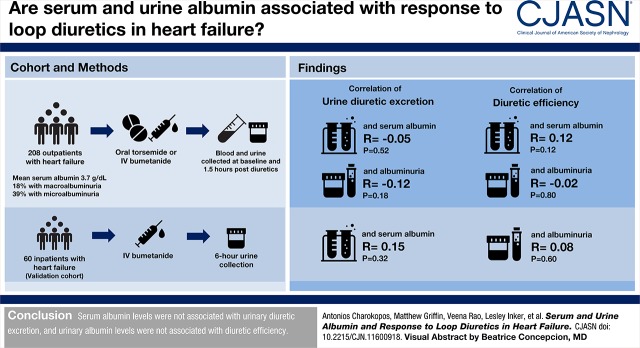
Visual Abstract
Keywords: diuretics; hypoalbuminemia; IL6 protein, human; Interleukin-6; albuminuria; Serum Albumin; creatinine; Sodium; Transitional Care; Sodium Potassium Chloride Symporter Inhibitors; Inpatients; Urinalysis; heart failure; Plasma; Inflammation; Cohort Studies
Abstract
Background and objectives
Diuretic resistance can limit successful decongestion of patients with heart failure. Because loop diuretics tightly bind albumin, low serum albumin and high urine albumin can theoretically limit diuretic delivery to the site of action. However, it is unknown if this represents a clinically relevant mechanism of diuretic resistance in human heart failure.
Design, setting, participants, & measurements
In total, 208 outpatients with heart failure at the Yale Transitional Care Center undergoing diuretic treatment were studied. Blood and urine chemistries were collected at baseline and 1.5 hours postdiuretic administration. Urine diuretic levels were normalized to urine creatinine and adjusted for diuretic dose administered, and diuretic efficiency was calculated as sodium output per doubling of the loop diuretic dose. Findings were validated in an inpatient heart failure cohort (n=60).
Results
Serum albumin levels in the outpatient cohort ranged from 2.4 to 4.9 g/dl, with a median of 3.7 g/dl (interquartile range, 3.5–4.1). Serum albumin had no association with urinary diuretic delivery (r=−0.05; P=0.52), but higher levels weakly correlated with better diuretic efficiency (r=0.17; P=0.02). However, serum albumin inversely correlated with systemic inflammation as assessed by plasma IL-6 (r=−0.35; P<0.001), and controlling for IL-6 eliminated the diuretic efficiency-serum albumin association (r=0.12; P=0.12). In the inpatient cohort, there was no association between serum albumin and urinary diuretic excretion (r=0.15; P=0.32) or diuretic efficiency (r=−0.16; P=0.25). In the outpatient cohort, 39% of patients had microalbuminuria, and 18% had macroalbuminuria. There was no correlation between albuminuria and diuretic efficiency after adjusting for kidney function (r=−0.02; P=0.89). Results were similar in the inpatient cohort.
Conclusions
Serum albumin levels were not associated with urinary diuretic excretion, and urinary albumin levels were not associated with diuretic efficiency.
Introduction
Loop diuretics are the foundation of decongestive therapy in heart failure (1). Resistance to loop diuretics is frequently encountered in patients with heart failure, and it is associated with poor outcomes (2–4). The underlying mechanisms of diuretic resistance in the contemporary heart failure population are not well understood. One mechanism described in nonheart failure populations involves reduction in serum albumin and/or an increase in urinary albumin. Because loop diuretics are 95%–99% albumin bound, changes in serum albumin levels can affect loop diuretic pharmacokinetics (5,6). Serum albumin levels dictate the volume of distribution of a loop diuretic; thus, diuretic delivery to the site of action should be proportional to the serum albumin level (5,7–10). The high-affinity binding between albumin and loop diuretics also suggests that increased urinary albumin in the tubular fluid might prevent the loop diuretic from binding its site of action (6,11–14)
These effects of abnormal serum and urinary albumin levels are biologically plausible but are not yet proven to be clinically relevant in human heart failure, particularly at concentrations of serum and urine albumin routinely encountered in clinical practice (15–17). Furthermore, at the relatively high doses of loop diuretic often administered to patients with heart failure, it is unclear if small changes in diuretic delivery or availability would be clinically relevant given that the majority of loop diuretic resistance in heart failure seems to be tubular in origin (18,19). The purpose of this study was to examine the effect of hypoalbuminemia and albuminuria on diuretic responsiveness. We hypothesize that aberrations in serum and urine albumin levels will not be strongly associated with diuretic resistance in contemporary patients with heart failure.
Materials and Methods
Study Design
All patients provided written informed consent, and the study was approved by the Yale Institutional Review Board.
Outpatient Cohort.
The main cohort of this study was composed of 208 consecutive outpatients with heart failure treated in the Yale Transitional Care Center who were prospectively enrolled. The Yale Transitional Care Center is an outpatient multidisciplinary heart failure center that focuses on volume management of ambulatory patients seen in posthospital follow-up or as referrals for specialty treatment (18). Patients were instructed to hold their morning diuretic dose. If the provider determined that the patients were euvolemic, they received oral torsemide (at a dose equivalent to their home loop diuretic dose) for “diuretic resistance testing.” If the provider determined that the patients were volume overloaded, they received intravenous bumetanide at a dose chosen by the provider. After the acquisition of baseline vitals and serum and urine chemistries, patient urine output was closely monitored and recorded. Repeat blood and urine samples were collected at the time of peak diuresis (approximately 1.5 hours after diuretic administration). Urine diuretic concentrations of bumetanide or torsemide were measured in these repeat samples.
