Abstract
Background
Diabetes mellitus (DM) is linked to an increased risk of lung cancer; however, the exact molecular basis is unclear.
Methods
We used a microarray method and found a group of microRNAs differently expressed in lung cancer cells at high or low glucose treatment.
Results
Among these, miR‐194 changed significantly, which indicated further analysis. miR‐194 was significantly downregulated in non‐small cell lung cancer (NSCLC) cells cultured in high glucose (HG) medium and clinical NSCLC tissues with DM. The introduction of miR‐194 significantly suppressed the proliferation, migration, and invasion of lung cancer cells induced by HG, suggesting that miR‐194 may be a suppressor during HG‐induced NSCLC progression. Further analysis indicated that NFAT5 was a direct target gene of miR‐194, evidenced by the direct binding of miR‐194 with the 3’untranslated region of NFAT5. MiR‐194 could decrease the expression of NFAT5 at both messenger RNA and protein levels, while overexpression of NFAT5 reversed the decreased proliferation, migration, and invasion ability mediated by miR‐194 in lung cancer cells.
Conclusion
Our findings provide new insight into the mechanism of NSCLC progression. Therapeutically, miR‐194 may serve as a potential target for the treatment of lung cancer patients with DM.
Keywords: HG, LG, MiR‐194, NFAT5, NSCLC
Introduction
Lung cancer, predominantly non‐small cell lung cancer (NSCLC), remains the leading cause of cancer‐related death worldwide.1 Although advances have been made in diagnosis and therapy in recent years, the prognosis of lung cancer patients remains unsatisfactory. To guide decisionmaking of therapeutic strategies for lung cancer patients and improve prognosis, a better understanding of the relevant factors affecting lung cancer prognosis is urgently needed.
Diabetes mellitus (DM) is a common disease worldwide.2 According to a 2014 World Health Organization (WHO) report, approximately 422 million individuals had DM, with the number expected to rise to 552 million by 2030.3 DM is a major cause of other diseases, including kidney failure, diabetes‐related heart diseases, lower limb amputation, and cancer.4, 5, 6 Increasing evidence has shown that DM could increase the risk of lung cancer, especially among female patients.7, 8, 9
MicroRNAs (miRNAs) are an abundant class of small, non‐coding RNAs, approximately 19–25 nucleotides. They negatively regulate gene expression at the posttranscription level by interacting with the 3’untranslated regions (UTRs) of target messenger RNA (mRNA). Emerging evidence indicates that miRNAs are aberrantly expressed in various tumors, and play an important role in the progression of cancer, such as tumor proliferation, invasion, and metastasis.10, 11, 12, 13 MiR‐194 is downregulated in hepatocellular carcinoma 14 and bladder,15 gastric,16, 17, 18 gallbladder,19 and lung cancers.20 The biological targets of miR‐194 have been partially identified, such as RAP2B, KDM5B, RBX1, FoxM1, FOXA1, and MAP4K4. However, little is known about the role of miR‐194 in HG‐induced lung cancer progression.
NFAT5 is a member of the NFAT protein family, which has a DNA binding domain structurally similar to the Rel‐homology region of NF‐Kb.21 NFAT5 plays important roles in embryonic development, cell differentiation, inflammatory processes, and cellular stress response in a tonicity‐independent manner in cells and tissues.22 Furthermore, accumulating evidence indicates that NFAT5 is implicated in tumor progression, metastasis, and tumor cell proliferation;23 for example, NFAT5 can promote renal carcinoma cell proliferation and invasion, and promote melanoma metastasis.24, 25, 26, 27, 28
We investigated the influence of HG on lung cancer cell proliferation, migration, and invasion.
Methods
Samples
Fresh samples of NSCLC tissue were obtained from 50 patients at the Second Affiliated Hospital of Tianjin Medical University between May 2012 and December 2017. The samples were immediately snap frozen in liquid nitrogen and stored at −80°C until RNA extraction. The tumors were classified according to the WHO classification. The hospital ethical committee approved the study, and all patients provided written informed consent.
Cell culture
Human lung cancer cell lines A549 and H1299 were cultured in RPMI‐1640 medium (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS, Gibco, Grand Island, NY, USA), 100 IU/mL penicillin, and 100 IU/mL streptomycin in humidified 5% CO2 at 37°C. The medium was supplemented with 25 mM glucose (high glucose [HG]) or 5.5 mM glucose (low glucose [LG]).
Transfection
The cells were plated on an antibiotic‐free growth medium at 30–50% confluence approximately 24 hours before transfection. RNA oligonucleotides were transfected at a final concentration of 100 nM, using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Further treatment proceeded 24 hours posttransfection.
