ABSTRACT
Background
During the Pregnancy and Birth to 24 Months Project, the USDA and Department of Health and Human Services initiated a review of evidence on diet and health in these populations.
Objectives
The aim of these systematic reviews was to examine the relation of 1) never versus ever feeding human milk, 2) shorter versus longer durations of any human milk feeding, 3) shorter versus longer durations of exclusive human milk feeding prior to infant formula introduction, 4) feeding a lower versus higher intensity of human milk to mixed-fed infants, and 5) feeding a higher intensity of human milk by bottle versus breast with food allergies, allergic rhinitis, atopic dermatitis, and asthma.
Methods
The Nutrition Evidence Systematic Review team conducted systematic reviews with external experts. We searched CINAHL, Cochrane, Embase, and PubMed for articles published between January 1980 and March 2016, dual-screened the results according to predetermined criteria, extracted data from and assessed the risk of bias for each included study, qualitatively synthesized the evidence, developed conclusion statements, and graded the strength of the evidence.
Results
The systematic reviews numbered 1–5 above included 44, 35, 1, 0, and 0 articles, respectively. Moderate, mostly observational, evidence suggests that 1) never versus ever being fed human milk is associated with higher risk of childhood asthma, and 2) among children and adolescents who were fed human milk as infants, shorter versus longer durations of any human milk feeding are associated with higher risk of asthma. Limited evidence does not suggest associations between 1) never versus ever being fed human milk and atopic dermatitis in childhood or 2) the duration of any human milk feeding and allergic rhinitis and atopic dermatitis in childhood.
Conclusions
Moderate evidence suggests that feeding human milk for short durations or not at all is associated with higher childhood asthma risk. Evidence on food allergies, allergic rhinitis, and atopic dermatitis is limited.
Keywords: breastfeeding, human milk, food allergy, atopic dermatitis, allergic rhinitis, asthma, infant, toddler, child, systematic review
Introduction
Pregnancy and the period from birth to 24 mo (B-24) are sensitive windows during which diet has a particularly strong influence on the life course health trajectory (1). The USDA and Department of Health and Human Services planned the Pregnancy and Birth to 24 Months (P/B-24) Project to begin examining evidence relating diet during pregnancy and the first 2 y of life with growth and health outcomes throughout the life span (2–4).
The systematic reviews (SRs) in this article examine the relationships between infant milk-feeding practices and food allergies, allergic rhinitis, atopic dermatitis, and asthma. According to the National Institute of Allergy and Infectious Diseases, food allergy affects ∼5% of children and ∼4% of adults in the United States; however, its prevalence is increasing (5). The Institute reports that atopic dermatitis affects ∼30% of the population (6), and the CDC reports that ∼8% of the population suffers from asthma (7) and from hay fever (8). Although all atopic diseases can affect quality of life, the prevention of food allergy and asthma is particularly important because these can be life-threatening diseases.
The purpose of this article is to summarize the results of 5 SRs conducted to answer the following questions:
What is the relationship between never versus ever feeding human milk and food allergies, allergic rhinitis, atopic dermatitis, and asthma?
What is the relationship between shorter versus longer durations of any human milk feeding and food allergies, allergic rhinitis, atopic dermatitis, and asthma?
What is the relationship between shorter versus longer durations of exclusive human milk feeding prior to the introduction of infant formula and food allergies, allergic rhinitis, atopic dermatitis, and asthma?
What is the relationship between feeding a lower versus higher intensity, proportion, or amount of human milk to mixed-fed infants and food allergies, allergic rhinitis, atopic dermatitis, and asthma?
What is the relationship between feeding a higher intensity, proportion, or amount of human milk by bottle versus by breast and food allergies, allergic rhinitis, atopic dermatitis, and asthma?
Methods
The Nutrition Evidence Systematic Review (NESR) team (previously the Nutrition Evidence Library, or NEL), which consisted of analysts and librarians who were trained in SR methodology and had advanced degrees in fields such as nutrition and library science, collaborated with a group of subject matter experts, called a Technical Expert Collaborative (TEC), to complete SRs using methods that are described in detail in this supplement (9). TEC members provided individual input on SR materials developed by the NESR staff but did not provide formal group advice or recommendations to the government.
Scope of the systematic review
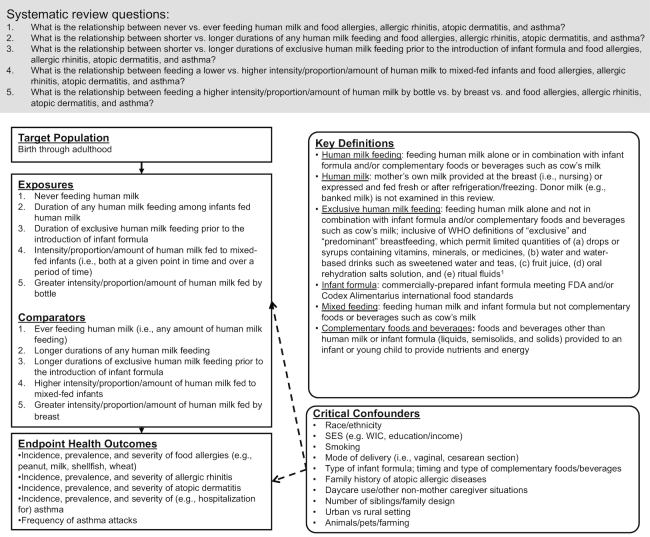
TEC members specified the target population, exposures and comparators, outcomes, critical confounding variables, and key definitions for the SRs according to the analytic framework shown in Figure 1. In the SRs, “infant milk-feeding practices” referred to the feeding of human milk or infant formula (or a combination). TEC members chose to use the term “human milk feeding” instead of “breastfeeding” for precision. “Breastfeeding” may be understood to mean feeding human milk at the breast when, in fact, feeding method was rarely distinguished by the authors of studies included in the SRs. TEC members intended to examine the feeding of human milk whether or not it was fed at the breast.
FIGURE 1.
Analytic framework for the systematic reviews on infant milk-feeding practices and food allergies, allergic rhinitis, atopic dermatitis, and asthma throughout the life span. This framework illustrates the overall scope of the project, including the population, exposures, and comparators and outcomes of interest. It also includes definitions for key terms and identifies key confounders considered in the systematic review. 1WHO. Indicators for assessing infant and young child feeding practices: conclusions of a consensus meeting held 6–8 November 2007 in Washington D.C. Geneva (Switzerland): WHO; 2008. FDA, Food and Drug Administration; SES, socioeconomic status; WIC, Special Supplemental Nutrition Program for Women, Infants, and Children.
For the comparison of never with ever feeding human milk, TEC members did not define any minimum amount for “ever feeding human milk.” Likewise, for the comparisons of shorter with longer durations of any and exclusive human milk feeding, TEC members did not define thresholds for “shorter duration” or “longer duration.” They examined all comparisons of never with ever feeding human milk (or vice versa) and of shorter with longer durations (or vice versa) as defined by the authors of the studies included in the SRs.
The SR question pertaining to the duration of exclusive human milk feeding only examined exclusive human milk feeding preceding the introduction of infant formula. It did not examine the duration of exclusive human milk feeding preceding the time of introduction of complementary foods and beverages (e.g., 4 compared with 6 mo). This was done to avoid overlap with another SR in the P/B-24 Project that examined the timing of the introduction of complementary foods and beverages and its relationship with food allergies, allergic rhinitis, atopic dermatitis, and asthma (10).
Literature search, screening, and selection
The librarians developed a literature search strategy that used exposure terminology but not outcome terminology (available at https://nesr.usda.gov) so that one search could be used to identify literature in support of SRs examining infant milk-feeding practices with several different outcomes (4). The librarians conducted a broad search in CINAHL, Cochrane, Embase, and PubMed using the search date range of January 1980–March 2016. The search excluded articles published before 1980 because the US Congress passed the Infant Formula Act in 1980, which established nutrient requirements for commercial infant formulas in the United States and thus health effects associated with formula consumption before 1980 might be different (11).
TEC members defined inclusion and exclusion criteria a priori (Table 1), which NESR analysts used to dual-screen the search results and the results of a manual search of the references of included articles and existing SRs. TEC members reviewed the search terms and list of included articles to ensure completeness of the body of evidence.
TABLE 1.
Inclusion and exclusion criteria established for the selection of studies to include in the systematic reviews on infant milk-feeding practices and food allergies, allergic rhinitis, atopic dermatitis, and asthma1
| Category | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Study design | Randomized controlled trials; nonrandomized controlled trials; prospective cohort studies; retrospective cohort studies; case-control studies | Cross-sectional studies; before-and-after studies; uncontrolled studies; narrative reviews; systematic reviews; meta-analyses |
| Publication status | Published in peer-reviewed journals | Gray literature, including unpublished data, manuscripts, reports, abstracts, and conference proceedings |
| Language | Published in English | Published in languages other than English |
| Date range | Published 1980–December 20152 | Published prior to 1980 |
| Source of foods, beverages, or nutrients | Human milk: mother's own milk (MOM), i.e., human milk fed at the breast or expressed and fed fresh or after refrigeration/freezing; infant formula: commercially prepared infant formula meeting FDA (87) or Codex Alimentarius (88) food standards | Human milk from third parties (e.g., banked/donor milk); infant formulas that are not commercially prepared or that do not meet FDA (87) or Codex Alimentarius (88) food standards |
| Study setting | Countries listed as Very High or High on the 2014 Human Development Index3 (86) | Countries listed as Medium or Low on the 2014 Human Development Index (86) |
| Study participants | Human participants; males, females | Nonhuman participants (e.g., animal studies, in vitro studies); hospitalized patients, not including birth and immediate postpartum hospitalization of healthy infants |
| Age of study participants | Exposure age: infants (0–12 mo), toddlers (12–24 mo); outcome age: infants (0–12 mo) and toddlers (12–24 mo) for food allergies, allergic rhinitis, and atopic dermatitis, and children (2–12 y), adolescents (13–18 y), and adults (≥19 y) for all outcomes | Outcome age: infants (0–12 mo) and toddlers (12–24 mo) for asthma outcomes, only, as outcomes in this age group may represent transient recurrent wheeze (89) |
| Size of study groups | Studies with ≥ 30 participants per study group or a power analysis indicating that the study is appropriately powered for the outcome(s) of interest | Studies with <30 participants per study group with no power analysis indicating that the study is appropriately powered for the outcome(s) of interest |
| Health status of study participants | Studies done in generally healthy populations; studies done in populations where infants were full term (≥37 and 0/7 wk gestational age); studies done in populations with elevated chronic disease risk, or that enroll some participants with a disease or with the health outcome of interest | Studies that exclusively enroll participants with a disease or the health outcome of interest; studies done in hospitalized participants (except for birth and immediate postpartum hospitalization of healthy infants) or malnourished participants; studies in exclusively preterm infants (gestational age <37 wk), exclusively infants who have low birth weight (<2500 g) or exclusively infants who are small for gestational age |
1FDA, Food and Drug Administration.
2In 1980 the Infant Formula Act was passed (11) and December 2015 was when the literature search was performed.
3When a country was not included in the Human Development Index ranking, country classification from the World Bank was used instead.
Data extraction and risk-of-bias assessment
NESR analysts assembled a table of systematically extracted data from each article included in the SRs (i.e., study characteristics, sample characteristics, exposures and outcomes, risks of bias, and funding sources). Two NESR analysts independently completed the NEL Bias Assessment Tool for each article to identify the risks of bias (9) (https://nesr.usda.gov).
Evidence synthesis, conclusion statement development, and grading the strength of the evidence
NESR analysts and TEC members engaged in a series of conference calls to review, discuss, and synthesize the evidence by age group. TEC members examined both significant and nonsignificant associations (e.g., ORs and CIs) for a thorough synthesis of the evidence. To answer the SR questions, conclusion statements were carefully constructed to accurately reflect the synthesis of evidence. Conclusion statements do not draw implications, nor should they be interpreted to be dietary guidance. The strength of the evidence underlying each conclusion statement was graded strong, moderate, limited, or grade not assignable according to the NESR grading rubric (9) (https://nesr.usda.gov), which takes into consideration the internal validity, consistency, adequacy, impact, and generalizability of the evidence. Finally, TEC members identified research recommendations.
Results
The literature search yielded 31,335 articles, and the bodies of evidence for the 5 SRs on infant milk-feeding practices and food allergies, allergic rhinitis, atopic dermatitis, and asthma comprise 73 articles. A table of articles excluded during full-text screening, with the rationale for exclusion, is available at https://nesr.usda.gov.
None of the included articles examined the intensity, proportion, or amount of human milk fed to mixed-fed infants or fed by bottle versus by breast, and only 1 article (12) examined the duration of exclusive human milk feeding prior to the introduction of infant formula. Additional information about these 3 SRs is available at https://nesr.usda.gov. Herein, we present evidence for the remaining 2 SRs:
What is the relationship between never versus ever feeding human milk and food allergies, allergic rhinitis, atopic dermatitis, and asthma?
What is the relationship between shorter versus longer durations of any human milk feeding and food allergies, allergic rhinitis, atopic dermatitis, and asthma?
Never versus ever feeding human milk and food allergies, allergic rhinitis, atopic dermatitis, and asthma throughout the life span
Forty-four articles met the inclusion criteria for this SR question (12–55). None examined food allergies or atopic dermatitis in adolescence or adulthood, allergic rhinitis in age groups other than childhood, or asthma in adulthood, and TEC members concluded that the scant evidence with methodologic limitations was insufficient to determine whether never versus ever being fed human milk is associated with food allergies from birth through childhood (13–17), allergic rhinitis in childhood (18, 19), or asthma in adolescence (20). Additional information about these topics is available at https://nesr.usda.gov. Evidence about asthma in childhood and atopic dermatitis from birth through childhood is presented below.
Asthma in childhood
Twenty-one articles presented evidence about never versus ever being fed human milk and asthma in childhood (17–19, 21–38) (Table 2). The evidence differed between the studies that included children only and the studies that included children as well as adolescents.
TABLE 2.
