:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bilban M, Haslinger P, Prast J, Klinglmüller F, Woelfel T, Haider S, Sachs A, Otterbein LE, Desoye G, Hiden U, Wagner O, Knöfler M. Identification of novel trophoblast invasion-related genes: heme oxygenase-1 controls motility via peroxisome proliferator-activated receptor gamma. Endocrinology 2009; 150:1000–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cawyer C, Afroze SH, Drever N, Allen S, Jones R, Zawieja DC, Kuehl T, Uddin MN. Attenuation of hyperglycemia-induced apoptotic signaling and anti-angiogenic milieu in cultured cytotrophoblast cells. Hypertens Pregnancy 2016; 35:159–169. [DOI] [PubMed] [Google Scholar]
- 29. McCarthy FP, Drewlo S, English FA, Kingdom J, Johns EJ, Kenny LC, Walsh SK. Evidence implicating peroxisome proliferator-activated receptor-gamma in the pathogenesis of preeclampsia. Hypertension 2011; 58:882–887. [DOI] [PubMed] [Google Scholar]
- 30. Xu Y, Romero R, Miller D, Kadam L, Mial TN, Plazyo O, Garcia-Flores V, Hassan SS, Xu ZH, Tarca AL, Drewlo S, Gomez-Lopez N. An M1-like macrophage polarization in decidual tissue during spontaneous preterm labor that is attenuated by rosiglitazone treatment. J Immunol 2016; 196:2476–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Capobianco E, Martínez N, Fornes D, Higa R, Di Marco I, Basualdo MN, Faingold MC, Jawerbaum A. PPAR activation as a regulator of lipid metabolism, nitric oxide production and lipid peroxidation in the placenta from type 2 diabetic patients. Mol Cell Endocrinol 2013; 377:7–15. [DOI] [PubMed] [Google Scholar]
- 32. Bolnick JM, Kilburn BA, Bolnick AD, Diamond MP, Singh M, Hertz M, Dai J, Armant DR. Sildenafil prevents apoptosis of human first-trimester trophoblast cells exposed to oxidative stress. Reprod Sci 2015; 22:718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marino J, Garcia Vior MC, Furmento VA, Blank VC, Awruch J, Roguin LP. Lysosomal and mitochondrial permeabilization mediates zinc(II) cationic phthalocyanine phototoxicity. Int J Biochem Cell Biol 2013; 45:2553–2562. [DOI] [PubMed] [Google Scholar]
- 34. Patel S, Kilburn B, Imudia A, Armant DR, Skafar DF. Estradiol elicits proapoptotic and antiproliferative effects in human trophoblast cells. Biol Reprod 2015; 93:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res 2009; 37:W202–W208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Becker J, Barysch SV, Karaca S, Dittner C, Hsiao HH, Berriel Diaz M, Herzig S, Urlaub H, Melchior F. Detecting endogenous SUMO targets in mammalian cells and tissues. Nat Struct Mol Biol 2013; 20:525–531. [DOI] [PubMed] [Google Scholar]
- 37. Rieusset J, Touri F, Michalik L, Escher P, Desvergne B, Niesor E, Wahli W. A new selective peroxisome proliferator-activated receptor gamma antagonist with antiobesity and antidiabetic activity. Mol Endocrinol 2002; 16:2628–2644. [DOI] [PubMed] [Google Scholar]
- 38. Redman CW, Sargent IL. The pathogenesis of pre-eclampsia. Gynécologie Obstétrique Fertilité 2001; 29:518–522. [DOI] [PubMed] [Google Scholar]
- 39. Jung JE, Kim GS, Narasimhan P, Song YS, Chan PH. Regulation of Mn-superoxide dismutase activity and neuroprotection by STAT3 in mice after cerebral ischemia. J Neurosci 2009; 29:7003–7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lanoix D, Lacasse AA, Reiter RJ, Vaillancourt C. Melatonin: the watchdog of villous trophoblast homeostasis against hypoxia/reoxygenation-induced oxidative stress and apoptosis. Mol Cell Endocrinol 2013; 381:35–45. [DOI] [PubMed] [Google Scholar]
