Abstract
Pulmonary pleomorphic carcinoma (PPC) is resistant to anticancer drug treatment, outcomes are poor, and no standard therapy has been established. High PD‐L1 expression has been found in PPCs, suggesting the possible efficacy of an immune checkpoint inhibitor (ICI) in cancer immunotherapy; however, this approach requires further investigation through case accumulation. Herein, we report a case of rapid recurrence and progression of PPC early after surgery in a 70‐year‐old male ex‐smoker. Surgery was performed for lung cancer of the right lower lobe, and a pathological examination indicated primary PPC with high PD‐L1 expression (tumor proportion score: 90%). Because systemic metastasis recurred only six weeks after surgery, nivolumab was administered as second‐line treatment. Marked tumor regression was observed on imaging after three cycles, revealing a near complete response. Palliative radiotherapy was applied to the bone metastasis region for pain relief before nivolumab was administered. This case suggests that an ICI can have an effect on PPC and that the efficacy of ICIs may be enhanced by radiotherapy‐induced abscopal effects.
Keywords: Abscopal effect, antitumor immunity, immune checkpoint inhibitor, nivolumab, pulmonary pleomorphic carcinoma
Introduction
Pulmonary pleomorphic carcinoma (PPC) is a rare disease that accounts for 0.1–0.4% of all cases of malignant lung tumors.1, 2 PPC is resistant to treatment, such as chemotherapy and radiotherapy, and prognosis is poor. Moreover, the recurrence rate after surgical resection is high and the biological malignancy is very high.3 PPCs express high levels of PD‐L1, which suggests that therapy with an immune checkpoint inhibitor (ICI) may be effective.4, 5 This approach has occasionally been reported,6, 7, 8 but further case accumulation is needed and the use of an ICI for PPC has not been established. We encountered a patient with rapid progressive systemic metastasis and recurrence of PPC early after surgery. Bone metastasis was treated with palliative radiotherapy followed by administration of an ICI, nivolumab, and a marked effect was noted after only three cycles, achieving a near complete response.
Case report
A 63‐year‐old male smoker (60 pack‐year) was referred to our department after detection of an abnormal shadow in the chest. The preoperative diagnosis was adenocarcinoma of the right lower lobe of the lung (cT4N1M0, stage IIIA) based on bronchoscopy and imaging (Fig 1a–d). Resection of the right lower lobe and lymph node dissection were performed via thoracotomy without preoperative adjuvant chemotherapy because of the need for immediate surgery after the clinical diagnosis was made. The tumor was EGFR mutation‐negative, ALK fusion gene‐negative, pleomorphic carcinoma (pT4N2M0 stage IIIB), and PD‐L1 expression had a tumor proportion score (TPS) of 90% (Fig 2a–c). Postoperative adjuvant chemotherapy was planned, but progressive systemic metastasis rapidly recurred six weeks after surgery (Fig 1e–h). The patient was treated with first‐line carboplatin (CBDCA) + nanoparticle albumin‐bound paclitaxel (nab‐PTX) for unresectable non‐small cell lung cancer, and palliative irradiation was applied at a total dose of 30 Gy to the right hip joint over the right femur for pain relief. As progressive aggravation and a lack of disease control was noted after one cycle of chemotherapy (Fig 1i,j), the drug was switched to nivolumab as second‐line therapy, and a near complete response was achieved after only three cycles (Fig 1k–n). Treatment continues at the time of writing. The patient provided consent of the use of his data for publication.
Figure 1.
