Abstract
Y‐box binding protein‐1 (YBX1), a multifunctional oncoprotein containing an evolutionarily conserved cold shock domain, dysregulates a wide range of genes involved in cell proliferation and survival, drug resistance, and chromatin destabilization by cancer. Expression of a multidrug resistance‐associated ATP binding cassette transporter gene, ABCB1, as well as growth factor receptor genes, EGFR and HER2/ErbB2, was initially discovered to be transcriptionally activated by YBX1 in cancer cells. Expression of other drug resistance‐related genes, MVP/LRP,TOP2A,CD44,CD49f,BCL2,MYC, and androgen receptor (AR), is also transcriptionally activated by YBX1, consistently indicating that YBX1 is involved in tumor drug resistance. Furthermore, there is strong evidence to support that nuclear localization and/or overexpression of YBX1 can predict poor outcomes in patients with more than 20 different tumor types. YBX1 is phosphorylated by kinases, including AKT, p70S6K, and p90RSK, and translocated into the nucleus to promote the transcription of resistance‐ and malignancy‐related genes. Phosphorylated YBX1, therefore, plays a crucial role as a potent transcription factor in cancer. Herein, a novel anticancer therapeutic strategy is presented by targeting activated YBX1 to overcome drug resistance and malignant progression.
Keywords: drug resistance, malignant progression, oncogenic effector, overcoming drug resistance, Y‐box binding protein‐1
1. Y‐BOX BINDING PROTEIN‐1 TRANSCRIPTIONALLY ACTIVATES ABC TRANSPORTER GENE CONFERRING CANCER‐ACQUIRED MULTIDRUG RESISTANCE
One of the most significant obstacles to anticancer therapy is the emergence of drug‐resistant tumors. Development of potent therapeutic drugs that can overcome drug resistance is a persistent challenge for cancer researchers. Over the past five decades, the classical approach of selecting drug‐resistant cancer cells through chronic exposure of drug‐sensitive cancer cells to anticancer agents has been used to elucidate how cells acquire drug resistance, in addition to how drug resistance‐reversal therapies are developed.1, 2
One representative ABC transporter gene, ABCB1, encodes a membrane glycoprotein catalyzing the ATP‐dependent efflux of multiple anticancer agents, and the efflux activity is closely associated with the acquisition of the most classical multidrug resistance (Figure 1A).3, 4 In one example, the same drug‐sensitive parental cell line was used to select two multidrug‐resistant cell lines; KB‐C15 was selected as colchicine‐resistant cells and KB/VJ3006 was selected as vincristine‐resistant cells. ABCB1 is amplified in resistant KB‐C1 cells,7 and the amplified region spans approximately 1‐2 Mb on the human chromosome 7q21.12.8 In contrast, overexpression of the ABCB1 protein was also observed in resistant KB/VJ300 cells without gene amplification.6 Therefore, ABCB1 is overexpressed through either gene amplification or transcriptional activation. Among 51 ABC transporters, ABCB1 is one of the major transporters of clinical significance.9 As a major mechanism of ABCB1 overexpression, transcriptional activation of ABCB1 is most often reported in various human malignancies and in cancer cell lines (Figure 1A). Y‐box binding protein‐1 (YBX1) binds to the Y‐box sequence in the 5′‐flanking region of ABCB1 in drug‐resistant cancer cells to promote its transcriptional activation (Figure 1A,B).10, 11, 12, 13 In addition, YBX1 binds to the Y‐box sequence in many other drug resistance‐related genes (Figure 1B).11, 14, 15, 16, 17, 18, 19, 20 Therefore, YBX1 drives the acquisition of drug resistance in cancer cells through its transcriptional activation.21, 22, 23
Figure 1.
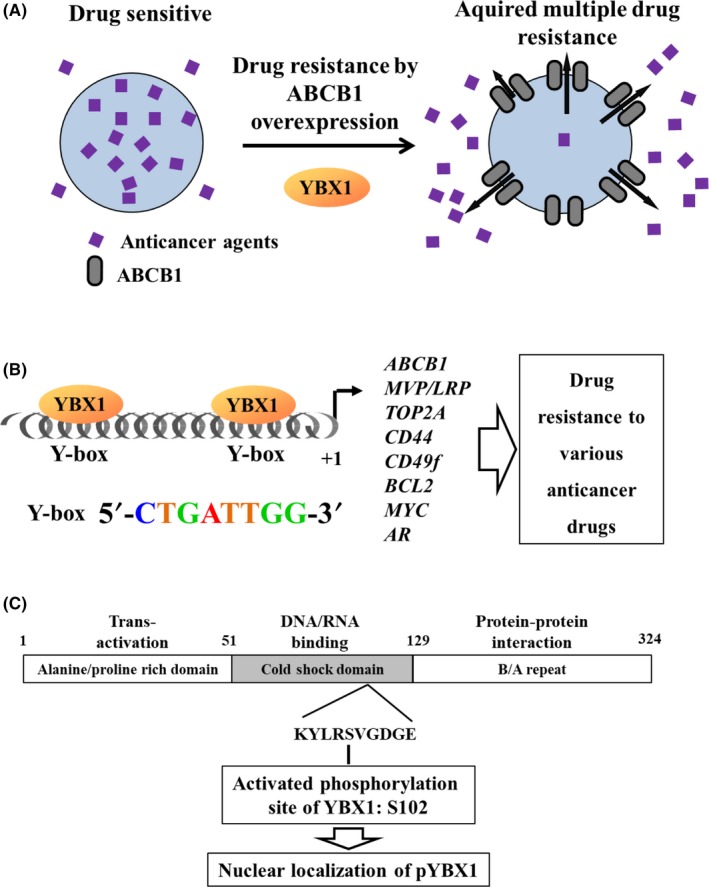
A, Among 51 ABC transporters, ABCB1 is a representative transporter that enhances outward efflux activity of anticancer drugs from the inside to the outside of cancer cells, resulting in acquired multidrug resistance. Enhanced transcriptional activation of ABCB1, either by chronic exposure to anticancer drugs or by acquired multidrug resistance, is mediated through the environmental stimuli‐naïve Y‐box binding protein‐1 (YBX1). B, YBX1‐induced activation of ABCB1 is first presented as a transcriptional mechanism of how tumor multidrug resistance is acquired during chemotherapeutic treatments in various human malignancies. YBX1 also induces activation of various other resistance‐related genes, including MVP/LRP,TOP2A,CD44,CD49f,BCL2,MYC, and androgen receptor (AR). C, Structure and phosphorylation sites of the YBX1 protein. Phosphorylation at Ser102 in the cold shock domain is essential for nuclear translocation and oncogenic activation of YBX1. B/A repeat, basic and acidic amino acid repeat sequences
YBX1 is a member of the cold shock domain protein superfamily, which is considered the most evolutionarily conserved nucleic acid‐binding protein from bacteria to human (Figure 1C).21, 24 YBX1 was initially identified as a transcription factor that binds to the Y‐box sequence of the 5′‐flanking region of the ovalbumin gene,25 the major histocompatibility complex class gene,26 and of epidermal growth factor receptor (EGFR).27 The consensus sequence binding site for YBX1 is 5′‐CTGATTGG‐3′, namely, the inverted CCAAT box (Figure 1B).