Evidence examining the relationship between never versus ever feeding human milk and asthma in childhood1
| First author, year (ref) | Study design (study/cohort name when applicable) | Country | Notable sample characteristics | Never vs. ever feeding human milk exposure2 | Significant associations with asthma | Nonsignificant associations with asthma |
|---|---|---|---|---|---|---|
| Arshad, 2005 (21) | Prospective cohort (IOW) | UK |
n = 1373 Baseline: birth Sex: NR Race/ethnicity: NR |
Exclusive FF vs. not | None | Current diagnosis of asthma at 10 y: NS (data NR) |
| Burr, 1993 (19) | Prospective cohort3 | UK |
n = 453 Baseline: birth Race/ethnicity: NR Risk: 100% family history (parent or sibling) |
Ever BF vs. never BF | None | Proportion of infants ever BF vs. never BF with asthma by 7 y (parents’ report): 23% vs. 20%, NS Proportion of infants ever BF vs. never BF with asthma by 7 y (diagnosis): 27% vs. 34%, NS |
| Colen, 2014 (37) | Prospective cohort (National Longitudinal Study of Youth 1979 Cohort) | USA |
n = 8237 in the full sample, 7319 in the sibling subsample, 1773 in the discordant sibling subsample (i.e., siblings fed differently in infancy) Baseline: birth Race/ethnicity: 74.49% non-Hispanic white, 17.28% non-Hispanic black, 8.23% Hispanic |
BF vs. not BF | Asthma at 4–14 y (full sample between-family estimate): β = 0.261 (SE = 0.106), P < 0.05 Asthma at 4–14 y (sibling subsample between-family estimate): β = 0.237 (SE = 0.117), P < 0.05 |
Proportion of infants BF vs. not BF with asthma at 4–14 y (discordant sibling subsample): 7.95% vs. 8.89%, NS Asthma at 4–14 y (sibling subsample within-family estimate): β = 0.023 (SE = 0.222), NS |
| Hillemeier, 2015 (22) | Prospective cohort (ECLS-B) | USA |
n = 6900 Baseline: birth Race/ethnicity: ∼53% non-Hispanic white, ∼11.5% Mexican with ≥ 1 foreign-born parent, ∼7% Mexican with 2 US-born parents, ∼7% other Hispanic, ∼14% African American, ∼2.5% Asian American, ∼4.5% other race |
BF vs. FF | None | Asthma diagnosis by age 60 mo: OR: 0.87 (95% CI: 0.70, 1.07) Number of asthma attacks from 24 to 60 mo among children with asthma: β = –0.20, NS Taking prescription medicine for asthma at 48 or 60 mo among children with asthma: OR: 0.90, NS Asthma hospitalization or emergency room visit from 24 to 60 mo among children with asthma: OR: 0.62, P < 0.1 |
| Infante-Rivard 1993 (23) | Case-control | Canada |
n = 457 cases, 457 controls Baseline: 3–4 y Race/ethnicity: NR |
No BF vs. BF | Asthma at age 3–4 y: OR: 1.47 (95% CI: 1.02, 2.13) | None |
| Infante-Rivard, 2001 (24) | Case-control | Canada |
n = 404 cases from the 1993 study (294 with persistent asthma, 110 with transient asthma), 457 controls Baseline: 3–4 y Race/ethnicity: NR |
No BF vs. any duration of BF | None | Persistent asthma from age 3–4 to 9–11 y: OR: 1.30 (95% CI: 0.85, 2.01) Transient asthma from age 3–4 to 9–11 y: OR: 1.17 (95% CI: 0.60, 2.28) |
| Larsson, 2008 (18) | Prospective cohort (DBH) | Sweden |
n = 4779 in the full sample without asthma at baseline, 3320 in the subsample without wheezing at baseline, 935 in the subsample with wheezing at baseline Baseline: 1–4 y Race/ethnicity: NR |
No BF vs. BF >6 mo | 5-y cumulative incidence of asthma by age 6–9 y in the full sample with no asthma at baseline: OR: 2.64 (95% CI: 1.28, 5.46) 5-y cumulative incidence of asthma by age 6–9 y in the subsample with no asthma and no wheezing ever at baseline: OR: 2.64 (95% CI: 1.18, 5.93) |
None |
| 5-y cumulative incidence of asthma by age 6–9 y in the subsample with no asthma, but with wheezing at baseline: OR: 4.08 (95% CI: 1.21, 13.72) | ||||||
| Leung, 2016 (25) | Prospective cohort (Children of 1997 Birth Cohort) | Hong Kong |
n = 8301 Baseline: first postnatal visit Race/ethnicity: NR |
Partially BF for any length of time or EBF <3 mo vs. never BF | None | Public hospital admissions for asthma from >3 mo to 12 y: HR: 1.12 (95% CI: 0.87, 1.43) Public hospital admissions for asthma from >3 mo to 2 y: HR: 0.63 (95% CI: 0.32, 1.25) Public hospital admissions for asthma from >2 to 6 y: HR: 1.14 (95% CI: 0.85, 1.53) Public hospital admissions for asthma from >3 mo to 6 y: HR: 1.03 (95% CI: 0.79, 1.35) Public hospital admissions for asthma from >6 to 12 y: HR: 0.87 (95% CI: 0.41, 1.83) |
| EBF ≥3 mo vs. never BF | None | Public hospital admissions for asthma from >3 mo to 12 y: HR: 1.27 (95% CI: 0.82, 1.98) Public hospital admissions for asthma from >3 mo to 2 y: HR: 1.69 (95% CI: 0.64, 4.50) Public hospital admissions for asthma from >2 to 6 y: HR: 1.11 (95% CI: 0.64, 1.90) Public hospital admissions for asthma from >3 mo to 6 y: HR: 1.19 (95% CI: 0.74, 1.92) Public hospital admissions for asthma from >6 to 12 y: HR: 1.05 (95% CI: 0.35, 3.19) |
||||
| Maas, 2011 (26) | Prospective cohort3 (PREVASC) | Netherlands |
n = 387 Baseline: Birth Race/ethnicity: NR |
BF 1–11 wk vs. BF 0 wk | None | Allergic asthma at 6 y: OR: 0.385 (95% CI: 0.145, 1.003) |
| BF 12–25 wk vs. BF 0 wk | Allergic asthma at 6 y: OR: 0.247 (95% CI: 0.093, 0.655) | None | ||||
| BF ≥26 wk vs. BF 0 wk | None | Allergic asthma at 6 y: OR: 0.523 (95% CI: 0.187, 1.458) | ||||
| Martel, 2009 (27) | Nested case-control | Canada |
n = 745 cases, 833 controls Baseline: birth Sex: NR Race/ethnicity: NR Risk: 85% family history (mothers) |
BF <6 mo vs. no BFBF ≥6 mo vs. no BF | Asthma in childhood (maximum age 10 y): OR: 0.70 (95% CI: 0.53, 0.92)None | NoneAsthma in childhood (maximum age 10 y): OR: 0.77 (95% CI: 0.57, 1.03) |
| Midodzi, 2010 (28) | Prospective cohort (NLSCY ECD) | Canada |
n = 8499 Baseline: <2 y Race/ethnicity: NR |
BF 0–3 mo vs. never BFBF >3 mo vs. never BF | NoneAsthma at 2–5 y: HR: 0.82 (95% CI: 0.69, 0.97) | Asthma at 2–5 y: HR: 0.85 (95% CI: 0.70, 1.00)None |
| Mihrshahi, 2007 (29) | Prospective cohort3 (CAPS) | Australia |
n = 516 Baseline: birth Sex: NR Race/ethnicity: NR |
Ever BF vs. never BF | None | Probable current asthma at 5 y: OR: 0.59 (95% CI: 0.30, 1.16) |
| Miller, 2001 (30) | Prospective or retrospective cohort depending on the analysis (NMIHS/LF) | USA |
n = 3883 Baseline: birth Race/ethnicity: 55.0% non-Hispanic white, 45.0% non-Hispanic black |
BF vs. no BF | Asthma by 3 y (maternal report): OR: 0.68 (95% CI: 0.49, 0.97) | Asthma by 3 y (physician report from retrospective medical chart review): OR: 0.94 (95% CI: 0.70, 1.26) |
| Milner, 2004 (17) | Prospective cohort (NMIHS/LF) | USA |
n = 8073 Baseline: birth Race/ethnicity: 51% black, 46% white, 3% other |
Ever BF vs. never BF | Asthma at 3 y (maternal report): OR: 0.61 (95% CI: 0.52, 0.71) | None |
| Nwaru, 2013 (31) | Prospective cohort (SEATON) | UK |
n = 934, subsamples with and without family history of atopy NR Baseline: birth Race/ethnicity: NR |
Ever BF vs. no BF | None | Asthma by 10 y: OR: 0.81 (95% CI: 0.59, 1.13) Asthma up to age 10 y in subsample with no family history of atopy: OR: 0.80 (95% CI: 0.42, 1.55) Asthma up to age 10 y in subsample with family history of atopy: OR: 0.78 (95% CI: 0.53, 1.14) |
| BF <2.25 mo vs. no BF | None | Asthma by 10 y: OR: 0.90 (95% CI: 0.61, 1.35) Asthma up to age 10 y in subsample with no family history of atopy: OR: 1.25 (95% CI: 0.59, 2.66) Asthma up to age 10 y in subsample with family history of atopy: OR: 0.77 (95% CI: 0.49, 1.23) |
||||
| BF ≥2.25 mo vs. no BF | None | Asthma by 10 y: OR: 0.76 (95% CI: 0.53, 1.09) Asthma up to age 10 y in subsample with no family history of atopy: OR: 0.58 (95% CI: 0.27, 1.23) Asthma up to age 10 y in subsample with family history of atopy: OR: 0.77 (95% CI: 0.51, 1.17) |
||||
| Orivuori, 2014 (32) | Prospective cohort (PASTURE) | Finland, France, Germany, Switzerland |
n = 853 Baseline: birth Race/ethnicity: NR |
Never BF vs. BF >6 mo | None | Asthma at 4 y: OR: 1.22 (95% CI: 0.32, 4.63) Asthma between 4 and 6 y: OR: 0.69 (95% CI: 0.22, 2.21) |
| Rosas-Salazar, 2015 (38) | Case-control | USA |
n = 509 cases, 618 controls Baseline: mean: 10 y (range: 6–14 y) Race/ethnicity: 100% Puerto Rican |
BF 0–6 mo vs. no BFBF >6 mo vs. no BFBF 0–2 mo vs. no BF | Asthma at 6–14 y: OR: 0.7 (95% CI: 0.5, 1.0), P = 0.04NoneAsthma at 6–14 y: OR: 0.6 (95% CI: 0.5, 0.9) | NoneAsthma at 6–14 y: OR: 1.5 (95% CI: 1.0, 2.4), P = 0.06None |
| BF 2–4 mo vs. no BF | None | Asthma at 6–14 y: OR: 0.9 (95% CI: 0.6, 1.3) | ||||
| BF 4–6 mo vs. no BF | None | Asthma at 6–14 y: OR: 0.6 (95% CI: 0.2, 1.8) | ||||
| BF 6–8 mo vs. no BF | None | Asthma at 6–14 y: OR: 1.6 (95% CI: 0.9, 3.0) | ||||
| BF 8–10 mo vs. no BF | None | Asthma at 6–14 y: OR: 2.9 (95% CI: 0.3, 25.2) | ||||
| BF 10–12 mo vs. no BF | None | Asthma at 6–14 y: OR: 1.5 (95% CI: 0.6, 3.4) | ||||
| BF >12 mo vs. no BF | None | Asthma at 6–14 y: OR: 1.4 (95% CI: 0.6, 3.1) | ||||
| Scholtens, 2009 (33) | Prospective cohort (PIAMA) | Netherlands |
n = 3115 in the full sample, 2238 in the subsample with nonallergic mothers, 877 in the subsample with allergic mothers Baseline: birth Race/ethnicity: NR |
BF 1–16 wk vs. no BFBF >16 wk vs. no BF | NoneAsthma at 3 y: OR: ∼0.65 (95% CI: ∼0.50, ∼0.85) Asthma at 3 y in subsample with nonallergic mothers: OR: ∼0.70 (95% CI: ∼0.50, ∼1.00) Asthma at 3 y in subsample with allergic mothers: OR: ∼0.55 (95% CI: ∼0.35, ∼0.90) Asthma at 5 y: OR: ∼0.50 (95% CI: ∼0.55, ∼0.90) Asthma at 5 y in subsample with nonallergic mothers: OR ∼0.70 (95% CI: ∼0.50, ∼1.00) Asthma at 6 y: OR: ∼0.60 (95% CI: ∼0.45, ∼0.80) Asthma at 6 y in subsample with nonallergic mothers: OR: ∼0.50 (95% CI: ∼0.35, ∼0.75) Asthma at 7 y: OR: ∼0.65 (95% CI: ∼0.50, ∼0.90) Asthma at 7 y in subsample with non-allergic mothers: OR: ∼0.65 (95% CI: ∼0.45, ∼0.95) Asthma at 8 y: OR: 0.57 (95% CI: 0.41, 0.80); chronic asthma by 8 y: OR: 0.65 (95% CI: 0.44, 0.96) Asthma at 8 y in subsample with nonallergic mothers: OR: ∼0.50 (95% CI: ∼0.35, ∼0.75) |
Asthma at 8 y: OR: 0.82 (95% CI: 0.61, 1.09) Chronic asthma by 8 y: OR: 0.87 (95% CI: 0.62, 1.24)Asthma at 4 y: OR: ∼0.80 (95% CI: ∼0.60, ∼1.05) Asthma at 4 y in subsample with nonallergic mothers: OR: ∼0.75 (95% CI: ∼0.55, ∼1.10) Asthma at 4 y in subsample with allergic mothers: OR: ∼0.85 (95% CI: ∼0.50, ∼1.35) Asthma at 5 y in subsample with allergic mothers: OR: ∼0.60 (95% CI: ∼0.40, ∼1.05) Asthma at 6 y in subsample with allergic mothers: OR: ∼0.75 (95% CI: ∼0.45, ∼1.30) Asthma at 7 y in subsample with allergic mothers: OR: ∼0.65 (95% CI: ∼0.40, ∼1.10) Asthma at 8 y in subsample with allergic mothers: OR: ∼0.80 (95% CI: ∼0.50, ∼1.35) |
| Sunyer, 2006 (34) | Prospective cohort | Spain |
n = 462 Baseline: birth Sex: NR Race/ethnicity: NR |
BF vs. no BF | Asthma at 6.5 y: OR: 0.33 (95% CI: 0.08, 0.87) | None |
| van Beijsterveldt, 2008 (35) | Prospective cohort | Netherlands |
n = 23,444 Baseline: birth Sex: NR Race/ethnicity: NR |
BF 0.5–3 mo vs. no BFBF >3 mo vs. no BF | NoneNone | Asthma by 5 y: OR: 0.96 (95% CI: 0.83, 1.09)Asthma by 5 y: OR: 0.92 (95% CI: 0.79, 1.08) |
| Wilson, 1998 (36) | Prospective cohort (Dundee Infant Feeding Study) | UK |
n = 545 Baseline: birth Race/ethnicity: NR |
Bottle feeding vs. partial BF | None | Percentage probability of asthma by 7 y: 18.6% (95% CI: 17.2%, 20.0%) vs. 21.7% (95% CI: 17.3%, 26.1%) |
| Bottle feeding vs. EBF | Percentage probability of asthma by 7 y: 18.6% (95% CI: 17.2%, 20.0%) vs. 12.1% (95% CI: 10.9%, 13.4%)4 | None |
1β, regression coefficient; BF, breastfed/breastfeeding; CAPS, Childhood Asthma Prevention Study; DBH, Dampness in Buildings and Health; EBF, exclusively breastfed/exclusive breastfeeding; ECLS-B, Early Childhood Longitudinal Study Birth Cohort; FF, formula fed/formula feeding; IOW, Isle of Wight; NLSCY ECD, Canadian National Longitudinal Study of Children and Youth Early Childhood Development Cohort; NMIHS/LF, 1988 National Maternal and Infant Health Survey and 1991 Longitudinal Follow-up; NR, not reported; PASTURE, Protection Against Allergy Study in Rural Environments; PIAMA, Prevention and Incidence of Asthma and Mite Allergy; PREVASC, Prevention of Asthma in Children; ref, reference; SEATON, Study of Eczema and Asthma to Observe the Influence of Nutrition; SR, systematic review.
2Exposures, as defined by the authors of the studies included in the body of evidence, which address never versus ever feeding human milk or vice versa.
3The cohort was sampled from a randomized controlled trial; however, the data of interest for this SR are unrelated to randomization.
4Study authors stated that there were no differences between feeding groups but the CIs do not overlap.
Evidence in children only
Nineteen articles examined asthma in children only (17–19, 21–36). These articles presented evidence from 14 prospective cohort studies (18, 19, 21, 22, 25, 26, 28, 29, 31–36), 1 additional cohort study with prospective and retrospective analyses in separate articles (17, 30), 1 nested case-control study (27), and 1 case-control study with unique evidence across 2 articles (23, 24) (i.e., there were 17 independent studies in total). Data about infant milk-feeding practices were collected from parent report by questionnaire, interview, or diary, and studies compared infants who never consumed human milk with infants ever fed human milk (17, 19, 21–25, 29–31, 34, 36), fed human milk for heterogeneous ranges of duration (18, 26–28, 31–33, 35), and fed human milk exclusively for ≥3 mo (25) or until weaning (36). Asthma outcomes were based on medical record (23–25, 27, 30), clinical assessment (19, 26, 29), parent report of a diagnosis or symptoms (17–19, 22, 28–30, 32–36), and parent report via the validated instrument (56) from the International Study of Asthma and Allergy in Childhood (ISAAC) (18, 31).
Statistically significant associations were reported by 9 studies across 11 articles (17, 18, 23, 24, 26–28, 30, 33, 34, 36). The studies provided consistent evidence of an association between never versus ever being fed human milk and higher asthma risk and suggested that the predominant difference between the statistically significant and nonsignificant associations was statistical power.
Seven of the studies that found significant associations were prospective cohort studies. Specifically, Larsson et al. (18) compared children never fed human milk with children fed human milk for >6 mo and found higher odds of asthma during the 5-y observation period in the full sample of children with no asthma at baseline (1–4 y of age; OR: 2.64; 95% CI: 1.28, 5.46) and in subsamples of children who did and did not have wheezing at baseline [OR (95% CI): 4.08 (1.21, 13.72) and 2.64 (1.18, 5.93), respectively]. Maas et al. (26) found lower odds of allergic asthma at 6 y of age when being fed human milk for 12–25 wk was compared with not being fed human milk (OR: 0.247; 95% CI: 0.093, 0.655); and when the exposure was being fed human milk for 1–11 wk, the upper limit of the CI was 1.003. A comparison of being fed human milk for ≥26 wk with not being fed human milk had a nonsignificant association in the same direction with a wide CI indicative of suboptimal statistical power. Midodzi et al. (28) reported a lower HR for asthma at 2–5 y of age when children who were fed human milk for >3 mo were compared with those who were never fed human milk (HR: 0.82; 95% CI: 0.69, 0.97). Further, when the exposure was being fed human milk for 0–3 mo, the upper limit of the CI around the nonsignificant association was 1.00. Miller et al. (30) and Milner et al. (17) examined the same cohort. In prospective analyses, ever being fed human milk was associated with lower odds of asthma by maternal report at (17) and by (30) 3 y of age [OR (95% CI): 0.61 (0.52, 0.71) and 0.68 (0.49, 0.97), respectively]. When Miller et al. (30) used physician reports from a retrospective medical chart review to determine whether children had been diagnosed with asthma by 3 y of age, the association was in the same direction but was not statistically significant (OR: 0.94; 95% CI: 0.70, 1.26). The authors described several plausible reasons for the discrepancy between maternal report and medical records, including response rate (i.e., the presence of maternal report when medical providers did not respond), health care utilization (i.e., the presence of maternal report when a medical record did not exist due to socioeconomic factors), and medical coding (e.g., for ease, providers may have coded reactive airway disease as asthma in medical charts); however, an additional explanation is that the analyses in both articles were unadjusted and may have been prone to confounding. Scholtens et al. (33) examined asthma in the full sample and subsamples of children with allergic and nonallergic mothers. The full-sample analyses found lower odds of asthma in 3-, 5-, 6-, 7-, and 8-y-old children (ORs between 0.50 and 0.65) and lower odds of chronic asthma by 8 y of age (OR: 0.65; 95% CI: 0.44, 0.96) in children fed human milk for >16 wk than in those not fed human milk, and the association at 4 y of age was in the same direction but not statistically significant. The subsample analyses of children with nonallergic mothers were very similar, although chronic asthma by 8 y of age was not assessed. In the subsample analyses of children with allergic mothers, the associations were in the same direction but not statistically significant, with the exception of a lower odds of asthma at 3 y of age. Sunyer et al. (34) found that children fed human milk had lower odds of asthma at 6.5 y of age than children never fed human milk (OR: 0.33; 95% CI: 0.08, 0.87). Wilson et al. (36) reported that the percentage probability of asthma by 7 y of age was higher in children never fed human milk (18.6%; 95% CI: 17.2%, 20.0%) than in children who were exclusively fed human milk (12.1%; 95% CI: 10.9%, 13.4%). However, a comparison of never with partial feeding of human milk was not statistically significant.
