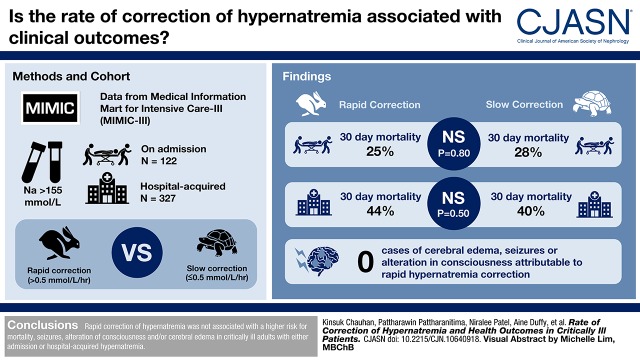
Visual Abstract
Keywords: hypernatremia, mortality, mortality risk, Critical Illness, Sodium, Brain Edema, Hospital Mortality, Patient Admission, hospitalization, Hospitals, Critical Care
Abstract
Background and objectives
Hypernatremia is common in hospitalized, critically ill patients. Although there are no clear guidelines on sodium correction rate for hypernatremia, some studies suggest a reduction rate not to exceed 0.5 mmol/L per hour. However, the data supporting this recommendation and the optimal rate of hypernatremia correction in hospitalized adults are unclear.
Design, setting, participants, & measurements
We assessed the association of hypernatremia correction rates with neurologic outcomes and mortality in critically ill patients with hypernatremia at admission and those that developed hypernatremia during hospitalization. We used data from the Medical Information Mart for Intensive Care-III and identified patients with hypernatremia (serum sodium level >155 mmol/L) on admission (n=122) and hospital-acquired (n=327). We calculated different ranges of rapid correction rates (>0.5 mmol/L per hour overall and >8, >10, and >12 mmol/L per 24 hours) and utilized logistic regression to generate adjusted odds ratios (aOR) with 95% confidence intervals (95% CIs) to examine association with outcomes.
Results
We had complete data on 122 patients with severe hypernatremia on admission and 327 patients who developed hospital-acquired hypernatremia. The difference in in-hospital 30-day mortality proportion between rapid (>0.5 mmol/L per hour) and slower (≤0.5 mmol/L per hour) correction rates were not significant either in patients with hypernatremia at admission with rapid versus slow correction (25% versus 28%; P=0.80) or in patients with hospital-acquired hypernatremia with rapid versus slow correction (44% versus 40%; P=0.50). There was no difference in aOR of mortality for rapid versus slow correction in either admission (aOR, 1.3; 95% CI, 0.5 to 3.7) or hospital-acquired hypernatremia (aOR, 1.3; 95% CI, 0.8 to 2.3). Manual chart review of all suspected chronic hypernatremia patients, which included all 122 with hypernatremia at admission, 128 of the 327 hospital-acquired hypernatremia, and an additional 28 patients with ICD-9 codes for cerebral edema, seizures and/or alteration of consciousness, did not reveal a single case of cerebral edema attributable to rapid hyprnatremia correction.
Conclusions
We did not find any evidence that rapid correction of hypernatremia is associated with a higher risk for mortality, seizure, alteration of consciousness, and/or cerebral edema in critically ill adult patients with either admission or hospital-acquired hypernatremia.
Introduction
Hypernatremia is defined as increased serum sodium concentration >145 mmol/L (1). It is a hyperosmolar state in which there is a deficit in total body volume in comparison to total body electrolytes (2). The incidence of hypernatremia is reported to be up to 3% in hospitalized patients and 9% in patients admitted to the intensive care unit (ICU) (3,4). Acute hypernatremia in ICU patients may have an independent association with higher mortality and length of stay, although the higher risk of mortality may reflect severity of related illness and comorbid conditions (5).
There are no evidence-based guidelines on the appropriate sodium correction rate for hypernatremia. However, expert opinion suggests a reduction rate no more than 0.5 mmol/L per hour, with an absolute change of <10 mmol/L per day to avoid cerebral edema, seizure, and permanent neurologic damage from rapid correction (1,6–8). Although prior literature has documented the association of cerebral edema and rapid hypernatremia correction in children, the literature in adults is scant (9,10). Contrary to the literature in children, two studies in adults demonstrated that excessively slow rates of correction are associated with a higher risk of mortality and those with a greater reduction rate of sodium had less mortality (11–13). Thus, the outcomes associated with hypernatremia correction rate in hospitalized adults are unclear.
We sought to determine the association between rates of hypernatremia correction with mortality and the incidence of neurologic outcomes in critically ill patients with either hypernatremia present on admission or in those who developed hypernatremia during hospitalization.
Materials and Methods
Study Design and Setting
We extracted data from the Medical Information Mart for Intensive Care-III (MIMIC-III) database to identify patients with hypernatremia. MIMIC-III is a publicly available critical care database of patients from a large, single-center tertiary care hospital (Beth Israel Deaconess Medical Center in Boston, MA) from 2001 to 2012 (14). This database includes patient demographics, vital signs, laboratory results, billing codes, and notes. We included patients in the analysis with severe hypernatremia, which was defined if they had serum sodium >155 mmol/L at any time point during their hospital admission. We excluded patients aged <18 years old and patients with no serum sodium values at any time points after the peak serum sodium level. The patients who had severe hypernatremia at hospital admission were labeled “admission hypernatremia” and patients who developed severe hypernatremia during their hospital stay were labeled “hospital-acquired hypernatremia.” We considered only the data from the patient’s first admission with hypernatremia. A study flow diagram is included in Supplemental Figure 1.
