ABSTRACT
Background
During the Pregnancy and Birth to 24 Months Project, the US Departments of Agriculture and Health and Human Services initiated a review of evidence on diet and health in these populations.
Objectives
The aim of these systematic reviews was to examine the relation of 1) never versus ever feeding human milk, 2) shorter versus longer durations of any human milk feeding, 3) shorter versus longer durations of exclusive human milk feeding, and 4) feeding a lower versus higher intensity of human milk to mixed-fed infants with acute childhood leukemia, generally, and acute lymphoblastic leukemia, specifically.
Methods
The Nutrition Evidence Systematic Review team conducted systematic reviews with external experts. We searched CINAHL, Cochrane, Embase, and PubMed for articles published January 1980 to March 2016, dual-screened the results using predetermined criteria, extracted data from and assessed risk of bias for each included study, qualitatively synthesized the evidence, developed conclusion statements, and graded the strength of the evidence.
Results
We included 24 articles from case-control or retrospective studies. Limited evidence suggests that never feeding human milk versus 1) ever feeding human milk and 2) feeding human milk for durations ≥6 mo are associated with a slightly higher risk of acute childhood leukemia, whereas evidence comparing never feeding human milk with feeding human milk for durations <6 mo is mixed. Limited evidence suggests that, among infants fed human milk, a shorter versus longer duration of human milk feeding is associated with a slightly higher risk of acute childhood leukemia. None of the included articles examined exclusive human milk feeding or the intensity of human milk fed to mixed-fed infants.
Conclusions
Feeding human milk for short durations or not at all may be associated with slightly higher acute childhood leukemia risk. The evidence could be strengthened with access to broadly generalizable prospective samples; therefore, we recommend linking surveillance systems that collect infant feeding and childhood cancer data.
Keywords: breastfeeding, breast milk, human milk, leukemia, infant, toddler, child, systematic review
Introduction
The Pregnancy and Birth to 24 Months Project was an initiative of the US Departments of Agriculture and Health and Human Services (1–3). During the Project, the USDA Nutrition Evidence Systematic Review (NESR) team (previously the Nutrition Evidence Library, or NEL) collaborated with external experts to conduct systematic reviews (SRs) on nutrition and health during pregnancy and from birth to 24 mo.
The SRs in this article examine the relationships of infant milk-feeding practices with acute childhood leukemia, generally, and acute lymphoblastic leukemia (ALL), specifically. Acute leukemia makes up nearly a third of cancers in children and teens, making it the most common cancer in those age groups, and about 75% of acute leukemia cases are ALL (4, 5). A 2007 review by the Agency for Healthcare Research and Quality (6) found that infectious etiologies may exist [e.g., Greaves’ delayed infection hypothesis (7)] and may be affected by feeding human milk. In a recent review, Greaves (8) proposed that, by modulating infants’ immune systems, feeding human milk for long durations can be a factor that potentially prevents some cases of ALL.
The purpose of this article is to summarize 4 SRs conducted to answer the following questions:
What is the relationship between shorter versus longer durations of any human milk feeding and childhood leukemia?
What is the relationship between shorter versus longer durations of exclusive human milk feeding and childhood leukemia?
What is the relationship between never versus ever feeding human milk and childhood leukemia?
What is the relationship between feeding a lower versus higher intensity, proportion, or amount of human milk to mixed-fed infants and childhood leukemia?
Methods
NESR analysts and librarians, who were trained in systematic review methodology and had advanced degrees in fields such as nutrition and library science, collaborated with a group of subject-matter experts, called a Technical Expert Collaborative (TEC), to complete SRs using methods that are described in detail in this supplement (9). TEC members provided individual input on SR materials developed by the NESR staff, but did not provide formal group advice or recommendations to the government.
Scope of the systematic review
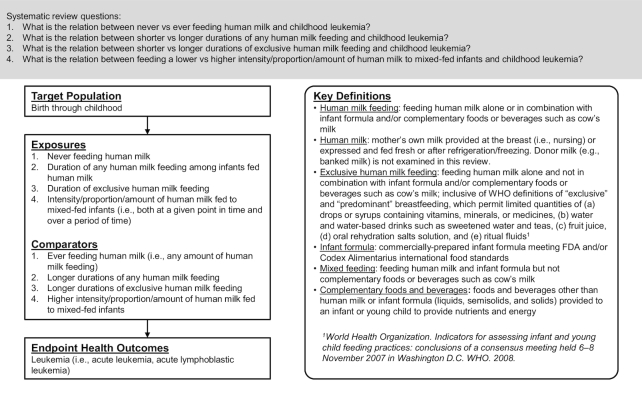
TEC members specified the target population, exposures and comparators, outcomes, and key definitions for these SRs using the analytic framework shown in Figure 1. In the SRs, infant milk-feeding practices referred to the feeding of human milk and/or infant formula. TEC members chose to use the term human milk feeding instead of breastfeeding for precision. Breastfeeding may be understood to mean feeding human milk at the breast when, in fact, feeding method was rarely distinguished by the authors of studies included in the SR. TEC members intended to examine the feeding of human milk whether or not it was fed at the breast.
FIGURE 1.
Analytic framework for systematic reviews conducted to examine the relation of infant milk-feeding practices with childhood leukemia. This framework illustrates the overall scope of the project, including the population, exposures, comparators, and outcomes of interest. It also includes definitions of key terms.
For the comparisons of shorter with longer durations of any and exclusive human milk feeding, TEC members did not define thresholds for shorter duration or longer duration. Likewise, for the comparison of never with ever feeding human milk, TEC members did not define any minimum amount for ever feeding human milk. They examined all comparisons of shorter with longer durations (or vice versa) and of never with ever feeding human milk (or vice versa) as defined by the authors of the studies included in the SRs.
Acute childhood leukemia was the outcome of interest, and we examined analyses that grouped all acute childhood leukemias together (referred to in this article as leukemia) as well as analyses of ALL specifically. We used this approach because some articles did not examine leukemia generally but did examine the most prevalent type of leukemia. We did not examine analyses of less prevalent forms of leukemia, which were less likely to have sufficient statistical power.
Literature search, screening, and selection
The librarians developed a literature search strategy that used exposure terminology but not outcome terminology (available at https://nesr.usda.gov) so that 1 search could be used to identify literature in support of SRs examining infant milk-feeding practices with several different outcomes (3). The librarians conducted a broad search in CINAHL, Cochrane, Embase, and PubMed using a search date range of January 1980 to March 2016. The search excluded articles published before 1980 because the US Congress passed the Infant Formula Act in 1980, which established nutrient requirements for commercial infant formulas in the US, and thus health effects associated with formula consumption before 1980 might be different (10).
TEC members defined inclusion and exclusion criteria a priori (Table 1), which NESR analysts used to dual-screen the search results and the results of a manual search of the references of included articles and existing SRs. TEC members reviewed the search terms and list of included articles to ensure completeness of the body of evidence.
TABLE 1.
