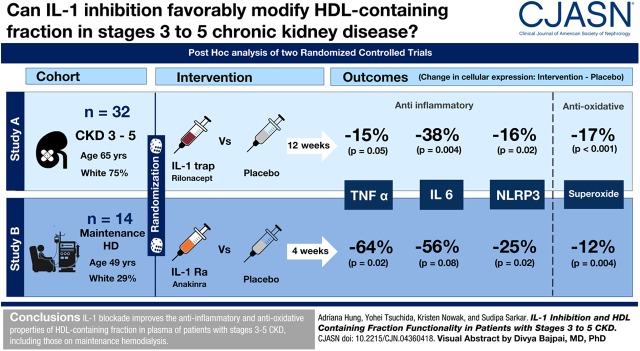
Visual Abstract
Keywords: Chronic inflammation; HDL; IL-1 inhibition; CKD; IL6 protein, human; IL1RN protein, human; Interleukin 1 Receptor Antagonist Protein; Superoxides; Macrophages, Peritoneal; Tumor Necrosis Factor-alpha; macrophages; Lipoproteins, HDL; Anti-Inflammatory Agents; Inflammation; Renal Insufficiency, Chronic; dialysis; Oxidants; Cholesterol; Cardiovascular Diseases; NLR Proteins; Receptors, Interleukin-1; oxidative stress
Abstract
Background and objectives
Systemic inflammation modulates cardiovascular disease risk and functionality of HDL in the setting of CKD. Whether interventions that modify systemic inflammation can improve HDL function in CKD is unknown.
Design, setting, participants, & measurements
We conducted a post hoc analysis of two randomized, clinical trials, IL-1 trap in participants with GFR 15–59 ml/min per 1.73 m2 (study A) and IL-1 receptor antagonist in participants on maintenance hemodialysis (study B), to evaluate if IL-1 blockade had improved the anti-inflammatory activity (IL-6, TNF-α, and Nod-like receptor protein 3), antioxidant function (superoxide production), and net cholesterol efflux capacity of HDL. HDL function was measured using LPS-stimulated THP-1 macrophages or peritoneal macrophages of apoE-deficient mice exposed to the apoB-depleted, HDL-containing fraction obtained from the plasma of the study participants, collected before and after the interventions to block IL-1 effects. Analysis of covariance was used for between group comparisons.
Results
The mean age of the participants was 60±13 years, 72% (n=33) were men, and 39% (n=18) were black. There were 32 CKD (16 IL-1 trap and 16 placebo) and 14 maintenance hemodialysis (7 IL-1 receptor antagonist and 7 placebo) participants. Compared with placebo, IL-1 inhibition, in study A and B reduced cellular expression of TNF-α by 15% (P=0.05) and 64% (P=0.02), IL-6 by 38% (P=0.004) and 56% (P=0.08), and Nod-like receptor protein 3 by 16% (P=0.01) and 25% (P=0.02), respectively. The intervention blunted superoxide production in the treated arm compared with placebo, with the values being higher by 17% in the placebo arm in study A (P<0.001) and 12% in the placebo arm in study B (P=0.004). Net cholesterol efflux capacity was not affected by either intervention.
Conclusions
IL-1 blockade improves the anti-inflammatory and antioxidative properties of the HDL-containing fraction of plasma in patients with stages 3–5 CKD, including those on maintenance hemodialysis.
Introduction
Systemic inflammation and oxidant stress prevail at all stages of CKD and are believed to be key mechanisms underlying many adverse consequences of CKD, including cardiovascular disease (1). Anti-inflammatory interventions, specifically anticytokine therapies, have been remarkably successful in several chronic diseases such as inflammatory bowel disease, rheumatoid arthritis, psoriasis, and most recently, atherosclerotic cardiovascular disease (2,3). A landmark study in patients with myocardial infarction demonstrated that administration of an mAb targeting IL-1β innate immunity pathway with canakinumab every 3 months for 4 years led to a significantly lower rate of recurrent cardiovascular events compared with placebo (2). The beneficial effect was observed with no reduction in lipid levels from baseline and have advanced the “inflammatory hypothesis of atherosclerotic cardiovascular disease” (2). These findings are highly relevant to the CKD population, who suffer from an accelerated atherosclerosis process. We have previously shown that short-term administration of an IL-1 receptor antagonist (IL-1ra) effectively reduced systemic inflammatory markers and increased circulating levels of adiponectin in patients on maintenance hemodialysis (4,5). We also reported that an intervention using an IL-1 trap reduced markers of systemic inflammation and vascular oxidative stress and improved endothelial function in patients with a GFR of 15–59 ml/min per 1.73 m2 (2,6). Consideration to inhibit another inflammatory pathway are underway. Thus, the Million Veteran Program, a biobank from the Veterans Administration recently revealed that a genetic variant that mimics the effect of an IL-6 blocker was associated with lower risk of cardiovascular disease, findings that have prompted randomized trials of IL-6 blockade in CKD (7).
HDL has a variety of beneficial actions in the general population (8–10). In addition to reverse cholesterol transport, whereby HDL transfers cholesterol from the periphery to the liver for excretion, HDL reduces inflammatory processes, limits oxidative stress, inhibits blood clotting mechanisms, and protects the endothelium. Although numerous studies have established that low levels of HDL are associated with increased cardiovascular disease (11,12), recently the emphasis has shifted from circulating levels to functionality of HDL as a better predictor of cardiovascular disease (13–15).
CKD impairs many of the protective functions of HDL, including anti-inflammatory and antioxidative activities (16–21). Noninfectious chronic inflammation is common in CKD (1). Whether interventions that reduce systemic inflammation and oxidative stress can improve HDL function in patients with CKD is underexplored. In this study, we aimed to determine whether IL-1 inhibition improves the anti-inflammatory and antioxidative effects of HDL particles in patients with moderate and severe CKD, including those on maintenance hemodialysis. We performed a post hoc analysis from patient samples from two previously completed randomized, controlled trials (RCTs) on IL-1 inhibition in CKD stages 3 and 4 (Clinicaltrials.gov identifiers NCT00897715 and NCT01663103) and maintenance hemodialysis (Clinicaltrials.gov identifier NCT00420290) to address these questions.
Materials and Methods
Study Population and Study Protocol
The primary results of the original RCTs have been published previously (5,6). In brief, in study A, patients with a GFR of 15–59 ml/min per 1.73 m2 were recruited at two clinical sites between 2012 and 2014 (University of Colorado Denver Anschutz Medical Campus and Tennessee Valley Healthcare System/Vanderbilt University Medical Center) (NCT00897715 and NCT01663103). The IL-1 trap rilonacept, an IL-1 decoy receptor that binds IL-1 and neutralizes it before it can bind to cell-surface receptors, was administered subcutaneously at 160 mg once weekly for 12 weeks, after a loading dose of 320 mg (6), versus placebo. The primary outcome for this study was changes in endothelial function measured as change in brachial artery flow-mediated dilation and the secondary outcome was the effect on high-sensitivity C-reactive protein (hsCRP). In study B, patients on maintenance hemodialysis were recruited from Tennessee Valley Healthcare System and Vanderbilt University Medical Center between 2008 and 2010 (NCT00420290). The human recombinant IL-1ra anakinra (100 mg administered subcutaneously; Amgen, Thousand Oaks, CA) or placebo was injected at each dialysis session for 4 weeks (5). For study B, the primary outcome was the effect of IL-1ra administration on hsCRP.