Definition of Rapid Correction and Categories
For each study participant, we identified peak serum sodium level and calculated the overall rate of correction using serum sodium values after the peak. We calculated rate of serum sodium correction was calculated using the following formula:
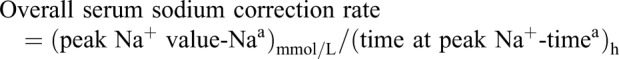 |
Naa is the first corrected serum sodium value <145 mmol/L or the last serum sodium value before discharge in those patients who did not correct down to 145mmol/L.
Timea is the time at first corrected serum sodium level <145 mmol/L or the time of last available value in those who did not achieve correction during their admission. We also calculated serum sodium correction rate at 24 hours using the same formula, where Naa is the serum sodium value regardless of their corrected level at 24-hour, and timea is the time at which Naa was recorded at 24 hours.
Rapid hypernatremia correction was defined as an overall serum sodium correction rate of >0.5 and ≤0.5 mmol/L per hour was considered slower hypernatremia correction rate. Additionally, we did several subanalysis with varying hypernatremia correction rates of >8, >10, and >12 mmol/L per 24 hours.
Definition of Neurologic Complications and Chronic Hypernatremia
To determine the incidence of cerebral edema, seizures, and alteration of consciousness in the study population, we used the International Classification of Diseases, Ninth Revision (ICD-9) diagnostic codes mentioned in Supplemental Table 1. We also identified patients who had serum sodium ≥145 mmol/L for >48 hours in patients with hospital-acquired hypernatremia and considered them as having chronic hypernatremia. We also presumed patients with hypernatremia at admission as having chronic hypernatremia because the onset of higher serum sodium was unknown. To identify the causes of these neurologic complications, we extracted medical resonance imaging, computerized tomography imaging reports, and discharge summary reports for the patients. Two independent clinicians manually reviewed all reports to identify cause of cerebral edema and to determine whether it was attributable to rapid hypernatremia correction.
Assessment of Covariates
Additional data extracted included demographic characteristics, additional comorbid conditions by ICD-9 codes (nonalcoholic liver disease, CKD, ESKD, congestive heart failure, diabetes mellitus type 2, depression, bipolar disorder, schizophrenia, epilepsy, stroke, myocardial infarction, AIDS, chronic obstructive pulmonary disease, hypertension, and peripheral arterial disease), ICU type during first admission, do-not-resuscitate (DNR) status, laboratory values during peak sodium level (serum: creatinine, potassium, phosphorus, magnesium, osmolality, bicarbonate, and albumin; urine: sodium, potassium, and osmolality), and diuretics use (thiazide and loop) (Table 1).
Table 1.
Characteristics of adults admitted to ICU with hypernatremia at admission and hospital-acquired hypernatremia by slower versus rapid correction rate
| Characteristic | Admission Hypernatremia, n=122; n (%) or Median (IQR) | Hospital-Acquired Hypernatremia, n=327; n (%) or Median (IQR) | ||
|---|---|---|---|---|
| ≤0.5 mmol/L per h | >0.5 mmol/L per h | ≤0.5 mmol/L per h | >0.5 mmol/L per h | |
| Total, n (%) | 90 (74) | 32 (26) | 225 (69) | 102 (31) |
| Age, yr, median (IQR) | 83 (72–90) | 80 (61–86) | 64 (48–75) | 58 (48–75) |
| Sex, n (%) | ||||
| Men | 48 (53) | 8 (25) | 149 (67) | 66 (65) |
| Women | 42 (47) | 24 (75) | 76 (34) | 36 (35) |
| ICU first service, n (%) | ||||
| Medical ICU | 73 (81) | 25 (78) | 61 (27) | 46 (45) |
| Surgical ICU | 8 (9) | 0 | 58 (26) | 17 (17) |
| Coronary care unit | 5 (6) | 3 (9) | 20 (9) | 9 (9) |
| Trauma/surgical ICU | 2 (2) | 3 (9) | 60 (27) | 23 (23) |
| Cardiac surgery recovery unit | 1 (1) | 0 | 26 (12) | 5 (5) |
| Admission type, n (%) | ||||
| Elective | 1 (1) | 0 | 18 (8) | 6 (6) |
| Urgent/emergency | 89 (99) | 32 (100) | 207 (92) | 96 (94) |
| Insurance, n (%) | ||||
| Government | 2 (2) | 1 (3) | 7 (3) | 6 (6) |
| Medicaid | 4 (4) | 1 (3) | 22 (10) | 9 (9) |
| Medicare | 75 (83) | 22 (69) | 105 (47) | 42 (41) |
| Private | 9 (10) | 8 (25) | 81 (36) | 39 (38) |
| Self-pay | 0 | 0 | 10 (4) | 6 (6) |
| Race/ethnicity, n (%) | ||||
| White | 56 (62) | 18 (56) | 128 (57) | 54 (53) |
| Black | 17 (19) | 4 (13) | 23 (10) | 19 (19) |
| Hispanic | 3 (3) | 3 (9) | 8 (4) | 3 (3) |
| Asian | 6 (7) | 3 (9) | 4 (2) | 4 (4) |
| Others/unknown | 8 (9) | 4 (13) | 62 (28) | 22 (22) |