YBX1 is localized in both the cytoplasm and nucleus of cancer cells (Figure 2A). YBX1 translocates from the cytoplasm to the nucleus when exposed to various environmental stimuli in vitro and in vivo,11, 12, 28, 29 as well as in cancer cells in patients treated with anticancer therapeutic drugs.30 YBX1 in the nucleus plays a major role as a transcription factor for various drug resistance‐related genes, including ABCB1, and in the repair of damaged DNA. However, in the cytoplasm, YBX1 is mainly involved in post‐transcriptional control of several mRNA splices, including epithelial‐to‐mesenchymal transition‐related genes.31, 32 In the present review, we focus on the oncogenic role of YBX1, its localization in the nucleus, and its association with drug resistance.
Figure 2.
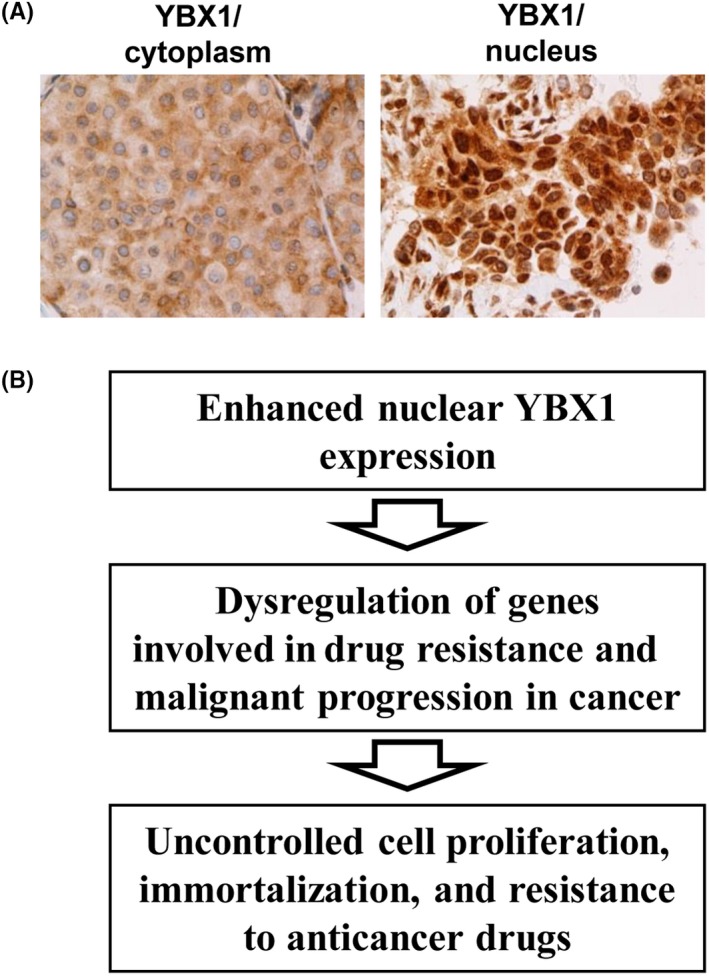
A, Representative immunohistochemistry images of Y‐box binding protein‐1 (YBX1) in the cytoplasm and nucleus of breast cancer tissues when stained with antibodies recognizing YBX1. B, Simplified model of how YBX1 induces resistance to anticancer drugs in cancer cells when it is located in the nucleus. Nuclear YBX1 induces activation of the genes involved in drug resistance and malignant progression
2. Y‐BOX BINDING PROTEIN‐1 DYSREGULATES GENES INVOLVED IN DRUG RESISTANCE AND TUMOR GROWTH PROMOTION
Immunohistochemistry (IHC) analyses have mainly been used to investigate whether nuclear and/or cytoplasmic expression of YBX1 is enhanced in cancer.28 Figure 2A presents typical IHC images of YBX1 expression in the cytoplasm and in the nucleus of cancer cells in tumor specimens from cancer patients.