The original trial for study A showed that the administration of IL-1 trap in CKD stages 3 and 4 improved flow mediated dilation by 3.36±2.06% and reduced hsCRP levels (baseline: median, 4.60 [interquartile range (IQR), 1.90–8.22] mg/L; 12 weeks: median, 2.16 [IQR, 0.92–7.38] mg/L). The original trial for study B showed that IL-1ra effectively reduced hsCRP by 50%.
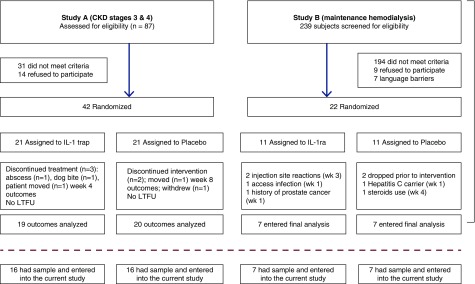
Exclusion criteria for both trials were active or history of chronic infection (HIV disease), hepatitis B, hepatitis C, positive tuberculosis/tuberculin test or positive QuantiFERON TB gold test, history of malignancy within the past 5 years, hospitalization in the prior month, immunosuppressive medication within the past year, or any investigational drug within 1 month before the study. Presence of a dialysis catheter was an exclusion criterion for the study on patients on maintenance hemodialysis. Both studies were approved by the respective institutional review boards. Signed informed consent was obtained from all study participants. A CONSORT flow chart is included (Figure 1). For study B (maintenance hemodialysis), the IL-1ra trial, all 14 participants who completed the original trial were included in this analysis (5). For study A (CKD stages 3 and 4), the IL-1 trap trial, 16 out of 21 (76%) patients in each arm had sufficient remaining blood samples for analysis for this post hoc study (6).
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram. LTFU, lost to follow up.
Procedures
Blood sample collection.
Baseline blood samples were collected during the baseline visit within the 2 days before starting the intervention. The intervention was initiated after the baseline samples and measurements were completed during the baseline visit for both studies. For the dialysis study, blood samples were collected before dialysis. End of study blood samples were collected within 1 week after the last dose of the study drug was administered.
HDL isolation and assessment of plasma profile.
Blood samples were collected by venipuncture into EDTA tubes, centrifuged at 1700×g for 15 minutes at 4°C. The samples were aliquoted and were stored at −80°C and thawed only once, which has been shown to have minimal effect on functionality (14,22,23). The HDL-containing fraction used in these studies was obtained after removal of apoB lipoproteins, which is the prevailent method in clinical studies examining HDL function. Patient specimen aliquots were treated with polyethylene glycol (PEG) solution to precipitate apoB-containing lipoproteins by adding 100 µl of PEG solution (20% PEG 8000 in 200 mM glycine, pH 7.4) to patient plasma (250 µl). After 15 minutes of incubation, the samples underwent high-speed centrifugation (1900×g for 15 minutes at 4°C). The supernatant was then removed and the apoB-depleted, HDL-enriched fraction (18,24,25) was used as in previous studies assessing HDL functionality. We chose this method of assessing HDL fraction functionality on the basis of its use in previous studies and to avoid the possible confounding effects of alternative approaches relying on density gradient separation, where added substances may alter the functionality of HDL (26) Plasma levels of total cholesterol, LDL, triglycerides, and HDL were measured enzymatically (Cliniqa, San Marcos, CA).
Macrophage inflammatory reaction with HDL.
HDL modulation of inflammatory effects was measured using the established cytokine response in LPS-activated cells (23). Briefly, THP-1 cells (American Type Culture Collection, Manassas, VA) were plated and differentiated using RPMI 1670 containing 10% fetal bovine plasma and 50 ng/ml phorbol 12-myristate 13-acetate. THP-1 macrophages were exposed to apoB-depleted HDL fraction (18 μg cholesterol/ml) and LPS (50 ng/ml) for 4 hours. Total RNA was extracted from cells with RNeasy Mini Kit (QIAGEN) as previously described (18,23). Quantification of human IL-6, TNF-α, Nod-like receptor protein 3 (NLRP3), and endogenous control human β-actin gene expression was performed by real-time RT-PCR using CFX96TM Real-Time System (Bio-Rad). Probes for IL-6 (Hs99999032_m1), TNF-α (Hs99999043_m1), NLRP3 (Hs00918082_m1), and β-actin (Hs01060665_g1) were obtained from Applied Biosystems (Foster City, CA). These inflammatory markers were chosen because of their well established participation in atherogenesis and critical roles in the IL-1β inflammatory pathway (2,27,28).
Macrophage generation of reactive oxygen species.
Cellular production of superoxide was measured as the formation of a superoxide specific product of dihydroethidium, 2-hydroxyethidium, using HPLC analysis in THP-1 cells exposed to apoB-depleted HDL fraction, as described in the inflammatory response studies (29).
Net cholesterol efflux assay.
Thioglycolate-elicited peritoneal macrophages were isolated from apoE-deficient mice and plated as previously described (30). After washing, DMEM with 40 μg acetylated-LDL/ml was added to each well (time 0) and incubated for 40 hours. Cells were exposed to 2% apoB-depleted HDL fraction (PEG 8000 precipitation) in DMEM for 24 hours and then washed, dried, and incubated with isopropanol to extract cellular lipid. To examine the ability of the apoB-depleted fraction to mediate the net efflux of cholesterol, cellular cholesterol mass was measured as described previously (31). Fluorescence intensity was measured at excitation wavelength 530 nm and emission wavelength 590 nm. Cellular cholesterol mass was calculated according to standard curves and corrected by protein (32). Net cholesterol efflux capacity was calculated as (cholesterol levels in To– cholesterol levels in efflux wells)/cholesterol levels in To×100%.
Study End Points
The primary outcomes were inflammatory biomarkers (IL-6, TNF-α, and NLRP3) response in LPS-stimulated THP-1 macrophages to patients’ apoB-depleted HDL fraction before and after the intervention for each trial. Secondary outcomes included the change in the production of superoxide in LPS-stimulated THP-1 macrophages and the changes in net cholesterol efflux capacity before and after the intervention for each trial. Exploratory outcomes included any effects on lipid profile. Covariates included demographics, body mass index, diabetes, serum albumin, and statin use.
