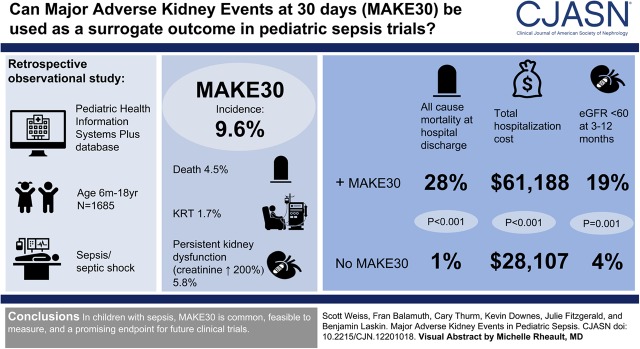
Visual Abstract
Keywords: pediatrics; outcomes; clinical trial; acute kidney failure; Length of Stay; Blood Culture; Incidence; Retrospective Studies; Hospital Costs; Patient Readmission; glomerular filtration rate; Inpatients; Logistic Models; Health Information Systems; Anti-Bacterial Agents; Confidence Intervals; Shock, Septic; Sepsis; Patient Discharge; Kidney Replacement Therapy
Abstract
Background and objectives
Major adverse kidney events, a composite of death, new kidney replacement therapy, or persistent kidney dysfunction, is a potential patient-centered outcome for clinical trials in sepsis-associated kidney injury. We sought to determine the incidence of major adverse kidney events within 30 days and validate this end point in pediatric sepsis.
Design, setting, participants, & measurements
We conducted a retrospective observational study using the Pediatric Health Information Systems Plus database of patients >6 months to <18 years old with a diagnosis of severe sepsis/septic shock; orders for bacterial blood culture, antibiotics, and at least one fluid bolus on hospital day 0/1; and known hospital disposition between January 2007 and December 2011. The primary outcome was incidence of major adverse kidney events within 30 days. Major adverse kidney events within 30 days were validated against all-cause mortality at hospital discharge, hospital length of stay, total hospital costs, hospital readmission within 30 days and 1 year, and lowest eGFR between 3 months and 1 year after discharge. We reported incidence of major adverse kidney events within 30 days with 95% confidence intervals using robust SEM and used multivariable logistic regression to test the association of major adverse kidney events within 30 days with hospital costs and mortality.
Results
Of 1685 admissions, incidence of major adverse kidney events within 30 days was 9.6% (95% confidence interval, 8.1% to 11.0%), including 4.5% (95% confidence interval, 3.5% to 5.4%) death, 1.7% (95% confidence interval, 1.1% to 2.3%) kidney replacement therapy, and 5.8% (95% confidence interval, 4.7% to 6.9%) persistent kidney dysfunction. Patients with versus without major adverse kidney events within 30 days had higher all-cause mortality at hospital discharge (28% versus 1%; P<0.001), higher total hospital costs ($61,188; interquartile range, $21,272–140,356 versus $28,107; interquartile range, $13,056–72,697; P<0.001), and higher proportion with eGFR<60 ml/min per 1.73 m2 between 3 months and 1 year after discharge (19% versus 4%; P=0.001). Major adverse kidney events within 30 days was not associated with length of stay or readmissions.
Conclusions
In children with sepsis, major adverse kidney events within 30 days are common, feasible to measure, and a promising end point for future clinical trials.
Introduction
Sepsis is life-threatening organ dysfunction caused by a dysregulated host response to infection, and it is a common cause of critical illness for children (1–3). AKI occurs in up to one third of children with severe sepsis or septic shock, and it is a risk factor for adverse outcomes (4–6). In the Sepsis Prevalence Outcomes and Therapies study, stage 2/3 AKI was evident in 21% of patients with sepsis, with an increased odds for death or new moderate disability (4). In the Assessment of Worldwide AKI, Renal Angina, and Epidemiology study, a stepwise increase in stage of AKI also conferred an incremental risk of death (7). Consequently, the potential to prevent, ameliorate, or reverse AKI has become an important therapeutic goal for children with sepsis.
Clinical trials require end points that are sufficiently common, feasible to measure with minimal risk of bias, and valued by patients and families. A recent systematic review concluded that pediatric sepsis trials should use composite outcomes capturing both mortality and long-term morbidity (8). For trials in which the intervention of interest is biologically mediated through the kidney, an outcome that reflects long-term morbidity due to the effect of AKI on patients is warranted. The composite outcome of major adverse kidney events (MAKE) has been proposed as a clinically meaningful, patient-centered end point (9). MAKE includes death, new kidney replacement therapy (KRT), and persistent kidney dysfunction, which are all measurable distinct events with little opportunity for misclassification bias that relate to long-term health and wellbeing (9). Although even transient AKI can progress to CKD and affect health-related quality of life (10–15), failure to recover from AKI before hospital discharge has been shown to identify the highest-risk subgroup (16). Moreover, new requirement for KRT during hospitalization increases risk of CKD and death beyond hospitalization (17,18). Finally, major adverse kidney events within 30 days (MAKE30) were recommended as a patient-centered outcome for phase 3 trials by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (19), and a reduction in MAKE30 with the use of balanced fluids compared with 0.9% saline recently confirmed the utility of this outcome in adult trials (20,21).
There are no data on the incidence of MAKE in pediatric sepsis or its validity as an end point in pediatric sepsis trials. We, therefore, sought to determine the incidence of MAKE30 and validate this end point in children with sepsis. We focused on MAKE30, because clinical outcomes are more likely attributable to sepsis the closer they occur to sepsis onset; also, shorter time intervals are more feasible and affordable, and they expose to less loss to follow-up.
Materials and Methods
Study Design
We conducted a retrospective observational study of patients with severe sepsis or septic shock included in the Pediatric Health Information Systems Plus (PHIS+) database between January 2007 and December 2011. The PHIS+ is an administrative database of inpatient hospitalizations from six children’s hospitals in the United States (22). Data quality id monitored through a joint effort between the Children’s Hospital Association (Overland Park, KS) and participating hospitals. The use of this deidentified dataset was not considered human subjects research in accordance with 45 CFR 46.102(f) and the policies of the Children’s Hospital of Philadelphia Institutional Review Board.