In a nested case-control study, Martel et al. (27) examined a sample in which ∼85% of both cases and controls had mothers with a history of asthma and found lower odds of childhood asthma up to 10 y of age when being fed human milk for <6 mo was compared with never being fed human milk (OR: 0.70; 95% CI: 0.53, 0.92). When being fed human milk for ≥6 mo was compared with never being fed human milk, the upper limit of the CI was 1.03.
Finally, in a case-control study, Infante-Rivard et al. (23) compared never with ever being fed human milk and reported higher odds of asthma at 3–4 y of age (OR: 1.47; 95% CI: 1.02, 2.13). When the study examined asthma that persisted from 3–4 y of age to 9–11 y of age, and transient asthma that was present at 3–4 y of age but not at 9–11 y of age (24), the associations were in the same direction but had wider CIs indicative of suboptimal statistical power [OR (95% CI): 1.30 (0.85, 2.01) and 1.17 (0.60, 2.28), respectively].
The majority of nonsignificant associations were consistent in direction with the significant associations (22, 24, 26–31, 33, 35), suggesting that never being fed human milk was associated with higher risk of asthma, and some of the nonsignificance was due to inadequate power. The minority of nonsignificant associations were discrepant (19, 25, 32) or did not report point estimates so direction could not be assessed (21). Some of the nonsignificant associations were from unadjusted analyses (19, 21, 35) and may have been prone to confounding.
Evidence from studies including both children and adolescents together
A prospective cohort study (37) and a case-control study (38) examined asthma in children and adolescents together. Both studies reported statistically significant associations, but the evidence was inconclusive. Colen et al. (37) found a significant association between ever compared with never being fed human milk and higher risk of asthma at 4–14 y in the full sample (β: 0.261; SE: 0.106; P < 0.05) and also among the subsample of children who were siblings (β: 0.237; SE: 0.117; P < 0.05). Of note, they also conducted a within-family analysis of siblings with discordant exposures (i.e., 1 sibling was fed human milk and the other was not), and this analysis found no difference in the proportion of siblings fed human milk compared with not being fed human milk who had asthma at 4–14 y, suggesting that residual confounding explained the significant difference observed in the between-family analyses. The case-control study by Rosas-Salazar et al. (38) reported conflicting results. When compared with children and adolescents never fed human milk, the children and adolescents fed human milk for <6 mo had lower odds of asthma (OR: 0.7; 95% CI: 0.5, 1.0; P = 0.04). On the other hand, there was a nonsignificant association between being fed human milk for ≥6 mo and higher odds of asthma that had a wide CI with a lower limit of 1.0.
To summarize, all 9 studies with statistically significant associations in childhood suggested that never versus ever being fed human milk was associated with higher risk of asthma (17, 18, 23, 24, 26–28, 30, 33, 34, 36). The nonsignificant associations provided further evidence of a relationship between never being fed human milk and asthma in childhood because they were mostly consistent in direction with the significant associations (22, 24, 26–31, 33, 35), and some of the analyses appeared to be underpowered. The evidence from studies examining children and adolescents together was mixed (37, 38), and it was difficult to determine whether or not the heterogeneous associations were due to the inclusion of adolescents in the samples because evidence in adolescents alone was scant.
Atopic dermatitis from birth to 24 mo
Sixteen articles presented inconclusive evidence on never versus ever being fed human milk and atopic dermatitis during the B-24 period (12, 13, 32, 39–51) (Table 3). The small number of significant associations was inconsistent in direction. Ivakhnenko et al. (13) found that a larger proportion of infants fed infant formula than fed human milk had atopic dermatitis by 18 mo of age (16.98% compared with 3.92%, P < 0.05), whereas Chuang et al. (40) found that being fed human milk for durations of ≤1, ≤2, ≤6, ≤12, and >12 mo (compared with not being fed human milk) was associated with higher odds of atopic dermatitis between 6 and 18 mo of age (ORs between 1.25 and 1.49). In a third study, Snijders et al. (49) found that being fed human milk for >9 mo (compared with never being fed human milk) was associated with lower odds of eczema by 2 y of age in a subsample of children whose mothers had no allergies or asthma (OR: 0.51; 95% CI: 0.29, 0.89). However, the significant association was limited to 1 (49) of the 3 articles by Snijders et al. (49–51) about the study, and to 1 of the 12 relevant analyses in the article. The nonsignificant associations were also inconsistent in direction, with no discernible trend in the direction of the point estimates (12, 32, 39, 41–48). In addition to the mixed evidence, TEC members had concerns about the specificity of the diagnosis of atopic dermatitis during the B-24 period.
TABLE 3.
Evidence examining the relationship between never versus ever feeding human milk and atopic dermatitis from birth to 24 mo1
| First author, year (ref) | Study design (study/cohort name when applicable) | Country | Notable sample characteristics | Never vs. ever feeding human milk exposure2 | Significant associations with atopic dermatitis | Nonsignificant associations with atopic dermatitis |
|---|---|---|---|---|---|---|
| Burr, 1989 (39) | Prospective cohort3 | UK |
n = 483 Baseline: birth Race/ethnicity: NR Risk: 100% family history (parent or sibling) |
BF at some time vs. never BF | None | Proportion of infants BF at some time vs. never BF with eczema symptoms by 12 mo: 35% vs. 40%, NS |
| Chuang, 2011 (40) | Prospective cohort (TBCS) | Taiwan |
n = 20,172 Baseline: birth Race/ethnicity: NR Risk: 0% with atopic dermatitis by 6 mo (exclusion criterion) |
BF ≤1 mo vs. no BFBF ≤2 mo vs. no BFBF ≤6 mo vs. no BF | Atopic dermatitis between 6 and 18 mo: OR: 1.25 (95% CI: 1.00, 1.56), P = 0.049Atopic dermatitis between 6 and 18 mo: OR: 1.37 (95% CI: 1.08, 1.74), P = 0.009Atopic dermatitis between 6 and 18 mo: OR: 1.47 (95% CI: 1.17, 1.84), P = 0.001 | NoneNoneNone |
| BF ≤12 mo vs. no BF | Atopic dermatitis between 6 and 18 mo: OR: 1.44 (95% CI: 1.11, 1.87), P = 0.006 | None | ||||
| BF >12 mo vs. no BF | Atopic dermatitis between 6 and 18 mo: OR: 1.49 (95% CI: 1.15, 1.93), P = 0.003 | None | ||||
| Harris, 2001 (41) | Prospective cohort | UK |
n = 624 Baseline: birth Race/ethnicity: NR |
Ever BF vs. never BF | None | Doctor-diagnosed eczema by 2 y: OR: 0.97 (95% CI: 0.64, 1.47), P = 0.89 Visible dermatitis by 2 y: OR: 1.25 (95% CI: 0.67, 2.35), P = 0.47 Maternally reported eczema by 2 y: OR: 0.93 (95% CI: 0.63, 1.36), P = 0.71 |
| Howie, 1990 (42) | Prospective cohort | UK |
n = 617 Baseline: birth Sex: NR Race/ethnicity: NR |
Bottle feeders (FF) vs. early weaners (BF <13 wk) | None | Eczema from birth to 13 wk: χ2: 2.36 (95% CI: NR), NS Eczema from 14 to 26 wk: χ2: 1.52 (95% CI: NR), NS Eczema from 27 to 39 wk: χ2: 0.02 (95% CI: NR), NS Eczema from 40 to 52 wk: χ2: 0.15 (95% CI: NR), NS |
| Bottle feeders (FF) vs. breast feeders (BF ≥13 wk) | None | Eczema from birth to 13 wk: χ2: 0.77 (95% CI: –3.2, 2.4) Eczema from 14 to 26 wk: χ2: 0.89 (95% CI: –1.3, 6.6) Eczema from 27–39 wk: χ2: 0.58 (95% CI: –3.6, 3.6) Eczema from 40 to 52 wk: χ2: 0.12 (95% CI: –3.5, 4.6) |
||||
| Ivakhnenko, 2013 (13) | Nonrandomized controlled trial4 | Ukraine |
n = 104 Baseline: birth Sex: NR Race/ethnicity: NR |
BF vs. standard FF | Proportion of infants BF vs. standard FF with atopic dermatitis during the first 18 mo: 3.92% vs. 16.98%, P < 0.05 | None |
| Mallet, 1992 (43) | Prospective cohort3 | France |
n = 139 Baseline: birth Sex: NR Race/ethnicity: NR Risk: 100% family history (≥1 parent or sibling) |
FF alone vs. FF with BF | None | Eczema at 2 y: NS (data NR) |
| Miskelly, 1988 (44) | Prospective cohort3 | UK |
n = 487 Baseline: birth Race/ethnicity: NR Risk: 100% family history (≥1 parent or sibling) |
Ever BF vs. never BF | None | Eczema in the first year of life: 35% vs. 40%, NS |
| Morales, 2012 (45) | Prospective cohort (INMA Project) | Spain |
n = 580 Baseline: birth Race/ethnicity: NR |
Predominant BF <2 mo vs. never BF | None | Atopic eczema from birth to 6 mo: OR: ∼0.7 (95% CI: ∼0.3, ∼1.5) Atopic eczema from 7 to 14 mo: OR: ∼0.6 (95% CI: ∼0.3, ∼1.3) Recurrent atopic eczema from birth–6 mo to 7–14 mo: OR: ∼0.4 (95% CI: ∼0.0, ∼1.3) Atopic eczema from 0 to 14 mo: OR: 0.67 (95% CI: 0.33, 1.36) Atopic eczema from 7 to 14 mo in subsample of infants with onset ≥7 mo: OR: 0.75 (95% CI: 0.27, 2.08) |
| Predominant BF 2–4 mo vs. never BF | None | Atopic eczema from birth to 6 mo: OR: ∼0.9 (95% CI: ∼0.4, ∼1.9) Atopic eczema from 7 to 14 mo: OR: ∼0.8 (95% CI: ∼0.4, ∼1.5) Recurrent atopic eczema from birth–6 mo to 7–14 mo: OR: ∼0.6 (95% CI: ∼0.2, ∼1.7) Atopic eczema from 0 to 14 mo: OR: 0.90 (95% CI: 0.48, 1.69) Atopic eczema from 7 to 14 mo in subsample of infants with onset ≥7 mo: OR: 0.95 (95% CI: 0.37, 2.44) |
||||
| Predominant BF 4–6 mo vs. never BF | None | Atopic eczema from birth to 6 mo: OR: ∼0.7 (95% CI: ∼0.4, ∼1.3) Atopic eczema from 7 to 14 mo: OR: ∼0.6 (95% CI: ∼0.3, ∼1.1) Recurrent atopic eczema from birth–6 mo to 7–14 mo: OR: ∼0.5 (95% CI: ∼0.2, ∼1.1) Atopic eczema from 0 to 14 mo: OR: 0.72 (95% CI: 0.42, 1.22) Atopic eczema from 7 to 14 mo in subsample of infants with onset ≥7 mo: OR: 0.74 (95% CI: 0.34, 1.63) |
||||
| Predominant BF >6 mo vs. never BF | None | Atopic eczema from 7 to 14 mo: OR: ∼0.6 (95% CI: ∼0.2, ∼1.4) Recurrent atopic eczema from birth–6 mo to 7–14 mo: OR: ∼0.7 (95% CI: ∼0.2, ∼2.0) Atopic eczema from 0 to 14 mo: OR: 0.64 (95% CI: 0.28, 1.44) Atopic eczema from 7 to 14 mo in subsample of infants with onset ≥7 mo: OR: 0.42 (95% CI: 0.11, 1.57) |
||||
| Orivuori, 2014 (32) | Prospective cohort (PASTURE) | Finland, France, Germany, Switzerland |
n = 853 Baseline: birth Race/ethnicity: NR |
Never BF vs. BF >6 mo | None | Atopic dermatitis up to 2 y: OR: 0.84 (95% CI: 0.40, 1.76) |
| Parazzini, 2014 (46) | Prospective cohort | Italy |
n = 796 Baseline: birth Race/ethnicity: NR |
BF only vs. no BFBF/other milks vs. no BF | NoneNone | 12-mo incidence of atopic dermatitis at 12 mo: RR: 1.34 (95% CI: 0.90, 2.02)12-mo incidence of atopic dermatitis at 12 mo: RR: 1.13 (95% CI: 0.71, 1.80) |
| BF only 1–4 mo vs. BF 0 mo | None | 12-mo incidence of atopic dermatitis at 12 mo: RR: 1.28 (95% CI: 0.70, 2.35) | ||||
| BF only ≥5 mo vs. BF 0 mo | None | 12-mo incidence of atopic dermatitis at 12 mo: RR: 1.46 (95% CI: 0.95, 2.25) | ||||
| BF/other milks 1–4 mo vs. BF 0 mo | None | 12-mo incidence of atopic dermatitis at 12 mo: RR: 1.15 (95% CI: 0.72, 1.84) | ||||
| BF/other milks ≥5 mo vs. BF 0 mo | None | 12-mo incidence of atopic dermatitis at 12 mo: RR: 1.39 (95% CI: 0.91, 2.12) | ||||
| Rothenbacher, 2005 (47) | Prospective cohort | Germany |
n = 803 Baseline: birth Race/ethnicity: NR (nationality: 90.0% German, 2.4% Turkish, 7.6% other) |
BF <3 mo vs. never BFBF 3 to <6 mo vs. never BF | NoneNone | Cumulative incidence of atopic dermatitis at 2 y: 27.5% vs. 13.2%, NSCumulative incidence of atopic dermatitis at 2 y: 22.1% vs. 13.2%, NS |
| BF 6 to <9 mo vs. never BF | None | Cumulative incidence of atopic dermatitis at 2 y: 17.9% vs. 13.2%, NS | ||||
| BF ≥9 mo vs. never BF | None | Cumulative incidence of atopic dermatitis at 2 y: 21.3% vs. 13.2%, NS | ||||
| Sariachvili, 2007 (48) | Prospective cohort (PIPO Project) | Belgium |
n = 976 Baseline: birth Race/ethnicity: NR |
Ever BF vs. never BFBF 1–6 mo vs. never BF | NoneNone | Eczema during the first year: OR: 1.3 (95% CI: 0.9, 1.8)Eczema during the first year: OR: 0.8 (95% CI: 0.4, 1.3) |
| BF 7–12 mo vs. never BF | None | Eczema during the first year: OR: 0.8 (95% CI: 0.5, 1.3) | ||||
| BF ≥13 mo vs. never BF | None | Eczema during the first year: OR: 1.0 (95% CI: 0.6, 1.5) | ||||
| Snijders, 2007 (49) | Prospective cohort (KOALA Birth Cohort) | Netherlands |
n = 2516, 1538 in the subsample with mothers with no allergy/no asthma, 652 in the subsample with mothers with allergy/no asthma, 217 in the subsample with mothers with asthma Baseline: birth Race/ethnicity: NR |
BF 0–3 mo vs. never BF | None | Eczema in the first 2 y in subsample with mothers with no allergy, no asthma: OR: 0.90 (95% CI: 0.61, 1.31) Eczema in the first 2 y in subsample with mothers with allergy, no asthma: OR: 0.90 (95% CI: 0.52, 1.57) Eczema in the first 2 y in subsample with mothers with asthma: OR: 1.39 (95% CI: 0.51, 3.78) |
| BF 4–6 mo vs. never BF | None | Eczema in the first 2 y in subsample with mothers with no allergy, no asthma: OR: 0.71 (95% CI: 0.42, 1.20) Eczema in the first 2 y in subsample with mothers with allergy, no asthma: OR: 0.86 (95% CI: 0.43, 1.71) Eczema in the first 2 y in subsample with mothers with asthma: OR: 1.40 (95% CI: 0.44, 4.42) |
||||
| BF 7–9 mo vs. never BF | None | Eczema in the first 2 y in subsample with mothers with no allergy, no asthma: OR: 0.69 (95% CI: 0.40, 1.19) Eczema in the first 2 y in subsample with mothers with allergy, no asthma: OR: 0.68 (95% CI: 0.34, 1.38) Eczema in the first 2 y in subsample with mothers with asthma: OR: 0.70 (95% CI: 0.22, 2.27) |
||||
| BF > 9 mo vs. never BF | Eczema in the first 2 y in subsample with mothers with no allergy, no asthma: OR: 0.51 (95% CI: 0.29, 0.89) | Eczema in the first 2 y in subsample with mothers with allergy, no asthma: OR: 0.63 (95% CI: 0.31, 1.29) Eczema in the first 2 y in subsample with mothers with asthma: OR: 1.67 (95% CI: 0.53, 5.21) |
||||
| Snijders, 2007 (50) | Prospective cohort (KOALA Birth Cohort) | Netherlands |
n = 2405 Baseline: birth Race/ethnicity: NR |
Ever BF vs. never BFBF 0–3 mo vs. never BF | NoneNone | Early development of eczema (0–3 mo): OR: 1.30 (95% CI: 0.96, 1.77)Eczema during first year of life: OR: 0.98 (95% CI: 0.71, 1.34) |
|
Eczema between 4 and 12 mo: OR: 0.98 (95% CI: 0.66, 1.46) |
||||||
| BF 4–6 mo vs. never BF | None | Eczema during first year of life: OR: 0.94 (95% CI: 0.66, 1.34) | ||||
| BF ≥7 mo vs. never BF | None | Eczema during first year of life: OR: 0.80 (95% CI: 0.57, 1.12) | ||||
| BF ≥4 mo vs. never BF | None | Eczema between 4 and 12 mo: OR: 0.77 (95% CI: 0.52, 1.15) | ||||
| Snijders, 2008 (51) | Prospective cohort (KOALA Birth Cohort) | Netherlands |
n = 2434 Baseline: birth Race/ethnicity: NR |
BF 0–3 mo vs. never BF | None | Eczema (parent report) by 2 y: OR: 1.00 (95% CI:0.75, 1.33) Atopic dermatitis (assessed) at 2 y: OR: 1.11 (95% CI: 0.51, 2.41) |
| BF 4–6 mo vs. never BF | None | Eczema (parent report) by 2 y: OR: 0.87 (95% CI: 0.55, 1.35) Atopic dermatitis (assessed) at 2 y: OR: 2.58 (95% CI: 0.77, 8.67) |
||||
| BF 7–9 mo vs. never BF | None | Eczema (parent report) by 2 y: OR: 0.74 (95% CI: 0.46, 1.20) Atopic dermatitis (assessed) at 2 y: OR: 1.58 (95% CI: 0.44, 5.66) |
||||
| BF >9 mo vs. never BF | None | Eczema (parent report) by 2 y: OR: 0.66 (95% CI: 0.40, 1.09) Atopic dermatitis (assessed) at 2 y: OR :1.30 (95% CI: 0.34, 4.95) |
||||
| Turati, 2016 (12) | Case-control | Italy |
n = 451 cases, 451 controls Baseline: median, 5 mo (range: 3–24 mo) Sex: 67% male Race/ethnicity: NR |
Never BF vs. ever BF | None | Atopic dermatitis at study inclusion (age 3–24 mo): OR: 0.68 (95% CI: 0.38, 1.24) |
1BF, breastfed/breastfeeding; FF, formula-fed/formula feeding; INMA, INfancia y Medio Ambiente; KOALA Birth Cohort, Child, Parent and Health: Lifestyle and Genetic Constitution; NR, not reported; PASTURE, Protection Against Allergy Study in Rural Environments; PIPO Project, Prospective Study on the Influence of Perinatal factors on the Occurrence of Asthma and Allergies; ref, reference; SR, systematic review; TBCS, Taiwan Birth Cohort Study.