Statistical Analyses
Data are presented as mean±SD or as median with IQRs, depending on the distribution of the particular variable or as proportions and compared using Mann–Whitney U or chi-squared tests when appropriate. Analysis of covariance (ANCOVA) was used to estimate the percent change (regression coefficient from the ANCOVA model) as a function of treatment group from baseline to end of the study for all outcomes, which refers to the difference in percent change between the treatment and the placebo groups (33). We did not generate the percent change at an individual level because within-patient change is affected strongly by regression to the mean and measurement error; instead, we selected ANCOVA as recommended by several authors for this setting. ANCOVA has additional advantages including control for baseline differences and incorporation of randomization strata as covariates. Outcome variables were log-transformed to improve normality in residuals, and the baseline value of the outcome variable was adjusted as a covariate. Because of the small number of participants, no adjustment of other variables was performed, as in the parent trials (5,6). A P value of <0.05 was considered statistically significant. All reported P values are two-sided. Analyses were performed using STATA version 15.
Results
Baseline Characteristics
There were 32 patients with CKD (16 active drug and 16 placebo) and 14 patients on maintenance hemodialysis (seven active drug and seven placebo). Baseline characteristics of both participants with CKD and those with ESKD have been described in detail in each of the parent trials (5,6). For patients with CKD, the mean age was 65±10 years, 28% were women (n=9), and 75% were white (n=24). The mean age for patients on maintenance hemodialysis was 49±13 years, 29% were women, and 71% were black. Table 1 shows baseline characteristics of the two study groups at the time of randomization. Patient enrollment, randomization, and completion flow diagram for both trials is shown in Figure 1.
Table 1.
Baseline characteristics of two clinical trials of IL-1 inhibition in CKD
| Variable | Study A | Study B | ||
|---|---|---|---|---|
| CKD Stages 3 and 4, n=16 | Maintenance Hemodialysis | |||
| IL-1 Trap, n=16 | Placebo, n=16 | IL-1ra, n=7 | Placebo, n=7 | |
| Demographics | ||||
| Men, n (%) | 11 (69) | 12 (75) | 5 (71) | 5 (71) |
| Age, yr, mean±SD | 62±12 | 67±8 | 51±14 | 48±12 |
| Black race, n (%) | 4 (25) | 4 (25) | 6 (86) | 4 (57) |
| Clinical characteristics | ||||
| Statin, n (%) | 8 (50) | 12 (75) | NA | NA |
| Diabetes, n (%) | 8 (50) | 9 (56) | 0 (0) | 3 (43) |
| BMI, kg/m2, mean±SD | 32±6.2 | 31±5 | 34±6 | 29±10 |
| Inflammatory biomarkers | ||||
| Serum albumin, g/dl | 4.0 (3.7, 4.0) | 3.9 (3.7, 4.1) | 4.1 (3.7, 4.3) | 3.8 (3.6, 4.5) |
| Plasma hsCRP, mg/L | 4.2 (2.1, 7.9) | 4.2 (1.45, 5.5) | 9.5 (6.8, 12.6) | 19.5 (5.2, 21.3) |
| Plasma IL-6, pg/ml | 1.6 (0.9, 2.3) | 1.6 (1.2, 3.6) | 4.6 (4.4, 8.4) | 6.2 (1.8, 17.2) |
| Plasma TNF-α, pg/ml | 1.7 (1.3, 3.4) | 2.1 (1.7, 3.6) | 21.6 (20.1, 23.1) | 20.7 (12.3, 66.6) |
| Lipids | ||||
| HDL, mg/dl | 43 (35, 60) | 37 (31, 40) | 47 (45, 50) | 39 (35, 42) |
| LDL, mg/dl | 83 (71, 123) | 78 (72, 97) | 95 (87, 100) | 65 (39, 78) |
| Triglycerides, mg/dl | 115 (72, 144) | 147 (97, 184) | 104 (94, 130) | 203 (132, 267) |
| Total cholesterol, mg/dl | 162 (129, 190) | 143 (134, 179) | 160 (158, 169) | 145 (126, 166) |
Statistical comparison done using chi-squared test for categorical variables and Kruskal–Wallis for continues variables. Data for inflammatory biomarkers and lipids is displayed as median (interquartile range). IL-1ra, IL-1 receptor antagonist; NA, not applicable; BMI, body mass index; hsCRP, high-sensitivity C-reactive protein.
IL-1 Inhibition Improves HDL Anti-Inflammatory Cytokine Response and of NLRP3 Inflammasome Expression
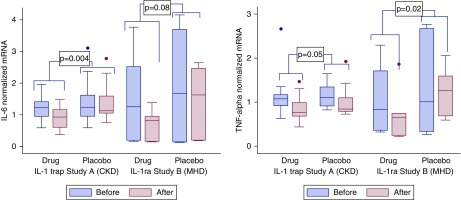
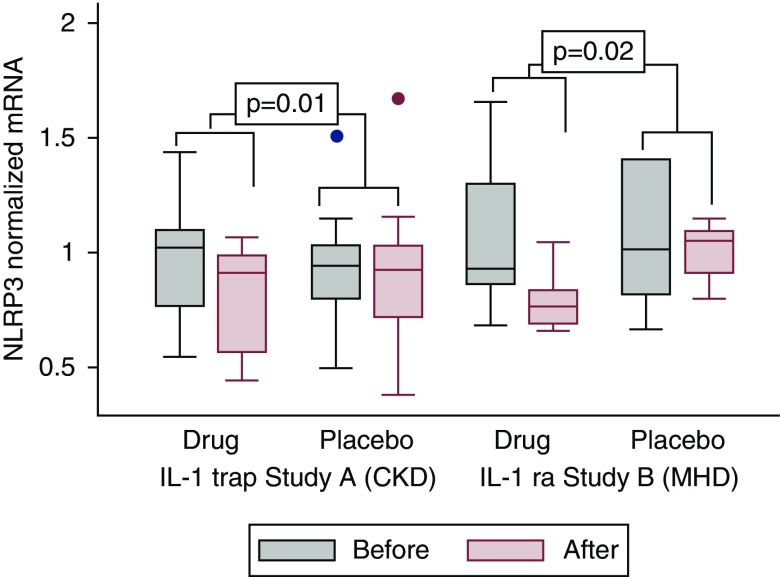
In study A (CKD stages 3 and 4), IL-1 trap effectively reduced the cellular expression of biomarkers of inflammation and oxidative stress (Figure 2A, Table 2). For IL-6, the intervention reduced the cellular IL-6 mRNA expression by 38% (P=0.004) compared with placebo. For TNF-α, mRNA expression was reduced by 15% (P=0.05) compared with placebo. For NLRP3, the intervention reduced the mRNA expression by 16% (P=0.01). In study B (maintenance hemodialysis), IL-1ra also reduced the cellular expression of biomarkers of inflammation and oxidative stress. However, statistical significance was not observed for all biomarkers (Figure 2B, Table 2). For IL-6, the intervention reduced the cellular mRNA expression by 56% (P=0.08) compared with placebo. For TNF-α, mRNA expression was reduced by 64% (P=0.02) compared with placebo. For NLRP3 (Figure 3, Table 2), the intervention reduced the mRNA expression by 25% (P=0.02) compared with placebo.
