Abstract
Mammalian cells are equipped with elaborate systems for protection against the toxicity of reactive oxygen and nitrogen species and electrophiles that are constant dangers to the integrity of their DNA. Phase 2 enzymes (e.g., glutathione transferases, NAD(P)H:quinone reductase) and glutathione synthesis are widely recognized as playing major protective roles against electrophilic carcinogens, but their antioxidant functions have attracted far less attention. The cytotoxicities of four oxidative stressors (menadione, tert-butyl hydroperoxide, 4-hydroxynonenal, and peroxynitrite) for human adult retinal pigment epithelial cells (ARPE-19) were quantified by measuring the concentration dependence of cell death and were expressed as the median effect dose (Dm) for each oxidant. After treatment of ARPE-19 cells for 24 h with 0–5 μM concentrations of sulforaphane (the powerful Phase 2 enzyme inducer isolated from broccoli), the toxicities of the oxidants were markedly reduced as shown by 1.5- to 3-fold increases in Dm values. The magnitude of protection was a function of the nature of the oxidants and the concentrations of both the oxidants and sulforaphane. Protection was prolonged and persisted for several days after removal of sulforaphane before returning to control levels. The sulforaphane-dependent increases in specific activities of cytosolic quinone reductase and the glutathione levels were highly significantly correlated with the degree of protection as measured by Dm values. Antioxidant protection was also demonstrated for human HaCaT keratinocytes and L1210 murine leukemia cells. It is therefore highly likely that the multifaceted and prolonged antioxidant protection provided by sulforaphane is a general phenomenon that is mediated through induction of the Phase 2 enzyme response.
Keywords: median effect plot‖tert-butylhydroperoxide‖peroxynitrite‖ glutathione‖quinone reductase‖4-hydroxynonenal
The toxicity of oxygen and more specifically its partial reduction products known as reactive oxygen species (ROS) is usually designated as oxidative stress. It arises from an imbalance of cellular prooxidant and antioxidant processes. Oxidative stress has been implicated in a variety of pathological and chronic degenerative processes including the development of cancer, atherosclerosis, inflammation, aging, neurodegenerative disorders, cataracts, retinal degeneration, drug action and toxicity, reperfusion injury after tissue ischemia, and defense against infection. Mammalian cells contribute to their own oxidative stress by generating ROS as part of normal aerobic metabolism, and have developed elaborate and overlapping mechanisms for combating these hazards (1). Nevertheless, protective mechanisms are not completely effective, especially during increased oxidative stress. The desirability of developing methods for augmenting these defenses is reflected in the widespread human consumption and perceived health benefits of plant-based antioxidants such as ascorbic acid, tocopherols, carotenoids, and polyphenols (2). These direct antioxidants neutralize free radicals and other chemical oxidants but are consumed in these reactions.
Recently, an alternative and possibly more effective strategy for combating the toxicities of ROS has attracted attention: the induction of a family of Phase 2 detoxification enzymes (3–6). Our initial interest in Phase 2 enzymes arose from the observation that the chemoprotective effects of many synthetic and natural substances that reduce the risk of cancer in animals could be attributed to induction of Phase 2 enzymes. The conclusion that elevation of Phase 2 enzymes is a major strategy for reducing the risk of cancer is supported by many lines of evidence and has gained widespread acceptance (4, 6–8).
In the past, enzymatic protection against oxidants focused largely on classical enzymes that are explicitly identified with inactivation of ROS, such as superoxide dismutases, catalase, and various types of peroxidases (1). Thus, although many Phase 2 enzymes display chemically versatile antioxidant properties, the potential of fortifying cellular antioxidant defenses by inducing Phase 2 enzymes has received relatively little attention (9), with a few notable exceptions (10, 11). Because oxidative stress is believed to be an important contributing factor in carcinogenesis, we became interested in the possibility that Phase 2 enzymes exerted their protective functions not only by inactivation of carcinogenic electrophiles but also through their antioxidant activities. Phase 2 enzymes were originally perceived as only promoting the conjugation of xenobiotics with endogenous ligands (e.g., glutathione, glucuronic acid) to generate more water-soluble and easily excretable products (12). This restricted view of the nature and functions of Phase 2 enzymes is gradually being expanded. There are now about two dozen genes that are considered part of the Phase 2 response. The enzymes encoded by these genes have chemically versatile antioxidant properties, share common regulatory mechanisms, and are highly inducible by a variety of agents including dietary components (6, 8, 9).†
In this paper we demonstrate that induction of Phase 2 enzymes is a powerful strategy for boosting antioxidant defense mechanisms and provides prolonged protection of human adult retinal pigment epithelial cells (ARPE-19) against chemically produced oxidative stress. We chose these cells because the retina is especially sensitive to oxidative damage (11, 17). To mimic the types of oxidative stresses that occur physiologically, we selected the following four oxidants: menadione, tert-butyl hydroperoxide, 4-hydroxynonenal, and peroxynitrite. The mechanisms by which these agents evoke oxidative damage, and how cells protect themselves against such damage are quite different, as described below (see Experimental Procedures).
