ABSTRACT
Background
During the Pregnancy and Birth to 24 Months Project, the US Departments of Agriculture and Health and Human Services initiated a review of evidence on diet and health in these populations.
Objectives
The aim of these systematic reviews was to examine the relation of 1) never versus ever feeding human milk, 2) shorter versus longer durations of any human milk feeding, 3) shorter versus longer durations of exclusive human milk feeding, and 4) feeding a lower versus higher intensity of human milk to mixed-fed infants with type 1 and type 2 diabetes in offspring.
Methods
The Nutrition Evidence Systematic Review team conducted systematic reviews with external experts. We searched CINAHL, Cochrane, Embase, and PubMed for articles published January 1980–March 2016, dual-screened the results according to predetermined criteria, extracted data from and assessed the risk of bias for each included study, qualitatively synthesized the evidence, developed conclusion statements, and graded the strength of the evidence.
Results
The 4 systematic reviews included 21, 37, 18, and 1 articles, respectively. Observational evidence suggests that never versus ever feeding human milk (limited evidence) and shorter versus longer durations of any (moderate evidence) and exclusive (limited evidence) human milk feeding are associated with higher type 1 diabetes risk. Insufficient evidence examined type 2 diabetes. Limited evidence suggests that the durations of any and exclusive human milk feeding are not associated with intermediate outcomes (e.g., fasting glucose, insulin resistance) during childhood.
Conclusions
Limited to moderate evidence suggests that feeding less or no human milk is associated with higher risk of type 1 diabetes in offspring. Limited evidence suggests no associations between the durations of any and exclusive human milk feeding and intermediate diabetes outcomes in children. Additional research is needed on infant milk-feeding practices and type 2 diabetes and intermediate outcomes in US populations, which may have distinct metabolic risk.
Keywords: breastfeeding, breast milk, human milk, diabetes, fasting glucose, insulin resistance, systematic review
Introduction
The Pregnancy and Birth to 24 Months Project was an initiative of the US Departments of Agriculture and Health and Human Services (1–3). During the Project, the US Department of Agriculture Nutrition Evidence Systematic Review (NESR) team [formerly the Nutrition Evidence Library (NEL)] collaborated with external experts to complete a series of systematic reviews (SRs) that examined food and nutrition topics relevant to women during pregnancy and offspring during the first 2 y of life.
The SRs in this article examine the relationships between infant milk-feeding practices and diabetes outcomes in offspring, including type 1 diabetes, type 2 diabetes, and some intermediate outcomes such as fasting glucose and insulin resistance. Primary prevention of diabetes is a public health priority in the United States (4), where the incidences of type 1 and type 2 diabetes are increasing in children and adolescents, especially among certain racial and ethnic minorities (5, 6).
The purpose of this article is to summarize the results of 4 SRs conducted to answer the following questions:
What is the relationship between never versus ever feeding human milk and diabetes outcomes in offspring?
What is the relationship between shorter versus longer durations of any human milk feeding and diabetes outcomes in offspring?
What is the relationship between shorter versus longer durations of exclusive human milk feeding and diabetes outcomes in offspring?
What is the relationship between feeding a lower versus higher intensity, proportion, or amount of human milk to mixed-fed infants and diabetes outcomes in offspring?
Methods
NESR analysts (DG, PN, CCL, CD) and librarians (YPW, NT), who were trained in SR methodology and had advanced degrees in fields such as nutrition and library science, collaborated with a group of subject matter experts (SAA, LB, TJ, KMJ, LAN-R, KOO'B, EO, RP-E, EEZ), called a Technical Expert Collaborative (TEC), to complete SRs through the use of the methods that are described in detail in this supplement (7). TEC members provided individual input on SR materials developed by the NESR staff, but did not provide formal group advice or recommendations to the government.
Scope of the systematic review
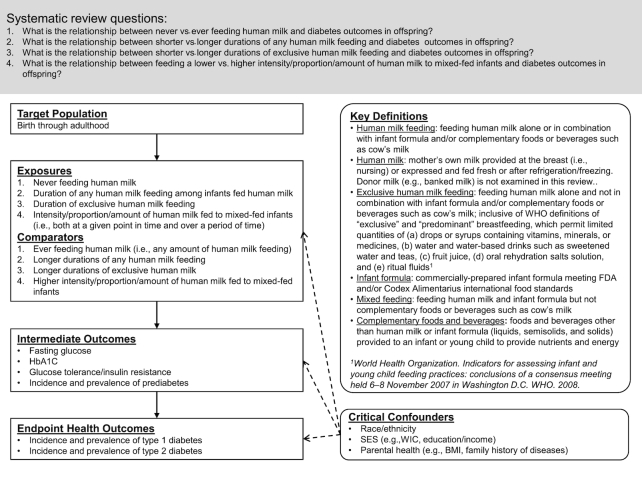
TEC members specified the target population, exposures and comparators, intermediate and endpoint health outcomes, critical confounding variables, and key definitions for the SRs according to the analytic framework shown in Figure 1. In the SRs, infant milk-feeding practices referred to the feeding of human milk, infant formula, or both. TEC members chose to use the term human milk feeding instead of breastfeeding for precision. Breastfeeding may be understood to mean feeding human milk at the breast when, in fact, feeding method was rarely distinguished by the authors of studies included in the SRs. TEC members intended to examine the feeding of human milk whether or not it was fed at the breast.
FIGURE 1.
The analytic framework for SRs conducted to examine the relation of infant milk-feeding practices with diabetes outcomes in offspring. This framework illustrates the overall scope of the project, including the population, exposures and comparators, and outcomes of interest. It also includes definitions for key terms and identifies key confounders considered in the SR. SR, systematic review.
For the comparison of never with ever feeding human milk, TEC members did not define any minimum amount for ever feeding human milk. Likewise, for the comparisons of shorter with longer durations of any and exclusive human milk feeding, TEC members did not define thresholds for shorter duration or longer duration. They examined all comparisons of never with ever feeding human milk (or vice versa) and of shorter with longer durations (or vice versa) as defined by the authors of the studies included in the SRs. TEC members specified both intermediate and endpoint outcomes, collectively referred to in this article as diabetes outcomes.
Literature search, screening, and selection
The librarians developed a literature search strategy that used exposure terminology but not outcome terminology (available at https://nesr.usda.gov) so that 1 search could be used to identify literature in support of SRs examining infant milk-feeding practices with several different outcomes (3). The librarians conducted a broad search in CINAHL, Cochrane, Embase, and PubMed using the search date range January 1980–March 2016. The search excluded articles published before 1980 because the US Congress passed the Infant Formula Act in 1980, which established nutrient requirements for commercial infant formulas in the United States and thus health effects associated with formula consumption before 1980 might be different (8).
TEC members defined inclusion and exclusion criteria a priori (Table 1), which NESR analysts used to dual-screen the search results and the results of a manual search of the references of included articles and existing SRs. TEC members reviewed the search terms and list of included articles to ensure completeness of the body of evidence.
TABLE 1.
Inclusion and exclusion criteria established for the selection of studies to include in SRs on infant milk-feeding practices and diabetes outcomes in offspring1
| Category | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Study design | Randomized controlled trials Nonrandomized controlled trials Prospective cohort studies Retrospective cohort studies Case-control studies |
Cross-sectional studies Before-and-after studies Uncontrolled studies Narrative reviews Systematic reviews Meta-analyses |
| Publication status | Published in peer-reviewed journals | Grey literature, including unpublished data, manuscripts, reports, abstracts, and conference proceedings |
| Language | Published in English | Published in languages other than English |
| Date range | Published from 1980 to December 20152 | Published prior to 1980 |
| Source of foods, beverages, or nutrients | Human milk: mother's own milk, i.e., human milk fed at the breast or expressed and fed fresh or after refrigeration/freezing Infant formula: commercially prepared infant formula meeting FDA (31) and/or Codex Alimentarius (32) food standards |
Human milk from third parties (e.g., banked/donor milk) Infant formulas that are not commercially prepared or that do not meet FDA (31) and/or Codex Alimentarius (32) food standards |
| Study setting | Countries listed as Very High or High on the 2014 Human Development Index3 (33, 34) | Countries listed as Medium or Low on the 2014 Human Development Index (33) |
| Study participants |
Human participants Males Females |
Nonhuman participants (e.g., animal studies, in vitro studies) Hospitalized patients, not including birth and immediate postpartum hospitalization of healthy babies |
| Age of study participants |
Exposure age: infants (0–12 mo), toddlers (12–24 mo) Outcome age: infants (0–12 mo), toddlers (12–24 mo), children (2–12 y), adolescents (13–18 y) adults (≥19 y) |
|
| Size of study groups | Studies with ≥30 participants per study group or a power analysis indicating that the study is appropriately powered for the outcome(s) of interest | Studies with <30 participants per study group with no power analysis indicating that the study is appropriately powered for the outcome(s) of interest |
| Health status of study participants | Studies done in generally healthy populations Studies done in populations where infants were full term (≥37 and 0/7 wk gestational age) Studies done in populations with elevated chronic disease risk, or that enroll some participants with a disease or with the health outcome of interest |
Studies that exclusively enroll participants with a disease or the health outcome of interest Studies done in hospitalized participants (except for birth and immediate post-partum hospitalization of healthy babies) or malnourished participants Studies of exclusively pre-term babies (gestational age <37 wk), exclusively babies that have low birth weight (<2500 g) and/or exclusively babies that are small for gestational age |
Data extraction and risk of bias assessment
NESR analysts assembled a table of systematically extracted data from each article included in the SRs (i.e., study characteristics, sample characteristics, exposures and outcomes, risks of bias, and funding sources). Two NESR analysts independently completed the NEL Bias Assessment Tool for each article to identify the risks of bias [(7), https://nesr.usda.gov].
Evidence synthesis, conclusion statement development, and grading the strength of the evidence
NESR analysts and TEC members engaged in a series of conference calls to review, discuss, and synthesize the evidence. TEC members examined both significant and nonsignificant associations (e.g., ORs and CIs) for a thorough synthesis of the evidence. To answer the SR questions, conclusion statements were carefully constructed to accurately reflect the synthesis of evidence. Conclusion statements do not draw implications, nor should they be interpreted to be dietary guidance. The strength of the evidence underlying each conclusion statement was graded strong, moderate, limited, or grade not assignable according to the NESR grading rubric [(7), https://nesr.usda.gov], which takes into consideration the internal validity, consistency, adequacy, impact, and generalizability of the evidence. Finally, TEC members identified research recommendations.
Results
The literature search yielded 31,335 articles, and the bodies of evidence for the 4 SRs on infant milk-feeding practices and diabetes outcomes in offspring comprise 53 articles. A table of articles excluded during full-text screening, with the rationale for exclusion, is available at https://nesr.usda.gov.
Only 1 of the included articles examined the intensity, proportion, or amount of human milk fed to mixed-fed infants (9). Additional information about this SR is available at https://nesr.usda.gov. Herein, we present evidence for the remaining 3 SRs:
What is the relationship between never versus ever feeding human milk diabetes outcomes in offspring?
What is the relationship between shorter versus longer durations of any human milk feeding and diabetes outcomes in offspring?
What is the relationship between shorter versus longer durations of exclusive human milk feeding and diabetes outcomes in offspring?
Never versus ever feeding human milk and diabetes outcomes in offspring
Twenty-one articles met the inclusion criteria for this SR question; 16 examined type 1 diabetes (10–25), 2 examined type 2 diabetes (26, 27), and 3 examined intermediate outcomes, i.e., fasting glucose (28, 29), hemoglobin A1c (HbA1c) (30) or insulin resistance (28). TEC members concluded that there was insufficient evidence to determine whether or not there is a relationship between never versus ever being fed human milk and type 2 diabetes, prediabetes, fasting glucose, HbA1c, and insulin resistance or glucose tolerance (Table 7). Evidence about type 1 diabetes is presented below.
TABLE 7.
