Abstract
Background
Crizotinib has demonstrated favorable efficacy in patients with advanced ALK‐positive non‐small cell lung cancer (NSCLC). Unfortunately, the majority of ALK‐positive patients ultimately develop acquired resistance within one year after the initiation of crizotinib treatment; however, the estimation of overall survival (OS) beyond crizotinib resistance has not yet been fully demonstrated. The purpose of this study was to identify favorable predictors affecting survival outcome.
Methods
In this single‐center retrospective study, the data of 136 patients with advanced ALK‐positive NSCLC beyond crizotinib resistance were analyzed between January 2013 and December 2017. Patients were divided into two groups according to intracranial or extracranial progression on crizotinib, and sequential therapies including crizotinib continuation with local therapy, next‐generation ALK inhibitors, and chemotherapy. The primary endpoint was the median OS duration from the start of crizotinib resistance to death or the last follow‐up. Univariate and multivariate Cox analyses of OS were carried out.
Results
At the time of analysis, 60 (41.1%) of the 136 patients had died. Median progression free survival (PFS) and OS from the metastatic diagnosis were 10.4 and 41.3 months, respectively. Sequential therapies administered beyond crizotinib treatment were: next‐generation ALK inhibitors (54 patients), chemotherapy (20 patients), and crizotinib continuation with local therapy (62 patients). Multivariate Cox analysis revealed that long PFS with crizotinib (≥ 10.4 months), intracranial progression, and next‐generation ALK inhibitors were significantly associated with a decreased risk of death.
Conclusion
Long PFS with crizotinib (≥10.4 months), intracranial progression, and use of next‐generation ALK inhibitors might be favorable predictors for OS in advanced ALK‐positive NSCLC patients.
Keywords: ALK, crizotinib, non‐small cell lung cancer, prognosis, resistance
Introduction
Lung cancer is the leading cause of cancer‐related mortality in the world, with an estimated 154 050 deaths in the United States by the end of 2018.1, 2 Adenocarcinoma has replaced squamous cell carcinoma as the most predominant histological subtype of lung cancer in China, which is consistent with the rate in developed countries. From 2002 to 2012, the relative frequency of adenocarcinoma increased from 21.96% to 43.36%.3 Furthermore, the majority of NSCLC patients are usually diagnosed at advanced stages or with metastatic disease, with a poor prognosis or median survival duration of 8–10 months.4
In 2007, Soda et al. discovered genomic rearrangement in ALK receptor tyrosine kinase, a specific molecular subtype of NSCLC, as a potential oncogenic driver.5 The incidence rate of ALK rearrangement ranges from 3% to 7% in East Asian NSCLC patients, and is observed predominantly in younger patients with no or a light smoking history and adenocarcinoma histology.6, 7 Because of the large number of patients with NSCLC, 40 000 new ALK‐positive cases are reported worldwide each year.8
Currently, crizotinib is approved for use in the first‐line setting for patients with locally advanced or metastatic ALK‐positive NSCLC on the basis of high response rates compared to chemotherapy (pemetrexed with either cisplatin or carboplatin) in the PROFILE 1014 phase 3 randomized trial.9 Despite the initial efficacy, ALK‐positive patients inevitably develop acquired resistance to crizotinib treatment within approximately seven months to a year,10, 11 which commonly manifests as intracranial or extracranial patterns of progression. Sequential therapy options beyond crizotinib resistance include crizotinib continuation plus local treatment, next‐generation ALK inhibitors or chemotherapy. However, the estimation of overall survival (OS) beyond crizotinib resistance in such patients has not yet been fully demonstrated.
Therefore, the purpose of this study was to identify favorable predictors that would impact survival outcomes in real‐world clinical practice.