Quantitative real‐time PCR
Quantitative real time (qRT) PCR was performed to validate the mRNA expression level using SYBR Premix Ex TaqTM (TaKaRa, Tokyo, Japan). PCR was performed in triplicate and results were analyzed using the ABI Prism 7900HT Fast Real‐Time PCR system (Applied Biosystems, Foster City, CA, USA). The relative quantification values for each gene were calculated by the 2‐ΔΔCt method using glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) as an internal reference. Primer sequences were as follows, NFAT5: forward 5′‐GAA GTG GAC ATT GAA GGC ACT‐3′, reverse 5′‐CTG GCT TCG ACA TCA GCA TT‐3′; GAPDH: forward 5′‐TGC ACC ACC AAC TGC TTA GC‐3′, reverse 5′‐GGC ATG CAC TGT GGT CAT GAG‐3′; miR‐194: forward 5′‐ATG GAC CTG GGG CCA GCG AAG ‐3′, reverse, 5′‐TCT GGC CTG GGA GCG TCG‐3′; and U6: forward 5′‐ CTC GCT TCG GCA GCA CA‐3′, reverse 5′‐ AAC GCT TCA CGA ATT TGC GT‐3′.
Cell viability assay
Cells transfected with miR‐194 or control miRNA mimics were harvested and plated in 96‐well plates at 2000 cells/well and incubated at 37°C. Cell viability was analyzed using Cell Counting Kit‐8 (CCK‐8, Beyotime Biotechnology, Shanghai, China) according to the manufacturer's protocol.
Cell proliferation assay
Cells transfected with miR‐194 or control miRNA mimics were harvested and plated in six‐well plates at 10000 cells/well. After incubation for 48 hours at 37°C, the cell proliferation ability was analyzed by cell counting.
Cell migration assay
The migration ability was determined using wound‐healing assay. Equivalent A549 and H1299 cells were plated into 12‐well plates without antibiotics. The cells were transfected with miR‐194 mimic (miR‐194) or mimic control (NC); 24 hours later, transfected cells were wounded with a sterile plastic 100 μL micropipette tip, the floating debris was washed with phosphate buffered saline, and the cells were then cultured in serum‐free medium. The width of the wound was measured at different time points. Three to four different locations were visualized and photographed under a phase‐contrast inverted microscope (Olympus, Tokyo, Japan).
Cell invasion assay
Transwell assay was used to examine cell invasion capability. Cells were transfected with miR‐ 194 mimic, mimic control, miR‐194 inhibitor, or inhibitor control (Ribobio, Guangzhou, China). Sixteen hours later the transfected cells were trypsinized and resuspended and 1.0 × 104 cells in 200 μL RPMI‐1640 medium were placed into the upper chambers (8 mm pore size; Millipore, Billerica, MA, USA). The lower chambers were filled with 600 μL complete medium with 10% FBS. After incubation for 12 hours at 37°C, the non‐invading cells were removed from the top of the chamber with a cotton swab. The invasion cells on the lower surface of the inserts were fixed and stained with 0.1% crystal violet, and five random fields for each insert were counted at 100× magnifications.
Western blotting
Cells were transfected with either pCMV‐NFAT5 or pCMV empty vector. Total cell extracts prepared from cells using radioimmunoprecipitation assay buffer (Beyotime Biotechnology) were resolved on 10% gradient sodium dodecyl sulfate‐polyacrylamide gel and transferred NC membranes. Membranes were blocked for one hour in 5% skim milk in tris‐buffered saline plus tween 20 and incubated with primary antibody overnight at 4°C, followed by incubation with appropriate horseradish peroxidase‐conjugated secondary antibody at optimized concentration. The primary antibodies used in this study were: anti‐NFAT5 antibody (1:1000, Abcam, Cambridge, UK) and anti‐β‐actin antibody (1:5000, Cell Signaling Technology, Danvers, MA, USA). The densitometry of Western blot results was measured using Image J software.
Dual‐luciferase reporter assay
Cells were seeded into 24‐well plates and co‐transfected with 200 ng of pMIR‐NFAT5 or pMIR‐NFAT5‐Mut vector and 100 ng of miR‐194 mimic or mimic control, and the pRL‐TK plasmid (Promega, Madison, WI, USA), which was used as internal normalization. After 48 hours, the cells were lysed using lysis buffer (Promega, Madison, WI, USA). Luciferase reporter gene assay was implemented using the Dual‐Luciferase Reporter Assay System (Promega) according to the manufacturer's instructions.