YBX1 dysregulates drug resistance‐related genes, including ABCB1, MVP/LRP, PCNA, MYC, TOP2A, CD44, CD49f, p53, BCL2, and androgen receptor (AR), conferring resistance to cancer cells against a wide range of anticancer therapeutic agents. For example, enhanced YBX1 expression is often correlated with cisplatin resistance, a representative cytotoxic anticancer drug.11, 12, 33 Conversely, YBX1 also dysregulates genes involved in cell proliferation and cell cycle, such as EGFR, human epidermal growth factor receptor 2 (HER2), cyclins A/B1/D1/E, CDC6, and AR. Overall, YBX1 enhances the expression of genes involved in cell proliferation, cell cycle, survival, and drug resistance and facilitates malignant progression as well as acquired resistance to anticancer chemotherapeutics by cancer cells (Figure 2B).
3. ENHANCED YBX1 EXPRESSION PREDICTS POOR OUTCOMES IN VARIOUS HUMAN MALIGNANCIES
Kamura et al34 first showed the clinical significance of nuclear YBX1 expression in predicting the expression of ABCB1 and poor outcomes in ovarian tumors. To date, based on expression levels of YBX1 in cancer cells, when assessed using IHC or qRT‐PCR, almost all 70 independent studies have consistently shown a close relationship between enhanced expression of YBX1 and poor outcomes in over 20 human tumor types (Table 1, Doc S1). Enhanced nuclear/cytoplasmic expression of YBX1 is negatively associated with overall survival or disease‐free survival in patients with various malignancies. In particular, nuclear YBX1 expression in cancer cells is significantly associated with poor outcomes in various malignancies, including breast, ovary, prostate, colon, liver, lung, bone, soft tissue, thyroid, skin, and nervous system, as well as osteosarcomas and hematological tumors (Table 1, Doc S1). In addition, enhanced expression of YBX1 is positively correlated with various biomarkers, including ABCB1, MVP/LRP, EGFR, HER2, AR, and CDC6, and negatively correlated with estrogen receptor α (ERα), in various tumor types. Therefore, the enhanced expression of YBX1 could be a prognostic biomarker for cancer progression, potentially because of its tumor‐promoting effects on cell proliferation, survival, and drug resistance in various tumor types. Similarly, carcinogenesis and further malignant progression of cancer are associated with the nine hallmarks of cancer, including cell proliferation, survival, immortalization, inflammation, invasion, metastasis, and others.35 In addition, most of the hallmarks are closely coupled with enhanced expression of YBX1.32
Table 1.
Association of YBX1 expression with outcomes and biomarkers in human malignanciesa
| Human malignancies | Biomarkers associated with YBX1 | No. of published papersb |
|---|---|---|
| Breast cancer | ABCB1, EGFR, HER2, E2F‐1, CDC6, ER, EGR‐1, ER effector genes | 19 |
| Lung cancer | PCNA, HER2, HER3, CDC25a, MACC1 | 7 |
| Ovarian cancer | CD44, ABCB1, LRP/MVP, amphiregulin | 7 |
| Prostate cancer | AR | 6 |
| Colorectal cancer | EGFR, topoisomerase IIα, PCNA | 5 |
| Gastric cancer | HER2, CDC6 | 4 |
| 16 different tumor typesc | ABCB1, EGFR, HER2, EXH2, Cyclin A1/D1, AKT, LRP/MVP | 1‐3/each cancer |
Augmented nuclear and/or total (nuclear/cytoplasmic) expression of YBX1 predicts poor outcomes in patients with human malignancies in close association with aberrant expression of various biomarkers and genes. Poor outcomes are mainly assessed using overall survival or disease‐free survival, but some reports referred to histological grade, tumor grade, and metastasis.
References are listed in Doc S1.
Additional 16 tumor types include diffuse large B‐cell lymphoma, uterine cervical cancer, hepatocellular carcinoma, renal cell carcinoma, osteosarcoma, glioblastoma, nasopharyngeal carcinoma, laryngeal squamous cell carcinoma, esophageal squamous cell carcinoma, head and neck squamous cell carcinoma, thyroid neoplasm, melanoma, rhabdomyosarcoma, synovial sarcoma, neuroblastoma, and non‐Hodgkin lymphoma.
AR, androgen receptor; EGFR, epidermal growth factor receptor; EGR‐1, early growth response protein 1; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PCNA, proliferating cell nuclear antigen; YBX1, Y‐box binding protein‐1.
4. Y‐BOX BINDING PROTEIN‐1 IS AN EFFECTIVE POTENT TARGET IN OVERCOMING ANTI‐ESTROGEN RESISTANCE BY BREAST CANCER
Among the numerous human tumor types, the clinical significance of YBX1 has been most extensively studied in breast cancer (Table 1, Doc S1). Enhanced expression of YBX1 is predictive of poor outcomes in patients with breast cancer.36, 37, 38, 39, 40, 41 Adjuvant endocrine therapy and chemotherapy treatments have been used extensively in the treatment of breast cancer.42, 43 However, the emergence of refractory tumors is a major complication for breast cancer patients treated with adjuvant endocrine therapy.44 Targeting YBX1 may facilitate overcoming anti‐estrogen resistance and malignant progression in breast cancer based on the following reasons: YBX1‐knock‐in mice induce breast cancers with diverse histological characteristics, implicating YBX1 as an oncoprotein for mammary tumors;45 YBX1 overexpression promotes tumorigenesis in mammary epithelial cells;46 YBX1 silencing induces marked downregulation of cell proliferation and cell cycle‐related genes, such as HER2, FGFR2, and CDC6, and upregulation of ERα in human breast cancer cell lines,36, 37, 47, 48, 49, 50 conferring enhanced responsiveness to endocrine therapeutics in breast cancer cells.50 In addition, YBX1 overexpression induces downregulation of ERα expression in breast cancer cells (Figure 3A) and acquired resistance to tamoxifen, a representative anti‐estrogen, in therapeutic experimental models in vivo (Figure 3B).47
Figure 3.