2Exposures, as defined by the authors of the studies included in the body of evidence, which address never versus ever feeding human milk or vice versa.
3The cohort was sampled from a randomized controlled trial; however, the data of interest for this SR are unrelated to randomization.
4Study was a randomized controlled trial; however, the data of interest for this SR used a nonrandomized comparison group.
Atopic dermatitis in childhood
Eight prospective cohort studies (18, 19, 29, 31, 32, 52, 53, 55) and 1 nested case-control study (54) examined the relationship between never versus ever being fed human milk and atopic dermatitis in childhood (Table 4). Data about infant milk-feeding practices were collected by diary, questionnaire, and interview. Infants never fed human milk were compared with infants categorized as ever fed human milk (19, 29, 31, 48, 52, 55) or fed human milk for heterogeneous ranges of duration (18, 31, 32, 53). Atopic dermatitis was defined based on parent responses to items from the ISAAC questionnaire (18, 31, 54), parent report of a physician's diagnosis (29, 32, 55), a positive Scoring Atopic Dermatitis (SCORAD) score (32), or physical examination plus parent-reported case history (19, 29, 52, 53). Bergman et al. (52), Burr et al. (19), and Mihrshahi et al. (29) examined atopic dermatitis in at-risk or “risk-enriched” samples based on family history or immunoglobulin E (IgE) concentrations. The comparisons of interest by Sariachvili et al. (54), Larsson et al. (18), and Burr et al. (19) were unadjusted, whereas the remaining studies considered a range of confounders.
TABLE 4.
Evidence examining the relationship between never versus ever feeding human milk and atopic dermatitis in childhood1
| First author, year (ref) | Study design (study/cohort name when applicable) | Country | Notable sample characteristics | Never vs. ever feeding human milk exposure2 | Significant associations with atopic dermatitis | Nonsignificant associations with atopic dermatitis |
|---|---|---|---|---|---|---|
| Bergmann, 2002 (52) | Prospective cohort (MAS) | Germany |
n = 939 Baseline: birth Sex NR Race/ethnicity: NR Risk: “risk enriched” 38% family history (≥2 first-degree relatives or IgE concentrations) |
Ever BF vs. never BF | None | Atopic eczema through 7 y: OR: 1.615 (95% CI: 0.933, 2.795) |
| Burr, 1993 (19) | Prospective cohort3 | UK |
n = 453 Baseline: birth Race/ethnicity: NR Risk: 100% family history (parent or sibling) |
Ever BF vs. never BF | None | Proportion of infants ever BF vs. never BF with eczema at age 7 y: 37% vs. 35%, NS |
| Larsson, 2008 (18) | Prospective cohort (DBH) | Sweden |
n = 4779 Baseline: 1–4 y Race/ethnicity: NR |
No BF vs. BF >6 mo | None | 5-y cumulative incidence of eczema by age 6–9 y: OR: 0.64 (95% CI: 0.33, 1.24) |
| Mihrshahi, 2007 (29) | Prospective cohort3 (CAPS) | Australia |
n = 516 Baseline: birth Sex: NR Race/ethnicity: NR Risk: 100% family history of asthma (parent or sibling) |
Ever BF vs. never BF | None | Eczema at 5 y: OR: 1.38 (95% CI: 0.61, 3.12) |
| Nwaru, 2013 (31) | Prospective cohort (SEATON) | UK |
n = 934, 770 in subsample with no eczema by 6 mo, 131 in subsample with eczema by 6 mo Baseline: birth Race/ethnicity: NR |
Ever BF vs. never BF | None | Eczema up to age 10 y: OR: 1.06 (95% CI: 0.83, 1.35) Eczema up to age 10 y in subsample with no eczema by 6 mo: OR: 1.22 (95% CI: 0.91, 1.63) Eczema up to age 10 y in subsample with eczema by 6 mo: OR: 0.87 (95% CI: 0.51, 1.49) |
| BF <2.25 mo vs. never BF | None | Eczema up to age 10 y: OR: 1.12 (95% CI: 0.84, 1.51) Eczema up to age 10 y in subsample with no eczema by 6 mo: OR: 1.25 (95% CI: 0.87, 1.79) Eczema up to age 10 y in subsample with eczema by 6 mo: OR: 0.95 (95% CI: 0.50, 1.81) |
||||
| BF ≥2.25 mo vs. never BF | None | Eczema up to age 10 y: OR: 1.04 (95% CI: 0.81, 1.35) Eczema up to age 10 y in subsample with no eczema by 6 mo: OR: 1.23 (95% CI: 0.90, 1.68) Eczema up to age 10 y in subsample with eczema by 6 mo: OR: 0.83 (95% CI: 0.46, 1.50) |
||||
| Orivuori, 2014 (32) | Prospective cohort (PASTURE) | Finland, France, Germany, Switzerland |
n = 853 Baseline: birth Race/ethnicity: NR |
Never BF vs. BF >6 mo | None | Atopic dermatitis up to 4 y: OR: 0.83 (95% CI: 0.40, 1.69) |
| Purvis, 2005 (53) | Prospective cohort4 (ABC Study) | New Zealand |
n = 550 Baseline: birth Race/ethnicity: NR; 100% New Zealanders of European descent Risk: ∼50% born SGA |
BF <6 mo vs. never BF | Atopic dermatitis at 3.5 y: OR: 6.12 (95% CI: 1.22, 30.7) | None |
| BF ≥6 mo vs. never BF | Atopic dermatitis at 3.5 y: OR: 12.0 (95% CI: 2.62, 54.8) | None | ||||
| Sariachvili, 2010 (54) | Nested case-control (PIPO Project) | Belgium |
n = 252 cases, 305 controls Baseline: birth Race/ethnicity: NR |
Ever BF vs. never BF | None | Eczema up to age 4 y: OR: 0.77 (95% CI: 0.50, 1.18) |
| Zutavern, 2004 (55) | Prospective cohort | UK |
n = 604 Baseline: birth Race/ethnicity: NR |
Ever BF vs. never BF | None | Eczema by age 5.5 y: OR: 0.71 (95% CI: 0.47, 1.1) |
1ABC, Auckland Birthweight Collaborative; BF, breastfed/breastfeeding; CAPS, Childhood Asthma Prevention Study; DBH, Dampness in Buildings and Health; IgE, immunoglobulin E; MAS, Multicentre Allergy Study; NR, not reported; PASTURE, Protection Against Allergy Study in Rural Environments; PIPO Project, Prospective Study on the Influence of Perinatal Factors on the Occurrence of Asthma and Allergies; ref, reference; SEATON, Study of Eczema and Asthma to Observe the Influence of Nutrition; SGA, small for gestational age; SR, systematic review.
2Exposures, as defined by the authors of the studies included in the body of evidence, which address never versus ever feeding human milk or vice versa.
3The cohort was sampled from a randomized controlled trial; however, the data of interest for this SR are unrelated to randomization.
4The cohort was sampled from a case-control study; however, the data of interest for this SR are unrelated to case/control status.
Most of the associations between never versus ever feeding human milk and atopic dermatitis in childhood were nonsignificant. The samples in the studies by Bergman et al. (52), Mihrshahi et al. (29), and Orivuori et al. (32) may have been too small for sufficient statistical power to examine the comparisons of interest for this SR, as they had wide CIs around their nonsignificant associations. The only study with statistically significant associations was by Purvis et al. (53). However, the associations from this study may not be generalizable because the study was originally intended to examine differences between infants born small and appropriate for gestational age and recruited a sample in which about half of the participants were born small for gestational age (although the study authors noted that this was accounted for in the statistical analysis). In the remaining studies, the nonsignificant associations were inconsistent in direction.
Shorter versus longer durations of any human milk feeding and food allergies, allergic rhinitis, atopic dermatitis, and asthma throughout the life span
Thirty-five articles met the inclusion criteria for this SR question (14, 18, 32, 37, 52, 54, 57–85). None of the articles examined food allergies, allergic rhinitis, or atopic dermatitis in adolescence or adulthood; and TEC members concluded that the scant evidence with methodologic limitations was insufficient to determine whether or not the duration of any human milk feeding was associated with food allergies from birth through childhood (14, 57, 58), allergic rhinitis during the B-24 period (59), or asthma in adulthood (79, 80). Additional information about these topics is available at https://nesr.usda.gov. Evidence on allergic rhinitis in childhood, asthma in childhood and adolescence, and atopic dermatitis from birth through childhood is presented below.
Allergic rhinitis in childhood
One cluster randomized controlled trial examined the relationship between shorter versus longer durations of any human milk feeding and allergic rhinitis in childhood (Table 5). Kramer et al. (60) presented evidence from the Promotion of Breastfeeding Intervention Trial (PROBIT), a cluster randomized controlled trial of an intervention to promote prolonged duration and exclusivity of human milk feeding among mothers who chose to feed human milk. Study pediatricians collected human milk-feeding data at well-baby medical appointments. The intervention group had higher rates of human milk feeding than the control group measured at 3, 6, 9, and 12 mo. There was no association between group status and ever having hay fever symptoms or having hay fever symptoms in the previous 12 mo, which were assessed by study pediatricians through the use of the ISAAC questionnaire (56).
TABLE 5.
Evidence examining the relationship between shorter versus longer durations of any human milk feeding and allergic rhinitis in childhood1
| First author, year (ref) | Study design (study/cohort name when applicable) | Country | Notable sample characteristics | Shorter vs. longer duration of any human milk feeding exposure2 | Significant associations with allergic rhinitis | Nonsignificant associations with allergic rhinitis |
|---|---|---|---|---|---|---|
| Codispoti, 2010 (61) | Prospective cohort (CCAAPS) | USA |
n = 80 African Americans, 218 non-African Americans Baseline: birth Race/ethnicity: 22.2% African American, 77.8% non–African American Risk: 100% family history (≥ 1 parent) |
BF duration (mo) | Allergic rhinitis at age 3 y in African-American subsample: OR: 0.8 (95% CI: 0.6, 0.9) | Allergic rhinitis at age 3 y in the non–African-American subsample: OR: 1.0 (95% CI: 0.96, 1.1) |
| Kramer, 2007 (60) | Cluster RCT3 (PROBIT) | Belarus |
n = 13,889 Baseline: birth Race/ethnicity: NR |
Experimental group (higher rates of any BF measured at 3, 6, 9, and 12 mo) vs. control group | None | Ever had hay fever symptoms by 6.5 y: OR: 1.1 (95% CI: 0.6, 1.9) Hay fever symptoms in the past 12 mo at 6.5 y: OR: 1.0 (95% CI: 0.6, 1.8) |
| Larsson, 2008 (18) | Prospective cohort (DBH) | Sweden |
n = 4779 Baseline: 1–4 y Race/ethnicity: NR |
BF duration <3 mo vs. >6 mo | None | 5-y cumulative incidence of rhinitis by age 6–9 y: OR: 0.96 (95% CI: 0.63, 1.46) 5-y cumulative incidence of any rhinitis symptoms by age 6–9 y: OR: 0.80 (95% CI: 0.59, 1.07) |
| BF duration 3–6 mo vs. >6 mo | None | 5-y cumulative incidence of rhinitis during by age 6–9 y: OR: 0.94 (95% CI: 0.69, 1.29) 5-y cumulative incidence of any rhinitis symptoms during by age 6–9 y: OR: 1.03 (95% CI: 0.84, 1.25) |
||||
| Nwaru, 2013 (63) | Prospective cohort (DIPP) | Finland |
n = 3112 Baseline: birth Race/ethnicity: NR Risk: 100% high-risk genotype for T1D |
Total BF <5 mo vs. >9.5 moTotal BF 5–9.5 mo vs. >9.5 mo | NoneNone | Allergic rhinitis at 5 y: OR: ∼1.3 (95% CI: ∼1.0, ∼1.8)Allergic rhinitis at 5 y: OR: ∼1.2 (95% CI: ∼0.9, ∼1.5) |
| Sandini, 2011 (64) | Prospective cohort4 | Finland |
n = 891 Baseline: birth Race/ethnicity: NR Risk: 100% family history (≥1 parent) |
BF duration ≥2 mo vs. <2 mo | None | Allergic rhinitis at 5 y: OR: 1.87 (95% CI: 0.55, 6.36) |
| von Kobyletzki, 2012 (62) | Prospective cohort (DBH) | Sweden |
n = 3124 Baseline: 1–2 y Race/ethnicity: NR |
BF ≤6 mo vs. > 6 mo | None | 5-y cumulative incidence of rhinitis by age 6–7 y: OR: 1.02 (95% CI: 0.73, 1.43) |
1BF, breastfeeding/breastfed; CCAAPS, Cincinnati Childhood Allergy and Air Pollution Study; DBH, Dampness in Buildings and Health; DIPP, Type 1 Diabetes Prediction and Prevention; NR, not reported; PROBIT, Promotion of Breastfeeding Intervention Trial; RCT, randomized controlled trial; ref, reference; SR, systematic review; T1D, type 1 diabetes.
2Exposures, as defined by the authors of the studies included in the body of evidence, which address shorter versus longer durations of any human milk feeding or vice versa.
3Cluster RCT of an intervention to promote prolonged duration and exclusivity of breastfeeding rather than an RCT of breastfeeding per se.
4The cohort was sampled from an RCT; however, the data of interest for this SR are unrelated to randomization.
There were also 4 prospective cohort studies that presented evidence across 5 articles (18, 61–64) (Table 5) [unique evidence from the Dampness in Buildings and Health (DBH) study was presented by Larsson et al. (18) and von Kobyletzki et al. (62)]. Two of the studies examined high-risk cohorts (based on family history of allergic disease) (61, 64), and a third study examined children who were at risk for type 1 diabetes (63). Data about the duration of human milk feeding were collected by parent questionnaire and assessed as a continuous variable by Codispoti et al. (61) and as heterogeneous categorical variables by the other studies (18, 62–64). Allergic rhinitis was defined based on parent responses to items from the ISAAC questionnaire (18, 61–63), parent report of physician diagnosis (18), or positive skin-prick test or allergen-specific IgE concentration ≥0.7 kU/L plus a history of symptoms (64). The comparisons of interest in 2 studies were unadjusted (18, 62, 63), and the remaining studies considered a range of confounders (61, 64).
Nearly all of the associations across the 4 prospective cohort studies were nonsignificant, with no discernible trend in the direction of the point estimates. The only statistically significant association was that reported by Codispoti et al. (61), who found that a longer duration of human milk feeding was associated with lower risk of allergic rhinitis in 3-y-old African Americans (OR: 0.8; 95% CI: 0.6, 0.9). There were no comparable analyses in other studies in this body of evidence that would allow TEC members to examine whether this association is typical among African American children.
Asthma in childhood and adolescence
One cluster randomized controlled trial examined the relationship between shorter versus longer durations of any human milk feeding and asthma in childhood (Table 6). In the PROBIT (described previously), Kramer et al. (60) found no significant association between group status and ever having asthma by 6.5 y, which was assessed by study pediatricians through the use of the ISAAC instrument.
TABLE 6.