| Charlson Comorbidity Index, n (%) | ||||
| 0 | 22 (24) | 9 (28) | 51 (23) | 24 (24) |
| 1 | 20 (22) | 15 (47) | 52 (23) | 32 (31) |
| 2 | 21 (23) | 3 (9) | 39 (17) | 15 (15) |
| ≥3 | 27 (30) | 5 (16) | 83 (37) | 31 (30) |
| Comorbidities, n (%) | ||||
| CKD | 19 (21) | 1 (3) | 20 (9) | 11 (11) |
| ESKD | 0 | 0 | 3 (1) | 2 (2) |
| Liver disease (NA) | 1 (1) | 0 | 0 | 1 (1) |
| Congestive heart failure | 22 (24) | 7 (22) | 47 (21) | 21 (21) |
| Diabetes mellitus type 2 | 14 (16) | 6 (19) | 42 (19) | 11 (11) |
| Depression | 10 (11) | 8 (25) | 13 (6) | 4 (4) |
| Bipolar disorder | 3 (3) | 1 (3) | 14 (6) | 4 (4) |
| Schizophrenia | 1 (1) | 0 | 1 (0.4) | 0 |
| Epilepsy | 5 (6) | 0 | 6 (3) | 1 (1) |
| Stroke (infarction, ICH, SAH) | 5 (6) | 2 (6) | 69 (31) | 20 (20) |
| Myocardial infarction | 11 (12) | 0 | 23 (10) | 6 (6) |
| HIV infection | 1 (1) | 1 (3) | 0 | 1 (1) |
| Chronic obstructive pulmonary disease | 6 (7) | 5 (16) | 22 (10) | 6 (6) |
| Hypertension | 27 (30) | 10 (31) | 76 (34) | 29 (28) |
| Peripheral arterial disease | 1 (1) | 0 | 3 (1) | 3 (3) |
| Laboratory values at peak sodium level, median (IQR) | ||||
| Creatinine, mg/dl | 1.9 (1.3–2.9) | 1.2 (0.8–2.1) | 1.1 (0.8–1.6) | 1.2 (1.0–2.0) |
| BUN, mg/dl | 62 (40–85) | 36 (27–64) | 34 (20–52) | 23 (15–54) |
| Potassium, mmol/L | 3.9 (3.6–4.2) | 3.8 (3.6–4.1) | 3.8 (3.6–4.0) | 3.8 (3.5–4.1) |
| Phosphorus, mg/dl | 3.2 (2.4–3.9) | 2.5 (2.2–3.8) | 2.9 (2.3–3.8) | 3.0 (2.3–3.9) |
| Magnesium, mg/dl | 2.3 (2.1–2.7) | 2.2 (2.0–2.4) | 2.3 (2.0–2.5) | 2.1 (1.9–2.3) |
| Serum osmolality, mOsm/kg | 354 (345–367) | 358 (352–365) | 328 (323–337) | 331 (320–345) |
| Serum bicarbonate, mmol/L | 22 (19–25) | 24 (20–28) | 26 (23–29) | 23 (19–26) |
| Serum bicarbonate ≤20 mmol/L, n (%) | 31 (34) | 11 (34) | 30 (14) | 29 (28) |
| Albumin, g/dl | 2.8 (2.5–3.3) | 3.0 (2.6–3.2) | 2.9 (2.6–3.2) | 2.9 (2.5–3.5) |
| Urine sodium, mmol/L | 47 (20–75) | 68 (46–109) | 54 (33–82) | 61 (30–112) |
| Urine potassium, mmol/L | 65 (44–81) | 43 (28–73) | 39 (31–49) | 25 (17–41) |
| Urine osmolality, mOsm/kg | 514 (440–618) | 632 (440–752) | 489 (355–674) | 440 (322–558) |
| Medications, n (%) | ||||
| Thiazide diuretics | 1 (1) | 0 | 30 (13) | 9 (9) |
| Loop diuretics | 23 (26) | 12 (38) | 117 (52) | 50 (49) |
| In-hospital mortality, n (%) | 25 (28) | 8 (25) | 90 (40) | 45 (44) |
| DNR, n (%) | 43 (48) | 12 (38) | 61 (27) | 23 (23) |
| Length of stay, d, median (IQR) | 3 (2–5) | 3 (2–9) | 7 (3–5) | 4 (2–9) |
ICU, intensive care unit; IQR, interquartile range; NA, non-alcoholic; ICH, intracerebral hemorrhage; SAH, subarachnoid hemorrhage; DNR, do not resuscitate.
Statistical Analyses
We conducted analysis to explore differences between patients who experienced overall slow correction (≤0.5 mmol/L per hour) versus rapid correction (>0.5 mmol/L per hour) stratified by two groups: admission hypernatremia and hospital-acquired hypernatremia. We further divided correction rate categories during hospital follow-up duration into patients with corrected sodium level (those who achieved serum sodium of <145 mmol/L) and patients with uncorrected sodium level (those who did not achieve a serum sodium level of <145 mmol/L). We used the Wilcoxon rank sum test for continuous variables and the Fisher exact test for categorical variables. We performed Kaplan–Meier curve to assess the survival rate difference in patients between different sodium correction rates. We then used unadjusted and adjusted logistic regression analysis to determine the influence of hypernatremia correction rate at different time points on mortality. An adjustment of age, sex, DNR status, and Charlson comorbidity index was included in the model. As a subgroup analyses, we identified patients who had serum sodium ≥145 mmol/L for >48 hours in patients with hospital-acquired hypernatremia and considered them as having chronic hypernatremia. Then we compared mortality proportions between acute and chronic hypernatremia groups within the hospital-acquired hypernatremia group and also between sex and median age groups among patients with hypernatremia at admission and those with hospital-acquired hypernatremia. A P value of 0.05 was considered statistically significant for all comparisons. As this study was done on publicly available, deidentified data, it was considered exempt from institutional review board approval. The analysis was done using SAS v9.4 (SAS Institute Inc., Cary, NC) and Stata/IC 15.1 software (StataCorp, College Station, TX).