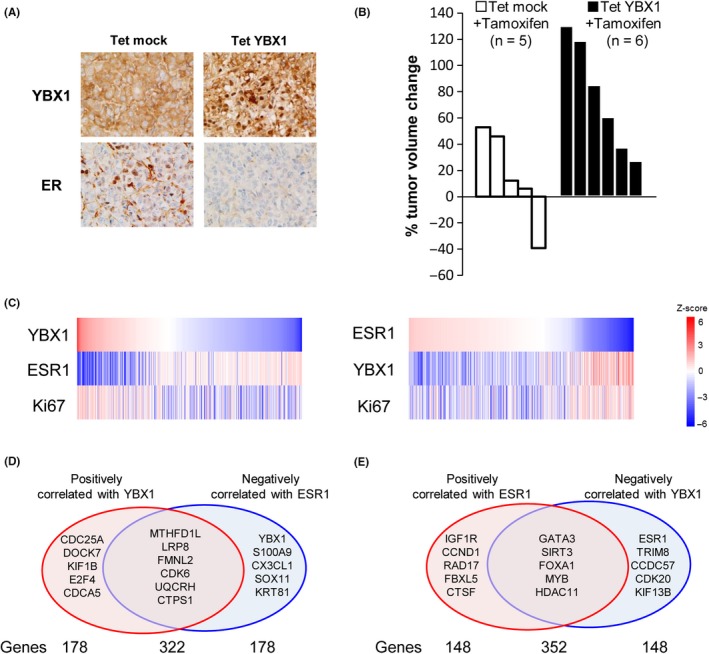
A, Immunohistochemistry images show reduced expression of estrogen receptor α (ERα) when Y‐box binding protein‐1 (YBX1) is overexpressed in mammary tumors induced by doxycycline in an experimental therapeutic model (Tet mock vs Tet YBX1). B, Overexpression of YBX1 in ERα‐positive breast cancer cells also induces resistance to endocrine therapy by tamoxifen in a xenograft experimental Tet YBX1 model compared with its Tet mock control. C, YBX1 expression is inversely correlated with ERα expression in human breast cancer. Heat map analysis of YBX1, ERα, and Ki67 in mammary tumors of breast cancer patients. D, Venn diagram showing the overlap of 322 genes between the top 500 genes that are positively correlated with YBX1 and the top 500 genes that are negatively correlated with estrogen receptor 1 (ESR1). Several representative genes are presented. E, Venn diagram showing the overlap of 352 genes between the top 500 genes that are positively correlated with ESR1 and the top 500 genes that are negatively correlated with YBX1. Several representative genes are presented
YBX1 expression is also negatively correlated with ERα expression in breast cancer cells in vitro and in vivo.36, 47 Expression levels of YBX1 mRNA are inversely correlated with expression levels of ERα mRNA and positively correlated with the expression of Ki67, a representative tumor growth marker, in breast cancer patients (The Cancer Genome Atlas [TCGA] database, n = 825)51 (Figure 3C). The top 500 genes that are positively or negatively correlated with YBX1 or estrogen receptor 1 (ESR1) have been identified based on RNA‐seq data from a cohort of 825 patients with invasive breast cancer (Figure 3D,E).37, 51 In the present study, 352 of the top 500 genes were shown to be both positively correlated with ESR1 and negatively correlated with YBX1 (Figure 3E).37, 51 Therefore, the enhanced expression of YBX1 is reciprocally associated with reduced expression of various ERα‐targeted genes, indicating that YBX1 preferentially promotes ERα‐independent tumor growth and survival. The findings suggest that YBX1 has oncogenic potential in breast cancer and that YBX1 could be a therapeutic target in breast cancer refractory to endocrine therapeutics.
5. PHOSPHORYLATION AND NUCLEAR LOCALIZATION OF YBX1 ARE REQUIRED FOR ITS ONCOGENIC DRIVER FUNCTIONS
Nuclear localization of YBX1 predicts poor outcomes in patients with various cancers (Table 1, Doc S1). Therefore, future studies should investigate the mechanisms by which YBX1 in the nucleus functions as an oncogenic transcription factor for various effector genes associated with malignant progression. Figure 4A presents representative images of phosphorylated YBX1 (pYBX1 Ser102) in the nucleus of cancer cells in malignantly progressive tumors from patients. Phosphorylation of YBX1 at Ser102 is essential for its nuclear translocation in cancer cells. YBX1 Ser102 phosphorylation is suppressed by inhibitors of PI3K, mTORC1, and p90 ribosomal S6 kinase (RSK).29, 52, 53, 54 Figure 4B shows the suppression of nuclear localization by a PI3K inhibitor (LY294002) or an mTORC1 inhibitor (everolimus). Consistent with Figure 4B, an Ser102 phosphorylation‐null mutant construct of YBX1 could not be translocated into the nucleus, which was accompanied by downregulated expression of EGFR and HER2, in breast cancer cells.52 Therefore, various kinases involved in AKT/mTOR and MEK/ERK signaling pathways influence both the phosphorylation and nuclear translocation of YBX1 (Figure 4C).
Figure 4.