Evidence examining the relationship between shorter versus longer durations of any human milk feeding and asthma in childhood and adolescence1
| First author, year (ref) | Study design (study/cohort name when applicable) | Country | Notable sample characteristics | Shorter vs longer duration of any human milk feeding exposure2 | Significant associations with asthma | Nonsignificant associations with asthma |
|---|---|---|---|---|---|---|
| Al-Mousawi, 2004 (76) | Case-control | Kuwait |
n = 160 cases, 303 controls Baseline: 8–15 y Sex: 73% male Race/ethnicity: NR |
BF >2 mo vs. <2 mo | Asthma diagnosis at age 8–15 y (in model that includes sensitization defined by SPT): OR: 0.54 (95% CI: 0.30, 0.96) Asthma diagnosis at age 8–15 y (in model that includes sensitization defined by IgE concentration): OR: 0.45 (95% CI: 0.26, 0.80) |
None |
| Bergmann, 2000 (65) | Prospective cohort (MAS) | Germany |
n = 880 Baseline: birth Sex: NR Race/ethnicity: NR Risk: “risk enriched” 38% family history (≥2 first-degree relatives or IgE concentrations) |
BF >6 mo vs. ≤6 mo | None | Asthma at 3–6 y: OR: 0.890 (95% CI: 0.580, 1.368) |
| Colen, 2014 (37) | Prospective cohort (National Longitudinal Study of Youth 1979 Cohort) | USA |
n = 8237 in the full sample, 7319 in the sibling subsample Baseline: birth Race/ethnicity: 74.49% non-Hispanic white, 17.28% non-Hispanic black, 8.23% Hispanic |
BF duration (wk) | Asthma at 4–14 y in the full sample (between-family estimate): β = 0.004 (SE = 0.002), P < 0.05 | Asthma at 4–14 y in the sibling subsample (within-family estimate): β = 0.006 (SE = 0.008) |
| Fredriksson, 2007 (77) | Prospective cohort | Finland |
n = 1933 Baseline: 1–7 y Race/ethnicity: NR |
BF 0–3 mo vs. 4–6 moBF 7–9 mo vs. 4–6 mo | NoneNone | Current asthma in participants ages 7–14 y: OR: 1.44 (95% CI: 0.78, 2.66)Current asthma in participants ages 7–14 y: OR: 1.16 (95% CI: 0.65, 2.08) |
| BF 10–12 mo vs. 4–6 mo | None | Current asthma in participants ages 7–14 y: OR: 1.72 (95% CI: 0.97, 2.08) | ||||
| BF > 12 mo vs. 4–6 mo | None | Current asthma in participants ages 7–14 y: OR: 1.60 (95% CI: 0.83, 2.08) | ||||
| Per 1-mo decrease in BF duration from 7 mo | None | Current asthma in participants ages 7–14 y: OR: 1.10 (95% CI: 0.92, 1.32) | ||||
| Per 1-mo increase in BF duration from 7 mo | None | Current asthma in participants ages 7–14 y: OR: 1.03 (95% CI: 1.00, 1.05) | ||||
| Grandjean, 2010 (66) | Prospective cohort | Denmark |
n = 464 Baseline: birth Race/ethnicity: NR |
BF duration (mo) in participants with asthma vs. no allergy | None | Current or past history of asthma by 5 or 7 y: 9.3 (IQR: 7, 12) vs. 9.9 (IQR: 6, 12), P = 0.58 |
| Hovland, 2015 (78) | Prospective cohort (Environment and Childhood Asthma Study) | Norway |
n = 322 with asthma never, 107 with pubertal asthma, 121 with asthma in remission in puberty, and 33 with pubertal onset of asthma Baseline: birth Race/ethnicity: NR |
BF >4 mo vs. ≤4 mo | Proportion of participants with asthma (0–10 y) in remission during puberty (10–16 y) vs. never had asthma who BF >4 mo: 80.2% vs. 91.2%, P < 0.01 | Proportion of participants with pubertal asthma at 10–16 y vs. never had asthma who BF >4 mo: 86.3% vs. 91.2% Proportion of participants with pubertal onset of asthma at 10–16 y vs. never had asthma who BF > 4 mo: 86.7% vs. 91.2% |
| BF >4 mo vs. ≤4 mo | Asthma (0–10 y) in remission during puberty (10–16 y): OR: 0.22 (95% CI: 0.08, 0.65) | None | ||||
| Karmaus, 2008 (67) | Prospective cohort (IOW) | UK |
n = 1224 Baseline: birth Sex: NR Race/ethnicity: NR |
BF ≥3 mo vs. <3 mo | None | Repeated measurement of asthma at ages 1, 2, 4, 10 y: RR: 0.83 (95% CI: 0.67, 1.02) Repeated measurement of asthma at ages 4 or 10 y: RR: 0.82 (95% CI: 0.64, 1.06) |
| Karunasekera, 2001 (68) | Case-control | Sri Lanka |
n = 300 cases, 300 controls Baseline: 1–10 y Sex: NR Race/ethnicity: NR |
BF ≤6 mo vs. >6 mo | Asthma at 1–10 y: OR: 2.0 (95% CI: 1.2, 3.2) | None |
| Klinnert, 2001 (69) | Prospective cohort | USA |
n = 145 Baseline: birth Sex: NR Race/ethnicity: “primarily Caucasians” Risk: 100% family history (mothers) |
BF duration | None | Asthma at 6–8 y: NS (data NR) |
| Kramer, 2007 (60) | Cluster RCT3 (PROBIT) | Belarus |
n = 13,889 Baseline: birth Race/ethnicity: NR |
Experimental group (higher rates of any BF measured at 3, 6, 9, and 12 mo) vs. control group | None | Ever had asthma by 6.5 y: OR: 1.2 (95% CI: 0.7, 1.9) |
| Kull, 2004 (70) | Prospective cohort (BAMSE) | Sweden |
n = 3670 Baseline: birth Race/ethnicity: NR |
EBF 0–2 mo + additional partial BF ≥ 3 mo vs. EBF 0–2 mo + additional partial BF 0–2 mo | None | Asthma at 4 y: OR: 0.90 (95% CI: 0.47, 1.73) |
| EBF 3–4 mo + additional partial BF 0–2 mo vs. EBF 0–2 mo + additional partial BF 0–2 mo | None | Asthma at 4 y: OR: 0.67 (95% CI 0.34, 1.32) | ||||
| EBF 3–4 mo + additional partial BF ≥3 mo vs. EBF 0–2 mo + additional partial BF 0–2 mo | Asthma at 4 y: OR: 0.44 (95% CI: 0.21, 0.87) | None | ||||
| EBF ≥5 mo + additional partial BF 0–2 mo vs. EBF 0–2 mo + additional partial BF 0–2 mo | None | Asthma at 4 y: OR: 0.64 (95% CI: 0.37, 1.09) | ||||
| EBF ≥5 mo + additional partial BF ≥3 mo vs. EBF 0–2 mo + additional partial BF 0–2 mo | Asthma at 4 y: OR: 0.43 (95% CI: 0.25, 0.74) | None | ||||
| Larsson, 2008 (18) | Prospective cohort (DBH) | Sweden |
n = 4483 in the full sample without asthma at baseline, 3320 in the subsample without wheezing at baseline, 935 in the subsample with wheezing at baseline Baseline: 1–4 y Race/ethnicity: NR |
BF duration <3 mo vs. >6 mo | 5-y cumulative incidence of asthma by age 6–9 y in the subsample with no asthma, but with wheezing at baseline: OR: 2.11 (95% CI: 1.12, 3.00) | 5-y cumulative incidence of asthma by age 6–9 y in the full sample with no asthma at baseline: OR: 1.54 (95% CI: 0.98, 2.43) 5-y cumulative incidence of asthma by age 6–9 y in the subsample with no asthma and no wheezing ever at baseline: OR: 1.31 (95% CI: 0.70, 2.45) |
| BF duration 3–6 mo vs. >6 mo | 5-y cumulative incidence of asthma by age 6–9 y in the subsample with no asthma, but with wheezing at baseline: OR: 1.84 (95% CI: 1.09, 3.11) | 5-y cumulative incidence of asthma by age 6–9 y in the full sample with no asthma at baseline: OR: 1.40 (95% CI: 0.98, 2.00) 5-y cumulative incidence of asthma by age 6–9 y in the subsample no asthma and no wheezing ever at baseline: OR: 1.18 (95% CI: 0.73, 1.91) |
||||
| Nwaru, 2013 (63) | Prospective cohort (DIPP) | Finland |
n = 3142 Baseline: birth Race/ethnicity: NR Risk: 100% high-risk genotype for T1D |
Total BF <5 mo vs. >9.5 moTotal BF 5–9.5 mo vs. >9.5 mo | Asthma at 5 y: HR: 1.91 (95% CI: 1.21, 3.02)Asthma at 5 y: HR: 1.97 (95% CI: 1.28, 3.02) | NoneNone |
| Oddy, 1999 (71) | Prospective cohort (Western Australia Pregnancy Cohort Study)4 | Australia |
n = 2187 Baseline: birth Race/ethnicity: 2.5% Aboriginal descent |
BF stopped by 3 mo vs. notBF stopped by 4 mo vs. not | NoneNone | Asthma diagnosed by a doctor by age 6 y: OR: 1.12 (95% CI: 0.91, 1.34)Asthma diagnosed by a doctor by age 6 y: OR: 1.14 (95% CI: 0.94, 1.40) |
| BF stopped by 5 mo vs. not | None | Asthma diagnosed by a doctor by age 6 y: OR: 1.20 (95% CI: 0.98, 1.47) | ||||
| BF stopped by 6 mo vs. not | None | Asthma diagnosed by a doctor by age 6 y: OR: 1.18 (95% CI: 0.97, 1.45) | ||||
| Orivuori, 2014 (32) | Prospective cohort (PASTURE) | Finland, France, Germany, Switzerland |
n = 853 Baseline: birth Race/ethnicity: NR |
BF ≤3 mo vs. >6 moBF 3–6 mo vs. 6 mo | NoneNone | Asthma at 4 y: OR: 1.32 (95% CI: 0.57, 3.05) Asthma between 4 and 6 y: OR: 0.76 (95% CI: 0.36, 1.62)Asthma at 4 y: OR: 0.79 (95% CI: 0.30, 2.06) |
| Asthma between 4 and 6 y: OR: 0.56 (95% CI: 0.25, 1.23) | ||||||
| Ronmark, 2002 (72) | Prospective cohort | Sweden |
n = 3247 Baseline: 7–8 y Race/ethnicity: NR |
BF ≤3 mo vs. unspecified longer duration | None | Ever asthma by 9–10 y: RR: 0.46 (95% CI: 0.20,1.04) |
| Sandini, 2011 (64) | Prospective cohort4 | Finland |
n = 891 Baseline: birth Race/ethnicity: NR Risk: 100% family history (≥1 parent) |
BF duration ≥2 mo vs. <2 mo | None | Asthma with IgE sensitization at 5 y: OR: 0.99 (95% CI: 0.22, 4.39) |
| Sigurs, 1995 (73) | Prospective cohort5 | Sweden |
n = 140 Baseline: <1 y Race/ethnicity: NR Risk: 33% infancy respiratory syncytial virus bronchiolitis |
Total BF duration (mo) | None | Asthma at 3 y: RR: 0.8 (95% CI: 0.65, 1.02) |
| Silvers, 2012 (74) | Prospective cohort (New Zealand Asthma and Allergy Cohort) | New Zealand |
n = 892 Baseline: birth Race/ethnicity: 14.6% Maori |
Duration of any BF (mo) | Current asthma at 3 y: OR: 0.94 (95% CI: 0.91, 0.97) Current asthma at 4 y: OR: 0.96 (95% CI: 0.92, 0.99) |
Current asthma at 5 y: OR: 0.98 (95% CI: 0.94, 1.00) Current asthma at 6 y: OR: 0.99 (95% CI: 0.96, 1.03) |
| Strassburger, 2010 (75) | Prospective cohort4 | Brazil |
n = 347 Baseline: birth Race/ethnicity: NR |
BF <6 mo vs. BF ≥6 mo | None | Asthma by 3–4 y: OR: 1.55 (95% CI: 0.61, 3.92) |
| von Kobyletzki, 2012 (62) | Prospective cohort (DBH) | Sweden |
n = 3124 in the subsample without asthma or wheezing at baseline Baseline: 1–2 y Race/ethnicity: NR |
BF ≤6 mo vs. BF >6 mo | None | 5-y cumulative incidence of asthma by age 6–7 y in the subsample with no asthma and no wheezing ever at baseline: OR: 1.14 (95%CI: 0.68, 1.90) |
1β, regression coefficient; BAMSE, Swedish abbreviation for Children, Allergy, Milieu, Stockholm, Epidemiology; BF, breastfed/breastfeeding; DBH, Dampness in Buildings and Health; DIPP, Type 1 Diabetes Prediction and Prevention; HLA, human leukocyte antigen; IgE, immunoglobulin E; IOW, Isle of Wight; MAS, Multicentre Allergy Study; NR, not reported; PASTURE, Protection Against Allergy Study in Rural Environments; PROBIT, Promotion of Breastfeeding Intervention Trial; RCT, randomized controlled trial; ref, reference; SPT, skin-prick test; SR, systematic review; T1D, type 1 diabetes.
2Exposures, as defined by the authors of the studies included in the body of evidence, which address shorter versus longer durations of any human milk feeding or vice versa.
3Cluster RCT of an intervention to promote prolonged duration and exclusivity of breastfeeding rather than an RCT of breastfeeding per se.
4The cohort was sampled from an RCT; however, the data of interest for this SR are unrelated to randomization.
5The cohort was sampled from a case-control study; however, the data of interest for this SR are unrelated to case/control status.
There were also 17 prospective cohort studies that presented evidence across 18 articles (18, 32, 37, 62–67, 69–75, 77, 78) (Table 6) [unique evidence from the DBH study was presented by Larsson et al. (18) and von Kobyletzki et al. (62)] and 2 case-control studies (68, 76). Data on human milk feeding were collected by interviews, diaries, or questionnaires given to parents and assessed as a continuous variable (37, 66, 69, 73, 74, 77) and heterogeneous categorical variables (18, 32, 62–65, 67, 68, 70–72, 75–78). The outcome was usually based on parent report of morbidity or physician diagnosis, or a combination of the 2 (18, 32, 37, 62, 63, 69, 72, 74, 75, 77, 78), although a few studies used physician diagnosis (66, 67, 73) or accessed medical records (64, 69).
Statistically significant associations were reported by 6 prospective cohort studies (18, 37, 63, 70, 74, 78). With 1 exception (37), these studies provided consistent evidence of an inverse association between the duration of any human milk feeding and asthma risk in children and adolescents and suggested that the predominant difference between the statistically significant and nonsignificant associations was statistical power.
Specifically, in the study by Hovland et al. (78) a larger proportion of the participants who never had asthma (n = 322) were fed human milk for >4 mo than participants who had asthma; however, the difference was only significant with the subsample of participants with asthma in remission during puberty (10–16 y of age; OR: 0.22; 95% CI: 0.08, 0.65) and not in the subsamples with asthma during puberty. Kull et al. (70) examined shorter compared with longer durations of exclusive plus additional partial human milk feeding. Being fed human milk for ≥3 mo after 3–4 mo of exclusive human milk feeding and being fed human milk for ≥5 mo after ≥3 mo of exclusive human milk feeding (i.e., longer durations) compared with being fed human milk for 0–2 mo after 0–2 mo of exclusive human milk feeding (i.e., a shorter duration) were associated with lower odds of asthma at 4 y of age [OR (95% CI): 0.44 (0.21, 0.87) and 0.43 (0.25, 0.74), respectively]. The nonsignificant associations were in the same direction but had wider CIs, indicative of suboptimal statistical power. In the DBH study, Larsson et al. (18) and von Kobyletzki et al. (62) examined the 5-y cumulative incidence of asthma by age 6–7 y (62) and by age 6–9 y (18) in a sample of children who did not have asthma at baseline and in subsamples of children who did (18) and did not (18, 62) have wheezing at baseline. In the subsample of children with wheezing at baseline, Larsson et al. (18) found higher odds of asthma in children fed human milk for <3 mo and for 3–6 mo compared with >6 mo [OR (95% CI): 2.11 (1.12, 3.00) and 1.84 (1.09, 3.11), respectively]. In analyses of the full sample and the subsample with no wheezing at baseline, the nonsignificant associations were in the same direction and had wide CIs, indicative of a lack of statistical power (18, 62). In a sample with high risk for type 1 diabetes, Nwaru et al. (63) found higher HRs among children fed human milk for <5 mo and 5–9.5 mo compared with >9.5 mo [HR (95% CI): 1.91 (1.21, 3.02) and 1.97 (1.28, 3.02), respectively]. Silvers et al. (74) examined the duration of any human milk feeding as a continuous variable and found lower odds of asthma at 3 and 4 y of age as the number of months of human milk feeding increased [OR (95% CI): 0.94 (0.91, 0.97) and 0.96 (0.92, 0.99), respectively]. At 5 y of age, the upper limit of the CI was 1.00, and at 6 y of age the CI included the null. Colen et al. (37) conducted the only prospective cohort study with a statistically significant association that showed that a longer compared with a shorter duration of any human milk feeding was associated with higher risk of asthma. It examined asthma from 4 to 14 y using a between-family estimate from the full sample as well as a within-family estimate from a subsample of sibling participants. In the full sample, each additional week of feeding human milk tended to increase asthma; however, the effect size was small (β: 0.004; SE: 0.002; P < 0.05) and a nonsignificant, and similarly small, effect size was found in the sibling subsample analysis.