ARPE-19 cells were treated with sulforaphane, an isothiocyanate isolated from broccoli on the basis of its Phase 2-inducing activity and the most potent naturally occurring Phase 2 enzyme inducer identified to date (3, 18, 19). Sulforaphane coordinately induces a family of Phase 2 detoxification enzymes and related proteins, and raises glutathione (GSH) levels by inducing γ-glutamylcysteine synthetase, the rate-limiting enzyme in GSH biosynthesis (20).
Cell viability measurements were analyzed by the median effect equation of Chou and Talalay (21) to obtain the median effect concentration (Dm) based on all the data points of the cytotoxicity-concentration curves. The Dm value for each oxidant was then compared with that for cells that had been treated with a range of concentrations of sulforaphane, thereby generating quantitative measures of protection.
Sulforaphane cannot react directly with free radicals or ROS; its “antioxidant” function is secondary to its ability to induce Phase 2 enzymes, and it is therefore an “indirect antioxidant.” The magnitude of the protective effects depends on concentrations of both oxidant stressors and inducers. Notably, unlike the effects of most direct antioxidants, the indirect antioxidant status persists for several days after sulforaphane is no longer present.
Parallel measurements of Phase 2 enzymes and GSH levels were obtained on cell extracts that had been exposed to sulforaphane under conditions identical with those used in protection experiments. When the degree of protection, quantified by increases in Dm values, was compared with elevations of these Phase 2 markers, remarkably close correlations were observed. Taken together, these results establish that protection against oxidative stress is quantitatively related to the indirect antioxidant action of sulforaphane, which results from elevations of Phase 2 enzymes and GSH.
Experimental Procedures
Chemicals.
tert-Butyl hydroperoxide, 3-morpholinosydnonimine (SIN-1), menadione sodium bisulfite (menadione), and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma. 4-Hydroxynonenal was obtained from Cayman Chemicals (Ann Arbor, MI)., and synthetic sulforaphane [1-isothiocyanato-(4R,S)-(methylsulfinyl)butane] was from LKT Laboratories (St. Paul).
Cell Culture.
Human adult retinal pigment epithelial cells (ARPE-19, TCC Catalog No. CRL-2302) were obtained from the American Type Culture Collection. These cells have structural and functional properties similar to the analogous retinal cells in vivo (22). They were cultured in a mixture of equal volumes of DMEM and Ham's F-12 medium plus 10% FBS that was heated for 90 min at 55°C with 1% (wt/vol) charcoal.
Human skin keratinocytes (HaCaT) were obtained from G. Tim Bowden (Arizona Cancer Center, Tucson, AZ) and grown in Eagle's minimum essential medium plus 8% FBS that had been treated with Chelex resin (Bio-Rad) to remove Ca2+ (23). Mouse L1210 leukemia cells, a gift from Joseph G. Cory (East Carolina State University, Greenville, NC), were grown in RPMI 1640 medium supplemented with 10% horse serum. All cultures were incubated in a humidified atmosphere of 5% CO2 at 37°C. Media and sera were obtained from Life Technologies (Rockville, MD).
Induction of Phase 2 Response by Sulforaphane.
All experiments were performed in 96-well microtiter plates. ARPE-19 and HaCaT cells were seeded at 10, 000 cells per well and grown for 24 h before addition of sulforaphane, whereas L1210 cells (5,000 cells per well) were not incubated before sulforaphane treatment. Solutions of sulforaphane (5 mM) in DMSO were diluted with the cognate culture medium to provide final inducer concentrations of 0.16–5.0 μM. The final DMSO concentrations were ≤0.1% by volume.
Choice of Oxidants.
tert-Butyl hydroperoxide differs from lipid hydroperoxides in being water-soluble, but unlike hydrogen peroxide, it is not metabolized by the peroxidative actions of catalase. It is principally inactivated by direct and glutathione transferase-promoted reduction of GSH (24).
Menadione causes necrotic cell death by participating in oxidative cycling, which generates superoxide and more reactive oxygen species by depletion of sulfhydryl groups and by accumulation of toxic intracellular levels of calcium (25). The relative toxicological importance of these processes probably depends on the tissue and local conditions. An important detoxification mechanism for menadione is the obligatory two-electron reduction to hydroquinones promoted by NAD(P)H:quinone reductase 1 (QR) (26). Mice in whom this gene has been disrupted are much more sensitive to the toxicity of menadione (27).
4-Hydroxynonenal is a highly cytotoxic and genotoxic alkenal that arises from peroxidation of polyunsaturated fatty acids such as arachidonic acid, and its tissue abundance is widely used as an index of lipid peroxidation (28, 29). The principal pathway for detoxification of 4-hydroxynonenal is conjugation with GSH by glutathione transferases leading to mercapturic acid formation (30, 31).