Statistical analysis
SPSS version 16.0 was used to complete data processing. Each experiment was repeated at least three times. Statistical significance was assessed by comparing mean values (± standard deviation) using a Student's t‐test for independent groups. The χ2 test was used to evaluate correlation between miR‐194 expression and clinical pathologic parameters. Results were considered statistically significant at P < 0.05(*).
Results
High‐glucose (HG) promotes non‐small cell lung cancer (NSCLC) cell proliferation, migration, and invasion
A549 and H1299 cells were cultured in 1640 medium with 5 mM LG or 25 mM HG for 48 hours. The cell proliferation ability was then tested by cell counting and CCK‐8 assays. The results showed that the proliferation ability of A549 and H1299 cells was increased in the HG group (Fig 1a,b). Wound healing assay was used to examine cell migration ability. The results showed that HG significantly promoted cell migration compared to LG (Fig 1c). We performed Transwell assay to investigate the effect of HG on cell invasion. As shown in Figure 1d,e, the invasion ability of A549 and H1299 cells was increased in the HG compared to the LG group. These results demonstrate that HG could accelerate the growth, proliferation, and migration ability of lung cancer cells.
Figure 1.
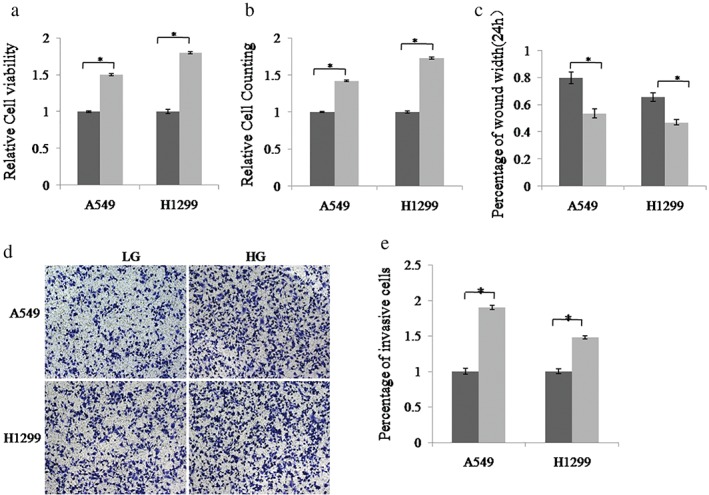
High glucose (HG)‐induced the proliferation, migration, and invasion of non‐small cell lung cancer (NSCLC) cells. A549 and H1299 cells were cultured in low glucose (LG, 5 mM) and HG (25 mM) medium, respectively. The cell proliferation ability was measured using (a) cell counting kit‐8 assays and (b) cell counting. (c) Cell migration ability was detected using wound‐healing assay. (d,e) The invasion ability of NSCLC cells was determined by Transwell assay.  LG,
LG,  HG. Data are reported as mean ± standard deviation of three independent experiments (*P < 0.05, t‐test).
HG. Data are reported as mean ± standard deviation of three independent experiments (*P < 0.05, t‐test).
MiRNA‐194 is down‐expressed in NSCLC cells and specimens in HG condition
A number of miRNAs are aberrantly expressed in DM patients. MiRNAs are involved in NSCLC proliferation and metastasis. Therefore, we analyzed the miRNA expression profile of A549 cells with HG and LG treatment by miRNA microarray analysis. A total of 20 miRNAs were downregulated in HG condition (Fig 2a). miR‐1255, miR‐518d, miR‐194‐1, and miR‐103a‐2 showed the lowest expression, while the role of miR‐194 in NSCLC with DM remains unknown. QRT‐PCR confirmed that miR‐194 expression was downregulated in lung cancer cell lines A549 and H1299 treated with HG culture medium (Fig 2b). Furthermore, we found that miR‐194 expression was significantly decreased in lung cancer tissues with DM compared to tissues without DM (P < 0.001) (Fig 2c).
Figure 2.