Inpatient Cohort.
A total of 60 inpatients admitted with acute decompensated heart failure to the cardiology service at Yale New Haven Hospital who required treatment with intravenous loop diuretics were used to validate our findings. Exclusion criteria were known bladder dysfunction, incontinence, or inability to comply with timed urine. Enrollment could occur at any time during hospitalization. Before receiving intravenous bumetanide at a dose determined by the treating physician, all patients were asked to completely empty their bladders. After administration, there was a timed 6-hour urine collection closely supervised by study staff. The cumulative urine produced was collected, and the collection was terminated with a forced void after 6 hours. Urine chemistries and urine concentration of bumetanide were measured from the cumulative sample. We chose a 6-hour collection period on the basis of prior data showing that natriuresis from a dose of intravenous bumetanide or furosemide is complete within that timeframe (20). In order to obtain more serum albumin low-values, we also utilized a second inpatient cohort in our Supplemental Scatterplots (Supplemental Material).
Assays
Urine and serum electrolytes were measured on a Randox RxDaytona automated clinical chemistry analyzer using ion-selective electrodes (Randox Laboratories). Commercially available urine and serum controls levels 2 and 3 from Randox Laboratories were run with each batch of samples analyzed. Lot-specific ranges were assigned to these controls, and the results were monitored to ensure that the values are within the mean±2-SD range. Bumetanide and torsemide in urine were measured using liquid chromatography mass spectrometry. Ultrahigh performance liquid chromatography was performed on the Agilent Infinity 1290 UPLC system. Chromatographic separation was achieved on the Zorbax Bonus RP 2.1×50-mm, 1.8-μm column at the flow rate of 0.6 ml/min, and the total run time was 10 minutes. The mobile phase consisted of 0.1% formic acid (Buffer A) and 80% acetonitrile in 0.1% formic acid (Buffer B). Mass spectrometry was performed on the Agilent Q-TOF system (Agilent). Detection was performed in positive ion mode. Urine samples were diluted tenfold with 0.1% formic acid, and 5 μl of the sample was injected on the column. Stock solution of torsemide was prepared in 100% DMSO at a concentration of 2 mg/ml. Bumetanide supplied as 0.25 mg bumetanide (intravenous) compounded with 0.85% sodium chloride and 0.4% ammonium acetate as buffers, 0.01% EDTA disodium, and 1% benzyl alcohol as preservative that was pH adjusted was used. The combined highest standard of 2000 ng/ml bumetanide and torsemide was prepared in 100% methanol, and this was further serially diluted to obtain 1000, 500, 250, 125, 62.5, 31.5 15.75, and 6.25 ng/ml. All samples and standards were run in duplicate, and the within-assay coefficient of variability was <5%. Quantitation was carried out using the Agilent MassHunter software.
Definitions and Calculations
Albuminuria was quantified by the albumin-to-creatinine ratio (ACR) (21,22). The Chronic Kidney Disease Epidemiology Collaboration equation was used to calculate eGFR (23). Loop diuretic doses were converted to furosemide equivalents with 1 mg bumetanide=20 mg torsemide=40 mg intravenous furosemide=80 mg oral furosemide. Diuretic efficiency was defined as the total sodium excreted per doubling of the diuretic dose as previously described (18,19,24). To account for the log-linear relationship between diuretic dose and sodium output, diuretic efficiency was calculated as the increase in sodium output per doubling of the loop diuretic dose centered on a dose of 40 mg of intravenous furosemide equivalents:
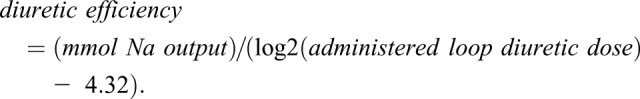 |
Doses ≤20 mg were winsorized to 40 mg to avoid negative numbers (18,19,24).
In our outpatient cohort, to approximate the concentration of diuretic reaching the luminal target, we used the ratio of loop diuretic to creatinine in the spot urine sample (expressed as nanograms of drug to milligrams of creatinine) at 1.5 hours postdiuretic administration. This value was divided by the dose of loop diuretic, and it was termed dose-adjusted estimated urine diuretic excretion. To allow for comparison across patients receiving different diuretics, urinary diuretic excretion was normalized to administered furosemide equivalents by dividing the quantity of loop diuretic in the urine by the published urinary clearance of the drug (50% for bumetanide and 17% for torsemide) (25,26) and then, converting to furosemide equivalents. In our inpatient cohort, we measured absolute urinary bumetanide clearance in the total urine collected over 6 hours. We termed this value dose-adjusted measured urine diuretic excretion.