Inclusion and exclusion criteria established for the selection of studies to include in systematic reviews on infant milk-feeding practices and childhood leukemia1
| Category | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Study design | Randomized controlled trials Nonrandomized controlled trials Prospective cohort studies Retrospective cohort studies Case-control studies |
Cross-sectional studies Before-and-after studies Uncontrolled studies Narrative reviews Systematic reviews Meta-analyses |
| Publication status | Published in peer-reviewed journals | Gray literature, including unpublished data, manuscripts, reports, abstracts, and conference proceedings |
| Language | Published in English | Published in languages other than English |
| Date range | Published from 1980 to December 20152 | Published before 1980 |
| Source of foods, beverages, or nutrients | Human milk: mothers’ own milk, that is, human milk at the breast (i.e., nursing) or expressed and fed fresh or after refrigeration/freezing Infant formula: commercially prepared infant formula meeting FDA (11) and/or Codex Alimentarius international food standards (12) |
Human milk from third parties (e.g., banked/donor milk) Infant formulas that are not commercially prepared or that do not meet FDA (11) and/or Codex Alimentarius international food standards (12) |
| Study setting | Countries listed as Very High or High on the 2014 Human Development Index (13) | Countries listed as Medium or Low on the 2014 Human Development Index (13) |
| Study participants | Human participants Males Females |
Nonhuman participants (e.g., animal studies, in vitro studies) Hospitalized patients, not including birth and immediate postpartum hospitalization of healthy infants |
| Age of study participants | Exposure age: infants (0–12 mo), toddlers (12–24 mo) Outcome age: children (2–12 y) (i.e., include studies with children within the sample) |
|
| Size of study groups | Studies with ≥30 participants per study group or a power analysis indicating that the study is appropriately powered for the outcome(s) of interest | Studies with <30 participants per study group with no power analysis indicating that the study is appropriately powered for the outcome(s) of interest |
| Health status of study participants | Studies done in generally healthy populations Studies done in populations where infants were full term (≥37 and 0/7 weeks of gestation) Studies done in populations with elevated chronic disease risk, or that enroll some participants with a disease or with the health outcome of interest |
Studies that exclusively enroll participants with a disease or the health outcome of interest Studies carried out in hospitalized participants (except for birth and immediate postpartum hospitalization of healthy infants) or malnourished participants Studies of exclusively preterm infants (gestational age <37 wk), exclusively infants that have low birth weight (<2500 g) and/or exclusively infants that are small for gestational age |
1FDA, Food and Drug Administration.
2In 1980, the Infant Formula Act was passed (14), and December 2015 was when the literature search was carried out.
Data extraction and risk of bias assessment
NESR analysts assembled a table of systematically extracted data from each article included in the SRs (i.e., study characteristics, sample characteristics, exposures and outcomes, risks of bias, and funding sources). Two NESR analysts independently completed the NEL Bias Assessment Tool for each article to identify the risks of bias [(9), https://nesr.usda.gov].
Evidence synthesis, conclusion statement development, and grading the strength of the evidence
NESR analysts and TEC members engaged in a series of conference calls to review, discuss, and synthesize the evidence. TEC members examined both significant and nonsignificant associations [e.g., odds ratios (ORs) and CIs] for a thorough synthesis of the evidence. To answer the SR questions, conclusion statements were carefully constructed to accurately reflect the synthesis of evidence. Conclusion statements do not draw implications, nor should they be interpreted to be dietary guidance. The strength of the evidence underlying each conclusion statement was graded strong, moderate, limited, or grade not assignable using the NESR grading rubric [(9), https://nesr.usda.gov], which takes into consideration the internal validity, consistency, adequacy, impact, and generalizability of the evidence. Finally, TEC members identified research recommendations.
Results
The literature search yielded 31,335 articles, and the bodies of evidence for the 4 SRs on infant milk-feeding practices and childhood leukemia comprise 24 articles (https://nesr.usda.gov). A table of articles excluded during full-text screening, with their rationale for exclusion, is available at https://nesr.usda.gov.
No articles met the inclusion criteria for the SRs (Table 1) that examined the relationships of shorter versus longer durations of exclusive human milk feeding or feeding lower versus higher intensities, proportions, or amounts of human milk to mixed-fed infants with childhood leukemia (Table 4). Herein, we present evidence for the remaining 2 SRs:
What is the relationship between never versus ever feeding human milk and childhood leukemia?
What is the relationship between shorter versus longer durations of any human milk feeding and childhood leukemia?
TABLE 4.
Systematic review questions, conclusion statements, and grades of the evidence supporting the conclusion statements within the context of these systematic reviews
| Systematic review question: what is the relationship between never versus ever feeding human milk and childhood leukemia? |
| Limited evidence suggests that never versus ever being fed human milk is associated with a slightly higher risk of childhood leukemia. The evidence comparing never being fed human milk with being fed human milk for short durations (i.e., <6 mo) and risk of childhood leukemia is mixed. However, the evidence comparing never being fed human milk with being fed human milk for long durations (i.e., ≥6 mo) is mostly consistent and is associated with a slightly higher risk of childhood leukemia. (grade: limited) |
| Systematic review question: what is the relationship between shorter versus longer durations of any human milk feeding and childhood leukemia? |
| Limited but consistent evidence suggests that, among infants fed some amount of human milk, a shorter versus longer duration of any human milk feeding is associated with a slightly higher risk of childhood leukemia. (grade: limited) |
| Systematic review question: what is the relationship between shorter versus longer durations of exclusive human milk feeding and childhood leukemia? |
| There is no evidence to determine whether or not there is a relationship between shorter versus longer durations of exclusive human milk feeding and childhood leukemia. (grade: grade not assignable) |
| Systematic review question: What is the relationship between feeding a lower versus higher intensity, proportion, or amount of human milk to mixed-fed infants and childhood leukemia? |
| There is no evidence to determine whether or not there is a relationship between feeding a lower versus higher intensity, proportion, or amount of human milk to mixed-fed infants and childhood leukemia. (grade: grade not assignable) |
What is the relationship between never versus ever feeding human milk and childhood leukemia?
Nineteen articles met the inclusion criteria for this SR question (Table 2), which presented evidence from 15 independent case-control studies and 1 retrospective cohort study. There were 4 independent case-control studies from France (14, 15, 21, 27, 29); 2 each from the US (17, 22, 31), Canada (20, 23), and the UK (16, 24, 25); and 1 each from the Netherlands (32), Greece (28), Australia (19), New Zealand (18), and China (30). The retrospective cohort was from the UK (26). The articles by Kwan et al. (22) and Urayama et al. (31), McKinney et al. (25) and Beral et al. (16), and the 2 articles by Perrillat et al. (14, 27) had overlapping samples but ran distinct analyses that met the inclusion criteria.
TABLE 2.