Systematic review questions, conclusion statements, and grades of the evidence supporting the conclusion statements
| Systematic review question | Conclusion statement and grade |
|---|---|
| What is the relationship between never versus ever feeding human milk and diabetes outcomes in offspring? | Limited evidence from observational studies suggests that never versus ever being fed human milk is associated with higher risk of type 1 diabetes. (Grade: Limited). There is insufficient evidence to determine whether or not there is a relationship between never versus ever feeding human milk and type 2 diabetes, prediabetes, fasting glucose, hemoglobin A1c, insulin resistance, and glucose tolerance throughout the lifespan. (Grade: Grade not assignable) |
| What is the relationship between shorter versus longer durations of any human milk-feeding and diabetes outcomes in offspring? | Moderate evidence from observational studies suggests that, among infants fed some amount of human milk, shorter versus longer durations of any human milk feeding are associated with higher risk of type 1 diabetes. (Grade: Moderate). Limited but consistent evidence suggests that the duration of any human milk feeding is not associated with fasting glucose or insulin resistance in childhood or during the transition from childhood into adolescence. (Grade: Limited). There is insufficient evidence to determine whether or not there is a relationship between shorter versus longer durations of any human milk feeding and type 2 diabetes, prediabetes, or hemoglobin A1c throughout the lifespan, and fasting glucose and insulin resistance in adulthood. (Grade: Grade not assignable) |
| What is the relationship between shorter versus longer durations of exclusive human milk feeding and diabetes outcomes in offspring? | Limited evidence from observational studies suggests that shorter versus longer durations of exclusive human milk feeding are associated with higher risk of type 1 diabetes. Limited evidence, from a single study that used a strong design, also suggests that the duration of exclusive human milk feeding is not associated with fasting glucose or insulin resistance at 11.5 y of age. (Grade: Limited). There is insufficient evidence to determine whether or not there is a relationship between shorter versus longer durations of any human milk feeding and type 2 diabetes, prediabetes, and hemoglobin A1c throughout the lifespan, and fasting glucose and insulin resistance at ages other than 11.5 y. (Grade: Grade not assignable) |
| What is the relationship between feeding a lower versus higher intensity, proportion, or amount of human milk to mixed-fed infants and diabetes outcomes in offspring? | There is insufficient evidence to determine whether or not there is a relationship between feeding a lower versus higher intensity, proportion, or amount of human milk to mixed-fed infants and diabetes outcomes in offspring. (Grade: Grade not assignable) |
Type 1 diabetes
The 16 articles that examined never versus ever being fed human milk and type 1 diabetes presented evidence from 15 independent studies (Table 2). There was 1 prospective cohort study (11) and there were 14 independent case-control studies (10, 12–25) because Dahlquist et al. (12) and Rami et al. (20) both presented evidence from the EURODIAB study.
TABLE 2.
Evidence examining the relationship between never versus ever feeding human milk and type 1 diabetes in offspring1
| Author and year | Study design (study/cohort name where applicable) | Country | Notable sample characteristics | Never vs. ever feeding human milk exposures2 | Significant associations with type 1 diabetes | Nonsignificant associations with type 1 diabetes |
|---|---|---|---|---|---|---|
| Alves 2012 (10) | Case control | Brazil |
n = 123 cases, 123 sibling controls Baseline: mean 9 y Race/ethnicity NR Risk: 100% of sibling controls had a sibling with T1D |
Proportion of cases vs. controls who BF | None | 94.3% vs. 99.1%, P = 0.070 |
| Chmiel 2015 (11) | Prospective cohort (BABYDIAB/BABYDIET) | Germany |
n = 2291 Baseline: birth to 3 mo Sex NR Race/ethnicity NR Risk: 100% family history of T1D (parent) |
Infant formula only before age 3 mo vs. EBF before age 3 mo | None | T1D by median 13 y: HR 0.72 (95% CI: 0.34, 1.53), P = 0.40 |
| Dahlquist 2002 (12) | Case control (EURODIAB) | Austria, Latvia, Lithuania, Luxembourg, UK |
n = 610 cases, 1616 controls Baseline: diagnosed <15 y but age at the study NR Sex NR Race/ethnicity NR |
BF vs. not BF | T1D: OR 0.59 (95% CI: 0.35, 0.97) | None |
| Esfarjani 2001 (13) | Case control | Iran |
n = 52 cases, 52 controls Baseline: <14 y Race/ethnicity NR Risk: 0% of controls with family history of IDDM |
Proportion of cases vs. controls who never BF | None | 17.3% vs. 23.1%, NS |
| Kostraba 1992 (14) | Case control | USA |
n = 211 cases, 211 controls Baseline: diagnosed <17 y but age at the study NR Race/ethnicity: 26.1% black, 73.9% white |
Ever BF vs. never BF | IDDM in white subsample: OR 0.5 (95% CI: 0.3, 0.9) | IDDM in black subsample: OR 0.5 (95% CI: 0.2, 1.4) |
| Kostraba 1993 (15) | Case control | USA |
n = 163 cases, 140 controls Baseline: diagnosed <18 y but age at the study NR Race/ethnicity: 43% Hispanic, 57% non-Hispanic white |
Proportion of cases vs. controls who were BF | None | 52.1% vs. 54.3%, NSIn Hispanic subsample: 38% vs. 35.1%, NS In non-Hispanic white subsample: 63% vs. 67.5%, NS |
| Malcova 2006 (16) | Case control | Czech Republic |
n = 868 cases, 1466 controls Baseline: ≤18 y, median 13 y (IQR: 10, 16) for cases, 12 y (IQR: 9, 15) for controls Race/ethnicity NR |
No BF vs. BF 1–3 mo | T1D: OR 1.93 (95% CI: 1.33, 2.80) | None |
| Marshall 2004 (17) | Case control | UK |
n = 196 cases, 381 controls Baseline NR Sex NR Race/ethnicity: ∼93% white |
BF vs. not BF | None | T1D: NS (data NR) |
| Mayer 1988 (18) | Case control | USA |
n = 268 cases, 479 controls Baseline: diagnosed ≤18 y but age at study NR Race/ethnicity: ∼91.5% white |
BF vs. no BF | IDDM: OR 0.70 (95% CI: 0.50, 0.97) | None |
| BF ≤0.99 mo vs. BF 0 mo | None | IDDM: OR 0.92 (95% CI: 0.47, 1.82) | ||||
| BF 1–2.99 mo vs. BF 0 mo | None | IDDM: OR 0.68 (95% CI: 0.39, 1.18) | ||||
| BF 3–5.99 mo vs. BF 0 mo | None | IDDM: OR 0.74 (95% CI: 0.46, 1.20) | ||||
| BF 6–11.99 mo vs. BF 0 mo | None | IDDM: OR 0.67 (95% CI: 0.43, 1.04) | ||||
| BF ≥12 mo vs. BF 0 mo | None | IDDM: OR 0.54 (95% CI: 0.27, 1.08) | ||||
| Meloni 1997 (19) | Case control | Italy |
n = 100 cases, 100 controls Baseline: diagnosed at 1–15 y, but age at study NR Race/ethnicity NR Risk: 0% family history of IDDM in controls, NR in cases |
No BF vs. BFBF 0 mo vs. BF >6 mo | IDDM: OR 0.41 (95% CI: 0.19, 0.91)IDDM: OR 0.36 (95% CI: 0.14, 0.94) | NoneNone |
| Rami 1999 (20) | Case control (EURODIAB ACE) | Austria |
n = 114 cases, 495 controls Baseline: <15 y Race/ethnicity NR |
Proportion of cases vs. controls who BF | None | 82.7% vs. 81%, P = 0.66 |
| Robertson 2010 (21) | Case control | UK |
n = 55 cases, 170 controls Baseline: <15 y Race/ethnicity NR |
BF vs. not BF | None | T1D: OR 1.62 (95% CI: 0.77, 3.44) |
| Siemiatycki 1989 (22) | Case control | Canada |
n = 128 cases, 255 controls Baseline: 5–14 y Race/ethnicity NR |
Never BF vs. BF | None | IDDM: OR 1.2 (95% CI: 0.6, 2.5) |
| Soltesz 1994 (23) | Case control | Hungary |
n = 130 cases, 175 controls Baseline: 0–14 y Race/ethnicity NR |
No BF vs. BF | None | IDDM: OR 1.76 (95% CI: 0.91, 3.41) |
| Tai 1998 (25) | Case control | China |
n = 117 cases, 193 controls Baseline: <30 y Sex: 36.9% male Race/ethnicity NR |
BF < 6 mo vs. never BFBF ≥ 6 mo vs. never BF | NoneT1D: OR 0.25 (95% CI: 0.09, 0.69) | T1D: OR 0.84 (95% CI: 0.45, 1.59)None |
| Thorsdottir 2000 (24) | Case control | Iceland |
n = 55 cases, 165 controls Baseline: 3–19 y, mean 12.5 y Sex NR Race/ethnicity NR |
Frequency of BF in cases vs. controls | None | P > 0.1 (data NR) |
1BF, breastfed; EBF, exclusively breastfed; EURODIAB, European Diabetes; EURODIAB ACE, European Diabetes: Aetiology of Childhood Diabetes on an Epidemiological Basis; IDDM, insulin-dependent diabetes mellitus; NR, not reported; NS, not significant; T1D, type 1 diabetes.
2Exposures, as defined by the authors of the studies included in the body of evidence, which address never versus ever feeding human milk or vice versa.
Six of the studies reported significant associations (12, 14, 16, 18, 19, 25). The primary difference between the studies that did and did not report significant associations was statistical power. For example, all 4 studies with >200 cases (12, 14, 16, 18) reported significant associations. On the other hand, the case-control studies with nonsignificant associations (10, 13, 15, 17, 21–24) had fewer cases. The 2 studies with high-risk samples (10, 11) (which can increase statistical power) did not find significant associations. However, there was nearly universal initiation of human milk feeding by cases and controls in the sample used by Alves et al. (10) (i.e., not a lot of variation), and the comparison of interest in the prospective cohort study by Chmiel et al. (11) had a wide CI around its nonsignificant association indicative of suboptimal statistical power.
With 1 exception (19), the statistically significant associations suggested that never, compared with ever, being fed human milk is associated with higher type 1 diabetes risk. There were significant associations across heterogeneous analyses that compared never feeding human milk with ever feeding human milk (12, 14, 18), feeding human milk for 1–3 mo (16), and feeding human milk for ≥6 mo (25), and that examined risk of type 1 diabetes at ages <15 y (12), <17 y (14), ≤18 y (16, 18), and <30 y (25). Three studies reported significant associations for some comparisons of interest and nonsignificant associations for other comparisons of interest (14, 18, 25), and the difference in significance was likely to be statistical power. For example, Kostraba et al. (14) reported that ever, compared with never, being fed human milk was associated with lower odds of type 1 diabetes in white participants (which comprised 74% of the sample), whereas the corresponding nonsignificant association in the smaller group of black participants was in the same direction but had a wide CI. Likewise, Mayer et al. (18) found that ever, compared with never, being fed human milk was associated with significantly lower odds of type 1 diabetes, whereas additional analyses that divided the group ever fed human milk into smaller groups fed human milk for ≤0.99, 1–2.99, 3–5.99, 6–11.99, and ≥12 mo had wide CIs around nonsignificant associations that were consistent in direction with the ever compared with never association. Finally, Tai et al. (25) reported that being fed human milk for ≥6 mo, compared with never being fed human milk, was associated with lower odds of type 1 diabetes by 30 y of age, whereas a nonsignificant association between being fed human milk for <6 mo, compared with never being fed human milk, was in the same direction but had a wide CI.
Shorter versus longer durations of any human milk feeding and diabetes outcomes in offspring
Thirty-seven articles met the inclusion criteria for this SR question; 30 examined type 1 diabetes (9, 14–20, 24, 36–56), 1 examined type 2 diabetes (27), and 6 examined the intermediate outcomes fasting glucose (28, 57, 58) and insulin resistance or glucose tolerance (28, 57–61). Intermediate outcome data were scant in adults (60, 61). TEC members concluded that there was insufficient evidence to determine the relationship between shorter versus longer durations of any human milk feeding and type 2 diabetes, prediabetes, or HbA1c throughout the lifespan, and fasting glucose, insulin resistance, or glucose tolerance in adulthood (Table 7). Evidence about type 1 diabetes and about fasting glucose, insulin resistance, and glucose tolerance in childhood and during the transition into adolescence is presented below.
Type 1 diabetes
The 30 articles that examined shorter versus longer durations of any human milk feeding and type 1 diabetes used prospective cohort (41, 45, 52, 56), nested case-control (9, 40, 53, 55), and case-control (14–20, 24, 36–39, 42–44, 46–51, 54) study designs (Table 3). There were 22 independent studies because 5 studies presented data across multiple articles [i.e., the Diabetes Autoimmunity Study in the Young with articles by Frederiksen et al. (9) and Hall et al. (56); the Swedish Childhood Diabetes Study with articles by Blom et al. (36) and Dahlquist et al. (38); the Diabetes and Environment around the Baltic Sea study with articles by Skrodeniene et al. (47) and Sadauskaite-Kuehne et al. (43); the European Diabetes: Aetiology of Childhood Diabetes on an Epidemiological Basis study with articles by Rami et al. (20) and Visalli et al. (54); and the Childhood Diabetes in Finland study with 5 articles by Virtanen et al. (49–53)].
TABLE 3.