Peroxynitrite is a much more powerful oxidant than either superoxide or nitric oxide and is formed in cells by the exceedingly rapid combination of these molecules. Although nitric oxide can protect cells against apoptosis, peroxynitrite is a much more toxic reagent and attacks many cellular components, reacting with thiols, iron-sulfur centers, and zinc fingers, and it initiates lipid peroxidation. It also nitrates tyrosine by a reaction catalyzed by superoxide dismutase (32). Peroxynitrite probably generates cellular oxidative stress by several mechanisms.
Treatment with Oxidants.
tert-Butyl hydroperoxide (1 M) and 4-hydroxynonenal (25 mM) were dissolved in DMSO and diluted 1000-fold with serum-free medium before addition of serial dilutions to the microtiter plate wells. The final concentrations of DMSO were therefore less than 0.1% (by volume). Menadione sodium bisulfite (0.5 M) and 3-morpholinosydnonimine (0.5 M) were dissolved and added in PBS. ARPE-19 cells were exposed to menadione for 2 h and to tert-butyl hydroperoxide for 16 h, washed with PBS, and cell viability was determined by the MTT test. ARPE-19 cells were exposed to peroxynitrite for 2 h and 4-hydroxynonenal for 4 h, and the cells were then incubated in serum-free media for 22 and 20 h, respectively, washed with PBS, and cell viability was determined. The additional incubation periods were required for peroxynitrite and 4-hydroxynonenal to evoke maximal cytotoxicity.
Cytotoxicity Measurements.
Cell viability was determined by spectroscopic measurement of the reduction of MTT (33). The culture media were discarded after the designated incubation periods, the cells were washed three times with PBS by use of a microtiter plate washer (Ultrawash Plus, Dynex Technologies, Chantilly, VA). Each well then received 150 μl of an MTT solution (0.5 mg/ml) in serum-free medium. The plates were incubated for 2 h at 37°C, the MTT solution was discarded, 100 μl of DMSO was added to each well, and the plates were shaken at 200 rpm on an orbital shaker for 5 min. The absorbances of the wells were determined at 555 nm with a microtiter plate reader (Spectra Max Plus, Molecular Devices). The absorbance of reduced MTT was then compared at each inducer and oxidant concentration with that of untreated control cells that received only the vehicle in which sulforaphane (DMSO) and menadione (DMSO or PBS) were dissolved. In each experiment three identical 96-well plates were used and the means of the absorbance values, the standard deviations of these means, and their coefficients of variation were calculated. The coefficients of variation ranged from 0.6% to 16.5%. The mean coefficients of variation were similar for treated and untreated cells and averaged 7.2 ± 4.2%.
Quantitative Analysis of Cytotoxicity.
Dose-effect analyses were performed according to the Median Effect Equation, by use of a computer program (34). The equation: fa/fu = [D/Dm]m, where fa is the fraction of cells affected by the oxidant, fu is the fraction unaffected (i.e., 1 − fa), D is the dose of oxidant required to produce the effect fa, Dm is the concentration of oxidant required to produce a 50% effect, i.e., when fa = fu, and the slope m is a measure of the sigmoidicity of the dose-response curve, and is therefore a measure of cooperativity. The results are analyzed by plotting log (fa/fu) with respect to log D of the oxidant. The computer program provides the slope (m) of the curves, and the goodness of fit (r2) to linearity.
Preparation of Cell Lysates.
Cells were lysed by addition of a digitonin solution (0.8 mg/ml digitonin in 2 mM EDTA, pH 7.8), incubated at 37°C for 20 min, gently shaken for 20 min at 25°C, and centrifuged at 1,500 × g for 20 min at 4°C.
Glutathione Analysis.
Total glutathione (oxidized and reduced) was determined by reduction of 5,5′-dithiobis-2-nitrobenzoic acid in a glutathione reductase-coupled assay in 96-well microtiter plates (35). Lysates (30 μl) were mixed with 60 μl of cold 2.5% metaphosphoric acid, stored at 4°C for 10 min, and centrifuged for 20 min at 1,500 × g at 4°C. In a new plate, 50 μl of the supernatant fraction of each sample were mixed with 50 μl of 1.26 mM 5,5′-dithiobis-2-nitrobenzoic acid, 50 μl of 200 mM sodium phosphate, pH 7.5, 5 mM EDTA, and 50 μl of a solution containing 3.1 units/ml of yeast glutathione reductase (Sigma). After 5 min incubation at 25°C, 50 μl of 0.72 mM NADPH were added to each well, and the initial reaction rates were determined at 412 nm. Calibration curves for pure GSH were included in each assay.
Enzyme Assays.
All measurements were made in 96-well microtiter plates at 25°C, and reaction rates were monitored with a microtiter plate reader. The QR activities of supernatant fractions were determined by procedures developed in our laboratory (3, 36). Specific activities were obtained by relating the reaction rates to protein concentrations determined with the bicinchoninic acid reagent (37). The dicumarol-inhibitable fraction of the total QR activity contributed more than 90% to the overall observed rates in the ARPE-19, HaCaT, and L1210 cells.
