Abstract
Increasing numbers of studies have confirmed that long noncoding RNA (lncRNA) play a critical role in epithelial ovarian cancer (EOC) progression. However, the potential function of the lncRNA tumor protein translationally controlled 1 (TPT1) antisense RNA 1 (TPT1‐AS1) in EOC is unclear. In this study, we aimed to uncover the biological roles and regulatory mechanisms of TPT1‐AS1 in EOC progression and metastasis. First, TPT1‐AS1 expression was significantly higher in EOC metastatic tissue and cell lines than in their respective control counterparts. In addition, ectopic TPT1‐AS1 expression was strongly associated with unfavorable EOC clinicopathological features, including FIGO stage, tumor size and tumor differentiation. TPT1‐AS1 overexpression remarkably induced cell proliferation, migration and invasion, and significantly attenuated cell adhesion ability in vitro and facilitated nude mouse subcutaneous xenograft growth and intraperitoneal metastasis in vivo, while the downregulation of TPT1‐AS1 expression produced the opposite effect in vitro. Mechanistically, TPT1‐AS1 was proven to be primarily distributed in EOC cell nuclei and positively modulated TPT1 promoter activity and transcription. Moreover, the oncogenic effects of TPT1‐AS1 could be reversed by TPT1 depletion, and the PI3K/AKT signaling pathway downstream of TPT1 was also altered. These results suggested that TPT1‐AS1 induced EOC tumor growth and metastasis through TPT1 and downstream PI3K/AKT signaling and that TPT1‐AS1 may be a promising therapeutic target for EOC.
Keywords: epithelial ovarian cancer, invasion and metastasis, proliferation, TPT1, TPT1‐AS1
1. INTRODUCTION
Epithelial ovarian cancer (EOC) is one of the most common gynecologic malignancies,1, 2 with 295 414 new cases and 184 799 deaths attributed to EOC during 2015 according to the 2018 global cancer statistics.3 Due to the asymptomatic nature of the early phase of disease and the limitations of sensitive clinical screening methods, most EOC patients are diagnosed at an advanced stage, with seeding metastases in peritoneal or distant organs. Devastating metastatic spread, high tumor recurrence rate and poor prognosis are common and serious problems in EOC cases4, 5, 6; therefore, exploring the underlying molecular mechanism of EOC progression and metastasis may help to improve the diagnosis of EOC and the development of novel therapeutic targets.
Long noncoding RNA (lncRNA) are a class of noncoding RNA more than 200 nucleotides long with no protein‐coding potential.7 Through regulating gene expression, lncRNA have crucial roles in multiple biological processes, such as development, differentiation and carcinogenesis.7 Antisense transcription is from the opposite strand of a protein‐coding gene or a sense strand‐derived RNA. Emerging studies show that antisense lncRNA (lncRNA‐AS) can function as positive or negative regulators of coding genes to further modulate cancer progression via miRNA decoys or competing endogenous RNA (ceRNA) and other mechanisms.8, 9
Studies have confirmed that lncRNA tumor protein translationally controlled 1 (TPT1) antisense RNA 1 (TPT1‐AS1) functions as a protective lncRNA in glioblastoma.10 In contrast, TPT1‐AS1 has been demonstrated to promote cell growth and metastasis by acting as a ceRNA for miR‐324‐5p in cervical cancers.11 However, the roles and mechanisms of TPT1‐AS1 in EOC tumorigenesis and progression remain to be elucidated. In this study, the expression of TPT1‐AS1 in EOC tissue and cells and the association between TPT1‐AS1 and EOC clinicopathological features were investigated. In addition, a series of gain‐ and loss‐of‐function experiments were performed to explore the potential roles of TPT1‐AS1 in EOC proliferation and metastasis as well as the correlation among TPT1‐AS1, TPT1 and the downstream signaling pathway. Our study sheds new light on the understanding of EOC progression and the development of novel drug targets for EOC therapy.
2. MATERIALS AND METHODS
2.1. Patients and tissue samples
This study was approved by the Ethical Committee of the Shanghai First Maternity and Infant Hospital. Informed consent was obtained for the studies involving human ovarian tissue samples. The study was performed according to the guidelines of the Declaration of Helsinki. EOC patients diagnosed at the Shanghai First Maternity and Infant Hospital between December 2014 and December 2016 were recruited. The available medical records, histological slides and paraffin‐embedded tissue blocks comprising 10 cases of FIGO Stage I human nonmetastatic EOC and 24 cases of FIGO Stage II‐IV primary EOC with paired metastatic EOC tissue samples were included in this study, and 20 samples of nonmalignant ovarian tissue were used as controls. None of the patients received adjuvant chemotherapy before surgery. All EOC samples were pathologically diagnosed according to the World Health Organization (WHO) classification guidelines (2014).
2.2. Cell culture
The human EOC cell lines ES‐2 and SKOV3 and normal human ovarian surface epithelium HOSEpiC cells obtained from our own laboratory were cultured and passaged according to the manufacturer's instructions. All the cell lines were cultured in RPMI‐1640 medium (HyClone) containing 10% FBS (Gibco), 100 units/mL of penicillin and 100 mg/mL streptomycin at 37°C in a humidified 5% CO2 incubator.
2.3. Transfection of lentiviral vectors and siRNA into epithelial ovarian cancer cells
For the ectopic expression of lncRNA TPT1‐AS1, ES‐2 and SKOV3 cells were transfected with an eGFP lentiviral vector encoding TPT1‐AS1 and a negative control vector (LV‐TPT1‐AS1 and LV‐vector, respectively, GeneChem, Shanghai, China) by using polybrene (5.0 μg/mL). The cells were selected with medium containing 0.2 mg/mL puromycin after 48 hours of transfection. To deplete the expression of TPT1‐AS1 and TPT1, ES‐2 and SKOV3 cells were treated with siRNA‐TPT1‐AS1, siRNA‐TPT1 and their corresponding control siRNA (RiboBio, Guangzhou, China) using X‐tremeGENE HP DNA Transfection Reagent (Roche, Basel, Switzerland). The cells were harvested at 48 hours posttransfection for future experiments. The dysregulation of TPT1‐AS1 expression was confirmed by using RT‐PCR.
2.4. Cell viability assay
Cell proliferation assays were performed using a Cell Counting Kit‐8 (CCK‐8, KeyGen BioTECH, Jiangsu, China) according to the manufacturer's instructions. EOC cells (2.0 × 104) were seeded in 96‐well plates with 100 μL maintenance medium. The number of viable cells was assessed by measuring the absorbance at 450 nm after a 2‐hour incubation with a Microplate Reader (BioTek Instruments, VT, USA). The viability rate was calculated as the experimental OD value/control OD value.
