Abstract
Hepatorenal syndrome is a severe complication of end-stage cirrhosis characterized by increased splanchnic blood flow, hyperdynamic state, a state of decreased central volume, activation of vasoconstrictor systems, and extreme kidney vasoconstriction leading to decreased GFR. The contribution of systemic inflammation, a key feature of cirrhosis, in the development of hepatorenal syndrome has been highlighted in recent years. The mechanisms by which systemic inflammation precipitates kidney circulatory changes during hepatorenal syndrome need to be clarified. Early diagnosis is central in the management and recent changes in the definition of hepatorenal syndrome help identify patients at an earlier stage. Vasoconstrictive agents (terlipressin in particular) and albumin are the first-line treatment option. Several controlled studies proved that terlipressin is effective at reversing hepatorenal syndrome and may improve short-term survival. Not all patients are responders, and even in responders, early mortality rates are very high in the absence of liver transplantation. Liver transplantation is the only curative treatment of hepatorenal syndrome. In the long term, patients transplanted with hepatorenal syndrome tend to have lower GFR compared with patients without hepatorenal syndrome. Differentiating hepatorenal syndrome from acute tubular necrosis (ATN) is often a challenging yet important step because vasoconstrictors are not justified for the treatment of ATN. Hepatorenal syndrome and ATN may be considered as a continuum rather than distinct entities. Emerging biomarkers may help differentiate these two conditions and provide prognostic information on kidney recovery after liver transplantation, and potentially affect the decision for simultaneous liver–kidney transplantation.
Keywords: hepatorenal; acute kidney injury; liver transplantation; Hepatorenal Syndrome; terlipressin; Vasoconstrictor Agents; Liver Transplantation; Vasoconstriction; kidney transplantation; Prognosis; glomerular filtration rate; Lypressin; Renal Circulation; Liver Cirrhosis; Kidney Tubular Necrosis, Acute; Albumins; Inflammation; Biomarkers; Early Diagnosis
Introduction
Advanced cirrhosis is a condition characterized by impaired liver function, portal hypertension, increased splanchnic blood volume, hyperdynamic state with increased cardiac output, systemic vasodilatation, a state of decreased central blood volume, and systemic inflammatory response. AKI is one of the most severe complications of cirrhosis, occurring in up to 50% of hospitalized patients, and has been associated with higher mortality, which increases with severity of AKI (1). Hepatorenal syndrome is one of the phenotypes of AKI that occurs in patients with advanced cirrhosis and is characterized by decreased kidney blood flow that is unresponsive to volume expansion. Hepatorenal syndrome is associated with significant health care resource utilization, with an estimated annual total direct medical cost in the United States of approximately $4 billion dollars (2). Refinements in the definitions have helped in the diagnosis of hepatorenal syndrome at an earlier stage during the course of cirrhosis. Recent advances in our understanding of the pathophysiology of hepatorenal syndrome suggest the involvement of systemic inflammation and circulatory changes in the kidney in parallel with systemic and splanchnic circulatory changes. Although treatment of hepatorenal syndrome with the use of vasoconstrictive agents in combination with albumin has improved outcomes, prognosis remains poor without liver transplantation. This review, using the most recent literature, will focus on the definitions, mechanisms, and management of hepatorenal syndrome.
Definition of Hepatorenal Syndrome and AKI in Cirrhosis
The definition of AKI in cirrhosis has undergone significant changes over the past several years. The common theme among the definitions is use of relative changes in serum creatinine instead of absolute cut-offs (e.g., >1.5 mg/dl) and identifying patients at highest risk for short- and long-term mortality on the basis of the escalating stages within each criterion (3,4). In 2012, the Acute Dialysis Quality Initiative (ADQI) recommended adaptation of the AKI Network serum creatinine criteria to define AKI in this patient population (3). These criteria were irrespective of the cause of AKI and as such, hepatorenal syndrome type 1 was categorized as a specific type of AKI and hepatorenal syndrome type 2 was categorized as a form of CKD. The International Club of Ascites (ICA) further modified the definition of AKI on the basis of the Kidney Disease Improving Global Outcomes serum creatinine criteria, using a baseline serum creatinine within the previous 3 months (1,4). Although oliguria is not included in the current definition of AKI in patients with cirrhosis, urine output has been found to be a sensitive and early marker for AKI in critically ill patients with cirrhosis and is associated with adverse outcomes (5). Therefore, regardless of any rise in serum creatinine, decrease in urine output or development of anuria should be considered as AKI in patients with cirrhosis until proven otherwise.
Changes in the definition of AKI in patients with cirrhosis has led to changes in the definition of hepatorenal syndrome such that the cut-off value of serum creatinine was removed and replaced with ICA AKI criteria, allowing for earlier diagnosis and treatment of patients with hepatorenal syndrome (4). A major limitation of the hepatorenal syndrome criteria is that it does not allow for the coexistence of other forms of acute or CKD, such as underlying diabetic nephropathy or glomerular diseases often associated in patients with liver disease. Patients with underlying kidney disease can still develop “hepatorenal physiology”; thus the term “hepatorenal disorders” has been proposed by ADQI to describe all patients with advanced cirrhosis and concurrent kidney dysfunction, which would allow these patients to be properly classified and treated while maintaining the term hepatorenal syndrome (3).