Glutathione reductase activity was assayed by mixing 50 μl of cell lysate with 25 μl of 1 mM NADPH, 25 μl of GSSG (20 mg/ml), and 150 μl of 50 mM sodium phosphate, pH 7.5. Initial reaction rates were obtained at 340 nm (38). Glucose-6-phosphate dehydrogenase was assayed by mixing 50 μl of cell lysate with 200 μl of assay buffer containing 2.0 mM glucose 6-phosphate, 20 mM MgCl2, and 150 μM NADP. Initial reaction rates were determined at 340 nm (39).
Results and Discussion
Quantitative Measurements of Menadione Toxicity to Human Retinal Pigment Epithelial Cells and Protection by Sulforaphane.
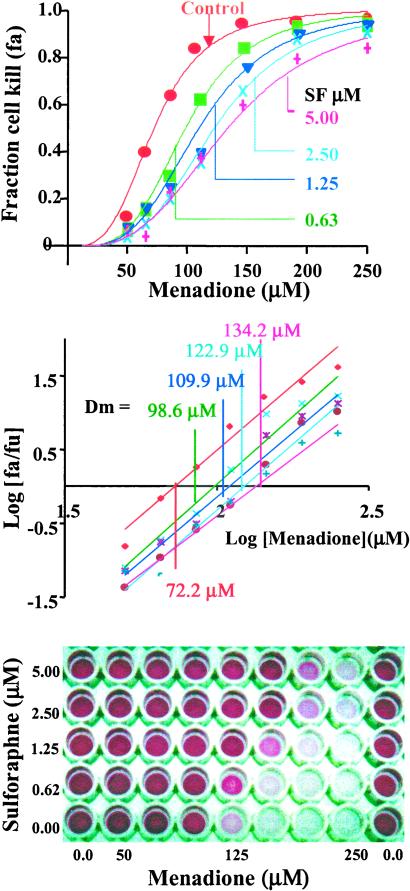
A standardized, highly reproducible system for quantitative determination of oxidant toxicity and protection by sulforaphane was developed for ARPE-19 cells grown in 96-well microtiter plates. The protective effect of 24-h prior incubation with 0–5 μM concentrations of sulforaphane on survival of ARPE-19 cells exposed for 2 h to 0–250 μM menadione is illustrated in Fig. 1, which shows the S-shaped dependence of cytotoxicity on increasing concentrations of menadione (plotted as the fractional cell kill, or fraction affected = fa). At the highest concentration of menadione almost no cells survive, but prior treatment with sulforaphane protected a substantial fraction of cells against oxidative death. Over the concentration ranges examined, cell survival decreases as the concentration of the oxidant menadione is increased, and increases as the concentration of sulforaphane is raised, as shown in Fig. 1.
Figure 1.
Protection of adult human retinal pigment epithelial (ARPE-19) cells against the toxicity of menadione (0–250 μM) by induction of Phase 2 enzymes by sulforaphane (SF) (0–5 μM). (Upper) Fractional killing of cells (fa) as a function of menadione concentration at a series of sulforaphane concentrations. (Center) Analysis of the data by the median effect plot. The median effect concentrations (Dm) at the sulforaphane concentrations above. (Lower) Photograph of a typical 96-well microtiter plate showing the protective effect of sulforaphane against the cytotoxicity of menadione for human ARPE-19 cells. The intensity of purple color is the reduced MTT formazan for a measure of cell viability.
Analysis of the data by the median effect equation of Chou and Talalay (21) provides: (i) a measure of the toxicity of the oxidant under each set of experimental conditions, expressed as the median effect concentration (Dm); (ii) compliance of the data with mass action principles that underlie the theoretical basis of the median effect equation i.e., the magnitudes of r2 values of plots of log [fa/fu] with respect to log D; and (iii) the Hill type coefficient (m), a measure of the sigmoidicity of the curves and hence of the cooperativity between the processes contributing to the biological endpoint (cell death). The median effect plots of the above data (Fig. 1) are a family of parallel and linear graphs (average of r2 for five plots = 0.976 ± 0.016) with average slopes (m) of 3.44 ± 0.22 (Table 1). These high slopes suggest that the processes contributing to cytotoxicity of menadione are highly cooperative. Notably, the Dm values rise asymptotically from 72.2 μM menadione under basal conditions to 134.2 μM for cells that had been treated with 5 μM sulforaphane for 24 h. In two other experiments, performed at intervals of many weeks, the control Dm values were 65.0 and 69.0 μM, respectively, and were therefore in good agreement.
Table 1.