Methods
Patients
In this retrospective single‐center study, 155 locally advanced or metastatic ALK‐positive NSCLC (stage IIIB–IV) patients resistant to crizotinib treatment at the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (Beijing, China) between January 2013 and December 2017 were enrolled. All patients who met the following criteria were registered: aged ≥ 18 years; histologically or cytologically confirmed locally advanced or metastatic disease; progression beyond crizotinib treatment at one site; ALK rearrangement determined by fluorescence in situ hybridization (FISH) or Ventana immunohistochemistry (IHC) with measurable target lesions assessed by computed tomography (CT) images of the chest and abdomen, magnetic resonance imaging (MRI), or positron emission tomography (PET)‐CT) according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; and an Eastern Cooperative Oncology Group performance status (ECOG PS) score of ≤ 2. Patients with untreated or treated brain metastasis were eligible regardless of whether they had been administered first‐line or second‐line crizotinib therapy.
Patients who received crizotinib treatment for less than a month, were administered any previous ALK inhibitor therapy other than crizotinib, or who experienced progression in multiple metastatic sites after crizotinib treatment were not included in the study. Additional local therapy (including whole brain radiotherapy [WBRT], stereotactic radiotherapy [SRT], surgical resection, and local ablation) was administered depending on the specialists’ assessment of symptoms and imaging examinations. Smokers were defined as current or former smokers, while non‐smokers referred to individuals who had smoked < 100 cigarettes in their lifetime. Data were collected from electronic medical records, including clinical data and survival outcomes. As this was an observational study, informed patient consent was not required. The institutional review board approved the study.
Treatment
Eligible patients received crizotinib at a dose of 250 mg twice daily at initiation. The patients were divided into two groups based on the site of disease progression beyond crizotinib treatment: extracranial (n = 72, 52.9%) and intracranial (n = 64, 47.1%) progression. Sequential therapies mainly included crizotinib continuation plus local therapy, next‐generation ALK inhibitors (ceritinib, alectinib, or AP26113), or chemotherapy. Patients were permitted to crossover to other therapies if they experienced progression during the course of treatment. All patients were followed‐up from the diagnosis of ALK rearrangement to 31 December 2017.
Outcomes and definitions
Disease was assessed at baseline after the first dose of study therapy until radiographic progressive disease (PD), determined by imaging examination or unacceptable toxicity. Imaging examinations included chest and abdomen CT, brain MRI, or PET‐CT. Scan intervals were set at approximately two months. Evaluations of response included complete response (CR), partial response (PR), stable disease (SD), or PD according to RECIST version 1.1. PFS was defined as the interval from the initiation of crizotinib treatment to PD, death, or last follow‐up. The primary endpoint was median OS from the start of crizotinib resistance to death or the last follow‐up.
Statistical analysis
SPSS version 16.0 was used for statistical analysis. Baseline characteristics were presented by applying descriptive statistics. The data for dichotomous variables were presented as the number of patients (n) and percentages (%), and continuous variables were presented as median and range values. Chi‐square or Fisher's exact tests were used for dichotomous data comparison between groups. The Kaplan–Meier method was used to calculate median OS from the start of crizotinib resistance. We analyzed hazard ratios (HR) and 95% CIs (confidence intervals) by a Cox model using the log‐rank test, and then all promising variables (P < 0.1) were entered into a multivariate Cox regression model. All statistical tests were two‐tailed, with P < 0.05 considered statistically significant. Variables included age, gender, smoking history, clinical stage, pathological and histological type, ECOG PS, lines of crizotinib therapy, PFS with crizotinib, progression patterns, and sequential therapy options. Graphpad 6.0 was used to present survival curves.
Results
Baseline characteristics
A total of 155 ALK‐positive advanced NSCLC patients were selected; 19 received no antitumor therapy beyond crizotinib resistance and died rapidly within a month. Thus, 136 patients were included in the final analysis. The baseline characteristics of the patients are summarized in Table 1: 73 (53.6%) were female; the median age was 50 years (range: 20–83); 77 (56.6%) had a good PS of 0–1; and the majority were non‐smokers with adenocarcinomas (n = 96, 70.5%). Thirty patients manifested brain metastasis before oral crizotinib treatment at baseline. Seventy‐two patients were administered crizotinib treatment in the first‐line setting; 64 patients were administered first‐line or multiple‐line chemotherapy, and were then subsequently treated with crizotinib after disease progression. Patient and disease characteristics were well balanced between the two groups, including distinct intracranial and extracranial progression patterns.