2.5. Colony formation assay
For colony formation assays, cells were plated in 6‐well plates at a concentration of 150 cells/well and incubated for approximately 2 weeks. Then, colonies of cells were observed, fixed with 100% methanol, and stained with hematoxylin.
2.6. Cell adhesion assay
ES‐2 and SKOV3 cells (1 × 105) were plated in 96‐well culture dishes precoated with 1:3 diluted Matrigel (Corning, NY, USA) and allowed to adhere for 0.45 hour. Subsequently, corresponding medium with non‐adhered cells was discarded and cells were gently washed twice with PBS to remove any loosely attached cells. Adhered cells were then counted using a 0.1% crystal violet staining solution at room temperature for 20 minutes subsequent to being fixed with methanol for 30 minutes at room temperature. The number of adherent cells was determined by counting the cells under a microscope at 100× magnification, and the adherent cells of each well were quantified as the mean number of cells in 5 random high‐power fields.
2.7. Cell migration and invasion assays
For cell migration and invasion assays, 1.0 × 105 cells in 150 μL RPMI‐1640 medium with 2% FBS were cultured in the upper chambers of an 8‐μm Transwell insert (Corning) with Matrigel (invasion) or without Matrigel (migration) in a 24‐well plate. After a 16‐28 hour incubation, the cells remaining in the upper side of each insert were gently removed. The cells that moved through the membrane were fixed with methanol and stained with hematoxylin. Migrated cells were photographed at 200× magnification under an inverted microscope, and the number of migratory/invasive cells was calculated for 10 randomly selected fields.
2.8. Construction of subcutaneous and orthotopic ovarian xenograft tumor models in nude mice
All animal experiments were approved by the Institutional Use and Care of Animals Committee and conducted according to the approved animal protocols of the Animal Center of Tongji University. Female nude mice, aged 4‐6 weeks (weighing approximately 20 g), were housed and cared for at the Animal Center of Tongji University (Shanghai, China).
For subcutaneous ovarian xenografts, 1 × 106 SKOV3 cells transfected with LV‐TPT1‐AS1 or LV‐vector in 100 μL PBS were subcutaneously injected into the left or right flank of mice, respectively. The tumor length (L) and width (W) were measured every 3 days using a digital Vernier caliper. The tumor volume was determined using the following formula: volume = L × W 2/2. At 45 days after the injection, the nude mice were sacrificed, and the xenografts were excised and weighed. The tumor grafts and peritoneal nodules were fixed in 10% formalin and subjected to routine histological examination and immunohistochemistry (IHC) staining of Ki‐67 (Abcam, Cambridge, UK) as previously described.12
For orthotopic ovarian xenografts, 5 × 105 SKOV3 cells transfected with LV‐TPT1‐AS1 or LV‐vector in 10 μL PBS were injected under the ovarian bursa of the nude mice. The inoculation method was performed as previously described.14 Xenograft growth was monitored by a NightOWL LB 983 In Vivo Imaging System (Berthold Technologies) every 2 days. Mice were sacrificed when animal weight loss (>15%), developed ascites metastasis or becoming moribund.
2.9. FISH
FISH probes for TPT1‐AS1 were purchased from RiboBio, and DAPI was used as a positive nuclear staining control. Briefly, ES‐2 and SKOV3 cells were grown on slides, rinsed gently in PBS, fixed in a 4% formaldehyde solution, permeabilized in PBS containing 0.5% Triton X‐100 for 5 minutes at 4°C, and then washed in PBS 3 times for 5 minutes each time. Hybridization was carried out with a FISH probe overnight at 37°C in the dark, and the cells were gradually washed by 4× SSC containing 1% Tween‐20, 2× SSC and 1× SSC for 5 minutes in the dark and then counterstained by DAPI to visualize the cell nucleus. Coverslips were mounted onto the glass slides with neutral gum, and the slides were observed by confocal laser scanning microscopy (TCS SP5, Leica).
2.10. Dual luciferase reporter assay
Sequences corresponding to the TPT1‐AS1 mRNA transcript and containing the wild‐type or mutated TPT1 binding sequence were synthesized by RiboBio (100 nM, RiboBio). These sequences were subcloned into the pGL3‐basic luciferase reporter vector (Promega, Madison, WI, USA). SKOV3 cells were cotransfected with the pGL3‐basic‐TPT1‐WT or pGL3‐basic‐TPT1‐MUT reporter plasmids together with the TPT1‐AS1 mimic or negative control oligoribonucleotides using X‐tremeGENE HP DNA Transfection Reagent (Roche). Firefly and Renilla luciferase activities were detected using a dual luciferase reporter system.
2.11. mRNA decay assay
To analyze the endogenous TPT1 mRNA decay rate, and upregulation or downregulation of TPT1‐AS1, SKOV3 cells were transfected with culture medium with the RNA transcription inhibitor Actinomycin D (Act D) (5 μg/mL, MedChem Express, NY, USA) for 1 hour, and RNA samples were harvested after 0, 4, 8, 12 and 24 hours. Because the mRNA levels for GAPDH were not changed after the Act D treatment, the GAPDH gene was used as the reference gene. The normalized TPT1 mRNA level at time 0 hour was set at 1.0.
2.12. Quantitative RT‐PCR
Total RNA from ES‐2 and SKOV3 cells and EOC tissue samples was extracted by Trizol Reagent (Invitrogen, CA, USA), and cDNA synthesis was performed by using a PrimeScript TM RT Master Mix Kit (TaKaRa BIO, Japan) according to the manufacturer's protocol. The mRNA level of TPT1‐AS1 was detected by using a Super Real PreMix Plus (SYBR Green) Kit (Tiangen Biotech, Beijing, China) and an Applied Biosystems Step One PlusTM Real‐Time PCR System. The TPT1‐AS1 and TPT1 mRNA relative expression levels were calculated using the method, and β‐actin was used as the positive control. The primers used for real‐time PCR were as follows:
β‐actin (forward): AACTCCATCATGAAGTGTGACG;
β‐actin (reverse): GATCCACATCTGCTGGAAGG;
TPT1‐AS1 (forward): AGGAGGCTATCCTTGCCCATC;
TPT1‐AS1 (reverse): AATTGGAGGCCAGTGCTCTGAA;
TPT1 (forward): GAAAGCACAGTAATCACTGGTGT;
TPT1 (reverse): GCAGCCCCTGTCATAAAAGGT;
GAPDH (forward): GCGACACCCACTCCTCCACCTTT; and
GAPDH (reverse): TGCTGTAGCCAAATTCGTTGTCATA.