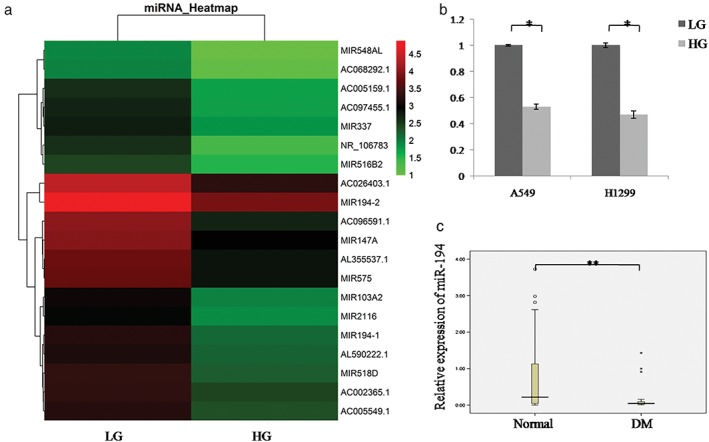
miR‐194 is downregulated in lung cancer cells and NSCLC tissue specimens with DM treated with high glucose (HG). (a) MicroRNA chips showed that miR‐194 was downregulated in HG treated A549 cells compared to low glucose (LG) treated cells. (b) MiR‐194 was detected in LG or HG treated A549 and H1299 cells using quantitative real time‐PCR assay. Data are reported as mean ± standard deviation of three independent experiments (*P < 0.05, t‐test).  LG,
LG,  HG. (c) Quantitative real‐time‐PCR analysis of miR‐194 expression in 50 cases of NSCLC tissues with or without DM (**P < 0.001, Wilcoxon signed‐rank test). DM, diabetes mellitus.
HG. (c) Quantitative real‐time‐PCR analysis of miR‐194 expression in 50 cases of NSCLC tissues with or without DM (**P < 0.001, Wilcoxon signed‐rank test). DM, diabetes mellitus.
MiRNA‐194 inhibits proliferation, migration, and invasion of NSCLC cells
We then tested the functional significance of miR‐194 in NSCLC cells. A549 and H1299 cells were transfected with miR‐194 mimic (miR‐194) or mimic control (NC), and wound healing assay was used to examine the cell migration ability. The results showed that miR‐194 overexpression significantly inhibited cell migration compared to the control group (Fig 3a). Furthermore, we performed Transwell assay to investigate the effect of miR‐194 on cell invasion. As shown in Figure 3b,c, when transfected with miR‐194 mimics, the invasion ability of A549 and H1299 cells was decreased compared to the control group. However, the cells showed increased invasion upon treatment with the miR‐194 inhibitor. Additionally, we investigated the effect of miR‐194 on cell proliferation. As shown in Figure 3d,e, when transfected with miR‐194 mimics, the proliferation ability of A549 and H1299 cells was downregulated compared to the control group. These results strongly suggest that miR‐194 can suppress the proliferation, migration, and invasion of NSCLC cells.
Figure 3.
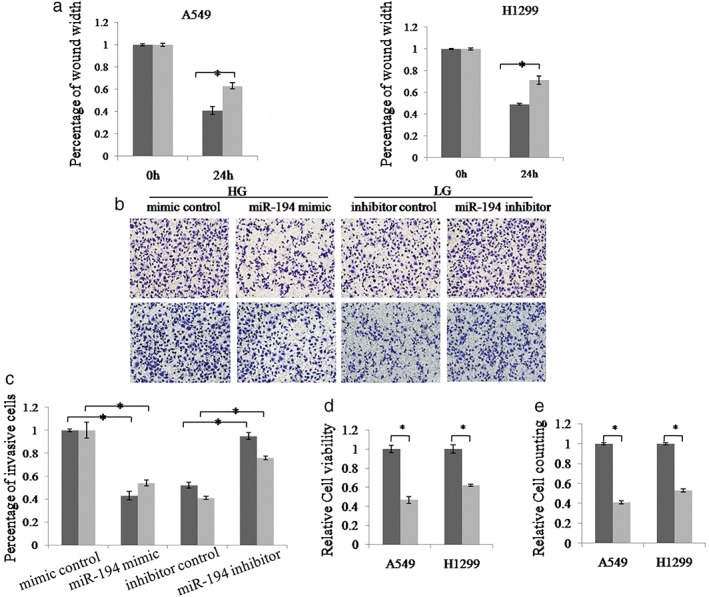
miR‐194 inhibits the proliferation, migration and invasion of non‐small cell lung cancer (NSCLC) cells. (a) Wound healing assay was used to detect the migration ability of A549 and H1299 cells. Cells were transfected with miR‐194 mimic or mimic control.  HG+NC,
HG+NC,  HG+miR‐194. (b, c) Transwell assay was employed to examine the invasion ability of A549 and H1299 cells. The invasive cell number in each group was normalized to the control. Cells were transfected with miR‐194 mimic or mimic control, and miR‐194 inhibitor or inhibitor control.
HG+miR‐194. (b, c) Transwell assay was employed to examine the invasion ability of A549 and H1299 cells. The invasive cell number in each group was normalized to the control. Cells were transfected with miR‐194 mimic or mimic control, and miR‐194 inhibitor or inhibitor control.  A549,
A549,  H1299. (d,e) Cell counting kit‐8 and cell counting assay were used to examine the proliferation ability of A549 and H1299 cells. Cells were transfected with miR‐194 mimic or mimic control.
