Abstract
Background
Malignant pleural mesothelioma (MPM) is a rare but aggressive tumor that originates from the pleura and has a poor prognosis. Eligible patients can benefit from surgery, but their survival is affected by many factors. Therefore, we created a graphic model that could predict the prognosis of surgically treated patients.
Methods
We retrospectively analyzed data from the Surveillance, Epidemiology, and End Results database from 2004 to 2014 to identify the key factors affecting the prognosis of surgically treated MPM patients. On this basis we built a nomogram to predict survival. We then evaluated the performance of the nomogram in a validation cohort.
Results
In a training cohort of 828 cases, independent prognostic factors, including age, gender, histological type, differentiation, N stage, chemotherapy, type of surgery, and lymph node dissection, were identified. We then developed a nomogram to evaluate individual patient survival. In Kaplan–Meier analysis, a higher score in the nomogram was associated with a worse prognosis. We also used a validation cohort consisting of 312 patients to evaluate the performance of the nomogram, which was well calibrated and had good discrimination ability, with concordance indices of 0.715 and 0.656 for the training and validation cohorts, respectively.
Conclusion
This study has improved our understanding of resected MPM and shown that key factors, including age and histological type, are associated with overall survival. The nomogram is a reliable tool that can help clinicians turn individualized prediction into reality and maximize patient benefit by identifying the most beneficial treatment approach.
Keywords: Malignant pleural mesothelioma, nomogram, SEER, surgery, survival
Introduction
Malignant pleural mesothelioma (MPM) is a rare tumor that originates in the pleura; it is very aggressive and generally has a poor prognosis.1, 2, 3 Surgery can be beneficial in patients who are healthy enough to tolerate it,4, 5, 6, 7 but postoperative survival varies, as many other factors also play a role. Previous studies have found that favorable prognostic factors include a lower age, epithelioid histology, good differentiation, negative lymph node status, and chemotherapy.4, 5, 6, 7 However, quantitative data based on large cohorts are lacking.
Recently Wang et al. built a nomogram based on data from the United States National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) database to predict the prognosis of patients with MPM,8 but this effort was complicated by the fact that the outcomes of patients treated with and without surgery differed significantly.9, 10, 11 In selected patients, surgery can lead to significantly better survival compared to non‐surgical therapy (18 vs. 12 months).12 Moreover, there are also some factors that specifically affect the survival of surgically treated patients, such as the type of surgery and postsurgical N status.10, 13 It is therefore necessary to study the prognosis of the two groups of MPM patients (surgery and non‐surgery) separately.
In this study, we analyzed the prognoses of MPM patients listed in the SEER database who had undergone surgery between 2004 and 2014. Our aim was to identify the key factors affecting prognosis and to use a population‐based database to develop a graphic tool that could enable clinicians to evaluate overall survival (OS), thus facilitating both individualized patient care and clinical research.
Methods
Study participants
Patients were selected using International Classification of Diseases for Oncology (ICD‐O)‐3 morphology codes 9050–9053. Patients aged > 18 years who had undergone surgery and were diagnosed with malignant mesothelioma between 2004 and 2014 were included. Surgical information was collected based on the SEER surgery code. The surgical procedures included palliative, radical, and not otherwise specified (NOS). We classified patients into groups based on site‐specific surgery using the primary site codes. Radical surgery was defined as radical resection (code 60); and palliative surgery as local tumor destruction (code 10), local tumor excision (code 20), simple/partial surgical removal of primary site (code 30), enucleation (code 40), and debulking (code 50). Patients who underwent surgery of an unknown type (code 90) were placed in the NOS surgery group.
A total of 4372 patients were identified using the code C38.4‐Pleura, NOS in the SEER database. Twenty‐one patients were excluded because no survival data had been recorded. We further excluded 3211 patients with SEER surgery codes indicating that either no cancer‐directed surgery was performed or it was unknown whether cancer‐directed surgery was performed. Finally, 1140 patients who had undergone surgery were enrolled (Fig 1).
Figure 1.