Statistical Analyses
Values are reported mean±SD if continuous or count (percentage) if categorical. The t test or the Mann–Whitney U test was used if variables were continuous, or the chi-squared test was used if they were categorical variables. Correlation coefficients reported are Spearman rho in the case of comparisons of two continuous variables. To adjust for other variables that may confound the association between two linear variables, partial Pearson correlations were used. Statistical significance was defined as two-tailed P<0.05. Statistical analysis was performed with IBM SPSS Statistics, version 24 (IBM Corp.). Missing values for serum albumin, albuminuria as measured by ACR, and diuretic efficiency are presented in Consort diagrams for the outpatient and inpatient cohorts (Supplemental Figure 1).
Results
Table 1 describes the baseline characteristics of the outpatient cohort stratified by median serum albumin level. Patients with serum albumin below the 50th percentile had higher levels of albuminuria (median ACR, 47 and 30 mg/g, respectively; P=0.01) and IL-6 (median, 2.6 and 1.7 pg/ml, respectively; P=0.001), were more likely to be treated with intravenous bumetanide rather than torsemide (66% versus 34%, respectively; P=0.02), and had lower diuretic efficiency (median, 26 and 41 mmol, respectively; P=0.02). Characteristics of the inpatient cohort are detailed in Supplemental Table 1.
Table 1.
Baseline patient characteristics according to serum albumin in the outpatient cohort
| Characteristic | Serum Albumin ≤ Median, n=99 | Serum Albumin > Median, n=94 |
|---|---|---|
| Demographics | ||
| Age, yr | 69±13 | 67±14 |
| Women, n (%) | 39 (39) | 38 (40) |
| Never smoker, n (%) | 28 (29) | 34 (36) |
| Comorbidities (%) | ||
| Diabetes | 53 (54) | 46 (49) |
| Stroke | 17 (17) | 9 (10) |
| CAD | 46 (47) | 43 (46) |
| Arrhythmia | 70 (71) | 49 (53) |
| Valvular heart disease | 24 (25) | 30 (32) |
| Ejection fraction | 44±18 | 44±16 |
| Hypertension | 81 (82) | 81 (86) |
| Sleep apnea | 33 (33) | 29 (31) |
| Baseline laboratory values | ||
| Serum albumin, g/dl | 3.4±0.3 | 4.1±0.3 |
| Urine ACR, mg/g Cr | 47 (IQR, 17–305) | 30 (IQR, 14–148) |
| Serum Cr, mg/dl | 1.6±0.8 | 1.6±0.8 |
| eGFR (CKD-EPI), ml/min per 1.73 m2 | 53±28 | 54±30 |
| BUN-to-Cr ratio | 24±9 | 23±7 |
| NT-proBNP, pg/ml | 2830 (IQR, 750–5250) | 1385 (IQR, 452–3700) |
| Serum sodium, mEq/L | 137±4 | 138±4 |
| Serum potassium, mEq/L | 4.1±0.5 | 4.2±0.5 |
| Serum chloride, mEq/L | 98±5 | 99±5 |
| Serum IL-6, pg/ml | 2.6 (IQR, 1.5–6.1) | 1.7 (IQR, 1.0–2.9) |
| Baseline medications (before study), n (%) | ||
| Aldosterone receptor antagonist | 21 (21) | 26 (28) |
| ACEI or ARB | 43 (43) | 46 (49) |
| Thiazide diuretic | 11 (11) | 12 (13) |
| Loop diuretic | 93 (94) | 89 (96) |
| Study medications | ||
| Study diuretic: bumetanide | 65 (66) | 45 (48) |
| Study diuretic: torsemide | 34 (34) | 49 (52) |
| Study loop diuretic dose (mg) in furosemide equivalents | 160 (IQR, 80–320) | 160 (IQR, 40–210) |
| Diuretic efficiency | ||
| Diuretic efficiency (mmol sodium per doubling of loop diuretic dose) | 26 (IQR, 15–43) | 41 (IQR, 15–67) |
Values reflect mean (±SD) if linearly distributed or number (percentage) if categorical. CAD, coronary artery disease; ACR, albumin-to-creatinine ratio; Cr, creatinine; IQR, interquartile range; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; NT-proBNP, N-terminal pro-brain natriuretic peptide; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Distribution of Serum Albumin Levels and Variables Associated with Serum Albumin in the Outpatient Cohort
Serum albumin levels in the outpatient cohort ranged between 2.4 and 4.9 g/dl, with a mean serum albumin of 3.7 g/dl (±0.4 SD) and a median serum albumin of 3.7 g/dl (interquartile range, 3.5–4.1). The percentage of patients with serum albumin ≤3.0 g/dl was 7%. Serum albumin correlated inversely with congestion estimated by N-terminal pro-brain natriuretic peptide (r=−0.21; P=0.004) and loop diuretic dose (r=−0.18; P=0.02). There was also an inverse relationship between albumin and the inflammatory cytokine plasma IL-6 (r=−0.35; P<0.001). There was no association of serum albumin with markers of decongestion, such as weight change, urine output, sodium output, and hemoconcentration (Table 2). There was also no association between serum albumin and eGFR (r=0.07; P=0.36), BUN (r=−0.02; P=0.83), BUN-to-creatinine ratio (r=−0.01; P=0.88), serum sodium (r=0.03; P=0.67), serum chloride (r=−0.01; P=0.92), or systolic BP (r=−0.02; P=0.77).
Table 2.