Study Population
Inclusion criteria were age >6 months and <18 years old; a principal or secondary diagnosis of severe sepsis or septic shock; orders for bacterial blood culture, broad-spectrum parenteral antibiotics, and at least one 0.9% saline or lactated Ringer fluid bolus on hospital day 0 or 1; and known disposition from the hospital. Age was limited to >6 months old to balance availability of baseline creatinine with inclusion of the infant age group at high risk for adverse sepsis outcomes. Day 0/1 culture, antibiotics, and fluid bolus were required to optimize the likelihood that sepsis was present at or near hospital admission. To identify severe sepsis and septic shock, we used previously published combinations of either (1) International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) codes for an invasive infection (Supplemental Table 1) plus acute organ dysfunction (Supplemental Table 2) or (2) ICD-9-CM codes for severe sepsis (785.52) or septic shock (995.92) (2,23,24). Patients transferred into or out of a PHIS+ site were excluded to ensure knowledge of acute therapies and outcomes.
Data Collection
Demographics, comorbid conditions, payer, and sepsis-related therapies were obtained from the PHIS+ database. Comorbid conditions were categorized using pediatric complex chronic conditions (CCCs) (25). Exposure to nephrotoxic injury negated by just-in-time action drugs (Supplemental Table 3) was also determined (26). AKI within the first 7 days of hospitalization was determined using the Kidney Disease Improving Global Outcomes classification (27).
Outcomes
The primary outcome was MAKE30, a composite of all-cause death, new KRT, or persistent kidney dysfunction at 30 days or hospital discharge, whichever occurred first. New KRT was defined as having (1) a procedure charge for dialysis catheter (38.95) with a charge for dialysis (39.95) or (2) PHIS+ charge codes for dialysis supplies (PHIS+ supply codes 143,000, 256,011, 241,361, 244,521, 255,119, 525,201, 525,205, 525,211, and 525,221) (28). Persistent kidney dysfunction was defined as a final inpatient serum creatinine value ≥200% of the baseline value (i.e., at least a doubling of baseline creatinine) and a minimum absolute increase of ≥0.3 mg/dl (9,20,21). We determined baseline serum creatinine for each patient by using the lowest recorded creatinine available between 12 months and 24 hours before the index admission. For patients without such data available, we imputed an estimated baseline creatinine using previously established median values for age and sex (Supplemental Table 4) (29). Although PHIS+ data collection began in 2007, only admissions available starting January 1, 2008 were included in this study to ensure that all patients had the potential to determine a measured baseline creatinine in the year before admission. Several sensitivity analyses were performed for baseline creatinine, including imputing baseline creatinine using upper limits of normal for age and sex (Supplemental Table 4) and using a multiple imputation strategy on the basis of age, sex, race, CCC, and minimum serum creatinine measured within the first 7 days of hospitalization (30). Patients with an ICD-9-CM code for ESKD undergoing dialysis (ICD-9-CM 585.6) present on admission or recorded during the most recent prior hospitalization were ineligible to meet the criteria for new KRT or persistent kidney dysfunction but could qualify for MAKE30 if they died in the hospital within 30 days.
Outcomes used to validate MAKE30 as an important end point included all-cause mortality through hospital discharge, hospital length of stay (LOS), total hospital costs, hospital readmission within 30 days and 1 year of discharge, and lowest eGFR in the period between 3 months and 1 year after discharge. Because height was not recorded in PHIS+, we used a validated height-independent eGFR equation (31). For hospital mortality, only KRT and persistent kidney dysfunction were considered to determine their association with death; thus, patients who would have met criteria for MAKE30 due to death within 30 days were excluded from this portion of the analysis. Total hospital costs were calculated as the overall Center for Medicare and Medicaid wage/price index–adjusted abstract-based charges multiplied by the overall hospital- and year-specific ratio-to-cost charge. For outcomes beyond hospital discharge (i.e., readmission and lowest eGFR), only hospital survivors within the first 4 years of data were included to ensure at least 1 year of follow-up. Lowest eGFR between 3 months and 1 year after discharge was determined from subsequent hospital admissions (if available), because PHIS+ does not contain data outside of the inpatient setting.
Statistical Analyses
All analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC). Data are presented as medians (interquartile ranges [IQRs]) or proportions and compared using Wilcoxon rank sum and or chi-squared tests, respectively. The incidence of MAKE30 was calculated for the primary and sensitivity analyses with the 95% confidence interval (95% CI) using robust SEM to account for repeat patient visits. To assess normative values for imputing baseline creatinine, we used linear regression to compare the measured creatinine with what would otherwise have been an imputed baseline in the subset of patients who had a measured baseline creatinine. Because measured creatinine would be expected to be higher than an imputed normal baseline value for patients with CKD, this correlation analysis excluded patients with kidney and urologic CCC. The creatinine values used for imputing baseline by age and sex were compared with the mean and 95% CI of measured values. Validation outcomes were compared between patients with MAKE30 and patients without MAKE30 using Wilcoxon rank sums for continuous outcomes and chi-squared tests for categorical outcomes. Lowest eGFR between 3 months and 1 year after discharge was analyzed as the proportion with eGFR<90 and <60 ml/min per 1.73 m2 to approximate CKD. We used multivariable logistic regression to differentiate the effect of AKI versus persistent kidney dysfunction on mortality at hospital discharge and test the independent association of the nonmortal components of MAKE30 with hospital mortality after adjusting for baseline differences. We also modeled the natural log of costs and adjusted for the same independent variables as with mortality. Costs were transformed back to the original scale for presentation. Statistical significance was defined as P<0.05.