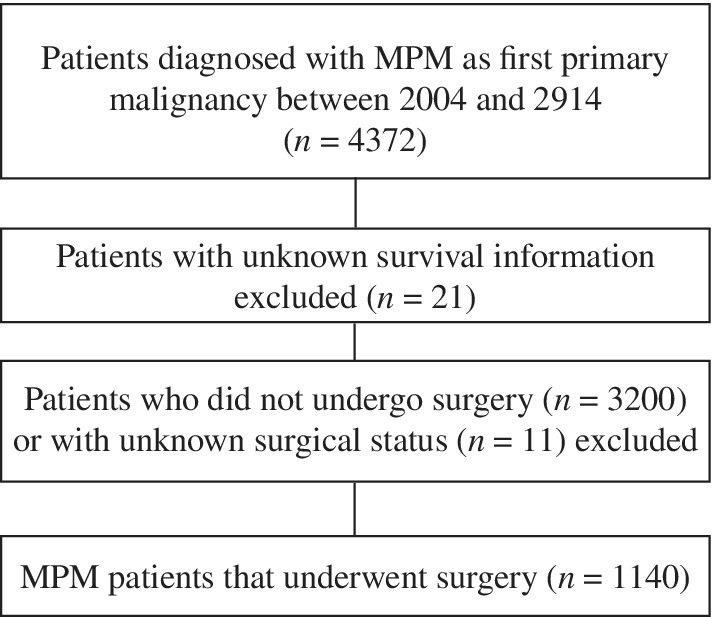
Flowchart showing the selection of study patients. MPM, malignant pleural mesothelioma.
The study was exempted from ethical review by the Beijing Cancer Hospital. We obtained the data agreement and downloaded the files directly from the SEER website in accordance with SEER requirements.
Statistical analysis
We used frequency tabulation and standard descriptive statistics to summarize all patient data. Medians and ranges were recorded as continuous variables, while frequencies and proportions were recorded for categorical variables.
Based on the data of 828 patients who were diagnosed between 2004 and 2011, we constructed a nomogram and then validated it using the data of 312 patients diagnosed between 2012 and 2014. We evaluated the clinicopathologic, demographic, and treatment data on each patient and examined the linearity assumption over continuous variables and proportional hazards (PH) using restricted cubic splines.14, 15 We transformed continuous variables into proper forms for fitting linearity and PH assumptions. We used log‐log survival plots for categorical variables to identify the PH assumption, and all variables were fitted to the PH assumption. Variables were entered into a multivariate Cox proportional hazards regression model using backward stepwise selection with the Akaike information criterion (AIC), and coefficients of the predictors were calculated. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated.16 We then constructed the nomogram using the identified prognostic factors to predict one‐year and three‐year survival rates.
The performance of the nomogram, including its discrimination and calibration, was tested using the validation cohort. A model's ability to separate subject outcomes is defined as discrimination and can be quantified by the Harrell C‐index17 while the comparison of actual and predicted survival is known as calibration, which can be measured with calibration plots. We used the validation cohort to compare the nomogram‐predicted probability of OS with the observed OS at one and three years. In a well‐calibrated model, the predictions should fall on a 45‐degree diagonal line. In addition, model performance was further evaluated by plotting Kaplan–Meier curves over the quartiles of prediction by nomogram.
We used R software version 3.3.3 for all statistical analyses. The nomogram was developed and modeled using rms of the R package. Reported significance levels were two‐sided, and P < 0.05 was taken to indicate statistical significance.
Results
Demographic, clinicopathologic, and treatment characteristics
The demographic, clinicopathologic, and treatment characteristics of the training (n = 828) and validation (n = 312) cohorts are shown in Table 1.
Table 1.