Correlation of urine and serum albumin with metrics of diuretic efficiency
| Parameters | Albuminuria (ACR) | Serum Albumin, g/dl | ||
|---|---|---|---|---|
| R | P Valuea | R | P Valuea | |
| Loop diuretic dosea | 0.25 | <0.001b | −0.18 | 0.02b |
| ΔWeight, kg | 0.15 | 0.07 | −0.10 | 0.19 |
| Urine output, ml | −0.06 | 0.50 | 0.07 | 0.33 |
| Net fluid loss, ml | −0.01 | 0.94 | 0.04 | 0.63 |
| ΔNT-proBNP, pg/ml | 0.03 | 0.78 | 0.002 | 0.98 |
| Hemoconcentration, % | 0.05 | 0.62 | 0.16 | 0.09 |
| Sodium output, mEq | −0.03 | 0.72 | 0.08 | 0.30 |
ΔWeight, urine output, and net fluid loss were calculated from the beginning to the end of the urine collection period. ΔNT-proBNP and hemoconcentration were calculated from the change in values from the beginning to peak diuresis (i.e., 1.5 hours). ACR, albumin-to-creatinine ratio; NT-proBNP, N-terminal pro-brain natriuretic peptide.
In furosemide equivalents.
Significant α <0.05.
Distribution of Albuminuria and Variables Associated with Albuminuria in the Outpatient Cohort
The prevalence of micro- and macroalbuminuria in the outpatient cohort was commensurate with prior studies (27,28); 43% of our patients had normal urinary albumin excretion (ACR=0–30 mg/g), 39% had microalbuminuria (ACR=30–300 mg/g), and 18% had macroalbuminuria (ACR≥300 mg/g). Patients with greater amounts of albuminuria were more likely to need a higher cumulative dose of loop diuretic (r=0.25; P=0.001) (Table 2). There was an inverse relationship between ACR and serum albumin levels (r=−0.24; P=0.003), and patients with a higher eGFR had lower ACR levels (r=−0.31; P<0.001). There was a positive correlation between ACR and systolic and diastolic BP (r=0.32; P<0.001 and r=0.16; P=0.05, respectively), venous congestion estimated by baseline N-terminal pro-brain natriuretic peptide (r=0.29; P<0.001), and the inflammatory cytokine IL-6 (r=0.19; P=0.03). There was no association between ACR and ejection fraction (r=0.06; P=0.44). There was also no association between ACR and markers of venous decongestion, such as urine output and hemoconcentration (Table 2).
Association of Hypoalbuminemia and Albuminuria with Diuretic Efficiency in the Outpatient Cohort
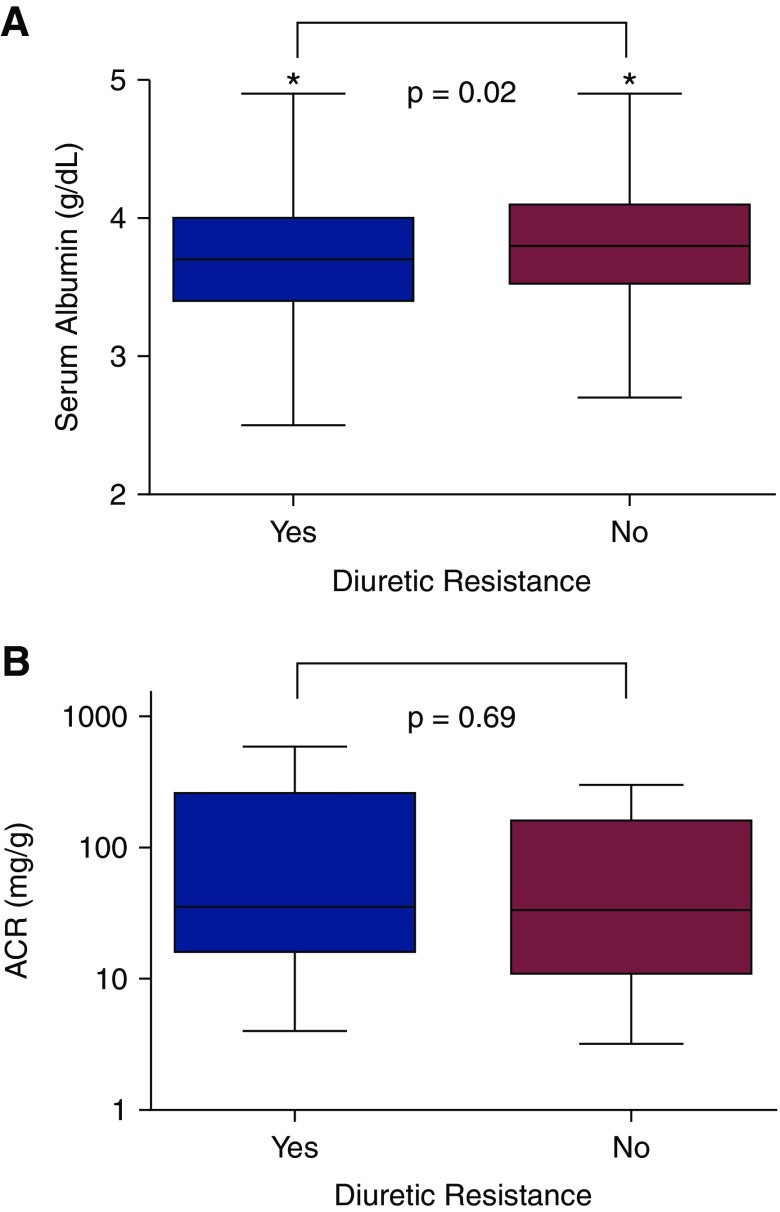
Serum albumin levels positively correlated with diuretic efficiency (r=0.17; P=0.02). Patients with higher diuretic efficiency had significantly higher serum albumin than patients who were diuretic resistant, but there was no difference in albuminuria (Figure 1). Controlling for IL-6 eliminates the association between diuretic efficiency and serum albumin (r=0.12; P=0.12). A scatterplot between the albumin and diuretic efficiency can be found in Supplemental Figure 2. There was a trend toward a negative correlation between ACR and diuretic efficiency, but this relationship did not reach statistical significance (r=−0.15; P=0.08), and it was eliminated after adjustment for eGFR (r=−0.02; P=0.80).