Table 1.
Baseline characteristics in ALK‐positive advanced NSCLC patients
| Characteristics | Total (n = 136) |
Intracranial (n = 64) |
Extracranial (n = 72) |
P |
|---|---|---|---|---|
| Age (years, %) | 0.810 | |||
| ≥ 60 | 31 (22.8) | 14 (21.9) | 17 (23.6) | |
| < 60 | 105 (77.2) | 50 (78.1) | 55 (76.4) | |
| Gender (%) | 0.903 | |||
| Male | 63 (46.3) | 30 (46.9) | 33 (45.8) | |
| Female | 73 (53.7) | 34 (53.1) | 39 (54.2) | |
| Smoker history (%) | 0.756 | |||
| Yes | 40 (29.4) | 18 (28.1) | 22 (30.6) | |
| No | 96 (70.6) | 46 (71.9) | 50 (69.4) | |
| Histological types (%) | 0.120 | |||
| ADC | 129 (94.8) | 63 (98.4) | 66 (91.7) | |
| Non‐ADC | 7 (5.2) | 1 (1.6) | 6 (8.3) | |
| Clinical stage (%) | 0.734 | |||
| IIIB | 9 (6.6) | 5 (7.8) | 4 (5.6) | |
| IV | 127 (93.4) | 59 (92.2) | 68 (94.4) | |
| ECOG scores (%) | 0.338 | |||
| 0–1 | 77 (56.6) | 39 (60.9) | 38 (52.8) | |
| ≥ 2 | 59 (43.4) | 25 (39.1) | 34 (47.2) | |
| Crizotinib therapy (%) | 0.078 | |||
| 1 line | 72 (52.9) | 39 (60.9) | 33 (45.8) | |
| ≥ 2 line | 64 (47.1) | 25 (39.1) | 39 (54.2) |
ADC, adenocarcinoma; ECOG, Eastern Cooperative Oncology Group; NSCLC, non‐small cell lung cancer.
Distribution of disease progression sites beyond crizotinib resistance
Brain metastasis and disease progression in the brain presented as resistance to crizotinib treatment in 47.0% of patients. Thirty patients had brain metastasis before oral crizotinib therapy at baseline, while 34 experienced brain metastases during the course of crizotinib treatment. Lung and liver metastasis developed in these patients at a rate of approximately 19.8% and 11.0%, respectively (Table 2).
Table 2.
Distribution of disease progression (%)
| Site of disease progression | Number (n = 136) | Ratio (%) |
|---|---|---|
| Brain | 64 | 47.0 |
| Lung | 27 | 19.8 |
| Liver | 15 | 11.0 |
| Pleura | 10 | 7.4 |
| Lymph node | 5 | 3.7 |
| Bone | 5 | 3.7 |
| Adrenal | 2 | 1.5 |
| Other | 8 | 5.9 |
Sequential therapy treatments according to progression sites
Twenty patients were administered chemotherapy, 62 were administered crizotinib continuation plus local therapy, and 54 were administered next‐generation ALK inhibitors.
For intracranial progression (n = 64), chemotherapy was administered to five patients, crizotinib continuation plus local therapy to 36 patients (24 WBRT, 9 SRT, and 3 surgical resection), and next‐generation ALK inhibitors were administered to 23 patients.
For extracranial progression (n = 72), chemotherapy (n = 15), crizotinib continuation plus local therapy (n = 26, 5 local ablation, 21 local radiotherapy), and next‐generation ALK inhibitors (n = 31) were administered.