Pathogenesis of Hepatorenal Syndrome
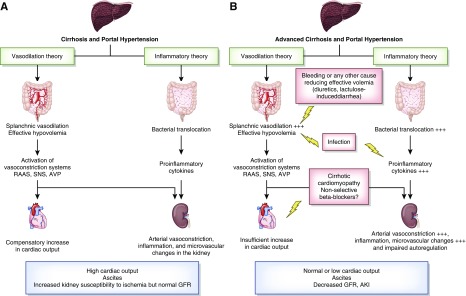
Cirrhosis is characterized by reduced systemic vascular resistance due to splanchnic arterial vasodilation (6). In early stages of the disease, splanchnic vasodilation is moderate and reduced systemic vascular resistance is balanced by increased cardiac output. In advanced stages, vasodilation is more pronounced because of increased synthesis of vasodilator factors, and cannot be balanced by the increase in cardiac output (Figure 1) (7). As a result, there is an effective arterial hypovolemia as a consequence of the disparity between the intravascular blood volume and the markedly dilated arterial circulation. Cirrhotic cardiomyopathy is a condition combining diastolic dysfunction, blunted increase in cardiac output after stimulations, and electromechanical abnormalities (7). Inflammatory response during cirrhosis with increased circulating levels of TNF-α may contribute to blunted cardiac response (8). In advanced stages of cirrhosis with ascites, decreased cardiac output seems to precede the occurrence of hepatorenal syndrome (9). Decreased cardiac output may precipitate the decline in kidney blood flow. Eventually, changes in hemodynamics in the kidney and altered autoregulation of kidney blood flow contribute to decreased GFR.
Figure 1.
Mechanisms involved in AKI in decompensated cirrhosis. (A) In decompensated cirrhosis, both vasodilation secondary to portal hypertension and systemic inflammation induced by gut bacterial translocation tend to induce kidney arterial vasoconstriction because of the activation of vasoconstrictive systems in response to decreased effective blood volume and inflammation in the kidney inducing microvascular changes. These changes result in a hyperdynamic state characterized by increased cardiac output, ascites, and normal GFR, but increase susceptibility of the kidney to AKI. (B) The onset of hepatorenal syndrome corresponds to the most advanced stages of these changes, with an intense kidney vasoconstriction and impaired kidney autoregulation leading to a decrease in GFR. Any event further decreasing hypovolemia (bleeding, diuretics overdose, lactulose-induced diarrhea), decreased cardiac output (e.g., cirrhotic cardiomyopathy, nonselective β-blockers) or systemic inflammation, with or without over sepsis, can precipitate hepatorenal syndrome. AVP, arginine vasopressin; RAAS, renin-angiotensin-aldosterone system; SNS, sympathetic nervous system.
To maintain arterial pressure, systemic vasoconstrictor systems (the renin-angiotensin-aldosterone system, sympathetic nervous system, and arginine vasopressin) are activated, which, along with increased cardiac output related to hyperdynamic state, help to preserve kidney blood flow. Although activation of these systems has positive effects by increasing arterial pressure, they result in kidney vasoconstriction, sodium retention leading to edema and ascites, and solute-free water excretion leading to hyponatremia and decreased GFR. In the most advanced stages of cirrhosis intense kidney vasoconstriction occurs and kidney perfusion is no longer compensated by increased cardiac output and GFR decreases, ultimately leading to the development of hepatorenal syndrome.
Recently, the concept of systemic inflammatory disease in cirrhosis has emerged, with growing evidence that inflammation plays a role in hepatorenal syndrome (10). Cirrhosis is associated with systemic inflammation, which correlates to the severity of liver disease and portal hypertension. The main mechanism is the translocation of bacteria and/or pathogen-associated molecular patterns from the gut due to altered intestinal permeability. Translocation induces a wide spectrum of genes encoding molecules responsible for inflammatory response via specific receptors called pattern recognition receptors (11). Toll-like receptor 4 (TLR4) is the main pattern recognition receptor that has been studied in this context. Overexpression of tubular TLR4 has been described in patients with cirrhosis and kidney dysfunction (12). A subset of patients with a diagnosis of hepatorenal syndrome showed both overexpression of TLR4 in tubular cells and evidence of tubular cell damage, suggesting that a diagnosis of hepatorenal syndrome does not exclude some degree of structural changes (12). Inflammatory components may extend to the systemic circulation and peripheral organs leading to extra hepatic organ dysfunction, including the kidney. Inflammation may contribute to systemic circulatory changes and compromised kidney perfusion. Patients with bacterial translocation have increased levels of proinflammatory cytokines (TNF-α and IL-6) as well as increased level of vasodilating factors (such as nitric oxide) (13). Bacterial infections represent a typical trigger of hepatorenal syndrome; however, about 30% of patients with hepatorenal syndrome have systemic inflammatory response syndrome without documented bacterial infection (10).