Results
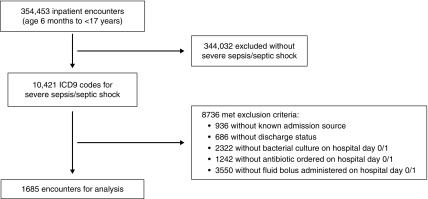
Of the 354,453 hospitalizations in the PHIS+ database, 1685 (0.5%) patient encounters were identified with severe sepsis or septic shock and met all eligibility criteria (representing 1460 unique patients) (Figure 1). Overall, stage 2/3 AKI occurred in 527 (31%) within 7 days of admission. Measured baseline creatinine was available for 988 (59%) encounters. The imputed median baseline creatinine was correlated with the measured baseline creatinine after excluding those with kidney and urologic CCC (r2=0.17; P<0.001) (Supplemental Figure 1), and imputed baseline creatinine was either within or above the 95% CI of measured values for all age and sex strata (Supplemental Table 5). The creatinine value used to determine persistent kidney dysfunction was measured at a median of hospital day 7 (IQR, 3–15) (Supplemental Figure 2).
Figure 1.
Patient identification and inclusion. ICD9, International Classification of Diseases, Ninth Edition.
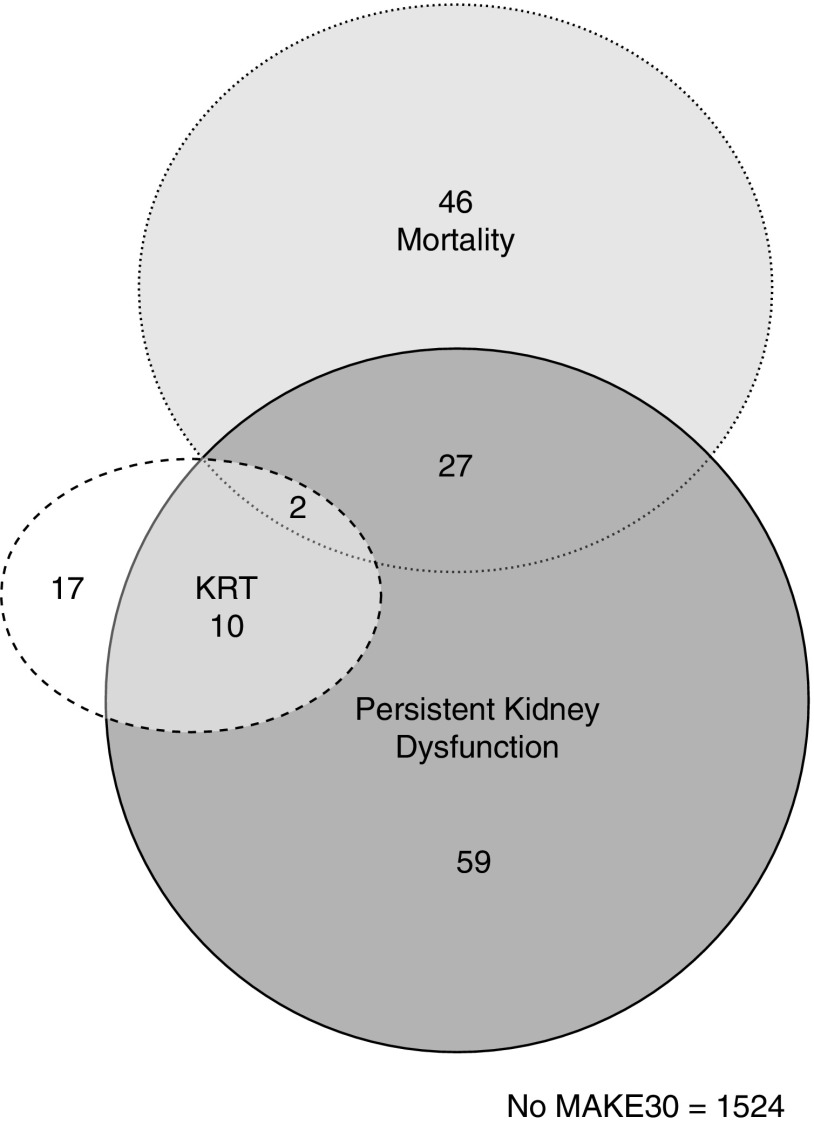
The incidence of MAKE30 was 9.6% (95% CI, 8.1% to 11.0%), including 4.5% (95% CI, 3.5% to 5.4%) with death, 1.7% (95% CI, 1.1% to 2.3%) with new KRT, and 5.8% (95% CI, 4.7% to 6.9%) with persistent kidney dysfunction. Characteristics for patient encounters with and without MAKE30 are shown in Table 1 (Supplemental Table 6 shows patient characteristics across components of MAKE30). Patient encounters with MAKE30 were more likely to have stage 2/3 AKI than patient encounters without MAKE30 (73% versus 27%; P<0.001), whereas encounters with stage 2/3 AKI were more likely than those without any AKI or with stage 1 AKI to have persistent kidney dysfunction (17% versus 0.8%; P<0.001) and develop MAKE30 (22% versus 4%; P<0.001). Of the 75 MAKE30 nonsurvivors, 48% had stage 2/3 AKI in the first 7 days, and 39% had either persistent kidney dysfunction or KRT (Figure 2, Supplemental Table 7 shows mutually exclusive combinations of MAKE30 components).
Table 1.