Evidence examining the relationship between never versus ever feeding human milk and childhood leukemia1
| Article, study design (study/cohort name when applicable), country |
Notable sample characteristics | Never vs. ever feeding human milk exposures2 |
Significant associations with childhood leukemia |
Nonsignificant associations with childhood leukemia |
|---|---|---|---|---|
| Ajrouche 2015 (15) Case control (ESTELLE) France |
N = 617 ALL cases, 1225 controls Baseline: 1–14 y Race/ethnicity NR |
Ever BF vs. never BF | ALL: OR 0.80 (95% CI: 0.66, 0.99) | None |
| BF <6 mo vs. never BF | None | ALL: OR 0.81 (95% CI: 0.65, 1.02) | ||
| BF ≥6 mo vs. never BF | None | ALL: OR 0.78 (95% CI: 0.59, 1.04) | ||
| Beral 2001 (16) Case control (UKCCS) UK |
N = 1637 leukemia cases (134 in the subsample age 1 y, 890 in the subsample ages 2–5 y, 613 in the subsample ≥6 y, 1401 in the ALL subsample), 6964 controls Baseline: 1–14 y Race/ethnicity NR Sex NR |
Ever BF vs. never BF | None | Leukemia: OR 0.89 (95% CI: 0.80, 1.00), P = 0.06 Leukemia in subsample age 1 y: OR 0.76 (95% CI: 0.51, 1.12) Leukemia in subsample ages 2–5 y: OR 0.95 (95% CI: 0.81, 1.11) Leukemia in subsample ages ≥6 y: OR 0.85 (95% CI: 0.70, 1.02) ALL subsample: OR 0.91 (95% CI: 0.81, 1.04) |
| BF <1 mo vs. never BF | None | Leukemia: OR 0.96 (95% CI: 0.81, 1.14) ALL subsample: OR 0.98 (95% CI: 0.82, 1.17) |
||
| BF 1–6 mo vs. never BF | None | Leukemia: OR 0.88 (95% CI: 0.77, 1.02) ALL subsample: OR 0.90 (95% CI: 0.77, 1.04) |
||
| BF ≥7 mo vs. never BF | None | Leukemia: OR 0.85 (95% CI: 0.73, 1.00) ALL subsample: OR 0.89 (95% CI: 0.75, 1.05) |
||
| Davis 1988 (17) Case control US |
N = 52 ALL cases, 181 controls Baseline: 1.5–15 y Race/ethnicity NR |
Artificial feeding vs. BF > 6 mo | None | ALL: OR 1.46 (95% CI: 0.68, 3.14) |
| Dockerty 1999 (18) Case control New Zealand |
N = 95 ALL cases, 303 controls Baseline: 0–14 y Race/ethnicity: 88.7% non-Maori 11.3% Maori Sex NR |
BF vs. no BF |
None |
ALL: OR 0.98 (95% CI: 0.39, 2.47) |
| BF 2 d to 6 mo vs. no BF | None | ALL: OR 1.24 (95% CI: 0.47, 3.23) | ||
| BF > 6 mo to 1 y vs. no BF | None | ALL: OR 0.82 (95% CI: 0.29, 2.27) | ||
| BF > 1 y vs. no BF | None | ALL: OR 0.47 (95% CI: 0.15, 1.43) | ||
| Greenop 2015 (19) Case control (Australian Study of the Causes of Acute Lymphoblastic Leukaemia) Australia |
N = 314 ALL cases, 663 controls Baseline: 0–14 y Race/ethnicity: 77% European Ethnicity 17.5% ≥50% European 2.7% ≥50% Non-European 2.8% Indeterminate |
BF vs. no BF |
ALL: OR 0.52 (95% CI: 0.32, 0.84) |
None |
| BF <3 mo vs. no BF | ALL: OR 0.49 (95% CI: 0.28, 0.86) | None | ||
| BF ≥3 to <6 mo vs. no BF | None | ALL: OR 0.62 (95% CI: 0.34, 1.12) | ||
| BF ≥6 mo vs. no BF | ALL: OR 0.51 (95% CI: 0.30, 0.84) | None | ||
| EBF <3 mo vs. no BF | ALL: OR 0.50 (95% CI: 0.29, 0.84) | None | ||
| EBF ≥3 to <6 mo vs. no BF | ALL: OR 0.52 (95% CI: 0.31, 0.87) | None | ||
| EBF ≥6 mo vs. no BF | ALL: OR 0.55 (95% CI: 0.31, 0.99) | None | ||
| Infante-Rivard 2000 (20) Case control Canada |
N = 491 ALL cases (249 in the subsample age <4 y, 242 in the subsample age ≥4 y), 491 controls Baseline: 0–9 y Race/ethnicity NR |
BF ≤3 mo vs. no BF | ALL: OR 0.68 (95% CI: 0.49, 0.95) | ALL in subsample age <4 y: OR 0.62 (95% CI: 0.37, 1.03) ALL in subsample age ≥4 y: OR 0.78 (95% CI: 0.50, 1.23) |
| BF >3 mo vs. no BF | ALL: OR 0.67 (95% CI: 0.47, 0.94) | ALL in subsample age <4 y: OR 0.63 (95% CI: 0.39, 1.03) ALL in subsample age ≥4 y: OR 0.68 (95% CI: 0.41, 1.14) |
||
| BF 1–6 mo vs. no BF | ALL in subsample age <4 y: OR 0.61 (95% CI: 0.39, 0.95) | ALL in subsample age ≥4 y: OR 0.72 (95% CI: 0.48, 1.09) | ||
| BF >6 mo vs. no BF | None | ALL in subsample age <4 y: OR 0.68 (95% CI: 0.36, 1.28) ALL in subsample age ≥4 y: OR 0.83 (95% CI: 0.42, 1.63) |
||
| Jourdan-Da Silva 2004 (21) Case control France |
N = 393 ALL cases, 221 leukemia cases age 2–6 y, 199 leukemia cases 6–15 y, 530 controls |
BF vs. no BF | None | ALL: OR 1.1 (95% CI: 0.9, 1.5) Leukemia age 2–6 y: OR 1.0 (95% CI: 0.7, 1.5) Leukemia age 6–15 y: OR 1.4 (95% CI: 0.9, 2.0) |
| Baseline: 1–14 y Race/ethnicity NR |
BF <3 mo vs. BF 0 mo | None | ALL: OR 1.2 (95% CI: 0.8, 1.7) Leukemia age 2–6 y: OR 0.8 (95% CI: 0.5, 1.3) Leukemia age 6–15 y: OR 1.7 (95% CI: 1.0, 2.9) |
|
| BF 3–6 mo vs. BF 0 mo | None | ALL: OR 1.1 (95% CI: 0.7, 1.6) | ||
| BF >6 mo vs. BF 0 mo | None | ALL: OR 1.4 (95% CI: 0.8, 2.5) | ||
| BF ≥3 mo vs. never BF | None | Leukemia age 2–6 y: OR 1.4 (95% CI: 0.8, 2.2) Leukemia age 6–15 y: OR 1.0 (95% CI: 0.6, 1.6) |
||
| Kwan 2005 (22) Case control (NCCLS) US |
N = 305 ALL cases (183 in the subsample age 2–5 y), 398 controls Baseline: 1–14 y |
Ever BF vs. never BF | None | ALL: OR 0.99 (95% CI: 0.64, 1.55) ALL in the subsample age 2–5 y: OR 1.49 (95% CI: 0.83, 2.65) |
| Race/ethnicity: 37.7% Hispanic 50% Non-Hispanic White |
BF ≤3 mo vs. never BF | None | ALL: OR 1.14 (95% CI: 0.68, 1.91) ALL in the subsample age 2–5 y: OR 1.67 (95% CI: 0.85, 3.28) |
|
| 2.8% Non-Hispanic Black 9.3% other |
BF 4–6 mo vs. never BF | None | ALL: OR 0.84 (95% CI: 0.48, 1.47) ALL in the subsample age 2–5 y: OR 1.07 (95% CI: 0.50, 2.25) |
|
| BF 7–12 mo vs. never BF | None | ALL: OR 0.88 (95% CI: 0.51, 1.53) ALL in the subsample age 2–5 y: OR 1.29 (95% CI: 0.63, 2.67) |
||
| BF ≥13 mo vs. never BF | None | ALL: OR 1.08 (95% CI: 0.61, 1.92) ALL in the subsample age 2–5 y: OR 1.87 (95% CI: 0.88, 3.95) |
||
| EBF ≤3 mo vs. FF only | None | ALL: OR 1.06 (95% CI: 0.65, 1.71) ALL in the subsample age 2–5 y: OR 1.75 (95% CI: 0.91, 3.34) |
||
| EBF 4–6 mo vs. FF only | None | ALL: OR 0.97 (95% CI: 0.55, 1.71) ALL in the subsample age 2–5 y: OR 1.32 (95% CI: 0.63, 2.77) |
||
| EBF 7–12 mo vs. FF only | None | ALL: OR 0.98 (95% CI: 0.55, 1.75) ALL in the subsample age 2–5 y: OR 1.14 (95% CI: 0.53, 2.44) |
||
| EBF ≥13 mo vs. FF only | None | ALL: OR 0.86 (95% CI: 0.38, 1.92) ALL in the subsample age 2–5 y: OR 2.04 (95% CI: 0.69, 6.07) |
||
| MacArthur 2008 (23) Case control (CCCLS) Canada |
N = 399 leukemia cases (351 in subsample with ALL), 399 controls Baseline: 0–14 y Race/ethnicity: 81.9% Caucasian 2.7% Asian 15.4% other |
BF vs. no BF at 0–3 mo | None | Leukemia: OR 1.26 (95% CI: 0.89, 1.79) ALL subsample: OR 1.33 (95% CI: 0.93, 1.91) |
| McKinney 1987 (24) Case control (IRESCC) UK |
N = 171 leukemia cases, 342 controls Baseline: 0–14 y Race/ethnicity: ∼93% White European ∼7% Indian/Pakistani/West Indian/Other |
