Abstract
Background
Crizotinib is associated with a favorable survival benefit in patients with ALK‐positive non‐small cell lung cancer (NSCLC); however, a subset of patients harboring ALK rearrangement shows a poor response.
Methods
We collected the clinical features and survival outcomes of 28 primary‐resistant responders (PRR) with progression‐free survival (PFS) of < 3 months on crizotinib and compared these with 78 long‐term responders (LTR) that achieved > 24 months PFS (control).
Results
Primary resistance was observed in 6.5% of the patients. The median PFS of the PRR and LTR groups was 1.2 months (95% confidence interval [CI] 0.70–1.73) and 47.0 months (95% CI 34.39–59.64), respectively. A better Eastern Cooperative Oncology Group performance status score was significantly associated with longer PFS (odds ratio 0.06, 95% CI 0.01–0.33; P = 0.001). The median overall survival (OS) of the PRR group was 8.4 months (95% CI 3.47–13.42) and crizotinib as first‐line treatment was an independent predictive factor for survival outcome (P = 0.005). Patients administered ALK‐tyrosine kinase inhibitors after crizotinib progression had significantly longer survival than the PRR group treated with best supportive care (P = 0.007), but no significant difference was found between ALK‐tyrosine kinase inhibitor treatment and single chemotherapy (P = 0.944).
Conclusion
Patients with primary resistance to crizotinib displayed unfavorable survival outcomes and the underlying mechanism cannot be identified in clinical features. Nevertheless, next‐generation ALK inhibitors and chemotherapy after crizotinib progression could confer a therapeutic and survival benefit in this population.
Keywords: Anaplastic lymphoma kinase, crizotinib, non‐small cell lung cancer, primary resistance
Introduction
Lung cancer is the leading cause of cancer‐related death worldwide.1 Approximately 40% of patients with non‐small cell lung cancer (NSCLC) present with metastatic or locally advanced disease.2 Over the past decade, therapies have been developed to inhibit irregular oncogenic pathways in lung cancer and these pathways represent promising potential targets for antitumor therapy. Indeed, the development of targeted therapy, such as EGFR and ALK tyrosine kinase inhibitors (TKIs), has led to different molecular pathology classifications in terms of targeted therapies for lung cancer.
ALK gene rearrangements are found in approximately 3–7% of NSCLC patients.3 Treatment with crizotinib, the first‐generation small molecule inhibitor of ALK kinase activity, has yielded high objective response rates (ORRs) of > 60% and median progression‐free survival (PFS) of seven months to one year in advanced ALK‐positive NSCLC patients, based on a series of PROFILE clinical trials.4, 5, 6, 7, 8 Although most patients with ALK‐positive NSCLC experience a substantial clinical benefit from crizotinib, disease relapse is inevitable as a result of acquired resistance. The mechanisms of acquired resistance have previously been reported, including secondary mutations in the ALK tyrosine kinase domain, copy number alterations of the ALK fusion gene, upregulation of bypass signaling pathways, and limited penetration of the central nervous system.9, 10, 11 However, despite the presence of ALK rearrangement, approximately 30% of patients do not respond to ALK‐TKIs and the underlying mechanisms of such poor responses have not been fully elucidated.12, 13 First‐line TKI treatment failure is rarely reported, contributing to the lack of comprehensive information about the clinical features and treatment outcome of this subset of ALK‐positive NSCLC patients.
We evaluated the clinical features and survival outcomes of primary‐resistant responders (PRR, PFS < 3 months) to crizotinib compared to long‐term responders (LTR, PFS > 24 months). We also analyzed the effects of subsequent treatments on survival following crizotinib progression and explored the possible mechanisms for primary resistance.