Patient encounter characteristics
| Variablea | No MAKE30 | MAKE30 |
|---|---|---|
| N | 1524 | 161 |
| Age, yr | 8 (3–13) | 10 (3–14) |
| Age categories | ||
| 6 mo to <1 yr | 89 (6) | 14 (9) |
| 1 to <6 yr | 530 (35) | 41 (25) |
| 6 to <13 yr | 479 (31) | 53 (33) |
| 13 to <18 yr | 426 (28) | 53 (33) |
| Sex, girls | 758 (50) | 71 (44) |
| Race | ||
| Non-Hispanic white | 1042 (68) | 115 (71) |
| Non-Hispanic black | 209 (14) | 16 (10) |
| Hispanic | 121 (8) | 11 (7) |
| Asian | 47 (3) | 4 (3) |
| Other | 105 (7) | 15 (9) |
| Payer | ||
| Private | 693 (45) | 76 (47) |
| Government | 696 (46) | 65 (41) |
| Other payer | 135 (9) | 20 (12) |
| Admission through EDb | 1284 (84) | 125 (78) |
| Pediatric ICU admissionb | 974 (64) | 126 (78) |
| Number of complex chronic conditionsb | ||
| None | 272 (18) | 10 (6) |
| One | 259 (17) | 30 (19) |
| Two | 231 (15) | 22 (14) |
| Three or more | 762 (50) | 99 (61) |
| Type of complex chronic condition | ||
| Cardiovascular | 325 (21) | 45 (28) |
| Respiratory | 255 (17) | 14 (9) |
| Neurologic/neuromuscular | 483 (32) | 51 (32) |
| Hematologic/immunodeficiencyb | 201 (13) | 34 (21) |
| Malignancy | 307 (20) | 42 (26) |
| Kidney/urologicb | 164 (11) | 57 (35) |
| Gastrointestinalb | 660 (43) | 51 (32) |
| Metabolicb | 231 (15) | 47 (30) |
| Other congenital/genetic | 194 (13) | 19 (12) |
| Neonatal | 50 (3) | 5 (3) |
| Technology dependency | 737 (48) | 88 (55) |
| Transplantationb | 214 (14) | 35 (22) |
| Creatinine, mg/dl, baselinec | 0.3 (0.3–0.5) | 0.4 (0.3–0.6) |
| eGFR, ml/min per 1.73 m2, baselined | 107 (107–177) | 115 (107–190) |
| AKIb,e | ||
| Stage 1 | 145 (10) | 10 (6) |
| Stage 2 | 289 (19) | 31 (19) |
| Stage 3 | 120 (8) | 87 (54) |
| Therapies | ||
| Noninvasive mechanical ventilation | 209 (14) | 26 (16) |
| Invasive mechanical ventilation, ETTb | 698 (46) | 111 (69) |
| Invasive mechanical ventilation, tracheostomy | 164 (11) | 10 (6) |
| Lactated Ringer fluid | 30 (2) | 3 (2) |
| Blood transfusionb | 49 (3) | 13 (8) |
| Vasoactive medicationb | 574 (38) | 107 (67) |
| Corticosteroidsb | 559 (37) | 88 (55) |
| Diureticsb | 537 (35) | 95 (59) |
| Extracorporeal membrane oxygenationb | 19 (1) | 12 (8) |
| NINJA drug exposure | ||
| Aminoglycosides | 491 (32) | 64 (40) |
| Two or more nonaminoglycoside NINJA drugs | 713 (47) | 71 (44) |
| Fewer than two NINJA drugs and no aminoglycoside | 320 (21) | 26 (16) |
MAKE30, major adverse kidney events within 30 days; ED, emergency department; ICU, intensive care unit; ETT, endotracheal tube; NINJA, nephrotoxic injury negated by just-in-time action (25).
Data are presented as n (percentage) or median (interquartile range).
Variable differed between patients with MAKE30 versus patients without MAKE30 at the P<0.05 level.
Baseline creatinine was measured for 988 (59%) patients and imputed for 697 (41%) patients.
Baseline eGFR was calculated using the height-independent equation: eGFR (milliliters per kilogram per 1.73 m2)=107.3/(measured or estimated baseline creatinine/Q), whereas Q is equal to the median serum creatinine concentration for children on the basis of age and sex (Supplemental Table 4) (30).
Stage 2 and 3 AKI within the first 7 days of admission was determined using the Kidney Disease Improving Global Outcomes classification system (26).
Figure 2.
Venn diagram of major adverse kidney events within 30 days (MAKE30) components. KRT, kidney replacement therapy.
Sensitivity analyses for incidence of MAKE30 and its components are shown in Table 2. Point estimates varied little, and the 95% CIs overlapped for all permutations.
Table 2.
Incidence of major adverse kidney events within 30 days by primary and sensitivity analyses
| Permutation | N | MAKE30 (95% CI) | Mortality, n (%) | KRT, n (%) | PKD, n (%) |
|---|---|---|---|---|---|
| Baseline creatinine measured or imputed using median normal value for age and sex | |||||
| Primary analysis | 1685 | 9.6% (8.1 to 11.0) | 75 (4.5) | 29 (1.7) | 98 (5.8) |
| Measured baseline creatinine only | 988 | 9.8% (7.9 to 11.7) | 44 (4.5) | 12 (1.2) | 68 (6.9) |
| Imputed baseline creatinine for all patients | 1685 | 8.7% (7.3 to 10.0) | 75 (4.5) | 29 (1.7) | 83 (4.9) |
| First hospitalization only | 1460 | 9.8% (8.4 to 11.4) | 67 (4.6) | 28 (1.9) | 85 (5.8) |
| Admitted through ED only | 1409 | 8.9% (7.4 to 10.4) | 55 (3.9) | 24 (1.7) | 73 (5.2) |
| Excluding patients with existing ESKD | 1665 | 9.5% (8.1 to 11.0) | 73 (4.4) | 29 (1.7) | 98 (5.9) |
| Baseline creatinine measured or imputed using upper limit of normal value for age and sex | 1685 | 9.1% (7.7 to 10.5) | 75 (4.5) | 29 (1.7) | 89 (5.3) |
| Baseline creatinine measured or imputed using multiple imputations | 1685 | 9.6% (8.2 to 11.0) | 75 (4.5) | 29 (1.7) | 95 (5.6) |
Multiple imputation strategy included the following variables: age, sex, race, chronic comorbid conditions, and minimum serum creatinine measured within the first 7 days of hospitalization. MAKE30, major adverse kidney events within 30 days; 95% CI, 95% confidence interval; KRT, kidney replacement therapy; PKD, persistent kidney dysfunction (defined as at least doubling of the baseline serum creatinine at hospital discharge or hospital day 30, whichever came first); ED, emergency department.