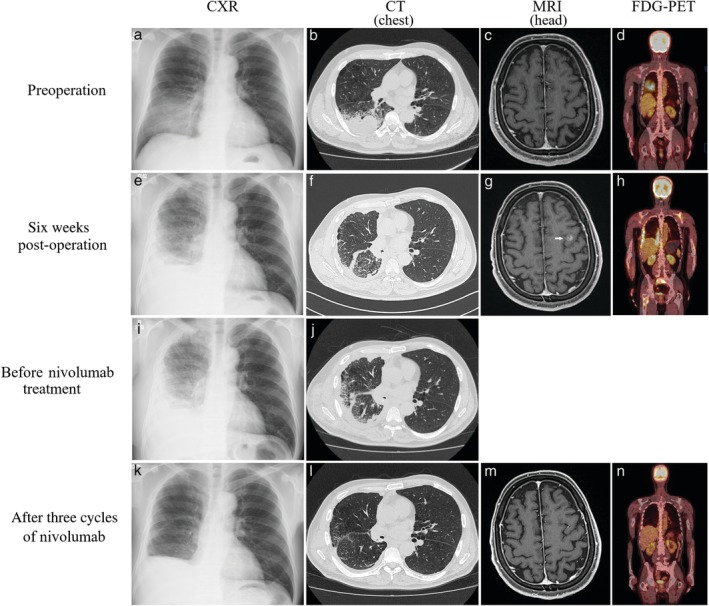
Course of imaging findings. (a) Chest X‐ray (CXR): A shadow was present in the right middle over the lower lung field. (b) Chest computed tomography (CT): A shadow of a mass was observed in the right lower lobe. (c) Contrast brain magnetic resonance imaging (MRI): No brain metastasis was noted. (d) Fluorodeoxyglucose (FDG)‐positron emission tomography (PET): FDG accumulation occurred that was consistent with the primary lesion. (e) CXR: Reduction of radiolucency of the entire right lung and pleural effusion were noted. (f) Chest CT: A feature of dissemination was present in the right pleura and findings suggesting carcinomatous lymphangiosis were observed in the lung parenchyma. (g) Contrast brain MRI: Brain metastasis was noted in the left frontal lobe (arrow). (h) FDG‐PET: FDG accumulated in the right pleura, left scapula, sacrum, and right femur. (i) CXR: Radiolucency of the lung field was progressive deterioration. (j) Chest CT: Dissemination in the right pleura and carcinomatous lymphangiosis were progressive deterioration. (k) CXR: The right lung showed only postoperative changes, and radiolucency of the lung field was improved. (l) Chest CT: Dissemination in the pleura of the right lung and carcinomatous lymphangiosis had mostly disappeared, and expansion of the right lung had occurred. (m) Contrast brain MRI: Brain metastasis noted in the left frontal lobe had disappeared. (n) FDG‐PET: Many FDG accumulation sites that were present after surgery had disappeared.
Figure 2.
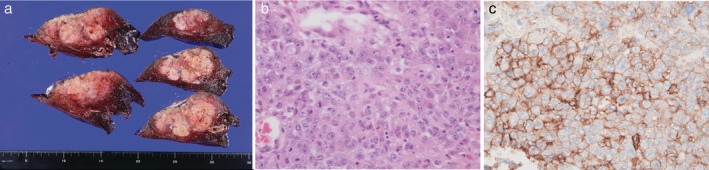
(a) Macroscopic image of the cut surface of the tumor measuring 78 × 42 × 60 mm in the resected lung. The inner region had partial necrosis. (b) Histopathology showed a pleomorphic tumor with strong nuclear atypia, such as a macronucleus and multinucleation, forming a solid alveolar lesion (hematoxylin and eosin staining: ×400 magnification). (c) Immunohistochemical analysis showed that the tumor cells strongly expressed PD‐L1 (Dako 22C3 clone staining: tumor proportion score 90%, ×400 magnification).
Discussion
PD‐L1 expression was high (TPS 90%) in our patient and has also been found to be high in previous reports of PPC.4, 5 The PD‐L1‐positive rate is regarded as a predictive marker of the efficacy of an ICI for lung cancer;9 however, in reports of three PPC cases with TPS ≥ 60%, the outcomes were partial response in two and stable disease in one.7 These results indicate that the high efficacy in our patient may not depend on high PD‐L1 expression alone.