2.13. Western blot analysis
Total protein from cells and tissue samples was extracted by lysing the samples in a Whole Cell Lysis Assay (Cat. KGP250, KeyGen BioTECH). A total of 30 μg protein per sample was resolved by 10% SDS‐PAGE and transferred to PVDF membranes. The membranes were first incubated overnight at 4°C in BSA in TBS containing 0.05% Tween 20 with primary antibodies against β‐actin and TPT1 (Proteintech, Chicago, USA), and PI3K, p‐PI3K, AKT and p‐AKT (Cell Signaling Technology, MA, USA), followed by incubation with secondary antibodies conjugated with HRP (KeyGen BioTECH) at room temperature for 1 hour. The protein bands were detected by using an enhanced Chemiluminescence Plus Kit (Millipore, MA, USA) as recommended by the manufacturer.
2.14. Statistical analysis
All experiments were repeated at least 3 times in duplicate. Data are presented as the mean ± SD. Differences between the treated and control groups were analyzed using Student's t‐test, and the associations between various demographic parameters were evaluated by Fisher's exact test. The level of significance was set at P < 0.05. All statistical analyses were performed with SPSS 24.0 (IBM, NY, USA).
3. RESULTS
3.1. Expression and clinical significance of lncRNA TPT1‐AS1 in epithelial ovarian cancer
TPT1‐AS1 expression in metastatic EOC tissue was significantly higher than that in paired primary EOC tissue and nonmalignant ovarian tissue (P < 0.05) (Figure 1A). In addition, the TPT1‐AS1 was gradually elevated in FIGO stage II, III and IV metastatic EOC tissues, with the highest TPT1‐AS1 expression in FIGO IV metastatic EOC tissues (P < 0.05); however, the TPT1‐AS1 expression in metastasis EOC tissues between FIGO II and III showed no statistical difference (P > 0.05; Figure 1B). Moreover, the clinicopathologic data showed that TPT1‐AS1 expression was significantly correlated with FIGO stage, tumor size and tumor differentiation (Table 1). Furthermore, the TPT1‐AS1 was significantly upregulated in EOC cell lines, and was expressed highest in SKOV3 cells, relative to ES‐2 and HOSEpiC cells (Figure 1C). In our pre‐experiments, SKOV3 cells with higher TPT1‐AS1 expression possessed more aggressive cell proliferation and invasion ability than ES‐2 cells (Figure S1); therefore, these 2 cells were chosen as experimental subjects. These data suggested that TPT1‐AS1 might play a crucial role in EOC tumorigenesis and metastasis. Subsequently, the TPT1‐AS1 stable overexpression and transient depletion EOC cell lines were constructed via lentiviral and siRNA transfection, respectively, and TPT1‐AS1 expression was effectively upregulated/downregulated in ES‐2 and SKOV3 cells as expected, compared with control cells (P < 0.05; Figure 1D‐F).
Figure 1.
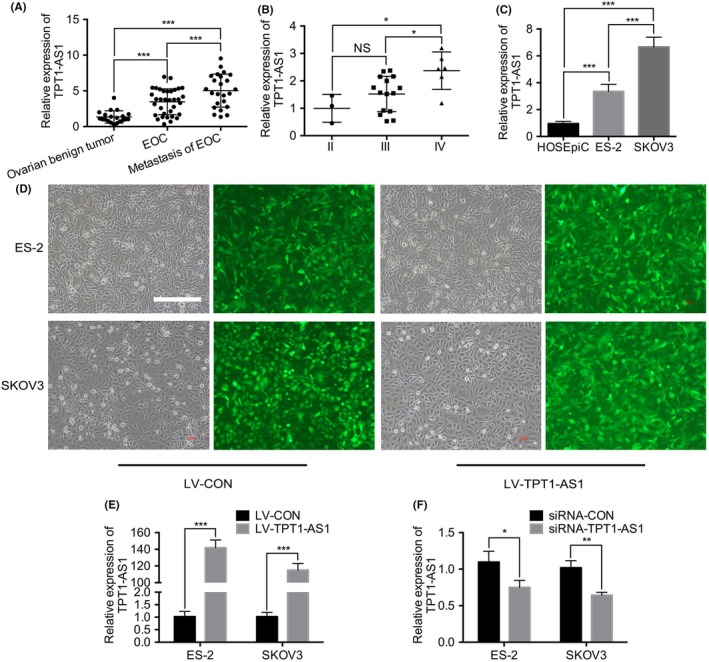
Expression of TPT1‐AS1 in epithelial ovarian cancer (EOC) tissue samples and parental cell lines. A, RT‐PCR analysis of TPT1‐AS1 expression in benign ovarian, primary and metastatic EOC tissue samples. B, RT‐PCR analysis of TPT1‐AS1 expression in FIGO II‐IV metastatic EOC tissue samples. C, RT‐PCR analysis of TPT1‐AS1 expression in the normal ovarian surface epithelium HOSEpiC and parental ES‐2 and SKOV3 cell lines. D, Successful construction of TPT1‐AS1‐overexpressing ES‐2 and SKOV3 cells. Scale bar, 400 μm. E, F, RT‐PCR analysis of TPT1‐AS1 expression in the TPT1‐AS1 overexpressing (E) or knockdown (F) ES‐2 and SKOV3 cell lines. Data represent mean ± SD from 3 independent experiments. NS, no significance. *P < 0.05, **P < 0.01 and ***P < 0.001
Table 1.
Correlation between TPT1‐AS1 expression level and patient clinicopathological features (N = 34)
| Clinicopathological parameters | High expression | Low expression |
|
P‐value | |
|---|---|---|---|---|---|
| Age (y) | |||||
| ≤60 | 7 | 8 | 0.119 | 0.730 | |
| >60 | 10 | 9 | |||
| FIGO stage | |||||
| I‐II | 3 | 10 | 6.103 | 0.013 | |
| III‐IV | 14 | 7 | |||
| Tumor size (cm) | |||||
| ≤5 | 2 | 8 | 5.100 | 0.024 | |
| >5 | 15 | 9 | |||
| Differentiation | |||||
| Well or moderate | 3 | 9 | 4.636 | 0.031 | |
| Poor | 14 | 8 | |||
| Lymph node metastasis | |||||
| Positive | 2 | 3 | 0.234 | 0.628 | |
| Negative | 15 | 14 | |||
| Serum CA‐125 level (U/mL) | |||||
| ≥35 | 17 | 17 | — | — | |
| <35 | 0 | 0 | |||
| Histological pathology | |||||
| Serous | 13 | 13 | 2.667 | 0.264 | |
| Mucinous | 2 | 4 | |||
| Endometrial | 2 | 0 | |||
3.2. TPT1‐AS1 promoted the proliferation of epithelial ovarian cancer cells in vitro and in vivo
To investigate the functional role of TPT1‐AS1 in cell proliferation and tumor growth, in vitro CCK‐8 and colony formation assays and in vivo nude mouse subcutaneous xenograft assays were performed. As the results show, the overexpression of TPT1‐AS1 significantly promoted cell proliferation and growth in ES‐2 and SKOV3 cells, while the knockdown of TPT1‐AS1 expression remarkably inhibited cell survival (P < 0.05; Figure 2A,B). Moreover, compared with the control groups, the overexpression of TPT1‐AS1 dramatically increased the tumor volume and weight of the subcutaneous xenografts in the nude mice (P < 0.05; Figure 2C), as well as the Ki‐67 expression in the xenograft tissues measured by IHC staining (P < 0.001; Figure 2D).