Methods
Patient eligibility and treatment
We retrospectively reviewed the medical records of 428 patients who were histologically or cytologically diagnosed with locally advanced, recurrent, or metastatic NSCLC at five cancer centers in China between January 2013 and November 2017 (each center enrolled at least 10 eligible patients). All enrolled patients tested ALK‐positive and received at least 21 days of crizotinib treatment. ALK translocation was determined by one of following methods: fluorescence in situ hybridization (FISH) assay, Ventana immunohistochemistry, anti‐ALK (D5F3), or real‐time reverse transcription PCR. In this study, crizotinib was administered at a dose of 250 mg twice daily, and proper dose adjustments were made. Clinical responses were evaluated one month after the first administration of crizotinib and then approximately every two months during crizotinib treatment until drug withdrawal. Routine hematology tests, biochemistry analyses, and electrocardiograms were also performed. The ethics committee of the Cancer Hospital, Chinese Academy of Medical Sciences, approved the study.
Data extraction
The demographics and clinical characteristics of the enrolled patients were collected, including age, gender, tumor stage, histological type, Eastern Cooperative Oncology Group performance status (ECOG PS) score, smoking history, and previous treatment regimens. The authors independently reviewed imaging data to evaluate the best treatment response and disease progression according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Survival information was obtained from clinical records or telephone follow‐up by investigators at each center.
Definitions and study endpoints
The study endpoints were PFS (from crizotinib initiation to the first RECIST‐defined progression or death from any cause) and OS (from crizotinib initiation to death or the last follow‐up). Patients that had not progressed at the time of analysis were censored at the date of their last contact with our institution. The groups were determined based on their tumor response to crizotinib treatment. The PRR group included patients who experienced disease progression within three months without any evidence of an objective response while receiving crizotinib treatment, while patients with PFS > 24 months were categorized as the LTR group. The subsequent drugs administered following crizotinib failure were also monitored.
Statistical analysis
All analyses were carried out at the final follow‐up date (30 November 2017) using SPSS version 19.0. For categorical variables, differences between the PRR and LTR groups were compared using two‐sided Fisher's exact or chi‐square tests. The Kaplan–Meier method was applied to estimate survival curves for OS, and the log‐rank test was performed to compare the survival outcomes between different subgroups. Cox proportional hazard models were used to evaluate independent prognostic factors and the differences in PFS were assessed by multivariate logistic regression. The results were presented as odds ratios (ORs) for logistic regression or hazard ratios (HRs) for Cox regression with their corresponding 95% confidence intervals (CIs). A two‐sided P value of < 0.05 was considered to indicate statistical significance.
Results
Patient characteristics and treatments
Primary resistance was observed in 6.5% of the patients (28/428). The cohort included 11 (39.3%) women and 17 (60.7%) never smokers. The median age of the PRR group was 52 years (range: 24–69) and all patients were at stage IV disease at crizotinib initiation. Most of the patients with primary resistance had good ECOG PS of 0–1 (20/28, 71.4%); 89.3% (25/28) of patients had adenocarcinoma; and 8 (28.6%) patients had brain metastasis at baseline. Thirteen (46.4%) patients received crizotinib as a first‐line regimen. A total of 78 patients with PFS > 24 months were included in the LTR group. The baseline clinical characteristics of the groups are summarized in Table 1.
Table 1.