We suggest that abscopal effects stimulated by local radiotherapy may have contributed to the effect of ICI, in addition to high PD‐L1 expression. The term “abscopal effect” was initially used by Mole et al. in 1953 to describe the phenomenon of enhanced immunity by strengthening tumor antigenicity in an irradiated region.10, 11 Cancer cells that are killed or weakened by irradiation release cancer antigens with immunostimulatory effects that lead to immunogenic cell death. Antigen‐presenting cells, such as macrophages, process these antigens and dendritic cells, through which tumor‐specific cytotoxic T lymphocytes (CTLs) are activated, migrate throughout the body, and find and attack cancer cells other than those that were directly irradiated. However, an increase in the total number of CTLs alone is not regarded as activation of cancer immunity.12, 13 Because activation cannot be shown without an increase in tumor‐specific CTLs killing cancer cells, Suzuki et al. suggested that the presence of an abscopal effect could be indirectly demonstrated by determining the number of tumor‐specific CTLs in the blood before and after irradiation, and observing an increase in the number of lymphocytes attacking cancer only.14 However, measurement of tumor‐specific CTLs is possible only at the research level, and could not be performed in our patient. Therefore, the presence of an abscopal effect was judged based on the clinical course alone. If measurement of tumor‐specific CTLs had been possible, this would have directly demonstrated an abscopal effect. With the development of ICIs, the synergistic effect of cancer immunotherapy and abscopal effects has recently attracted attention, and combination treatment with radiotherapy and an ICI may be effective.15 Radiotherapy at one site may induce the effect of the drug, even in patients with multiple metastases – our patient had received palliative radiotherapy before nivolumab administration. An abscopal effect in combination with ipilimumab has been reported and is currently being investigated in clinical studies.15 Therefore, prior radiotherapy may increase the effect of an ICI on treatment‐resistant PPC.
Our case and previous findings suggest the need to investigate PPC as a therapeutic target of ICIs and to examine whether greater efficacy can be obtained by irradiation before ICI administration, including palliative irradiation, to induce the abscopal effect. Future studies including analysis of accumulated cases and formal clinical trials are desired.
Disclosure
No authors report any conflict of interest.
References
- 1. Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization Classification of Lung Tumors. Eur Respir J 2001; 18: 1059–68. [DOI] [PubMed] [Google Scholar]
- 2. Travis WD. Pathology of lung cancer. Clin Chest Med 2011; 32: 669–92. [DOI] [PubMed] [Google Scholar]
- 3. Mochizuki T, Ishii G, Nagai K et al. Pleomorphic carcinoma of the lung: Clinicopathologic characteristics of 70 cases. Am J Surg Pathol 2008; 32: 1727–35. [DOI] [PubMed] [Google Scholar]
- 4. Chang YL, Yang CY, Lin MW, Wu CT, Yang PC. High co‐expression of PD‐L1 and HIF‐1α correlates with tumour necrosis in pulmonary pleomorphic carcinoma. Eur J Cancer 2016; 60: 125–35. [DOI] [PubMed] [Google Scholar]
- 5. Kim S, Kim MY, Koh J et al. Programmed death‐1 ligand 1 and 2 are highly expressed in pleomorphic carcinomas of the lung: Comparison of sarcomatous and carcinomatous areas. Eur J Cancer 2015; 51: 2698–707. [DOI] [PubMed] [Google Scholar]
- 6. Ito K, Hataji O, Katsuta K et al. “Pseudoprogression” of pulmonary pleomorphic carcinoma during nivolumab therapy. J Thorac Oncol 2016; 11: e117–9. [DOI] [PubMed] [Google Scholar]
- 7. Kanazu M, Uenami T, Yano Y et al. Case series of pleomorphic carcinomas of the lung treated with nivolumab. Thorac Cancer 2017; 8: 724–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Okamura K, Fukuda Y, Soda H et al. Pulmonary pleomorphic carcinoma with few PD‐1‐positive immune cells and regulatory T cells that showed a complete response to nivolumab. Thorac Cancer 2018; 9: 193–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shukuya T, Carbone DP. Predictive markers for the efficiency of anti‐PD‐1/PD‐L1 antibodies in lung cancer. J Thorac Oncol 2016; 11: 976–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol 1953; 26: 234–41. [DOI] [PubMed] [Google Scholar]
- 11. Brix N, Tiefenthaller A, Anders H, Belka C, Lauber K. Abscopal, immunological effects of radiotherapy: Narrowing the gap between clinical and preclinical experiences. Immunol Rev 2017; 280: 249–79. [DOI] [PubMed] [Google Scholar]
- 12. Kono K, Mimura K, Kiessling R. Immunogenic tumor cell death induced by chemoradiotherapy: Molecular mechanisms and a clinical translation. Cell Death Dis 2013; 4: e688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer 2018; 18: 313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suzuki Y, Mimura K, Yoshimoto Y et al. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer Res 2012; 72: 3967–76. [DOI] [PubMed] [Google Scholar]
- 15. Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer 2016; 4: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]