Results
Study Cohort Characteristics
We identified 512 patients with severe hypernatremia during their first admission to the ICU from 2001 to 2012. A total of 449 patients were included in the main analysis after excluding patients without follow-up serum sodium values beyond their peak level. We also segregated patients into those that presented with severe hypernatremia on admission (n=122) and those who developed hospital-acquired hypernatremia (n=327), respectively (Supplemental Figure 1). The baseline characteristics between admission hypernatremia and hospital-acquired hypernatremia groups are shown in Supplemental Table 2. Both groups were significantly different with respect to key characteristics, including age, sex, and ICU first service. The baseline characteristics by correction rates in both groups are available in Table 1. A total of 32 (26%) and 102 (31%) patients had correction >0.5 mmol/L per hour in admission and hospital-acquired hypernatremia groups, respectively.
In the admission hypernatremia group, those with a rapid correction had a higher proportion of female patients (75% versus 47%; P=0.01), Charlson Comorbidity Index score of 1 (47% versus 22%; P=0.03), comorbid conditions such as depression (25% versus 11%; P=0.06), and a lower proportion of CKD (3% versus 21%; P=0.02).
Although patients in the hospital-acquired hypernatremia group who experienced rapid correction had no difference in sex or CKD status, they had a shorter median length of stay (4 days [interquartile range (IQR), 2.2–8.8] versus 7 days [IQR 3.1–14.7]; P=0.002) and lower proportion of stroke (20% versus 31%; P=0.04) (Table 1) compared with those in the slower correction group. In patients with hospital-acquired hypernatremia with rapid correction rate, the median serum bicarbonate was significantly lower (26 versus 23 mmol/L; P=0.02) and the number of patients with serum bicarbonate ≤20 mmol/L were significantly higher in the rapid correction group (28%) compared with slower correction group (14%; P=0.002).
There were some missing values for first care unit, marital status, and laboratory values at the time of peak sodium level, but they were used only for descriptive purposes.
Serum Sodium Level and Correction of Hypernatremia
In the rapid serum sodium correction group of patients with hospital-acquired hypernatremia, the time to correction to serum sodium <145 from peak serum sodium was 14.7 hours (IQR, 9.2–18.9). The median rate of correction was higher in patients with hospital-acquired hypernatremia (0.9; IQR, 0.6–1.6 mmol/L per hour) compared with those with admission hypernatremia (0.7; IQR, 0.6–1.4) (Table 2). The peak serum sodium concentration in patients with admission hypernatremia was 163 mmol/L (IQR, 159–168), which was significantly higher than the peak serum sodium in those with hospital-acquired hypernatremia (158 mmol/L; IQR, 156–161; P<0.001). Similarly, rate of correction was higher at 24 hours in patients with admission hypernatremia (0.5 mmol/h; IQR, 0.3–0.7 mmol/h) compared with those with hospital-acquired hypernatremia (0.4 mmol/h; IQR, 0.2–0.7 mmol/h; P=0.05) (Supplemental Table 3).
Table 2.
Distribution of the sodium level, difference, and correction time of adults admitted to ICU with hypernatremia at admission and hospital-acquired
| Variable | Admission Hypernatremia, n=122; Median (IQR) | Hospital-Acquired Hypernatremia, n=327; Median (IQR) | ||||
|---|---|---|---|---|---|---|
| ≤0.5 mmol/L per h | >0.5 mmol/L per h | P Value | ≤0.5 mmol/L per h | >0.5 mmol/L per h | P Value | |
| Total, n (%) | 90 (74) | 32 (26) | 225 (69) | 102 (31) | ||
| Serum sodium, mmol/L | ||||||
| at peak | 162.0 (159.0–168.0) | 166.0 (158.5–169.5) | 0.4 | 157.0 (156.0–160.0) | 159.0 (157.0–164.0) | <0.001 |
| at 24 h | 156.0 (153.0–160.0) | 152.0 (145.5–157.0) | 0.1 | 153.0 (150.0–156.0) | 146.0 (143.0–152.0) | <0.001 |
| after correction or last available | 144.0 (143.0–145.0) | 144.0 (141.0–145.0) | 0.9 | 145.0 (143.0–149.0) | 144.0 (142.0–145.0) | 0.02 |
| Serum sodium difference, mmol/L | ||||||
| at 24 h | 7.5 (4.0–10.0) | 13.0 (11.0–15.0) | <0.001 | 5.0 (3.0–7.0) | 13.0 (8.0–19.0) | <0.001 |
| at correction or last available | 18.0 (14.0–24.0) | 25.0 (13.0–31.5) | 0.1 | 13.0 (10.0–15.0) | 16.0 (13.0–21.0) | <0.001 |
| Serum sodium time difference, h | ||||||
| at 24 h | 19.4 (16.0–21.8) | 18.0 (15.0–20.5) | 0.2 | 18.2 (11.9–21.9) | 14.7 (9.2–18.9) | <0.001 |
| at correction or last available | 69.2 (45.3–89.4) | 24.0 (16.2–41.1) | <0.001 | 56.7 (34.1–88.8) | 14.2 (6.7–23.4) | <0.001 |
| Rate of sodium correction, mmol/L per h | ||||||
| at 0–24 h | 0.4 (0.2–0.6) | 0.7 (0.6–1.0) | <0.001 | 0.3 (0.2–0.4) | 0.9 (0.6–1.6) | <0.001 |
| Peak to normal level or last available | 0.3 (0.2–0.4) | 0.7 (0.6–1.4) | <0.001 | 0.2 (0.1–0.3) | 0.9 (0.6–1.6) | <0.001 |
ICU, intensive care unit; IQR, interquartile range.