Baseline characteristics of the population at the time of crizotinib initiation (n = 106)
| Characteristics, n (%) | All patients | PRR group | LTR group | P | |
|---|---|---|---|---|---|
| Age, years | 50 ± 10.7 | 52 ± 9.7 | 49 ± 10.9 | ||
| < 60 | 83 (78.3) | 20 (71.4) | 63 (80.8) | 0.304 | |
| ≥ 60 | 23 (21.7) | 8 (28.6) | 15 (19.2) | ||
| Gender | |||||
| Male | 56 (52.8) | 17 (60.7) | 39 (50.0) | 0.330 | |
| Female | 50 (47.2) | 11 (39.3) | 39 (50.0) | ||
| Smoking status | |||||
| Never smoker | 72 (67.9) | 17 (60.7) | 55 (70.5) | 0.341 | |
| Former smoker | 34 (32.1) | 11 (39.3) | 23 (29.5) | ||
| Histology | |||||
| Adenocarcinoma | 100 (94.3) | 25 (89.3) | 75 (96.2) | 0.383 | |
| Other† | 6 (5.7) | 3 (10.7) | 3 (3.8) | ||
| ECOG PS | |||||
| 0–1 | 96 (90.6) | 20 (71.4) | 76 (97.4) | < 0.001 | |
| 2–3 | 10 (9.4) | 8 (28.6) | 2 (2.6) | ||
| Line of therapy before crizotinib | |||||
| 0 | 64 (60.4) | 13 (46.4) | 51 (65.4) | 0.079 | |
| ≥ 1 | 42 (39.6) | 15 (53.6) | 27 (34.6) | ||
| Metastasis site | |||||
| Brain | 25 (23.6) | 8 (28.6) | 17 (21.8) | 0.469 | |
| Lung | 47 (44.3) | 11 (39.3) | 36 (46.2) | 0.530 | |
| Pleural | 42 (39.6) | 10 (35.7) | 32 (41.0) | 0.622 | |
| Liver | 11 (10.4) | 6 (21.4) | 5 (6.4) | 0.061 | |
| Bone | 28 (26.4) | 10 (35.7) | 18 (23.1) | 0.193 | |
| Lymph node | 64 (60.4) | 19 (67.9) | 45 (57.7) | 0.346 | |
| Others‡ | 8 (7.5) | 1 (3.6) | 7 (9.0) | 0.609 |
Includes squamous, adenosquamous, and large cell carcinomas.
Includes adrenal and subcutaneous metastases. BM, brain metastasis; ECOG PS, Eastern Cooperative Oncology Group performance status; LTR, long‐term responder; NSCLC, non‐small cell lung cancer; PRR, primary‐resistant responder.
The demographic and clinical features were compared between the groups, and multivariate analyses of logistic regression revealed that better ECOG PS was significantly associated with longer PFS (OR 0.06, 95% CI 0.01–0.33; P = 0.001). Other features, including age (P = 0.814), gender (P = 0.722), brain metastases (P = 0.805), and lines of crizotinib treatment (P = 0.308), were not significantly different between the groups (Table 2).
Table 2.
Logistic regression analysis of factors associated with primary resistance and long‐term PFS underlying crizotinib treatment
| Risk factor | OR | 95% CI | P |
|---|---|---|---|
| Age | |||
| < 60 (Reference) | 0.87 | (0.26–2.85) | 0.814 |
| ≥ 60 | |||
| Gender | |||
| Male (Reference) | 1.27 | (0.34–4.67) | 0.722 |
| Female | |||
| Smoking status | |||
| Never smoker (Reference) | 0.53 | (0.15–1.91) | 0.334 |
| Former smoker | |||
| ECOG PS | |||
| 0–1 (Reference) | 0.06 | (0.01–0.33) | 0.001 |
| 2–3 | |||
| Brain metastasis | |||
| No (Reference) | 0.87 | (0.29–2.62) | 0.805 |
| Yes | |||
| Line of therapy before crizotinib | |||
| 0 (Reference) | 0.60 | (0.22–1.61) | 0.308 |
| ≥ 1 |
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; OR, odds ratio; PFS, progression‐free survival.
Survival analysis
The median PFS of the PRR and LTR groups was 1.2 months (95% CI 0.70–1.73, range: 0.9–3.0) and 47.0 months (95% CI 34.39–59.64, range: 24.2–53.3), respectively. The median OS of the PRR group was 8.4 months (95% CI 3.47–13.42) and 6 patients were still alive at the last follow‐up. Notably, OS was significantly shorter in the PRR than in the LTR group (8.4 months vs. not reached; P < 0.001) (Fig 1a).
Figure 1.