Univariate and multivariate analyses by Cox regression model
Univariate analysis showed that OS in patients with ALK‐positive advanced NSCLC was significantly associated with the following factors: age (≥ 60 vs. < 60 years), ECOG PS (0–1 vs. ≥ 2), PFS with crizotinib (≥ 10.4 vs. < 10.4 months), pattern of progression on crizotinib treatment (intracranial vs. extracranial), and sequential therapy options (next‐generation ALK inhibitor therapy or chemotherapy vs. crizotinib continuation) (Table 3). The line of crizotinib therapy did not impact OS beyond crizotinib resistance. Statistically significant variables in univariate analysis were entered into a Cox proportional hazard regression model. Multivariate analysis revealed that long PFS with crizotinib (≥ 10.4 months), a pattern of intracranial metastases, and the use of next‐generation ALK inhibitors were favorable predictors of OS from the start of crizotinib resistance in advanced ALK‐positive NSCLC patients (Table 4).
Table 3.
Univariate analysis of overall survival
| Variable | B | SE | HR | 95% CI | P |
|---|---|---|---|---|---|
| Age (≥ 60 vs. < 60) | 0.604 | 0.281 | 1.830 | 1.055–3.172 | 0.031 |
| Gender (Male vs. female) | −0.007 | 0.260 | 0.933 | 0.596–1.653 | 0.979 |
| Smoking history (Yes vs. no) | 0.273 | 0.271 | 1.314 | 0.772–2.236 | 0.314 |
| Histological type (ADC vs. non‐ADC) | −0.309 | 0.598 | 0.734 | 0.227–2.371 | 0.605 |
| Clinical stage (IIIB vs. IV) | −0.455 | 0.721 | 0.635 | 0.154–2.610 | 0.528 |
| ECOG PS (0–1 vs. ≥ 2) | −0.621 | 0.261 | 0.537 | 0.322–0.897 | 0.017 |
| Crizotinib therapy lines (1 line vs. ≥ 2 lines) | −0.142 | 0.259 | 0.868 | 0.522–1.441 | 0.583 |
| PFS with crizotinib (≥ 10.4 vs. < 10.4 m) | −0.931 | 0.280 | 0.394 | 0.228–0.682 | 0.001 |
| Progressive pattern (intracranial vs. extracranial) | −0.620 | 0.266 | 0.538 | 0.319–0.907 | 0.020 |
| Sequential therapy crizotinib continuation chemotherapy | 0.693 | 0.329 | 1.999 | 1.048–3.813 | 0.035 |
| next‐generation ALKi | −0.523 | 0.304 | 0.593 | 0.327–1.076 | 0.086 |
ADC, adenocarcinoma; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; PFS, progression‐free survival; SE, standard error.
Table 4.
Predictors of overall survival analyzed by a Cox regression model
| Variable | B | SE | HR | 95% CI | P |
|---|---|---|---|---|---|
| Age (≥ 60 vs. < 60) | −0.123 | 0.369 | 0.884 | 0.429–1.822 | 0.739 |
| ECOG PS (0–1 vs. ≥ 2) | −0.639 | 0.342 | 0.528 | 0.270–1.033 | 0.062 |
| PFS with crizotinib (≥ 10.4 vs. < 10.4 m) | −0.785 | 0.290 | 0.456 | 0.258–0.804 | 0.007 |
| Progression pattern (intracranial vs. extracranial) | −0.605 | 0.289 | 0.546 | 0.310–0.962 | 0.036 |
| Sequential therapy crizotinib continuation chemotherapy | 0.138 | 0.359 | 1.148 | 0.568–2.322 | 0.700 |
| next‐generation ALKi | −0.752 | 0.337 | 0.436 | 0.225–0.845 | 0.014 |
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; PFS, progression‐free survival; SE, standard error.