Demographic, clinicopathologic, and treatment characteristics of study patients
| Training cohort (n = 828) | Validation cohort (n = 312) | |||
|---|---|---|---|---|
| Characteristics | No. of patients | % | No. of patients | % |
| Age, years | ||||
| Median | 67 | 70 | ||
| Range | 26–95 | 32–94 | ||
| Gender | ||||
| Male | 647 | 78.1 | 234 | 75.0 |
| Female | 181 | 21.9 | 78 | 25.0 |
| Race | ||||
| White | 767 | 92.6 | 288 | 92.3 |
| Black | 31 | 3.7 | 13 | 4.2 |
| Other | 30 | 3.6 | 11 | 3.5 |
| Histology | ||||
| Sarcomatoid | 226 | 27.3 | 63 | 20.2 |
| Fibrous | 80 | 9.7 | 27 | 8.7 |
| Epithelioid | 412 | 49.8 | 165 | 52.9 |
| Biphasic | 110 | 13.3 | 58 | 18.6 |
| Differentiation | ||||
| Well or moderately | 28 | 3.4 | 7 | 2.2 |
| Poorly | 49 | 5.9 | 18 | 5.8 |
| Undifferentiated | 18 | 2.2 | 5 | 1.6 |
| NOS | 733 | 88.5 | 282 | 90.4 |
| Chemotherapy | ||||
| Yes | 491 | 59.3 | 195 | 62.5 |
| No | 337 | 40.7 | 117 | 37.5 |
| Radiotherapy | ||||
| Yes | 222 | 26.8 | 63 | 20.2 |
| No | 606 | 73.2 | 249 | 79.8 |
| Primary tumor location | ||||
| Bilateral | 15 | 1.8 | 3 | 1.0 |
| Left‐sided | 313 | 37.8 | 109 | 34.9 |
| Right‐sided | 500 | 60.4 | 200 | 64.1 |
| Clinical stage | ||||
| I | 128 | 15.5 | 59 | 18.9 |
| II | 151 | 18.2 | 53 | 17.0 |
| III | 267 | 32.2 | 91 | 29.2 |
| IV | 282 | 34.1 | 109 | 34.9 |
| N stage | ||||
| N0 | 540 | 65.2 | 201 | 64.4 |
| N1 | 111 | 13.4 | 100 | 32.1 |
| N2 | 142 | 17.1 | 4 | 1.3 |
| N3 | 11 | 1.3 | 2 | 0.6 |
| NX | 24 | 2.9 | 5 | 1.6 |
| T stage | ||||
| T1 | 164 | 19.8 | 73 | 23.4 |
| T2 | 218 | 26.3 | 90 | 28.8 |
| T3 | 220 | 26.6 | 67 | 21.5 |
| T4 | 222 | 26.8 | 79 | 25.3 |
| TX | 4 | 0.5 | 3 | 1.0 |
| M stage | ||||
| M0 | 718 | 86.7 | 256 | 84.9 |
| M1 | 102 | 12.3 | 47 | 15.1 |
| MX | 8 | 1 | 0 | 0.0 |
| Type of surgery | ||||
| Palliative | 568 | 68.6 | 235 | 75.3 |
| Radical | 218 | 26.3 | 71 | 22.8 |
| NOS | 42 | 5.1 | 6 | 1.9 |
| Lymph node dissection | ||||
| 1–3 removed | 65 | 7.9 | 21 | 6.7 |
| ≥ 4 removed | 271 | 32.7 | 89 | 28.5 |
| None/unknown | 492 | 59.4 | 202 | 64.7 |
NOS, not otherwise specified.
The majority of patients were white (92.5%), male (77.3%), and had right‐sided lesions (61.4%). Based on the American Joint Committee on Cancer staging system, 187 stage I cases (16.4%), 204 stage II cases (17.89%), 358 stage III cases (31.40%), and 391 stage IV cases (34.30%) were enrolled. Because > 60% of the patients were stage III–IV, most surgical procedures were palliative.
In total, 577 patients had epithelioid histology (50.6%), 289 had sarcomatoid histology (25.4%), 168 had biphasic histology (14.7%), and 107 had fibrous histology (9.4%). Because the differentiation of resected tumors was not regularly recorded in the SEER database, most patient differentiations were NOS. Of these patients, 686 (60.18%) were administered cytotoxic chemotherapy and 285 (25.0%) were administered radiotherapy. Among the entire cohort, median OS was 14 months (95% CI 13–15 months). The one, three, and five‐year OS rates were 53.9%, 17.1%, and 8.5%, respectively.