Prevention of Hepatorenal Syndrome
Strategies to prevent the development of hepatorenal syndrome include preventing progression of liver disease in the well compensated patient, reversing decompensation in patients who have advanced cirrhosis, avoiding agents known to exacerbate AKI, and preventing factors that further impair circulatory status and reduce kidney perfusion. Prophylactic antibiotics to prevent spontaneous bacterial peritonitis and, after variceal bleed, intravenous albumin in patients with spontaneous bacterial peritonitis (1.5 g/kg on day 1 followed by 1 g/kg on day 3) (14) and patients undergoing large-volume paracentesis (>5 L) (15) have been shown to decrease the incidence of hepatorenal syndrome (14). There is no evidence that albumin in addition to antibiotics reduces the incidence of AKI in patients with bacterial infection other than spontaneous bacterial peritonitis (14). In a controlled trial, long-term administration of albumin in patients with decompensated cirrhosis was associated with reduced rates of spontaneous bacterial peritonitis, hepatorenal syndrome, and improved survival (16).
β-Blockers are very effective at preventing variceal bleeding and are widely used in patients with cirrhosis and significant portal hypertension. A recent meta-analysis suggests that the use of β-blockers is not associated with a significant increase in mortality in patients with ascites or refractory ascites (17). However, in some series increased mortality has been observed in patients with refractory ascites receiving β-blockers compared with patients without β-blockers. It has been suggested that the decrease in cardiac output caused by β-blockers could precipitate AKI (18). Clinicians should weigh the risks and benefits of continuation of nonselective β-blockers on an individual basis in patients with refractory ascites.
Management and Treatment of Hepatorenal Syndrome
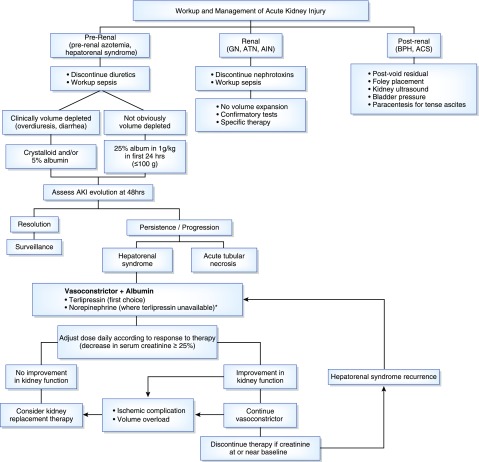
The etiology of AKI should be investigated quickly to prevent further worsening of AKI, because progression to advanced stage AKI has been associated with a higher mortality rate (Figure 2) (1,5). This is particularly important in those with hepatorenal syndrome because early initiation of treatment may increase the likelihood of hepatorenal syndrome resolution, possibly improving short-term survival. Albumin is an important step in the treatment and diagnosis of hepatorenal syndrome; however, it is important to exercise caution when administrating fluids in patients with AKI so as to avoid development of significant fluid retention and pulmonary edema, given the presence of reduced kidney sodium and water excretion in patients with cirrhosis.
Figure 2.
Algorithm for workup and management of AKI. *A trial of octreotide/midodrine (maximum 3 days) can be attempted before initiation of norepinephrine. ACS, abdominal compartment syndrome; AIN, acute interstitial nephritis; ATN, acute tubular necrosis; BPH, benign prostatic hypertrophy.
Pharmacologic Therapy
Vasoconstrictive agents in combination with albumin represent the first-line option to treat hepatorenal syndrome (Table 1) (19–23). Terlipressin is the most commonly used vasopressin analog; however, it has not been approved in all countries. The efficacy of terlipressin plus albumin in the treatment of hepatorenal syndrome has been proven in a large number of studies, with a response rate ranging from 25% to 75%. Terlipressin can be administered by intravenous boluses at starting dose of 0.5–1 mg every 4–6 hours, with a progressive increase to a maximum dose of 2 mg every 4 hours in cases of nonresponse, namely a reduction of baseline serum creatinine of <25%. Treatment should be maintained until complete response or for a maximum of 14 days in cases of partial response or nonresponse. Continuous infusion of terlipressin at a dose of 2–12 mg/d has been shown in a single study to be as efficacious as bolus administration but with lower rates of adverse events (24). The most serious side effects of terlipressin are related to vasoconstriction with a risk of myocardial infarction and intestinal ischemia. Baseline serum creatinine and acute-on-chronic liver failure grade are associated with response to terlipressin (25,26). However, studies in the use of vasoconstrictors with lower serum creatinine and in early stages of hepatorenal syndrome are lacking.
Table 1.
Summary of randomized, controlled studies of vasoconstrictor therapy in patients with type 1 hepatorenal syndrome
| Author | Year | Treatment | Patients | Hepatorenal Syndrome Reversal, % | Mortality without Transplantation, % | Transplantation, % |
|---|---|---|---|---|---|---|
| Alessandria et al. (27) | 2007 | Terlipressin | 12 | 83 | 90 | 66 |
| Norepinephrine | 10 | 70 | 100 | 70 | ||
| Sharma et al. (28) | 2008 | Terlipressin | 20 | 50 | 45 | — |
| Norepinephrine | 20 | 50 | 45 | — | ||
| Sanyal et al. (21) | 2008 | Terlipressin | 56 | 34 | 87 | — |
| Placebo | 56 | 13 | 91 | — | ||
| Martin-Llahí et al. (20) | 2008 | Terlipressin | 23 | 44 | 73 | 0 |
| Albumin | 23 | 9 | 81 | 4 | ||
| Singh et al. (29) | 2012 | Terlipressin | 23 | 39 | 61 | 0 |
| Norepinephrine | 23 | 43 | 52 | 0 | ||
| Cavallin et al. (19) | 2015 | Terlipressin | 27 | 70 | 41 | 0 |
| Midodrine | 22 | 29 | 57 | 4 | ||
| Boyer et al. (23) | 2016 | Terlipressin | 97 | 24 | 42 | — |
| Albumin | 99 | 15 | 46 | — | ||
| Arora et al. (22) | 2018 | Terlipressin | 60 | 40 | 52 | — |
| Noradrenaline | 60 | 17 | 80 | — |
All treatment arms included albumin. —, not available.