- 41. Wang L, Zheng Q, Yuan Y, Li Y, Gong X. Effects of 17beta-estradiol and 2-methoxyestradiol on the oxidative stress-hypoxia inducible factor-1 pathway in hypoxic pulmonary hypertensive rats. Exp Ther Med 2017; 13:2537–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Iriyama T, Wang W, Parchim NF, Song A, Blackwell SC, Sibai BM, Kellems RE, Xia Y. Hypoxia-independent upregulation of placental hypoxia inducible factor-1alpha gene expression contributes to the pathogenesis of preeclampsia. Hypertension 2015; 65:1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Blum JI, Bijli KM, Murphy TC, Kleinhenz JM, Hart CM. Time-dependent PPARgamma modulation of HIF-1alpha signaling in hypoxic pulmonary artery smooth muscle cells. Am J Med Sci 2016; 352:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aris A, Benali S, Ouellet A, Moutquin JM, Leblanc S. Potential biomarkers of preeclampsia: inverse correlation between hydrogen peroxide and nitric oxide early in maternal circulation and at term in placenta of women with preeclampsia. Placenta 2009; 30:342–347. [DOI] [PubMed] [Google Scholar]
- 45. Jung TW, Lee JY, Shim WS, Kang ES, Kim SK, Ahn CW, Lee HC, Cha BS. Rosiglitazone protects human neuroblastoma SH-SY5Y cells against MPP+ induced cytotoxicity via inhibition of mitochondrial dysfunction and ROS production. J Neurol Sci 2007; 253:53–60. [DOI] [PubMed] [Google Scholar]
- 46. Khoo NK, Hebbar S, Zhao W, Moore SA, Domann FE, Robbins ME. Differential activation of catalase expression and activity by PPAR agonists: implications for astrocyte protection in anti-glioma therapy. Redox Biol 2013; 1:70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yi JH, Park SW, Brooks N, Lang BT, Vemuganti R. PPARgamma agonist rosiglitazone is neuroprotective after traumatic brain injury via anti-inflammatory and anti-oxidative mechanisms. Brain Res 2008; 1244:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Watson AL, Palmer ME, Jauniaux E, Burton GJ. Variations in expression of copper/zinc superoxide dismutase in villous trophoblast of the human placenta with gestational age. Placenta 1997; 18:295–299. [DOI] [PubMed] [Google Scholar]
- 49. Watson AL, Skepper JN, Jauniaux E, Burton GJ. Changes in concentration, localization and activity of catalase within the human placenta during early gestation. Placenta 1998; 19:27–34. [DOI] [PubMed] [Google Scholar]
- 50. Ghneim HK, Al-Sheikh YA, Alshebly MM, Aboul-Soud MA. Superoxide dismutase activity and gene expression levels in Saudi women with recurrent miscarriage. Mol Med Rep 2016; 13:2606–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Roland L, Beauchemin D, Acteau G, Fradette C, St-Pierre I, Bilodeau JF. Effects of labor on placental expression of superoxide dismutases in preeclampsia. Placenta 2010; 31:392–400. [DOI] [PubMed] [Google Scholar]
- 52. McCarthy CM, Kenny LC. Mitochondrial [dys]function; culprit in pre-eclampsia? Clin Sci 2016; 130:1179–1184. [DOI] [PubMed] [Google Scholar]
- 53. Doonan F, Wallace DM, O’Driscoll C, Cotter TG. Rosiglitazone acts as a neuroprotectant in retinal cells via up-regulation of sestrin-1 and SOD-2. J Neurochem 2009; 109:631–643. [DOI] [PubMed] [Google Scholar]