Evidence examining the relationship between shorter versus longer durations of any human milk feeding and type 1 diabetes in offspring1
| Author and year | Study design (study/cohort name where applicable) | Country | Notable sample characteristics | Shorter vs. longer duration of any human milk feeding exposure2 | Significant associations with type 1 diabetes | Nonsignificant associations with type 1 diabetes |
|---|---|---|---|---|---|---|
| Blom 1989 (36) | Case control (Swedish Childhood Diabetes Study) | Sweden |
n = 339 cases, 527 controls Baseline: 0–14 y Race/ethnicity NR |
Median BF duration in cases vs. controls | In subsample w/onset at 0–6 y: 5 mo vs. 6 mo, P = 0.03 | 4 mo vs. 4 mo, NS In subsample w/onset at 7–14 y: 3 mo vs. 3 mo, NS |
| BF <3 mo vs. BF ≥3 mo | T1D in subsample w/onset at 0–6 y subsample: OR 1.7 (95% CI: 1.02, 2.89) | None | ||||
| Borch-Johnsen 1984 (37) | Case control | Denmark |
n = 266 cases, 230 controls Baseline: diagnosis <15 y, age at study NR Sex NR Race/ethnicity NR Risk: 100% sibling controls have a sibling with IDDM |
Average3 BF duration in cases vs. sibling controls | 2.69 mo vs. 3.41 mo, P < 0.01 | None |
| Dahlquist 1991 (38) | Case control (Swedish Childhood Diabetes Study) | Sweden |
n = 339 cases, 528 controls Baseline: 0–14 y Sex NR Race/ethnicity NR |
BF <3 mo vs. ≥3 mo | T1D in subsample w/onset at 0–4 y: OR: 3.81 (95% CI: 1.10, 13.29), P = 0.035 | T1D: P ≥ 0.20 (data NR) |
| Fort 1986 (39) | Case control | USA |
n = 95 cases, 194 sibling controls, 95 peer controls Baseline: mean 14.8 y (SD = 5.5) Sex NR Race/ethnicity NR Risk: 100% of sibling controls had a sibling with T1D |
BF duration in cases vs. sibling controlsBF duration in cases vs. peer controls | NoneNone | 4.6 mo vs. 4.2 mo, NS4.6 mo vs. 3.3 mo, NS |
| Frederiksen 2013 (9) | Nested case control4 (DAISY) | USA |
n = 53 cases, 1782 controls Baseline: Birth Race/ethnicity: 70.1% non-Hispanic white Risk: 100% at-risk genotype or family history of T1D (first-degree relative) |
Mean BF duration in cases vs. controls | None | 5.8 mo (SD = 7.0) vs. 6.4 mo (SD = 6.9); T1D: HR 0.97 (95% CI: 0.92, 1.01), P = 0.17 |
| Hall 2015 (56) | Prospective cohort (DAISY) | USA |
n = 1783 Baseline: birth Race/ethnicity: 70.2% non-Hispanic white Risk: 100% at-risk genotype or family history of T1D (first-degree relative) |
BF duration (mo) as a continuous variable | T1D: HR 0.95 (CI: 0.90, 1.00), P = 0.05 | None |
| Kostraba 1992 (14) | Case control | USA |
n = 211 cases, 211 controls Baseline: diagnosed <17 y but age at the study NR Race/ethnicity: 26.1% black, 73.9% white |
BF duration in cases vs. controls | None | In white subsample: 23 wk vs. 25 wk, P = 0.8 In black subsample: 17 wk vs. 28 wk, P = 0.13 |
| Kostraba 1993 (15) | Case control | USA |
n = 163 cases, 145 controls Baseline: diagnosed <18 y but age at the study NR Race/ethnicity: 43% Hispanic, 57% non-Hispanic white |
BF duration in cases vs. controls | None | 24.8 wk (SD = 20.4) vs. 27.8 wk (SD = 25.0), NS In Hispanic subsample: 24.3 wk (SD = 25.0) vs. 26.2 wk (SD = 18.3), NS In non-Hispanic white subsample: 25.0 wk (SD = 18.4) vs.. 28.3 wk (SD = 27.1), NS |
| Kyvik 1992 (40) | Nested case control | Denmark |
n = 76 cases (including some deceased), 154 controls Baseline: birth Sex: 100% male Race/ethnicity NR |
BF 0–1 mo vs. BF ≥5 mo | None | T1D (including deaths) by age 20 y: OR 0.93 (95% CI: 0.46, 1.89) |
| BF 1–2 mo vs. BF ≥5 mo | T1D (including deaths) by age 20 y: OR 0.51 (95% CI: 0.29, 0.91) | None | ||||
| BF 2–3 mo vs. BF ≥5 mo | None | T1D (including deaths) by age 20 y: OR 0.58 (95% CI: 0.33, 1.02) | ||||
| BF 3–4 mo vs. BF ≥5 mo | None | T1D (including deaths) by age 20 y: OR 0.95 (95% CI: 0.51, 1.78) | ||||
| BF 4–5 mo vs. BF ≥5 mo | None | T1D (including deaths) by age 20 y: OR 0.91 (95% CI: 0.47, 1.77) | ||||
| Lund-Blix 2015 (41) | Prospective cohort (MIDIA) | Norway |
n = 726 Baseline: birth Race/ethnicity NR Risk: 100% at-risk genotype |
BF duration (mo) as a continuous variable | None | T1D by age 7.70 (SD = 1.58) y: HR 0.99 (95% CI: 0.88, 1.11) |
| Any BF ≥12 mo vs. <12 mo | T1D by age 7.70 (SD = 1.58) y: HR 0.37 (95% CI: 0.15, 0.93) | None | ||||
| Malcova 2006 (16) | Case control | Czech Republic |
n = 868 cases, 1466 controls Baseline: ≤18 y, median 13 y (IQR: 10, 16) for cases, 12 y (IQR: 9, 15) for controls Race/ethnicity NR |
BF 4–6 mo vs. BF 1–3 mo | None | T1D: OR 1.11 (95% CI: 0.82, 1.50) |
| BF 7–9 mo vs. BF 1–3 mo | None | T1D: OR .96 (95% CI: 0.65, 1.41) | ||||
| BF 10–12 mo vs. BF 1–3 mo | None | T1D: OR 0.94 (95% CI: 0.57, 1.56) | ||||
| BF >12 mo vs. BF 1–3 mo | T1D: OR 0.42 (95% CI: 0.22, 0.81) | None | ||||
| Marshall 2004 (17) | Case control | UK |
n = 196 cases, 381 controls Baseline: NR Sex NR Race/ethnicity: ∼93% white |
BF duration (mo) as a continuous variable | None | T1D: NS (data NR) |
| Mayer 1988 (18) | Case control | USA |
n = 268 cases, 479 controls Baseline: diagnosed ≤18 y but age at study NR Race/ethnicity: ∼91.5% white |
BF duration in cases vs. controls | None | 6.39 mo (range: 0.01–23.97 mo) vs. 7.06 mo (range: 0.20–30.33), P = 0.11 |
| Meloni 1997 (19) | Case control | Italy |
n = 100 cases, 100 controls Baseline: diagnosed 1–15 y, but age at study NR Race/ethnicity NR Risk: 0% family history of IDDM in controls, NR in cases |
BF 3–5 mo vs. BF >6 moBF 1–2 mo vs. BF >6 moPer 1 mo increase in BF duration | NoneNoneNone | IDDM: OR 1.18 (95% CI: 0.52, 2.68)IDDM: OR 0.48 (95% CI: 0.19, 1.24)IDDM: OR 1.10 (95% CI: 0.99 , 1.22) |
| Rami 1999 (20) | Case control (EURODIAB ACE) | Austria |
n = 114 cases, 495 controls Baseline: <15 y Race/ethnicity NR |
Median BF duration in cases vs. controls | None | 2 mo (range: 0–24) vs. 2 mo (range: 0–72), P = 0.54 |
| Rosenbauer 2008 (42) | Case control | Germany |
n = 719 cases, 1735 controls Baseline: <6 y Race/ethnicity NR |
BF <5 mo vs. ≥5 mo | T1D: OR 1.40 (95% CI: 1.13, 1.73), P = 0.002 | None |
| BF 2–6 wk vs. BF <2 wk | None | T1D: OR 0.97 (95% CI: 0.72, 1.31) | ||||
| BF 7 wk-4 mo vs. BF <2 wk | None | T1D: OR 0.85 (95% CI: 0.63, 1.13) | ||||
| BF ≥5 mo vs. BF <2 wk | T1D: OR 0.71 (95% CI: 0.54, 0.93), P = 0.012 | None | ||||
| BF duration trend including categories <2 wk, 2–6 wk, 7 wk–4 mo, ≥5 mo | T1D: OR 0.89 (95% CI: 0.82, 0.97), P = 0.008 | None | ||||
| Sadauskaite-Kuehne 2004 (43) | Case control (Diabetes and Environment around the Baltic Sea) | Sweden |
n = 517 cases, controls NR (∼2 controls per case) Baseline: 0–15 y Race/ethnicity NR |
Total BF ≥7 mo vs. <7 mo | T1D in subsample ages 5–9 y: OR 0.56 (95% CI: 0.38, 0.84) | NR5 |
| Total BF ≥9 mo vs. <9 mo | T1D in subsample ages 5–9 y: OR 0.61 (95% CI: 0.41, 0.92) | NR | ||||
| Samuelsson 1993 (44) | Case control | Sweden |
n = 297 cases, 792 controls Baseline: 0–14 y Sex NR Race/ethnicity NR |
Mean duration of complete and partial BF in cases vs. controls | None | NS (data NR) In subsample <5 y: 8.50 mo (SD = 1.72) vs. 6.08 mo (SD = 1.06), P = 0.33 In subsample ages 5–9 y: 6.60 mo (SD = 0.58) vs. 6.28 mo (SD = 0.43), P = 0.62 In subsample >10 y: 3.85 mo (SD = 0.22) vs. 4.23 mo (SD = 0.20), P = 0.36 |
| Savilahti 2009 (45) | Prospective cohort6 | Finland |
n = 4444 Baseline: birth Sex NR Race/ethnicity NR |
Total BF ≤5.8 mo vs. >5.8 mo | None | T1D by mean 11.5 y: OR 0.95 (95% CI: 0.63, 1.42) |
| Sipetic 2005 (46) | Case control | Serbia |
n = 105 cases (68 in sibling subsample), 210 outpatient controls, 68 sibling controls Baseline: 0–16 y Sex NR Race/ethnicity NR Risk: 100% of sibling controls had a sibling with T1D by 16 y |
BF <4 mo vs. BF ≥4 mo | T1D: OR 2.09 (95% CI: 1.30, 3.36) | T1D in sibling subsample: OR 1.39 (95% CI: 0.75, 2.56) |
| Skrodenienė 2010 (47) | Case control (Diabetes and Environment around the Baltic Sea) | Lithuania |
n = 191 cases, controls NR Baseline: 0–15 y Race/ethnicity NR |
Total BF <3 mo vs. ≥3 mo | T1D: OR 3.46 (95% CI: 1.14, 10.50) | None |
| Thorsdottir 2000 (24) | Case control | Iceland |
n = 55 cases, 165 controls Baseline: 3–19 y, mean 12.5 y Sex NR Race/ethnicity NR |
BF duration in cases vs. controls | None | P > 0.1 (data NR) |
| Verge 1994 (48) | Case control | Australia |
n = 217 cases, 258 controls Baseline: 0–15 y, median 9.2 y Race/ethnicity NR |
Median BF duration in cases vs. controls | 3 mo (IQR: 1, 9) vs. 4 mo (IQR: 1, 10), P = 0.03 | None |
| BF 2–7 mo vs. <2 mo | IDDM: OR 0.63 (95% CI: 0.40, 0.99)IDDM in subsample age <9.2 y: OR 0.33 (95% CI: 0.16, 0.69) | IDDM in subsample age ≥9.2 y: OR 0.86 (95% CI: 0.46, 1.60) | ||||
| BF >7 mo vs. <2 mo | IDDM in subsample age <9.2 y: OR 0.39 (95% CI: 0.19, 0.79) | IDDM: OR 0.68 (95% CI: 0.42, 1.09) IDDM in subsample age ≥9.2 y: OR 1.06 (95% CI: 0.51, 2.18) | ||||
| Virtanen 1991 (49) | Case control (DiMe) | Finland |
n = 103 cases, 103 controls Baseline: 0–6 y Race/ethnicity NR |
Median BF duration in cases vs. controls | None | 8 mo vs. 9 mo, NS |
| BF ≥1 mo vs. <1 mo | None | IDDM: ∼97% in cases vs. ∼100% in controls | ||||
| BF ≥2 mo vs. <2 mo | None | IDDM: ∼94% in cases vs. ∼99% in controls | ||||
| BF ≥3 mo vs. <3 mo | None | IDDM: ∼82% in cases vs. ∼92% in controls, OR 0.45 (95% CI: 0.15, 1.32) | ||||
| BF ≥4 mo vs. <4 mo | None | IDDM: ∼79% in cases vs. ∼89% in controls, OR 0.53 (95% CI: 0.22, 1.26) | ||||
| BF ≥5 mo vs. <5 mo | None | IDDM: ∼75% in cases vs. ∼85% in controls, OR 0.68 (95% CI: 0.32, 1.44) | ||||