Association of Serum Sodium Correction Rate with In-Hospital Mortality
The in-hospital mortality proportion was not significantly different between patients with admission hypernatremia with rapid correction versus slow correction (25% versus 28%; P=0.80) (Table 1). Similarly, the in-hospital mortality rate was not significantly different between patients with hospital-acquired hypernatremia with rapid correction versus slow correction (44% versus 40%; P=0.50) (Table 1). In multivariable analysis, rapid correction and 24-hour serum sodium correction rate was not associated with mortality in patients with admission hypernatremia (adjusted odds ratio [aOR], 1.3; 95% confidence interval [95% CI], 0.5 to 3.7; and aOR, 0.7; 95% CI, 0.3 to 1.6, respectively) (Table 3). Similarly, among patients with hospital-acquired hypernatremia there was no association between higher overall and 24-hour serum sodium correction rate and mortality (aOR, 1.3; 95% CI, 0.8 to 2.3; and aOR, 1.4; 95% CI, 0.9 to 2.4, respectively) (Table 3).
Table 3.
Measures of association and 95% CIs for overall correction rate and at 24 hours with the mortality outcome
| Variables | Admission Hypernatremia | Hospital-Acquired Hypernatremia | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | |||||
| Events, N (%) | Unadjusted | Adjusteda | Events, N (%) | Unadjusted | Adjusteda | |
| Overall sodium correction rate | ||||||
| ≤0.5 | 90 (74) | Ref | Ref | 225 (69) | Ref | Ref |
| >0.5 | 32 (26) | 0.9 (0.3 to 2.2) | 1.3 (0.5 to 3.7) | 102 (31) | 1.2 (0.7 to 1.9) | 1.3 (0.8 to 2.3) |
| At 24-h sodium correction rate | ||||||
| ≤0.5 | 62 (23) | Ref | Ref | 203 (77) | Ref | Ref |
| >0.5 | 58 (33) | 0.5 (0.2 to 1.3) | 0.7 (0.3 to 1.6) | 118 (67) | 1.3 (0.8 to 2.1) | 1.4 (0.9 to 2.4) |
95% CI, 95% confidence interval; OR, odds ratio; Ref, reference.
Adjusted by age, sex, do-not-resuscitate status, and Charlson Comorbidity Index score.
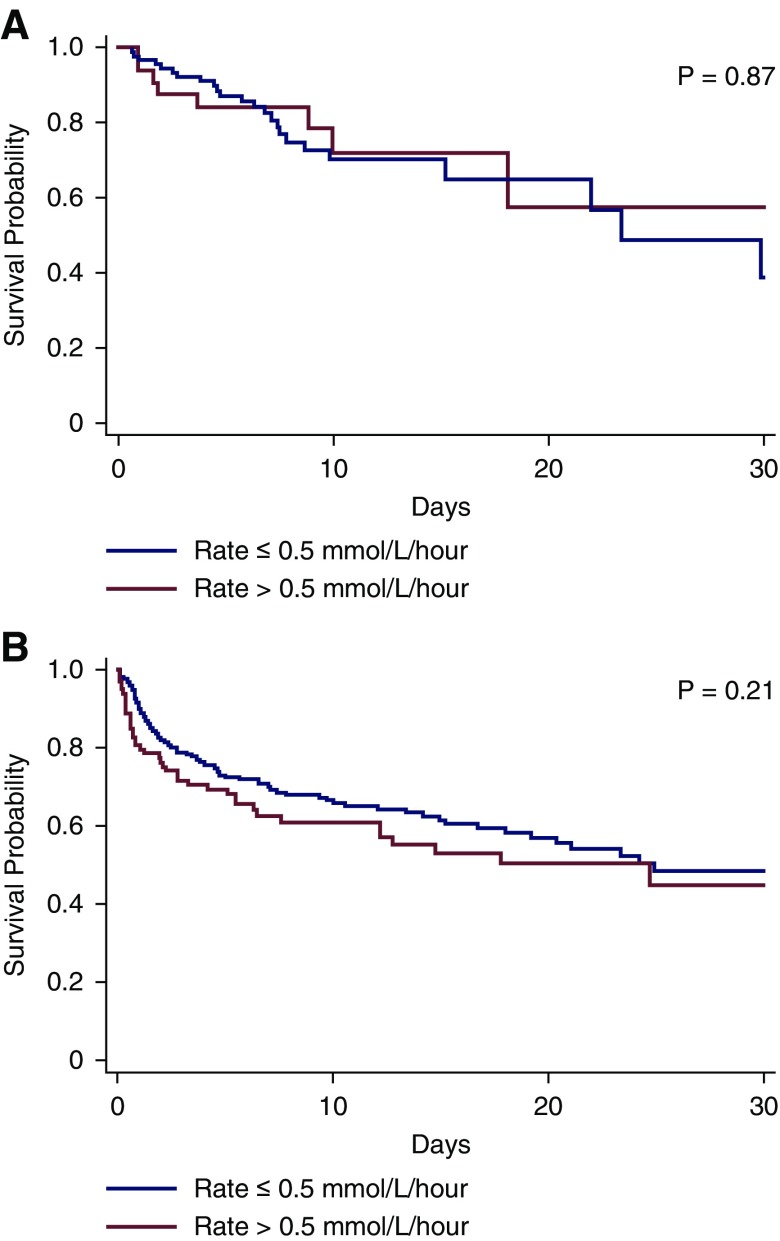
The Kaplan–Meier curves for 30-day survival for the rapid versus slow correction rate groups are shown in Figure 1, A and B and none of these curves were significantly different.
Figure 1.
Thirty-day survival curves for the rapid versus slow correction rate groups are not significantly different. Figure 1A and 1B represents admission and hospital-acquired hypernatremia patients respectively. Thirty-day survival patterns by rapid versus slow correction rate in patients with (A) admission and (B) hospital-acquired hypernatremia.