Figure 2.
Cytokine response in LPS-stimulated THP-1 macrophages to patients’ HDL before and after treatment with IL-1 trap versus placebo for study A (CKD stages 3 and 4), and with IL-1ra versus placebo for study B (maintenance hemodialysis). (A) IL-6 and (B) TNF-α mRNA expression response before and after intervention in each trial measured by real-time RT-PCR. *Statistical comparison of the intervention effect between groups for drug versus placebo for each trial was done using ANCOVA (P values are derived from the ANCOVA). MHD, maintenance hemodialysis.
Table 2.
Change in mRNA expression for cytokine and superoxide production by LPS-stimulated THP-1 macrophages
| Biomarkers | IL-1 Inhibition | Placebo | Difference in Change by Treatment Assignment (P Value)a |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| Study A, CKD stages 3 and 4 | |||
| Baseline TNF-α | 1.07 (0.92–1.17) | 1.10 (0.91–1.27) | −15% (P=0.05) |
| Post-treatment TNF-α | 0.76 (0.68–0.99) | 0.85 (0.79–1.10) | |
| Baseline IL-6 | 1.24 (0.96–1.42) | 1.24 (0.96–1.62) | −38% (P=0.004) |
| Post-treatment IL-6 | 0.93 (0.61–1.17) | 1.13 (1.05–1.59) | |
| Baseline NLRP3 | 1.02 (0.77–1.10) | 0.94 (0.80–1.03) | −16% (P=0.01) |
| Post-treatment NLRP3 | 0.91 (0.57–0.99) | 0.93 (0.72–1.03) | |
| Baseline superoxide | 490 (471–520) | 502 (469–513) | −17% (P<0.001) |
| Post-treatment superoxide | 513 (482–526) | 595 (574–610) | |
| Study B, maintenance hemodialysis | |||
| Baseline TNF-α | 0.83 (0.36–1.71) | 1.01 (0.34–2.67) | −64% (P=0.02) |
| Post-treatment TNF-α | 0.66 (0.25–0.74) | 1.26 (0.69–1.60) | |
| Baseline IL-6 | 1.26 (0.21–2.52) | 1.69 (0.15–3.69) | −56% (P=0.08) |
| Post-treatment IL-6 | 0.83 (0.17–0.95) | 1.63 (0.20–2.47) | |
| Baseline NLRP3 | 0.93 (0.86–1.30) | 1.01 (0.82–1.41) | −25% (P=0.02) |
| Post-treatment NLRP3 | 0.77 (0.69–0.84) | 1.05 (0.91–1.10) | |
| Baseline superoxide | 790 (766–813) | 780 (748–782) | −12% (P=0.004) |
| Post-treatment superoxide | 816 (788–840) | 921 (899–924) |
Cytokine response in LPS-stimulated THP-1 macrophages to HDL of patients with CKD stages 3 and 4 treated with IL-1 trap (study A) versus placebo and patients on maintenance hemodialysis treated with IL-1 receptor antagonist (study B) versus placebo. IL-6 and TNF-α response before and after intervention is shown for each trial. Values are expressed as median and interquartile range. mRNA expression measured by real-time RT-PCR. The units for these biomarkers represent normalized mRNA in arbitrary units (AU). IQR, interquartile range; NLRP3, Nod-like receptor protein 3.
Statistical comparison of the intervention effect between groups for drug versus placebo for each trial was done using analysis of covariance to estimate the percent change. All variables were log transformed.
Figure 3.
NLRP3 mRNA expression in LPS-stimulated THP-1 macrophages exposed to patients’ HDL before and after treatment with IL-1 trap versus placebo for study A (CKD stages 3 and 4), and with IL-1ra versus placebo for study B (maintenance hemodialysis). NLRP3 mRNA expression was measured by real-time RT-PCR. *Statistical comparison of the intervention effect between groups for drug versus placebo for each trial was done using ANCOVA (P values are derived from the ANCOVA). MHD, maintenance hemodialysis.
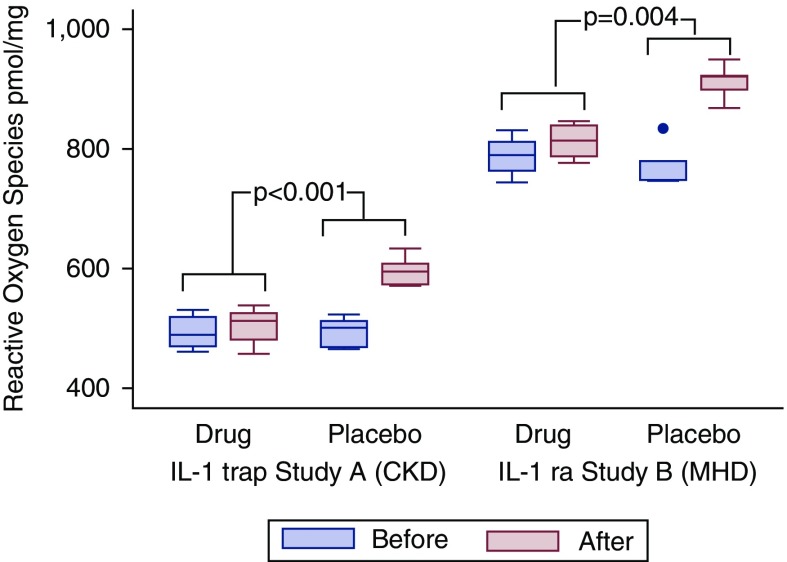
IL-1 Inhibition Improves HDL Capacity to Decrease Cellular Reactive Oxygen Species Generation
To further investigate the effects of IL-1 inhibition on HDL fraction function, we measured superoxide production in LPS-stimulated THP-1 macrophages exposed to HDL fraction before and after the intervention (Figure 4). Compared with placebo, IL-1 inhibition led to a significant blunting in reactive oxygen species generation in response to HDL.
Figure 4.
Cellular production of reactive oxygen species in LPS-stimulated THP-1 macrophages exposed to patients; HDL before and after treatment with IL-1 trap versus placebo for study A (CKD stages 3 and 4), and with IL-1ra versus placebo for study B (maintenance hemodialysis). *Statistical comparison of the intervention effect between groups for drug versus placebo for each trial was done using ANCOVA (P values are derived from the ANCOVA). MHD, maintenance hemodialysis.
In study A, reactive oxygen species production increased to a lesser extent in the IL-1 trap arm, from a median of 490 pmol/mg (IQR, 471–520) to a median of 513 pmol/mg (IQR, 482–526) at the end of the study, whereas in the placebo group, reactive oxygen species production increased from a median of 502 pmol/mg (IQR, 469–513) to a median of 595 pmol/mg (IQR, 574–610) at the end of the study. The comparison between groups for the effect of the intervention was statistically significant (P<0.001) with the intervention blunting superoxide production in the treated arm, with the values being higher by 17% in the placebo arm.