| BF ≥6 mo vs. <6 mo | None | IDDM: ∼69% in cases vs. ∼79% in controls, OR 0.69 (95% CI: 0.36, 1.32) | ||||
| BF ≥7 mo vs. <7 mo | IDDM: ∼52% in cases vs. ∼71% in controls, OR 0.48 (95% CI: 0.25, 0.92), P < 0.01 | None | ||||
| BF ≥8 mo vs. <8 mo | None | IDDM: ∼50% in cases vs. ∼65% in controls, OR 0.62 (95% CI: 0.33, 1.17) | ||||
| BF ≥9 mo vs. <9 mo | None | IDDM: ∼40% in cases vs. ∼50% in controls, OR 0.73 (95% CI: 0.36, 1.50) | ||||
| BF ≥10 mo vs. <10 mo | None | IDDM: ∼33% in cases vs. ∼41% in controls | ||||
| BF ≥11 mo vs. <11 mo | None | IDDM: ∼30% in cases vs. ∼37% in controls | ||||
| BF ≥12 mo vs. <12 mo | None | IDDM: ∼23% in cases vs. ∼27% in controls | ||||
| Virtanen 1992 (50) | Case control (DiMe) | Finland |
n = 426 cases, 426 controls Baseline: 7–14 y |
Median BF duration in cases vs. controls | None | 4 mo vs. 5 mo, NS |
| Race/ethnicity NR | BF ≥1 mo vs. <1 mo | None | IDDM: ∼95% in cases vs. ∼93% in controls; OR ∼1.2 (95% CI: ∼0.6, ∼2.3) | |||
| BF ≥2 mo vs. <2 mo | IDDM: ∼83% in cases vs. ∼89% in controls, OR 0.64 (95% CI: 0.42, 0.98), P < 0.05 | None | ||||
| BF ≥3 mo vs. <3 mo | IDDM: ∼70% in cases vs. ∼78% in controls, OR 0.67 (95% CI: 0.48, 0.95), P < 0.05 | None | ||||
| BF ≥4 mo vs. <4 mo | None | IDDM: ∼53% in cases vs. ∼60% in controls, OR ∼0.8 (95% CI: ∼0.6, ∼1.1) | ||||
| BF ≥5 mo vs. <5 mo | None | IDDM: ∼45% in cases vs. ∼50% in controls, OR ∼0.8 (95% CI: ∼0.6, ∼1.1) | ||||
| BF ≥6 mo vs. <6 mo | None | IDDM: ∼38% in cases vs. ∼40% in controls, OR ∼0.8 (95% CI: ∼0.6, ∼1.2) | ||||
| BF ≥7 mo vs. <7 mo | None | IDDM: ∼29% in cases vs.. ∼29% in controls, OR ∼1.0 (95% CI: ∼0.8, ∼1.4) | ||||
| BF ≥8 mo vs. <8 mo | None | IDDM: ∼23% in cases vs. ∼21% in controls, OR ∼1.1 (95% CI: ∼0.8, ∼1.6) | ||||
| BF ≥9 mo vs. <9 mo | None | IDDM: ∼20% in cases vs. ∼17% in controls, OR ∼1.3 (95% CI: ∼0.8, ∼1.9) | ||||
| BF ≥10 mo vs. <10 mo | None | IDDM: ∼12% in cases vs. ∼10% in controls, OR ∼1.4 (95% CI: ∼0.8, ∼2.2) | ||||
| Virtanen 1994 (51) | Case control (DiMe) | Finland |
n = 415 cases, 415 sibling controls Baseline: 3–14 ySex NR Race/ethnicity NRRisk: 100% sibling controls have a sibling with IDDM |
Median BF duration in cases vs. sibling controlsBF duration ≥4 mo vs. <4 mo | 5 mo vs. 6 mo, P = 0.008None | NoneIDDM: OR 1.68 (95% CI: 0.33, 8.72) |
| Virtanen 1998 (52) | Prospective cohort (DiMe) | Finland |
n = 725 Baseline: 0.4–24.9 y, median 9.4 y Race/ethnicity NR Risk: 100% family risk of T1D (sibling) |
Duration of total BF ≥2 mo vs. <2 mo | None | T1D within 4 y of T1D diagnosis in a sibling: HR 0.53 (95% CI: 0.2, 1.6) |
| Virtanen 2000 (53) | Nested Case Control (DiMe) | Finland |
n = 33 cases, 254 controls Baseline: 1.6–16.9 y Race/ethnicity NR Risk: 100% family risk of T1D (sibling) |
Duration of total BF ≥2 mo vs. <2 mo | None | T1D: OR 1.11 (95% CI: 0.3, 3.7) |
| Visalli 2003 (54) | Case control (EURODIAB ACE) | Italy |
n = 150 cases, 750 controls Baseline: 6–18 y Race/ethnicity NR |
BF duration <3 mo vs. ≥3 mo | T1D: OR 1.74 (95% CI: 1.40, 2.45) | None |
| Welander 2014 (55) | Nested case control7 (ABIS Study) | Sweden |
n = 46 cases, 9368 reference Baseline: birthRace/ethnicity NR |
BF 0–2 mo vs. ≥11 mo | None | T1D by ∼13.5 y: HR 0.7 (95% CI: 0.2, 3.1) |
| BF 3–4 mo vs. ≥11 mo | None | T1D by ∼13.5 y: HR 0.7 (95% CI: 0.2, 3.2) | ||||
| BF 5–6 mo vs. ≥11 mo | None | T1D by ∼13.5 y: HR 1.2 (95% CI: 0.4, 3.5) | ||||
| BF 7–8 mo vs. ≥11 mo | None | T1D by ∼13.5 y: HR 1.2 (95% CI: 0.5, 2.6) | ||||
| BF 9–10 mo vs. ≥11 mo | None | T1D by ∼13.5 y: HR 1.4 (95% CI: 0.7, 3.0) |
1ABIS, All Infants in Southeast Sweden; BF, breastfed; DAISY, Diabetes Autoimmunity Study in the Young; DiMe, Childhood Diabetes in Finland; EURODIAB ACE, European Diabetes: Aetiology of Childhood Diabetes on an Epidemiological Basis; IDDM, insulin-dependent diabetes mellitus; MIDIA – Environmental Triggers of Type 1 Diabetes; NR, not reported; NS, not significant; T1D, type 1 diabetes.
2Exposures, as defined by the authors of the studies included in the body of evidence, which address shorter versus longer durations of any human milk feeding or vice versa.
3Authors do not specify what type of average this is (e.g., mean, median).
4Authors call the study a prospective cohort; however, the assessment grouped participants by outcome status rather than infant feeding exposure.
5Authors only reported significant associations; information about nonsignificant findings were NR.
6Original study was a randomized controlled trial; however, the data of interest are pooled and unrelated to randomization.
7Authors call the study a prospective cohort; however, the assessment grouped participants by outcome status rather than infant feeding exposure.
Twelve studies reported significant associations across 16 articles (16, 36–38, 40–43, 46–51, 54, 56). A primary difference between the studies that did and did not report significant associations was statistical power. For example, 7 (16, 36–38, 42, 43, 48, 50, 51) of 10 studies with >200 cases (14, 16, 18, 36–38, 42–44, 48, 50, 51) and 4 (37, 41, 51, 56) of 6 studies that examined high-risk samples (9, 37, 39, 41, 46, 51–53, 56) found significant associations. In contrast, the 10 studies that did not find significant associations (14, 15, 17–19, 24, 39, 44, 45, 55) tended to have fewer cases (15, 17, 19, 24, 39, 55).
With 1 exception (40), the significant associations between the duration of any human milk feeding and type 1 diabetes risk were inverse associations. The significant associations were consistent in direction across prospective cohort (41, 56) and case-control study designs (16, 36–38, 42, 43, 46–51, 54), and across heterogeneous analyses that examined duration as a continuous variable (56), compared the average duration of human milk feeding in cases and controls (36, 37, 48, 51), compared heterogeneous ranges of duration [i.e., <2 wk compared with ≥5 mo (42), 1–3 compared with >12 mo (16), <2 compared with ≥2 mo (50), <2 compared with 2–7 mo (48), <2 compared with >7 mo (48), <3 compared with ≥3 mo (36, 38, 47, 50, 54), <4 compared with ≥4 mo (46), <5 compared with ≥5 mo (42), <7 compared with ≥7 mo (43, 49), <9 compared with ≥9 mo (43), and <12 compared with ≥12 mo (41)], and assessed the trend across multiple categories of duration (42). They examined risk of type 1 diabetes at ages 3–14 y (51), ≤4 y (38), <6 y (42), ≤6 y (36, 49), 6–18 y (54), <7.7 y (41), 7–14 y (50), <15 y (37), ≤15 y (43, 47, 48), ≤16 y (46), and ≤18 y (16).
The study by Kyvik et al. (40) was the only one with a significant association in a discrepant direction. However, this study has limited external validity as it included a male-only sample of cases who were both living and deceased (participants were identified from records of rejection from the mandatory military conscription or death certificates), and there were no comparable analyses in other studies in this body of evidence that would allow TEC members to examine whether the reported associations are typical among males.
Fasting glucose, insulin resistance, and glucose tolerance in childhood and the transition into adolescence
One cluster randomized controlled trial (57) and 3 prospective cohort studies (28, 58, 59) found no associations between the duration of any human milk feeding and fasting glucose, insulin resistance, or glucose tolerance in childhood and the transition from childhood into adolescence (Table 4). Martin et al. (57) presented evidence from the Promotion of Breastfeeding Intervention Trial (PROBIT), a cluster randomized controlled trial of an intervention to promote prolonged duration and exclusivity of breastfeeding among mothers who chose to feed human milk. The primary, intention-to-treat, analysis compared the intervention group (which had higher rates of any human milk feeding measured at 3, 6, 9, and 12 mo) to the control group, and found no significant differences in fasting glucose or homeostasis model assessment of insulin resistance (HOMA-IR) at 11.5 y. Prospective cohort analyses of PROBIT study data, which compared children fed human milk 3–<6 mo and ≥6 mo with children fed human milk <3 mo and examined the trend across the 3 categories of duration (<3, 3–<6, and ≥6 mo), were also nonsignificant. Jeffery et al. (59) reported that HOMA-IR at 8 y of age was not significantly associated with the duration of any human milk feeding in girls or in boys. Rodekamp et al. (58) found no significant associations between the duration of human milk feeding and fasting glucose, 2-h oral glucose tolerance test results, or impaired glucose tolerance [based on National Diabetes Data Group criteria for children (62)] at 2 y of age in a sample of children whose mothers had type 1 diabetes or gestational diabetes. Davis et al. (28) also enrolled a high-risk sample; participants were 8–13 y of age, Latino, had a BMI ≥85th percentile according to CDC growth standards, and had a family history of type 2 diabetes. This study found no significant associations between the duration of human milk feeding and fasting glucose, 2-h oral glucose tolerance test results, insulin sensitivity, acute insulin response, or disposition index (a measure of pancreatic β-cell function) at Tanner pubertal stage 1 or across the pubertal transition from Tanner pubertal stage 1 to 5.
TABLE 4.