Figure 1.
Diuretic resistant patients (DE <50th percentile) had lower serum albumin, but no difference in albuminuria. Comparison of serum (A) and urine (B) albumin levels as stratified by median diuretic efficiency in the outpatient cohort. ACR, albumin-to-creatinine ratio. *Significant α<0.05.
Association of Hypoalbuminemia and Albuminuria with Estimated Urine Diuretic Excretion in the Outpatient Cohort
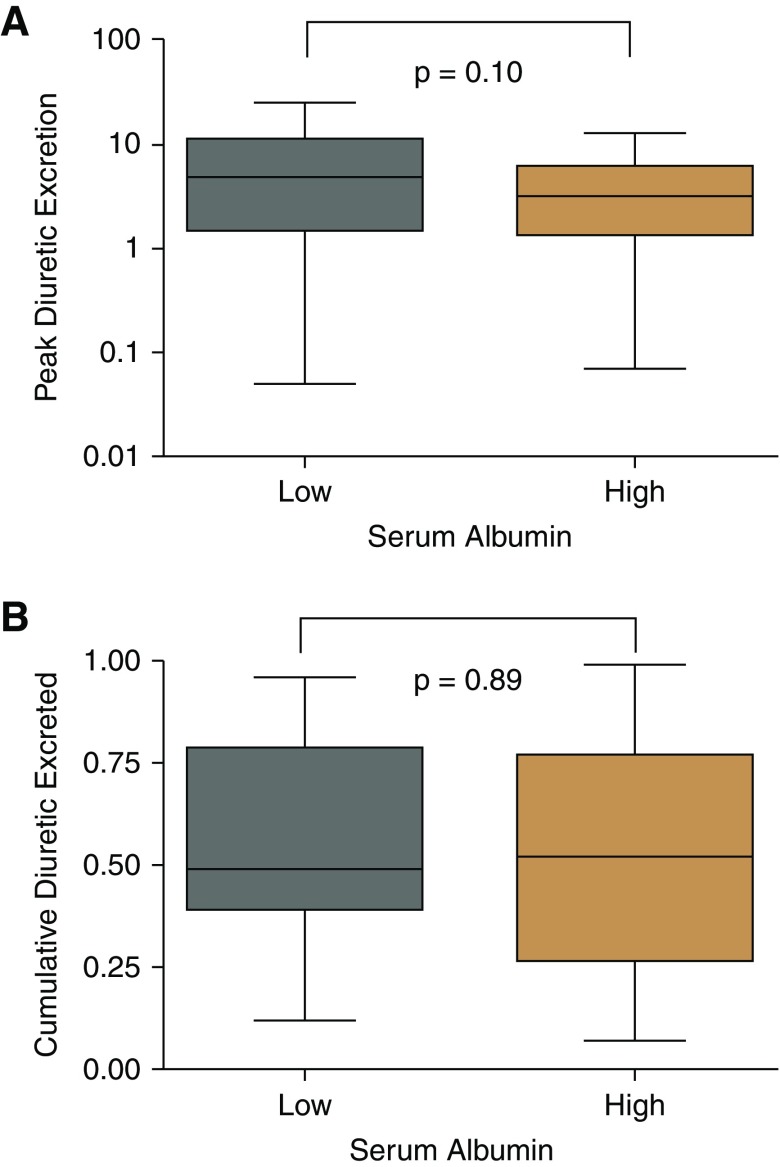
In the outpatient cohort, there was no relationship between dose-adjusted estimated loop diuretic urinary excretion and serum albumin levels (r=−0.05; P=0.52) (Figure 2A) or levels of urine albumin (r=−0.12; P=0.18).
Figure 2.
Both estimated and measured urine diuretic excretion was not significantly associated with serum albumin in the outpatient and inpatient cohorts, respectively. (A) Dose-adjusted estimated spot urine diuretic excretion (nanograms per milligram creatinine per milligram administered diuretic) as stratified by serum albumin (median, 3.7 g/dl; interquartile range, 3.5–4.1 g/dl). This was measured 1.5 hours postdiuretic administration in the outpatient cohort. (B) Dose-adjusted measured urine diuretic excretion (milligrams urine diuretic per milligram administered diuretic) as stratified by median serum albumin (low <3.6 g/dl; high ≥3.6 g/dl; interquartile range, 3.3–3.9 g/dl) in the inpatient cohort.