Figure 2.
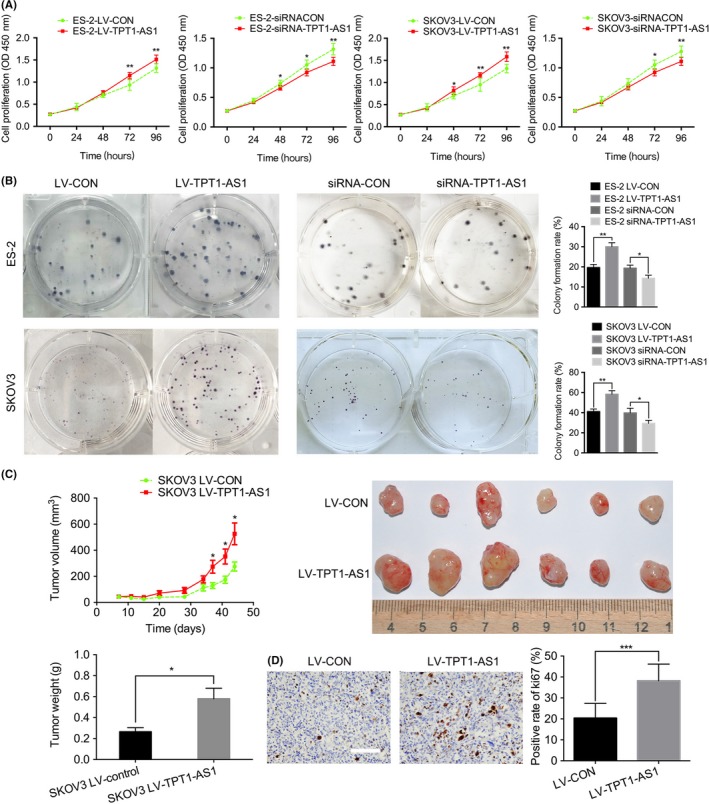
Overexpression of TPT1‐AS1 significantly promoted epithelial ovarian cancer (EOC) cell proliferation in vitro and in vivo. A, B, Cell Counting Kit‐8 (A) and colony formation assays (B) using the TPT1‐AS1‐overexpressing or knockdown ES‐2 and SKOV3 cells. C, The tumor growth curve and weights of the TPT1‐AS1‐overexpressing SKOV3 subcutaneous ovarian xenografts. D, Representative immunohistochemical (IHC) staining for Ki‐67 in subcutaneous xenograft tumor tissues. Scale bar, 50 μm. Data represent mean ± SD from 3 independent experiments. *P < 0.05, **P < 0.01 and ***P < 0.001
3.3. TPT1‐AS1 promoted epithelial ovarian cancer cell invasion and metastasis in vitro and in vivo
Cell adhesion assay showed that overexpression of TPT1‐AS1 significantly attenuated cell adhesion ability, while downregulation of TPT1‐AS1 remarkably increased cell adhesion in ES‐2 and SKOV3 cells (P < 0.05) (Figure 3A). Transwell migration and invasion assays revealed that the overexpression of TPT1‐AS1 significantly increased the number of penetrating ES‐2 and SKOV3 cells, while the depletion of TPT1‐AS1 dramatically inhibited both the cell migratory and invasive capabilities (P < 0.001; Figure 3B). In addition, the metastasis and dissemination of the xenografts were significantly promoted by TPT1‐AS1 expression, as detected with a NightOWL LB 983 In Vivo Imaging System (Figure 3C). The nodular lesions were confirmed to be of EOC tissue origin by pathology experts via H&E staining after dissection (data not shown), and overexpressing TPT1‐AS1 could significantly increase the tumor burden and number of metastatic nodule lesions in the omentum, mesentery and peritoneum compared with the control expression of TPT1‐AS1 (Figure 3D). Moreover, the numbers and weights of the metastatic nodules (diameter >2 mm) were also remarkably increased by TPT1‐AS1 overexpression (P < 0.05; Figure 3E).
Figure 3.
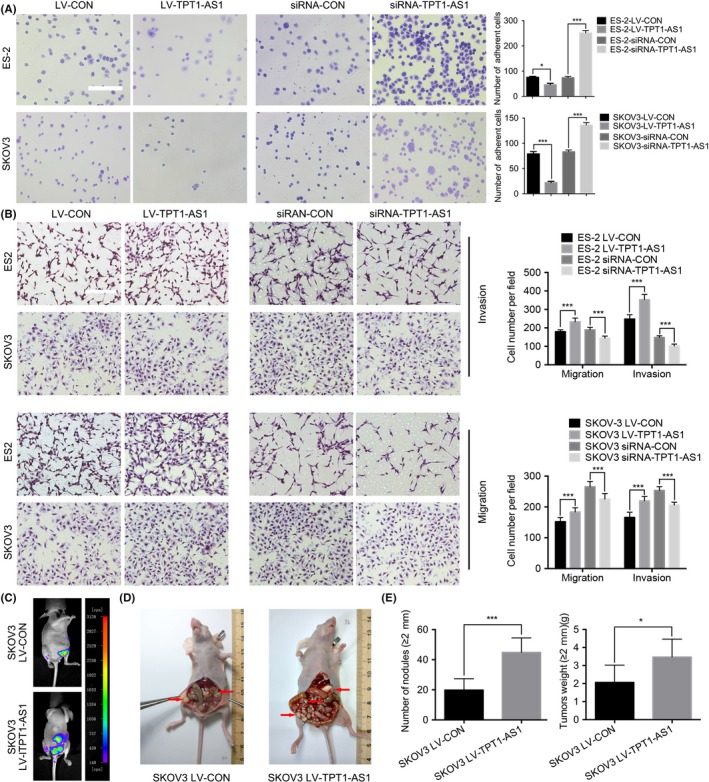
Overexpression of TPT1‐AS1 remarkably enhanced the invasive and metastatic abilities of epithelial ovarian cancer (EOC) cells in vitro and in vivo. A, Adhesion assays in EOC cells with dysregulated TPT1‐AS1 expression in vitro. Scale bar, 100 μm. B, Transwell migration and invasion assays in EOC cells with dysregulated TPT1‐AS1 expression in vitro. Scale bar, 200 μm. C, The growth of the TPT1‐AS1‐overexpressing SKOV3 orthotopic ovarian xenografts was detected with a NightOWL LB 983 In Vivo Imaging System. D, After sacrifice, the ovarian tumors in the nude mice were removed, and these tumors are indicated by the red arrows in the images. E, The average number of peritoneal tumor nodules and the average weight of the tumors from each group were quantified. Data represent mean ± SD from 3 independent experiments. *P < 0.05 and ***P < 0.001
3.4. TPT1‐AS1 promoted TPT1 expression by inducing the transcriptional activity of the TPT1 promoter
The FISH experiment was performed to identify the distribution of TPT1‐AS1 in the ES‐2 and SKOV3 cells, and by comparing with the DAPI staining, the results show that TPT1‐AS1 was principally localized in the nucleus, with slight expression in the cytoplasm (Figure 4A). Because an antisense lncRNA might play a vital role in regulating sense mRNA, whether TPT1‐AS1 regulates TPT1 expression was subsequently assessed. As the results show, TPT1 expression was positively correlated with TPT1‐AS1 expression in the metastatic EOC tissue samples, TPT1‐AS1‐overexpressing and depleted EOC cells, and TPT1‐AS1‐overexpressing orthotopic xenografts (P < 0.05; Figure 4B‐E). The above results suggested that TPT1 expression might be regulated by TPT1‐AS1 in EOC.