Evidence examining the relationship between shorter versus longer durations of any human milk feeding and fasting glucose, insulin resistance, and glucose tolerance in offspring in childhood and the transition from childhood into adolescence1
| Author and year | Study design (study/cohort name where applicable) | Country | Notable sample characteristics | Shorter vs. longer duration of any human milk feeding exposure2 | Significant associations with intermediate outcomes | Nonsignificant associations with intermediate outcomes |
|---|---|---|---|---|---|---|
| Davis 2007 (28) | Prospective cohort (University of Southern California longitudinal SOLAR) | USA |
n = 150 Baseline: 8–13 y Race/ethnicity: 100% Latino Risk: 100% family history of T2D (≥1 parent, sibling, or grandparent); 100% BMI ≥85th percentile |
BF duration | None | Fasting glucose at Tanner pubertal stage 1: P ≥ 0.20 (data NR) Fasting glucose across pubertal transition from Tanner pubertal stage 1 to 5: P ≥ 0.20 (data NR) 2-h glucose during OGTT at Tanner pubertal stage 1: P ≥ 0.20 (data NR) 2-h glucose during OGTT across pubertal transition from Tanner pubertal stage 1 to 5: P ≥ 0.20 (data NR) Insulin sensitivity at Tanner pubertal stage 1: P ≥ 0.20 (data NR) Insulin sensitivity across pubertal transition from Tanner pubertal stage 1 to 5: P ≥ 0.20 (data NR) Acute insulin response at Tanner pubertal stage 1: P ≥ 0.20 (data NR) Acute insulin response across pubertal transition from Tanner pubertal stage 1 to 5: P ≥ 0.20 (data NR) Disposition index at Tanner pubertal stage 1: P ≥ 0.20 (data NR) Disposition index across pubertal transition from Tanner pubertal stage 1 to 5: P ≥ 0.20 (data NR) |
| Jeffery 2006 (59) | Prospective cohort (EarlyBird Diabetes Study) | UK |
n = 235 Baseline: 5 y Race/ethnicity: 98% white |
BF duration | None | HOMA-IR for boys at 8 y: NS (data NR) HOMA-IR for girls at 8 y: NS (data NR) |
| Martin 2014 (57) | RCT3 or prospective cohort, depending on the analysis (PROBIT) | Belarus |
n = 13,616 Baseline: birth Race/ethnicity NR |
Intervention group (higher rate of any BF at 3, 6, 9, and 12 mo) vs. control group | None | Fasting glucose (mmol/L) at 11.5 y: mean difference –0.03 (95% CI: –0.16, 0.10) HOMA-IR at 11.5 y: ratio of geometric means 1.05 (95% CI: 0.85, 1.30) |
| BF 3–<6 mo vs. <3 mo | None | Fasting glucose (mmol/L) at 11.5 y: mean difference 0.00 (95% CI: –0.03, 0.02) HOMA-IR at 11.5 y: ratio of geometric means 1.00 (95% CI: 0.95, 1.05) |
||||
| BF ≥6 mo vs. <3 mo | None | Fasting glucose (mmol/L) at 11.5 y: mean difference 0.01 (95% CI: –0.01, 0.03) HOMA-IR at 11.5 y: ratio of geometric means 0.97 (0.93, 1.02) |
||||
| BF duration trend according to the categories <3, 3–<6, and ≥6 mo | None | Fasting glucose (mmol/L) at 11.5 y: P = 0.27 HOMA-IR at 11.5 y: P = 0.66 |
||||
| Rodekamp 2005 (58) | Prospective cohort (Kaulsdorf Cohort Study) | Germany |
n = 112 Baseline: birth Race/ethnicity NR Risk: 100% family history of T1D or GDM (mothers) |
BF duration (wk) | None | Fasting glucose at 2 y: NS (data NR) 2-h glucose during OGTT at 2 y: β = 0.15, P = 0.13 Impaired glucose tolerance at 2 y: OR 0.99 (95% CI: 0.94, 1.06) |
1BF, breastfed; GDM, gestational diabetes mellitus; NR, not reported; NS, not significant; OGTT, oral-glucose-tolerance test; PROBIT, Promotion of Breastfeeding Intervention Trial; RCT, randomized controlled trial; SOLAR, Study Of Latino Adolescents at Risk for Diabetes; T1D, type 1 diabetes; T2D, type 2 diabetes.
2Exposures, as defined by the authors of the studies included in the body of evidence, which address shorter versus longer durations of any human milk feeding or vice versa.
3RCT of an intervention to promote prolonged duration and exclusivity of breastfeeding rather than an RCT of breastfeeding per se.
Shorter versus longer durations of exclusive human milk and diabetes outcomes in offspring
Eighteen articles met the inclusion criteria for this SR question; 17 articles examined type 1 diabetes (9, 10, 13, 14, 16, 20, 41, 43, 44, 48–50, 35, 63–66), and 1 article examined fasting glucose and insulin resistance at 11.5 y of age (57). TEC members concluded that there was insufficient evidence to determine whether or not there is a relationship between shorter versus longer durations of exclusive human milk feeding and type 2 diabetes, prediabetes, HbA1c, fasting glucose at ages other than 11.5 y, and insulin resistance at ages other than 11.5 y (Table 7). Evidence about type 1 diabetes and about fasting glucose and insulin resistance at 11.5 y of age is presented below.
Type 1 diabetes
The 17 articles that examined shorter versus longer durations of exclusive human milk feeding and type 1 diabetes presented evidence from 15 independent studies (Table 5). There was 1 prospective cohort study (41), 1 nested case-control study (9), and 13 independent case-control studies (10, 13, 14, 16, 20, 43, 44, 48–50, 35, 63–66) because Samuelsson et al. (44, 65) and Virtanen et al. (49, 50) each presented data for a single study across 2 articles.
TABLE 5.
Evidence examining the relationship between shorter versus longer durations of exclusive human milk feeding and type 1 diabetes in offspring1
| Author and year Country | Study design (study/cohort name where applicable) | Country | Notable sample characteristics | Shorter vs. longer durations of exclusive human milk feeding exposures2 | Significant associations with type 1 diabetes | Nonsignificant associations with type 1 diabetes |
|---|---|---|---|---|---|---|
| Alves 2012 (10) | Case control | Brazil |
n = 123 cases, 123 sibling controls Baseline: mean 9 y Race/ethnicity NR Risk: 100% of sibling controls had a sibling with T1D |
Mean difference in EBF duration in cases vs. controls | –0.9 mo (95% CI: –1.2, –0.6), P < 0.001 | None |
| Esfarjani 2001 (13) | Case control | Iran |
n = 52 cases, 52 controls Baseline: <14 y Race/ethnicity NR Risk: 0% of controls with family history of IDDM |
Mean duration of EBF in cases vs. controls | None | 4.5 mo (SD = 3.1) vs. 4.1 mo (SD = 3.9), NS |
| Frederiksen 2013 (9) | Nested case control3 (DAISY) | USA |
n = 53 cases, 1782 controls Baseline: birth Race/ethnicity: 70.1% non-Hispanic white Risk: 100% at-risk genotype or family history of T1D (first-degree relative) |
Mean EBF duration in cases vs. controls | None | 1.4 mo (SD = 2.0) vs. 1.3 mo (SD = 1.7); T1D HR 0.97 (95% CI: 0.83, 1.14), P = 0.73 |
| Gimeno 1997 (63) | Case control | Brazil |
n = 346 cases, 346 controls Baseline: <18 y Sex NR Race/ethnicity NR |
EBF 0–7 d vs. >60 dEBF 8–60 d vs. > 60 d | IDDM: OR 2.13 (95% CI: 1.28, 3.55)None | NoneIDDM: OR 1.14 (95% CI: 0.82, 1.58) |
| Kostraba 1992 (14) | Case control | USA |
n = 211 cases, 211 controls Baseline: diagnosed <17 y but age at the study NR Race/ethnicity: 26.1% black, 73.9% white |
EBF duration in cases vs. controls | None | In white subsample: 18 wk vs. 13 wk, P = 0.4 In black subsample: 13 wk vs. 27 wk, P = 0.16 |
| Lund-Blix 2015 (41) | Prospective cohort (MIDIA) | Norway |
n = 726 Baseline: birth Race/ethnicity NR Risk: 100% at-risk genotype |
Full BF duration (mo) as a continuous variable | None | T1D by age 7.70 (SD = 1.58) y: HR 0.96 (95% CI: 0.78, 1.18) |
| Full BF 4–5.9 mo vs. <4 mo | None | T1D by age 7.70 (SD = 1.58) y: HR 0.79 (95% CI: 0.32, 1.94) | ||||
| Full BF ≥6 mo vs. <4 mo | None | T1D by age 7.70 (SD = 1.58) y: HR 0.84 (95% CI: 0.26, 2.73) | ||||
| Full BF ≤2 wk vs. >2 wk | None | T1D by age 7.70 (SD = 1.58) y: HR 1.10 (95% CI: 0.36, 3.41) | ||||
| Malcova 2006 (16) | Case control | Czech Republic |
n = 868 cases, 1466 controls Baseline: ≤18 y, median 13 y (IQR: 10, 16) for cases, 12 y (IQR: 9, 15) for controls Race/ethnicity NR |
Introduced to formula or other supplementary feeding at 1–3 mo vs. 4–6 mo | None | T1D: OR 1.11 (95% CI: 0.83, 1.50) |
| Introduced to formula or other supplementary feeding at 7–9 mo vs. 4–6 mo | None | T1D: OR 0.96 (95% CI: 0.69, 1.34) | ||||
| Introduced to formula or other supplementary feeding at ≥ 10 mo vs. 4–6 mo | None | T1D: OR 0.90 (95% CI: 0.49, 1.67) | ||||
| Perez-Bravo 1996 (35) | Case control | Chile |
n = 80 cases, 85 controls Baseline: mean: 15.1 y (SD = 5.6) Race/ethnicity: strata III of the sociogenetic classification (40% indigenous admixture with European genetic pools of mostly Spanish origin) |
Mean EBF duration in cases vs. controls | 21.55 wk (SD = 15.05) vs. 33.95 wk (SD = 20.40), P = 0.01 | None |
| Perez-Bravo 2003 (64) | Case control | Chile |
n = 143 cases, 107 controls Baseline: mean ∼8 y (SD ∼4 y) Race/ethnicity: 100% 2 Hispanic surnames and no Amerindian background |
Mean EBF duration in cases vs. controls | 5.4 mo (SD = 3.5) vs. 7.6 mo (SD = 3.6), P < 0.02 | None |
| Rami 1999 (20) | Case control (EURODIAB ACE) | Austria |
n = 114 cases, 495 controls Baseline: <15 y Race/ethnicity NR |
Median EBF duration in cases vs. controls | None | 2 mo (range: 0–7) vs. 2 mo (range: 0–18), P = 0.40 |
| Sadauskaite-Kuehne 2004 (43) | Case control (Diabetes and Environment around the Baltic Sea) | Sweden, Lithuania |
n = 517 Swedish cases, 286 Lithuanian cases, controls NR (∼2 controls per case) Baseline: 0–15 y Race/ethnicity NR |
EBF ≥5 mo vs. <5 mo | T1D in Swedish subsample ages 5–9 y: OR 0.54 (95% CI: 0.36, 0.81) | NR4 |
| EBF ≥2 mo vs. <2 mo | T1D in Lithuanian subsample ages 5–9 y: OR 0.58 (95% CI: 0.34, 0.99) | NR | ||||
| Samuelsson 1993 (44) | Case control | Sweden |
n = 297 cases, 792 controls Baseline: 0–14 y Sex NR Race/ethnicity NR |
Mean duration of complete BF in cases vs. controls | None | NS (data NR) In subsample age <5 y: 4.50 mo (SD = 0.68) vs. 3.02 mo (SD = 0.47), P = 0.17 In subsample age 5–9 y: 3.18 mo (SD = 0.26) vs. 3.55 mo (SD = 0.25), P = 0.34 In subsample age >10 y: 2.17 mo (SD = 0.16) vs. 2.40 mo (SD = 0.11) |
| Samuelsson 2001 (65) | Case control | Sweden |
n = 297 cases, 736 controls Baseline: 0–14 y Sex NR Race/ethnicity NR |
Mean EBF duration in cases vs. controls | None | 2.5 mo (95% CI: 2.2, 2.7) vs. 2.6 mo (95% CI: 2.5, 2.8) |
| Verge 1994 (48) | Case control | Australia |
n = 217 cases, 258 controls Baseline: 0–15 y, median 9.2 y Race/ethnicity NR |
EBF ≥3 mo vs. EBF <3 mo | IDDM: OR 0.66 (95% CI: 0.45, 0.97) IDDM in subsample age <9.2 y: OR 0.50 (95% CI: 0.28, 0.87) |
IDDM in subsample age ≥9.2 y: OR 0.80 (95% CI: 0.46, 1.39) |
| Virtanen 1991 (49) | Case control (DiMe) | Finland |
n = 103 cases, 103 controls Baseline: 0–6 y Race/ethnicity NR |
Median EBF duration in cases vs. controls | 3 mo vs. 4 mo, P = 0.02 | None |
| EBF ≥1 mo vs. <1 mo | None | IDDM: ∼90% in cases vs. ∼99% in controls, NS | ||||
| EBF ≥2 mo vs. <2 mo | None | IDDM: ∼88% in cases vs. ∼95% in controls, NS | ||||
| EBF ≥3 mo vs. <3 mo | IDDM: OR 0.36 (95% CI: 0.14, 0.93) ∼73% in cases vs. ∼90% in controls, P < 0.05 |
None | ||||
| EBF ≥4 mo vs. <4 mo | IDDM: OR 0.41 (95% CI: 0.21, 0.83) ∼41% in cases vs. ∼61% in controls, P < 0.05 |
None | ||||
| EBF ≥5 mo vs. <5 mo | None | IDDM: OR 0.77 (95% CI: 0.36, 1.64) ∼28% in cases vs. ∼30% in controls, NS |
||||
| EBF ≥6 mo vs. <6 mo | None | IDDM: ∼15% in cases vs. ∼14% in controls, NS | ||||
| Virtanen 1992 (50) | Case control (DiMe) | Finland |
n = 426 cases, 426 controls Baseline: 7–14 y Race/ethnicity NR |
Median EBF duration in cases vs. controls | 2 mo vs. 2 mo,5P = 0.04 | None |
| EBF ≥1 mo vs. <1 mo | None | IDDM: OR ∼0.8 (95% CI: ∼0.5, ∼1.4) ∼88% in cases vs. ∼90% in controls | ||||
| EBF ≥2 mo vs. <2 mo | IDDM: OR 0.60 (95% CI: 0.41, 0.89) ∼65% in cases vs. ∼75% in controls, P < 0.05 |
None | ||||
| EBF ≥3 mo vs. <3 mo | IDDM: OR 0.63 (95% CI: 0.43, 0.93) ∼41% in cases vs. ∼51% in controls, P < 0.05 |
None | ||||
| EBF ≥4 mo vs. <4 mo | None | IDDM: OR ∼0.7 (95% CI: ∼0.4, ∼1.1) ∼14% in cases vs. ∼20% in controls |
||||
| EBF ≥5 mo vs. <5 mo | IDDM: ∼7% in cases vs. ∼13% in controls, P < 0.05 | IDDM: OR ∼0.6 (95% CI: ∼0.3, ∼1.2) | ||||
| EBF ≥6 mo vs. <6 mo | None | IDDM: OR ∼0.7 (95% CI: ∼0.3, ∼1.7) ∼4% in cases vs. ∼7% in controls |
||||
| Wadsworth 1997 (66) | Case control | UK |
n = 218 cases, 324 controls Baseline: <5 y Sex NR Race/ethnicity NR |
First introduction of artificial milk formula 2–6 wk vs. <2 wk | None | T1D: OR 1.42 (95% CI: 0.75, 2.70) |
| First introduction of artificial milk formula 6 wk–4 mo vs. <2 wk | None | T1D: OR 0.71 (95% CI: 0.40, 1.23) | ||||
| First introduction of artificial milk formula >4 mo vs. <2 wk | None | T1D: OR 1.41 (95% CI: 0.70, 2.80) |
1BF, breastfeeding; DAISY, Diabetes Autoimmunity Study in the Young; DiMe; Childhood Diabetes in Finland; EBF, exclusively breastfed; IDDM, insulin-dependent diabetes mellitus; MIDIA, Environmental Triggers of Type 1 Diabetes; NR, not reported; NS, not significant; T1D, type 1 diabetes.