Analysis by Median Effect Equation of protection by sulforaphane of human retinal pigment epithelial cells (ARPE-19), keratinocytes (HaCaT), and murine leukemia (L 1210) cells, against toxicities of menadione, tert-butyl hydroperoxide, 4-hydroxynonenal, and peroxynitrite
| Oxidants | Sulforaphane, μM | Dm, μM | m | r2 |
|---|---|---|---|---|
| ARPE-19 cells | ||||
| Menadione | 0.00 | 72.2 | 3.35 | 0.952 |
| 0.63 | 98.6 | 3.69 | 0.979 | |
| 1.25 | 110 | 3.49 | 0.983 | |
| 2.50 | 123 | 3.58 | 0.994 | |
| 5.00 | 134 | 3.12 | 0.972 | |
| tert-Butyl hydroperoxide | 0.00 | 95.8 | 2.52 | 0.923 |
| 0.63 | 140 | 2.17 | 0.964 | |
| 1.25 | 163 | 1.79 | 0.980 | |
| 2.50 | 165 | 1.26 | 0.953 | |
| 4-Hydroxynonenal | 0.00 | 8.70 | 2.85 | 0.885 |
| 0.63 | 14.1 | 2.51 | 0.931 | |
| 1.25 | 25.8 | 2.78 | 0.981 | |
| 2.50 | 26.8 | 2.51 | 0.993 | |
| Peroxynitrite | 0.00 | 1440 | 6.07 | 0.958 |
| 0.63 | 2780 | 6.30 | 0.984 | |
| 1.25 | 2820 | 6.19 | 0.982 | |
| 2.50 | 2890 | 6.62 | 0.977 | |
| HaCaT cell | ||||
| tert-Butyl hydroperoxide | 0.00 | 63.5 | 0.899 | 0.955 |
| 0.63 | 113 | 0.894 | 0.965 | |
| 1.25 | 166 | 0.921 | 0.971 | |
| 2.50 | 200 | 0.768 | 0.974 | |
| L1210 cell | ||||
| Menadione | 0.00 | 12.2 | 0.725 | 0.967 |
| 0.16 | 19.6 | 0.864 | 0.986 | |
| 0.31 | 26.5 | 1.00 | 0.987 | |
| 0.63 | 36.2 | 1.17 | 0.977 | |
The cells were treated with the oxidants and their viability determined by the MTT reduction measurements under conditions described in the text. Dm values were obtained from a series of plots of log (fa/fu) with respect to log oxidant concentration at each concentration of sulforaphane. The m values are the slopes of these plots, and r2 is the linear correlation coefficient.
Correlation Between Protection of ARPE-19 Against Menadione Toxicity by Sulforaphane and Elevations of Glutathione Levels and Quinone Reductase Specific Activities.
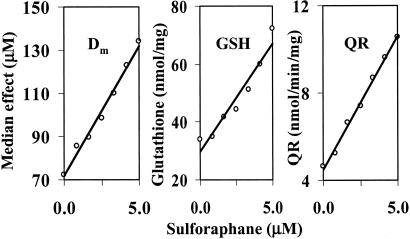
The specific activities of QR and concentrations of GSH were measured in cytosols of ARPE-19 cells that had been treated with 0–5.0 μM sulforaphane for 24 h, under conditions identical with those used above to determine the Dm values for menadione toxicity. As expected, both indicators of Phase 2 induction rose with exposure to increasing concentrations of sulforaphane (Fig. 2). The responses were linearly correlated with the sulforaphane concentration (r2 = 0.995 and 0.935, respectively). More importantly, a multivariate regression analysis showed a high correlation between sulforaphane concentrations and QR activities, GSH levels and Dm values (P = 0.0095, 0.0004, 0.0038, respectively). A highly significant quantitative association therefore exists between the degree of protection afforded by sulforaphane against menadione toxicity and the elevations of QR activities and GSH levels, suggesting that the changes in these variables may be causally related.
Figure 2.
Comparison of the effects of treatment of human ARPE-19 cells with a series of concentrations of sulforaphane (0–5 μM) for 24 h on the toxicity of exposure for 2 h to menadione. (Left) Cytotoxicity expressed as the median effect concentration (Dm). (Center) Glutathione concentrations expressed as nanomoles per milligram of cytosolic protein. (Right) Quinone reductase specific activity, expressed as nanomoles per minute per milligram of cytosolic protein. The multivariate regression correlations between sulforaphane concentrations and the other three variables all had P values of <0.01.
Sulforaphane Provides Prolonged Antioxidant Protection Against Menadione Oxidant Stress.