BF vs. not BF | None | Leukemia: NS (RR <2 and nonsignificant statistical test; data NR) |
| McKinney 1999 (25) Case control (UKCCS) UK |
N = 144 leukemia cases (124 in the subsample with ALL), 271 controls Baseline: 3 mo to 14 y Race/ethnicity NR Sex NR |
Initially BF vs. not initially BF | None | Leukemia: OR 0.96 (95% CI: 0.62, 1.49) ALL subsample: OR 0.92 (95% CI: 0.58, 1.47) |
| Murray 2002 (26) Retrospective cohort (Northern Ireland Child Health System Cohort) UK |
N = 434,933 Baseline: 0–15 y Race/ethnicity NR |
BF vs. no BF | None | ALL: RR 0.98 (95% CI: 0.68, 1.42) |
| Perrillat 2002 (14) Case control France |
N = 247 leukemia cases, 237 controls Baseline: 2–15 y Race/ethnicity: 84.5% Caucasian 7% North African 1.6% Caribbean 1.4% African 1.4% Asian/Middle Eastern 4% mixed/others |
BF <6 mo vs. no BF |
None |
Leukemia: OR 1.1 (95% CI: 0.7, 1.7) |
| BF ≥6 mo vs. no BF | Leukemia: OR 0.5 (95% CI: 0.2, 1.0)3 | None | ||
| Perrillat 2002 (27) Case control |
N = 247 leukemia cases (219 in the subsample with ALL), 237 controls |
Any BF duration vs. never BF | None | Leukemia: OR 0.8 (95% CI: 0.6, 1.2) ALL subsample: OR 0.8 (95% CI: 0.6, 1.2) |
| France | Baseline: 2–15 y Race/ethnicity: 84.5% Caucasian 7% North African 1.6% Caribbean 1.4% African 1.4% Asian/Middle Eastern 4% mixed/others |
BF <3 mo vs. never BF |
None |
Leukemia: OR 1.0 (95% CI: 0.6, 1.7) ALL subsample: OR 1.0 (95% CI: 0.6, 1.6) |
| BF 3–5 mo vs. never BF | None | Leukemia: OR 1.3 (95% CI: 0.8, 2.3) ALL subsample: OR 1.3 (95% CI: 0.8, 2.4) |
||
| BF 6–11 mo vs. never BF | Leukemia: OR 0.4 (95% CI: 0.2, 1.0)3 | ALL subsample: OR 0.5 (95% CI: 0.2, 1.1) | ||
| BF ≥12 mo vs. never BF | None | Leukemia: OR 0.6 (95% CI: 0.2, 2.7) ALL subsample: OR 0.5 (95% CI: 0.1, 2.5) |
||
| Petridou 1997 (28) Case control Greece |
N = 153 leukemia cases, 300 controls Baseline: 0–14 y Race/ethnicity NR |
BF vs. no BF | None | Leukemia: OR 0.85 (95% CI: 0.52, 1.41) |
| Rudant 2010 (29) |
N = 634 ALL cases, 1494 controls | BF vs. no BF | None | ALL: OR 1.0 (95% CI: 0.8, 1.2) |
| Case control (ESCALE) |
Baseline: 1–14 y | BF <6 mo vs. no BF | None | ALL: OR 1.1 (95% CI: 0.9, 1.4) |
| France | Race/ethnicity NR | BF ≥6 mo vs. no BF | ALL: OR 0.7 (95% CI: 0.5, 1.0), P < 0.05 | None |
| BF ≤2 mo vs. no BF | None | ALL: OR 1.3 (95% CI: 1.0, 1.6) | ||
| BF 3–5 mo vs. no BF | None | ALL: OR 0.9 (95% CI: 0.7, 1.2) | ||
| BF 6–11 mo vs. no BF | None | ALL: OR 0.7 (95% CI: 0.5, 1.1) | ||
| BF ≥12 mo vs. no BF | None | ALL: OR 0.6 (95% CI: 0.3, 1.0) | ||
| Shu 1995 (30) Case control China |
N = 159 leukemia cases (99 in subsample age ≤5 y, 60 in subsample age >5 y, 108 in subsample with ALL), 159 controls Baseline: 1–14 y Race/ethnicity: NR Sex NR |
Ever BF vs. never BF | None | Leukemia: OR 1.14 (95% CI: 0.7,1.9) Leukemia in subsample age ≤5 y: OR 1.48 (95% CI: 0.8, 2.8) Leukemia in subsample age >5 y: OR 0.76 (95% CI: 0.3, 2.0) ALL subsample: OR 1.12 (95% CI: 0.6, 2.1) |
| BF 1–6 mo vs. never BF | None | Leukemia: OR 1.20 (95% CI: 0.6, 2.3) Leukemia in subsample age ≤5 y: OR 1.54 (95% CI: 0.7, 3.4) Leukemia in subsample age >5 y: OR 0.82 (95% CI: 0.2, 2.9) ALL subsample: OR 1.10 (95% CI: 0.5, 2.5) |
||
| BF >6 mo vs. never BF | None | Leukemia: OR 1.11 (95% CI: 0.6,1.9) Leukemia in subsample age ≤5 y: OR 1.44 (95% CI: 0.7, 2.9) Leukemia in subsample age >5 y: OR 0.74 (95% CI: 0.3–2.0) ALL subsample: OR 1.12 (95% CI: 0.6, 2.2) |
||
| Urayama 2012 (31) Case control (NCCLS) US |
N = 507 ALL cases (231 in the Non-Hispanic White subsample, 276 in the Hispanic subsample), 762 controls Baseline: 1–14 y Race/ethnicity: 47.0% Non-Hispanic White 53% Hispanic |
BF vs. no BF | None | ALL: OR 0.88 (95% CI: 0.62, 1.23) ALL in non-Hispanic white subsample: OR 0.97 (95% CI: 0.55, 1.69) ALL in Hispanic subsample: OR 0.89 (95% CI: 0.57, 1.38) |
| van Steensel-Moll 1986 (32) Case control (Dutch Childhood Leukemia Study) Netherlands |
N = 516 ALL cases, 500 controls Baseline: 0–14 y Race/ethnicity NR Sex NR |
BF vs. no BF | None | ALL: RR 1.1 (95% CI: 0.8, 1.4) |
1ALL, acute lymphoblastic leukemia; BF, breastfeeding/breastfed; CCCLS, Cross-Canada Childhood Leukemia Study; EBF, exclusive breastfeeding/exclusively breastfed; ESCALE, Etude Sur les Cancers et les Leucémies de l'Enfant; ESTELLE, Etude Sur les Tumeurs Embryonnaires; Leucémies et Lymphomes de l'Enfant; FF, formula fed/formula feeding; IRESCC, Inter-Regional Epidemiological Study of Childhood Cancer; NCCLS, Northern California Childhood Leukemia Study; NR, not reported; RR, relative risk; UKCCS, UK Childhood Cancer Study.
2Exposures, as defined by the authors of the studies included in the body of evidence, which address never compared with ever feeding human milk or vice versa.
3Although the CI includes the null, the authors indicated the association was significant.
Participants were up to 15 y of age at the time of the study, although several studies excluded infants to minimize reverse causality or to account for the possiblity that leukemia diagnosed in infancy has a different etiology (14–17, 21, 22, 27, 29–31). The studies from the United Kingdom, France, Canada, and Australia that reported race and ethnicity had participants who were primarily Caucasian, white European, or of European descent (14, 19, 23, 24, 27); the study from New Zealand reported that most participants were Non-Maori (18); and 1 US study ran analyses on participants who were primarily (22) or entirely (31) white (both Hispanic and Non-Hispanic). The remaining studies did not report participants’ race or ethnicity. Some studies did not report participants’ sex (16, 18, 25,30, 32), but all other samples included both males and females.
In almost all of the studies, infant milk-feeding data were collected retrospectively by maternal recall; however, 2 studies accessed infant-feeding data collected during infancy from medical records (25) or the Department of Health, Social Services and Public Safety database (26). The outcome was medically diagnosed. All studies included matching variables, and all but a few (17, 24–26) included additional adjustment variables. Every study matched cases with controls using participants’ sex and age. Most studies also matched participants using geographic location (14, 16, 17, 19–23, 25, 27, 28, 30, 32), and a few in addition used race or ethnicity or both (14, 22, 27, 31).