2Exposures, as defined by the authors of the studies included in the body of evidence, which address shorter versus longer durations of exclusive human milk feeding or vice versa
3The authors call the study a prospective cohort; however, the assessment grouped participants by outcome status rather than infant feeding exposure.
4Authors only reported significant associations; information about nonsignificant findings was not reported.
5Although the medians are the same, the authors describe this as significantly shorter EBF duration in cases than controls
Seven of the studies reported significant associations across 8 articles (10, 43, 48–50, 35, 63, 64). It is notable that the study by Alves et al. (10), which is the only study in the body of evidence that paired cases with sibling controls to minimize confounding from shared genetic and environmental factors, found a significant association. On the other hand, the studies that were more likely to have sufficient statistical power (i.e., studies with the largest numbers of cases and studies that recruited high-risk samples) reported both significant and nonsignificant associations. For example, 4 (43, 48, 50, 63) of 8 studies with >200 cases (14, 16, 43, 44, 48, 50, 63, 65, 66) found significant associations, and 1 (10) of 3 studies that examined high-risk samples (9, 10, 41) found significant associations.
All of the significant associations in the body of evidence were consistent in direction, suggesting that shorter versus longer durations of any human milk feeding are associated with higher risk of type 1 diabetes. The significant associations were from case-control studies that compared the average duration of exclusive human milk feeding in cases and controls (10, 49, 50, 35, 64) or compared heterogeneous ranges of duration [i.e., ≤7 compared with >60 d (63), <2 compared with ≥2 mo (43, 50), <3 compared with ≥3 mo (48–50), <4 compared with ≥4 mo (49), and <5 compared with ≥5 mo (43, 50)], and that examined risk of type 1 diabetes at ages ≤6 y (49), 7–14 y (50), a mean of 8 y (64), a mean of 9 y (10), a mean of 15.1 y (35), ≤15 y (43, 48) and <18 y (63).
The remaining 8 studies found nonsignificant associations between the duration of exclusive human milk feeding and type 1 diabetes (9, 13, 14, 16, 20, 41, 44, 65, 66). As noted above, some of these studies included a large number of cases (14, 16, 44, 65, 66) or high-risk samples (9, 41). The nonsignificant associations were inconsistent in direction.
Fasting glucose and insulin resistance at 11.5 y of age
The cluster randomized controlled trial, PROBIT (described previously), was the only study to provide evidence about the duration of exclusive human milk feeding and fasting glucose and insulin resistance in childhood (Table 6). In the intention-to-treat analysis, Martin et al. (57) reported no significant differences in fasting glucose or HOMA-IR at 11.5 y of age between the intervention group (which had higher rates of exclusive human milk feeding measured at 3 and 6 mo) and the control group. Prospective cohort analyses of PROBIT study data found a slightly higher, but significant, fasting glucose level in children fed human milk exclusively 3–<6 mo compared with <3 mo, but no significant difference in HOMA-IR. There were no differences in fasting glucose or HOMA-IR between children fed human milk exclusively at ≥6 mo in comparison with <3 mo, and the trends across the 3 categories of duration (<3, 3–<6, and ≥6 mo) were nonsignificant.
TABLE 6.
Evidence examining the relationship between shorter versus longer durations of exclusive human milk feeding and fasting glucose and insulin resistance in offspring at 11.5 y of age1
| Author and year | Study design (study/cohort name where applicable) | Country | Notable sample characteristics | Shorter vs. longer durations of exclusive human milk feeding exposures2 | Significant associations with intermediate outcomes | Nonsignificant associations with intermediate outcomes |
|---|---|---|---|---|---|---|
| Martin 2014 (57) | RCT3 or prospective cohort, depending on the analysis (PROBIT) | Belarus |
n = 13,616 Baseline: birth Race/ethnicity NR |
Intervention group (higher rate of EBF at 3 and 6 mo) vs. control group | None | Fasting glucose (mmol/L) at 11.5 y: mean difference –0.03 (95% CI: –0.16, 0.10) HOMA-IR at 11.5 y: Ratio of geometric means 1.05 (95% CI: 0.85, 1.30) |
| EBF 3 to <6 mo vs. <3 mo | Fasting glucose (mmol/L) at 11.5 y (prospective cohort analysis): mean difference 0.02 (95% CI: 0.01, 0.04) | Fasting glucose (mmol/L) at 11.5 y (instrumental variable analysis): mean difference –0.09 (95% CI: –0.46, 0.29) HOMA-IR at 11.5 y (instrumental variable analysis): ratio of geometric means 1.17 (95% CI: 0.58, 2.37) HOMA-IR at 11.5 y (prospective cohort analysis): ratio of geometric means 1.00 (95% CI: 0.95, 1.05) |
||||
| EBF ≥6 mo vs. <3 mo | None | Fasting glucose (mmol/L) at 11.5 y (instrumental variable analysis): mean difference –0.15 (95% CI: –0.72, 0.42) Fasting glucose (mmol/L) at 11.5 y (prospective cohort analysis): mean difference 0.00 (95% CI: –0.05, 0.04) HOMA-IR at 11.5 y (instrumental variable analysis): ratio of geometric means 1.28 (95% CI: 0.44, 3.76) HOMA-IR at 11.5 y (prospective cohort analysis): ratio of geometric means 1.01 (95% CI: 0.91, 1.12) |
||||
| EBF duration trend according to the categories <3, 3–<6, and ≥6 mo EBF | None | Fasting glucose (mmol/L) at 11.5 y: P = 0.38 HOMA-IR at 11.5 y: P = 0.94 |
1EBF, exclusively breastfed; NR, not reported; PROBIT, Promotion of Breastfeeding Intervention Trial; RCT randomized controlled trial.
2Exposures, as defined by the authors of the studies included in the body of evidence, which address shorter versus longer durations of exclusive human milk feeding or vice versa.
3RCT of an intervention to promote prolonged duration and exclusivity of breastfeeding rather than an RCT of breastfeeding per se.
Discussion
The conclusion statements that answer the 4 SR questions related to infant milk-feeding practices and diabetes outcomes in offspring, and the grades of the evidence underlying the conclusion statements, are listed in Table 7. TEC members used the NESR grading rubric to consider the aspects of the adequacy, consistency, generalizability, impact, and internal validity of the evidence discussed below.
The majority of evidence examined type 1 diabetes rather than type 2 diabetes or intermediate outcomes. Therefore, the adequacy of the evidence underlying the conclusion statements about the relationships of never versus ever being fed human milk (i.e., 15 studies) and shorter versus longer durations of any (i.e., 22 studies) and exclusive (i.e., 15 studies) human milk feeding with type 1 diabetes was good. On the other hand, given the low prevalence of type 1 diabetes, some of the studies likely had inadequate statistical power. For example, 7 (13, 15, 17, 21–24) of 9 (10, 11, 13, 15, 17, 21–24) studies with nonsignificant associations between never versus ever being fed human milk and type 1 diabetes, and 6 (15, 17, 19, 24, 45, 55) of 10 (14, 15, 17–19, 24, 39, 44, 45, 55) studies with nonsignificant associations between the duration of any human milk feeding and type 1 diabetes, were prospective studies or small case-control studies (i.e., <200 cases) and did not recruit high-risk samples. A similar pattern did not emerge in the body of evidence that examined the duration of exclusive human milk feeding and type 1 diabetes.
The evidence related to type 1 diabetes was consistent. With 1 exception (19), the studies with significant associations between never versus ever being fed human milk and type 1 diabetes suggested that never being fed human milk is associated with higher risk of type 1 diabetes (12, 14, 16, 18, 25). Likewise, with 1 exception (40), the studies with significant associations between shorter versus longer durations of any human milk feeding and type 1 diabetes suggested that shorter durations are associated with higher risk of type 1 diabetes (16, 36–38, 41–43, 46–51, 54, 56). Notably, the only study with a significant association in the opposite direction (40) examined a sample that was entirely male and comprised of both living and deceased individuals, which hindered TEC members’ ability to judge its consistency with other analyses. In the body of evidence examining shorter versus longer durations of exclusive human milk feeding and type 1 diabetes, all of the studies that reported statistically significant associations were consistent in suggesting that shorter durations are associated with higher risk of type 1 diabetes (10, 43, 48–50, 35, 63, 64). The consistency in the direction of the significant associations across these bodies of evidence is noteworthy given that the independent variables were heterogeneous, which was a feature of not defining longer duration, shorter duration, or ever feeding human milk, and instead considering all analyses that compared shorter with longer durations of any or exclusive human milk feeding and never with ever feeding human milk in the synthesis of the evidence.
In the NESR grading rubric, the impact of the evidence takes into consideration the directness with which the study designs examined the link between the exposure and outcome of interest in the SR question, and the clinical significance of the evidence. Only 3 studies described objectives to examine interventions or exposures outside of the scope of these SRs: Kostraba et al. (15) and Thorsdottir et al. (24) stated intentions to examine the consumption of cow's milk or solid foods, and the study by Savilahti et al. (45) was originally an experimental study to compare pasteurized human milk and extensively hydrolyzed formula with cow's milk formula to reduce cow's milk allergy. Qualitative methods were not used to judge clinical significance in terms of the magnitude of the risk of being fed human milk for short durations or not at all on type 1 diabetes. However, given the increasing incidence of type 1 diabetes in the United States and the social and economic consequences of this disease (5, 6), even small decreases in the risk for type 1 diabetes have the potential to be of public health importance.
The generalizability of the evidence to US populations was sound overall. There were a number of US studies that presented evidence about never versus ever being fed human milk (14, 15, 18), shorter versus longer durations of any human milk feeding (9, 14, 15, 18, 39, 56), and shorter versus longer durations of exclusive human milk feeding (9, 14) and type 1 diabetes, and they included some racial and ethnic diversity. Furthermore, all of the evidence came from countries that met the inclusion criterion of being high or very high on the Human Development Index (33), and therefore having a level of human development likely generalizable to the United States. Some of the studies recruited high-risk samples that may not be generalizable (9–11, 28, 37, 39, 41, 46, 51–53, 56, 58); yet, as previously mentioned, this had the effect of increasing the studies’ statistical power, which is important given the low incidence of type 1 diabetes.