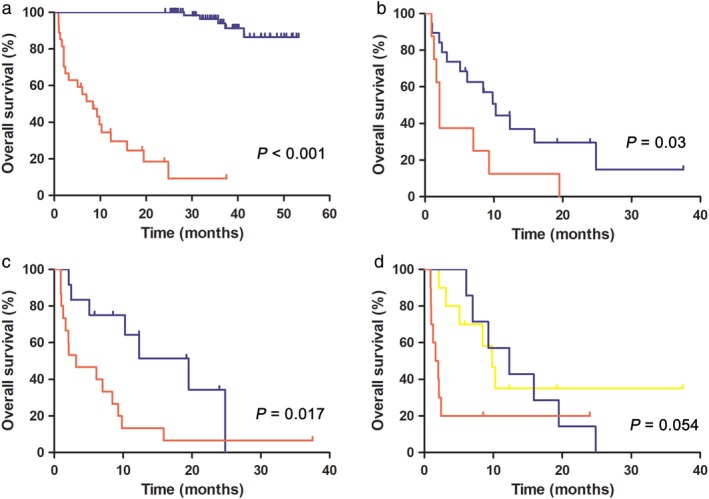
Overall survival (OS) of patients with ALK‐positive non‐small cell lung cancer (NSCLC) treated with crizotinib. Kaplan–Meier curves of OS (a) in the primary‐resistant responder (PRR) versus long‐term responder (LTR) group, and (b–d) in the PRR group according to Eastern Cooperative Oncology Group performance status (ECOG PS) score, line of crizotinib treatment, and subsequent therapy after crizotinib progression, respectively, including ALK inhibitors (ALKis), chemotherapy, and best supportive care (BSC).  PRR group,
PRR group,  LTR group,
LTR group,  ECOG 0‐1,
ECOG 0‐1,  ECOG 2‐3,
ECOG 2‐3,  First line,
First line,  Non‐first line,
Non‐first line,  ALKis,
ALKis,  Chemotherapy,
Chemotherapy,  BSC.
BSC.
We further analyzed the effects of clinical factors on OS in the PRR group. A log‐rank test demonstrated that better ECOG PS (P = 0.030) (Fig 1b) and first‐line crizotinib treatment (P = 0.017) (Fig 1c) were significantly associated with favorable survival outcomes. Multivariate Cox analyses revealed that crizotinib as a first‐line regimen was an independent predictive factor of OS in ALK‐positive patients with primary resistance (HR 5.24, 95% CI 1.64–16.74; P = 0.005) (Table 3).
Table 3.
Cox multivariate analysis of survival from the first crizotinib dose in patients with primary resistance (n = 28)
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Median OS (95% CI) | Log‐rank test | HR | 95% CI | P | HR | 95% CI | P |
| Age | ||||||||
| < 60 (Reference) | 9.3 (4.19–14.40) | 0.146 | 2.02 | (0.77–5.32) | 0.155 | |||
| ≥ 60 | 1.6 (0.72–2.57) | |||||||
| Gender | ||||||||
| Male (Reference) | 7.0 (2.43–11.57) | 0.911 | 0.95 | (0.4–2.29) | 0.911 | |||
| Female | 9.8 (0–21.53) | |||||||
| Smoking status | ||||||||
| Never smoker (Reference) | 7.0 (0–14.73) | 0.532 | 0.75 | (0.30–1.86) | 0.535 | |||
| Former smoker | 8.4 (1.22–15.66) | |||||||
| ECOG PS | ||||||||
| 0–1 (Reference) | 10.3 (6.88–13.69) | 0.03 | 2.63 | (1.06–6.53) | 0.037 | 2.77 | (0.90–8.50) | 0.075 |
| 2–3 | 2.1 (1.49–2.72) | |||||||
| Brain metastases | ||||||||
| No (Reference) | 8.4 (4.40–12.49) | 0.402 | 0.65 | (0.24–1.80) | 0.406 | |||
| Yes | 3.2 (0.00–19.51) | |||||||
| Line of therapy before crizotinib | ||||||||
| 0 (Reference) | 19.5 (8.81–30.23) | 0.017 | 2.93 | (1.16, 7.39) | 0.023 | 5.24 | (1.64, 16.74) | 0.005 |
| ≥ 1 | 3.2 (0–8.30) | |||||||
| Subsequent therapy after crizotinib PD | ||||||||
| ALKis (Reference) | 9.8 (7.26–12.39) | 0.054 | 0.069 | 0.007 | ||||
| Chemotherapy | 12.3 (4.56–20.08) | 1.26 | (0.42–3.77) | 0.682 | 0.96 | (0.27–3.42) | 0.944 | |
| BSC | 1.6 (0.47–2.81) | 3.31 | (1.11–9.86) | 0.032 | 6.98 | (1.71–28.58) | 0.007 | |
ALKis, ALK inhibitors; BSC, best supportive care; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; OS, overall survival; PD, progressive disease.