Figure 4.
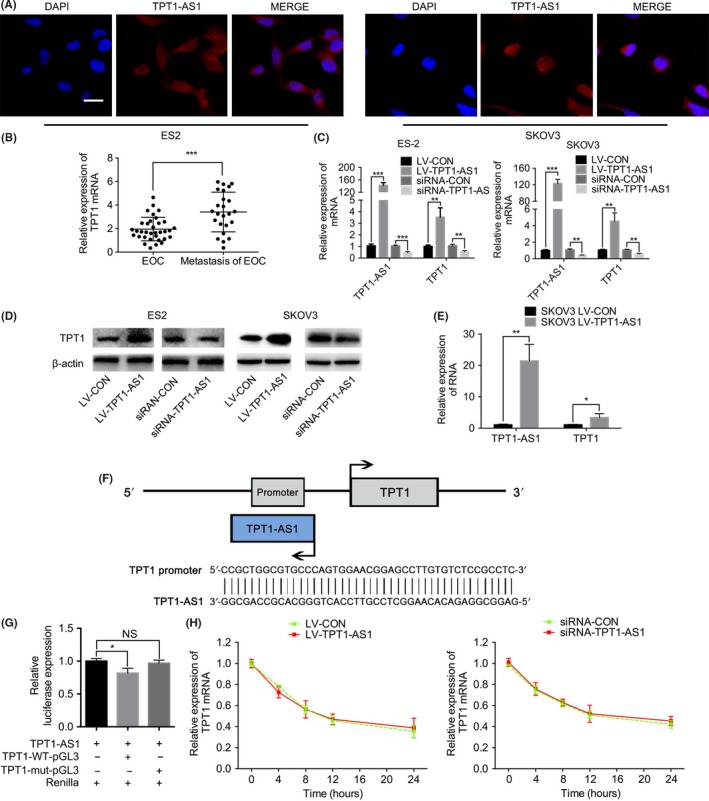
TPT1‐AS1 promoted TPT1 expression by inducing the transcriptional activity of the TPT1 promoter. A, FISH analysis of the subcellular localization of TPT1‐AS1 in the ES‐2 and SKOV3 cell lines. Scale bar, 50 μm. B, RT‐PCR analysis of TPT1 expression in the primary and paired metastatic epithelial ovarian cancer (EOC) tissue samples. C, D, RT‐PCR (C) and western blot (D) analysis of TPT1 expression in the dysregulated TPT1‐AS1 ES‐2 and SKOV3 cells. E, RT‐PCR analysis of TPT1 expression in the TPT1‐AS1‐overexpressing SKOV3 orthotopic xenografts. F, TPT1‐AS1 was found to contain a sequence that was reverse complementary to the TPT1 promoter, according to the prediction by the USCS genome browser. G, Dual luciferase reporter assay of TPT1‐AS1 and TPT1 promoter activity in SKOV3 cells. H, The mRNA degradation assay for TPT1 levels in the TPT1‐AS1‐overexpressing and knockdown SKOV3 cells. Data represent mean ± SD from 3 independent experiments. *P < 0.05, **P < 0.01 and ***P < 0.001
Because of the positive association between TPT1‐AS1 and TPT1 expression, the mechanism by which TPT1‐AS1 regulates TPT1 was further explored. According to the USCS genome browser bioinformatics analysis, TPT1‐AS1 transcripts were located close to the genomic location of TPT1, and 43 nucleotides of TPT1‐AS1 were found to be reverse complementary to the TPT1 promoter (Figure 4F). In addition, the results of the dual luciferase reporter assay showed that rather than TPT1‐MUT, TPT1‐AS1 remarkably modulated the TPT1‐WT promoter activity in SKOV3 cells (P < 0.05; Figure 4G). Moreover, the results of the mRNA degradation analysis showed that the upregulation/downregulation of TPT1‐AS1 expression did not alter the TPT1 mRNA degradation ratio in SKOV3 cells compared with the control groups (Figure 4H). These results indicated that TPT1‐AS1 promoted TPT1 expression by inducing the transcriptional activity of the TPT1 promoter but did not inhibit TPT1 mRNA degradation.
3.5. TPT1‐AS1 promoted epithelial ovarian cancer tumorigenesis and metastasis through TPT1 regulation
To further elucidate the mechanisms of TPT1‐AS1 in the EOC proliferation and dissemination mediated by TPT1, corresponding rescue experiments were performed. As the results show, the TPT1‐specific siRNA could significantly reduce cell viability, colony formation, migration and invasion, and restore adhesion ability in TPT1‐AS1‐overexpressing ES‐2 and SKOV3 cells in vitro compared with the respective control groups (P < 0.05; Figure 5A‐D). Because studies have validated that TPT1 promotes tumor metastasis through the PI3K/AKT signaling pathway,13, 33, 34 the phosphorylation levels of PI3K and AKT were measured by western blotting. As the results show, concordant with TPT1 downregulation, the phosphorylation levels of PI3K and AKT were obviously decreased in the knockdown of TPT1 in TPT1‐AS1 overexpression EOC cells, compared with control groups (Figure 5E). Thus, these results suggested that TPT1‐AS1 promoted EOC tumorigenesis and metastasis via the TPT1/PI3K/AKT signaling pathway.
Figure 5.