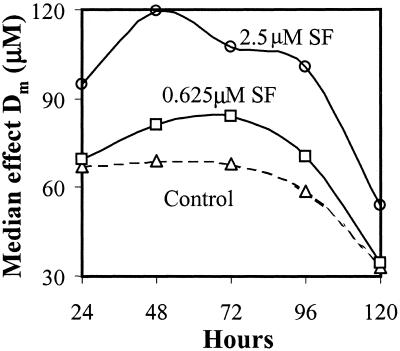
Because sulforaphane, like other isothiocyanates, does not usually participate in oxidation/reduction reactions, its antioxidant mechanism must be indirect, presumably through induction of Phase 2 proteins. Consequently, it seemed likely that the protective effects of sulforaphane should be catalytic and persist for several days (in relation to the half-lives of the cognate proteins) after removal of the inducer, unlike direct antioxidants (e.g., ascorbic acid, tocopherols) which are consumed stoichiometrically in radical quenching reactions. Therefore, we treated ARPE-19 cells for 24 h with two concentrations of sulforaphane (0.625 and 2.5 μM) and then incubated them for an additional 96 h in medium without FBS (to minimize the complications of cell growth and the difficulties of distinguishing the effects of cell mass increases on specific biochemical indices). Triplicate sets of identical plates were evaluated for menadione toxicity (2-h exposure) immediately after sulforaphane exposure and at 24-h intervals thereafter. The median effect concentration (Dm) for menadione of control cells was 66.8 μM, and the Dm values for cells treated with 0.625 and 2.5 μM sulforaphane were 69.2 and 94.5 μM, respectively. Control cell resistance remained unchanged for 48 h, whereas the resistance to menadione toxicity of the cells treated with sulforaphane continued to increase during this period, and then declined during the subsequent 48 h, finally approaching control cell levels (Fig. 3).
Figure 3.
Prolonged protection of ARPE-19 cells against menadione toxicity by sulforaphane (SF) expressed as median effect concentrations (Dm, μM). The menadione toxicity was determined immediately after induction (time = 0), and 24, 48, 96, and 120 h later. Note that protection continued to rise for 24–48 h, and then declined during the next 48 h.
These experiments establish that the protection evoked by sulforaphane at the end of the 24-h induction treatment is maintained or exceeded for at least 3 days in culture (Fig. 3). The specific activities of QR, glucose-6-phosphate dehydrogenase, and glutathione reductase in the cytosols of cells treated in an identical manner also continued to rise for 48 h after removal of sulforaphane from the medium and then remained high (glucose-6-phosphate dehydrogenase and glutathione reductase) or declined modestly (QR) during the ensuing 48–72 h (Fig. 4). The GSH levels after 24-h treatment with 2.5 μM sulforaphane were increased about 50%, remained at this level for another 24 h, and then declined to control cell levels in the ensuing 96 h. In ARPE-19 cells that have been exposed to sulforaphane for 24 h, and are then maintained in serum-free culture media for several days, the protective status remains substantially elevated, in parallel with higher levels of GSH and elevated Phase 2 enzyme markers.
Figure 4.
Persistent induction of quinone reductase (QR), glucose-6-phosphate dehydrogenase (G6PD), glutathione reductase (GR) (nanomoles per minute per milligram of cytosolic protein) and elevation of GSH levels (expressed as nanomoles per milligram of cytosolic protein) in human ARPE-19 cells after exposure to sulforaphane [0 (▴), 0.625 (□), and 2.5 (●) μM] for 24 h.
Protection of ARPE-19 Cells by Sulforaphane Against the Oxidative Stress of tert-Butyl Hydroperoxide, Peroxynitrite, and 4-Hydroxynonenal by Sulforaphane.
Treatment of ARPE-19 cells with a range of concentrations of sulforaphane (0, 0.625, 1.25, and 2.5 μM) for 24 h, also provided protection against other oxidants with mechanisms of action that differed from that of menadione. Thus the cytotoxicities of tert-butyl hydroperoxide (0.5–1.0 mM for 16 h), peroxynitrite (generated from 3-morpholinosydnonimine, 0.25–4.0 mM for 2 h), and 4-hydroxynonenal (1.56–25 μM for 4 h) were also significantly ameliorated by treatment with sulforaphane. This protection, like that against menadione, depended on concentration of both the oxidants and sulforaphane (Fig. 5 and Table 1).
Figure 5.
Protection of human ARPE-19 cells against the toxicity of menadione, tert-butyl hydroperoxide (0.5, 0.75, 1 mM for 16 h), 4-hydroxynonenal (6.25, 12.5, 25 μM for 4 h), and peroxynitrite (1, 2, 4 mM for 2 h) as a function of prior exposure for 24 h to 0–2.5 μM sulforaphane. The bar graphs show that cell viability is a function of both the concentrations of the oxidant and of the sulforaphane inducer. The front, center, and rear series of bars refer to the highest, middle, and lowest concentration of oxidants, respectively.
More detailed examination of the protective effects by the median effect equation reveals: (i) the slopes m for the cytotoxicities of these oxidants are quite different (means of 1.93, 2.66, and 6.29 for tert-butyl hydroperoxide, 4-hydroxynonenal, and peroxynitrite, respectively), and different from the m value (3.45) for menadione; (ii) the degree of protection provided by comparable concentrations of sulforaphane against different antioxidants ranged from 2- to 3-fold.
Protection of Human Keratinocytes (HaCaT) and Murine Leukemia (L1210) Cells Against Oxidative Stress.