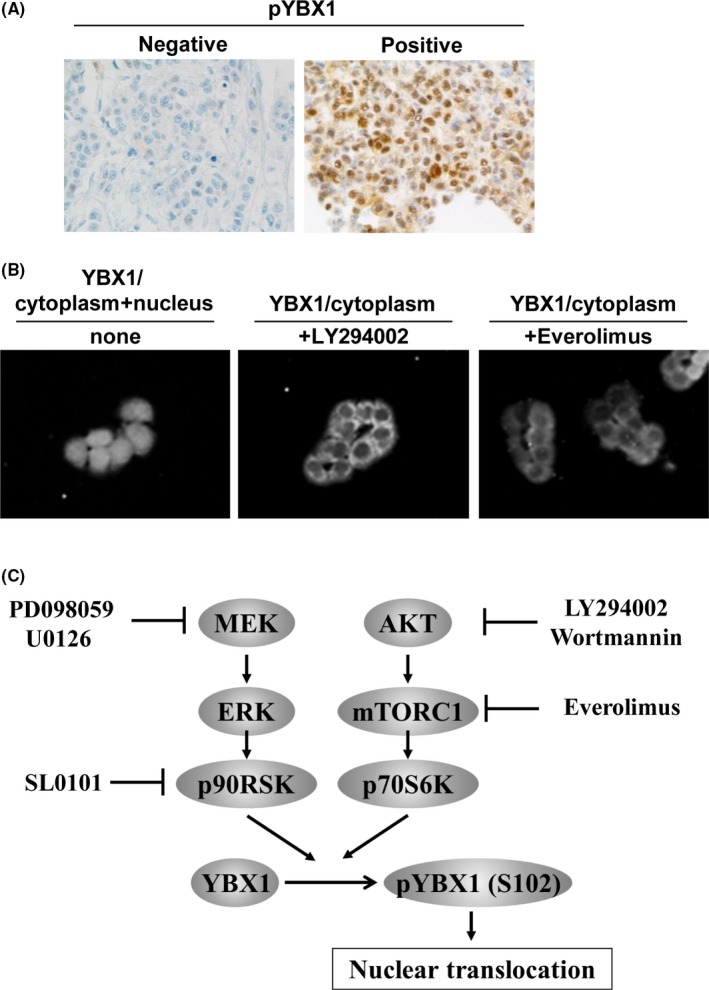
A, Representative immunohistochemistry images of expression of phosphorylated Y‐box binding protein‐1 (pYBX1) with an antibody recognizing pYBX1 Ser102 in breast cancer tissues. pYBX1 is preferentially localized in the nucleus of cancer cells in patients with progressive cancer. Left panel shows the negative control. B, Inhibitors of both PI3K/AKT (LY294002) and mTORC1 (everolimus) suppress nuclear localization of YBX1 in cancer cells. C, Hypothetical model of how YBX1 is activated through its phosphorylation at Ser102. YBX1 is phosphorylated and activated by PI3K/AKT/mTORC1 and/or MEK/ERK signaling pathways, promoting nuclear translocation
6. TARGETING YBX1 CONTRIBUTES TO FURTHER DEVELOPMENT OF EFFECTIVE ANTICANCER THERAPEUTICS
Preclinical therapeutic studies targeting YBX1 itself have been assessed. A molecular decoy cell permeable peptide of nine amino acids that flank the YBX1 Ser102 site induced cytotoxicity in cancer cells in vitro.55 Other preclinical therapeutic approaches involving the silencing of YBX1 with siRNAs or miRNAs have been carried out. A Dectin‐1‐targeting vehicle delivering YBX1 antisense DNA has been developed by making use of a novel polysaccharide drug delivery system, and the YBX1 silencing approach showed some suppression effects on cell viability in vitro.56 Another study has reported that silencing of YBX1 expression using long noncoding RNA induces cell growth suppression in lung cancer cells.57 Such preclinical trials show some cytotoxic effects on cancer cells in vitro, and more therapeutic experiments in vivo are required.
The next approach would be to develop drugs that specifically target YBX1 activation processes in cancer cells (Figure 5). One crucial role of YBX1 with regard to its oncogenic potential involves transcriptional activation of various effector genes that are closely associated with cell proliferation, survival, and drug resistance. Tumor growth and survival most often depend on AKT/mTOR and/or RAF/MEK signaling pathways in various human malignancies, and their downstream effector, YBX1, functions as a potent oncogenic driver (Figure 5). Ichikawa and colleagues recently developed a novel multikinase inhibitor that targets AKT, p90RSK, and p70S6K, and the compound shows potent antitumor activities against various tumor types with YBX1 Ser102 phosphorylation.58 Targeting AKT/mTOR/p70S6K and/or MEK/ERK/p90RSK signaling pathways results in the inhibition of the activation of YBX1, and such targeting would be useful in the development of novel therapeutics that are effective in overcoming drug resistance (Figure 5).
Figure 5.