Statistically significant associations were also reported by both case-control studies (68, 76), which provided additional evidence of an inverse association between the duration of any human milk feeding and asthma risk in children and adolescents. Karunasekera et al. (68) reported that being fed human milk for ≤6 mo compared with >6 mo was associated with higher odds of asthma at age 1–10 y (OR: 2.0; 95% CI: 1.2, 3.2), and Al-Mousawi et al. (76) reported that being fed human milk for >2 mo compared with <2 mo was associated with lower odds of asthma at age 8–15 y (OR: 0.54; 95% CI: 0.30, 0.96).
To summarize, 7 of 8 studies with statistically significant associations had findings that suggested that shorter versus longer durations of any human milk feeding are associated with higher relative risk of asthma in childhood and adolescence (18, 63, 68, 70, 74, 76, 78), and the remaining study found a small positive association between duration and asthma (37). In these studies, and across most of the observational studies in the body of evidence, the nonsignificant associations provided further evidence of an inverse relationship between the duration of any human milk feeding and asthma because they were consistent in direction with the significant associations (18, 62, 64–67, 70, 71, 73–75, 78) and some of the nonsignificance could be attributed to inadequate power. The minority of studies had nonsignificant associations that were discrepant (32, 60, 72, 77) or did not report point estimates, so that direction could not be assessed (69).
Atopic dermatitis from birth to 24 mo
Eight articles presented inconclusive evidence on shorter versus longer durations of any human milk feeding and atopic dermatitis during the B-24 period (32, 57, 59, 64, 81–84) (Table 7). Kramer et al. (84) provided compelling evidence from the PROBIT (a cluster randomized controlled trial described previously) that the experimental group had lower risk of atopic dermatitis at 12 mo of age than the control group (OR: 0.54; 95% CI: 0.31, 0.95). However, evidence from 7 observational studies (32, 57, 59, 64, 81–83) was inconsistent with evidence from the PROBIT study. Miyake et al. (82) found that feeding human milk for ≥6 mo, compared with <6 mo, was associated with significantly higher odds of atopic dermatitis in the absence of parental atopic history (OR: 3.39; 95% CI: 1.20, 12.36), and evidence from the remaining studies lacked statistical significance and had point estimates that were inconsistent in direction. Furthermore, TEC members had concerns about reverse causality and the specificity of detecting atopic dermatitis during the B-24 period.
TABLE 7.
Evidence examining the relationship between shorter versus longer durations of any human milk feeding and atopic dermatitis from birth to 24 mo1
| First author, year (ref) | Study design (study/cohort name when applicable) | Country | Notable sample characteristics | Shorter vs. longer duration of any human milk feeding exposure2 | Significant associations with atopic dermatitis | Nonsignificant associations with dermatitis |
|---|---|---|---|---|---|---|
| Hesselmar, 2010 (57) | Prospective cohort (ALLERGYFLORA) | Sweden |
n = 184 Baseline: 1–3 d Race/ethnicity: NR Risk: 80% family history (≥1 parent) |
Median duration of partial BF (mo) in participants with and without eczema | None | Eczema by 6 mo: 8 mo (IQR: 6.0, 9.5) vs. 7 mo (IQR: 4.5, 9.0), P = 0.619 Eczema by 18 mo: 6.7 mo (IQR: 6.0, 9.0) vs. 7.2 mo (IQR: 4.0, 10.0), P = 0.818 |
| Kerkhof, 2003 (81) | Nested case control (PIAMA) | Netherlands |
n = 76 cases, 228 controls Baseline: birth Race/ethnicity: NR Risk: 100% family history (mothers) |
BF duration (wk) | None | Expected probability of atopic dermatitis at 12 mo: NS (data NR) |
| Kramer, 2001 (84) | Cluster RCT3 (PROBIT) | Belarus |
n = 16,491 Baseline: birth Race/ethnicity: NR |
Experimental group (higher rates of any BF measured at 3, 6, 9, and 12 mo) vs. control group | Atopic eczema by 12 mo: OR: 0.54 (95% CI: 0.31, 0.95) | None |
| Kull, 2002 (59) | Prospective cohort (BAMSE) | Sweden |
n = 3791 Baseline: birth Race/ethnicity: NR |
Partial BF ≥6 mo vs. <6 mo | None | Atopic dermatitis by age 2 y: OR: 0.88 (95% CI: 0.72, 1.05) |
| Miyake, 2009 (82) | Prospective cohort (Osaka Maternal and Child Health Study) | Japan |
n = 763, 313 in the subsample with negative parental atopic history, and 450 in the subsample with positive parental atopic history Baseline: birth Race/ethnicity: NR |
Partial BF ≥6 mo vs. <6 mo | Atopic eczema at 16–24 mo among children without suspected atopic eczema at 2–9 mo in the subsample with negative parental atopic history: OR: 3.39 (95% CI: 1.20, 12.36) | Atopic eczema at 16–24 mo among children without suspected atopic eczema at 2–9 mo: OR: 1.66 (95% CI: 0.99, 2.92) Atopic eczema at 16–24 mo among children without suspected atopic eczema at 2–9 mo in the subsample with positive parental atopic history: OR: 1.33 (95% CI: 0.72, 2.55) |
| Orivuori, 2014 (32) | Prospective cohort (PASTURE) | Finland, France, Germany, Switzerland |
n = 853 Baseline: birth Race/ethnicity: NR |
BF ≤3 mo vs. >6 moBF 3–6 mo vs. >6 mo | NoneNone | Atopic dermatitis up to 2 y: OR: 1.15 (95% CI: 0.69, 1.89)Atopic dermatitis up to 2 y: OR: 1.05 (95% CI: 0.65, 1.70) |
| Sandini, 2011 (64) | Prospective cohort4 | Finland |
n = 891 Baseline: birth Race/ethnicity: NR Risk: 100% family history (≥1 parent) |
BF ≥2 mo vs. <2 mo | None | Atopic eczema at 2 y: OR: 1.77 (95% CI: 0.52, 6.02) |
| Silvers, 2009 (83) | Prospective cohort (New Zealand Asthma and Allergy Cohort) | New Zealand |
n = 1011 Baseline: birth Race/ethnicity: 14.6% Maori |
BF duration (mo) | None | Ever had eczema by 15 mo: OR: 1.00 (95% CI: 0.98, 1.03) |
1BAMSE, Swedish abbreviation for Children, Allergy, Milieu, Stockholm, Epidemiology; BF, breastfeeding/breastfed; NR, not reported; PASTURE, Protection Against Allergy Study in Rural Environments; PIAMA, Prevention and Incidence of Asthma and Mite Allergy; PROBIT, Promotion of Breastfeeding Intervention Trial; RCT, randomized controlled trial; ref, reference; SR, systematic review.
2Exposures, as defined by the authors of the studies included in the body of evidence, which address shorter versus longer durations of any human milk feeding or vice versa.
3Cluster RCT of an intervention to promote prolonged duration and exclusivity of breastfeeding rather than an RCT of breastfeeding per se.
4The cohort was sampled from an RCT; however, the data of interest for this SR are unrelated to randomization.
Atopic dermatitis in childhood
One cluster randomized controlled trial examined the relationship between shorter versus longer durations of any human milk feeding and atopic dermatitis in childhood (Table 8). In the PROBIT (described previously), Kramer et al. (60) found no association between group status and ever having eczema by 6.5 y, which was assessed by study pediatricians through the use of the ISAAC instrument.
TABLE 8.
Evidence examining the relationship between shorter versus longer durations of any human milk feeding and atopic dermatitis in childhood1
| First author, year (ref) | Study design (study/cohort name when applicable) | Country | Notable sample characteristics | Shorter vs. longer duration of any human milk feeding exposure2 | Significant associations with atopic dermatitis | Nonsignificant associations with atopic dermatitis |
|---|---|---|---|---|---|---|
| Bergmann, 2000 (65) | Prospective cohort (MAS) | Germany |
n = 880 Baseline: birth Sex: NR Race/ethnicity: NR Risk: “risk enriched” 38% family history (≥2 first-degree relatives or IgE concentrations) |
BF >6 mo vs. ≤6 mo | None | Atopic dermatitis at 3–6 y: OR: 1.410 (95% CI: 0.959, 2.072) |
| Bergmann, 2002 (52) | Prospective cohort (MAS) | Germany |
n = 939 Baseline: birth Sex: NR Race/ethnicity: NR Risk: “risk enriched” 38% family history (≥2 first-degree relatives or IgE concentrations) |
BF duration (mo)BF ≥1 mo vs. BF <1 moBF ≥2 mo vs. BF <2 mo | Atopic eczema through 7 y: OR: 1.029 (95% CI: 1.002, 1.057), P = 0.034NoneAtopic eczema through 7 y: OR: 1.384 (95% CI: 1.025, 1.869) | NoneAtopic eczema through 7 y: OR: 1.187 (95% CI: 0.854, 1.648)None |
| BF ≥3 mo vs. BF <3 mo | None | Atopic eczema through 7 y: OR: 1.192 (95% CI: 0.899, 1.580) | ||||
| BF ≥4 mo vs. BF <4 mo | None | Atopic eczema through 7 y: OR: 1.292 (95% CI: 0.991, 1.685) | ||||
| BF ≥5 mo vs. BF <5 mo | None | Atopic eczema through 7 y: OR: 1.273 (95% CI: 0.977, 1.658) | ||||
| BF ≥6 mo vs. BF <6 mo | None | Atopic eczema through 7 y: OR: 1.183 (95% CI: 0.907, 1.543) | ||||
| BF ≥7 mo vs. BF <7 mo | None | Atopic eczema through 7 y: OR: 1.318 (95% CI: 0.988, 1.759) | ||||
| BF ≥8 mo vs. BF <8 mo | None | Atopic eczema through 7 y: OR: 1.294 (95% CI: 0.943, 1.776) | ||||
| BF ≥9 mo vs. BF <9 mo | None | Atopic eczema through 7 y: OR: 1.318 (95% CI: 0.943, 1.842) | ||||
| Grandjean, 2010 (66) | Prospective cohort | Denmark |
n = 464 Baseline: birth Race/ethnicity: NR |
BF duration (mo) in participants with current or past history of atopic dermatitis by 5 or 7 y vs. no allergy | None | 9.5 mo (IQR: 6, 12) vs. 9.9 mo (IQR: 6, 12), P = 0.63 |
| Kramer, 2007 (60) | Cluster RCT3 (PROBIT) | Belarus |
n = 13,889 Baseline: birth Race/ethnicity: NR |
Experimental group (higher rates of any BF measured at 3, 6, 9, and 12 mo) vs. control group | None | Ever had eczema by 6.5 y: OR: 1.0 (95% CI: 0.5, 1.8) |
| Kusel, 2005 (85) | Prospective cohort | Australia |
n = 198 Baseline: birth Race/ethnicity: NR Risk: 100% family history (≥1 parent) |
BF duration (wk) in participants with nonatopic eczema vs. atopic eczema by 5 y | None | ∼22.2 wk (95% CI: ∼19.7, ∼25.0) vs. ∼26.0 wk (95% CI: ∼23.7, ∼28.5), P = 0.06 |
| Larsson, 2008 (18) | Prospective cohort (DBH) | Sweden |
n = 4779 Baseline: 1–4 y Race/ethnicity: NR |
BF 3–6 mo vs. BF >6 mo | None | 5-y cumulative incidence of eczema by 6–9 y: OR: 0.90 (95% CI: 0.71, 1.15) |
| BF <3 mo vs. >6 mo | None | 5-y cumulative incidence of eczema by 6–9 y: OR: 0.88 (95% CI: 0.64, 1.23) | ||||
| Orivuori, 2014 (32) | Prospective cohort (PASTURE) | Finland, France, Germany, Switzerland |
n = 853 Baseline: birth Race/ethnicity: NR |
BF ≤3 mo vs. BF >6 moBF 3–6 mo vs. BF >6 mo | NoneNone | Atopic dermatitis up to 4 y: OR: 1.14 (95% CI: 0.71, 1.85)Atopic dermatitis up to 4 y: OR: 1.25 (95% CI: 0.79, 1.98) |
| Sandini, 2011 (64) | Prospective cohort4 | Finland |
n = 891 Baseline: birth Race/ethnicity: NR Risk: 100% family history (≥1 parent) |
BF ≥2 mo vs. BF <2 mo | None | Atopic eczema at 5 y: OR: 2.70 (95% CI: 0.79, 9.16) |
| Sariachvili, 2010 (54) | Nested case-control (PIPO Project) | Belgium |
n = 252 cases, 305 controls Baseline: birth Race/ethnicity: NR |
BF >4 mo vs. BF ≤4 moMean BF duration (wk) in cases vs. controls | NoneNone | Eczema up to age 4 y: OR: 0.97 (95% CI: 0.67, 1.41)Eczema up to age 4 y: 13.8 wk (SE = 0.8) vs. 15.0 wk (SE = 0.8), P = 0.27 |
1BF, breastfeeding; DBH, Dampness in Buildings and Health; IgE, immunoglobulin E; MAS, Multicentre Allergy Study; NR, not reported; PASTURE, Protection Against Allergy Study in Rural Environments; PIPO Project, Prospective Study on the Influence of Perinatal factors on the Occurrence of Asthma and Allergies; PROBIT, Promotion of Breastfeeding Intervention Trial; RCT, randomized controlled trial; ref, reference; SR, systematic review.
2Exposures, as defined by the authors of the studies included in the body of evidence, which address shorter versus longer durations of any human milk feeding or vice versa.
3RCT of an intervention to promote prolonged duration and exclusivity of breastfeeding rather than an RCT of breastfeeding per se.
4The cohort was sampled from an RCT; however, the data of interest for this SR are unrelated to randomization.
There were also 6 prospective cohort studies that presented evidence across 7 articles (18, 32, 52, 64–66, 85) (Table 8) [Bergmann et al. (52, 65) presented evidence from the Multicentre Allergy Study (MAS) across 2 articles] and 1 nested case-control study (54). Data on human milk feeding were collected by parent report via interview, questionnaire, or diary. The duration of human milk feeding was assessed as a continuous variable (52, 54, 66, 85) and heterogeneous categorical variables (18, 32, 52, 54, 64, 65). Atopic dermatitis was defined based on parent responses to items from the ISAAC questionnaire (18, 54); by parent report of a physician's diagnosis or a positive SCORAD score (32); and by physical examination plus parent-reported case history (52, 65, 66), a positive skin-prick test on ≥1 occasion (64, 85) or an allergen-specific IgE concentration ≥0.7 kU/L (64). Bergmann et al. (52, 65), Sandini et al. (64), and Kusel et al. (85) specifically recruited high-risk or “risk-enriched” samples based on a combination of family history of allergic disease and IgE concentrations. The comparisons of interest by Sariachvili et al. (54), Larsson et al. (18), and Kusel et al. (85) were unadjusted, whereas the remaining studies considered a range of confounders.
Most of the associations between the duration of any human milk feeding and atopic dermatitis in childhood were nonsignificant. The only study with statistically significant associations was the MAS. Bergmann et al. (52) found a positive association between the duration of any human milk feeding (assessed as a continuous variable) and atopic eczema through 7 y of age (OR: 1.029; 95% CI: 1.002, 1.057) and between being fed human milk for ≥2 mo, compared with <2 mo, and higher odds of atopic eczema through 7 y of age (OR: 1.384; 95% CI: 1.025, 1.869). However, the significant associations were limited to 1 (52) of 2 articles (52, 65) with data from the MAS, and to 2 of the 10 relevant analyses in the article.
Discussion
TEC members graded the evidence underlying their conclusions about 1) never versus ever being fed human milk and asthma in childhood and 2) shorter versus longer durations of any human milk feeding and asthma in childhood and adolescence as moderate (Table 9) after considering the adequacy, consistency, impact, generalizability, and internal validity of the evidence. Seventeen studies examined never versus ever being fed human milk and asthma in children, and the statistically significant associations all showed that never being fed human milk was associated with higher risk of asthma (17, 18, 23, 26–28, 30, 33, 34, 36, 62). Similarly, 20 studies examined the duration of any human milk feeding and asthma in childhood and adolescence and, with 1 exception (37), the statistically significant associations showed that shorter versus longer durations were associated with higher risk of asthma (18, 63, 68, 70, 74, 76, 78). The majority of nonsignificant associations were also consistent in suggesting higher risk with never versus ever feeding human milk and shorter versus longer durations of any human milk feeding (18, 22, 24, 26, 27, 29–31, 33, 35, 62, 64–67, 70, 71, 73–75, 78), and some of the inconsistency in statistical significance may be explained by insufficient statistical power resulting in wide CIs (18, 24, 62, 64, 75). Evidence was consistent despite heterogeneous independent variables resulting from not defining “longer,” “shorter,” or “ever” for the SRs and instead including all relevant comparisons. However, the consistency was limited to observational studies because the single experimental study had a nonsignificant association (60).
TABLE 9.