Six patients (21.4%) in the PRR group continued crizotinib after disease progression, four of which experienced disease re‐progression. Four patients were administered next‐generation ALK‐TKIs and nine patients were administered chemotherapy as the first subsequent regimen after crizotinib withdrawal. The ORRs of next‐generation ALK‐TKIs and chemotherapy were 25.0% (1 partial response [PR], 2 stable disease [SD], and 1 progressive disease [PD]) and 11.1% (1 PR, 5 SD, 2 PD, and 1 unassessable), and the median PFS rates were 2.9 months (95% CI 0.61–5.11) and 4.0 months (95% CI 1.56–6.39), respectively. Statistical analysis showed that patients administered ALK‐TKIs (including crizotinib and next‐generation ALK inhibitors [ALKis], n = 10, median OS 9.8 months, 95% CI 7.26–12.39) had significantly better survival outcomes than those treated with best supportive care (n = 11, median OS 1.6 months, 95% CI 0.47–2.81), but there was no significant difference to patients who received single chemotherapy (n = 7, median OS 12.3 months, 95% CI 4.56–20.08) (Fig 1d, Table 3).
Next‐generation sequencing
Next‐generation sequencing was performed in two patients in tumor or blood plasma specimens. New additional intracranial involvement was discovered in a 58‐year old man treated with crizotinib as first‐line treatment after 2.6 months. The baseline lymph node formalin‐fixed and paraffin‐embedded specimen was detected as FISH‐positive and a 509‐panel NGS platform was used to analyze genomic profiling in the baseline tumor and plasma. The tumor specimen harbored EML4_E13:ALK_E19 fusion and the matched blood plasma was detected with a novel LTBP1_E2:ALK_E11 fusion variant with EML4_E13:ALK_E19 and concomitant L1196M mutation at disease progression. Another patient, a 24‐year old woman administered crizotinib as a second‐line regimen, developed intracranial progression within one month. The baseline lymph node was detected as Ventana immunohistochemistry‐positive and the NGS results of baseline blood plasma showed a TP53 mutation concurrent with EML4_E20:ALK_E19 fusion.
Discussion
Currently, little data is available concerning primary resistance to ALK‐TKIs. To our knowledge, our study is the first to include a relatively large sample to investigate the clinical features and survival outcomes of ALK‐positive NSCLC patients with a poor response to crizotinib. Primary resistance was observed in 6.5% of the patients, which is consistent with 5–7% reported in previous crizotinib trials.4, 6, 7 The clinical characteristics of the ALK‐positive patients with primary resistance in our study were similar to the general ALK population (i.e. adenocarcinoma histology, never smokers, and young age).14
Because long‐term PFS can be translated into favorable survival prognoses, it is critically important to identify factors that can effectively discriminate PRRs from LTRs prior to crizotinib treatment. However, the results of our study showed no specific baseline clinicopathologic factors between the groups, with the exception of ECOG PS. Thus, our findings emphasize the need for further studies investigating reliable biomarkers that can predict the therapeutic efficacy of ALK‐TKIs and explore the mechanisms underlying primary resistance.