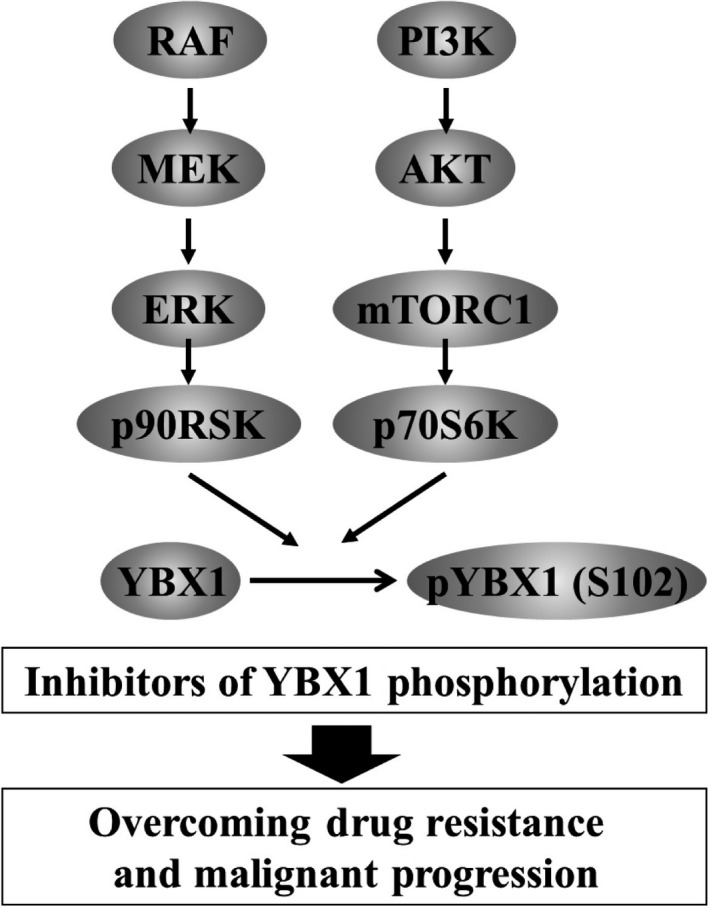
Model depicting how novel drugs targeting the Y‐box binding protein‐1 (YBX1) activation pathway overcome drug resistance. YBX1 is phosphorylated by Ser/Thr kinases involving AKT/mTOR/p70S6K and/or MEK/ERK/p90RSK signaling pathways. Phosphorylated YBX1 induces oncogenic transcriptional activation of various YBX1 targeted genes and plays crucial roles in cell proliferation, survival, and drug resistance. Development of inhibitors of the activation pathways of YBX1 would facilitate overcoming intrinsic and acquired resistance in addition to malignant progression
In conclusion, YBX1 plays essential roles in innate and acquired tumor drug resistance through the upregulation of a wide range of resistance‐related genes. Development of therapeutic drugs by targeting YBX1 phosphorylation would further facilitate overcoming tumor resistance to anticancer therapeutics in various progressive cancers. A future critical step would be the development and clinical evaluation of YBX1‐targeted therapeutics in patients with progressive cancers.
CONFLICTS OF INTEREST
Authors declare no conflicts of interest for this article.
Supporting information
ACKNOWLEDGMENTS
We thank all colleagues who have joined the YBX1 project in our lab and other labs, who greatly contributed to our understanding of the intrinsic important knowledge on YBX1. In particular, we acknowledge our colleagues, Dr Kimitoshi Kohno (University of Occupational and Environmental Health), Dr Yuji Basaki (Taiho Pharmaceutical Co. Ltd), Dr Yoshinao Oda (Kyushu University) and Dr Akihiko Kawahara (Kurume University Hospital). The continuous encouragement from Mr Yoshio Ide (Chairman, St. Mary's Hospital) is gratefully acknowledged. We dedicate this review to Dr Yutaka Ariyoshi (the late Chairman, Aichi Cancer Center Aichi Hospital).
Kuwano M, Shibata T, Watari K, Ono M. Oncogenic Y‐box binding protein‐1 as an effective therapeutic target in drug‐resistant cancer. Cancer Sci. 2019;110:1536–1543. 10.1111/cas.14006
REFERENCES
- 1. Kuwano M, Sonoda K, Murakami Y, Watari K, Ono M. Overcoming drug resistance to receptor tyrosine kinase inhibitors: learning from lung cancer. Pharmacol Ther. 2016;161:97‐110. [DOI] [PubMed] [Google Scholar]
- 2. Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug‐resistant cancer. Nat Rev Cancer. 2018;18(7):452‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Debenham PG, Kartner N, Siminovitch L, Riordan JR, Ling V. DNA‐mediated transfer of multiple drug resistance and plasma membrane glycoprotein expression. Mol Cell Biol. 1982;2(8):881‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tsuruo T, Naito M, Tomida A, et al. Molecular targeting therapy of cancer: drug resistance, apoptosis and survival signal. Cancer Sci. 2003;94(1):15‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Akiyama S, Fojo A, Hanover JA, Pastan I, Gottesman MM. Isolation and genetic characterization of human KB cell lines resistant to multiple drugs. Somat Cell Mol Genet. 1985;11(2):117‐126. [DOI] [PubMed] [Google Scholar]
- 6. Kohno K, Kikuchi J, Sato S, et al. Vincristine‐resistant human cancer KB cell line and increased expression of multidrug‐resistance gene. Jpn J Cancer Res. 1988;79(11):1238‐1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roninson IB, Chin JE, Choi KG, et al. Isolation of human mdr DNA sequences amplified in multidrug‐resistant KB carcinoma cells. Proc Natl Acad Sci USA. 1986;83(12):4538‐4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Torigoe K, Sato S, Kusaba H, et al. A YAC‐based contig of 1.5 Mb spanning the human multidrug resistance gene region and delineating the amplification unit in three human multidrug‐resistant cell lines. Genome Res. 1995;5(3):233‐244. [DOI] [PubMed] [Google Scholar]
- 9. Leonard GD, Fojo T, Bates SE. The role of ABC transporters in clinical practice. Oncologist. 2003;8(5):411‐424. [DOI] [PubMed] [Google Scholar]
- 10. Kohno K, Sato S, Takano H, Matsuo K, Kuwano M. The direct activation of human multidrug resistance gene (MDR1) by anticancer agents. Biochem Biophys Res Commun. 1989;165:1415‐1421. [DOI] [PubMed] [Google Scholar]
- 11. Ohga T, Koike K, Ono M, et al. Role of the human Y box‐binding protein YB‐1 in cellular sensitivity to the DNA‐damaging agents cisplatin, mitomycin C, and ultraviolet light. Cancer Res. 1996;56(18):4224‐4228. [PubMed] [Google Scholar]
- 12. Ohga T, Uchiumi T, Makino Y, et al. Direct involvement of the Y‐box binding protein YB‐1 in genotoxic stress‐induced activation of the human multidrug resistance 1 gene. J Biol Chem. 1998;273(11):5997‐6000. [DOI] [PubMed] [Google Scholar]
- 13. Morrow CS, Nakagawa M, Goldsmith ME, Madden MJ, Cowan KH. Reversible transcriptional activation of mdr1 by sodium butyrate treatment of human colon cancer cells. J Biol Chem. 1994;269:10739‐10746. [PubMed] [Google Scholar]
- 14. Morgan SE, Beck WT. Role of an inverted CCAAT element in human topoisomerase IIalpha gene expression in ICRF‐187‐sensitive and ‐resistant CEM leukemic cells. Mol Pharmacol. 2001;59(2):203‐211. [DOI] [PubMed] [Google Scholar]