Other vasoconstrictive agents in combination with albumin have been proposed (Table 1). Norepinephrine (given intravenously at a dose of 0.5–3 mg/h) is an alternative agent that has been shown in small studies to be effective in increasing arterial pressure and reversal of kidney impairment in patients with hepatorenal syndrome (27–29); however, a recent controlled trial suggests that norepinephrine is inferior to terlipressin in reversal of hepatorenal syndrome, kidney replacement therapy (KRT) requirement, and overall survival (22). The combination of midodrine plus octreotide, used in countries where terlipressin is not yet available, has been shown in a single-center study to be less effective than terlipressin (19).
Transjugular Intrahepatic Portosystemic Shunt
Theoretically, a transjugular intrahepatic portosystemic shunt may improve kidney function in hepatorenal syndrome by decreasing portal hypertension and reducing and reversing the circulatory changes (and possibly systemic inflammation) that precipitate hepatorenal syndrome. Small studies have shown that a transjugular intrahepatic portosystemic shunt is associated with a decrease in serum creatinine, with possible survival benefit in patients with hepatorenal syndrome, but with high incidence of hepatic encephalopathy and further deterioration in patients with advanced liver disease (30).
Kidney Replacement Therapy
Initiation of KRT in patients with hepatorenal syndrome is controversial and has typically been viewed as a bridge to transplantation in listed patients. Recent studies have demonstrated that the severity of illness and number of organ failure in patients with acute-on-chronic liver failure is more predictive of 28-day mortality than cause of AKI (31,32). Therefore, it seems reasonable to consider a trial of KRT in select patients regardless of transplant candidacy. The ideal timing for initiation of KRT has not been studied in patients with cirrhosis and so should be individualized and made on clinical grounds, such as worsening kidney function coupled with electrolyte disturbances not responding to medical management, or diuretic intolerance/resistance. KRT should also be considered if the daily fluid balance cannot be maintained or is negative, regardless of their urine output, to prevent fluid accumulation.
Liver Support System
Although preliminary results suggested that albumin dialysis with the molecular adsorbent recirculating system could improve the outcome of patients with hepatorenal syndrome, this has not been confirmed in larger randomized trials. In a randomized trial of patients with acute on chronic liver failure, there was no significant difference in 28-day mortality between patients with hepatorenal syndrome who underwent molecular adsorbent recirculating system therapy compared with standard medical therapy (33). At this time, there is no evidence that albumin dialysis is superior to conventional filtration in patients requiring KRT.
Liver versus Simultaneous Liver–Kidney Transplantation
Predicting the recovery of impaired kidney function and the extent of that recovery after liver transplantation is challenging because of difficulties in delineating the relative contribution of preexisting comorbidities, unrecognized intrinsic kidney disease, perioperative events, and post-transplant immunosuppression to kidney dysfunction after liver transplant. The development of AKI before liver transplantation has been shown to be associated with higher risk of CKD and ESKD in the long-term after liver transplantation, but has also been associated with increased risk of mortality (34,35). Using the Scientific Registry of Transplant Recipients, in a Centers for Medicare and Medicaid Services ESKD program cohort of 2112 liver transplant recipients who received acute KRT for ≤90 days before liver transplantation, only 9% had kidney nonrecovery and needed chronic KRT within 6 months after liver transplantation; however, the postliver-transplantation mortality was high in this cohort (36). The treatment of choice for patients with hepatorenal syndrome is liver transplantation, and in theory, kidney function is fully reversible post-transplant. Kidney recovery and patient survival after liver transplantation in patients with hepatorenal syndrome was shown in a single-center study to be significantly higher than patients with acute tubular necrosis and comparable with those with no AKI or stage 1 AKI regardless of their dialysis status before transplantation (35).
The introduction of organ allocation on the basis of the Model for End-Stage Liver Disease in 2002 resulted in a dramatic increase in the number of simultaneous liver–kidney transplantations because of priority in allocation to liver transplantation candidates with kidney dysfunction (37). Simultaneous liver–kidney transplantation now represents 10% of all liver transplants in the United States, with approximately 5% of transplanted deceased donor kidneys drawn away from kidney-transplant-only candidates, raising concern in the kidney transplant community, especially given the uncertain benefit of simultaneous liver–kidney transplantation (38). The decision to perform simultaneous liver–kidney transplantation versus liver transplantation alone is driven not only by the concern of increased mortality post-transplant, but also by the concern of lack of kidney recovery, which is felt to contribute to the increased mortality. Studies have shown that postliver-transplantation patients on the kidney waitlist have a higher mortality compared with patients waiting for kidney transplant only (39). Recently, listing criteria for simultaneous liver-kidney transplantation were developed by the Organ Procurement and Transplantation Network on the basis of prior consensus recommendations, which include factors such as duration of AKI and dialysis and evidence of CKD (Table 2) (40–42). Factors such as age, comorbidities, or cause of AKI, which could affect kidney recovery, are currently not included in the criteria.