H1299. (d,e) Cell counting kit‐8 and cell counting assay were used to examine the proliferation ability of A549 and H1299 cells. Cells were transfected with miR‐194 mimic or mimic control.  NC,
NC,  miR‐194. Data are reported as mean ± standard deviation of three independent experiments (*P < 0.05, t‐test).
miR‐194. Data are reported as mean ± standard deviation of three independent experiments (*P < 0.05, t‐test).
MiR‐194 directly inhibits the expression of NFAT5 through its 3’untranslated region
To gain further insight into the molecular mechanism by which miR‐194 suppresses the migration and proliferation of lung cancer cells, we searched for genes targeted by miR‐194 using biological target prediction software, TargetScan. The gene NFAT5 harbored a potential miR‐194 binding site within its 3’UTR (Fig 4a). To detect whether NFAT5 is regulated by miR‐194, the wild type or mutant 3’UTR sequence of NFAT5 was cloned into pMIR reporter vector, respectively (Fig 4b). The luciferase activity of the pMIR‐NFAT5‐3’UTR‐wt construct was significantly decreased upon miR‐194 overexpression in A549 and H1299 cells, whereas its mutant counterpart was not (Fig 4c). In addition, the messenger RNA (mRNA) and protein level of NFAT5 in A549 and H1299 cells was dramatically reduced by miR‐194 (Fig 4d–f). These results indicate that NFAT5 is a direct target of miR‐194 in lung cancer.
Figure 4.
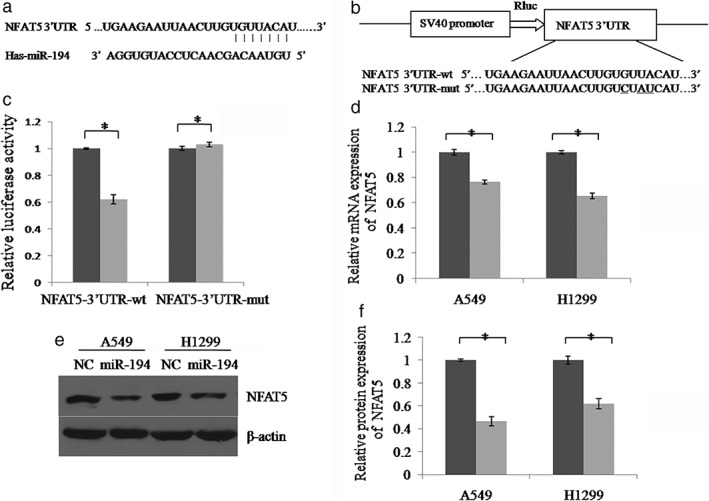
MiR‐194 directly inhibits the expression of NFAT5 through its 3’untranslated region (UTR). (a) The miR‐194 binding site predicted in the 3’UTR of NFAT5 messenger RNA (mRNA). (b) Mutant was generated at the seed region of NFAT5 3’UTR, indicated by the underline. A 3’UTR fragment of NFAT5 mRNA containing wild‐type or mutant of the miR‐194 binding sequence was cloned into the downstream of the luciferase gene in pMIR vector. (c) A549 cells were transfected with pMIR reporter vectors containing either wild‐type or mutant NFAT5 3’UTR (NFAT5‐3’UTR‐wt and NFAT5‐3’UTR‐mut) with either miR‐194 mimics (miR‐194) or miR‐194 mimic control (NC). Luciferase activity was determined 48 hours after transfection. (d) The expression level of NFAT5 mRNA was examined by real‐time PCR.  NC,
NC,  miR‐194. (e) The NFAT5 protein was examined by Western blot. (f) The relative gray values of each band (normalized to β‐actin). Protein bands from three independent Western blot assays were quantified using Quantity One software (Bio‐Rad, USA).
miR‐194. (e) The NFAT5 protein was examined by Western blot. (f) The relative gray values of each band (normalized to β‐actin). Protein bands from three independent Western blot assays were quantified using Quantity One software (Bio‐Rad, USA).  NC,
NC,  miR‐194. Data are reported as mean ± standard deviation of three independent experiments (*P < 0.05, t‐test).
miR‐194. Data are reported as mean ± standard deviation of three independent experiments (*P < 0.05, t‐test).