Five of the 16 studies reported statistically significant associations (14, 15, 19, 20, 27, 29). This evidence consistently suggested that never compared with ever being fed human milk (i.e., any amount of human milk feeding) was associated with a higher risk of childhood leukemia.
Specifically, Ajrouche et al. (15) found that ever, compared with never, being fed human milk was associated with significantly lower odds of ALL. This study also divided the group ever fed human milk into 2 smaller groups fed human milk <6 and ≥6 mo. The resulting associations between being fed human milk <6 and ≥6 mo, compared with never being fed human milk, and lower odds of ALL were nonsignificant with slightly wider CIs that included the null.
Greenop et al. (19) also found a significant association between ever, compared with never, being fed human milk and lower odds of ALL. Additional analyses divided the group ever fed human milk into smaller groups fed human milk <3, ≥3 to <6, and ≥6 mo, and fed human milk exclusively <3, ≥3 to <6, and ≥6 mo. In comparison with never being fed human milk, all of the human milk-feeding variables were associated with lower odds of ALL, and in all but 1 instance (i.e., being fed human milk ≥3 to <6 mo compared with never) the associations were statistically significant.
Infante-Rivard et al. (20) compared being fed human milk ≤3 and >3 mo with never being fed human milk and found that both durations were associated with significantly lower odds of ALL in the full sample. Additional analyses divided the full sample into subsamples <4 and ≥4 y of age. In these smaller groups, the associations between being fed human milk ≤3 and >3 mo, compared with never being fed human milk, and lower odds of ALL had wider CIs that included the null. Subsample analyses comparing being fed human milk 1–6 and >6 mo with never being fed human milk also found associations with lower odds of ALL, although the only association with statistical significance was the comparison of 1–6 mo with never in the subsample <4 y of age.
In the study by Perrillat et al. (14, 27), the associations between ever compared with never being fed human milk and lower odds of leukemia and of ALL were not statistically significant; however, dividing the group ever fed human milk by the length of participants’ human milk exposure made it evident that duration mattered. In general, when compared with never being fed human milk, longer durations of human milk feeding were associated with lower odds of leukemia and of ALL, whereas shorter durations of human milk feeding were not. Specifically, with 2 categorical durations of human milk feeding, there was a significant association between being fed human milk ≥6 mo, compared with never being fed human milk, and lower odds of leukemia, and a nonsignificant association between being fed human milk <6 mo, compared with never being fed human milk, and higher odds of leukemia. The authors also divided the group ever fed human milk into 4 categories of duration. Being fed human milk 6–11 mo compared with never was associated with significantly lower odds of leukemia. The odds of ALL, specifically, were also lower but had a slightly wider CI that included the null. Likewise, the associations between being fed human milk ≥12 mo, compared with never being fed human milk, and lower odds of leukemia and of ALL were nonsignificant with wide CIs. On the other hand, the authors found no associations between being fed human milk <3 mo compared with never and the odds of leukemia or of ALL specifically (i.e., the ORs were both 1.0), and nonsignificant associations between being fed human milk 3–5 mo, compared with never being fed human milk, and higher odds of leukemia and of ALL.
Finally, the study by Rudant et al. (29) found no association between never compared with ever being fed human milk and ALL (i.e., the OR was 1.0); however, like the study by Perrillat et al. (14, 27), the duration of the human milk exposure mattered. In general, when compared with never being fed human milk, longer-term intake of human milk was associated with lower odds of leukemia, whereas shorter-term intake of human milk was not. When the group ever fed human milk was divided into 2 categories of duration, the authors found that being fed human milk ≥6 mo compared with never was associated with significantly lower odds of ALL, but there was a nonsignificant association between being fed human milk <6 mo and higher odds of ALL. The group ever fed human milk was also divided into 4 categories of duration. There were nonsignificant associations between being fed human milk 3–5 and 6–11 mo, compared with never being fed human milk, and lower odds of ALL, and, for the association between being fed human milk ≥12 mo compared with never and odds of ALL, the upper limit of the CI was at the null. On the other hand, for the association between being fed human milk ≤2 mo compared with never and ALL, the lower limit of the CI was at the null.
Next, TEC members looked across the entire body of evidence to see whether there were any distinct associations between never compared with longer-term (i.e., ≥6 mo) and never compared with shorter-term (i.e., <6 mo) feeding of human milk and childhood leukemia, because some of the associations described above depended on the duration of ever (14, 27, 29). Eleven studies compared never being fed human milk with being fed human milk ≥6 mo (14–22, 27, 29, 30); the majority (i.e., 8 studies) found consistent evidence that never being fed human milk is associated with higher odds of leukemia or of ALL (14–18, 20, 27, 29), and in 3 of the studies the association was statistically significant (14, 19, 27, 29). On the other hand, out of 10 studies that compared never being fed human milk with being fed human milk <6 mo (14–16, 18–22, 27, 29, 30), only 4 found consistent evidence that never being fed human milk is associated with higher odds of leukemia or of ALL (15, 16, 19, 20).
In summary, the evidence from the 5 studies with statistically significant associations (14, 15, 19, 20, 27, 29) is consistent and suggests that never being fed human milk compared with ever being fed human milk (i.e., any amount of human milk feeding) is associated with a higher risk of childhood leukemia. Some of the studies in the body of evidence compared never being fed human milk with being fed human milk for specific durations. Upon closer examination of these analyses, TEC members concluded that the evidence comparing never being fed human milk with being fed human milk for shorter-term durations (i.e., <6 mo) and risk of childhood leukemia is mixed. However, the evidence comparing never being fed human milk with being fed human milk for longer-term durations (i.e., ≥6 mo) is mostly consistent and is associated with a slightly higher risk of childhood leukemia (Table 4).
What is the relationship between shorter versus longer durations of any human milk feeding and childhood leukemia?
Eight articles met the inclusion criteria for this SR question (Table 3), which presented evidence from 8 case-control studies. Three studies were from the US (17, 22, 34), and there was 1 study each from the UK (16), Germany (35), Oman (37), Russia (36), and the United Arab Emirates (33). Participants were up to 15 y of age at the time of the study, although 3 studies excluded infants to minimize reverse causality or to account for the possibility that leukemia diagnosed in infancy has a different etiology (16, 17, 22). Two US samples had primarily white participants who were both Hispanic and non-Hispanic (22, 34) and the study from the United Arab Emirates (33) reported having a sample that was 100% Bedouin Arab. No other studies reported race or ethnicity. One study did not report participants’ sex (16), but all other samples included both males and females.
TABLE 3.