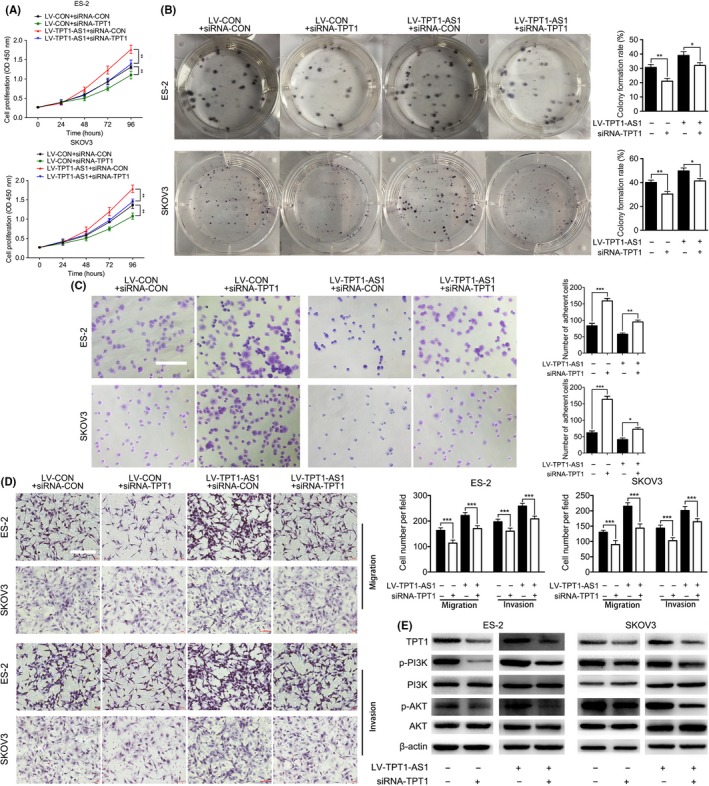
TPT1‐AS1 enhanced epithelial ovarian cancer (EOC) cell proliferation, migration and invasion via the TPT1/PI3K/AKT signaling pathway in vitro. A, B, Cell Counting Kit‐8 (A) and colony formation assays (B) after TPT1 depletion in the TPT1‐AS1‐overexpressing ES‐2 and SKOV3 cells. C, Adhesion assays after TPT1 depletion in the TPT1‐AS1‐overexpressing ES‐2 and SKOV3 cells in vitro. Scale bar, 100 μm. D, Transwell assays after TPT1 depletion in the TPT1‐AS1‐overexpressing ES‐2 and SKOV3 cells in vitro. Scale bar, 200 μm. E, Western blot analysis of the TPT1, p‐PI3K, PI3K, p‐AKT, AKT and β‐actin levels in the TPT1‐AS1‐overexpressing‐TPT1‐depletion EOC cells. Data represent mean ± SD from 3 independent experiments. *P < 0.05, **P < 0.01 and ***P < 0.001
4. DISCUSSION
Emerging studies have validated that numerous lncRNA are aberrantly expressed in EOC and play crucial roles in tumorigenesis and progression.15, 16 For instance, the expression of the lncRNA human ovarian cancer‐specific transcript 2 (HOST2) was found to be upregulated in EOC tissue, promoting cell proliferation and metastasis by sponging miRNA let‐7b.17 In addition, highly expressed homeobox (HOX) transcript antisense intergenic RNA (HOTAIR) was found to be closely related to poor EOC prognosis and the promotion of EOC migration and invasion through matrix metalloproteinases (MMP) and epithelial‐mesenchymal transition (EMT) in vitro and in vivo.18
In our previous study, β‐hCG was verified to promote the malignant transformation of human ovarian epithelial cells, and significantly facilitate EOC cell migration and invasion through the ERK/MMP2 signaling pathway.12, 14, 19 In addition, to investigate the underlying mechanisms of β‐hCG on EOC dissemination, 1708 lncRNA and 2758 mRNA were found to be differentially expressed in β‐hCG dysregulated cell lines by a gene chip (GEO accession number GSE122147, Figure S2A). During these dysregulated lncRNA, TPT1‐AS1 was found to be one of the most upregulated genes, and the functional roles and downstream signaling pathways of TPT1‐AS1 were mainly related to cell adhesion and junction (Figure S2B). Therefore, we focused on exploring the function role of TPT1‐AS1 in EOC progression.
In the present research, TPT1‐AS1 expression was confirmed to be significantly elevated in metastatic EOC tissues and cells, and remarkably associated with unfavorable EOC clinicopathological features, including III‐IV FIGO stages, >5 cm tumor size and poor differentiation. In addition, the TPT1‐AS1 was significantly upregulated in EOC cell lines, and parental SKOV3 cells with higher TPT1‐AS1 expression possessed a more aggressive malignancy relative to parental ES‐2 cells. Moreover, TPT1‐AS1 was proved to remarkably promote EOC proliferation, migration and invasion, and inhibit cell adhesion ability in vitro and in vivo. Therefore, TPT1‐AS1 might play an oncogenic role in EOC tumorigenesis and metastasis.
Antisense lncRNA, which comprise transcripts with sequence complementarity to other RNA, have been proven to be widely expressed in various tumors and cell lines,20 and have pivotal regulatory roles in sense mRNA stability, nuclear processing and exporting, and sense‐encoded protein translation through trans‐antisense RNA (nonoverlapping complementary sequences from remote loci) and cis‐antisense RNA (overlapping complementary sequences from the same genomic region).21, 22, 23 For instance, the lncRNA metastasis‐associated in colon cancer‐1 (MACC1) antisense RNA 1 (MACC1‐AS1) promotes gastric cancer cell metabolic plasticity by promoting MACC1 expression through mRNA stabilization.24 Moreover, some antisense transcripts inhibit sense protein expression by stalling translation. For example, the lncRNA Wilms' tumor 1 (WT1) antisense RNA (WT1‐AS) can promote hepatocellular carcinoma (HCC) apoptosis by binding to the WT1 promoter region and downregulating the expression of WT1.25 In our study, TPT1‐AS1 was validated to be primarily distributed in EOC cell nuclei and could positively regulate TPT1 expression in EOC cell lines, xenografts and EOC tissue samples. In addition, TPT1‐AS1 significantly stimulated TPT1 promoter activity and positively strengthened TPT1 transcription.
Tumor protein translationally controlled 1 is a multifunctional protein implicated in a diverse array of biological processes related to cell growth and tumor development26; in addition, elevated TPT1 expression is strongly associated with aggressive tumor biological behavior, metastatic potential and poor prognosis27 . 28, 29 The PI3K/AKT signaling pathway has been observed in various cancers and can frequently modulate EOC metastasis, angiogenesis and chemoresistance,30, 31 and corresponding inhibitors targeting this pathway, such as BKM120, GSK2110183 and AZD5363, are currently being evaluated as effective treatment strategies for EOC.32 Because the PI3K/AKT signaling pathway has been reported as a downstream factor for the maintenance of tumor malignancy by TPT1,29, 33, 34 we preliminarily proved that the knockdown of TPT1 in TPT1‐AS1‐overexpressing EOC cells could significantly impede tumor cell proliferation and metastasis in vitro and decrease the phosphorylation of PI3K and AKT, which indicated that TPT1‐AS1 might promote EOC tumorigenesis and dissemination through the TPT1/PI3K/AKT signaling pathway.