In study B, reactive oxygen species production was also blunted in the IL-1ra administration arm, from a baseline median of 790 pmol/mg (IQR, 766–813) to a median of 816 pmol/mg (IQR, 788–840) at the end of the study, whereas in the placebo group, reactive oxygen species production increased from a baseline median of 780 pmol/mg (IQR, 748–782) to a median of 921 pmol/mg (IQR, 899–924) at the end of the study. The comparison between groups for the effect of the intervention was statistically significant (P<0.004) with the intervention blunting superoxide production in the treated arm, with the values being higher by 12% in the placebo arm (Figure 4).
IL-1 Inhibition and HDL Net Cholesterol Efflux Capacity
There were no significant changes in net cholesterol efflux capacity with either intervention or placebo in either study. In study A, net cholesterol efflux capacity change from a median of 26% (IQR, 25%–27%) before intervention and 29% (IQR, 27%–31%) after intervention, compared with a median of 32% (IQR, 30%–33%) before intervention and 38% (IQR, 31%–43%) after intervention in the placebo group (P=0.3). In study B, there were no significant changes in either group, from a median 38% (IQR, 30%–48%) to a median 36% (IQR, 29%–47%) in the intervention group and from a median 37% (IQR, 29%–45%) to a median 38% (IQR, 29%–46%), in the placebo group (P=0.5).
IL-1 Inhibition Effects on Plasma Lipids
There were no significant changes in plasma lipid profiles, including LDL, HDL-C, and triglycerides, in response to IL-1 blockade in study A (CKD stages 3 and 4). In study B (maintenance hemodialysis), there was a decrease in HDL of 15% driven by changes in the placebo arm (P=0.02) (Table 3).
Table 3.
Change in plasma lipid profile in the intervention group compared with the placebo group
| Lipid Profile | IL-1 Inhibition | Placebo | P Valuea |
|---|---|---|---|
| Study A, CKD stages 3 and 4, mg/dl | |||
| Baseline total cholesterol | 162 (129, 190) | 143 (134, 179) | 0.22 |
| Post-treatment total cholesterol | 168 (138, 201) | 144 (134, 174) | |
| Baseline HDL | 43 (35, 60) | 37 (31, 40) | 0.06 |
| Post-treatment HDL | 47 (43, 50) | 35 (30, 46) | |
| Baseline LDL | 83 (71, 123) | 78 (72, 97) | 0.35 |
| Post-treatment LDL | 95 (74, 137) | 84 (71, 102) | |
| Baseline triglycerides | 115 (72, 144) | 147 (97, 184) | 0.91 |
| Post-treatment triglycerides | 112 (83, 177) | 137 (102, 198) | |
| Study B, maintenance hemodialysis, mg/dl | |||
| Baseline total cholesterol | 160 (158, 169) | 145 (126, 166) | 0.51 |
| Post-treatment total cholesterol | 170 (159, 185) | 152 (146, 152) | |
| Baseline HDL | 47 (45, 50) | 39 (35, 42) | 0.02 |
| Post-treatment HDL | 48 (42, 56) | 35 (34, 35) | |
| Baseline LDL | 95 (87, 100) | 65 (39, 78) | 0.31 |
| Post-treatment LDL | 92 (89, 101) | 61 (40, 74) | |
| Baseline triglycerides | 104 (94, 130) | 203 (132, 267) | 0.94 |
| Post-treatment triglycerides | 113 (109, 178) | 213 (148, 353) |
Data are displayed as median (interquartile range).
Statistical comparison of the intervention effect between groups for drug versus placebo for each trial was done using analysis of covariance to estimate the percent change. All variables were log transformed.
Discussion
CKD is associated with dysfunctional HDL, which acquires a proinflammatory and pro-oxidative phenotype that can promote adverse consequences of CKD, including cardiovascular disease risk (17,19,34–38). Our study examined whether blockade of IL-1 activity by a direct inhibitor or a receptor antagonist could improve HDL fraction function in patients with CKD stage 3–5, including individuals on maintenance hemodialysis. Compared with HDL of participants who received placebo, HDL fraction of patients with CKD who received IL-1 trap or IL-1ra had significantly improved cellular anti-inflammatory capacity, reflected by reduced mRNA expression of cytokines and NLRP3. Additionally, there was an amelioration of enhanced oxidant effects of HDL, reflected by reduced superoxide production in LPS-stimulated macrophages of the treated patients. Lipid handling, assessed by net cholesterol efflux capacity of HDL, was not affected by either intervention, compared with placebo.
Although multiple epidemiologic studies have firmly established the inverse relationship between HDL cholesterol concentration and cardiovascular disease risk, treatments that raise HDL cholesterol concentration have not reduced cardiovascular events (11,12,39). These observations have given rise to the new concept that HDL function is a better predictor of risk than HDL concentration (21). Although these results have stimulated intense interest in factors affecting HDL functionality (13–15,40), it is currently uncertain which particular HDL functionality or panel of functionalities is most important, and some of the pleiotropic actions of HDL are under intense investigation. Nonetheless, recent reports indicate that beneficial functions of HDL can indeed be restored (7,25,41,42). Anti-inflammatory treatment of patients with rheumatoid arthritis with methotrexate and infliximab improved HDL-directed functions in endothelial cells, including nitric oxide bioavailability and superoxide production (42). These results are highly relevant to patients with CKD who have consistently demonstrated HDL dysfunction (16–21), and have a high prevalence of chronic noninfectious inflammation, which contributes to their cardiovascular risk (1,27,28,40). Whether HDL functionality can be improved in this high-risk population is unknown.
To test the hypothesis that IL-1β blockade can improve HDL functionality in the setting of advanced CKD, our study used HDL fraction isolated from participants of two RCTs (stages 3 and 4 CKD and maintenance hemodialysis) (5,6). IL-1 blockade reduced macrophage expression of IL-6 and TNF-α exposed to the HDL fraction of treated participants. Thus, despite different levels of kidney function (stages 3 and 4 CKD and maintenance hemodialysis), different IL-1 inhibitors (IL-1 trap and IL-1ra), and different duration of treatment (12 weeks in CKD and 4 weeks in maintenance hemodialysis), IL-1 blockade effectively improved HDL fraction anti-inflammatory actions. In addition, HDL fraction of patients treated with IL-1 blockade also had blunted cellular superoxide production in LPS-exposed macrophages compared with exposure to HDL fraction in the placebo-treated group of each study. These results complement our previous observations that anti-inflammatory therapy targeting IL-1β benefits vascular function in patients with CKD stages 3 and 4, including improved brachial artery flow-mediated dilation, an index of impairment of endothelium-dependent dilation, and endothelial cell NADPH oxidase expression (6). We have also observed increased levels of adiponectin in both studies, suggesting that the metabolic benefits of IL-1 blockade is remarkably consistent and broad (4,5). Because patients with advanced CKD requiring dialysis do not consistently respond to conventional lipid-lowering treatments (43,44), these results suggest that cytokine-based therapy could represent a novel, complementary, nonlipid-lowering intervention to reduce the high cardiovascular disease risk in this vulnerable population.