Evidence examining the relationship between shorter versus longer durations of any human milk feeding and childhood leukemia1
| Article, study design (study name when applicable), country |
Notable sample characteristics | Shorter vs. longer duration of any human milk feeding exposures2 |
Significant associations with childhood leukemia |
Nonsignificant associations with childhood leukemia |
|---|---|---|---|---|
| Bener 2008 (33) Case control UAE |
N = 107 male ALL cases, 62 female ALL cases, 169 controls Baseline: 0–15 y Race/ethnicity: 100% Bedouin Arab 63% male |
Mean BF duration in cases vs. controls | ALL in males: 9.1 mo (95% CI: 7.9, 10.4) vs. 12.2 mo (95% CI: 11.0, 13.4), P = 0.001 ALL in females: 8.4 mo (95% CI: 6.9, 10.1) vs. 11.5 mo (95% CI: 10.0–13.0), P = 0.002 |
None |
| Beral 2001 (16) Case control (UKCCS) UK |
N = 1637 leukemia cases (1401 in the ALL subsample), 6964 controls Baseline: 1–14 y Race/ethnicity NR Sex NR |
Duration of BF trend using the categories <1 mo, 1–6 mo, ≥7 mo | None | Leukemia: P = 0.29 (15.3% cases and 14.6% controls BF <1 mo, 27.7% cases and 29.2% controls BF 1–6 mo, 18.5% cases and 20.3% controls BF ≥7 mo) ALL subsample: P = 0.48 (15.5% cases and 14.6% controls BF <1 mo, 27.7% cases and 29.2% controls BF 1–6 mo, 19.0% cases and 20.3% controls BF ≥7 mo) |
| Davis 1988 (17) Case control US |
N = 52 ALL cases, 181 controls Baseline: 1.5–15 y Race/ethnicity NR |
BF ≤6 mo vs. BF >6 mo | None | ALL: OR 1.95 (95% CI: 0.86, 4.40) |
| Kwan 2005 (22) Case control (NCCLS) US |
N = 305 ALL cases (183 in the subsample age 2–5 y), 398 controls Baseline: 1–14 y Race/ethnicity: 37.7% Hispanic 50% non-Hispanic white 2.8% non-Hispanic black 9.3% other |
BF duration (mo) | None | ALL: OR 1.00 (95% CI: 0.98, 1.02) ALL in the 2–5 y subsample: OR 1.02 (95% CI: 0.99, 1.05) |
| Schraw 2014 (34) Case control US |
N = 142 ALL cases, 284 controls Baseline: 0–14 y Race/ethnicity: 83.3% white 11.8% African American 4.9% other 49.3% Hispanic 50.5% non-Hispanic |
BF duration (mo) | None | ALL: OR 1.01 (95% CI: 0.94, 1.08) |
| Schuz 1999 (35) Case control Germany |
N = 1683 leukemia cases (686 in the subsample with ALL), 3575 controls Baseline: 0–14 y Race/ethnicity NR |
BF ≤1 mo vs. >6 mo |
None |
Leukemia: OR 1.2 (95% CI: 0.9, 1.6) ALL subsample: OR 1.3 (95% CI: 1.0, 1.7) |
| BF 2–6 mo vs. >6 mo | None | Leukemia: OR 1.2 (95% CI: 0.9, 1.5) ALL subsample: OR 1.2 (95% CI: 0.9, 1.6) |
||
| Smulevich 1999 (36) Case control |
N = 199 leukemia cases, 398 controls |
BF <1 mo vs. BF >12 mo | Leukemia: OR 9.2 (95% CI: 3.1, 28.1) | None |
| Russia | Baseline: 0–14 y | BF 1–2 mo vs. BF >12 mo | None | Leukemia: OR 1.0 (95% CI: 0.4, 2.3) |
| Race/ethnicity NR | BF 3–4 mo vs. BF >12 mo | None | Leukemia: OR 1.6 (95% CI: 0.8, 2.0) | |
| BF 5–6 mo vs. BF >12 mo | None | Leukemia: OR 1.5 (95% CI: 0.8, 3.1) | ||
| BF 7–12 mo vs. BF >12 mo | None | Leukemia: OR 1.1 (95% CI: 0.6, 2.1) | ||
| Waly 2011 (37) Case control Oman |
N = 70 ALL cases, 70 controls Baseline: range NR, mean 13.2 y Race/ethnicity NR |
Duration of BF trend using the categories <6 mo, 6–12 mo, 12–24 mo, >24 mo | None | ALL: P = 0.282 (8% cases and 3% controls BF <6 mo, 14% cases and 9% controls BF 6–12 mo, 75% cases and 81% controls BF 12–24 mo, 4% cases, and 7% controls BF >24 mo) |
1ALL, acute lymphoblastic leukemia; BF, breastfeeding/breastfed; NCCLS, Northern California Childhood Leukemia Study; NR, not reported; UAE, United Arab Emirates; UKCCS, UK Childhood Cancer Study.
2Exposures, as defined by the authors of the studies included in the body of evidence, which address shorter compared with longer durations of any human milk feeding or vice versa.
The studies collected data about the duration of any human milk feeding retrospectively by maternal recall. The outcome was medically diagnosed. All studies included matching variables and most included additional adjustment variables (16, 22, 34–36). Every study matched cases with controls using participants’ sex and age. The 2 US studies (22, 34) in addition used race and ethnicity as matching variables, whereas 4 studies (16, 17, 35, 36) used geographic location as an additional matching variable, and 1 study (37) in addition matched cases with controls by family or neighborhood to minimize differences in socio-economic, genetic, and environmental exposures including diet.
Two studies reported statistically significant associations (33, 36). The evidence from these studies suggested that shorter compared with longer durations of any human milk feeding are associated with higher risk of childhood leukemia. Most of the studies had nonsignificant associations that were also in the direction of shorter compared with longer durations of any human milk feeding being associated with a higher risk of childhood leukemia (16, 17, 35–37) or had ORs at or close to the null (i.e., ORs 1.00–1.02) (22, 34).
The studies that reported statistically significant associations between shorter compared with longer durations of any human milk feeding and higher risk of childhood leukemia were by Bener et al. (33) and Smulevich et al. (36). Bener et al. (33) assessed male and female participants separately and found that, in both sexes, the mean duration of any human milk feeding was significantly shorter for cases with ALL than for controls. Smulevich et al. (36) compared <1, 1–2, 3–4, 5–6, and 7–12 mo with >12 mo of human milk feeding and found that being fed human milk <1 mo was associated with significantly higher odds of leukemia.
The studies that reported nonsignificant associations between shorter and longer durations of any human milk feeding and higher risk of childhood leukemia were by Beral et al. (16), Davis et al. (17), Schuz et al. (35), Smulevich et al. (36), and Waly et al. (37). Some of the nonsignificant associations were likely underpowered because, among the studies that reported ORs, some had wide CIs (17, 36). As described above, Smulevich et al. (36) compared multiple shorter durations with >12 mo of human milk feeding. Durations of 3–4, 5–6, and 7–12 mo had nonsignificant associations with higher odds of childhood leukemia that had wide CIs. Likewise, Davis et al. (17) reported that being fed human milk ≤6 mo, in comparison with >6 mo, had a nonsignificant association with higher odds of ALL, and the CI around the OR was wide. Schuz et al. (35) found that ≤1 mo and 2–6 mo of human milk feeding, compared with >6 mo, had nonsignificant associations with higher odds of leukemia generally and of ALL specifically, and the lower limit of the CI around the OR for ≤1 mo versus >6 mo and ALL was at the null. The studies by Beral et al. (16) and Waly et al. (37) examined the proportions of cases and controls within several categories of duration. Both studies found higher proportions of cases than controls in the shortest duration categories and higher proportions of controls than cases in the longer duration categories.
In summary, the notable feature of this body of evidence was its consistency in the direction of the associations across most of the studies. Both studies with statistically significant associations (36, 33) found that shorter compared with longer durations of any human milk feeding were associated with higher risk of childhood leukemia. Further, the majority of nonsignificant associations (16, 17, 35–37), some of which were likely underpowered, were consistent in direction with the significant associations. Therefore, TEC members concluded that limited but consistent evidence suggests that shorter versus longer durations of any human milk feeding are associated with a slightly higher risk of childhood leukemia (Table 4).
Discussion
The conclusion statements that answer the 4 SR questions, and the grades of the evidence underlying the conclusion statements, are listed in Table 4. For 2 of the 4 SR questions, the conclusion statements and their grades reflect that no articles met the inclusion criteria for these SRs (Table 1); TEC members concluded that there is insufficient evidence to determine whether or not there is a relationship between shorter versus longer durations of exclusive human milk feeding, and between feeding a lower versus higher intensity, proportion, or amount of human milk to mixed-fed infants, and childhood leukemia. TEC members answered the SR questions about shorter versus longer durations of any human milk feeding and never versus ever feeding human milk and childhood leukemia. After assessing the adequacy, consistency, impact, generalizability, and internal validity of the evidence (i.e., elements of the NESR grading rubric), TEC members graded the conclusion statements for both SR questions as limited.