Table 2.
Previously proposed and existing Organ Procurement and Transplantation Network selection criteria for simultaneous liver and kidney transplantation
| Author (yr) | Eligibility Criteria for Simultaneous Liver–Kidney Transplantation |
|---|---|
| Davis et al. (2007) (41) | 1. CKD with CrCl≤30 ml/min (preferentially iothalamate) for >3 mo |
| 2. AKI and/or hepatorenal syndrome on hemodialysis for ≥6 wk | |
| 3. AKI with kidney biopsy showing fixed kidney damage | |
| 4. Simultaneous liver–kidney not recommended in AKI not requiring hemodialysis | |
| 5. Metabolic diseases | |
| Eason et al. (2008) (40) | 1. CKD with GFR≤30 ml/min for >3 mo |
| 2. AKI with serum creatinine ≥2 and on hemodialysis for ≥8 wk | |
| 3. Kidney biopsy with >30% glomerulosclerosis or 30% fibrosis | |
| 4. Metabolic diseases | |
| • Criteria recommended to be considered: diabetes, hypertension, age >65 yr, kidney size, and duration of serum creatinine ≥2 mg/dl | |
| Nadim et al. (2012) (42) | 1. AKI ≥ 4 wk with one of the following: |
| • Stage 3 AKI: three times the baseline serum creatinine or on KRT | |
| • eGFR≤35 ml/min (MDRD-6) or GFR≤25 ml/min (iothalamate) | |
| 2. CKD with one of the following: | |
| • eGFR≤40 ml/min (MDRD-6) or GFR≤30 ml/min (iothalamate) | |
| • Proteinuria ≥2 g/d | |
| • Kidney biopsy >30% glomerulosclerosis and/or >30% interstitial fibrosis | |
| 3. Metabolic diseases | |
| Organ Procurement and Transplantation Network (2017) (37) | 1. AKI for ≥6 consecutive wk with one or a combination of both (weekly documentation) |
| • Dialysis | |
| • eGFR/CrCl ≤25 ml/min | |
| 2. CKD with eGFR≤60 ml/min for >90 d with one of the following: | |
| • ESKD | |
| • eGFR/CrCl ≤30 ml/min at the time or after registration on kidney waiting list | |
| 3. Metabolic diseases | |
| 4. Safety net: | |
| • Any patient who is registered on the kidney waitlist between 60–365 d after liver transplantation and is either on chronic hemodialysis or has an eGFR<20 ml/min will qualify for increased priority | |
| • Documentation required by transplant nephrologist |
CrCl, creatinine clearance; KRT, kidney replacement therapy; MDRD, Modification of Diet in Renal Disease.
Biomarkers
Early diagnosis and identification of the phenotype of AKI is crucial as management differs according to different causes. Conventional tools such as urine output or fractional excretion of sodium or urea have been shown to have significant limitation in patients with advanced cirrhosis and poor correlation with biopsy findings (43). Recently, several innovative biomarkers have been studied, with neutrophil gelatinase-associated lipocalin, kidney injury molecule-1, liver fatty acid-binding protein, and IL-18 being the most extensively studied. These specific biomarkers typically reflect the earliest markers of ischemia-related events and may play a role in the diagnosis of AKI before liver transplantation (44,45). These biomarkers are not specific to kidney injury, may be influenced by inflammation or infection, do not comprehensively discriminate dichotomous outcomes, and have not been validated using kidney biopsy as a gold standard. In addition, substantial overlap has been observed between different phenotypes and no clear cut-off value separates hepatorenal syndrome from acute tubular necrosis. Biomarkers predictive of recovery from AKI after liver transplantation could enhance decision algorithms regarding the need for liver–kidney transplant or kidney-sparing regimens (46). Tissue inhibitor of metalloproteinase-1 and osteopontin, along with patient characteristics (e.g., age, diabetes), have been shown in a single-center study to differentiate between recipients that developed reversible versus irreversible AKI after liver transplantation (46).
Imaging Studies
Kidney ultrasonography is a useful noninvasive test to help exclude structural causes of AKI, such as obstructive uropathy and intrinsic parenchymal kidney disease, which would rule out the diagnosis of hepatorenal syndrome. Assessment of arterial kidney-resistive indexes by Doppler ultrasonography (47,48), contrast-enhanced ultrasonography (49), and magnetic resonance elastography (50) have been shown in very small studies to be associated with the development of hepatorenal syndrome. Whether these techniques help in early diagnosis of hepatorenal syndrome, differentiation of hepatorenal syndrome from other phenotypes of AKI, or prediction of response to vasoconstrictors needs to be further explored in larger trials.