Patients who met the KRT or persistent kidney dysfunction criteria for MAKE30 compared with patients who did not had significantly higher mortality when followed out to hospital discharge (28% versus 1%; P<0.001). Moreover, although stage 2/3 AKI itself was associated with mortality at hospital discharge (odds ratio, 2.9; 95% CI, 1.9 to 4.4; P<0.001), this relationship was largely mediated through persistent kidney dysfunction, because AKI was no longer associated with mortality after controlling for persistent kidney dysfunction (adjusted odds ratio, 1.5; 95% CI, 0.9 to 2.5; P=0.12) (Supplemental Figure 3). Patient encounters with MAKE30 by any of the three components compared with those without MAKE30 also had no difference in hospital LOS (10; IQR, 5–20 versus 9; IQR, 5–18 days; P=0.53), but they did have higher total hospital costs ($61,188; IQR, $21,272–140,356 versus $28,107; IQR, $13,056–72,697; P<0.001).
Twenty-nine percent of encounters had follow-up data available beyond hospital discharge, including 21 of 83 survivors with MAKE30 and 431 of 1502 survivors without MAKE30 (median durations from discharge to follow-up were 121; IQR, 117–138 and 134; IQR, 106–194 days, respectively). Of the 452 hospital survivors with follow-up data available, there were no differences between readmission within 30 days (37% versus 31%; P=0.32) or 1 year of discharge (55% versus 58%; P=0.68), but patients with MAKE30 were more likely to have eGFR<90 ml/min per 1.73 m2 (33% versus 11%; P=0.003) and <60 ml/min per 1.73 m2 (19% versus 4%; P=0.001) between 3 months and 1 year after discharge.
In multivariable analyses, KRT or persistent kidney dysfunction was independently associated with mortality at hospital discharge (Table 3). Total hospital costs also remained higher in patients with versus without MAKE30 after adjusting for age, sex, race, number of CCCs, and payer ($68,914; IQR, $45,386–75,663 versus $41,166; IQR, $21,837–43,512; P<0.001).
Table 3.
Multivariable analysis of the association of nonfatal components of major adverse kidney events within 30 days with all-cause hospital mortality
| Variable | aOR | 95% CI | P Value |
|---|---|---|---|
| Nonmortal components of MAKE30 | |||
| No RRT or persistent kidney dysfunction | Reference | ||
| KRT or persistent kidney dysfunction | 7.7 | 4.7 to 12.6 | <0.001 |
| Age categories | |||
| 6 mo to <1 yr | 1.5 | 0.6 to 3.4 | 0.38 |
| 1 to <6 yr | 1.2 | 0.7 to 2.1 | 0.49 |
| 6 to <13 yr | 0.99 | 0.6 to 1.7 | 0.96 |
| 13 to <18 yr | Reference | ||
| Sex | |||
| Girls | Reference | ||
| Boys | 1.3 | 0.8 to 1.9 | 0.30 |
| Race | |||
| Non-Hispanic white | Reference | ||
| Non-Hispanic black | 0.5 | 0.2 to 1.1 | 0.08 |
| Hispanic | 1.1 | 0.5 to 2.4 | 0.85 |
| Asian | 0.8 | 0.2 to 3.3 | 0.71 |
| Other | 1.5 | 0.8 to 3.0 | 0.28 |
| Number of complex chronic conditions | |||
| None | Reference | ||
| One | 21.1 | 2.8 to 160 | 0.003 |
| Two | 15.0 | 1.9 to 115 | 0.01 |
| Three or more | 20.3 | 2.8 to 148 | 0.003 |
| Payer | |||
| Private | Reference | ||
| Government | 0.8 | 0.5 to 1.3 | 0.39 |
| Other payer | 1.4 | 0.7 to 3.0 | 0.31 |
aOR, adjusted odds ratio; 95% CI, 95% confidence interval; MAKE30, major adverse kidney events within 30 days; KRT, kidney replacement therapy.
Discussion
Nearly one in ten children presenting with severe sepsis or septic shock developed an MAKE, defined as death, new KRT, or persistent kidney dysfunction, within 30 days of admission. MAKE30 was associated with higher hospital resource utilization (estimated by costs) and low eGFR after hospital discharge. In addition, the nonmortal components of MAKE30 were strongly associated with mortality at hospital discharge. These data support MAKE30 as common, feasible to measure, and a promising end point for future clinical trials in pediatric sepsis testing therapies biologically mediated through the kidney.
Mortality as an end point in pediatric sepsis trials is limited by low incidence and fails to account for the potential effect of therapies on organ-specific morbidity (8). Composite outcomes that include both mortality and measures of morbidity more fully capture the health effect of sepsis on children and have the advantage of being more common with increased statistical efficiency (32). To be most useful, composite outcomes should be coherent without complete coincidence (i.e., each component should reflect related but different aspects of pathobiology), and each component must be of high importance to patients (33).
A workgroup convened by the NIDDK recommended a composite of death, KRT, and “a sustained loss of kidney function at a discrete time point (e.g., 30 days)” as a clinically meaningful end point for trials (19,34). This guidance was later expanded to include persistent kidney dysfunction “at hospital discharge truncated at 30 days” (35). Subsequently, clinical trials in adults have adopted this revised definition of MAKE30 to minimize costs and loss to follow-up after hospital discharge (20,21). The incidence of MAKE30 is 5%–16% in hospitalized adults and up to 35% in adult sepsis (20,21,36,37). Although MAKE30 was less frequent in pediatric sepsis, an incidence near 10% among children with community-acquired sepsis renders MAKE30 more common than mortality and as common as new or progressive multiple organ dysfunction syndrome (38). Although new or progressive multiple organ dysfunction syndrome has also been suggested as a composite end point for pediatric sepsis trials, it requires complex data abstraction, lacks sensitivity for improvement in any particular organ system, and has not been validated as a composite outcome (39).