Interestingly, TPT1‐AS1 has been validated to exercise various roles in different tumors. In our study, TPT1‐AS1 was proved to promote EOC tumor growth and metastasis through TPT1 and the downstream PI3K/AKT signaling pathway. In cervical cancers, TPT1‐AS1 was verified to facilitate cell proliferation, migration and invasion via sponge for miR‐324‐5p.11 However, in glioblastoma, TPT1‐AS1 was demonstrated to be a protective lncRNA, and to be highly expressed in a low risk group, through binding with inhibitory targets, including AGO2, CPSF7, ELAVL1 and FUS, predicted by CLIP‐seq data. 10 The reason lncRNA plays opposite functions in different tumor cells may be due to the different targets it is binding to.35, 36, 37, 38
Because siRNA mainly exert their gene‐silencing effects in cytoplasm,39, 40 the RNA interference in nuclei does not seem as effective as in cytoplasm. However, in some studies, siRNA were utilized to silence lncRNA and mRNA expression in the nuclei, and the downstream genes and effects were efficaciously regulated.41, 42, 43, 44 In our study, the TPT1‐AS1 depletion EOC cell lines were constructed by siRNA transfection, and were further utilized for in vitro function experiments. As the results showed, the knockdown efficiency of TPT1‐AS1 was approximately 30%‐40%, and the downregulation of TPT1‐AS1 dramatically attenuated EOC cell proliferation, migration and invasion ability, and promoted cell adhesion ability, which are significantly opposite to the oncogenic effects of TPT1‐AS1 overexpression. Therefore, despite the low depletion efficiency of siRNA, the tumor inhibitory function was observed; thus, siRNA might work against nuclei TP1‐AS1. Efforts have been made to modify antisense oligonucleotide (ASO) drugs to target nuclear lncRNA via RNase H–dependent degradation.45, 46, 47 Studies have proven that the systemic administration of MALAT‐1 ASO inhibitors slows breast tumor growth, which is accompanied by a significant reduction in metastasis.48 Thus, ASO targeting TPT1‐AS1 are worthy of exploration as a potential therapy for inhibiting EOC malignant progression in the future.
Collectively, our study revealed that the lncRNA TPT1‐AS1 facilitated EOC tumor growth and metastasis, and inhibited cell adhesion by inducing TPT1 expression and the phosphorylation of molecules of the PI3K and AKT signaling pathway in vitro and in vivo. Our findings suggest that TPT1‐AS1 may function as a tumor oncogene in EOC and may be a potential target for inhibiting EOC progression.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest.
Supporting information
Wu W, Gao H, Li X, et al. LncRNA TPT1‐AS1 promotes tumorigenesis and metastasis in epithelial ovarian cancer by inducing TPT1 expression. Cancer Sci. 2019;110:1587–1598. 10.1111/cas.14009
Wu and Gao contributed equally to this work.
Contributor Information
Na Liu, Email: Na_Liu@tongji.edu.cn.
Xiaoqing Guo, Email: Xiaoqing_Guo@tongji.edu.cn.
REFERENCES
- 1. Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376‐1388. [DOI] [PubMed] [Google Scholar]
- 2. May T, Yang J, Shoni M, et al. BRCA1 expression is epigenetically repressed in sporadic ovarian cancer cells by overexpression of C‐terminal binding protein 2. Neoplasia. 2013;15:600‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 4. Naora H, Montell DJ. Ovarian cancer metastasis: integrating insights from disparate model organisms. Nat Rev Cancer. 2005;5:355‐366. [DOI] [PubMed] [Google Scholar]
- 5. Bast RC Jr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9:415‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yeung TL, Leung CS, Yip KP, Au YC, Wong ST, Mok SC. Cellular and molecular processes in ovarian cancer metastasis. A review in the theme: cell and molecular processes in cancer metastasis. Am J Physiol Cell Physiol. 2015;309:C444‐C456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tsai MC, Spitale RC, Chang HY. Long intergenic noncoding RNAs: new links in cancer progression. Cancer Res. 2011;71:3‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Q, Zhang J, Liu Y, et al. A novel cell cycle‐associated lncRNA, HOXA11‐AS, is transcribed from the 5‐prime end of the HOXA transcript and is a biomarker of progression in glioma. Cancer Lett. 2016;373:251‐259. [DOI] [PubMed] [Google Scholar]
- 9. Chan JJ, Tay Y. Noncoding RNA:RNA regulatory networks in cancer. Int J Mol Sci. 2018;19:1310-1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang W, Yang F, Zhang L, et al. LncRNA profile study reveals four‐lncRNA signature associated with the prognosis of patients with anaplastic gliomas. Oncotarget. 2016;7:77225‐77236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jiang H, Huang G, Zhao N, et al. Long non‐coding RNA TPT1‐AS1 promotes cell growth and metastasis in cervical cancer via acting AS a sponge for miR‐324‐5p. J Exp Clin Cancer Res. 2018;37:169. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12. Liu N, Peng SM, Zhan GX, et al. Human chorionic gonadotropin beta regulates epithelial‐mesenchymal transition and metastasis in human ovarian cancer. Oncol Rep. 2017;38:1464‐1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jung J, Kim HY, Kim M, Sohn K, Kim M, Lee K. Translationally controlled tumor protein induces human breast epithelial cell transformation through the activation of Src. Oncogene. 2011;30:2264‐2274. [DOI] [PubMed] [Google Scholar]
- 14. Wu W, Gao H, Li X, et al. β‐hCG promotes epithelial ovarian cancer metastasis through ERK/MMP2 signaling pathway‐hCG promotes epithelial ovarian cancer metastasis through ERK/MMP2 signaling pathway. Cell Cycle. 2019;18:46‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Y, Wang Y, Fu X, Lu Z. Long non‐coding RNA NEAT1 promoted ovarian cancer cells' metastasis through regulation of miR‐382‐3p/ROCK1 axial. Cancer Sci. 2018;109:2188‐2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang K, Geng J, Wang J. Long non‐coding RNA RP11‐552M11.4 promotes cells proliferation, migration and invasion by targeting BRCA2 in ovarian cancer. Cancer Sci. 2018;109:1428‐1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gao Y, Meng H, Liu S, et al. LncRNA‐HOST2 regulates cell biological behaviors in epithelial ovarian cancer through a mechanism involving microRNA let‐7b. Hum Mol Genet. 2015;24:841‐852. [DOI] [PubMed] [Google Scholar]