Replication of Findings in the Inpatient Cohort
Serum albumin levels in the inpatient cohort ranged between 2.4 and 4.6 g/dl, with a mean serum albumin of 3.5 g/dl (±0.5 SD) and a median serum albumin of 3.6 g/dl (interquartile range, 3.3–3.9). Using the albuminuria categories defined earlier, 45% of the inpatients with heart failure had normal urinary albumin excretion, 35% had microalbuminuria, and 20% had macroalbuminuria. There was no correlation between diuretic efficiency and levels of albumin in the serum (r=−0.16; P=0.25) or urine (r=0.08; P=0.60). Furthermore, there was no relationship between measured dose-adjusted urine diuretic excretion and serum albumin (r=0.15; P=0.32) (Figure 2B) or urine albumin (r=−0.20; P=0.20).
Discussion
Loop diuretics are highly albumin bound, and thus, it follows that low serum albumin levels could reduce diuretic delivery and that high urine levels could sequester diuretics from their tubular site of action. Both of these mechanisms could theoretically be a cause for diuretic resistance. However, in the two independent cohorts analyzed in this study, we found that differences in serum or urine albumin levels between patients were not meaningfully associated with diuretic responsiveness. Furthermore, serum albumin levels were not associated with the quantity of secreted diuretic. Overall, these results suggest that urine albumin and serum albumin are not associated with diuretic resistance in patients with heart failure.
The hypothesis that albumin could be associated with diuretic resistance is supported by studies that demonstrated that effective delivery of furosemide to the rat kidney is dependent on albumin binding and that infusion of an albumin-furosemide solution improved the diuretic response in both animals and humans (7,9,10). However, studies in human heart failure have not identified a clear association between serum albumin and diuretic response. (15,16). Thus, the role of serum albumin in contemporary patients with heart failure remains unclear. In our outpatient cohort, patients who were diuretic resistant tended to have lower serum albumin levels, although the magnitude of this relationship was extremely weak and eliminated by adjustment for serum IL-6 levels. Given that IL-6 levels have been shown to correlate with features of diuretic resistance and neurohormonal activation (29), it is plausible that the above findings are explained by confounding by the acute-phase reactant properties of albumin. Most importantly, in both cohorts, there was no correlation between serum albumin and urinary loop diuretic delivery, the proposed mechanism by which hypoalbuminemia would influence diuretic response. These data suggest that serum albumin levels are not associated with tubular diuretic delivery across the spectrum of albumin levels typically seen in a contemporary heart failure population.
The role of albuminuria in diuretic response has also been examined; in normal rats, using in vivo loop segment microperfusion, addition of albumin to kidney tubular fluid attenuated the response to furosemide, and inhibition of albumin-furosemide binding restored furosemide potency (12,13). In proteinuric rats, the diuretic response to furosemide correlated inversely with the degree of proteinuria independent of serum protein levels (11). It has, therefore, been proposed that, irrespective of serum albumin levels, the presence of albuminuria and resulting albumin-diuretic binding may explain the reduced diuretic effectiveness observed in patients who are albuminuric. However, we were unable to find such associations in either of the cohorts tested, despite a relatively high prevalence of albuminuria. These findings are corroborated by a study in human subjects with nephrotic syndrome, wherein despite heavy proteinuria, displacement of furosemide from albumin in the urinary space did not affect furosemide excretion (30). A possible explanation for these null findings is that the strength of loop diuretic binding to albumin is eclipsed by the far greater affinity of loop diuretics for the human sodium-potassium-chloride cotransporter. Notably, the Kd for bumetanide-albumin binding has been reported in the millimolar range (31), but the Kd for bumetanide–sodium-potassium-chloride cotransporter is in the micromolar range (32).
Our findings add to the growing body of literature examining the association of albumin on loop diuretic efficiency in heart failure. Post hoc analysis of data from the Diuretic Strategies in Patients with Acute Decompensated Heart Failure trial and the Low-Dose Dopamine or Low-Dose Nesiritide in Acute Heart Failure with Renal Dysfunction trial found no association between baseline serum albumin levels and diuretic efficiency (16), and analysis of data from the Placebo-Controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload trial demonstrated only a weak positive correlation between serum albumin and diuretic efficiency (17). These findings are concordant with the null results from our study and extend them by demonstrating that differences in serum albumin levels do not meaningfully influence urinary diuretic excretion.
The findings of our study must be interpreted in the context of several limitations. First, we measured serum albumin and urine albumin at a single timepoint, and we are, therefore, unable to track how these levels change over days of decongestive therapy. Second, both our outpatient and inpatient cohorts had relatively mild hypoalbuminemia and albuminuria, which may limit our ability to assess the degree to which extreme changes in serum or urine albumin affects diuretic delivery and response.
In conclusion, serum and urinary levels of albumin across the range of values typically found in contemporary patients with heart failure do not seem to be associated with diuretic delivery or resistance.
Disclosures
Dr. Rao has a patent Precision treatment of heart failure and cardiorenal syndrome with royalties paid to Yale University, Dr. Rao, and Dr. Testani.