The components of MAKE largely reflect the severity of kidney injury sustained during sepsis (i.e., coherence). Numerous studies show an association of AKI and KRT with hospital death in pediatric sepsis (4–6,40), and 10%–17% of children with moderate to severe AKI who survive hospitalization develop CKD within 3 years (11,12). Moreover, those with enduring kidney dysfunction have worse outcomes than patients who recover from AKI (41). Yet, we acknowledge that the operational definition of MAKE that we and others reported is truncated at hospital discharge. Although this feature has practical advantages for a trial, it is distinguished from the concept of “acute kidney disease” or nonrecovery of AKI (42), and it is likely that some who fulfill MAKE30 will recover kidney function after discharge. However, in adult sepsis, failure to recover serum creatinine to within 150% of baseline by hospital discharge (median LOS of 7 days) was associated with increased ESKD and long-term mortality (16).
Another potential concern with MAKE30 is that some deaths may not be attributable to or involve kidney injury. In our study, only 48% of MAKE30 deaths had stage 2/3 AKI, and only 39% also had at least one of the kidney-specific MAKE components. Nonetheless, because patients with MAKE30 incurred higher hospital costs and were more likely to have low eGFR after discharge, the components of this end point are associated with meaningful effects on patients’ lives. Moreover, because 78% of patients with stage 2/3 AKI did not develop persistent kidney dysfunction, require new KRT, or die and because the effect of AKI on hospital mortality was largely mediated through persistent kidney dysfunction, MAKE30 more specifically captured meaningful events in children with sepsis than AKI itself.
The validity and value of MAKE30 to children with sepsis and their families are of critical importance. There is clear face validity, with death a traditional end point for pediatric sepsis trials and kidney dysfunction linked to increased mortality. For construct validity, need for KRT and persistence of kidney dysfunction are both associated with the more patient-centered outcome of CKD. Concurrent validity is supported by the strong association of KRT and persistent kidney dysfunction with hospital costs and hospital mortality, and predictive validity is suggested by the association of MAKE30 with low eGFR after discharge. Numerous studies also suggest that adults value kidney function and independence from dialysis higher than survival. Although data are limited in pediatrics, severe AKI has been associated with lower health-related quality of life in children, especially a decline in physical function (15). Although it is reasonable to extrapolate that MAKE30 would, therefore, be meaningful to children and their families, definitive evidence that reducing the nonmortal components of MAKE will translate into improved long-term kidney outcomes requires confirmation (43).
There are several limitations. First, only patients who were retrospectively coded as severe sepsis/septic shock were analyzed. In a clinical trial, enrollment will occur prospectively, perhaps before an infection or sepsis can be confirmed. If MAKE30 occurs at a higher rate in sepsis compared with “sepsis mimickers,” then the incidence of MAKE30 could be lower than we observed here. Second, baseline creatinine was imputed in 41% of patients, and even measured creatinine before admission may fail to accurately capture kidney dysfunction present before sepsis. However, our data show that median creatinine for age and sex often slightly exceeded the upper confidence limit of measured baseline creatinine (and thus, provides a conservative estimate of MAKE30), with no difference in MAKE30 incidence when using higher imputed levels or multiple imputation. Still, we acknowledge that imputed baselines were on the basis of normative values in patients of white race (29). Third, only 29% of patients had data available to determine follow-up eGFR, and only inpatient laboratory data were available; although we evaluated lowest creatinine, we could not distinguish between CKD and a repeat episode of AKI as the cause of low eGFR after hospital discharge. Additional investigation into a potential causal link between MAKE30 and CKD, long-term morbidity and mortality, and postdischarge health services utilization are needed. Fourth, deaths incorporated within MAKE30 without AKI or concurrence of persistent kidney dysfunction or KRT likely include “nonkidney events.” Fifth, although data in PHIS+ were contributed by six centers over several years, all were large academic children’s hospitals, and the data were collected 7–11 years ago, which may limit generalizability across settings and time.
In summary, MAKE30 is common, feasible to measure, and a promising end point that could be used in future clinical trials for pediatric sepsis in which the intervention of interest may be biologically mediated through the kidney. The ease of collection and additional prognostic value compared with AKI suggest MAKE30 as a useful study end point. Additional efforts to validate the effect of MAKE30 in larger studies of pediatric sepsis with more complete postdischarge data are warranted.
Disclosures
Dr. Weiss has received honorarium from Thermo Fisher Scientific and has served as an advisory panel member for Bristol-Myers Squibb for work unrelated to this study. Dr. Downes reports grants from Pfizer, Inc., Merck, Inc., and the National Institute of Child Health and Human Development outside the submitted work. Dr. Fitzgerald reports grants from the National Institutes of Health outside the submitted work. Dr. Laskin reports grants from the National Institute of Diabetes and Digestive and Kidney Diseases during the conduct of the study and personal fees from Jazz Pharmaceuticals outside the submitted work. Dr. Balamuth and Dr. Thurm have nothing to disclose.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.12201018/-/DCSupplemental.
Supplemental Figure 1. Imputed versus measured baseline creatinine values.
Supplemental Figure 2. Hospital day on which serum creatinine to define persistent kidney dysfunction was measured.
Supplemental Figure 3. Mediation analysis of the association of stage 2/3 AKI with mortality at hospital discharge.
Supplemental Table 1. International Classification of Diseases, Ninth Edition, Clinical Modification codes for invasive infection.
Supplemental Table 2. International Classification of Diseases, Ninth Edition, Clinical Modification codes for organ dysfunction.
Supplemental Table 3. Nephrotoxic injury negated by just-in-time action (NINJA) drugs.
Supplemental Table 4. Creatinine values to determine baseline kidney function for patients without measured baseline creatinine.
Supplemental Table 5. Imputed versus measured baseline creatinine values for patients with known measured baseline creatinine after excluding those with kidney and urologic chronic comorbid conditions.
Supplemental Table 6. Patient encounter characteristics by components of major adverse kidney events within 30 days.
Supplemental Table 7. Distribution across components of major adverse kidney events within 30 days.