Analysis of progression‐free and overall survival
By the time of analysis, 60 of the ALK‐positive advanced or metastatic NSCLC patients (41.1%) had died. Median PFS with crizotinib therapy and OS from the time of metastatic diagnosis in all patients were 10.4 months (95% CI 9.1–11.6) and 41.3 months (95% CI 31.5–51.2), respectively.
The PFS and OS from the start of crizotinib resistance were analyzed according to different progression patterns. There was no significant difference in PFS between different progression patterns (median, intracranial progression 11.8 months vs. extracranial progression 9.3 months; P = 0.358) (Fig 1a). The median OS from the time of crizotinib resistance was significantly longer in patients with intracranial progression compared to those with extracranial progression (median, 25.4 vs. 13.3 months; P = 0.018) (Fig 1b).
Figure 1.
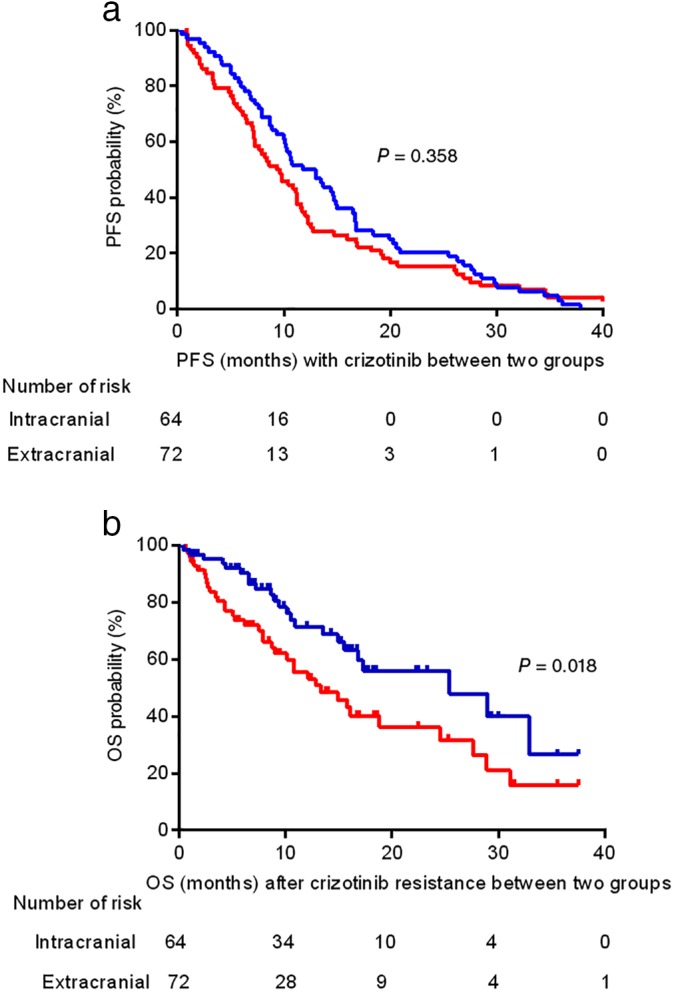
Kaplan–Meier curves of progression‐free survival (PFS) and overall survival (OS) from the time of crizotinib resistance according to different progression patterns. (a) No significant difference was observed in median PFS between different progression patterns (intracranial 11.8 months vs. extracranial 9.3 months; P = 0.358). (b) The median OS was significantly longer in patients with intracranial progression compared to those with extracranial progression (25.4 vs. 13.3 months; P = 0.018). CI, confidence interval. ( ) Intracranial and (
) Intracranial and ( ) Extracranial.
) Extracranial.
The patients were separated into two groups to analyze the effect of PFS with crizotinib or sequential treatment on OS. Patients with long PFS with crizotinib (median, ≥ 10.4 months) achieved a longer median OS than those with short PFS (median, 28.9 vs. 10.8 months; P = 0.001) (Fig 2a). Median OS from the start of crizotinib resistance was 16.8 months (95% CI 9.1–24.4). A significant difference in OS from the start of crizotinib resistance was also found among distinct sequential therapy (median, next‐generation ALK inhibitors 27.6 months vs. crizotinib continuation plus local therapy 13.3 months vs. chemotherapy 9.0 months; P = 0.002) (Fig 2b).