TEC members did have some concerns about internal validity related to study design. Most of the studies were case-control studies. TEC members recognized the importance of case-control studies in this area because they are useful for examining low-incident outcomes such as type 1 diabetes. However, because case-control studies rely on the retrospective collection of exposure data, differential or nondifferential misclassification of the exposure may have introduced bias. Differential misclassification from recall bias (i.e., if mothers of children with type 1 diabetes recalled or reported infant milk-feeding practices differently from mothers of children without type 1 diabetes) could have resulted in over- or underestimations of the associations, whereas nondifferential misclassification would have tended to bias the reported associations toward the null. There was no such concern related to the outcome, which was medically diagnosed and unlikely to misclassify cases or controls. Although all of the case-control studies included matching variables, and many included additional adjustment variables, residual confounding from other variables related to infant-feeding and type 1 diabetes risk may have occurred. Residual confounding may have been less of a concern from the small number of studies that compared individuals who had type 1 diabetes with their siblings, with whom they shared genetic and environmental factors. Four such studies examined the duration of any human milk feeding (37, 39, 46, 51); 2 found significant associations between shorter compared with longer durations of any human milk feeding and higher risk of type 1 diabetes (37, 51) and a third had a nonsignificant association in the same direction with a wide CI indicative of suboptimal statistical power (46). One such study examined the duration of exclusive human milk feeding as well as never versus ever feeding human milk (10); this study found that children with type 1 diabetes were fed human milk exclusively for a significantly shorter duration from their healthy siblings but, with nearly universal initiation of human milk feeding, there was not a significant association between never versus ever being fed human milk and type 1 diabetes. Another potential source of bias was multiple comparison bias; in particular, in the bodies of evidence examining shorter versus longer durations of any and exclusive human milk feeding and type 1 diabetes, Virtanen et al. (49–53) assessed multiple comparisons across 5 articles. In addition, Sadauskaite-Kuehne et al. (43) only reported significant associations and therefore it is not possible to know how many comparisons were assessed.
TEC members graded the evidence underlying their conclusions about shorter versus longer durations of any and exclusive human milk feeding and fasting glucose, insulin resistance, and glucose tolerance during childhood and into adolescence as limited. The intention-to-treat analyses of the PROBIT study, which is a large randomized trial of an intervention to promote prolonged duration and exclusivity of breastfeeding (57), formed the basis for these conclusion statements. The PROBIT study was likely to have good internal validity because randomization mitigates selection bias and confounding. In addition, detection bias may have been reduced by collecting infant-feeding data prospectively, and an audit by PROBIT researchers found that a random subset of infant-feeding data had close agreement with data obtained by maternal interview. The bodies of evidence were small. Four studies (including PROBIT) examined shorter versus longer durations of any human milk feeding and fasting glucose, insulin resistance, and glucose tolerance; yet, the direction of the associations across the studies was consistent. The PROBIT study, alone, provided evidence about shorter versus longer durations of exclusive human milk feeding and fasting glucose and insulin resistance. TEC members had some doubts about the generalizability of the evidence to generally healthy US populations. Just 1 US sample (28) provided evidence for shorter versus longer durations of any human milk feeding and fasting glucose and insulin resistance. US populations may have higher metabolic risk than the populations from which participants were sampled in the remaining studies (e.g., the Belarusian population from which the PROBIT study was sampled). Regarding the impact of the evidence, TEC members concluded there was evidence of no association between the durations of any or exclusive human milk feeding and fasting glucose, insulin resistance, and glucose tolerance during childhood and into adolescence, which would mean there is no clinical significance.
Research recommendations
TEC members identified several areas for future research. There was insufficient evidence to answer 1 of the 4 SR questions (Table 7) because only 1 article examined the intensity, proportion, or amount of human milk fed to mixed-fed infants (9). In addition, scant evidence examined type 2 diabetes, and evidence examining intermediate outcomes tended to be from samples outside of the United States that may differ in metabolic risk from the US population. Therefore, the primary research recommendations are for future research to examine the following: 1) the relationship between the intensity of human milk fed to mixed-fed infants and diabetes outcomes, 2) the relationship between infant milk-feeding practices and type 2 diabetes, and 3) intermediate and endpoint outcomes in representative and well-powered US samples. Large prospective samples could perhaps be acquired by linking surveillance systems that collect data about infant feeding and diabetes outcomes, or through the use of electronic medical record data. Infant-feeding research will continue to rely on observational designs; however, researchers should endeavor to minimize bias through sound research design and conduct. For example, baseline differences in critical confounding variables (e.g., race and ethnicity, socioeconomic status, and family history of diabetes) should be assessed. Study designs that further minimize confounding include sib-pair analyses (e.g., comparisons of associations within sibling pairs compared with associations irrespective of sibship), analyses of cohorts with different confounding structures, and use of instrumental variables such as Mendelian randomization approaches. Researchers should incorporate effect modification into their study design whenever possible (e.g., participant race and ethnicity) because different social, demographic, and biological characteristics are likely to modify the impact of infant milk-feeding practices on the outcomes. Infant milk-feeding research should also move toward collecting infant-feeding data consistently through the use of validated methods, and we propose that researchers study the duration of human milk feeding among infants fed human milk (i.e., assess infants who were never fed human milk separately from infants who were fed human milk).
ACKNOWLEDGEMENTS
We thank Katherine Kortsmit and Kelly Mannherz for their assistance with extracting data and assessing risk of bias for included studies. We also thank Sue Anderson for serving as a TEC member until December 2015.
The authors’ responsibilities were as follows: DG, PN, SAA, LB, KMJ, LAN-R, KOO'B, EO, RP-E, EEZ, and JMS: participated in establishing the research questions, analytic framework, and study inclusion and exclusion criteria; YPW and NT: developed the literature search and conducted the literature search; PN, CD, and DG: screened search results and identified studies for inclusion; CCL, PN, and DG: conducted a manual search, extracted data, and assessed risk of bias for included studies; SAA, LB, TJ, KMJ, LAN-R, KOO'B, EO, RP-E, and EEZ: reviewed and provided substantive feedback on all SR materials, including the synthesis of the body of evidence, conclusion statement, and grade of the strength of the evidence; DG, PN, and CCL: wrote the manuscript; DG: has primary responsibility for the final content; JMS: provided oversight of the Project; and all authors: critically reviewed and approved the final manuscript. The authors declare no conflict of interest.
Notes
Published in a supplement to The American Journal of Clinical Nutrition. This article is one in a series of systematic reviews completed with support from the USDA’s Nutrition Evidence Systematic Review team as part of the Pregnancy and Birth to 24 Months Project. The supplement is sponsored by the USDA Food and Nutrition Service. The Supplement Coordinator for the supplement publication was Joanne M Spahn, USDA Center for Nutrition Policy and Promotion, Alexandria, VA. Supplement Coordinator disclosure: No conflicts of interest or financial ties to disclose. The Guest Editor for this supplement was Pieter J Sauer. Guest Editor disclosure: No conflicts of interest to disclose.
Publication costs for this supplement were defrayed in part by the payment of page charges. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of The American Journal of Clinical Nutrition.
Abbreviations used: HbA1c, hemoglobin A1c; NESR, Nutrition Evidence Systematic Review; SR, systematic review; TEC, Technical Expert Collaborative
References
- 1. Obbagy JE, Blum-Kemelor DM, Essery EV, Lyon JMG, Spahn JM. USDA Nutrition Evidence Library: methodology used to identify topics and develop systematic review questions for the birth-to-24-mo population. Am J Clin Nutr. 2014;99(3): S 692–6. [DOI] [PubMed] [Google Scholar]
- 2. Raiten DJ, Raghavan R, Porter A, Obbagy JE, Spahn JM. Executive summary: evaluating the evidence base to support the inclusion of infants and children from birth to 24 mo of age in the Dietary Guidelines for Americans—“the B-24 Project.” Am J Clin Nutr. 2014;99(3):S663–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stoody EE, Spahn JM, Casavale KO. The Pregnancy and Birth to 24 Months Project:a series of systematic reviews on diet and health. Am J Clin Nutr. 2019;109(7):685S–97S. [DOI] [PubMed] [Google Scholar]
- 4. US Department of Health & Human Services. Office of Disease Prevention and Health Promotion. [Internet]. https://www.healthypeople.gov/2020/topics-objectives/topic/diabetes#top [cited 2 May, 2018]. [Google Scholar]
- 5. Centers for Disease Control and Prevention. CDC Newsroom. Version 13 April 2017. [Internet]. https://www.cdc.gov/media/releases/2017/p0412-diabtes-rates.html [cited 26 April, 2018]. [Google Scholar]
- 6. Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, Imperatore G, Linder B, Marcovina S, Pettitt DJ et al.. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med. 2017;376(15):1419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Obbagy JE, Spahn JM, Wong YP, Psota TL, Spill MK, Dreibelbis C, Güngör D, Nadaud P, Raghavan R, Callahan EH et al.. Systematic review methodology used in the Pregnancy and Birth to 24 Months Project. Am J Clin Nutr. 2019;109(7):698S–704S. [DOI] [PubMed] [Google Scholar]
- 8. Infant Formula Act of 1980. 96–359. [Google Scholar]
- 9. Frederiksen B, Kroehl M, Lamb MM, Seifert J, Barriga K, Eisenbarth GS, Rewers M, Norris JM. Infant exposures and development of type 1 diabetes mellitus: the Diabetes Autoimmunity Study in the Young (DAISY). JAMA Pediatr. 2013;167(9):808–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alves J G, Figueiroa JN, Meneses J, Alves GV. Breastfeeding protects against type 1 diabetes mellitus: a case-sibling study. Breastfeed Med. 2012;7(1):25–8. [DOI] [PubMed] [Google Scholar]
- 11. Chmiel R, Beyerlein A, Knopff A, Hummel S, Ziegler AG, Winkler C. Early infant feeding and risk of developing islet autoimmunity and type 1 diabetes. Acta Diabetol. 2015;52(3):621–4. [DOI] [PubMed] [Google Scholar]
- 12. Dahlquist G, Patterson C, Soltesz G, Grp ESS. Rapid early growth is associated with increased risk of childhood type 1 diabetes in various European populations. Diabetes Care. 2002;25(10):1755–60. [DOI] [PubMed] [Google Scholar]
- 13. Esfarjani F, Azar MR, Gafarpour M. IDDM and early exposure of infant to cow's milk and solid food. Indian J Pediatr. 2001;68(2):107–10. [DOI] [PubMed] [Google Scholar]
- 14. Kostraba JN, Dorman JS, LaPorte RE, Scott FW, Steenkiste AR, Gloninger M, Drash AL. Early infant diet and risk of IDDM in blacks and whites. A matched case-control study. Diabetes Care. 1992;15(5):626–31. [DOI] [PubMed] [Google Scholar]
- 15. Kostraba JN, Cruickshanks KJ, Lawler-Heavner J, Jobim LF, Rewers MJ, Gay EC, Chase HP, Klingensmith G, Hamman RF. Early exposure to cow's milk and solid foods in infancy, genetic predisposition, and risk of IDDM. Diabetes. 1993;42(2):288–95. [PubMed] [Google Scholar]
