Cardiovascular death is the leading cause of mortality in patients undergoing hemodialysis. However, traditional risk factors, such as diabetes, hypertension, and other comorbidities, do not fully account for this excess risk. Uremic toxins, substances that accumulate in patients with kidney failure, may be responsible for accelerated cardiovascular disease. However, these uremic toxins remain largely undefined. The goal of our pilot study was to determine if an untargeted metabolomic approach could identify metabolites associated with cardiac death in patients on dialysis.
We designed a matched patient-control study (n=94) nested in the Hemodialysis study, a trial of dialysis dose and flux (1). We defined cases as patients who had cardiac death within 1 year of study enrollment and matched them 1:1 to controls, defined as patients who were alive at the end of year 1. Both cases and controls were patients without residual kidney function, and they were matched on age within 5 years, sex, race, diabetes, cardiac disease, and albumin within 0.25 g/dl. Predialysis serum samples frozen at −80°C from the 4-month follow-up visit were sent to Metabolon, Inc. (Durham, NC) for metabolite profiling. We used the standardized methods for data cleaning detailed previously (2). There were 1266 metabolites detected (847 named and 419 unnamed). After excluding unnamed metabolites (n=419), drugs (n=56), and metabolites with >80% missing across samples (n=14), 777 metabolites remained for analysis. We scaled all metabolites to a median of one and log2 transformed. We used logistic regression to assess the association between each metabolite and cardiac death, adjusting for the matching factors (age, sex, race, diabetes, cardiovascular disease, and albumin), Index of Coexistent Disease score, duration of prior dialysis, and systolic BP. Because this was a discovery study, we set the threshold for significance at P<0.005. In a complementary analysis, we explored associations using a false discovery rate threshold of 0.1.
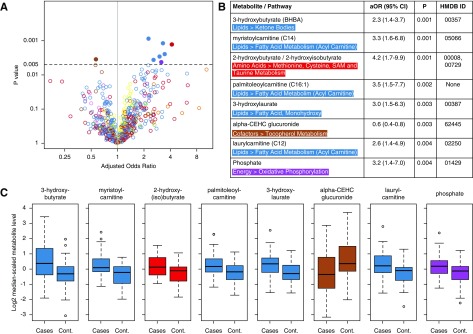
The 94 participants had a mean age of 63 (±13) years old, 62% were women, 53% had diabetes, and 96% had cardiac disease. The participants had received an average of 4 years of hemodialysis before study enrollment. The untargeted metabolites had high correlation (rho range, 0.80–0.95) with previous targeted measurements of selected metabolites measured at the same time point. Figure 1 displays the results for the association between the 777 metabolites and cardiac death. Using the P<0.005 threshold, higher odds for cardiac death were noted with higher levels of several lipid metabolites (3-hydroxybutyrate, myristoylcarnitine, palmitoleoylcarnitine, 3-hydroxylaurate, and laurylcarnitine), an amino acid metabolite (2-hydroxybutyrate/2-hydroxyisobutyrate), and phosphate. Lower odds of cardiac death were noted with higher levels of a vitamin E metabolite (α-carboxyethyl hydrochroman glucuronide). None of the metabolites had significant associations using the false discovery rate threshold.
Figure 1.
A metabolomic approach identifies potential metabolites associated with cardiac death in the Hemodialysis (HEMO) study. (A) Volcano plot and table depicting odds ratios and confidence intervals of the associations between individual metabolites (m=777) and cardiac death at 1 year in the HEMO Study (n=94). Each open circle represents a single metabolite plotted by its odds ratio (x axis) and P value (y axis). The y-axis horizontal line at 0.005 represents the P value threshold used in the primary analysis. Closed circles represent metabolites with potential associations. (B) Cardiac death–related metabolites are presented with superpathways, subpathways, adjusted odds ratios (aORs) with 95% confidence intervals (95% CIs), P values for association on logistic regression (Ps), and Human Metabolome Database (HMDB) identification. (C) Tukey boxplots of log2-transformed and median-scaled metabolite levels of each of the cardiac death–related metabolites in patients and controls. Boxes indicate 25th, 50th, and 75th percentiles of data, with whiskers indicating values within 1.5 times the interquartile range above and below the median. Metabolites are color coded by pathway, whereby red denotes amino acids, green denotes carbohydrates, brown denotes cofactors, purple denotes energy metabolites, blue denotes lipids, orange denotes nucleotides, pink denotes peptides, and yellow denotes nondrug xenobiotics. The HEMO study was a patient-control design. Logistic regression models were adjusted for age, sex, race, diabetes, cardiovascular disease, albumin, Index of Coexistent Disease score, duration of prior dialysis, and systolic BP. cont, control.