Supplementary Material
Acknowledgments
Financial support was provided by the Departments of Anesthesiology and Critical Care and Emergency Medicine at the Children’s Hospital of Philadelphia. Additionally, Dr. Weiss is supported by National Institute of General Medical Sciences grant K23GM110496, Dr. Downes is supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development grant K23HD091365, and Dr. Laskin is supported by National Institute of Diabetes and Digestive and Kidney Diseases grant K23DK101600.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Hartman ME, Linde-Zwirble WT, Angus DC, Watson RS: Trends in the epidemiology of pediatric severe sepsis. Pediatr Crit Care Med 14: 686–693, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Ruth A, McCracken CE, Fortenberry JD, Hall M, Simon HK, Hebbar KB: Pediatric severe sepsis: Current trends and outcomes from the Pediatric Health Information Systems database. Pediatr Crit Care Med 15: 828–838, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Schlapbach LJ, Straney L, Alexander J, MacLaren G, Festa M, Schibler A, Slater A; ANZICS Paediatric Study Group : Mortality related to invasive infections, sepsis, and septic shock in critically ill children in Australia and New Zealand, 2002-13: A multicentre retrospective cohort study. Lancet Infect Dis 15: 46–54, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald JC, Basu RK, Akcan-Arikan A, Izquierdo LM, Piñeres Olave BE, Hassinger AB, Szczepanska M, Deep A, Williams D, Sapru A, Roy JA, Nadkarni VM, Thomas NJ, Weiss SL, Furth S; Sepsis PRevalence, OUtcomes, and Therapies Study Investigators and Pediatric Acute Lung Injury and Sepsis Investigators Network : Acute kidney injury in pediatric severe sepsis: An independent risk factor for death and new disability. Crit Care Med 44: 2241–2250, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzgerald JC, Ross ME, Thomas NJ, Weiss SL, Balamuth F, Anderson AH: Risk factors and inpatient outcomes associated with acute kidney injury at pediatric severe sepsis presentation. Pediatr Nephrol 33: 1781–1790, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Akcan Arikan A, Williams EA, Graf JM, Kennedy CE, Patel B, Cruz AT: Resuscitation bundle in pediatric shock decreases acute kidney injury and improves outcomes. J Pediatr 167: 1301–1305 e1, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL; AWARE Investigators : Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 376: 11–20, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menon K, McNally JD, Zimmerman JJ, Agus MS, O’Hearn K, Watson RS, Wong HR, Duffett M, Wypij D, Choong K: Primary outcome measures in pediatric septic shock trials: A systematic review. Pediatr Crit Care Med 18: e146–e154, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Billings FT 4th, Shaw AD: Clinical trial endpoints in acute kidney injury. Nephron Clin Pract 127: 89–93, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberg JH, Coca S, Parikh CR: Long-term risk of chronic kidney disease and mortality in children after acute kidney injury: A systematic review. BMC Nephrol 15: 184, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mammen C, Al Abbas A, Skippen P, Nadel H, Levine D, Collet JP, Matsell DG: Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: A prospective cohort study. Am J Kidney Dis 59: 523–530, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Chawla LS, Kimmel PL: Acute kidney injury and chronic kidney disease: An integrated clinical syndrome. Kidney Int 82: 516–524, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Bucaloiu ID, Kirchner HL, Norfolk ER, Hartle JE 2nd, Perkins RM: Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int 81: 477–485, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Jones J, Holmen J, De Graauw J, Jovanovich A, Thornton S, Chonchol M: Association of complete recovery from acute kidney injury with incident CKD stage 3 and all-cause mortality. Am J Kidney Dis 60: 402–408, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson KL, Watson RS, Hingorani S: Quality of life following hospitalization-associated acute kidney injury in children. J Nephrol 31: 249–256, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiorentino M, Tohme FA, Wang S, Murugan R, Angus DC, Kellum JA: Long-term survival in patients with septic acute kidney injury is strongly influenced by renal recovery. PLoS One 13: e0198269, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirwan CJ, Blunden MJ, Dobbie H, James A, Nedungadi A, Prowle JR: Critically ill patients requiring acute renal replacement therapy are at an increased risk of long-term renal dysfunction, but rarely receive specialist nephrology follow-up. Nephron 129: 164–170, 2015 [DOI] [PubMed] [Google Scholar]
- 18.De Corte W, Dhondt A, Vanholder R, De Waele J, Decruyenaere J, Sergoyne V, Vanhalst J, Claus S, Hoste EA: Long-term outcome in ICU patients with acute kidney injury treated with renal replacement therapy: A prospective cohort study. Crit Care 20: 256, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palevsky PM, Molitoris BA, Okusa MD, Levin A, Waikar SS, Wald R, Chertow GM, Murray PT, Parikh CR, Shaw AD, Go AS, Faubel SG, Kellum JA, Chinchilli VM, Liu KD, Cheung AK, Weisbord SD, Chawla LS, Kaufman JS, Devarajan P, Toto RM, Hsu CY, Greene T, Mehta RL, Stokes JB, Thompson AM, Thompson BT, Westenfelder CS, Tumlin JA, Warnock DG, Shah SV, Xie Y, Duggan EG, Kimmel PL, Star RA: Design of clinical trials in acute kidney injury: Report from an NIDDK workshop on trial methodology. Clin J Am Soc Nephrol 7: 844–850, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Self WH, Semler MW, Wanderer JP, Wang L, Byrne DW, Collins SP, Slovis CM, Lindsell CJ, Ehrenfeld JM, Siew ED, Shaw AD, Bernard GR, Rice TW; SALT-ED Investigators : Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med 378: 819–828, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, Stollings JL, Kumar AB, Hughes CG, Hernandez A, Guillamondegui OD, May AK, Weavind L, Casey JD, Siew ED, Shaw AD, Bernard GR, Rice TW; SMART Investigators and the Pragmatic Critical Care Research Group : Balanced crystalloids versus saline in critically ill adults. N Engl J Med 378: 829–839, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narus SP, Srivastava R, Gouripeddi R, Livne OE, Mo P, Bickel JP, de Regt D, Hales JW, Kirkendall E, Stepanek RL, Toth J, Keren R: Federating clinical data from six pediatric hospitals: Process and initial results from the PHIS+ consortium. AMIA Annu Symp Proc 2011: 994–1003, 2011 [PMC free article] [PubMed] [Google Scholar]