The mechanisms by which blocking the actions of IL-1 leads to improvements in HDL fraction functionality in the setting of advanced CKD are unclear. The current understanding involves a signaling cascade that moves upstream from C-reactive protein to IL-6 to IL-1 (28,40,45). Our previously reported findings of reduced circulating hsCRP and IL-6, together with blunted cellular IL-6 response to HDL with IL-1 inhibitor treatment, fits well with this pathway. Critically, IL-1β is controlled by a cytosolic multiprotein complex, the inflammasome, which includes NLRP3. HDL can downregulate NLRP3, which in turn reduces secretion of IL-1β (46). It is therefore possible that the interaction between the inflammasome and HDL is abnormal when HDL is dysfunctional, as is the case in the CKD population. In particular, because activation of NLRP3 depends on the production and binding of reactive oxygen species to NLRP3, decreasing reactive oxygen species can inhibit the NLRP3 inflammasome. Our findings that IL-1 blockade significantly reduces cellular reactive oxygen species production by HDL fraction and lowers NLRP3 expression in patients with CKD and patients on maintenance hemodialysis are consistent with this possibility. These results reiterate the central role played by the inflammasome and support the growing interest in directly targeting NLRP3 to treat atherosclerosis (2,3,28,40,45).
In contrast to the beneficial effects on the anti-inflammatory and antioxidant capacity of HDL, IL-1 blockade did not influence net cholesterol efflux capacity. It is possible that a larger sample size is needed to evaluate if IL-1 trap or IL-1ra improves cholesterol efflux capacity. However, we previously observed this lack of a parallel response between efflux capacity and heightened inflammatory response in patients on maintenance hemodialysis, as well as children with moderate CKD or ESKD requiring dialysis (18,23). Studies in other populations also reiterate that HDL net efflux capacity and other vasoprotective functions are not necessarily linked (42,47). These observations suggest that anti-inflammatory therapies have a greater effect on the vascular functions involving inflammation and oxidative stress than lipid-handling functions of HDL. Indeed, several studies have shown HDL-associated oxidant stress markers correlate with clinical outcomes in the CKD population (19,48).
It is worth emphasizing that IL-1 inhibition did not improve the plasma lipid profile in our study in CKD stages 3 and 4. We did observe an effect on HDL in patients on maintenance hemodialysis (study B) driven by a decrease in the HDL levels in the placebo arm. Notably, however, in the much longer Canakinumab Anti-inflammatory Thrombosis Outcomes Study trial (1875 patients with GFR<60 ml/min followed for 48 months of canakinumab in three different doses), there was no effect on plasma lipids (HDL and LDL) (2,27). However, canakinumab significantly reduced hsCRP and significantly lowered cardiovascular events compared with placebo, even in patients with CKD and in the absence of any effects on atherogenic lipids (2,27). Indeed, systemic inflammation, which prevails at all stages of CKD, may be highly relevant to the recently advanced “inflammatory hypothesis of atherosclerotic cardiovascular disease” (2,27). These observations represent a departure from the previous focus on increasing the levels of circulating HDL to targeting HDL function with metabolic benefits of HDL (7,25,41).
HDL has well described benefits to protect the endothelium. In patients with CKD, HDL strongly inhibits nitric oxide production, promotes superoxide production, and reduces HDL capacity to protect endothelial cells against monocyte adhesion molecules (49). Unfortunately, we were not able to test the effect of HDL fraction on these parameters before and after the intervention. Nonetheless, in study A, IL-1 blockade improved endothelial function measured as brachial artery flow-mediated dilation, suggesting a possible role for HDL in this response (6).
Our study has several strengths, including the randomized, placebo-controlled trial design of the parent studies and drug administration performed under direct supervision. The experiments in this study were all done in a blinded fashion, albeit in a post hoc analysis without a specific a priori power analysis. In addition, the effectiveness of IL-1 blockade in reducing the systemic inflammatory response paralleled the effectiveness in improving HDL functionality across a spectrum of CKD (i.e., stages 3–5, including individuals on maintenance hemodialysis). Our study also has several limitations. Both parent studies were short term, mechanistic in nature, and both had relatively small sample sizes consistent with the power calculations needed for the primary outcomes of the parent studies. Accordingly, we were underpowered for some of the measurements and we assessed multiple markers in several pathways generating multiple comparisons. These studies were performed in patients with mild to moderate chronic inflammation and may not be generalizable to patients that are not inflamed. However, inflammation is highly prevalent in both patients with CKD and patients with ESKD. The study lacked the ability to examine the association with hard end points such as cardiovascular events or mortality, as well as safety parameters such as increased risk of infections and thrombocytopenia, as was observed in the canakinumab trial. Notably, we observed two episodes of infection, three injection site reactions, and one episode of thrombocytopenia. Finally, there is no universally accepted standard method of HDL isolation or functional assay, and the in vitro methodology used in our study may not completely recapitulate the in vivo effects of disease on HDL composition, metabolism, or macrophage function (26). However, a number of clinical studies used this approach to assay cholesterol efflux capacity and the anti-inflammatory functions of HDL, and these measures of HDL function have been shown to add independent incremental value in cardiovascular disease risk prediction models (14,15). Indeed, the apoB-depleted serum fraction is the most often used method in clinical studies to assay cholesterol efflux capacity and anti-inflammatory functions. The efflux assay relies on measuring cell cholesterol mass, which assures that the net flux of cholesterol is assessed, thereby controlling for the influx side (i.e., free cholesterol influx, cholesteryl esters selective uptake, HDL uptake, and degradation) that may differ between populations. Nonetheless, the in vitro method has inherent limitations, including that it does not precisely reflect the in vivo HDL metabolism or macrophage function.
In conclusion, our results suggest that IL-1 blockade improves HDL-mediated anti-inflammatory and antioxidant function in patients with stage 3–5 CKD, including individuals on maintenance hemodialysis. These findings suggest potential utility and possible mechanisms in antagonizing IL-1 in this population to reduce the atherosclerotic burden and improve cardiovascular outcomes in the setting of moderate to advanced CKD. Larger studies of longer duration are required to confirm our study findings and evaluate the effects of these interventions in cardiovascular morbidity and mortality in patients with moderate to advanced CKD.