Although this study does not necessarily suggest causality, some of the observed associations implicate interesting metabolic pathways. Elevated ketone bodies, such as 3-hydroxybutyrate (also known as β-hydroxybutyrate), are well described in patients with heart failure and may reflect increased sympathetic nervous system activity (3). Elevated ketone body levels were associated with cardiovascular mortality in a recent report from a Japanese dialysis cohort (4). Elevated acylcarnitines could be an indicator of mitochondrial dysfunction and were associated with cardiovascular mortality in an incident United States dialysis cohort (5). The association between elevated phosphate and cardiovascular disease is well known. The protective effect seen with higher levels of vitamin E metabolites could reflect the potential antioxidant effect of vitamin E, or it could be a marker of better nutritional status. Randomized trials of vitamin E supplementation in patients undergoing dialysis have shown mixed results in reduction of cardiovascular events and mortality.
In summary, this hypothesis-generating study suggests the potential of metabolomics as a tool for uremic toxin discovery. Disrupted metabolic pathways in uremia may inform the science of uremic toxin discovery and the development of targeted therapies. Such discoveries can change the way that we treat kidney failure, a major goal of the KidneyX initiative.
Disclosures
Dr. Kovesdy reports personal fees from Amgen, personal fees from Sanofi-Aventis, personal fees from Fresenius Medical Care, personal fees from Keryx, grants from Shire, personal fees from Bayer, personal fees from Abbott, personal fees from Abbvie, personal fees from Dr. Schar, personal fees from GSK, personal fees from Astra Zeneca, personal fees from Takeda, outside the submitted work.
Dr. Shafi reports personal fees from Siemens, personal fees from University of California Irvine, personal fees from University of Mississippi Medical Center, personal fees from Hershey Medical Center, personal fees from University of Tennessee, outside the submitted work.
Dr. Coresh, Dr. Grams, Dr. Guallar, Dr. Hu, Mr. Hwang, and Dr. Rhee have nothing to disclose.
Acknowledgments
Dr. Coresh reports grants from NIH, grants from NKF, during the conduct of the study.
Dr. Guallar reports grants from NIH/NHLBI (CAMARO-ESRD: Cardiac Arrhythmia Monitoring and Related Outcomes in End Stage Renal Disease, R01HL132372), during the conduct of the study.
Dr. Shafi reports grants from NIDDK, during the conduct of the study.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Greene T, Beck GJ, Gassman JJ, Gotch FA, Kusek JW, Levey AS, Levin NW, Schulman G, Eknoyan G: Design and statistical issues of the hemodialysis (HEMO) study. Control Clin Trials 21: 502–525, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Hu J-R, Coresh J, Inker LA, Levey AS, Zheng Z, Rebholz CM, Tin A, Appel LJ, Chen J, Sarnak MJ, Grams ME: Serum metabolites are associated with all-cause mortality in chronic kidney disease. Kidney Int 94: 381–389, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lommi J, Kupari M, Koskinen P, Näveri H, Leinonen H, Pulkki K, Härkönen M: Blood ketone bodies in congestive heart failure. J Am Coll Cardiol 28: 665–672, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Obokata M, Negishi K, Sunaga H, Ishida H, Ito K, Ogawa T, Iso T, Ando Y, Kurabayashi M: Association between circulating ketone bodies and worse outcomes in hemodialysis patients. J Am Heart Assoc 6: pii:e006885, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalim S, Clish CB, Wenger J, Elmariah S, Yeh RW, Deferio JJ, Pierce K, Deik A, Gerszten RE, Thadhani R, Rhee EP: A plasma long-chain acylcarnitine predicts cardiovascular mortality in incident dialysis patients. J Am Heart Assoc 2: e000542, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]