There were limitations to the adequacy of the evidence for both SR questions. The number of studies in the body of evidence for shorter versus longer durations of any human milk feeding was small; only 8 studies met the inclusion criteria. For the SR question related to never versus ever feeding human milk, there were limitations related to the independence of the studies because across 20 included articles, there were 15 independent samples. For both SR questions, TEC members identified issues with statistical power indicating that there were sample-size limitations.
TEC members did not have any concerns about the generalizability of the evidence to the US. The studies were conducted in the US or several other countries categorized as high or very high on the 2014 Human Development Index (13), according to the a priori inclusion criteria.
TEC members did have some concerns about internal validity related to study design. Nearly all of the studies were case-control studies. TEC members recognized the importance of case-control studies because they are useful for examining low-incident outcomes such as leukemia. However, because case-control studies rely on the retrospective collection of exposure data, differential or nondifferential misclassification of the exposure may have introduced bias. Differential misclassification from recall bias (i.e., if mothers of children with leukemia recalled or reported infant milk-feeding practices differently from mothers of children without leukemia) could have resulted in over- or underestimations of the associations, whereas nondifferential misclassification would have tended to bias the reported associations toward the null. There was no such concern related to the outcome, which was medically diagnosed and unlikely to misclassify cases or controls. Multiple comparisons used by some of the studies (14, 19, 21, 22, 27, 29, 36) could have resulted in finding statistically significant associations by chance; however, TEC members considered all associations (i.e., significant and nonsignificant) during their evidence synthesis.
The consistency of the evidence was particularly important when evaluating the strength of the evidence. Only 2 of the 8 studies examining shorter compared with longer durations of any human milk feeding and 5 of 16 studies examining never compared with ever feeding human milk reported any statistically significant associations (14, 15, 19, 20, 27, 29, 33). However, the small number of statistically significant associations was consistent in showing that shorter compared with longer durations of any human milk feeding and never compared with ever feeding human milk are associated with a higher risk of childhood leukemia. TEC members examined the bodies of evidence in their totality, including significant and nonsignificant associations, to provide a more thorough synthesis related to consistency. It was clear that the majority of significant and nonsignificant associations between shorter compared with longer durations of any human milk feeding and childhood leukemia risk were consistent in direction (16, 17, 33, 35–37), suggesting that shorter durations are associated with with higher risk of childhood leukemia. It was also clear that the majority of significant and nonsignificant associations between never compared with ever being fed human milk, and especially never versus ≥6 mo of human milk feeding, and childhood leukemia were consistent in direction (14–16, 18–20, 27, 29), suggesting that never being fed human milk is associated with higher risk of childhood leukemia. Evidence was consistent in direction despite heterogeneous independent variables resulting from not defining longer, shorter, or ever for the SRs and instead including all relevant comparisons. Some of the inconsistency in statistical significance was explainable because several comparisons were likely underpowered. In addition, as described previously, there was the potential for differential and nondifferential misclassification of the exposure to bias the associations toward the null. Therefore, TEC members concluded that limited evidence suggests that there are associations between shorter versus longer durations of any human milk feeding and between never versus ever feeding human milk and higher risk of childhood leukemia.
Regarding impact, the higher leukemia risk associated with being fed human milk for short durations or not at all is likely to be small. Still, small changes in risk are important due to the seriousness of the disease.
Research recommendations
None of the articles that met the inclusion criteria for these SRs (Table 1) examined shorter versus longer durations of exclusive human milk feeding or the intensity, proportion, or amount of human milk fed to mixed-fed infants and childhood leukemia. Therefore, studies need to be designed and conducted to examine these relationships. To better understand the broader relationships between early diet and childhood leukemia risk, additional SRs could examine the timing of the introduction of, and the types and amounts of, human milk substitutes (e.g., infant formula) and complementary foods and beverages in infants’ diets. If no data are available, future research could address these topics.
In general, infant-feeding researchers should move toward collecting data consistently using valid and reliable methods and increase the precision with which they define their infant-feeding variables. Researchers should incorporate effect modification into their study design whenever possible in case different biological or environmental characteristics modify the impact of infant feeding on the outcomes. Finally, TEC members recommend linking surveillance systems that capture information about infant feeding and childhood cancer in order to explore the relationship with adequately powered, broadly generalizable, prospective samples. Electronic medical records may be another source of prospectively collected infant-feeding and leukemia data.
ACKNOWLEDGEMENTS
We thank Katherine Kortsmit for her assistance with extracting data and assessing risk of bias for included studies. We also thank Sue Anderson for serving as a TEC member until December 2015.
The authors’ responsibilities were as follows—DG, PN, SAA, LB, KMJ, LAN-R, KOO'B, EO, RP-E, EEZ, and JMS: participated in establishing the research questions, analytic framework, and study inclusion and exclusion criteria; YPW and NT: developed the literature search strategy and conducted the literature search; PN, CD, and DG: screened search results, and identified studies for inclusion. DG and PN: extracted data and assessed the risk of bias for the included studies. SAA, LB, TJ, KMJ, LAN-R, KOO'B, EO, RP-E, and EEZ: reviewed and provided substantive feedback on all SR materials, including the synthesis of the body of evidence, conclusion statement, and grade of the strength of the evidence. DG, PN, and CCL: wrote the manuscript; DG: has primary responsibility for final content. JMS: oversaw the Project; and all authors critically reviewed and approved the final manuscript. None of the other authors report a conflict of interest related to research presented in this article.
Notes
Published in a supplement to The American Journal of Clinical Nutrition. This article is one in a series of systematic reviews completed with support from the USDA’s Nutrition Evidence Systematic Review team as part of the Pregnancy and Birth to 24 Months Project. The supplement is sponsored by the USDA Food and Nutrition Service. The Supplement Coordinator for the supplement publication was Joanne M Spahn, USDA Center for Nutrition Policy and Promotion, Alexandria, VA. Supplement Coordinator disclosure: No conflicts of interest or financial ties to disclose. The Guest Editor for this supplement was Pieter J Sauer. Guest Editor disclosure: No conflicts of interest to disclose.
Publication costs for this supplement were defrayed in part by the payment of page charges. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of The American Journal of Clinical Nutrition.
Abbreviations used: ALL, acute lymphoblastic leukemia; NESR, Nutrition Evidence Systematic Review; SR, Systematic review; TEC, Technical Expert Collaborative.