Disclosures
Dr. Chonchol reports grants from American Heart Association, during the conduct of the study; grants and personal fees from Corvidia, personal fees from Amgen, grants and personal fees from Genzyme, grants from Otsuka, outside the submitted work. Dr. Ikizler reports grants and nonfinancial support from Sobi Pharmaceuticals, outside the submitted work. Dr. Linton reports grants from Regeneron, grants from Sanofi, grants from Regenxbio, grants from National Institutes of Health, other from Amgen, grants from Merck, outside the submitted work. In addition, Dr. Linton has a patent, Dicarbonyl Scavengers, for the prevention of atherosclerosis pending. Dr. Nowak reports grants from American Heart Association, grants and nonfinancial support from Regeneron Pharmaceuticals, during the conduct of the study. Dr. Hung reports grants from the Department of Veterans Affairs and National Institute of Diabetes and Digestive and Kidney Diseases, and non-financial support from Regeneron. Dr. Dikalova, Dr. Huang, Dr. Kon, Dr. Salas, Dr. Sarkar, Dr. Tsuchida, Dr. Whitfield, and Dr. Yancey have nothing to disclose.
Acknowledgments
In study A, the study drug and matching placebo were kindly provided by Regeneron Pharmaceuticals (Tarrytown, NY). In study B, the study drug (anakinra) and matching placebo were kindly provided by Amgen (Thousand Oaks, CA).
Study A: This work and Dr. Hung were supported by a Department of Veterans Affairs Clinical Science Research & Development (CSR&D) Service Office merit review award “Dysmetabolism of CKD and Vascular Health” (CX000982 to Dr. Hung). The parent studies were supported by career development award “Inflammation in CKD and CVD—the Role of Genetics and IL-1ra” (CDA-CSR&D 2-031-09S to Dr. Hung) and by an American Heart Association postdoctoral fellowship award (12POST11920023 to Dr. Nowak). Study B: This work was supported in part by grant R21 DK077373 from the National Institute of Diabetes, Digestive and Kidney Diseases (to Dr. Hung and Dr. Ikizler). Additional support was provided by the National Center for Advancing Translational Sciences (grants UL1 TR000445 and UL1 RR025780) and the National Heart, Lung and Blood Institute (grant P01HL116263 to Dr. Kon, Dr. Ikizler, and Dr. Linton). Dr. Ikizler was supported in part by the CSR&D merit grant 1I01CX000414.
The sponsors had no influence on the design, execution, and analysis of the results of these trials.
Because Dr. Chonchol is a Deputy Editor of the Clinical Journal of the American Society of Nephrology, he was not involved in the peer review process for this manuscript. Another editor oversaw the peer review and decision-making process for this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimbürger O, Massy Z: Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: How do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol 3: 505–521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group : Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N Engl J Med 377: 1119–1131, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Abbate A, Van Tassell BW, Biondi-Zoccai G, Kontos MC, Grizzard JD, Spillman DW, Oddi C, Roberts CS, Melchior RD, Mueller GH, Abouzaki NA, Rengel LR, Varma A, Gambill ML, Falcao RA, Voelkel NF, Dinarello CA, Vetrovec GW: Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study]. Am J Cardiol 111: 1394–1400, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hung AM, Limkunakul C, Placido JS, Siew ED, Ellis CD, Shintani A, Ikizler TA: Administration of IL-1ra improves adiponectin levels in chronic hemodialysis patients. J Nephrol 27: 681–688, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Hung AM, Ellis CD, Shintani A, Booker C, Ikizler TA: IL-1β receptor antagonist reduces inflammation in hemodialysis patients. J Am Soc Nephrol 22: 437–442, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nowak KL, Chonchol M, Ikizler TA, Farmer-Bailey H, Salas N, Chaudhry R, Wang W, Smits G, Tengesdal I, Dinarello CA, Hung AM: IL-1 inhibition and vascular function in CKD. J Am Soc Nephrol 28: 971–980, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao KP, Playford MP, Frits M, Coblyn JS, Iannaccone C, Weinblatt ME, Shadick NS, Mehta NN: The association between reduction in inflammation and changes in lipoprotein levels and HDL cholesterol efflux capacity in rheumatoid arthritis. J Am Heart Assoc 4, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Annema W, von Eckardstein A: High-density lipoproteins. Multifunctional but vulnerable protections from atherosclerosis. Circ J 77: 2432–2448, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Soran H, Hama S, Yadav R, Durrington PN: HDL functionality. Curr Opin Lipidol 23: 353–366, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Williams KJ: What does HDL do? A new mechanism to slow atherogenesis--but a new problem in type 2 diabetes mellitus. Atherosclerosis 225: 36–38, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Castelli WP, Anderson K: A population at risk. Prevalence of high cholesterol levels in hypertensive patients in the Framingham Study. Am J Med 80[2A]: 23–32, 1986 [DOI] [PubMed] [Google Scholar]
- 12.Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J; Emerging Risk Factors Collaboration : Major lipids, apolipoproteins, and risk of vascular disease. JAMA 302: 1993–2000, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, Lukmanova D, Mucksavage ML, Luben R, Billheimer J, Kastelein JJ, Boekholdt SM, Khaw KT, Wareham N, Rader DJ: Association of HDL cholesterol efflux capacity with incident coronary heart disease events: A prospective case-control study. Lancet Diabetes Endocrinol 3: 507–513, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW: HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med 371: 2383–2393, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ: Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 364: 127–135, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaziri ND: HDL abnormalities in nephrotic syndrome and chronic kidney disease. Nat Rev Nephrol 12: 37–47, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Speer T, Rohrer L, Blyszczuk P, Shroff R, Kuschnerus K, Kränkel N, Kania G, Zewinger S, Akhmedov A, Shi Y, Martin T, Perisa D, Winnik S, Müller MF, Sester U, Wernicke G, Jung A, Gutteck U, Eriksson U, Geisel J, Deanfield J, von Eckardstein A, Lüscher TF, Fliser D, Bahlmann FH, Landmesser U: Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity 38: 754–768, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Kaseda R, Jabs K, Hunley TE, Jones D, Bian A, Allen RM, Vickers KC, Yancey PG, Linton MF, Fazio S, Kon V: Dysfunctional high-density lipoproteins in children with chronic kidney disease. Metabolism 64: 263–273, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalantar-Zadeh K, Kopple JD, Kamranpour N, Fogelman AM, Navab M: HDL-inflammatory index correlates with poor outcome in hemodialysis patients. Kidney Int 72: 1149–1156, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Holzer M, Birner-Gruenberger R, Stojakovic T, El-Gamal D, Binder V, Wadsack C, Heinemann A, Marsche G: Uremia alters HDL composition and function. J Am Soc Nephrol 22: 1631–1641, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kronenberg F: HDL in CKD-the devil is in the detail. J Am Soc Nephrol 29: 