Predictors of overall survival and model specifications
We first selected 14 clinically relevant candidate variables from the database: age at diagnosis, race, gender, differentiation, histology, radiotherapy, chemotherapy, primary tumor location, clinical stage, tumor node metastasis (TNM) stage, surgery type, and lymph node dissection. In univariate analysis, the factors significantly associated with reduced OS were: advanced age, male gender, fibrous histology, poor differentiation, undifferentiated, and treatment without chemotherapy or radiotherapy (Table 2). Backward stepwise selection using the AIC in Cox proportional hazards regression modeling identified eight variables that were included in the final model. Table 2 presents the HRs and 95% CIs for the multivariate Cox proportional hazards regression analysis for variables selected by the AIC. Gender (HR 1.486, 95% CI 1.241–1.779), differentiation (HR 2.312, 95% CI 1.362–3.921), histology (HR 1.478, 95% CI 1.088–2.005), lymph node metastasis (HR 1.409, 95% CI 1.155–1.718), treatment without chemotherapy (HR 1.319, 95% CI 1.130–1.538), and lymph node dissection (HR 1.350, 95% CI 1.025–1.776) were each independently associated with OS (all P < 0.05), while the surgery type (HR 1.234, 95% CI 0.868–1.753) tended to be associated with prognosis.
Table 2.
Factor and overall survival associations via the Cox proportional hazards regression model in the training cohort (n = 828)
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| Prognostic factor | HR | 95% CI | P | HR | 95% CI | P |
| Factors selected | ||||||
| Age | 1.004 | 0.992–1.017 | 0.500 | 1.003 | 0.990–1.017 | 0.600 |
| Age’† | 1.030 | 1.014–1.047 | < 0.001 | 1.027 | 1.009–1.044 | 0.002 |
| Gender | ||||||
| Female | Ref | — | — | Ref | — | — |
| Male | 1.427 | 1.196–1.703 | < 0.001 | 1.486 | 1.241–1.779 | < 0.001 |
| Histology | ||||||
| Biphasic | Ref | — | — | Ref | — | — |
| Epithelioid | 0.689 | 0.554–0.859 | < 0.001 | 0.682 | 0.543–0.855 | < 0.001 |
| Fibrous | 1.675 | 1.244–2.254 | < 0.001 | 1.478 | 1.088–2.005 | 0.012 |
| Mesothelioma | 0.878 | 0.693–1.113 | 0.283 | 0.734 | 0.574–0.939 | 0.014 |
| Differentiation | ||||||
| Well or moderately | Ref | — | — | Ref | — | — |
| Poorly | 2.463 | 1.469–4.131 | < 0.001 | 2.312 | 1.362–3.921 | 0.002 |
| Undifferentiated | 2.228 | 1.147–4.328 | 0.018 | 1.468 | 0.738–2.916 | 0.273 |
| NOS | 1.868 | 1.209–2.887 | 0.005 | 1.735 | 1.113–2.704 | 0.015 |
| Chemotherapy | ||||||
| Yes | Ref | — | — | Ref | — | — |
| No | 1.350 | 1.168–1.561 | < 0.001 | 1.319 | 1.130–1.538 | < 0.001 |
| N stage | ||||||
| N0 | Ref | — | — | Ref | — | — |
| N1 | 0.950 | 0.766–1.178 | 0.640 | 1.342 | 1.068–1.685 | 0.012 |
| N2 | 1.122 | 0.928–1.357 | 0.235 | 1.409 | 1.155–1.718 | < 0.001 |
| N3 | 1.333 | 0.732–2.426 | 0.348 | 1.480 | 0.809–2.707 | 0.203 |
| NX | 1.356 | 0.884–2.080 | 0.162 | 1.006 | 0.647–1.561 | 0.980 |
| Lymph node dissection | ||||||
| 1–3 removed | Ref | — | — | Ref | — | — |
| ≥ 4 removed | 0.880 | 0.661–1.171 | 0.380 | 0.968 | 0.722–1.296 | 0.827 |
| None/unknown | 1.382 | 1.053–1.814 | 0.019 | 1.350 | 1.025–1.776 | 0.032 |
| Type of surgery | ||||||
| NOS | Ref | — | — | Ref | — | — |
| Palliative | 1.093 | 0.789–1.514 | 0.592 | 1.234 | 0.868–1.753 | 0.240 |
| Radical | 0.819 | 0.581–1.155 | 0.255 | 1.077 | 0.739–1.572 | 0.698 |
| Factors not selected | ||||||
| Race | ||||||
| White | Ref | — | — | |||
| Black | 0.981 | 0.672–1.431 | 0.920 | |||
| Other | 0.689 | 0.458–1.035 | 0.073 | |||
| Radiotherapy | ||||||
| Yes | Ref | — | — | |||
| No | 1.278 | 1.088–1.501 | 0.003 | |||
| Primary tumor location | ||||||
| Bilateral | Ref | — | — | |||
| Left‐sided | 1.171 | 0.657–2.087 | 0.593 | |||
| Right‐sided | 1.326 | 0.748–2.353 | 0.334 | |||
| Clinical stage | ||||||
| I | Ref | — | — | |||
| II | 0.988 | 0.772–1.265 | 0.924 | |||
| III | 0.920 | 0.737–1.148 | 0.460 | |||
| IV | 1.202 | 0.966–1.467 | 0.099 | |||
| T stage | ||||||
| T1 | Ref | — | — | |||
| T2 | 1.056 | 0.855–1.305 | 0.611 | |||
| T3 | 0.930 | 0.752–1.151 | 0.503 | |||
| T4 | 1.177 | 0.953–1.454 | 0.130 | |||
| TX | 1.085 | 0.402–2.930 | 0.873 | |||
| M stage | ||||||
| M0 | Ref | — | — | |||
| M1 | 1.191 | 0.958–1.480 | 0.115 | |||
| MX | 1.512 | 0.717–3.188 | 0.277 | |||
Age’ is constructed as a spline variable (when k = 3). A model selection technique based on the Akaike information criteria was used. NOS, not otherwise specified.