- 15. Stein U, Bergmann S, Scheffer GL, et al. YB‐1 facilitates basal and 5‐fluorouracil‐inducible expression of the human major vault protein (MVP) gene. Oncogene. 2005;24(22):3606‐3618. [DOI] [PubMed] [Google Scholar]
- 16. Schittek B, Psenner K, Sauer B, Meier F, Iftner T, Garbe C. The increased expression of Y box‐binding protein 1 in melanoma stimulates proliferation and tumor invasion, antagonizes apoptosis and enhances chemoresistance. Int J Cancer. 2007;120(10):2110‐2118. [DOI] [PubMed] [Google Scholar]
- 17. To K, Fotovati A, Reipas KM, et al. Y‐box binding protein‐1 induces the expression of CD44 and CD49f leading to enhanced self‐renewal, mammosphere growth, and drug resistance. Cancer Res. 2010;70(7):2840‐2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kang Y, Hu W, Ivan C, et al. Role of focal adhesion kinase in regulating YB‐1‐mediated paclitaxel resistance in ovarian cancer. J Natl Cancer Inst. 2013;105(19):1485‐1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bommert KS, Effenberger M, Leich E, et al. The feed‐forward loop between YB‐1 and MYC is essential for multiple myeloma cell survival. Leukemia. 2013;27(2):441‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shiota M, Fujimoto N, Imada K, et al. Potential role for YB‐1 in castration‐resistant prostate cancer and resistance to enzalutamide through the androgen receptor V7. J Natl Cancer Inst. 2016;108(7):djw005. [DOI] [PubMed] [Google Scholar]
- 21. Kohno K, Izumi H, Uchiumi T, Ashizuka M, Kuwano M. The pleiotropic functions of the Y‐box‐binding protein, YB‐1. BioEssays. 2003;25(7):691‐698. [DOI] [PubMed] [Google Scholar]
- 22. Kuwano M, Uchiumi T, Hayakawa H, et al. The basic and clinical implications of ABC transporters, Y‐box‐binding protein‐1 (YB‐1) and angiogenesis‐related factors in human malignancies. Cancer Sci. 2003;94(1):9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kuwano M, Oda Y, Izumi H, et al. The role of nuclear Y‐box binding protein 1 as a global marker in drug resistance. Mol Cancer Ther. 2004;3(11):1485‐1492. [PubMed] [Google Scholar]
- 24. Ladomery M, Sommerville J. A role for Y‐box proteins in cell proliferation. BioEssays. 1995;17(1):9‐11. [DOI] [PubMed] [Google Scholar]
- 25. Bradshaw MS, Tsai MJ, O'Malley BW. A far upstream ovalbumin enhancer binds nuclear factor‐1‐like factor. J Biol Chem. 1988;263(17):8485‐8490. [PubMed] [Google Scholar]
- 26. Didier DK, Schiffenbauer J, Woulfe SL, Zacheis M, Schwartz BD. Characterization of the cDNA encoding a protein binding to the major histocompatibility complex class II Y box. Proc Natl Acad Sci USA. 1988;85(19):7322‐7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sakura H, Maekawa T, Imamoto F, Yasuda K, Ishii S. Two human genes isolated by a novel method encode DNA‐binding proteins containing a common region of homology. Gene. 1988;73(2):499‐507. [DOI] [PubMed] [Google Scholar]
- 28. Koike K, Uchiumi T, Ohga T, et al. Nuclear translocation of the Y‐box binding protein by ultraviolet irradiation. FEBS Lett. 1997;417(3):390‐394. [DOI] [PubMed] [Google Scholar]
- 29. Basaki Y, Hosoi F, Oda Y, et al. Akt‐dependent nuclear localization of Y‐box‐binding protein 1 in acquisition of malignant characteristics by human ovarian cancer cells. Oncogene. 2007;26(19):2736‐2746. [DOI] [PubMed] [Google Scholar]
- 30. Fujita T, Ito K, Izumi H, et al. Increased nuclear localization of transcription factor Y‐box binding protein 1 accompanied by up‐regulation of P‐glycoprotein in breast cancer pretreated with paclitaxel. Clin Cancer Res. 2005;11(24 Pt 1):8837‐8844. [DOI] [PubMed] [Google Scholar]
- 31. Kosnopfel C, Sinnberg T, Schittek B. Y‐box binding protein 1‐a prognostic marker and target in tumour therapy. Eur J Cell Biol. 2014;93(1‐2):61‐70. [DOI] [PubMed] [Google Scholar]
- 32. Maurya PK, Mishra A, Yadav BS, et al. Role of Y box protein‐1 in cancer: as potential biomarker and novel therapeutic target. J Cancer. 2017;8(10):1900‐1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ise T, Nagatani G, Imamura T, et al. Transcription factor Y‐box binding protein 1 binds preferentially to cisplatin‐modified DNA and interacts with proliferating cell nuclear antigen. Cancer Res. 1999;59(2):342‐346. [PubMed] [Google Scholar]
- 34. Kamura T, Yahata H, Amada S, et al. Is nuclear expression of Y box‐binding protein‐1 a new prognostic factor in ovarian serous adenocarcinoma? Cancer. 1999;85(11):2450‐2454. [DOI] [PubMed] [Google Scholar]
- 35. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646‐674. [DOI] [PubMed] [Google Scholar]
- 36. Fujii T, Kawahara A, Basaki Y, et al. Expression of HER2 and estrogen receptor alpha depends upon nuclear localization of Y‐box binding protein‐1 in human breast cancers. Cancer Res. 2008;68(5):1504‐1512. [DOI] [PubMed] [Google Scholar]