Systematic review questions, conclusion statements, and grades of the evidence supporting the conclusion statements
| Systematic review questions | Conclusion statements |
|---|---|
| What is the relationship between never versus ever feeding human milk and food allergies, allergic rhinitis, atopic dermatitis, and asthma throughout the life span? | Moderate evidence suggests that never, in comparison to ever, being fed human milk is associated with higher risk of childhood asthma. (Grade: moderate)Limited evidence does not suggest a relationship between never versus ever being fed human milk and atopic dermatitis in childhood. (Grade: limited)Evidence about the relationship between never versus ever being fed human milk and atopic dermatitis from birth to 24 mo is inconclusive, and there is insufficient evidence to determine the relationship of never versus ever being fed human milk with food allergies throughout the life span, allergic rhinitis throughout the life span, asthma in adolescence or in adulthood, and atopic dermatitis in adolescence or in adulthood. (Grade: grade not assignable) |
| What is the relationship between shorter versus longer durations of any human milk feeding and food allergies, allergic rhinitis, atopic dermatitis, and asthma throughout the life span? | Moderate evidence, mostly from observational studies, suggests that, among infants fed human milk, shorter versus longer durations of any human milk feeding are associated with higher risk of asthma in childhood and adolescence. (Grade: moderate)Limited evidence does not suggest a relationship between the duration of any human milk feeding and allergic rhinitis or atopic dermatitis in childhood. (Grade: limited)Evidence about the relationship between shorter versus longer durations of any human milk feeding and atopic dermatitis from birth to 24 mo is inconclusive, and there is insufficient evidence to determine the relationship of shorter versus longer durations of any human milk feeding with food allergies throughout the life span; allergic rhinitis from birth to 24 mo, in adolescence, or in adulthood; asthma in adulthood; and atopic dermatitis in adolescence or in adulthood. (Grade: grade not assignable) |
| What is the relationship between shorter versus longer durations of exclusive human milk feeding prior to the introduction of infant formula and food allergies, allergic rhinitis, atopic dermatitis, and asthma throughout the life span? | There is insufficient evidence to determine the relationship between shorter versus longer durations of exclusive human milk feeding prior to the introduction of infant formula and food allergies, allergic rhinitis, atopic dermatitis, and asthma throughout the life span. (Grade: grade not assignable) |
| What is the relationship between feeding a lower versus higher intensity, proportion, or amount of human milk to mixed-fed infants and food allergies, allergic rhinitis, atopic dermatitis, and asthma throughout the life span? | There is no evidence to determine the relationship between feeding a lower versus higher intensity, proportion, or amount of human milk to mixed-fed infants and food allergies, allergic rhinitis, atopic dermatitis, and asthma throughout the life span. (Grade: grade not assignable) |
| What is the relationship between feeding a higher intensity, proportion, or amount of human milk by bottle versus by breast and food allergies, allergic rhinitis, atopic dermatitis, and asthma throughout the life span? | There is no evidence to determine the relationship between feeding a higher intensity, proportion, or amount of human milk by bottle versus by breast and food allergies, allergic rhinitis, atopic dermatitis, and asthma throughout the life span. (Grade: grade not assignable) |
In the NESR grading rubric, the impact of the evidence takes into consideration the directness with which the study designs examined the link between the exposure and outcome of interest in the SR question, and the clinical significance of the evidence. Although some studies’ original objectives were not explicitly stated, most studies described objectives related to examining the link between feeding human milk and asthma. Six studies with evidence about shorter versus longer durations of human milk feeding and asthma in childhood (18, 62–64, 71, 73, 75) and 4 studies with evidence about never versus ever being fed human milk and asthma in childhood (18, 19, 26, 29, 62) were indirect. Regarding clinical significance, asthma affects the quality of life for millions of children in the United States and can be life threatening (7). Therefore, even small decreases in the risk for asthma have the potential to be of public health importance.
The generalizability of the evidence to US populations had a few limitations but was sound overall. There were 2 US studies of shorter versus longer durations of any human milk feeding and asthma in childhood and adolescence; however, they lacked racial and ethnic diversity. In addition, 1 sample was high risk for type 1 diabetes and the evidence of interest for this SR did not include any corresponding model adjustments (63). There were 5 North American studies with evidence on never versus ever feeding human milk and childhood asthma, and the 2 US studies used racially and ethnically diverse samples. Across both bodies of evidence, the samples were from countries that were high or very high on the Human Development Index (86), and therefore had a level of human development likely generalizable to the United States.
There were some concerns about internal validity. Infant milk-feeding research can be prone to detection bias because infant milk-feeding data are often collected through the use of parent-reporting methods that may not be valid and reliable; however, most studies collected these data prospectively, which reduces recall bias. Confounding can arise because differences between feeding groups are rarely mitigated by randomization (due to ethical issues around allocating infants to be fed less or no human milk) and infant-feeding decisions can be strongly socially patterned. However, most studies adjusted for confounding variables deemed important and feasible to control, although the specific adjustment variables varied between studies. Reverse causation can be a major concern because parents may decide, or receive medical advice, to continue or discontinue feeding human milk based on infants’ symptoms and because atopic disease in parents or older siblings may influence parents’ feeding decisions as they try to prevent asthma. However, the majority of studies found no baseline differences in family history of atopic disease between groups or included family history of atopic disease as an adjustment variable (18, 22–25, 27–29, 31–34, 36, 60, 62–65, 67, 68, 70, 73–78). Attrition bias, due to high attrition, differential attrition, or both, may have existed among some of the studies in the body of evidence (18, 22, 27, 31, 33, 36, 37, 62, 65, 66, 71, 77, 78); however, these studies were not more concentrated among the studies with significant compared with nonsignificant associations.
TEC members graded the evidence underlying their conclusions on 1) never versus ever being fed human milk and atopic dermatitis in childhood, 2) longer versus shorter durations of any human milk feeding and allergic rhinitis in childhood, and 3) longer versus shorter durations of any human milk feeding and atopic dermatitis in childhood as limited (Table 9). Evidence underlying all 3 conclusion statements was consistent, with nonsignificant associations across all but 1 of the observational studies in each body of evidence (52, 53, 61). Furthermore, the inconsistency may be explainable because the significant associations were found in 1) a study that may have had limited generalizability because approximately half of the participants were born small for gestational age (53), 2) a subsample analysis of African Americans (61) with no similar analyses that TEC members could examine to assess whether the association was typical in African Americans, and 3) a study with 11 comparisons across 2 articles (52, 65) that may have been prone to multiple comparison bias.
The evidence underlying all 3 conclusion statements had limitations related to adequacy, impact, generalizability, and internal validity. The bodies of evidence were small (9, 5, and 8 studies, respectively), and some samples may have been too small for sufficient statistical power to examine the comparisons of interest (29, 32, 52, 64, 65). Regarding impact, the evidence does not suggest relationships between never versus ever being fed human milk and atopic dermatitis in childhood or between the duration of human milk feeding and allergic rhinitis or atopic dermatitis in childhood, and with no relationship there would be no clinical significance. There are some doubts about generalizability to US populations because there was only 1 US sample with evidence about the duration of any human milk feeding and allergic rhinitis in childhood and it lacked racial and ethnic diversity (61), and there were no US samples with evidence on the duration of any human milk feeding or never versus ever being fed human milk and atopic dermatitis in childhood. In addition, in the evidence base for shorter versus longer durations of any human milk feeding and allergic rhinitis, 1 sample was from a cohort at risk for type 1 diabetes (not atopic disease) and the comparison of interest for this SR was not adjusted for any type 1 diabetes risk–related variables (63). Finally, the evidence had internal validity limitations because, as previously described, detection and selection bias may pervade infant milk-feeding research and some studies had high or differential attrition (18, 31, 52, 53, 62, 65, 66, 85).
Research recommendations
TEC members identified several research recommendations. There was insufficient evidence to answer 3 of the 5 SR questions (Table 9). In addition, there was evidence to answer 2 of the 5 SR questions for specific age groups but not throughout the life span. Therefore, studies need to be designed and conducted to examine these gaps in evidence. Studies with representative US samples are needed to confirm current evidence. Researchers should move toward collecting infant-feeding data consistently using validated methods. We propose that researchers study the duration of human milk feeding among infants fed human milk (i.e., assess infants who were never fed human milk separately from humans who were fed human milk). Another consideration is that there may be a large degree of overlap between current literature examining the duration of exclusive human milk feeding (which may terminate with complementary feeding) and the timing of introduction of complementary foods and beverages. However, the degree of overlap is difficult to ascertain because infant-feeding variables often lack explicit and clear definitions. Precise information about what infants are fed needs to be collected and presented more consistently by the research community. Infant-feeding research will continue to rely on observational designs; however, researchers should endeavor to minimize bias through sound research design and conduct. For example, baseline differences in critical confounding variables should be assessed between comparison groups, and statistical adjustments should be made, as necessary. Studies of outcomes in the B-24 population should also address temporality. Finally, researchers should incorporate effect modification into their study design whenever possible (e.g., family history of atopic disease) because different environmental and biological characteristics may modify the impact of infant milk-feeding practices on the outcomes.
ACKNOWLEDGEMENTS
We thank Katherine Kortsmit and Kelly Mannherz for their assistance with extracting data and assessing the studies’ risk of bias. We also thank Sue Anderson for serving as a TEC member until December 2015.
The authors’ responsibilities were as follows—DG, PN, SAA, LB, KMJ, LAN-R, KOO'B, EO, RP-E, EEZ, and JMS: participated in establishing the research questions, analytic framework, and study inclusion and exclusion criteria; YPW and NT: developed and conducted the literature search; PN, CD, and DG: screened search results and identified studies for inclusion; CCL, PN, and DG: conducted a manual search, extracted data, and assessed risk of bias for included studies; SAA, LB, TJ, KMJ, LAN-R, KOO'B, EO, RP-E, and EEZ: reviewed and provided substantive feedback on all SR materials, including the synthesis of the body of evidence, conclusion statement, and grade of the strength of the evidence; DG, PN, and CCL: wrote the manuscript; DG: had primary responsibility for final content: JMS: provided oversight of the project; and all authors: critically reviewed and read and approved the final manuscript. The authors declared no conflicts of interest.
Notes
Published in a supplement to The American Journal of Clinical Nutrition. This article is one in a series of systematic reviews completed with support from the USDA’s Nutrition Evidence Systematic Review team as part of the Pregnancy and Birth to 24 Months Project. The supplement is sponsored by the USDA Food and Nutrition Service. The Supplement Coordinator for the supplement publication was Joanne M Spahn, USDA Center for Nutrition Policy and Promotion, Alexandria, VA. Supplement Coordinator disclosure: No conflicts of interest or financial ties to disclose. The Guest Editor for this supplement was Pieter J Sauer. Guest Editor disclosure: No conflicts of interest to disclose.
Publication costs for this supplement were defrayed in part by the payment of page charges. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of The American Journal of Clinical Nutrition.
Abbreviations used: B-24, birth to 24 months; DBH, Dampness in Buildings and Health; IgE, immunoglobulin E; ISAAC, International Study of Asthma and Allergy in Childhood; MAS, Multicentre Allergy Study; NESR, Nutrition Evidence Systematic Review; PROBIT, Promotion of Breastfeeding Intervention Trial; SCORAD, Scoring Atopic Dermatitis; SR, systematic review; TEC, Technical Expert Collaborative.
References
- 1. Halfon N, Larson K, Lu M, Tullis E, Russ S. Lifecourse health development: past, present and future. Matern Child Health J. 2014;18(2):344–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Obbagy JE, Blum-Kemelor DM, Essery EV, Lyon JMG, Spahn JM. USDA Nutrition Evidence Library: methodology used to identify topics and develop systematic review questions for the birth-to-24-mo population. Am J Clin Nutr. 2014;99(3):S 692–6. [DOI] [PubMed] [Google Scholar]
- 3. Raiten DJ, Raghavan R, Porter A, Obbagy JE, Spahn JM. Executive summary: evaluating the evidence base to support the inclusion of infants and children from birth to 24 mo of age in the Dietary Guidelines for Americans—“the B-24 Project.” Am J Clin Nutr. 2014;99(3):S663–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stoody EE, Spahn JM, Casavale KO. The Pregnancy and Birth to 24 Months Project: a series of systematic reviews on diet and health. Am J Clin Nutr. 2019;109(Supplement_7):685S–97S. [DOI] [PubMed] [Google Scholar]
- 5. National Institute of Allergy and Infectious Diseases. Food allergy. [Internet]. Version 27 [March 2017; cited 2018 Jan 31]. Available from: https://www.niaid.nih.gov/diseases-conditions/food-allergy. [Google Scholar]
- 6. National Institute of Allergy and Infectious Diseases. Eczema (atopic dermatitis). [Internet]. Version 19 [April 2017; cited 2018 Jan 31]. Available from: https://www.niaid.nih.gov/diseases-conditions/eczema-atopic-dermatitis. [Google Scholar]
- 7. Centers for Disease Control and Prevention. National Center for Health Statistics. Asthma [Internet]. Version 31 [March 2017; cited 2018 Jan 31]. Available from: https://www.cdc.gov/nchs/fastats/asthma.htm. [Google Scholar]
- 8. Centers for Disease Control and Prevention. National Center for Health Statistics. Allergies [Internet]. Version 30 [March 2017; cited 2018 Jan 31]. Available from: https://www.cdc.gov/nchs/fastats/allergies.htm. [Google Scholar]
- 9. Obbagy JE, Spahn JM, Wong YP, Psota TL, Spill MK, Dreibelbis C, Güngör D, Nadaud P, Raghavan R, Callahan EH et al.. Systematic review methodology used in the Pregnancy and Birth to 24 Months Project. Am J Clin Nutr. 2019;109(Supplement_7):698S–704S. [DOI] [PubMed] [Google Scholar]
- 10. Obbagy JE, English LK, Wong YP, Butte NF, Dewey KG, Fleischer DM, Fox MK, Greer FR, Krebs NF, Scanlon KS et al.. Complementary feeding and food allergies, atopic dermatitis and eczema, asthma, and allergic rhinitis: a systematic review. Am J Clin Nutr. 2019;109(Supplement_7):890S–934S. [DOI] [PubMed] [Google Scholar]
- 11. Infant Formula Act of 1980, Public Law 96–359., 96th Congress, Session 2. [Google Scholar]
- 12. Turati F, Bertuccio P, Galeone C, Pelucchi C, Naldi L, Bach JF, La Vecchia C, Chatenoud L. Early weaning is beneficial to prevent atopic dermatitis occurrence in young children. Allergy. 2016;71(6):878–88. [DOI] [PubMed] [Google Scholar]
- 13. Ivakhnenko OS, Nyankovskyy SL. Effect of the specific infant formula mixture of oligosaccharides on local immunity and development of allergic and infectious disease in young children: randomized study. Pediatria Polska. 2013;88(5):398–404. [Google Scholar]
- 14. Grimshaw KE, Maskell J, Oliver EM, Morris RC, Foote KD, Mills EN, Roberts G, Margetts BM. Introduction of complementary foods and the relationship to food allergy. Pediatrics. 2013;132(6):e1529–38. [DOI] [PubMed] [Google Scholar]
- 15. Host A. Importance of the first meal on the development of cow's milk allergy and intolerance. Allergy Proc. 1991;12(4):227–32. [DOI] [PubMed] [Google Scholar]