Conclusion and Perspectives
Significant improvements have been achieved in the diagnosis and management of hepatorenal syndrome in recent years. Even with the use of vasoconstrictive agents and albumin, 3-month mortality rates remain especially high in the absence of liver transplantation. In addition to splanchnic and systemic circulatory changes, inflammation may play an important role in the development of hepatorenal syndrome. Therapeutic interventions aimed at controlling inflammation may help prevent or reverse hepatorenal syndrome. Novel biomarker in combination with imaging studies may improve diagnostic performance of AKI in patients with cirrhosis. Irreversible kidney changes are probably underestimated; in the future, novel biomarkers and imaging studies may provide further information on the potential of kidney recovery after liver transplantation (along with cause), and potentially affect the decision to allocate a simultaneous liver–kidney transplantation.
Disclosures
Dr. Nadim reports receiving personal fees from Mallinckrodt and Baxter, outside the submitted work. Dr. Durand, Dr. Francoz, Dr. Genyk, and Dr. Kahn have nothing to disclose.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Tandon P, James MT, Abraldes JG, Karvellas CJ, Ye F, Pannu N: Relevance of new definitions to incidence and prognosis of acute kidney injury in hospitalized patients with cirrhosis: A retrospective population-based cohort study. PLoS One 11: e0160394, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rice JB, White AG, Galebach P, Korenblat KM, Wagh A, Lovelace B, Wan GJ, Jamil K: The burden of hepatorenal syndrome among commercially insured and Medicare patients in the United States. Curr Med Res Opin 33: 1473–1480, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Nadim MK, Kellum JA, Davenport A, Wong F, Davis C, Pannu N, Tolwani A, Bellomo R, Genyk YS; ADQI Workgroup: Hepatorenal syndrome: The 8th International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 16: R23, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, Moreau R, Jalan R, Sarin SK, Piano S, Moore K, Lee SS, Durand F, Salerno F, Caraceni P, Kim WR, Arroyo V, Garcia-Tsao G; International Club of Ascites: Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites. Gut 64: 531–537, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Amathieu R, Al-Khafaji A, Sileanu FE, Foldes E, DeSensi R, Hilmi I, Kellum JA: Significance of oliguria in critically ill patients with chronic liver disease. Hepatology 66: 1592–1600, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J: Peripheral arterial vasodilation hypothesis: A proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology 8: 1151–1157, 1988 [DOI] [PubMed] [Google Scholar]
- 7.Ruiz-del-Arbol L, Monescillo A, Arocena C, Valer P, Ginès P, Moreira V, Milicua JM, Jiménez W, Arroyo V: Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology 42: 439–447, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Yang YY, Liu H, Nam SW, Kunos G, Lee SS: Mechanisms of TNFalpha-induced cardiac dysfunction in cholestatic bile duct-ligated mice: Interaction between TNFalpha and endocannabinoids. J Hepatol 53: 298–306, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krag A, Bendtsen F, Henriksen JH, Møller S: Low cardiac output predicts development of hepatorenal syndrome and survival in patients with cirrhosis and ascites. Gut 59: 105–110, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V: Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol 63: 1272–1284, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Albillos A, Lario M, Álvarez-Mon M: Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J Hepatol 61: 1385–1396, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Shah N, Mohamed FE, Jover-Cobos M, Macnaughtan J, Davies N, Moreau R, Paradis V, Moore K, Mookerjee R, Jalan R: Increased renal expression and urinary excretion of TLR4 in acute kidney injury associated with cirrhosis. Liver Int 33: 398–409, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Du Plessis J, Vanheel H, Janssen CE, Roos L, Slavik T, Stivaktas PI, Nieuwoudt M, van Wyk SG, Vieira W, Pretorius E, Beukes M, Farré R, Tack J, Laleman W, Fevery J, Nevens F, Roskams T, Van der Merwe SW: Activated intestinal macrophages in patients with cirrhosis release NO and IL-6 that may disrupt intestinal barrier function. J Hepatol 58: 1125–1132, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Fernández J, Navasa M, Planas R, Montoliu S, Monfort D, Soriano G, Vila C, Pardo A, Quintero E, Vargas V, Such J, Ginès P, Arroyo V: Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology 133: 818–824, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Bernardi M, Caraceni P, Navickis RJ, Wilkes MM: Albumin infusion in patients undergoing large-volume paracentesis: A meta-analysis of randomized trials. Hepatology 55: 1172–1181, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Caraceni P, Riggio O, Angeli P, Alessandria C, Neri S, Foschi FG, Levantesi F, Airoldi A, Boccia S, Svegliati-Baroni G, Fagiuoli S, Romanelli RG, Cozzolongo R, Di Marco V, Sangiovanni V, Morisco F, Toniutto P, Tortora A, De Marco R, Angelico M, Cacciola I, Elia G, Federico A, Massironi S, Guarisco R, Galioto A, Ballardini