References
- 1. Obbagy JE, Blum-Kemelor DM, Essery EV, Lyon JMG, Spahn JM. USDA Nutrition Evidence Library: methodology used to identify topics and develop systematic review questions for the birth-to-24-mo population. Am J Clin Nutr. 2014;99(3):692s–96s. [DOI] [PubMed] [Google Scholar]
- 2. Raiten DJ, Raghavan R, Porter A, Obbagy JE, Spahn JM. Executive summary: evaluating the evidence base to support the inclusion of infants and children from birth to 24 mo of age in the Dietary Guidelines for Americans—“the B-24 Project.”. Am J Clin Nutr. 2014;99(3):663s–91s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stoody EE, Spahn JM, Casavale KO. The Pregnancy and Birth to 24 Months Project: a series of systematic reviews on diet and health. Am J Clin Nutr. 2019;109(7):685S–97S. [DOI] [PubMed] [Google Scholar]
- 4. American Cancer Society. Version 3 February 2016. [Internet]. Available from: https://www.cancer.org/cancer/leukemia-in-children/about/key-statistics.html [cited 16 March 2018]. [Google Scholar]
- 5. National Cancer Institute. Version 5 April 2018. [Internet]. Available from: https://www.cancer.gov/types/leukemia/hp/child-all-treatment-pdq [cited 9 April 2018]. [Google Scholar]
- 6. Ip S, Chung M, Raman G, Chew P, Magula N, DeVine D, Trikalinos T, Lau J. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep). 2007;April(153):1–186. [PMC free article] [PubMed] [Google Scholar]
- 7. Greaves MF. Speculations on the cause of childhood acute lymphoblastic leukemia. Leukemia. 1988;2(2):120–25. [PubMed] [Google Scholar]
- 8. Greaves M. A causal mechanism for childhood acute lymphoblastic leukaemia. Nat Rev Cancer. 2018;18(8):471–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Obbagy JE, Spahn JM, Wong YP, Psota TL, Spill MK, Dreibelbis C, Güngör D, Nadaud P, Raghavan R, Callahan EH et al.. Systematic review methodology used in the Pregnancy and Birth to 24 Months Project. Am J Clin Nutr. 2019;109(7):698S–704S. [DOI] [PubMed] [Google Scholar]
- 10. Infant Formula Act of 1980. 96–359. [Google Scholar]
- 11. US Food and Drug Administration. Version 19 December 2013. [Internet]. Available from: https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/InfantFormula/ucm136118.htm#manufacture [cited 23 March 2018]. [Google Scholar]
- 12. Food and Agriculture Organization of the United Nations. World Health Organization. Codex Alimentarius. International Food Standards. Standard for infant formula and formulas for special medical purposes intended for infants. Codex Stan 72–1981 2007. [Google Scholar]
- 13. United Nations Development Programme. Human Development Report 2014. Sustaining human progress: reducing vulnerabilities and building resilience. New York: United Nations Development Programme; 2014. [Google Scholar]
- 14. Perrillat F, Clavel J, Auclerc MF, Baruchel A, Leverger G, Nelken B, Philippe N, Schaison G, Sommelet D, Vilmer E et al.. Day-care, early common infections and childhood acute leukaemia: a multicentre French case-control study. Br J Cancer. 2002;86(Supplement_7):1064–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ajrouche R, Rudant J, Orsi L, Petit A, Baruchel A, Lambilliotte A, Gambart M, Michel G, Bertrand Y, Ducassou S et al.. Childhood acute lymphoblastic leukaemia and indicators of early immune stimulation: the Estelle study (SFCE). Br J Cancer. 2015;112(6):1017–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beral V, Fear NT, Alexander F, Appleby P; Investigat UCCS. Breastfeeding and childhood cancer. Br J Cancer. 2001;85(11):1685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davis MK, Savitz DA, Graubard BI. Infant feeding and childhood cancer. Lancet. 1988;2(8607):365–68. [DOI] [PubMed] [Google Scholar]
- 18. Dockerty JD, Skegg DC, Elwood JM, Herbison GP, Becroft DM, Lewis ME. Infections, vaccinations, and the risk of childhood leukaemia. Br J Cancer. 1999;80(9):1483–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greenop KR, Bailey HD, Miller M, Scott RJ, Attia J, Ashton LJ, Downie P, Armstrong BK, Milne E. Breastfeeding and nutrition to 2 years of age and risk of childhood acute lymphoblastic leukemia and brain tumors. Nutr Cancer. 2015;67(3):431–41. [DOI] [PubMed] [Google Scholar]
- 20. Infante-Rivard C, Fortier I, Olson E. Markers of infection, breast-feeding and childhood acute lymphoblastic leukaemia. Br J Cancer. 2000;83(11):1559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jourdan-Da Silva N, Perel Y, Mechinaud F, Plouvier E, Gandemer V, Lutz P, Vannier JP, Lamagnere JL, Margueritte G, Boutard P et al.. Infectious diseases in the first year of life, perinatal characteristics and childhood acute leukaemia. Br J Cancer. 2004;90(1):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kwan ML, Buffler PA, Wiemels JL, Metayer C, Selvin S, Ducore JM, Block G. Breastfeeding patterns and risk of childhood acute lymphoblastic leukaemia. Br J Cancer. 2005;93(3):379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. MacArthur AC, McBride ML, Spinelli JJ, Tamaro S, Gallagher RP, Theriault GP. Risk of childhood leukemia associated with vaccination, infection, and medication use in childhood: the Cross-Canada Childhood Leukemia Study. Am J Epidemiol. 2008;167(5):598–606. [DOI] [PubMed] [Google Scholar]
- 24. McKinney PA, Cartwright RA, Saiu JM, Mann JR, Stiller CA, Draper GJ, Hartley AL, Hopton PA, Birch JM, Waterhouse JA et al.. The inter-regional epidemiological study of childhood cancer (IRESCC): a case control study of aetiological factors in leukaemia and lymphoma. Arch Dis Child. 1987;62(3):279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McKinney PA, Juszczak E, Findlay E, Smith K, Thomson CS. Pre- and perinatal risk factors for childhood leukaemia and other malignancies: a Scottish case control study. Br J Cancer. 1999;80(11):1844–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murray L, McCarron P, Bailie K, Middleton R, Davey Smith G, Dempsey S, McCarthy A, Gavin A. Association of early life factors and acute lymphoblastic leukaemia in childhood: historical cohort study. Br J Cancer. 2002;86(3):356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perrillat F, Clavel J, Jaussent I, Baruchel A, Leverger G, Nelken B, Philippe N, Schaison G, Sommelet D, Vilmer E et al.. Breast-feeding, fetal loss and childhood acute leukaemia. Eur J Pediatr. 2002;161(4):235–37. [DOI] [PubMed] [Google Scholar]
- 28. Petridou E, Trichopoulos D, Kalapothaki V, Pourtsidis A, Kogevinas M, Kalmanti M, Koliouskas D, Kosmidis H, Panagiotou JP, Piperopoulou F et al.. The risk profile of childhood leukaemia in Greece: a nationwide case-control study. Br J Cancer. 1997;76(9):1241–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rudant J, Orsi L, Menegaux F, Petit A, Baruchel A, Bertrand Y, Lambilliotte A, Robert A, Michel G, Margueritte G et al.. Childhood acute leukemia, early common infections, and allergy: the ESCALE Study. Am J Epidemiol. 2010;172(9):1015–27. [DOI] [PubMed] [Google Scholar]
- 30. Shu XO, Clemens J, Zheng W, Ying DM, Ji BT, Jin F. Infant breastfeeding and the risk of childhood lymphoma and leukaemia. Int J Epidemiol. 1995;24(1):27–32. [DOI] [PubMed] [Google Scholar]
- 31. Urayama KY, Chokkalingam AP, Metayer C, Ma X, Selvin S, Barcellos LF, Wiemels JL, Wiencke JK, Taylor M, Brennan P et al.. HLA-DP genetic variation, proxies for early life immune modulation and childhood acute lymphoblastic leukemia risk. Blood. 2012;120(15):3039–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Steensel-Moll HA, Valkenburg HA, van Zanen GE. Childhood leukemia and infectious diseases in the first year of life: a register-based case-control study. Am J Epidemiol. 1986;124(4):590–94. [DOI] [PubMed] [Google Scholar]
- 33. Bener A, Hoffmann GF, Afify Z, Rasul K, Tewfik I. Does prolonged breastfeeding reduce the risk for childhood leukemia and lymphomas?. Minerva Pediatr. 2008;60(2):155–61. [PubMed] [Google Scholar]
- 34. Schraw JM, Dong YQ, Okcu MF, Scheurer ME, Forman MR. Do longer formula feeding and later introduction of solids increase risk for pediatric acute lymphoblastic leukemia?. Cancer Causes Control. 2014;25(1):73–80. [DOI] [PubMed] [Google Scholar]
- 35. Schuz J, Kaletsch U, Meinert R, Kaatsch P, Michaelis J. Association of childhood leukaemia with factors related to the immune system. Br J Cancer. 1999;80(3–4):585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smulevich VB, Solionova LG, Belyakova SV. Parental occupation and other factors and cancer risk in children: I. Study methodology and non-occupational factors. Int J Cancer. 1999;83(6):712–17. [DOI] [PubMed] [Google Scholar]
- 37. Waly MI, Ali A, Al-Saadoon M, Al-Mukhaini YK, Wali YA. Breastfeeding is not associated with risk of developing childhood leukemia in the Sultanate of Oman. Asian Pac J Cancer Prev. 2011;12(8):2087–91. [PubMed] [Google Scholar]