NFAT5 contributes to miR‐194 mediated proliferation, migration, and invasion of NSCLC cells
We examined the expression of NFAT5 in lung cancer cells and NSCLC tissues in HG and LG groups. NFAT5 was markedly upregulated in the HG compared to the LG group, both at mRNA and protein levels (Fig 5a,b). NFAT5 expression was significantly increased in lung cancer tissues with DM compared to tissues without DM (P < 0.05) (Fig 5c). Additionally, we investigated whether NFAT5 contributed to the proliferation, migration, and invasion of NSCLC cells by miR‐194. The functional experiments showed that NFAT5 overexpression could reverse the decreased proliferation, migration, and invasion ability induced by miR‐194 mimics in A549 cells (Fig 5d‐g). The protein level of NFAT5 was determined by Western blot (Fig 5e).
Figure 5.
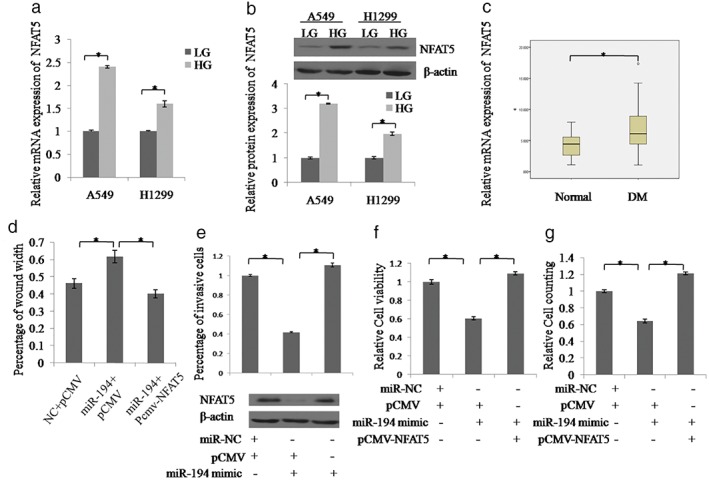
NFAT5 contributes to miR‐194 mediated proliferation, migration, and invasion of non‐small cell lung cancer (NSCLC) cells. (a) NFAT5 messenger RNA and (b) NFAT5 protein expression were examined in A549 and H1299 cells under high glucose (HG) or low glucose (LG) treatment by real‐time PCR and Western blot, respectively.  LG,
LG,  HG. (c) Quantitative real‐time‐PCR analysis of NFAT5 expression in 50 cases of NSCLC tissues with or without diabetes mellitus (DM) (**P < 0.05, Wilcoxon signed‐rank test). (d–g). Wound healing, Transwell, CCK8, or cell counting assays were used to detect the proliferation, migration, or invasion ability of A549 cells with different treatments, respectively. Additionally, the protein levels of NFAT5 were examined by Western blot. Data are reported as mean ± standard deviation of three independent experiments (*P < 0.05, t‐test).
HG. (c) Quantitative real‐time‐PCR analysis of NFAT5 expression in 50 cases of NSCLC tissues with or without diabetes mellitus (DM) (**P < 0.05, Wilcoxon signed‐rank test). (d–g). Wound healing, Transwell, CCK8, or cell counting assays were used to detect the proliferation, migration, or invasion ability of A549 cells with different treatments, respectively. Additionally, the protein levels of NFAT5 were examined by Western blot. Data are reported as mean ± standard deviation of three independent experiments (*P < 0.05, t‐test).
Discussion
DM is linked to an increased risk of lung cancer. Several miRNAs are reported to be dysregulated in the serum of DM patients. Growing evidence indicates that miRNAs play a crucial role in tumorigenesis and tumor progression; therefore, it is important to identify the miRNAs involved in the progression of DM turn to cancer.
In this study, we confirmed that HG promoted the proliferation, migration, and invasion of NSCLC cells. We performed miRNA profiling of A549 cells treated with HG or LG to identify the miRNAs that might be involved in HG‐induced lung cancer. MiR‐194 was markedly decreased in NSCLC cell lines with HG culture. Furthermore, we found that miR‐194 was significantly decreased in lung cancer tissues with DM. Thus, we hypothesize that miR‐194 may be involved in HG‐induced lung cancer occurrence and progression.
In addition, we investigated the role of miR‐194 in NSCLC biological function. Our data showed that miR‐194 upregulation dramatically suppressed lung cancer cell proliferation, migration, and invasion ability. Therefore, we speculated that downregulation of miR‐194 could contribute to the advanced development of NSCLC. A single miRNA can modulate a signaling network by targeting genes with multiple functions. Several miR‐194 targets have been identified in different cell contexts and organs, for example, MAP4K4 is a target of miR‐194, which mediates an effect on cell proliferation in hepatocellular carcinoma.18 Zhang et al. indicated that miR‐194 could inhibit cell proliferation and invasion via repression of RAP2B in bladder cancer.14 MiR‐194 directly targets FoxM1to inhibit epithelial–mesenchymal transition in gastric cancer cells. 17 Using luciferase reporter gene assays and Western blot, we found that NFAT5 was a functional target of miR‐194.