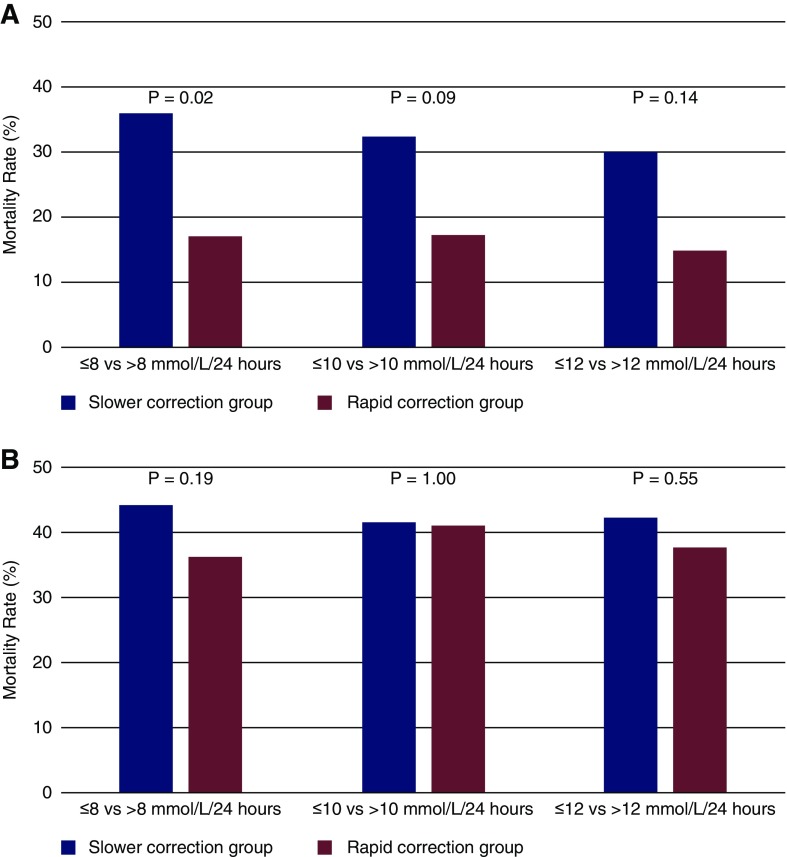
Incidence of in-hospital mortality by rapid versus slow correction rates by different cut-offs (>8, >10, and >12 mmol/L in 24 hours) for both groups of patients are presented in Figure 2, A and B. In subanalysis using different cut-offs of rapid correction (>8, >10, and >12 mmol/L at 24 hours), results for 30-day survival estimates were also largely consistent in both groups of patients (Supplemental Figure 2, A–C). In fact, there was a trend toward lower mortality in some of the rapid correction rate groups that did not reach statistical significance (Supplemental Figure 2, A and B). The mortality proportions in admission and hospital-acquired hypernatremia groups were not significantly different among sexes in both slower and rapid correction groups.
Figure 2.
Incidence of in-hospital mortality rates are lower in rapid correction rate group versus slow correction rate group but not significantly different by various cut-offs of correction rates Figure 2A and 2B represents admission and hospital-acquired hypernatremia patients respectively. In-hospital mortality proportions in patients with (A) admission and (B) hospital-acquired hypernatremia.
In subgroup analyses, the mortality rates in admission and hospital-acquired hypernatremia groups were not significantly different among sex and age (by median) categories (≤69 versus >69 years) in both slower and rapid correction groups (Supplemental Table 4). In patients with hospital-acquired hypernatremia, the mortality proportion was higher in patients who had acute hypernatremia, specifically in a group defined by a 48-hour interval (47% versus 32%; P=0.01). There is no significant difference in mortality proportion between slower and rapid correction group in any duration of hypernatremia development (Supplemental Table 4).
Manual Review of Potential Neurologic Complications due to Correction of Hypernatremia
We conducted a manual chart review of all available imaging reports, physician progress notes, and discharge summaries among the admission hypernatremia group and found no patients that had documented worsening mental status, seizures, or generalized cerebral edema due to correction of serum sodium. Among 327 hospital-acquired hypernatremia group, 128 were defined to have chronic hypernatremia. Similarly, in the manual chart reviews of patients with chronic hypernatremia, no patients experienced worsening mental status, seizures, or generalized cerebral edema due to correction of serum sodium. Moreover, among the 327 patients with hospital-acquired hypernatremia, there were 28 additional patients in addition to the 128 defined as having chronic hypernatremia that had ICD-9 codes for cerebral edema, seizures, and/or alteration of consciousness. None of these 128 patients had these symptoms secondary to hypernatremia correction. Additionally, 47 out of 122 patients with admission hypernatremia and 128 patients with hospital-acquired hypernatremia had progress notes available in the database from nursing staff, intensivists, physician residents, and physician attendings. We manually reviewed all these notes starting from the peak serum sodium level through discharge and were unable to find any documentation of an adverse event related to serum sodium correction. Rather than correction of hypernatremia, our manual chart review revealed that the top five major primary causes of worsening mental status, seizures, or cerebral edema were intracerebral hemorrhage, stroke, epilepsy, brain tumors, and brain trauma.
Discussion
In the largest cohort published to date to determine the effect of hypernatremia correction rate in critically ill patients (11–13), we found that rapid correction of both admission and hospital-acquired hypernatremia occurred in a third of patients, and that rapid correction >0.5 mmol/L per hour or >12 mmol/L per day was not associated with in-hospital mortality or cerebral edema. In fact, there were no cases of cerebral edema in the 78 patients who had serum sodium correction of >12 mmol/L per day.
The target rate of sodium reduction in hypernatremia treatment that is widely used in clinical practice is 0.5 mmol/L per hour, with a maximum rate of 10 mmol/L per day (1,6–8). However, the data to support this rate of correction is negligible (9). The studies in adults with hypernatremia showed that rapid sodium correction rates associated with less mortality (11,13). Alshayeb et al. (12) and Ates et al. (13) used >0.134 and ≥0.25 mmol/L per hour as a rapid hypernatremia correction rates, respectively. However, the definitions of rapid correction rates in these studies are <0.5 mmol/L per hour and could be considered as slow correction rates.