To examine the generality of protection by Phase 2 induction we looked at the effects of 24-h treatment with sulforaphane on the toxicity to human keratinocytes (HaCaT) and mouse leukemia (L1210) cells of tert-butyl hydroperoxide and menadione, respectively (Fig. 6). The slopes of the median effect plots for both oxidants in these cell lines are in the 0.8–1.2 range, indicating lack of significant cooperativity among the processes contributing to cell death in these cell lines. The effects of these oxidants on ARPE-19 cells are quite different (Table 1). It appears therefore that the cooperativity between lethal processes depends on the cell line. Nevertheless, the substantial protection observed in the untransformed human keratinocyte cell line and in the highly neoplastic murine leukemia cell line indicates that the protection provided by sulforaphane is a more general phenomenon, not restricted to retinal epithelial pigment cells.
Figure 6.
Protection of human keratinocytes (HaCaT) against oxidative cytotoxicity of tert-butyl hydroperoxide (0.313, 0.625, 1 mM for 8 h) (Left) and mouse leukemia (L1210) cells against oxidative cytotoxicity of menadione (15.6, 31.3, 62.5 μM for 2 h) (Right) by treatment with sulforaphane (0–2.5 μM for 24 h). The Dm and m values obtained from the median effect plots are shown in Table 1. The front, center, and rear series of bars refer to the highest, middle, and lowest concentration of oxidants, respectively.
In conclusion, prior treatment of human adult retinal epithelial cells and two unrelated types of cells with sulforaphane, a potent dietary inducer of Phase 2 enzymes, provides highly effective protection against the toxicity of several very different types of oxidant stressors. The protection is chemically versatile, long-lasting, catalytic, and unlikely to evoke prooxidant effects.
Acknowledgments
We acknowledge helpful discussions with Dean P. Jones of Emory University, Atlanta, who drew our attention to the special vulnerability of the retina to oxidative damage, and with whom we first discussed this problem in relation to the use of dimethyl fumarate (10) and sulforaphane as Phase 2 enzyme inducers and protectors. Dr. T.-C. Chou, Memorial Sloan–Kettering Cancer Center, New York, provided valuable insight into the application of the Median Effect Plot. These studies were supported by generous gifts from the Barbara Lubin Goldsmith Foundation, the Four Friends Foundation, and the McMullan Family Fund, all of New York.
Abbreviations
- ARPE-19
human adult retinal pigment epithelial cells
- Dm
dose (concentration) required to produce 50% cell mortality
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- HaCaT cells
human keratinocytes
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PBS
Dulbecco's phosphate-buffered saline, pH 7.1
- QR (NQO1)
NAD(P)H:quinone acceptor oxidoreductase (EC 1.6.99.2.)
- sulforaphane
1-isothiocyanato-4(RS)-methylsulfinylbutane
- ROS
reactive oxygen species
Footnotes
These enzymes include glutathione transferases, glucuronosyltransferases, NAD(P)H:quinone reductase 1, heme oxygenase 1, and γ-glutamylcysteine synthetase which catalyze the rate-limiting step in the synthesis of GSH, as well as several other protective proteins (4–6). Although no generally accepted definition of Phase 2 proteins can be given, the view is evolving that Phase 2 proteins share the following properties: (i) coordinate induction by several classes of inducers (13, 14) that also induce classical Phase 2 enzymes such as glutathione transferases; (ii) regulation by molecular mechanisms that are very similar and probably involve common promoter elements (e.g., the Antioxidant Responsive Element or ARE) (15, 16); and (iii) catalysis of a wide variety of reactions that protect cells against the toxicities of electrophiles and ROS (4–6).