NFAT5 is a member of the NFAT protein family that has a DNA binding domain with structural similarity to the Rel‐homology region of NF‐Kb. NFAT5 is overexpressed in several human cancers, including renal carcinoma, melanoma cancer, and NSCLC. In our study, NFAT5 was frequently highly expressed in HG cultured NSCLC cells and was responsible for miR‐194‐modulated proliferation, migration, and invasion of NSCLC cells.
In summary, we investigated the role of miR‐194 in HG‐induced progression of NSCLC and found that miR‐194 was downregulated in lung cancer cells with HG condition and clinical tissues, and miR‐194 expression was associated with clinicopathologic characteristics in NSCLC. Moreover, we identified that NFAT5 was one of direct target genes of miR‐194. Downregulation of miR‐194 by HG significantly enhanced cell proliferation, migration, and invasion in NSCLC cells by targeting NFAT5. Our data provide new insight into the mechanism responsible for HG‐induced progression of NSCLC. Targeting miR‐194 could be a promising therapeutic strategy in diabetic NSCLC.
Acknowledgments
This study was funded by the financial support from the Youth Science Foundation of the Second Hospital Central Laboratory of Tianjin Medical University (No. 2018ydey18), National Natural Science Foundation of China (No. 81600643, No. 91746205), the Tianjin Health Industry Key Research Projects (No. 15KG101), Tianjin Science and Technology Support Project (No. 17JCYBJC27000), Key Projects of Tianjin Natural Science Foundation (No. 18JCZDJC32900), Scientific Research Funding of Tianjin Medical University Chu Hsien‐I Memorial Hospital (No. 2016DX01) Science foundation of Tianjin Medical University (No. 2016KYZQ19).
References
- 1. Travis WD. Pathology of lung cancer. Clin Chest Med 2002; 23: 65–81 viii. [DOI] [PubMed] [Google Scholar]
- 2. Roglic G, Norris SL. Medicines for treatment intensification in type 2 diabetes and type of insulin in type 1 and type 2 diabetes in low‐resource settings: Synopsis of the World Health Organization guidelines on second‐ and third‐line medicines and type of insulin for the control of blood glucose levels in nonpregnant adults with diabetes mellitus. Ann Intern Med 2018; 169 (6): 394–7. [DOI] [PubMed] [Google Scholar]
- 3. Giovannucci E, Harlan DM, Archer MC et al. Diabetes and cancer: A consensus report. CA Cancer J Clin 2010; 60: 207–21. [DOI] [PubMed] [Google Scholar]
- 4. Shimoyama S. Diabetes mellitus carries a risk of gastric cancer: A meta‐analysis. World J Gastroenterol 2013; 19: 6902–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cai H, Xu Z, Xu T, Yu B, Zou Q. Diabetes mellitus is associated with elevated risk of mortality amongst patients with prostate cancer: A meta‐analysis of 11 cohort studies. Diabetes Metab Res Rev 2015; 31: 336–43. [DOI] [PubMed] [Google Scholar]
- 6. Chen L, Li H, Gu L et al. The impact of diabetes mellitus on renal cell carcinoma prognosis: A meta‐analysis of cohort studies. Medicine 2015; 94: e1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu L, Cao H, Zhang T et al. The effect of diabetes mellitus on lung cancer prognosis: A PRISMA‐compliant meta‐analysis of cohort studies. Medicine 2016; 95 (17): e3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luo H, Qiao L, Liang N, Zhang J. Risk factors for recurrence in patients with resected N1 non‐small cell lung cancer: A systematic review and meta‐analysis. J BUON 2015; 20 (3): 791–9. [PubMed] [Google Scholar]