Figure 2.
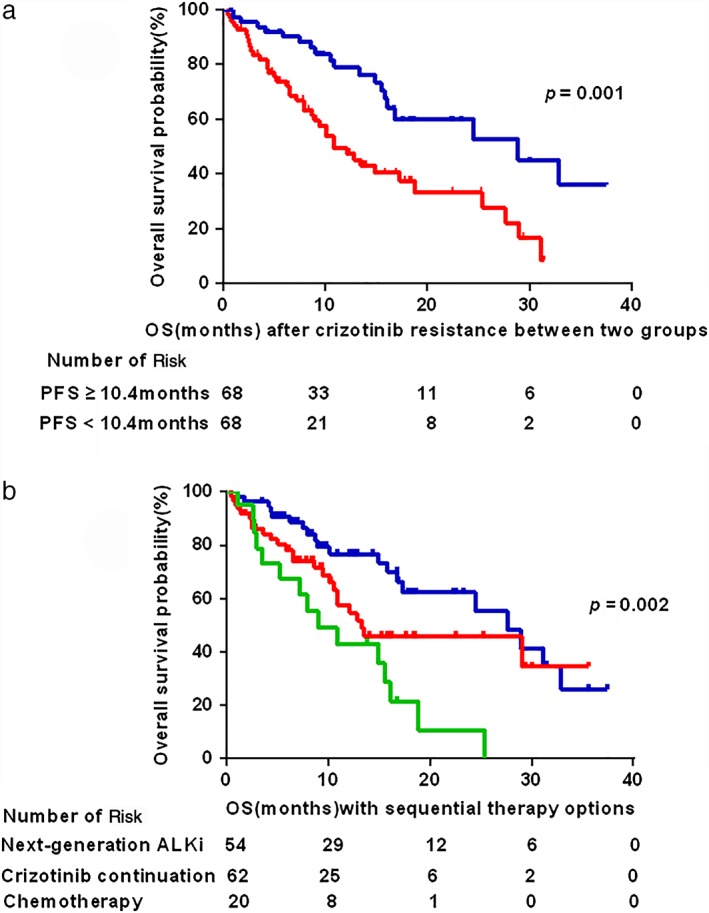
Kaplan–Meier curves of overall survival (OS) from the beginning of crizotinib resistance. (a) Patients with long progression‐free survival (PFS, ≥ 10.4 months) with crizotinib achieved longer median OS than those with short PFS (median, 28.9 vs. 10.8 months; P = 0.001). (b) A significant difference was observed in OS according to sequential therapy options (median, next‐generation ALK inhibitors 27.6 months vs. crizotinib continuation plus local therapy 13.3 months vs. chemotherapy 9.0 months; P = 0.002). CI, confidence interval. (a) ( ) PFS ≥ 10.4 months, (
) PFS ≥ 10.4 months, ( ) PFS < 10.4 months. (b) (
) PFS < 10.4 months. (b) ( ) Next‐generation ALKi, (
) Next‐generation ALKi, ( ) Crizotinib continuation, (
) Crizotinib continuation, ( ) Chemotherapy.
) Chemotherapy.