- 16. McGowan EC, Bloomberg GR, Gergen PJ, Visness CM, Jaffee KF, Sandel M, O'Connor G, Kattan M, Gern J, Wood RA. Influence of early-life exposures on food sensitization and food allergy in an inner-city birth cohort. J Allergy Clin Immunol. 2015;135(1):171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Milner JD, Stein DM, McCarter R, Moon RY. Early infant multivitamin supplementation is associated with increased risk for food allergy and asthma. Pediatrics. 2004;114(1):27–32. [DOI] [PubMed] [Google Scholar]
- 18. Larsson M, Hagerhed EL, Sigsgaard T, Janson S, Sundell J, Bornehag CG. Incidence rates of asthma, rhinitis and eczema symptoms and influential factors in young children in Sweden. Acta Paediatr. 2008;97(9):1210–5. [DOI] [PubMed] [Google Scholar]
- 19. Burr ML, Limb ES, Maguire MJ, Amarah L, Eldridge BA, Layzell JC, Merrett TG. Infant feeding, wheezing, and allergy: a prospective study. Arch Dis Child. 1993;68(6):724–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burgess SW, Dakin CJ, O'Callaghan MJ. Breastfeeding does not increase the risk of asthma at 14 years. Pediatrics. 2006;117(4):e787–92. [DOI] [PubMed] [Google Scholar]
- 21. Arshad SH, Kurukulaaratchy RJ, Fenn M, Matthews S. Early life risk factors for current wheeze, asthma, and bronchial hyperresponsiveness at 10 years of age. Chest. 2005;127(2):502–8. [DOI] [PubMed] [Google Scholar]
- 22. Hillemeier MM, Landale NS, Oropesa RS. Asthma in US Mexican-origin children in early childhood: differences in risk and protective factors by parental nativity. Acad Pediatr. 2015;15(4):421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Infante RC. Childhood asthma and indoor environmental risk factors. Am J Epidemiol. 1993;137(8):834–44. [DOI] [PubMed] [Google Scholar]
- 24. Infante RC, Amre D, Gautrin D, Malo JL. Family size, day-care attendance, and breastfeeding in relation to the incidence of childhood asthma. Am J Epidemiol. 2001;153(Supplement_7):653–8. [DOI] [PubMed] [Google Scholar]
- 25. Leung JY, Kwok MK, Leung GM, Schooling CM. Breastfeeding and childhood hospitalizations for asthma and other wheezing disorders. Ann Epidemiol. 2016;26(1):21–7 e1-3. [DOI] [PubMed] [Google Scholar]
- 26. Maas T, Dompeling E, Muris JW, Wesseling G, Knottnerus JA, van Schayck OC. Prevention of asthma in genetically susceptible children: a multifaceted intervention trial focussed on feasibility in general practice. Pediatr Allergy Immunol. 2011;22(8):794–802. [DOI] [PubMed] [Google Scholar]
- 27. Martel MJ, Rey E, Malo JL, Perreault S, Beauchesne MF, Forget A, Blais L. Determinants of the incidence of childhood asthma: a two-stage case-control study. Am J Epidemiol. 2009;169(2):195–205. [DOI] [PubMed] [Google Scholar]
- 28. Midodzi WK, Rowe BH, Majaesic CM, Saunders LD, Senthilselvan A. Early life factors associated with incidence of physician-diagnosed asthma in preschool children: results from the Canadian Early Childhood Development cohort study. J Asthma. 2010;47(1):7–13. [DOI] [PubMed] [Google Scholar]
- 29. Mihrshahi S, Ampon R, Webb K, Almqvist C, Kemp AS, Hector D, Marks GB. The association between infant feeding practices and subsequent atopy among children with a family history of asthma. Clin Exp Allergy. 2007;37(5):671–9. [DOI] [PubMed] [Google Scholar]
- 30. Miller JE. Predictors of asthma in young children: does reporting source affect our conclusions? Am J Epidemiol. 2001;154(3):245–50. [DOI] [PubMed] [Google Scholar]
- 31. Nwaru BI, Craig LC, Allan K, Prabhu N, Turner SW, McNeill G, Erkkola M, Seaton A, Devereux G. Breastfeeding and introduction of complementary foods during infancy in relation to the risk of asthma and atopic diseases up to 10 years. Clin Exp Allergy. 2013;43(11):1263–73. [DOI] [PubMed] [Google Scholar]
- 32. Orivuori L, Loss G, Roduit C, Dalphin JC, Depner M, Genuneit J, Lauener R, Pekkanen J, Pfefferle P, Riedler J et al.. Soluble immunoglobulin A in breast milk is inversely associated with atopic dermatitis at early age: the PASTURE cohort study. Clin Exp Allergy. 2014;44(1):102–12. [DOI] [PubMed] [Google Scholar]
- 33. Scholtens S, Wijga AH, Brunekreef B, Kerkhof M, Hoekstra MO, Gerritsen J, Aalberse R, de Jongste JC, Smit HA. Breast feeding, parental allergy and asthma in children followed for 8 years. The PIAMA birth cohort study. Thorax. 2009;64(Supplement_7):604–9. [DOI] [PubMed] [Google Scholar]
- 34. Sunyer J, Torrent M, Garcia ER, Ribas FN, Carrizo D, Romieu I, Anto JM, Grimalt JO. Early exposure to dichlorodiphenyldichloroethylene, breastfeeding and asthma at age six. Clin Exp Allergy. 2006;36(10):1236–41. [DOI] [PubMed] [Google Scholar]
- 35. van Beijsterveldt TC, Boomsma DI. An exploration of gene-environment interaction and asthma in a large sample of 5-year-old Dutch twins. Twin Res Hum Genet. 2008;11(2):143–9. [DOI] [PubMed] [Google Scholar]
- 36. Wilson AC, Forsyth JS, Greene SA, Irvine L, Hau C, Howie PW. Relation of infant diet to childhood health: seven year follow up of cohort of children in Dundee infant feeding study. BMJ. 1998;316(7124):21–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Colen CG, Ramey DM. Is breast truly best? Estimating the effects of breastfeeding on long-term child health and wellbeing in the United States using sibling comparisons. Soc Sci Med. 2014;109:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rosas-Salazar C, Forno E, Brehm JM, Han YY, Acosta-Perez E, Cloutier MM, Wakefield DB, Alvarez M, Colon-Semidey A, Canino G et al.. Breastfeeding duration and asthma in Puerto Rican children. Pediatr Pulmonol. 2015;50(6):527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Burr ML, Miskelly FG, Butland BK, Merrett TG, Vaughan WE. Environmental factors and symptoms in infants at high risk of allergy. J Epidemiol Community Health. 1989;43(2):125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chuang CH, Hsieh WS, Chen YC, Chang PJ, Hurng BS, Lin SJ, Chen PC. Infant feeding practices and physician diagnosed atopic dermatitis: a prospective cohort study in Taiwan. Pediatr Allergy Immunol. 2011;22(1 Part 1):43–9. [DOI] [PubMed] [Google Scholar]
- 41. Harris JM, Cullinan P, Williams HC, Mills P, Moffat S, White C, Newman Taylor AJ. Environmental associations with eczema in early life. Br J Dermatol. 2001;144(4):795–802. [DOI] [PubMed] [Google Scholar]
- 42. Howie PW, Forsyth JS, Ogston SA, Clark A, Florey C. Protective effect of breastfeeding against infection. BMJ 1990;300(6716):11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mallet E, Henocq A. Long-term prevention of allergic diseases by using protein hydrolysate formula in at-risk infants. J Pediatr. 1992;121(5 Pt 2):S95–100. [DOI] [PubMed] [Google Scholar]
- 44. Miskelly F G, Burr M L, Vaughan WE, Fehily A M, Butland BKMerrett TG. Infant feeding and allergy. Arch Dis Child. 1988;63(4):388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Morales E, Garcia ER, Guxens M, Guerra S, Mendez M, Molto-Puigmarti C, Lopez-Sabater MC, Sunyer J. Effects of prolonged breastfeeding and colostrum fatty acids on allergic manifestations and infections in infancy. Clin Exp Allergy. 2012;42(6):918–28. [DOI] [PubMed] [Google Scholar]
- 46. Parazzini F, Cipriani S, Zinetti C, Chatenoud L, Frigerio L, Amuso G, Ciammella M, Di Landro A, Naldi L. Perinatal factors and the risk of atopic dermatitis: a cohort study. Pediatr Allergy Immunol. 2014;25(1):43–50. [DOI] [PubMed] [Google Scholar]
- 47. Rothenbacher D, Weyermann M, Beermann C, Brenner H. Breastfeeding, soluble CD14 concentration in breast milk and risk of atopic dermatitis and asthma in early childhood: birth cohort study. Clin Exp Allergy. 2005;35(8):1014–21. [DOI] [PubMed] [Google Scholar]
- 48. Sariachvili M, Droste J, Dom S, Wieringa M, Vellinga A, Hagendorens M, Bridts C, Stevens W, Sprundel MV, Desager K et al.. Is breast feeding a risk factor for eczema during the first year of life? Pediatr Allergy Immunol. 2007;18(5):410–7. [DOI] [PubMed] [Google Scholar]
- 49. Snijders BE, Thijs C, Dagnelie PC, Stelma F F, Mommers M, Kummeling I, Penders J, van Ree R, van den Brandt PA. Breast-feeding duration and infant atopic manifestations, by maternal allergic status, in the first 2 years of life (KOALA study). J Pediatr. 2007;151(4):347–51., 51 e1–2. [DOI] [PubMed] [Google Scholar]
- 50. Snijders BE, Thijs C, Kummeling I, Penders J, van den Brandt PA. Breastfeeding and infant eczema in the first year of life in the KOALA birth cohort study: a risk period-specific analysis. Pediatrics. 2007;119(1):e137–41. [DOI] [PubMed] [Google Scholar]
- 51. Snijders BE, Thijs C, van Ree R, van den Brandt PA. Age at first introduction of cow milk products and other food products in relation to infant atopic manifestations in the first 2 years of life: the KOALA Birth Cohort Study. Pediatrics. 2008;122(1):e115–22. [DOI] [PubMed] [Google Scholar]
- 52. Bergmann RL, Diepgen TL, Kuss O, Bergmann KE, Kujat J, Dudenhausen JW, Wahn U. Breastfeeding duration is a risk factor for atopic eczema. Clin Exp Allergy. 2002;32(2):205–9. [DOI] [PubMed] [Google Scholar]
- 53. Purvis DJ, Thompson JM, Clark PM, Robinson E, Black PN, Wild CJ, Mitchell EA. Risk factors for atopic dermatitis in New Zealand children at 3.5 years of age. Br J Dermatol. 2005;152(4):742–9. [DOI] [PubMed] [Google Scholar]
- 54. Sariachvili M, Droste J, Dom S, Wieringa M, Hagendorens M, Stevens W, van Sprundel M, Desager K, Weyler J. Early exposure to solid foods and the development of eczema in children up to 4 years of age. Pediatr Allergy Immunol. 2010;21(1 Pt 1):74–81. [DOI] [PubMed] [Google Scholar]
- 55. Zutavern A, von Mutius E, Harris J, Mills P, Moffatt S, White C, Cullinan P. The introduction of solids in relation to asthma and eczema. Arch Dis Child. 2004;89(4):303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. International Study of Asthma and Allergies in Childhood. Childhood Acute Lymphoblastic Leukemia Treatment (PDQ®)—Health Professional Version. [Internet]. Version 5 [April 2017; cited 13 Apr 2018]. Available from: https://www.cancer.gov/types/leukemia/hp/child-all-treatment-pdq. [Google Scholar]
- 57. Hesselmar B, Saalman R, Rudin A, Adlerberth I, Wold A. Early fish introduction is associated with less eczema, but not sensitization, in infants. Acta Paediatr. 2010;99(12):1861–7. [DOI] [PubMed] [Google Scholar]
- 58. Poole JA, Barriga K, Leung DY, Hoffman M, Eisenbarth GS, Rewers M, Norris JM. Timing of initial exposure to cereal grains and the risk of wheat allergy. Pediatrics. 2006;117(6):2175–82. [DOI] [PubMed] [Google Scholar]
- 59. Kull I, Wickman M, Lilja G, Nordvall SL, Pershagen G. Breast feeding and allergic diseases in infants—a prospective birth cohort study. Arch Dis Child. 2002;87(6):478–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kramer MS, Matush L, Vanilovich I, Platt R, Bogdanovich N, Sevkovskaya Z, Dzikovich I, Shishko G, Mazer B. Effect of prolonged and exclusive breast feeding on risk of allergy and asthma: cluster randomised trial. BMJ. 2007;335(7624):815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Codispoti CD, Levin L, LeMasters GK, Ryan P, Reponen T, Villareal M, Burkle J, Stanforth S, Lockey JE, Khurana Hershey GK et al.. Breast-feeding, aeroallergen sensitization, and environmental exposures during infancy are determinants of childhood allergic rhinitis. J Allergy Clin Immunol. 2010;125(5):1054–60 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. von Kobyletzki LB, Bornehag CG, Hasselgren M, Larsson M, Lindstrom CB, Svensson A. Eczema in early childhood is strongly associated with the development of asthma and rhinitis in a prospective cohort. BMC Dermatol. 2012;12:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nwaru BI, Takkinen HM, Niemela O, Kaila M, Erkkola M, Ahonen S, Haapala AM, Kenward MG, Pekkanen J, Lahesmaa R et al.. Timing of infant feeding in relation to childhood asthma and allergic diseases. J Allergy Clin Immunol. 2013;131(1):78–86. [DOI] [PubMed] [Google Scholar]
- 64. Sandini U, Kukkonen AK, Poussa T, Sandini L, Savilahti E, Kuitunen M. Protective and risk factors for allergic diseases in high-risk children at the ages of two and five years. Int Arch Allergy Immunol. 2011;156(3):339–48. [DOI] [PubMed] [Google Scholar]
- 65. Bergmann RL, Edenharter G, Bergmann K E, Lau S, Wahn U. Socioeconomic status is a risk factor for allergy in parents but not in their children. Clin Exp Allergy. 2000;30(12):1740–5. [DOI] [PubMed] [Google Scholar]
- 66. Grandjean P, Poulsen LK, Heilmann C, Steuerwald U, Weihe P. Allergy and sensitization during childhood associated with prenatal and lactational exposure to marine pollutants. Environ Health Perspect. 2010;118(10):1429–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Karmaus W, Dobai AL, Ogbuanu I, Arshard SH, Matthews S, Ewart S. Long-term effects of breastfeeding, maternal smoking during pregnancy, and recurrent lower respiratory tract infections on asthma in children. J Asthma. 2008;45(8):688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Karunasekera KA, Jayasinghe JA, Alwis L W. Risk factors of childhood asthma: a Sri Lankan study. J Trop Pediatr. 2001;47(3):142–5. [DOI] [PubMed] [Google Scholar]
- 69. Klinnert MD, Nelson HS, Price MR, Adinoff AD, Leung DY, Mrazek DA. Onset and persistence of childhood asthma: predictors from infancy. Pediatrics. 2001;108(4):E69. [DOI] [PubMed] [Google Scholar]
- 70. Kull I, Almqvist C, Lilja G, Pershagen G, Wickman M. Breast-feeding reduces the risk of asthma during the first 4 years of life. J Allergy Clin Immunol. 2004;114(4):755–60. [DOI] [PubMed] [Google Scholar]
- 71. Oddy WH, Holt PG, Sly PD, Read AW, Landau LI, Stanley FJ, Kendall GE, Burton PR. Association between breast feeding and asthma in 6 year old children: findings of a prospective birth cohort study. BMJ. 1999;319(7213):815–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ronmark E, Perzanowski M, Platts-Mills T, Lundback B. Incidence rates and risk factors for asthma among school children: a 2-year follow-up report from the obstructive lung disease in Northern Sweden (OLIN) studies. Respir Med. 2002;96(12):1006–13. [DOI] [PubMed] [Google Scholar]
- 73. Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B, Bjorksten B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95(4):500–5. [PubMed] [Google Scholar]
- 74. Silvers KM, Frampton CM, Wickens K, Pattemore PK, Ingham T, Fishwick D, Crane J, Town GI, Epton MJ et al.. Breastfeeding protects against current asthma up to 6 years of age. J Pediatr. 2012;160(6):991–6 e1. [DOI] [PubMed] [Google Scholar]
- 75. Strassburger SZ, Vitolo MR, Bortolini GA, Pitrez PM, Jones MH, Stein RT. Nutritional errors in the first months of life and their association with asthma and atopy in preschool children. J Pediatr (Rio J). 2010;86(5):391–9. [DOI] [PubMed] [Google Scholar]
- 76. Al-Mousawi MS, Lovel H, Behbehani N, Arifhodzic N, Woodcock A, Custovic A. Asthma and sensitization in a community with low indoor allergen levels and low pet-keeping frequency. J Allergy Clin Immunol. 2004;114(6):1389–94. [DOI] [PubMed] [Google Scholar]
- 77. Fredriksson P, Jaakkola N, Jaakkola JJ. Breastfeeding and childhood asthma: a six-year population-based cohort study. BMC Pediatr. 2007;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hovland V, Riiser A, Mowinckel P, Carlsen KH, Lodrup Carlsen KC. Early risk factors for pubertal asthma. Clin Exp Allergy. 2015;45(1):164–76. [DOI] [PubMed] [Google Scholar]
- 79. da Costa LR, Victora CG, Menezes AM, Barros FC. Do risk factors for childhood infections and malnutrition protect against asthma? A study of Brazilian male adolescents. Am J Public Health. 2003;93(11):1858–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Grabenhenrich LB, Gough H, Reich A, Eckers N, Zepp F, Nitsche O, Forster J, Schuster A, Schramm D, Bauer CP et al.. Early-life determinants of asthma from birth to age 20 years: a German birth cohort study. J Allergy Clin Immunol. 2014;133(4):979–88. [DOI] [PubMed] [Google Scholar]
- 81. Kerkhof M, Koopman LP, van Strien RT, Wijga A, Smit HA, Aalberse RC, Neijens HJ, Brunekreef B, Postma DS, Gerritsen J. Risk factors for atopic dermatitis in infants at high risk of allergy: the PIAMA study. Clin Exp Allergy. 2003;33(10):1336–41. [DOI] [PubMed] [Google Scholar]
- 82. Miyake Y, Tanaka K, Sasaki S, Kiyohara C, Ohya Y, Fukushima W, Yokoyama T, Hirota Y. Breastfeeding and atopic eczema in Japanese infants: The Osaka Maternal and Child Health Study. Pediatr Allergy Immunol. 2009;20(3):234–41. [DOI] [PubMed] [Google Scholar]
- 83. Silvers KM, Frampton CM, Wickens K, Epton MJ, Pattemore PK, Ingham T, Fishwick D, Crane J, Town GI. Breastfeeding protects against adverse respiratory outcomes at 15 months of age. Matern Child Nutr. 2009;5(3):243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kramer MS, Chalmers B, Hodnett ED, Sevkovskaya Z, Dzikovich I, Shapiro S, Collet JP, Vanilovich I, Mezen I, Ducruet T et al.. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285(4):413–20. [DOI] [PubMed] [Google Scholar]
- 85. Kusel MM, Holt PG, de Klerk N, Sly PD. Support for 2 variants of eczema. J Allergy Clin Immunol. 2005;116(5):1067–72. [DOI] [PubMed] [Google Scholar]
- 86. United Nations Development Programme. Human Development Report 2014. Sustaining human progress: reducing vulnerabilities and building resilience New York; 2014. [Google Scholar]
- 87. US Food and Drug Administration. Regulations and information on the manufacture and distribution of infant formula. [Internet]. Version 19 [December 2013; cited 23 March 2018]. Available from: https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/InfantFormula/ucm136118.htm#manufacture. [Google Scholar]
- 88. Food and Agriculture Organization of the United Nations; World Health Organization. Codex Alimentarius. International Food Standards. Standard for infant formula and formulas for special medical purposes intended for infants. Codex Stan 72-1981 2007. [Google Scholar]
- 89. Stein RT, Holberg CJ, Morgan WJ, Wright AL, Lombardi E, Taussig L, Martinez FD. Peak flow variability, methacholine responsiveness and atopy as markers for detecting different wheezing phenotypes in childhood. Thorax. 1997;52(11):946–52. [DOI] [PMC free article] [PubMed] [Google Scholar]