G, Rendina M, Nardelli S, Piano S, Elia C, Prestianni L, Cappa FM, Cesarini L, Simone L, Pasquale C, Cavallin M, Andrealli A, Fidone F, Ruggeri M, Roncadori A, Baldassarre M, Tufoni M, Zaccherini G, Bernardi M; ANSWER Study Investigators: Long-term albumin administration in decompensated cirrhosis (ANSWER): An open-label randomised trial. Lancet 391: 2417–2429, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Chirapongsathorn S, Valentin N, Alahdab F, Krittanawong C, Erwin PJ, Murad MH, Kamath PS: Nonselective β-blockers and survival in patients with cirrhosis and ascites: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 14: 1096–1104.e9, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Sersté T, Melot C, Francoz C, Durand F, Rautou PE, Valla D, Moreau R, Lebrec D: Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology 52: 1017–1022, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Cavallin M, Kamath PS, Merli M, Fasolato S, Toniutto P, Salerno F, Bernardi M, Romanelli RG, Colletta C, Salinas F, Di Giacomo A, Ridola L, Fornasiere E, Caraceni P, Morando F, Piano S, Gatta A, Angeli P; Italian Association for the Study of the Liver Study Group on Hepatorenal Syndrome: Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: A randomized trial. Hepatology 62: 567–574, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Martín-Llahí M, Pépin MN, Guevara M, Díaz F, Torre A, Monescillo A, Soriano G, Terra C, Fábrega E, Arroyo V, Rodés J, Ginès P; TAHRS Investigators: Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: A randomized study. Gastroenterology 134: 1352–1359, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Sanyal AJ, Boyer T, Garcia-Tsao G, Regenstein F, Rossaro L, Appenrodt B, Blei A, Gülberg V, Sigal S, Teuber P; Terlipressin Study Group: A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology 134: 1360–1368, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arora V, Maiwall R, Rajan V, Jindal A, Muralikrishna Shasthry S, Kumar G, Jain P, Sarin SK: Terlipressin is superior to noradrenaline in the management of acute kidney injury in acute on chronic liver failure [published online ahead of print August 3, 2018]. Hepatology doi: 10.1002/hep.30208 [DOI] [PubMed] [Google Scholar]
- 23.Boyer TD, Sanyal AJ, Wong F, Frederick RT, Lake JR, O'Leary JG, Ganger D, Jamil K, Pappas SC, Investigators RS: Terlipressin plus albumin is more effective than albumin alone in improving renal function in patients with cirrhosis and hepatorenal syndrome type 1. Gastroenterology 150: 1579–1589.e2, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Cavallin M, Piano S, Romano A, Fasolato S, Frigo AC, Benetti G, Gola E, Morando F, Stanco M, Rosi S, Sticca A, Cillo U, Angeli P: Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: A randomized controlled study. Hepatology 63: 983–992, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Boyer TD, Sanyal AJ, Garcia-Tsao G, Blei A, Carl D, Bexon AS, Teuber P; Terlipressin Study Group: Predictors of response to terlipressin plus albumin in hepatorenal syndrome (HRS) type 1: Relationship of serum creatinine to hemodynamics. J Hepatol 55: 315–321, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piano S, Schmidt HH, Ariza X, Amoros A, Romano A, Husing-Kabar A, Sola E, Gerbes A, Bernardi M, Alessandria C, Scheiner B, Tonon M, Maschmeier M, Sole C, Trebicka J, Gustot T, Nevens F, Arroyo V, Gines P, Angeli P; EASL CLIF Consortium, European Foundation for the Study of Chronic Liver Failure (EF Clif): Association between grade of acute on chronic liver failure and response to terlipressin and albumin in patients with hepatorenal syndrome. Clin Gastroenterol Hepatol 16: 1792–1800.e3, 2018 [DOI] [PubMed] [Google Scholar]
- 27.Alessandria C, Ottobrelli A, Debernardi-Venon W, Todros L, Cerenzia MT, Martini S, Balzola F, Morgando A, Rizzetto M, Marzano A: Noradrenalin vs terlipressin in patients with hepatorenal syndrome: A prospective, randomized, unblinded, pilot study. J Hepatol 47: 499–505, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Sharma P, Kumar A, Shrama BC, Sarin SK: An open label, pilot, randomized controlled trial of noradrenaline versus terlipressin in the treatment of type 1 hepatorenal syndrome and predictors of response. Am J Gastroenterol 103: 1689–1697, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Singh V, Ghosh S, Singh B, Kumar P, Sharma N, Bhalla A, Sharma AK, Choudhary NS, Chawla Y, Nain CK: Noradrenaline vs. terlipressin in the treatment of hepatorenal syndrome: A randomized study. J Hepatol 56: 1293–1298, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Song T, Rossle M, He F, Liu F, Guo X, Qi X: Transjugular intrahepatic portosystemic shunt for hepatorenal syndrome: A systematic review and meta-analysis. Dig Liver Dis 50: 323–330, 2018 [DOI] [PubMed] [Google Scholar]