Studies on the mechanism of primary resistance are relatively rare. Several studies have revealed that concurrent gene alterations in ALK‐rearranged tumors may negatively impact the PFS of crizotinib in patients with ALK‐rearranged NSCLC. Yu et al. demonstrated that concurrent ALK activation mutations were more common in patients administered multiple lines of TKI treatment compared to single agent crizotinib, and a co‐existing TP53 mutation was correlated to unfavorable survival in ALK‐positive NSCLC patients treated with crizotinib.15 We also found a TP53 mutation in the baseline blood plasma of one patient with primary resistance, which might be related to her poor response to crizotinib. Furthermore, a small number of case reports revealed that the intrinsic factors in ALK‐rearranged lung cancer cells, such as KRAS mutations,16, 17 MYC amplification18 and the Bim deletion polymorphism,19 might be responsible for primary resistance to crizotinib. Different EML4‐ALK translocation variants might generate a distinct response to ALKis3, 20 and the significance of diverse ALK fusion partners has not yet been fully elucidated.21, 22, 23, 24 In our study, a novel LTBP1‐ALK fusion was detected by NGS technology at the baseline specimen of a patient with primary resistance, and his “gold standard” FISH assay result was ALK‐positive. This fusion gene has not been reported previously, but an EML4‐ALK fusion with atypical LTBP1 insertion could responded well to crizotinib.25 In future research, we will explore the underlying mechanism for this result. Because NGS technology has displayed impressive capability for identifying the underlying molecular profile of cancers, its clinical application as a molecular screening test might favorably alter the clinical outcomes of ALK‐positive NSCLC patients.26
To further explore the therapeutic options that can overcome primary resistance to crizotinib, we investigated the clinical efficacy of subsequent therapies after crizotinib failure and analyzed their impacts on survival outcomes. The results demonstrated that ALK‐positive patients with primary resistance to crizotinib can obtain therapeutic and survival benefits from second‐generation ALKis or chemotherapy, and no significant differences were found between these two regimens. Previous research has indicated that next‐generation ALKis, such as alectinib, ceritinib, and lorlatinib, show a favorable response to crizotinib resistance.27, 28, 29, 30 These ALK‐TKIs can overcome the acquired resistance caused by “ALK‐dependent” alterations, such as ALK tyrosine domain mutations or amplification of the ALK gene. However, the efficacy of novel ALKis in patients with primary resistance to crizotinib has not been fully documented. Facchinetti et al. first reported a case of an ALK‐rearranged NSCLC patient with primary resistance to crizotinib who experienced a partial and durable response to ceritinib.31 Preclinical evidence has also confirmed the potential benefit of ceritinib in overcoming crizotinib‐resistant mutations.27 It may therefore be presumed that the presence of resistance mutations in the ALK kinase domain at baseline may give rise to the lack of crizotinib efficacy, which can be successfully interrupted by the more potent compound next‐generation ALKis. However, other resistance mechanisms, including pharmacokinetic issues and interpatient variability in drug bioavailability, may also have an impact on crizotinib efficacy. The current availability of a wide spectrum of ALK‐TKIs makes it imperative to obtain multiplex molecular genetic profiling for lung cancer before making final therapeutic decisions.
There were some limitations to the current study that cannot be ignored. First, this was a retrospective study. The patient sample was small and the characteristics of the groups were partially imbalanced because of selection bias. Second, limited patients received sequencing treatment after crizotinib progression, and we were not able to compare the efficacy of different ALKis and chemotherapy regimens. Given the crossover of ALKis and chemotherapy in subsequent treatment, the results on survival outcomes need to be interpreted with caution. Third, only two patients had adequate specimens to conduct NGS testing, thus the association between gene alterations and primary resistance to crizotinib remains unknown.