Dr. Inker has a patent Precise estimation of GFR from multiple biomarkers (PCT/US2015/044567) issued; funding from Retrophin, Omeros, and Reata Pharmaceuticals for research; contracts with Tufts Medical Center; and consulting agreements with Tricida and Omeros Corp. Dr. Inker is on the medical advisory board for the Alport Syndrome Foundation. Dr. Cox reports grants from Otsuka Pharmaceuticals and grants from Cumberland Emerging Technologies outside the submitted work; in addition, Dr. Cox has a patent PCT/US16/18055 Milrinone composition and method for administering same pending. Dr. Testani reports grants and personal fees from Sequana Medical, grants and personal fees from BMS, personal fees from AZ, personal fees from Novartis, grants and personal fees from 3ive labs, personal fees from cardionomic, personal fees from Bayer, grants and personal fees from Boehringer Ingelheim, personal fees from MagentaMed, grants from Otsuka, personal fees from Renalguard, grants and personal fees from Sanofi, grants and personal fees from FIRE1, grants from Abbott, and personal fees from W.L. Gore outside the submitted work. Dr. Charokopos, Dr. Griffin, Dr. Sury, Dr. Asher, Dr. Turner, Dr. Mahoney, and Dr. Wilson have nothing to disclose.
Supplementary Material
Acknowledgments
Dr. Inker reports funding from the National Institutes of Health (NIH), the National Kidney Foundation, and grants from the Paul Teschan Research Fund outside the submitted work. This work was also supported by NIH grants K23HL114868, L30HL115790, R01HL139629, R21HL143092, R01HL128973 (to Dr. Testani), K23DK097201 (to Dr. Wilson), and 5T32HL007950 (to Dr. Griffin).
The funding sources had no role in study design, data collection, analysis, or interpretation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Mechanistic Insights into Loop Diuretic Responsiveness in Heart Failure,” on pages 650–652.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi: 10.2215/CJN.11600918/-/DCSupplemental.
Supplemental Figure 1. Consort diagrams for treatment allocation and missing values for the (A) outpatient and (B) inpatient cohorts, respectively.
Supplemental Figure 2. (A) Scatterplot of the relationship between serum albumin and diuretic efficiency in the outpatient cohort. (B) Scatterplot of the relationship between serum albumin and diuretic efficiency in a second inpatient cohort. Diuretic efficiency is expressed in millimoles of sodium per doubling of loop diuretic dose. Overall correlation between albumin and diuretic efficiency was r=0.06 (P=0.32).
Supplemental Table 1. Baseline patient characteristics according to serum albumin in the inpatient cohort. Values reflect mean (±SD) if linearly distributed or number (percentage) if categorical.
References
- 1.Ellison DH: Diuretic therapy and resistance in congestive heart failure. Cardiology 96: 132–143, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Testani JM, Brisco MA, Turner JM, Spatz ES, Bellumkonda L, Parikh CR, Tang WH: Loop diuretic efficiency: A metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ Heart Fail 7: 261–270, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh D, Shrestha K, Testani JM, Verbrugge FH, Dupont M, Mullens W, Tang WH: Insufficient natriuretic response to continuous intravenous furosemide is associated with poor long-term outcomes in acute decompensated heart failure. J Card Fail 20: 392–399, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verbrugge FH, Dupont M, Bertrand PB, Nijst P, Penders J, Dens J, Verhaert D, Vandervoort P, Tang WH, Mullens W: Determinants and impact of the natriuretic response to diuretic therapy in heart failure with reduced ejection fraction and volume overload. Acta Cardiol 70: 265–273, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Cox ZL, Lenihan DJ: Loop diuretic resistance in heart failure: Resistance etiology-based strategies to restoring diuretic efficacy. J Card Fail 20: 611–622, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Wilcox CS: New insights into diuretic use in patients with chronic renal disease. J Am Soc Nephrol 13: 798–805, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Inoue M, Okajima K, Itoh K, Ando Y, Watanabe N, Yasaka T, Nagase S, Morino Y: Mechanism of furosemide resistance in analbuminemic rats and hypoalbuminemic patients. Kidney Int 32: 198–203, 1987 [DOI] [PubMed] [Google Scholar]
- 8.Besseghir K, Mosig D, Roch-Ramel F: Facilitation by serum albumin of renal tubular secretion of organic anions. Am J Physiol 256: F475–F484, 1989 [DOI] [PubMed] [Google Scholar]
- 9.Pichette V, Geadah D, du Souich P: The influence of moderate hypoalbuminaemia on the renal metabolism and dynamics of furosemide in the rabbit. Br J Pharmacol 119: 885–890, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pichette V, Geadah D, du Souich P: Role of plasma protein binding on renal metabolism and dynamics of furosemide in the rabbit. Drug Metab Dispos 27: 81–85, 1999 [PubMed] [Google Scholar]
- 11.Green TP, Mirkin BL: Resistance of proteinuric rats to furosemide: Urinary drug protein binding as a determinant of drug effect. Life Sci 26: 623–630, 1980 [DOI] [PubMed] [Google Scholar]