1356–1371, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kekulawala JR, Murphy A, D’Souza W, Wai C, Chin-Dusting J, Kingwell B, Sviridov D, Mukhamedova N: Impact of freezing on high-density lipoprotein functionality. Anal Biochem 379: 213–215, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto S, Yancey PG, Ikizler TA, Jerome WG, Kaseda R, Cox B, Bian A, Shintani A, Fogo AB, Linton MF, Fazio S, Kon V: Dysfunctional high-density lipoprotein in patients on chronic hemodialysis. J Am Coll Cardiol 60: 2372–2379, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH: The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol 30: 796–801, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ormseth MJ, Yancey PG, Solus JF, Bridges SL Jr., Curtis JR, Linton MF, Fazio S, Davies SS, Roberts LJ 2nd, Vickers KC, Kon V, Michael Stein C; TETRAD Investigators : Effect of drug therapy on net cholesterol efflux capacity of high-density lipoprotein-enriched serum in rheumatoid arthritis. Arthritis Rheumatol 68: 2099–2105, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anastasius M, Kockx M, Jessup W, Sullivan D, Rye KA, Kritharides L: Cholesterol efflux capacity: An introduction for clinicians. Am Heart J 180: 54–63, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Ridker PM, MacFadyen JG, Glynn RJ, Koenig W, Libby P, Everett BM, Lefkowitz M, Thuren T, Cornel JH: Inhibition of interleukin-1β by canakinumab and cardiovascular outcomes in patients with Chronic Kidney disease. J Am Coll Cardiol 71: 2405–2414, 2018 [DOI] [PubMed] [Google Scholar]
- 28.Ridker PM, Lüscher TF: Anti-inflammatory therapies for cardiovascular disease. Eur Heart J 35: 1782–1791, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI: Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107: 106–116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yancey PG, Jerome WG, Yu H, Griffin EE, Cox BE, Babaev VR, Fazio S, Linton MF: Severely altered cholesterol homeostasis in macrophages lacking apoE and SR-BI. J Lipid Res 48: 1140–1149, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Robinet P, Wang Z, Hazen SL, Smith JD: A simple and sensitive enzymatic method for cholesterol quantification in macrophages and foam cells. J Lipid Res 51: 3364–3369, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sankaranarayanan S, de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Weibel GL, Rothblat GH: Importance of macrophage cholesterol content on the flux of cholesterol mass. J Lipid Res 51: 3243–3249, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Breukelen GJ: ANCOVA versus CHANGE from baseline in nonrandomized studies: The difference. Multivariate Behav Res 48: 895–922, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Zewinger S, Speer T, Kleber ME, Scharnagl H, Woitas R, Lepper PM, Pfahler K, Seiler S, Heine GH, März W, Silbernagel G, Fliser D: HDL cholesterol is not associated with lower mortality in patients with kidney dysfunction. J Am Soc Nephrol 25: 1073–1082, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tölle M, Pawlak A, Schuchardt M, Kawamura A, Tietge UJ, Lorkowski S, Keul P, Assmann G, Chun J, Levkau B, van der Giet M, Nofer JR: HDL-associated lysosphingolipids inhibit NAD(P)H oxidase-dependent monocyte chemoattractant protein-1 production. Arterioscler Thromb Vasc Biol 28: 1542–1548, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tölle M, Huang T, Schuchardt M, Jankowski V, Prüfer N, Jankowski J, Tietge UJ, Zidek W, van der Giet M: High-density lipoprotein loses its anti-inflammatory capacity by accumulation of pro-inflammatory-serum amyloid A. Cardiovasc Res 94: 154–162, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Navab M, Anantharamaiah GM, Fogelman AM: The effect of apolipoprotein mimetic peptides in inflammatory disorders other than atherosclerosis. Trends Cardiovasc Med 18: 61–66, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morena M, Cristol JP, Dantoine T, Carbonneau MA, Descomps B, Canaud B: Protective effects of high-density lipoprotein against oxidative stress are impaired in haemodialysis patients. Nephrol Dial Transplant 15: 389–395, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Lincoff AM, Nicholls SJ, Riesmeyer JS, Barter PJ, Brewer HB, Fox KAA, Gibson CM, Granger C, Menon V, Montalescot G, Rader D, Tall AR, McErlean E, Wolski K, Ruotolo G, Vangerow B, Weerakkody G, Goodman SG, Conde D, McGuire DK, Nicolau JC, Leiva-Pons JL, Pesant Y, Li W, Kandath D, Kouz S, Tahirkheli N, Mason D, Nissen SE; ACCELERATE Investigators : Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med 376: 1933–1942, 2017 [DOI] [PubMed] [Google Scholar]
- 40.Ridker PM: How common is residual inflammatory risk? Circ Res 120: 617–619, 2017 [DOI] [PubMed] [Google Scholar]
- 41.Ronda N, Greco D, Adorni MP, Zimetti F, Favari E, Hjeltnes G, Mikkelsen K, Borghi MO, Favalli EG, Gatti R, Hollan I, Meroni PL, Bernini F: Newly identified antiatherosclerotic activity of methotrexate and adalimumab: Complementary effects on lipoprotein function and macrophage cholesterol metabolism. Arthritis Rheumatol 67: 1155–1164, 2015 [DOI] [PubMed] [Google Scholar]
- 42.O’Neill F, Charakida M, Topham E, McLoughlin E, Patel N, Sutill E, Kay CWM, D’Aiuto F, Landmesser U, Taylor PC, Deanfield J: Anti-inflammatory treatment improves high-density lipoprotein function in rheumatoid arthritis. Heart 103: 766–773, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, Ritz E; German Diabetes and Dialysis Study Investigators : Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 353: 238–248, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellström B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Grönhagen-Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R; SHARP Investigators : The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (study of heart and renal protection): A randomised placebo-controlled trial. Lancet 377: 2181–2192, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abbate A, Dinarello CA: Anti-inflammatory therapies in acute coronary syndromes: Is IL-1 blockade a solution? Eur Heart J 36: 337–339, 2015 [DOI] [PubMed] [Google Scholar]
- 46.Thacker SG, Zarzour A, Chen Y, Alcicek MS, Freeman LA, Sviridov DO, Demosky SJ Jr., Remaley AT: High-density lipoprotein reduces inflammation from cholesterol crystals by inhibiting inflammasome activation. Immunology 149: 306–319, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh N, Jacobs F, Rader DJ, Vanhaecke J, Van Cleemput J, De Geest B: Impaired cholesterol efflux capacity and vasculoprotective function of high-density lipoprotein in heart transplant recipients. J Heart Lung Transplant 33: 499–506, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Honda H, Ueda M, Kojima S, Mashiba S, Michihata T, Takahashi K, Shishido K, Akizawa T: Oxidized high-density lipoprotein as a risk factor for cardiovascular events in prevalent hemodialysis patients. Atherosclerosis 220: 493–501, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Shroff R, Speer T, Colin S, Charakida M, Zewinger S, Staels B, Chinetti-Gbaguidi G, Hettrich I, Rohrer L, O’Neill F, McLoughlin E, Long D, Shanahan CM, Landmesser U, Fliser D, Deanfield JE: HDL in children with CKD promotes endothelial dysfunction and an abnormal vascular phenotype. J Am Soc Nephrol 25: 2658–2668, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]