- 9. Zhang J, Wu J, He Q, Liang W, He J. The prognostic value of metformin for advanced non‐small cell lung cancer: A systematic review and meta‐analysis. Transl Lung Cancer Res 2018; 7 (3): 389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luo W, Huang B, Li Z et al. MicroRNA‐449a is downregulated in non‐small cell lung cancer and inhibits migration and invasion by targeting c‐Met. PLoS One 2013; 8: e64759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang J, Gu Z, Ni P et al. NF‐kappaB P50/P65 hetero‐dimer mediates differential regulation of CD166/ALCAM expression via interaction with micoRNA‐9 after serum deprivation, providing evidence for a novel negative auto‐regulatory loop. Nucleic Acids Res 2011; 39: 6440–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xiao H, Zeng J, Li H et al. MiR‐1 downregulation correlates with poor survival in clear cell renal cell carcinoma where it interferes with cell cycle regulation and metastasis. Oncotarget 2015; 6: 13201–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Y, Chen P, Zu L, Liu B, Wang M, Zhou Q. MicroRNA‐338‐3p suppresses metastasis of lung cancer cells by targeting the EMT regulator Sox4. Am J Cancer Res 2016; 6 (2): 127–40. [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao Y, Li F, Zhang X et al. MicroRNA‐194 acts as a prognostic marker and inhibits proliferation in hepatocellular carcinoma by targeting MAP4K4. Int J Clin Exp Pathol 2015; 8 (10): 12446–54. [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang M, Zhuang Q, Cui L. MiR‐194 inhibits cell proliferation and invasion via repression of RAP2B in bladder cancer. Biomed Pharmacother 2016; 80: 268–75. [DOI] [PubMed] [Google Scholar]
- 16. Bao J, Zou JH, Li CY, Zheng GQ. miR‐194 inhibits gastric cancer cell proliferation and tumorigenesis by targeting KDM5B. Eur Rev Med Pharmacol Sci 2016; 20 (21): 4487–93. [PubMed] [Google Scholar]
- 17. Chen X, Wang Y, Zang W, Du Y, Li M, Zhao G. miR‐194 targets RBX1 gene to modulate proliferation and migration of gastric cancer cells. Tumour Biol 2015; 36 (4): 2393–401. [DOI] [PubMed] [Google Scholar]
- 18. Li Z, Ying X, Chen H et al. MicroRNA‐194 inhibits the epithelial‐mesenchymal transition in gastric cancer cells by targeting FoxM1. Dig Dis Sci 2014; 59 (9): 2145–52. [DOI] [PubMed] [Google Scholar]
- 19. Wang SH, Wu XC, Zhang MD, Weng MZ, Zhou D, Quan ZW. Long noncoding RNA H19 contributes to gallbladder cancer cell proliferation by modulated miR‐194 targeting AKT2. Tumour Biol 2016; 37 (7): 9721–30. [DOI] [PubMed] [Google Scholar]
- 20. Zhu X, Li D, Yu F et al. miR‐194 inhibits the proliferation, invasion, migration, and enhances the chemosensitivity of non‐small cell lung cancer cells by targeting forkhead box A1 protein. Oncotarget 2016; 7 (11): 13139–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muller MR, Rao A. NFAT, immunity and cancer: A transcription factor comes of age. Nat Rev Immunol 2010; 10: 645e656. [DOI] [PubMed] [Google Scholar]
- 22. Halterman JA1, Kwon HM, Wamhoff BR. Tonicity‐independent regulation of the osmosensitive transcription factor TonEBP (NFAT5). Am J Physiol Cell Physiol 2012; 302: C1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jauliac S, López‐Rodriguez C, Shaw LM, Brown LF, Rao A, Toker A. The role of NFAT transcription factors in integrin‐mediated carcinoma invasion. Nat Cell Biol 2002; 4 (7): 540–4. [DOI] [PubMed] [Google Scholar]
- 24. Chen M, Sinha M, Luxon BA, Bresnick AR, O'Connor KL. Integrin alpha6beta4 controls the expression of genes associated with cell motility, invasion, and metastasis, including S100A4/metastasin. J Biol Chem 2009; 284 (3): 1484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Germann S, Gratadou L, Zonta E et al. Dual role of the ddx5/ddx17 RNA helicases in the control of the pro‐migratory NFAT5 transcription factor. Oncogene 2012; 31 (42): 4536–49. [DOI] [PubMed] [Google Scholar]
- 26. Guo K, Jin F. NFAT5 promotes proliferation and migration of lung adenocarcinoma cells in part through regulating AQP5 expression. Biochem Biophys Res Commun 2015; 465 (3): 644–9. [DOI] [PubMed] [Google Scholar]
- 27. Küper C, Beck FX, Neuhofer W. NFAT5‐mediated expression of S100A4 contributes to proliferation and migration of renal carcinoma cells. Front Physiol 2014; 8 (5): 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim DH, Kim KS, Ramakrishna S. NFAT5 promotes in vivo development of murine melanoma metastasis. Biochem Biophys Res Commun 2018; 505 (3): 748–54. [DOI] [PubMed] [Google Scholar]