- 12.Kirchner KA, Voelker JR, Brater DC: Intratubular albumin blunts the response to furosemide-A mechanism for diuretic resistance in the nephrotic syndrome. J Pharmacol Exp Ther 252: 1097–1101, 1990 [PubMed] [Google Scholar]
- 13.Kirchner KA, Voelker JR, Brater DC: Binding inhibitors restore furosemide potency in tubule fluid containing albumin. Kidney Int 40: 418–424, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Green TP, Mirkin BL: Furosemide disposition in normal and proteinuric rats: Urinary drug-protein binding as a determinant of drug excretion. J Pharmacol Exp Ther 218: 122–127, 1981 [PubMed] [Google Scholar]
- 15.Bleske BE, Clark MM, Wu AH, Dorsch MP: The effect of continuous infusion loop diuretics in patients with acute decompensated heart failure with hypoalbuminemia. J Cardiovasc Pharmacol Ther 18: 334–337, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Grodin JL, Lala A, Stevens SR, DeVore AD, Cooper LB, AbouEzzeddine OF, Mentz RJ, Groarke JD, Joyce E, Rosenthal JL, Vader JM, Tang WH: Clinical implications of serum albumin levels in acute heart failure: Insights from DOSE-AHF and ROSE-AHF. J Card Fail 22: 884–890, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ter Maaten JM, Valente MA, Metra M, Bruno N, O’Connor CM, Ponikowski P, Teerlink JR, Cotter G, Davison B, Cleland JG, Givertz MM, Bloomfield DM, Dittrich HC, van Veldhuisen DJ, Hillege HL, Damman K, Voors AA: A combined clinical and biomarker approach to predict diuretic response in acute heart failure. Clin Res Cardiol 105: 145–153, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao VS, Planavsky N, Hanberg JS, Ahmad T, Brisco-Bacik MA, Wilson FP, Jacoby D, Chen M, Tang WHW, Cherney DZI, Ellison DH, Testani JM: Compensatory distal reabsorption drives diuretic resistance in human heart failure. J Am Soc Nephrol 28: 3414–3424, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ter Maaten JM, Rao VS, Hanberg JS, Perry Wilson F, Bellumkonda L, Assefa M, Sam Broughton J, D’Ambrosi J, Wilson Tang WH, Damman K, Voors AA, Ellison DH, Testani JM: Renal tubular resistance is the primary driver for loop diuretic resistance in acute heart failure. Eur J Heart Fail 19: 1014–1022, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilcox CS, Mitch WE, Kelly RA, Skorecki K, Meyer TW, Friedman PA, Souney PF: Response of the kidney to furosemide. I. Effects of salt intake and renal compensation. J Lab Clin Med 102: 450–458, 1983 [PubMed] [Google Scholar]
- 21.Jensen JS, Clausen P, Borch-Johnsen K, Jensen G, Feldt-Rasmussen B: Detecting microalbuminuria by urinary albumin/creatinine concentration ratio. Nephrol Dial Transplant 12[Suppl 2]: 6–9, 1997 [PubMed] [Google Scholar]
- 22.Keane WF, Eknoyan G: Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): A position paper of the national kidney foundation. Am J Kidney Dis 33: 1004–1010, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanberg JS, Rao V, Ter Maaten JM, Laur O, Brisco MA, Perry Wilson F, Grodin JL, Assefa M, Samuel Broughton J, Planavsky NJ, Ahmad T, Bellumkonda L, Tang WH, Parikh CR, Testani JM: Hypochloremia and diuretic resistance in heart failure: Mechanistic insights. Circ Heart Fail 9: pii:e003180, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vargo DL, Kramer WG, Black PK, Smith WB, Serpas T, Brater DC: Bioavailability, pharmacokinetics, and pharmacodynamics of torsemide and furosemide in patients with congestive heart failure. Clin Pharmacol Ther 57: 601–609, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Ward A, Heel RC: Bumetanide. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use. Drugs 28: 426–464, 1984 [DOI] [PubMed] [Google Scholar]
- 27.Koyama S, Sato Y, Tanada Y, Fujiwara H, Takatsu Y: Early evolution and correlates of urine albumin excretion in patients presenting with acutely decompensated heart failure. Circ Heart Fail 6: 227–232, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Jackson CE, Solomon SD, Gerstein HC, Zetterstrand S, Olofsson B, Michelson EL, Granger CB, Swedberg K, Pfeffer MA, Yusuf S, McMurray JJ; CHARM Investigators and Committees : Albuminuria in chronic heart failure: Prevalence and prognostic importance. Lancet 374: 543–550, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Hanberg JS, Rao VS, Ahmad T, Chunara Z, Mahoney D, Jackson K, Jacoby D, Chen M, Wilson FP, Tang WHW, Kakkar R, Testani JM: Inflammation and cardio-renal interactions in heart failure: A potential role for interleukin-6. Eur J Heart Fail 20: 933–934, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agarwal R, Gorski JC, Sundblad K, Brater DC: Urinary protein binding does not affect response to furosemide in patients with nephrotic syndrome. J Am Soc Nephrol 11: 1100–1105, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Robertson A, Karp W: Albumin binding of bumetanide. Dev Pharmacol Ther 9: 241–248, 1986 [DOI] [PubMed] [Google Scholar]
- 32.Isenring P, Forbush B 3rd: Ion and bumetanide binding by the Na-K-Cl cotransporter. Importance of transmembrane domains. J Biol Chem 272: 24556–24562, 1997 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.