- 37. Shibata T, Tokunaga E, Hattori S, et al. Y‐box binding protein YBX1 and its correlated genes as biomarkers for poor outcomes in patients with breast cancer. Oncotarget. 2018;9(98):37216‐37228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Habibi G, Leung S, Law JH, et al. Redefining prognostic factors for breast cancer: YB‐1 is a stronger predictor of relapse and disease‐specific survival than estrogen receptor or HER‐2 across all tumor subtypes. Breast Cancer Res. 2008;10(5):R86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lasham A, Samuel W, Cao H, et al. YB‐1, the E2F pathway, and regulation of tumor cell growth. J Natl Cancer Inst. 2012;104(2):133‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu K, Chen K, Wang C, et al. Cell fate factor DACH1 represses YB‐1‐mediated oncogenic transcription and translation. Cancer Res. 2014;74(3):829‐839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lasham A, Mehta SY, Fitzgerald SJ, et al. A novel EGR‐1 dependent mechanism for YB‐1 modulation of paclitaxel response in a triple negative breast cancer cell line. Int J Cancer. 2016;139(5):1157‐1170. [DOI] [PubMed] [Google Scholar]
- 42. Clarke CA, Keegan TH, Yang J, et al. Age‐specific incidence of breast cancer subtypes: understanding the black‐white crossover. J Natl Cancer Inst. 2012;104(14):1094‐1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Graham J, Pitz M, Gordon V, Grenier D, Amir E, Niraula S. Clinical predictors of benefit from fulvestrant in advanced breast cancer: a meta‐analysis of randomized controlled trials. Cancer Treat Rev. 2016;45:1536‐6. [DOI] [PubMed] [Google Scholar]
- 44. Ma CX, Reinert T, Chmielewska I, Ellis MJ. Mechanisms of aromatase inhibitor resistance. Nat Rev Cancer. 2015;15(5):261‐275. [DOI] [PubMed] [Google Scholar]
- 45. Bergmann S, Royer‐Pokora B, Fietze E, et al. YB‐1 provokes breast cancer through the induction of chromosomal instability that emerges from mitotic failure and centrosome amplification. Cancer Res. 2005;65(10):4078‐4087. [DOI] [PubMed] [Google Scholar]
- 46. Davies AH, Reipas KM, Pambid MR, et al. YB‐1 transforms human mammary epithelial cells through chromatin remodeling leading to the development of basal‐like breast cancer. Stem Cells. 2014;32:1437‐1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shibata T, Watari K, Izumi H, et al. Breast cancer resistance to antiestrogens is enhanced by increased ER degradation and ERBB2 expression. Cancer Res. 2017;77(2):545‐556. [DOI] [PubMed] [Google Scholar]
- 48. Wu J, Lee C, Yokom D, et al. Disruption of the Y‐box binding protein‐1 results in suppression of the epidermal growth factor receptor and HER‐2. Cancer Res. 2006;66(9):4872‐4879. [DOI] [PubMed] [Google Scholar]
- 49. Basaki Y, Taguchi K, Izumi H, et al. Y‐box binding protein‐1 (YB‐1) promotes cell cycle progression through CDC6‐dependent pathway in human cancer cells. Eur J Cancer. 2010;46(5):954‐965. [DOI] [PubMed] [Google Scholar]
- 50. Campbell TM, Castro MAA, de Oliveira KG, Ponder BAJ, Meyer KB. ERα binding by transcription factors NFIB and YBX1 enables FGFR2 signaling to modulate estrogen responsiveness in breast cancer. Cancer Res. 2018;78(2):410‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cancer Genome Atlas Network . Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sutherland BW, Kucab J, Wu J, et al. Akt phosphorylates the Y‐box binding protein 1 at Ser102 located in the cold shock domain and affects the anchorage‐independent growth of breast cancer cells. Oncogene. 2005;24(26):4281‐4292. [DOI] [PubMed] [Google Scholar]
- 53. Evdokimova V, Ruzanov P, Anglesio MS, et al. Akt‐mediated YB‐1 phosphorylation activates translation of silent mRNA species. Mol Cell Biol. 2006;26(1):277‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stratford AL, Fry CJ, Desilets C, et al. Y‐box binding protein‐1 serine 102 is a downstream target of p90 ribosomal S6 kinase in basal‐like breast cancer cells. Breast Cancer Res. 2008;10(6):R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Law JH, Li Y, To K, et al. Molecular decoy to the Y‐box binding protein‐1 suppresses the growth of breast and prostate cancer cells whilst sparing normal cell viability. PLoS ONE. 2010;5(9):e12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Izumi H, Nagao S, Mochizuki S, Fujiwara N, Sakurai K, Morimoto Y. Optimal sequence of antisense DNA to silence YB‐1 in lung cancer by use of a novel polysaccharide drug delivery system. Int J Oncol. 2016;48(6):2472‐2478. [DOI] [PubMed] [Google Scholar]
- 57. Su W, Feng S, Chen X, et al. Silencing of long noncoding RNA MIR22HG triggers cell survival/death signaling via oncogenes YBX1, MET, and p21 in lung cancer. Cancer Res. 2018;78(12):3207‐3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ichikawa K, Ito S, Machida T, et al. TAS0612, a novel and highly potent RSK, AKT, and S6K inhibitor, exhibited strong antitumor effect in preclinical tumor models with deregulated RAS and PI3K pathway activities. Eur J Cancer. 2018;103(Suppl 13):430 (PB‐093). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