- 31.Allegretti AS, Parada XV, Eneanya ND, Gilligan H, Xu D, Zhao S, Dienstag JL, Chung RT, Thadhani RI: Prognosis of patients with cirrhosis and AKI who initiate RRT. Clin J Am Soc Nephrol 13: 16–25, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angeli P, Rodríguez E, Piano S, Ariza X, Morando F, Solà E, Romano A, García E, Pavesi M, Risso A, Gerbes A, Willars C, Bernardi M, Arroyo V, Ginès P; CANONIC Study Investigators of EASL-CLIF Consortium: Acute kidney injury and acute-on-chronic liver failure classifications in prognosis assessment of patients with acute decompensation of cirrhosis. Gut 64: 1616–1622, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Bañares R, Nevens F, Larsen FS, Jalan R, Albillos A, Dollinger M, Saliba F, Sauerbruch T, Klammt S, Ockenga J, Pares A, Wendon J, Brünnler T, Kramer L, Mathurin P, de la Mata M, Gasbarrini A, Müllhaupt B, Wilmer A, Laleman W, Eefsen M, Sen S, Zipprich A, Tenorio T, Pavesi M, Schmidt HH, Mitzner S, Williams R, Arroyo V; RELIEF study group: Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: The RELIEF trial. Hepatology 57: 1153–1162, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM: Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 349: 931–940, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Nadim MK, Genyk YS, Tokin C, Fieber J, Ananthapanyasut W, Ye W, Selby R: Impact of the etiology of acute kidney injury on outcomes following liver transplantation: Acute tubular necrosis versus hepatorenal syndrome. Liver Transpl 18: 539–548, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Sharma P, Goodrich NP, Zhang M, Guidinger MK, Schaubel DE, Merion RM: Short-term pretransplant renal replacement therapy and renal nonrecovery after liver transplantation alone. Clin J Am Soc Nephrol 8: 1135–1142, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.U.S. Department of Health & Human Services: Organ procurement and transplantation network data. Available at: https://optn.transplant.hrsa.gov/data. Accessed December 31, 2018
- 38.Asch WS, Bia MJ: New organ allocation system for combined liver-kidney transplants and the availability of kidneys for transplant to patients with stage 4-5 CKD. Clin J Am Soc Nephrol 12: 848–852, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srinivas TR, Stephany BR, Budev M, Mason DP, Starling RC, Miller C, Goldfarb DA, Flechner SM, Poggio ED, Schold JD: An emerging population: Kidney transplant candidates who are placed on the waiting list after liver, heart, and lung transplantation. Clin J Am Soc Nephrol 5: 1881–1886, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eason JD, Gonwa TA, Davis CL, Sung RS, Gerber D, Bloom RD: Proceedings of Consensus Conference on Simultaneous Liver Kidney Transplantation (SLK). Am J Transplant 8: 2243–2251, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Davis CL, Feng S, Sung R, Wong F, Goodrich NP, Melton LB, Reddy KR, Guidinger MK, Wilkinson A, Lake J: Simultaneous liver-kidney transplantation: Evaluation to decision making. Am J Transplant 7: 1702–1709, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Nadim MK, Sung RS, Davis CL, Andreoni KA, Biggins SW, Danovitch GM, Feng S, Friedewald JJ, Hong JC, Kellum JA, Kim WR, Lake JR, Melton LB, Pomfret EA, Saab S, Genyk YS: Simultaneous liver-kidney transplantation summit: Current state and future directions. Am J Transplant 12: 2901–2908, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Trawalé JM, Paradis V, Rautou PE, Francoz C, Escolano S, Sallée M, Durand F, Valla D, Lebrec D, Moreau R: The spectrum of renal lesions in patients with cirrhosis: A clinicopathological study. Liver Int 30: 725–732, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Belcher JM, Sanyal AJ, Peixoto AJ, Perazella MA, Lim J, Thiessen-Philbrook H, Ansari N, Coca SG, Garcia-Tsao G, Parikh CR; TRIBE-AKI Consortium: Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology 60: 622–632, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fagundes C, Pépin MN, Guevara M, Barreto R, Casals G, Solà E, Pereira G, Rodríguez E, Garcia E, Prado V, Poch E, Jiménez W, Fernández J, Arroyo V, Ginès P: Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J Hepatol 57: 267–273, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Levitsky J, Baker TB, Jie C, Ahya S, Levin M, Friedewald J, Al-Saden P, Salomon DR, Abecassis MM: Plasma protein biomarkers enhance the clinical prediction of kidney injury recovery in patients undergoing liver transplantation. Hepatology 60: 2017–2026, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Platt JF, Ellis JH, Rubin JM, Merion RM, Lucey MR: Renal duplex Doppler ultrasonography: A noninvasive predictor of kidney dysfunction and hepatorenal failure in liver disease. Hepatology 20: 362–369, 1994 [PubMed] [Google Scholar]
- 48.Mindikoglu AL, Dowling TC, Wong-You-Cheong JJ, Christenson RH, Magder LS, Hutson WR, Seliger SL, Weir MR: A pilot study to evaluate renal hemodynamics in cirrhosis by simultaneous glomerular filtration rate, renal plasma flow, renal resistive indices and biomarkers measurements. Am J Nephrol 39: 543–552, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schneider AG, Schelleman A, Goodwin MD, Bailey M, Eastwood GM, Bellomo R: Contrast-enhanced ultrasound evaluation of the renal microcirculation response to terlipressin in hepato-renal syndrome: A preliminary report. Ren Fail 37: 175–179, 2015 [DOI] [PubMed] [Google Scholar]
- 50.Low G, Owen NE, Joubert I, Patterson AJ, Graves MJ, Alexander GJ, Lomas DJ: Magnetic resonance elastography in the detection of hepatorenal syndrome in patients with cirrhosis and ascites. Eur Radiol 25: 2851–2858, 2015 [DOI] [PubMed] [Google Scholar]