References
- 1.Halliwell B, Gutteridge J M C. Free Radicals in Biology and Medicine. New York: Oxford Univ. Press; 1999. pp. 1–36. [Google Scholar]
- 2.Pokorny J, Yanishlieva M, Gordon M. Antioxidants in Food: Practical Applications. Cambridge, U.K.: Woodhead Publishing, Ltd.; 2001. [Google Scholar]
- 3.Fahey J W, Zhang Y, Talalay P. Proc Natl Acad Sci USA. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kensler T W. Environ Health Perspect. 1997;105:965–970. doi: 10.1289/ehp.97105s4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talalay P, Fahey J W, Holtzclaw W D, Prestera T, Zhang Y. Toxicol Lett. 1995;82/83:173–179. doi: 10.1016/0378-4274(95)03553-2. [DOI] [PubMed] [Google Scholar]
- 6.Talalay P. BioFactors. 2000;12:5–11. doi: 10.1002/biof.5520120102. [DOI] [PubMed] [Google Scholar]
- 7.Talalay P. Proc Am Philos Soc. 1999;143:52–72. [Google Scholar]
- 8.Ramos-Gomez M, Kwak M K, Dolan P M, Itoh K, Yamamoto M, Talalay P, Kensler T W. Proc Natl Acad Sci USA. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fahey J W, Talalay P. Food Chem Toxicol. 1999;37:973–979. doi: 10.1016/s0278-6915(99)00082-4. [DOI] [PubMed] [Google Scholar]
- 10.Nelson K C, Carlson J L, Newman M L, Sternberg P, Jr, Jones D P, Kavanaugh T J, Diaz D, Cai J, Wu M. Invest Ophthalmol Visual Sci. 1999;40:1927–1935. [PubMed] [Google Scholar]
- 11.Cai J, Nelson K C, Wu M, Sternberg P, Jones D P. Prog Retinal Eye Res. 2000;19:205–221. doi: 10.1016/s1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- 12.Williams R T. Fed Proc. 1967;26:1027–1039. [PubMed] [Google Scholar]
- 13.Talalay P, De Long M J, Prochaska H J. Proc Natl Acad Sci USA. 1988;85:8261–8265. doi: 10.1073/pnas.85.21.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prestera T, Holtzclaw W D, Zhang Y, Talalay P. Proc Natl Acad Sci USA. 1993;90:2965–2969. doi: 10.1073/pnas.90.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaiswal A K. Biochem Pharmacol. 1994;48:439–444. doi: 10.1016/0006-2952(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 16.Rushmore T H, Morton M R, Pickett C B. J Biol Chem. 1991;266:11632–11639. [PubMed] [Google Scholar]
- 17.Winkler B S, Boulton M E, Gottsch J D, Sternberg P. Mol Vis. 1999;5:32–42. [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Talalay P, Cho C G, Posner G H. Proc Natl Acad Sci USA. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Kensler T W, Cho C C, Posner G H, Talalay P. Proc Natl Acad Sci USA. 1994;91:3147–3150. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulcahy R T, Wartman M A, Bailey H H, Gipp J J. J Biol Chem. 1997;272:7445–7454. doi: 10.1074/jbc.272.11.7445. [DOI] [PubMed] [Google Scholar]
- 21.Chou T-C, Talalay P. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 22.Dunn K C, Aotaki-Keen A E, Putkey F R, Hjelmeland L M. Exp Eye Res. 1996;62:155–159. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 23.Boukamp P, Petrussevska R T, Breitkreutz D, Hornung J, Markham A, Fusening N E. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurst R, Bao Y, Jemth P, Mannervik B, Williamson G. Biochem J. 1998;332:97–100. doi: 10.1042/bj3320097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith M T, Evans C G, Thor H, Orrenius S. In: Oxidative Stress. Sies H, editor. London: Academic; 1985. pp. 91–113. [Google Scholar]
- 26.Dinkova-Kostova A T, Talalay P. Free Radical Biol Med. 2000;29:231–240. doi: 10.1016/s0891-5849(00)00300-2. [DOI] [PubMed] [Google Scholar]
- 27.Radjendirane V, Joseph P, Lee Y-H, Kimura S, Klein-Szanto A J P, Gonzalez F J, Jaiswal A K. J Biol Chem. 1998;273:7382–7389. doi: 10.1074/jbc.273.13.7382. [DOI] [PubMed] [Google Scholar]
- 28.Prior W A, Porter N A. Free Radical Biol Med. 1990;8:541–543. doi: 10.1016/0891-5849(90)90153-a. [DOI] [PubMed] [Google Scholar]
- 29.Esterbauer H, Schauer R J, Zollner H. Free Radical Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 30.Alary J, Bravais E, Cravedi J-P, Debrauwer L, Rao D, Bories G. Chem Res Toxicol. 1995;8:34–39. doi: 10.1021/tx00043a004. [DOI] [PubMed] [Google Scholar]
- 31.Hubatsch I, Ridderström M, Mannervik B. Biochem J. 1998;330:175–179. doi: 10.1042/bj3300175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Estévez A G, Spear N, Pelluffo H, Kamaid A, Barbeito L, Beckman J S. Methods Enzymol. 1999;301:393–402. doi: 10.1016/s0076-6879(99)01103-9. [DOI] [PubMed] [Google Scholar]
- 33.Carmichael J, DeGraff W G, Gazdar A F, Minna J D, Mitchell J B. Cancer Res. 1987;47:936–942. [PubMed] [Google Scholar]
- 34.Chou T-C, Hayball M. CalcuSyn for Windows 3.1 and 95: Multiple Dose Effect Analyzer and Manual for IBM-PC. Cambridge, U.K.: Biosoft; 1996. [Google Scholar]
- 35.Ritchie J P, Jr, Skowronski L, Abraham P, Leutzinger Y. Clin Chem. 1996;42:64–70. [PubMed] [Google Scholar]
- 36.Prochaska H J, Santamaria A B, Talalay P. Proc Natl Acad Sci USA. 1992;89:2394–2398. doi: 10.1073/pnas.89.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gratner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 38.Carlberg I, Mannervik B. Methods Enzymol. 1985;113:484–490. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- 39.Kornberg A, Horecker B L. Methods Enzymol. 1955;1:323–327. [Google Scholar]