In conclusion, this study demonstrated that clinical variables cannot successfully predict the survival outcomes of patients with primary resistance to crizotinib treatment. This subset of patients can obtain therapeutic and survival benefits from next‐generation ALKis and chemotherapy. The mechanism of primary resistance to crizotinib requires further investigation, and NGS technology might be a good complementary method for screening genetic alterations and making final treatment decisions.
Disclosure
No authors report any conflict of interest.
Acknowledgment
We thank all of the patients and their families, as well as the investigators and staff at each center for their participation in data collection, monitoring, and computing.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Blumenthal GM, Karuri SW, Zhang H et al. Overall response rate, progression‐free survival, and overall survival with targeted and standard therapies in advanced non‐small‐cell lung cancer: US Food and Drug Administration trial‐level and patient‐level analyses. J Clin Oncol 2015; 33: 1008–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Soda M, Choi YL, Enomoto M et al. Identification of the transforming EML4‐ALK fusion gene in non‐small‐cell lung cancer. Nature 2007; 448: 561–6. [DOI] [PubMed] [Google Scholar]
- 4. Camidge DR, Bang YJ, Kwak EL et al. Activity and safety of crizotinib in patients with ALK‐positive non‐small‐cell lung cancer: Updated results from a phase 1 study. Lancet Oncol 2012; 13: 1011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim DWAMJ, Shi Y et al. Results of a global phase II study with crizotinib in advanced ALK‐positive non‐small cell lung cancer (NSCLC). Ann Oncol 2012; 30: 32–3. [Google Scholar]
- 6. Shaw AT, Kim DW, Nakagawa K et al. Crizotinib versus chemotherapy in advanced ALK‐positive lung cancer. Published erratum appears in N Engl J Med 2015;373:1582). N Engl J Med 2013; 368: 2385–94. [DOI] [PubMed] [Google Scholar]
- 7. Solomon BJ, Mok T, Kim DW et al. First‐line crizotinib versus chemotherapy in ALK‐positive lung cancer. N Engl J Med 2014; 371: 2167–77. [DOI] [PubMed] [Google Scholar]
- 8. Lu S, Mok T, Lu Y et al. Phase 3 study of first‐line crizotinib vs pemetrexed−cisplatin/carboplatin (PCC) in East Asian patients (pts) with ALK+ advanced non‐squamous non‐small cell lung cancer (NSCLC). J Clin Oncol 2016; 34: 9058–8. [Google Scholar]
- 9. Choi YL, Soda M, Yamashita Y et al. EML4‐ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med 2010; 363: 1734–9. [DOI] [PubMed] [Google Scholar]
- 10. Katayama R, Shaw AT, Khan TM et al. Mechanisms of acquired crizotinib resistance in ALK‐rearranged lung cancers. Sci Transl Med 2012; 4: 120ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doebele RC, Pilling AB, Aisner DL et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non‐small cell lung cancer. Clin Cancer Res 2012; 18: 1472–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gainor JF, Dardaei L, Yoda S et al. Molecular mechanisms of resistance to first‐ and second‐generation ALK inhibitors in ALK‐rearranged lung cancer. Cancer Discov 2016; 6: 1118–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Steuer CE, Ramalingam SS. ALK‐positive non‐small cell lung cancer: Mechanisms of resistance and emerging treatment options. Cancer 2014; 120: 2392–402. [DOI] [PubMed] [Google Scholar]
- 14. Shaw AT, Yeap BY, Mino‐Kenudson M et al. Clinical features and outcome of patients with non‐small‐cell lung cancer who harbor EML4‐ALK. J Clin Oncol 2009; 27: 4247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yu Y, Ou Q, Wu X et al. Concomitant resistance mechanisms to multiple tyrosine kinase inhibitors in ALK‐positive non‐small cell lung cancer. Lung Cancer 2019; 127: 19–24. [DOI] [PubMed] [Google Scholar]