Unlike hyponatremia, where the risks of osmotic demyelination syndrome are well described and often studied in patients with rapid correction of serum sodium in all age groups (15), there have been no convincing reports of cerebral edema after rapid correction of hypernatremia in adults. Clinicians and trainees often extrapolate the data from hyponatremia and apply it to hypernatremia. Most often, correction of the serum sodium is a matter of increasing the rate of free water administration, especially in vulnerable patients who do not have access to or the capacity to ask for free water. Trepidation in the rate of correction leads to longer length of stay without any balancing benefit or evidence-based justification.
We performed multiple sensitivity analysis to determine the effect of varying rates of hypernatremia correction on in-hospital mortality. For the admission hypernatremia group, mortality rates were consistently lower in those with rapid correction (>8, >10, and >12 mmol/L) at 24 hours, with no difference in mortality overall. For the hospital-acquired hypernatremia group, there was no clinically important difference in in-hospital mortality in all rate groups at 24 hours and at overall. All previous studies have been done in the patients with hypernatremia at admission, and our results are consistent with them (11–13). However, patients who developed hospital-acquired hypernatremia were significantly different from those with admission hypernatremia with higher comorbidity burden and DNR status. The median serum bicarbonate in the hospital-acquired hypernatremia group with rapid correction group was significantly lower than the patients in slower correction group. Although we did not evaluate the fluid administration in our study, it is possible that there are differences in bicarbonate therapy in rapid versus slower serum sodium correction groups in patients with hospital-acquired hypernatremia. However, Zhang et al. (15) showed that the bicarbonate therapy in patients with metabolic acidosis was not associated with the mortality outcome using the same MIMIC-III database. Another randomized controlled study by Jaber et al. (16) showed similar results. Thus, we hypothesized that the differences in bicarbonate level in hospital-acquired hypernatremia would not affect the mortality outcome.
We found no neurologic complications associated with rapid hypernatremia correction. This is in contrast to the study in neonates, which reported seizures due to cerebral edema in the rapid correction group. There is a possible explanation for this conflicting outcome. The human brain volume rapidly increases during the first 6 years of life, and then progressively increases until age 15 years. In general, the ratio of brain volume to cranial vault size was greatest around age 6 years. In adults, the brain volume gradually reduces from age 45 years and reaches the lowest volume at age 86 years (17–19). These differences between children and adults limit brain adaptation and can potentially explain the edema associated with rapid hypernatremia correction seen only in infants.
Our study has several advantages. First, to the best of our knowledge, this is the largest adult cohort study focusing on the neurologic complications and mortality after hypernatremia correction in critically ill adults. Second, we conducted a comprehensive manual review of neurologic outcomes where the imaging reports and discharge summaries were available. Third, the previous studies were done entirely in patients with hypernatremia present on admission, whereas we included patients with both admission and hospital-acquired hypernatremia and demonstrated the distinct differences in cohort characteristics and outcomes. Finally, we accounted for many factors (including comorbidity burden and DNR status) that may confound the association between hypernatremia correction and mortality.
These results, however, should be interpreted in light of some limitations. First, we were unable to identify the exact timing of the onset of hypernatremia among patients with admission hypernatremia. Animal studies have shown that increasing concentration of idiogenic osmoles plays an important role in the regulation of intracellular osmolality during the course of hypernatremia. However, this change occurs only in chronic hypernatremia (20). Therefore, the patients with chronic hypernatremia were theoretically more susceptible for neurologic complication and their outcomes may differ from acute hypernatremia after rapid sodium reduction. Nevertheless, we could not find any instances of neurologic complications from hypernatremia correction regardless of chronicity of the onset. Second, we included patients with sodium level ≥155 mmol/L and patients with lower sodium levels were not included. Theoretically, the adverse effects of rapid hypernatremia correction in patients with mild hypernatremia is less than what we found in our study; however, we could not find any neurologic complications associated with rapid correction. Third, the types of fluids used to correct hypernatremia was not evaluated in this study, and it could be one of the confounding factors. However, the evidence to suggest that treatment with different fluid administration strategies would have an influence on outcomes in critically ill patients with hypernatremia is limited. Fourth, only 47 patients had progress notes available for a detailed manual chart review to assess potential complications. However, we did manually review all imaging reports and discharge summaries for patients with chronic hypernatremia. Finally, we lacked an external validation cohort or a cohort with noncritically ill patients.
In conclusion, we did not find any evidence that rapid correction of hypernatremia was associated with a higher risk for mortality, seizure, alteration of consciousness, and/or cerebral edema in critically ill adult patients with either admission or hospital-acquired hypernatremia. These findings point toward the fact that the fears of rapid correction of hypernatremia may be overstated and clinicians should consider correcting the serum sodium level with free-water administration to shorten the length of stay in this vulnerable patient population.
Disclosures
Dr. Coca reports personal fees and other from RenalytixAI, personal fees from CHF Solutions, personal fees from Quark, personal fees from Takeda, personal fees from Janssen, personal fees and other from pulseData, and personal fees from Goldfinch, outside the submitted work. Dr. Nadkami reports personal fees and other funding from RenalytixAI, personal fees and other funding from pulseData, research funding from Goldfinch, outside the submitted work, and personal fees from BioVie, outside the submitted work. Dr. Van Vleck reports personal fees from Clinithink, outside the submitted work. Dr. Chan, Dr. Chauhan, Dr. Chaudhary, Dr. Debnath, Dr. Duffy, Dr. Patel, Dr. Pattharanitima, and Dr. Saha have nothing to disclose.