- 54. Vandewalle B, Moerman E, Lefebvre B, Defrance F, Gmyr V, Lukowiak B, Kerr Conte J, Pattou F. PPARgamma-dependent and -independent effects of rosiglitazone on lipotoxic human pancreatic islets. Biochem Biophys Res Commun 2008; 366:1096–1101. [DOI] [PubMed] [Google Scholar]
- 55. Li J, Ke W, Zhou Q, Wu Y, Luo H, Zhou H, Yang B, Guo Y, Zheng Q, Zhang Y. Tumour necrosis factor-alpha promotes liver ischaemia-reperfusion injury through the PGC-1alpha/Mfn2 pathway. J Cell Mol Med 2014; 18:1863–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chiang MC, Cheng YC, Lin KH, Yen CH. PPARgamma regulates the mitochondrial dysfunction in human neural stem cells with tumor necrosis factor alpha. Neuroscience 2013; 229:118–129. [DOI] [PubMed] [Google Scholar]
- 57. Okuno Y, Matsuda M, Miyata Y, Fukuhara A, Komuro R, Shimabukuro M, Shimomura I. Human catalase gene is regulated by peroxisome proliferator activated receptor-gamma through a response element distinct from that of mouse. Endocr J 2010; 57:303–309. [DOI] [PubMed] [Google Scholar]
- 58. Corona JC, Duchen MR. PPARgamma as a therapeutic target to rescue mitochondrial function in neurological disease. Free Radic Biol Med 2016; 100:153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gharesi-Fard B, Zolghadri J, Kamali-Sarvestani E. Proteome differences of placenta between pre-eclampsia and normal pregnancy. Placenta 2010; 31:121–125. [DOI] [PubMed] [Google Scholar]
- 60. Benirschke K, Kaufmann P, Baergen R. Pathology of the Human Placenta. New York: Springer; 2006. [Google Scholar]
- 61. Kikuchi M, Kaku K, Odawara M, Momomura S, Ishii R. Efficacy and tolerability of rosiglitazone and pioglitazone in drug-naive Japanese patients with type 2 diabetes mellitus: a double-blind, 28 weeks' treatment, comparative study. Curr Med Res Opin 2012; 28:1007–1016. [DOI] [PubMed] [Google Scholar]
- 62. Rodie VA, Young A, Jordan F, Sattar N, Greer IA, Freeman DJ. Human placental peroxisome proliferator-activated receptor delta and gamma expression in healthy pregnancy and in preeclampsia and intrauterine growth restriction. J Soc Gynecol Investig 2005; 12:320–329. [DOI] [PubMed] [Google Scholar]
- 63. Holdsworth-Carson SJ, Permezel M, Riley C, Rice GE, Lappas M. Peroxisome proliferator-activated receptors and retinoid X receptor-alpha in term human gestational tissues: tissue specific and labour-associated changes. Placenta 2009; 30:176–186. [DOI] [PubMed] [Google Scholar]
- 64. He P, Chen Z, Sun Q, Li Y, Gu H, Ni X. Reduced expression of 11beta-hydroxysteroid dehydrogenase type 2 in preeclamptic placentas is associated with decreased PPARgamma but increased PPARalpha expression. Endocrinology 2014; 155:299–309. [DOI] [PubMed] [Google Scholar]
- 65. Parast MM, Yu H, Ciric A, Salata MW, Davis V, Milstone DS. PPARgamma regulates trophoblast proliferation and promotes labyrinthine trilineage differentiation. PLoS One 2009; 4:e8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Finck BN, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation 2007; 115:2540–2548. [DOI] [PubMed] [Google Scholar]
- 67. Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature 1998; 391:82–86. [DOI] [PubMed] [Google Scholar]
- 68. Lecarpentier Y, Vallee A. Opposite interplay between PPAR gamma and canonical Wnt/beta-catenin pathway in amyotrophic lateral sclerosis. Front Neurol 2016; 7:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.