- 23.Balamuth F, Weiss SL, Neuman MI, Scott H, Brady PW, Paul R, Farris RW, McClead R, Hayes K, Gaieski D, Hall M, Shah SS, Alpern ER: Pediatric severe sepsis in U.S. children’s hospitals. Pediatr Crit Care Med 15: 798–805, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss SL, Parker B, Bullock ME, Swartz S, Price C, Wainwright MS, Goodman DM: Defining pediatric sepsis by different criteria: Discrepancies in populations and implications for clinical practice. Pediatr Crit Care Med 13: e219–e226, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD: Deaths attributed to pediatric complex chronic conditions: National trends and implications for supportive care services. Pediatrics 107: E99, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Goldstein SL, Mottes T, Simpson K, Barclay C, Muething S, Haslam DB, Kirkendall ES: A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int 90: 212–221, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Sutherland SM, Byrnes JJ, Kothari M, Longhurst CA, Dutta S, Garcia P, Goldstein SL: AKI in hospitalized children: Comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol 10: 554–561, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss SL, Keele L, Balamuth F, Vendetti N, Ross R, Fitzgerald JC, Gerber JS: Crystalloid fluid choice and clinical outcomes in pediatric sepsis: A matched retrospective cohort study. J Pediatr 182: 304–310.e310, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pottel H, Vrydags N, Mahieu B, Vandewynckele E, Croes K, Martens F: Establishing age/sex related serum creatinine reference intervals from hospital laboratory data based on different statistical methods. Clin Chim Acta 396: 49–55, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Siew ED, Peterson JF, Eden SK, Moons KG, Ikizler TA, Matheny ME: Use of multiple imputation method to improve estimation of missing baseline serum creatinine in acute kidney injury research. Clin J Am Soc Nephrol 8: 10–18, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blufpand HN, Westland R, van Wijk JA, Roelandse-Koop EA, Kaspers GJ, Bökenkamp A: Height-independent estimation of glomerular filtration rate in children: An alternative to the Schwartz equation. J Pediatr 163: 1722–1727, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Weiss SL: Clinical trials in pediatric sepsis: What’s the (end) point? Pediatr Crit Care Med 18: 296–297, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCoy CE: Understanding the use of composite endpoints in clinical trials. West J Emerg Med 19: 631–634, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kellum JA, Zarbock A, Nadim MK: What endpoints should be used for clinical studies in acute kidney injury? Intensive Care Med 43: 901–903, 2017 [DOI] [PubMed] [Google Scholar]
- 35.Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, Bihorac A, Birkhahn R, Cely CM, Chawla LS, Davison DL, Feldkamp T, Forni LG, Gong MN, Gunnerson KJ, Haase M, Hackett J, Honore PM, Hoste EA, Joannes-Boyau O, Joannidis M, Kim P, Koyner JL, Laskowitz DT, Lissauer ME, Marx G, McCullough PA, Mullaney S, Ostermann M, Rimmelé T, Shapiro NI, Shaw AD, Shi J, Sprague AM, Vincent JL, Vinsonneau C, Wagner L, Walker MG, Wilkerson RG, Zacharowski K, Kellum JA: Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 17: R25, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Semler MW, Rice TW, Shaw AD, Siew ED, Self WH, Kumar AB, Byrne DW, Ehrenfeld JM, Wanderer JP: Identification of major adverse kidney events within the electronic health record. J Med Syst 40: 167, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Semler MW, Wanderer JP, Ehrenfeld JM, Stollings JL, Self WH, Siew ED, Wang L, Byrne DW, Shaw AD, Bernard GR, Rice TW; SALT Investigators * and the Pragmatic Critical Care Research Group; SALT Investigators : Balanced crystalloids versus saline in the intensive care unit: The SALT randomized trial. Am J Respir Crit Care Med 195: 1362–1372, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Workman JK, Ames SG, Reeder RW, Korgenski EK, Masotti SM, Bratton SL, Larsen GY: Treatment of pediatric septic shock with the surviving sepsis campaign guidelines and PICU patient outcomes. Pediatr Crit Care Med 17: e451–e458, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Lin JC, Spinella PC, Fitzgerald JC, Tucci M, Bush JL, Nadkarni VM, Thomas NJ, Weiss SL; Sepsis Prevalence, Outcomes, and Therapy Study Investigators : New or progressive multiple organ dysfunction syndrome in pediatric severe sepsis: A sepsis phenotype with higher morbidity and mortality. Pediatr Crit Care Med 18: 8–16, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deep A, Sagar H, Goonasekera C, Karthikeyan P, Brierley J, Douiri A: Evolution of acute kidney injury and its association with systemic hemodynamics in children with fluid-refractory septic shock. Crit Care Med 46: e677–e683, 2018 [DOI] [PubMed] [Google Scholar]
- 41.Pannu N, James M, Hemmelgarn B, Klarenbach S; Alberta Kidney Disease Network : Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol 8: 194–202, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, Bittleman D, Cruz D, Endre Z, Fitzgerald RL, Forni L, Kane-Gill SL, Hoste E, Koyner J, Liu KD, Macedo E, Mehta R, Murray P, Nadim M, Ostermann M, Palevsky PM, Pannu N, Rosner M, Wald R, Zarbock A, Ronco C, Kellum JA; Acute Disease Quality Initiative Workgroup 16. : Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative (ADQI) 16 workgroup. Nat Rev Nephrol 13: 241–257, 2017 [DOI] [PubMed] [Google Scholar]
- 43.Coca SG: Is it AKI or nonrecovery of renal function that is important for long-term outcomes? Clin J Am Soc Nephrol 8: 173–176, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.