- 16. Malcova H, Sumnik Z, Drevinek P, Venhacova J, Lebl J, Cinek O. Absence of breast-feeding is associated with the risk of type 1 diabetes: a case-control study in a population with rapidly increasing incidence. Eur J Pediatr. 2006;165(2):114–9. [DOI] [PubMed] [Google Scholar]
- 17. Marshall AL, Chetwynd A, Morris JA, Placzek M, Smith C, Olabi A, Thistlethwaite D. Type 1 diabetes mellitus in childhood: a matched case control study in Lancashire and Cumbria, UK. Diabet Med. 2004;21(9):1035–40. [DOI] [PubMed] [Google Scholar]
- 18. Mayer EJ, Hamman RF, Gay EC, Lezotte DC, Savitz DA, Klingensmith GJ. Reduced risk of IDDM among breast-fed children. The Colorado IDDM Registry. Diabetes. 1988;37(12):1625–32. [DOI] [PubMed] [Google Scholar]
- 19. Meloni T, Marinaro AM, Mannazzu MC, Ogana A, La VC, Negri E, Colombo C. IDDM and early infant feeding. Sardinian case-control study. Diabetes Care. 1997;20(3):340–2. [DOI] [PubMed] [Google Scholar]
- 20. Rami B, Schneider U, Imhof A, Waldhor T, Schober E. Risk factors for type I diabetes mellitus in children in Austria. Eur J Pediatr. 1999;158(5):362–6. [DOI] [PubMed] [Google Scholar]
- 21. Robertson L, Harrild K. Maternal and neonatal risk factors for childhood type 1 diabetes: a matched case-control study. BMC Public Health. 2010;10:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siemiatycki J, Colle E, Campbell S, Dewar RA, Belmonte MM. Case-control study of IDDM. Diabetes Care. 1989;12(3):209–16. [DOI] [PubMed] [Google Scholar]
- 23. Soltesz G, Jeges S, Dahlquist G. Hungarian Childhood Diabetes Epidemiology Study Group. Non-genetic risk determinants for type 1 (insulin-dependent) diabetes mellitus in childhood. Acta Paediatr. 1994;83(Supplement_7):730–5. [DOI] [PubMed] [Google Scholar]
- 24. Thorsdottir I, Birgisdottir BE, Johannsdottir IM, Harris DP, Hill J, Steingrimsdottir L, Thorsson AV. Different beta-casein fractions in Icelandic versus Scandinavian cow's milk may influence diabetogenicity of cow's milk in infancy and explain low incidence of insulin-dependent diabetes mellitus in Iceland. Pediatrics. 2000;106(4):719–24. [DOI] [PubMed] [Google Scholar]
- 25. Tai TY, Wang CY, Lin LL, Lee LT, Tsai ST, Chen CJ. A case-control study on risk factors for type 1 diabetes in Taipei City. Diabetes Res Clin Pract. 1998;42(3):197–203. [DOI] [PubMed] [Google Scholar]
- 26. Mayer D E J, Dabelea D, Lamichhane AP, D'Agostino R B Jr., Liese AD, Thomas J, McKeown RE, Hamman RF. Breast-feeding and type 2 diabetes in the youth of three ethnic groups: the SEARCH for diabetes in youth case-control study. Diabetes Care. 2008;31(3):470–5. [DOI] [PubMed] [Google Scholar]
- 27. Young TK, Martens PJ, Taback SP, Sellers EA, Dean HJ, Cheang M, Flett B. Type 2 diabetes mellitus in children: prenatal and early infancy risk factors among Native Canadians. Arch Pediatr Adolesc Med. 2002;156(Supplement_7):651–5. [DOI] [PubMed] [Google Scholar]
- 28. Davis JN, Weigensberg MJ, Shaibi GQ, Crespo NC, Kelly LA, Lane CJ, Goran MI. Influence of breastfeeding on obesity and type 2 diabetes risk factors in Latino youth with a family history of type 2 diabetes. Diabetes Care. 2007;30(4):784–9. [DOI] [PubMed] [Google Scholar]
- 29. Plancoulaine S, Charles MA, Lafay L, Tauber M, Thibult N, Borys JM, Eschwege E, Fleurbaix Laventie Ville Sante Study Group. Infant-feeding patterns are related to blood cholesterol concentration in prepubertal children aged 5–11 y: the Fleurbaix-Laventie Ville Sante study. Eur J Clin Nutr. 2000;54(2):114–9. [DOI] [PubMed] [Google Scholar]
- 30. Shultis WA, Leary SD, Ness AR, Scott J, Martin RM, Whincup PH, Smith GD, ALSPAC Study Team. Haemoglobin A1c is not a surrogate for glucose and insulin measures for investigating the early life and childhood determinants of insulin resistance and Type 2 diabetes in healthy children. An analysis from the Avon Longitudinal Study of Parents and Children (ALSPAC). Diabet Med. 2006;23(12):1357–63. [DOI] [PubMed] [Google Scholar]
- 31. US Food and Drug Administration. Version 19 December 2013. [Internet]. https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/InfantFormula/ucm136118.htm#manufacture [cited 23 March, 2018]. [Google Scholar]
- 32. Food and Agriculture Organization of the United Nations. World Health Organization. Codex Alimentarius. International food standards. Standard for infant formula and formulas for special medical purposes intended for infants. Codex Stan 72-1981 2007. [Google Scholar]
- 33. United Nations Development Programme. Human Development Report 2014. Sustaining human progress: reducing vulnerabilities and building resilience. New York: United Nations; 2014. [Google Scholar]
- 34. The World Bank. [Internet]. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups [cited 27 March, 2018]. [Google Scholar]
- 35. Perez BF, Carrasco E, Gutierrez LMD, Martinez MT, Lopez G, de los Rios MG. Genetic predisposition and environmental factors leading to the development of insulin-dependent diabetes mellitus in Chilean children. J Mol Med (Berl). 1996;74(2):105–9. [DOI] [PubMed] [Google Scholar]
- 36. Blom L, Dahlquist G, Nystrom L, Sandstrom A, Wall S. The Swedish childhood diabetes study—social and perinatal determinants for diabetes in childhood. Diabetologia. 1989;32(1):7–13. [DOI] [PubMed] [Google Scholar]
- 37. Borch-Johnsen K, Joner G, Mandrup-Poulsen T, Christy M, Zachau-Christiansen B, Kastrup K, Nerup J. Relation between breast-feeding and incidence rates of insulin-dependent diabetes mellitus. A hypothesis. Lancet. 1984;2(8411):1083–6. [DOI] [PubMed] [Google Scholar]
- 38. Dahlquist G, Blom L, Lonnberg G. The Swedish Childhood Diabetes Study—a multivariate analysis of risk determinants for diabetes in different age groups. Diabetologia. 1991;34(10):757–62. [DOI] [PubMed] [Google Scholar]
- 39. Fort P, Lanes R, Dahlem S, Recker B, Weyman-Daum M, Pugliese M, Lifshitz F. Breast feeding and insulin-dependent diabetes mellitus in children. J Am Coll Nutr. 1986;5:439–41. [DOI] [PubMed] [Google Scholar]
- 40. Kyvik KO, Green A, Svendsen A, Mortensen K. Breast feeding and the development of type 1 diabetes mellitus. Diabet Med. 1992;9(3):233–5. [DOI] [PubMed] [Google Scholar]
- 41. Lund B, N A, Stene LC, Rasmussen T, Torjesen PA, Andersen LF, Rønningen KS. Infant feeding in relation to islet autoimmunity and type 1 diabetes in genetically susceptible children: the MIDIA Study. Diabetes Care. 2015;38(2):257–63. [DOI] [PubMed] [Google Scholar]
- 42. Rosenbauer J, Herzig P, Giani G. Early infant feeding and risk of type 1 diabetes mellitus-a nationwide population-based case-control study in pre-school children. Diabetes Metab Res Rev. 2008;24(3):211–22. [DOI] [PubMed] [Google Scholar]
- 43. Sadauskaite KV, Ludvigsson J, Padaiga Z, Jasinskiene E, Samuelsson U. Longer breastfeeding is an independent protective factor against development of type 1 diabetes mellitus in childhood. Diabetes Metab Res Rev. 2004;20(2):150–7. [DOI] [PubMed] [Google Scholar]
- 44. Samuelsson U, Johansson C, Ludvigsson J. Breast-feeding seems to play a marginal role in the prevention of insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1993;19(3):203–10. [DOI] [PubMed] [Google Scholar]
- 45. Savilahti E, Saarinen KM. Early infant feeding and type 1 diabetes. Eur J Nutr. 2009;48(4):243–9. [DOI] [PubMed] [Google Scholar]
- 46. Sipetic S, Vlajinac H, Kocev N, Bjekic M, Sajic S. Early infant diet and risk of type 1 diabetes mellitus in Belgrade children. Nutrition. 2005;21(4):474–9. [DOI] [PubMed] [Google Scholar]
- 47. Skrodeniene E, Marčiulionyte D, Padaiga Z, Jašinskiene E, Sadauskaite KV, Sanjeevi CB, Vitkauskiene A, Ludvigsson J. Associations between HLA class II haplotypes, environmental factors and type 1 diabetes mellitus in Lithuanian children with type 1 diabetes and controls. Pol Ann Med. 2010;17(1):7–15. [Google Scholar]
- 48. Verge CF, Howard NJ, Irwig L, Simpson JM, Mackerras D, Silink M. Environmental factors in childhood IDDM. A population-based, case-control study. Diabetes Care. 1994;17(12):1381–9. [DOI] [PubMed] [Google Scholar]
- 49. Virtanen SM, Rasanen L, Aro A, Lindstrom J, Sippola H, Lounamaa R, Toivanen L, Tuomilehto J, Akerblom HK, Childhood Diabetes in Finland Study Group. Infant feeding in Finnish children less than 7 yr of age with newly diagnosed IDDM. Diabetes Care. 1991;14(5):415–7. [DOI] [PubMed] [Google Scholar]
- 50. Virtanen SM, Rasanen L, Aro A, Ylonen K, Lounamaa R, Tuomilehto J, Akerblom HK, Childhood Diabetes in Finland Study Group. Feeding in infancy and the risk of type 1 diabetes mellitus in Finnish children. Diabet Med. 1992;9(9):815–9. [DOI] [PubMed] [Google Scholar]
- 51. Virtanen SM, Saukkonen T, Savilahti E, Ylonen K, Rasanen L, Aro A, Knip M, Tuomilehto J, Akerblom HK, Childhood Diabetes in Finland Study Group. Diet, cow's milk protein antibodies and the risk of IDDM in Finnish children. Diabetologia. 1994;37(4):381–7. [DOI] [PubMed] [Google Scholar]
- 52. Virtanen SM, Hypponen E, Laara E, Vahasalo P, Kulmala P, Savola K, Rasanen L, Aro A, Knip M, Akerblom HK. Childhood Diabetes in Finland Study Group. Cow's milk consumption, disease-associated autoantibodies and type 1 diabetes mellitus: a follow-up study in siblings of diabetic children. Diabet Med. 1998;15(9):730–8. [DOI] [PubMed] [Google Scholar]
- 53. Virtanen SM, Laara E, Hypponen E, Reijonen H, Rasanen L, Aro A, Knip M, Ilonen J, Akerblom HK, Childhood Diabetes in Finland Study Group. Cow's milk consumption, HLA-DQB1 genotype, and type 1 diabetes: a nested case-control study of siblings of children with diabetes. Diabetes. 2000;49(6):912–7. [DOI] [PubMed] [Google Scholar]
- 54. Visalli N, Sebastiani L, Adorisio E, Conte A, De C A L, D'Elia R, Manfrini S, Pozzilli P, IMDIAB Group. Environmental risk factors for type 1 diabetes in Rome and province. Arch Dis Child. 2003;88(8):695–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Welander A, Montgomery SM, Ludvigsson J, Ludvigsson JF. Infectious disease at gluten introduction and risk of childhood diabetes mellitus. J Pediatr. 2014;165(2):326–31 e1. [DOI] [PubMed] [Google Scholar]
- 56. Hall K, Frederiksen B, Rewers M, Norris JM. Daycare attendance, breastfeeding, and the development of type 1 diabetes: the diabetes autoimmunity study in the young. Biomed Res Int. 2015;2015:203947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Martin RM, Patel R, Kramer MS, Vilchuck K, Bogdanovich N, Sergeichick N, Gusina N, Foo Y, Palmer T, Thompson J et al.. Effects of promoting longer-term and exclusive breastfeeding on cardiometabolic risk factors at age 11.5 years: a cluster-randomized, controlled trial. Circulation. 2014;129(3):321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rodekamp E, Harder T, Kohlhoff R, Franke K, Dudenhausen JW, Plagemann A. Long-term impact of breast-feeding on body weight and glucose tolerance in children of diabetic mothers: role of the late neonatal period and early infancy. Diabetes Care. 2005;28(6):1457–62. [DOI] [PubMed] [Google Scholar]
- 59. Jeffery AN, Metcalf BS, Hosking J, Murphy MJ, Voss LD, Wilkin TJ. Little evidence for early programming of weight and insulin resistance for contemporary children: EarlyBird Diabetes Study report 19. Pediatrics. 2006;118(3):1118–23. [DOI] [PubMed] [Google Scholar]
- 60. Pirila S, Taskinen M, Viljakainen H, Makitie O, Kajosaari M, Saarinen-Pihkala UM, Turanlahti M. Breast-fed infants and their later cardiovascular health: a prospective study from birth to age 32 years. Br J Nutr. 2014;111(6):1069–76. [DOI] [PubMed] [Google Scholar]
- 61. Pearce MS, Unwin NC, Parker L, Alberti KG. Life course determinants of insulin secretion and sensitivity at age 50 years: the Newcastle thousand families study. Diabetes Metab Res Rev. 2006;22(2):118–25. [DOI] [PubMed] [Google Scholar]
- 62. National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28(12):1039–57. [DOI] [PubMed] [Google Scholar]
- 63. Gimeno SG, de SJM. IDDM and milk consumption. A case-control study in Sao Paulo, Brazil. Diabetes Care. 1997;20(8):1256–60. [DOI] [PubMed] [Google Scholar]
- 64. Perez BF, Oyarzun A, Carrasco E, Albala C, Dorman JS, Santos JL. Duration of breast feeding and bovine serum albumin antibody levels in type 1 diabetes: a case-control study. Pediatr Diabetes. 2003;4(4):157–61. [DOI] [PubMed] [Google Scholar]
- 65. Samuelsson U, Ludvigsson J. Seasonal variation of birth month and breastfeeding in children with diabetes mellitus. J Pediatr Endocrinol Metab. 2001;14(1):43–6. [DOI] [PubMed] [Google Scholar]
- 66. Wadsworth EJ, Shield JP, Hunt LP, Baum JD. A case-control study of environmental factors associated with diabetes in the under 5s. Diabet Med. 1997;14(5):390–6. [DOI] [PubMed] [Google Scholar]