- 16. Schmid S, Gautschi O, Rothschild S et al. Clinical outcome of ALK‐positive non‐small cell lung cancer (NSCLC) patients with De Novo EGFR or KRAS Co‐mutations receiving tyrosine kinase inhibitors (TKIs). J Thorac Oncol 2017; 12: 681–8. [DOI] [PubMed] [Google Scholar]
- 17. Mengoli MC, Barbieri F, Bertolini F, Tiseo M, Rossi G. K‐RAS mutations indicating primary resistance to crizotinib in ALK‐rearranged adenocarcinomas of the lung: Report of two cases and review of the literature. Lung Cancer 2016; 93: 55–8. [DOI] [PubMed] [Google Scholar]
- 18. Rihawi K, Alfieri R, Fiorentino M et al. MYC amplification as a potential mechanism of primary resistance to crizotinib in ALK‐rearranged non‐small cell lung cancer: A brief report. Transl Oncol 2019; 12: 116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang L, Jiang T, Li X et al. Clinical features of Bim deletion polymorphism and its relation with crizotinib primary resistance in Chinese patients with ALK/ROS1 fusion‐positive non‐small cell lung cancer. Cancer 2017; 123: 2927–35. [DOI] [PubMed] [Google Scholar]
- 20. Yoshida T, Oya Y, Tanaka K et al. Differential crizotinib response duration among ALK fusion variants in ALK‐positive non‐small‐cell lung cancer. J Clin Oncol 2016; 34: 3383–9. [DOI] [PubMed] [Google Scholar]
- 21. Wong DW, Leung EL, Wong SK et al. A novel KIF5B‐ALK variant in nonsmall cell lung cancer. Cancer 2011; 117: 2709–18. [DOI] [PubMed] [Google Scholar]
- 22. Togashi Y, Soda M, Sakata S et al. KLC1‐ALK: A novel fusion in lung cancer identified using a formalin‐fixed paraffin‐embedded tissue only. PLoS One 2012; 7: e31323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fang DD, Zhang B, Gu Q et al. HIP1‐ALK, a novel ALK fusion variant that responds to crizotinib. J Thorac Oncol 2014; 9: 285–94. [DOI] [PubMed] [Google Scholar]
- 24. Du X, Shao Y, Gao H et al. CMTR1‐ALK: An ALK fusion in a patient with no response to ALK inhibitor crizotinib. Cancer Biol Ther 2018; 19 (11): 962–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aguado C, Gil MD, Yeste Z et al. Response to crizotinib in a non‐small‐cell lung cancer patient harboring an EML4‐ALK fusion with an atypical LTBP1 insertion. Onco Targets Ther 2018; 11: 1117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dagogo‐Jack I, Brannon AR, Ferris LA et al. Tracking the evolution of resistance to alk tyrosine kinase inhibitors through longitudinal analysis of circulating tumor DNA. JCO Precis Oncol 2018; 2018: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Friboulet L, Li N, Katayama R et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non‐small cell lung cancer. Cancer Discov 2014; 4: 662–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zou HY, Friboulet L, Kodack DP et al. PF‐06463922, an ALK/ROS1 inhibitor, overcomes resistance to first and second generation ALK inhibitors in preclinical models. Cancer Cell 2015; 28: 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ou SH, Ahn JS, De Petris L et al. Alectinib in crizotinib‐refractory ALK‐rearranged non‐small‐cell lung cancer: A phase II global study. J Clin Oncol 2016; 34: 661–8. [DOI] [PubMed] [Google Scholar]
- 30. Metro G, Tazza M, Matocci R, Chiari R, Crino L. Optimal management of ALK‐positive NSCLC progressing on crizotinib. Lung Cancer 2017; 106: 58–66. [DOI] [PubMed] [Google Scholar]
- 31. Facchinetti F, Caramella C, Auger N et al. Crizotinib primary resistance overcome by ceritinib in a patient with ALK‐rearranged non‐small cell lung cancer. Tumori 2016; 102 (Suppl 2): S46–9 [DOI] [PubMed] [Google Scholar]