Supplementary Material
Acknowledgments
Dr. Chan reports grants from the National Institutes of Health (5T32DK007757-18), outside the submitted work. Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Evidence for Managing Hypernatremia: Is It Just Hyponatremia in Reverse?,” on pages 645–647.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10640918/-/DCSupplemental.
Supplemental Table 1. List of International Classification of Diseases, Ninth Revision (ICD-9) codes used.
Supplemental Table 2. Characteristics of adults admitted to ICU with hypernatremia at admission versus hospital acquired.
Supplemental Table 3. Distribution of the sodium level, difference, and correction time of adults admitted to ICU with hypernatremia at admission versus hospital‐acquired.
Supplemental Table 4. Mortality rates of adults admitted to ICU with hypernatremia at admission and patients with hospital-acquired hypernatremia by slower versus rapid correction rate.
Supplemental Figure 1. Selection of patients with hypernatremia from the MIMIC‐III database.
Supplemental Figure 2. Thirty‐day survival patterns by rapid versus slow correction rate in admission and hospital-acquired hypernatremia groups.
References
- 1.Adrogué HJ, Madias NE: Hypernatremia. N Engl J Med 342: 1493–1499, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Chumlea WC, Guo SS, Zeller CM, Reo NV, Siervogel RM: Total body water data for white adults 18 to 64 years of age: The Fels Longitudinal Study. Kidney Int 56: 244–252, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Palevsky PM, Bhagrath R, Greenberg A: Hypernatremia in hospitalized patients. Ann Intern Med 124: 197–203, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Funk G-C, Lindner G, Druml W, Metnitz B, Schwarz C, Bauer P, Metnitz PG: Incidence and prognosis of dysnatremias present on ICU admission. Intensive Care Med 36: 304–311, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Lindner G, Funk G-C, Schwarz C, Kneidinger N, Kaider A, Schneeweiss B, Kramer L, Druml W: Hypernatremia in the critically ill is an independent risk factor for mortality. Am J Kidney Dis 50: 952–957, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Braun MM, Barstow CH, Pyzocha NJ: Diagnosis and management of sodium disorders: Hyponatremia and hypernatremia. Am Fam Physician 91: 299–307, 2015 [PubMed] [Google Scholar]
- 7.Al-Absi A, Gosmanova EO, Wall BM: A clinical approach to the treatment of chronic hypernatremia. Am J Kidney Dis 60: 1032–1038, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Sterns RH: Disorders of plasma sodium--causes, consequences, and correction. N Engl J Med 372: 55–65, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Kahn A, Brachet E, Blum D: Controlled fall in natremia and risk of seizures in hypertonic dehydration. Intensive Care Med 5: 27–31, 1979 [DOI] [PubMed] [Google Scholar]
- 10.Bolat F, Oflaz MB, Güven AS, Özdemir G, Alaygut D, Doğan MT, Içağasoğlu FD, Cevit Ö, Gültekin A: What is the safe approach for neonatal hypernatremic dehydration? A retrospective study from a neonatal intensive care unit. Pediatr Emerg Care 29: 808–813, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Bataille S, Baralla C, Torro D, Buffat C, Berland Y, Alazia M, Loundou A, Michelet P, Vacher-Coponat H: Undercorrection of hypernatremia is frequent and associated with mortality. BMC Nephrol 15: 37, 2014. Available at: http://bmcnephrol.biomedcentral.com/articles/10.1186/1471-2369-15-37. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alshayeb HM, Showkat A, Babar F, Mangold T, Wall BM: Severe hypernatremia correction rate and mortality in hospitalized patients. Am J Med Sci 341: 356–360, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Ates I, Özkayar N, Toprak G, Yılmaz N, Dede F: Factors associated with mortality in patients presenting to the emergency department with severe hypernatremia. Intern Emerg Med 11: 451–459, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Johnson AEW, Pollard TJ, Shen L, Lehman LW, Feng M, Ghassemi M, Moody B, Szolovits P, Celi LA, Mark RG: MIMIC-III, a freely accessible critical care database. Sci Data 3: 160035, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Zhu C, Mo L, Hong Y: Effectiveness of sodium bicarbonate infusion on mortality in septic patients with metabolic acidosis. Intensive Care Med 44: 1888–1895, 2018. [Internet] [DOI] [PubMed] [Google Scholar]
- 16.Jaber S, Paugam C, Futier E, Lefrant J-Y, Lasocki S, Lescot T, Pottecher J, Demoule A, Ferrandière M, Asehnoune K, Dellamonica J, Velly L, Abback P-S, de Jong A, Brunot V, Belafia F, Roquilly A, Chanques G, Muller L, Constantin J-M, Bertet H, Klouche K, Molinari N, Jung B; BICAR-ICU Study Group : Sodium bicarbonate therapy for patients with severe metabolic acidaemia in the intensive care unit (BICAR-ICU): A multicentre, open-label, randomised controlled, phase 3 trial. Lancet 392: 31–40, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Dekaban AS: Changes in brain weights during the span of human life: Relation of brain weights to body heights and body weights. Ann Neurol 4: 345–356, 1978 [DOI] [PubMed] [Google Scholar]
- 18.Hafkemeijer A, Altmann-Schneider I, de Craen AJ, Slagboom PE, van der Grond J, Rombouts SA: Associations between age and gray matter volume in anatomical brain networks in middle-aged to older adults. Aging Cell 13: 1068–1074, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourisly AK, El-Beltagi A, Cherian J, Gejo G, Al-Jazzaf A, Ismail M: A voxel-based morphometric magnetic resonance imaging study of the brain detects age-related gray matter volume changes in healthy subjects of 21-45 years old. Neuroradiol J 28: 450–459, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lien YH, Shapiro JI, Chan L: Effects of hypernatremia on organic brain osmoles. J Clin Invest 85: 1427–1435, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.