Discussion
We retrospectively examined the different progression patterns and OS predictors in locally advanced or metastatic ALK‐positive NSCLC patients with crizotinib resistance. Our findings revealed that brain metastasis was the most common site of disease progression following resistance to crizotinib, accounting for 47%, which was similar to the results of previous studies.12, 13, 14 Inadequate penetration of the central nervous system (CNS) and a low cerebrospinal fluid (CSF)‐to‐plasma ratio of crizotinib may be the main reasons for brain metastases.15 Kaneda et al. also reported a low CSF‐to‐plasma ratio of crizotinib, at only 0.0026.16 In addition, crizotinib is a good P‐glycoprotein substrate, which limits its accumulation in the CNS as a result of a drug efflux membranous transporter.17
In our study, the median OS from the beginning of crizotinib resistance was longer in patients with intracranial progression than in those with extracranial progression (median, 25.4 vs. 13.3 months, respectively). However, Jo et al. revealed that the presence of intracranial metastasis was an independent poor prognostic factor of OS, which is inconsistent with our results.18 One reason for the long OS in patients with intracranial progression in our study was the proportion of the study population administered crizotinib continuation plus local therapy (56.2%). These patients also had extra survival time after developing crizotinib resistance, particularly those amenable to local therapy, including WBRT, SRT, or surgical resection. Another reasonable explanation is the poor drug penetration into the CNS in patients whose disease was sensitive to crizotinib therapy – if adequate drug concentrations can be delivered into the CNS. Therefore, patients who experience isolated CNS progression should not be considered as having systemically acquired resistance to crizotinib. Platinum‐based regimens did not show good intracranial activity in such patients, thus the prognosis was poor for advanced ALK‐positive patients with intracranial metastases. Therefore, the management failure of patients with isolated CNS progression should be distinguished from that of patients with other progression patterns.
Another important finding provides real‐world survival analysis in ALK‐positive NSCLC patients with crizotinib resistance. In this study, long PFS with crizotinib (≥ 10.4 months) correlated with better survival outcomes, and the line of crizotinib therapy did not impact OS beyond crizotinib treatment. Additionally, using next‐generation ALK inhibitors beyond crizotinib resistance was a favorable predictor of OS in ALK‐positive NSCLC patients. Comparing crizotinib continuation plus local therapy as a standard treatment, 54 patients subsequently administered next‐generation ALK inhibitors achieved OS of 27.6 months from the start of crizotinib resistance, while 20 patients administered chemotherapy had poorer survival outcomes, with median OS of only 9.0 months. The results of our study further indicate the high activity of next‐generation ALK inhibitors in crizotinib‐resistant patients, as reported by Shaw et al. and Ou et al. 19, 20 Therefore, next‐generation ALK inhibitors are regarded as a favorable treatment because of their ability to overcome crizotinib resistance and significantly improve survival as a sequential therapy option.
Several limitations of the present study must be noted. Firstly, as a single‐center, retrospective study with a relatively small sample, there is the possibility of bias. Secondly, we included patients with distinct progression patterns administered different local treatments, including SRT, ablative treatment, or surgical resection, thus the survival outcomes could also be affected by partial bias. Thirdly, we did not perform either post‐progression biopsy beyond crizotinib treatment or comprehensive genomic analysis of progression sites, especially when the progressing metastatic lesions occurred in the brain. Depending on the results of repeated biopsies and sequential therapy options according to specific molecular mechanisms of crizotinib resistance would not be considered personalized therapy.
In conclusion, multivariate analysis revealed that long PFS with crizotinib (≥ 10.4 months), intracranial progression on crizotinib, and next‐generation ALK inhibitors might be favorable predictors for OS in advanced ALK‐positive NSCLC patients after crizotinib treatment. Although tissue biopsies are not always feasible at disease progression sites, liquid biopsy is an alternative method to detect drug resistance mechanisms. Large‐scale, prospective, multicenter evaluation is warranted to determine the optimal sequential options for ALK‐positive NSCLC patients beyond crizotinib resistance according to specific molecular profile of the tumor at the time of progression.
Disclosure
No authors report any conflict of interest.
Acknowledgments
We would like to thank the patients, their families, and all of the research members.