- 18. Qiu JJ, Lin YY, Ye LC, et al. Overexpression of long non‐coding RNA HOTAIR predicts poor patient prognosis and promotes tumor metastasis in epithelial ovarian cancer. Gynecol Oncol. 2014;134:121‐128. [DOI] [PubMed] [Google Scholar]
- 19. Guo X, Liu G, Schauer IG, et al. Overexpression of the beta subunit of human chorionic gonadotropin promotes the transformation of human ovarian epithelial cells and ovarian tumorigenesis. Am J Pathol. 2011;179:1385‐1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. He Y, Vogelstein B, Velculescu VE, Papadopoulos N, Kinzler KW. The antisense transcriptomes of human cells. Science. 2008;322:1855‐1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morrow EL, Erwin VG, Smith TL. [3H]inositol trisphosphate specific binding in cerebella of mouse lines selectively bred for differential ethanol sensitivity. Proc West Pharmacol Soc. 1988;31:269‐271. [PubMed] [Google Scholar]
- 22. Munroe SH, Zhu J. Overlapping transcripts, double‐stranded RNA and antisense regulation: a genomic perspective. Cell Mol Life Sci. 2006;63:2102‐2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Faghihi MA, Wahlestedt C. Regulatory roles of natural antisense transcripts. Nat Rev Mol Cell Biol. 2009;10:637‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao Y, Liu Y, Lin L, et al. The lncRNA MACC1‐AS1 promotes gastric cancer cell metabolic plasticity via AMPK/Lin28 mediated mRNA stability of MACC1. Mol Cancer. 2018;17:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lv L, Chen G, Zhou J, Li J, Gong J. WT1‐AS promotes cell apoptosis in hepatocellular carcinoma through down‐regulating of WT1. J Exp Clin Cancer Res. 2015;34:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Acunzo J, Baylot V, So A, Rocchi P. TCTP as therapeutic target in cancers. Cancer Treat Rev. 2014;40:760‐769. [DOI] [PubMed] [Google Scholar]
- 27. Chan TH, Chen L, Guan XY. Role of translationally controlled tumor protein in cancer progression. Biochem Res Int. 2012;2012:369384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Amson R, Karp JE, Telerman A. Lessons from tumor reversion for cancer treatment. Curr Opin Oncol. 2013;25:59‐65. [DOI] [PubMed] [Google Scholar]
- 29. Bommer UA. The translational controlled tumour protein TCTP: biological functions and regulation. Results Probl Cell Differ. 2017;64:69‐126. [DOI] [PubMed] [Google Scholar]
- 30. Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cai Y, Tan X, Liu J, et al. Inhibition of PI3K/Akt/mTOR signaling pathway enhances the sensitivity of the SKOV3/DDP ovarian cancer cell line to cisplatin in vitro. Chin J Cancer Res. 2014;26:564‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mabuchi S, Kuroda H, Takahashi R, Sasano T. The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol Oncol. 2015;137:173‐179. [DOI] [PubMed] [Google Scholar]
- 33. Kim M, Jung J, Lee K. Roles of ERK, PI3 kinase, and PLC‐gamma pathways induced by overexpression of translationally controlled tumor protein in HeLa cells. Arch Biochem Biophys. 2009;485:82‐87. [DOI] [PubMed] [Google Scholar]
- 34. Bommer UA, Iadevaia V, Chen J, Knoch B, Engel M, Proud CG. Growth‐factor dependent expression of the translationally controlled tumour protein TCTP is regulated through the PI3‐K/Akt/mTORC1 signalling pathway. Cell Signal. 2015;27:1557‐1568. [DOI] [PubMed] [Google Scholar]
- 35. Han Y, Wu Z, Wu T, et al. Tumor‐suppressive function of long noncoding RNA MALAT1 in glioma cells by downregulation of MMP2 and inactivation of ERK/MAPK signaling. Cell Death Dis. 2016;7:e2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang JK, Ma L, Song WH, et al. LncRNA‐MALAT1 promotes angiogenesis of thyroid cancer by modulating tumor‐associated macrophage FGF2 protein secretion. J Cell Biochem. 2017;118:4821‐4830. [DOI] [PubMed] [Google Scholar]
- 37. Peng F, Li TT, Wang KL, et al. H19/let‐7/LIN28 reciprocal negative regulatory circuit promotes breast cancer stem cell maintenance. Cell Death Dis. 2017;8:e2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang DM, Lin ZY, Yang ZH, et al. IncRNA H19 promotes tongue squamous cell carcinoma progression through beta‐catenin/GSK3beta/EMT signaling via association with EZH2. Am J Transl Res. 2017;9:3474‐3486. [PMC free article] [PubMed] [Google Scholar]
- 39. Wittrup A, Lieberman J. Knocking down disease: a progress report on siRNA therapeutics. Nat Rev Genet. 2015;16:543‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chi X, Gatti P, Papoian T. Safety of antisense oligonucleotide and siRNA‐based therapeutics. Drug Discov Today. 2017;22:823‐833. [DOI] [PubMed] [Google Scholar]
- 41. Zhang T, Wang H, Li Q, Fu J, Huang J, Zhao Y. MALAT1 activates the P53 signaling pathway by regulating MDM2 to promote ischemic stroke. Cell Physiol Biochem. 2018;50:2216‐2228. [DOI] [PubMed] [Google Scholar]
- 42. Zhang R, Hardin H, Huang W, Buehler D, Lloyd RV. Long non‐coding RNA Linc‐ROR is upregulated in papillary thyroid carcinoma. Endocr Pathol. 2018;29:1587‐8. [DOI] [PubMed] [Google Scholar]
- 43. Zhang B, Lu HY, Xia YH, Jiang AG, Lv YX. Long non‐coding RNA EPIC1 promotes human lung cancer cell growth. Biochem Biophys Res Commun. 2018;503:1342‐1348. [DOI] [PubMed] [Google Scholar]
- 44. Hu M, Wang R, Li X, et al. LncRNA MALAT1 is dysregulated in diabetic nephropathy and involved in high glucose‐induced podocyte injury via its interplay with beta‐catenin. J Cell Mol Med. 2017;21:2732‐2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kole R, Krainer AR, Altman S. RNA therapeutics: beyond RNA interference and antisense oligonucleotides. Nat Rev Drug Discov. 2012;11:125‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Adams BD, Parsons C, Walker L, Zhang WC, Slack FJ. Targeting noncoding RNAs in disease. J Clin Invest. 2017;127:761‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Matsui M, Corey DR. Non‐coding RNAs as drug targets. Nat Rev Drug Discov. 2017;16:167‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Arun G, Diermeier S, Akerman M, et al. Differentiation of mammary tumors and reduction in metastasis upon Malat1 lncRNA loss. Genes Dev. 2016;30:34‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