References
- 1. Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol 2016; 893: 1–19. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 3. Zou XN, Lin DM, Wan X et al. Histological subtypes of lung cancer in Chinese males from 2000 to 2012. Biomed Environ Sci 2014; 27: 3–9. [DOI] [PubMed] [Google Scholar]
- 4. Schiller JH, Harrington D, Belani CP et al. Comparison of four chemotherapy regimens for advanced non‐small‐cell lung cancer. N Engl J Med 2002; 346: 92–8. [DOI] [PubMed] [Google Scholar]
- 5. Soda M, Choi YL, Enomoto M et al. Identification of the transforming EML4‐ALK fusion gene in non‐small‐cell lung cancer. Nature 2007; 448: 561–6. [DOI] [PubMed] [Google Scholar]
- 6. Fukui T, Yatabe Y, Kobayashi Y et al. Clinicoradiologic characteristics of patients with lung adenocarcinoma harboring EML4‐ALK fusion oncogene. Lung Cancer 2012; 77: 319–25. [DOI] [PubMed] [Google Scholar]
- 7. Camidge DR, Doebele RC. Treating ALK‐positive lung cancer: Early successes and future challenges. Nat Rev Clin Oncol 2012; 9: 268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shaw AT, Engelman JA. ALK in lung cancer: Past, present, and future. J Clin Oncol 2013; 31: 1105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Solomon BJ, Mok T, Kim DW et al. First‐line crizotinib versus chemotherapy in ALK‐positive lung cancer. N Engl J Med 2014; 371: 2167–77. [DOI] [PubMed] [Google Scholar]
- 10. Camidge DR, Bang YJ, Kwak EL et al. Activity and safety of crizotinib in patients with ALK‐positive non‐small‐cell lung cancer: Update results from a phase 1 study. Lancet Oncol 2012; 13: 1011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kwak EL, Bang YJ, Camidge DR et al. Anaplastic lymphoma kinase inhibition in non‐small‐cell lung cancer. N Engl J Med 2010; 363: 1693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Costa DB, Shaw AT, Ou SH et al. Clinical experience with crizotinib in patients with advanced ALK‐rearranged non‐small‐cell lung cancer and brain metastasis. J Clin Oncol 2015; 33: 1881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Camidge DR. Taking aim at ALK across the blood‐brain barrier. J Thorac Oncol 2013; 8: 389–90. [DOI] [PubMed] [Google Scholar]
- 14. Metro G, Lunardi G, Floridi P et al. CSF concentration of crizotinib in two ALK‐positive non‐small‐cell lung cancer patients with CNS metastases deriving clinical benefit from treatment. J Thorac Oncol 2015; 10: e26–7. [DOI] [PubMed] [Google Scholar]
- 15. Costa DB, Kobayashi S, Pandya SS et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 2011; 29: e443–5. [DOI] [PubMed] [Google Scholar]
- 16. Kaneda H, Okamoto I, Nakagawa K. Rapid response of brain metastasis to crizotinib in a patient with ALK rearrangement‐positive non‐small‐cell lung cancer. J Throac Oncol 2013; 8: e32–3. [DOI] [PubMed] [Google Scholar]
- 17. Giroux‐Leprieur E, Fallet V, Cadranel J, Wislez M. Spotlight on crizotinib in the first‐line treatment of ALK‐positive advanced non‐small‐cell lung cancer: Patients selection and perspectives. Lung Cancer (Auckl) 2016; 7: 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jo J, Kim SH, Kim YJ et al. Efficacy of pemetrexed‐based chemotherapy in comparison to non‐pemetrexed‐based chemotherapy in advanced ALK+ non‐small cell lung cancer. Yonsei Med J 2018; 59: 202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shaw AT, Kim DW, Mehra R et al. Ceritinib in ALK‐rearranged non‐small‐cell lung cancer. N Engl J Med 2014; 370: 1189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ou S‐H, Ahn JS, De Petris L et al. Alectinib in crizotinib‐refractory ALK‐rearranged non‐small‐cell lung cancer: A phase II global study. J Clin Oncol 2016; 34: 661–8. [DOI] [PubMed] [Google Scholar]