To construct the final model, we used restricted cubic splines to examine the continuous variable effects. The variable of age at diagnosis had nonlinear effects on the log of the hazard ratio of OS (Fig 2); age was thus optimally modeled with three knots, with the extremes showing the highest mortality risk.
Figure 2.
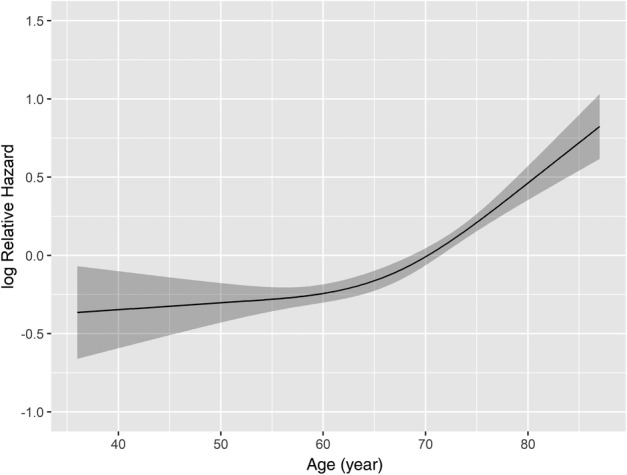
Continuous variable transformation in univariate analysis via restricted cubic splines concerning age.
Nomogram
A nomogram model was created to predict the OS of MPM patients who underwent surgery (Fig 3). A high score in the nomogram was associated with a poor prognosis. We divided the predicted nomogram scores into quartiles and plotted their survival curves (Fig 4). The nomogram was able to stratify patients into four distinct prognostic groups (quartile 1: 1‐year survival rate, 74.9%; quartile 2: 1‐year survival rate, 63.5%; quartile 3: 1‐year survival rate, 52.4%; and quartile 4: 1‐year survival rate, 13.3%; P < 0.001).
Figure 3.
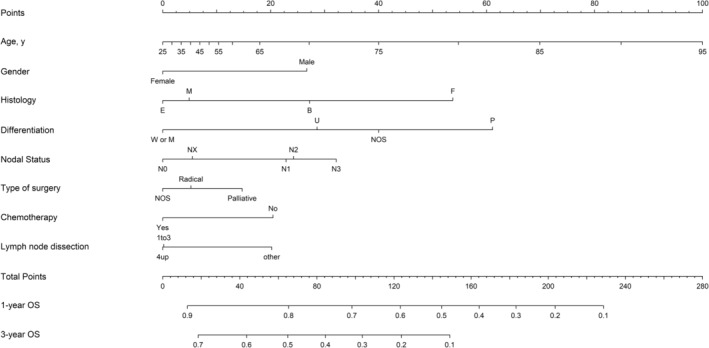
Prediction of overall survival (OS) of patients who underwent surgery according to the nomogram. Histology: B, biphasic mesothelioma; E, epithelioid mesothelioma; F, fibrous mesothelioma; M, mesothelioma. Differentiation: M, moderately differentiated; P, poorly differentiated; NOS, not otherwise specified; U, undifferentiated; W, well differentiated.
Figure 4.
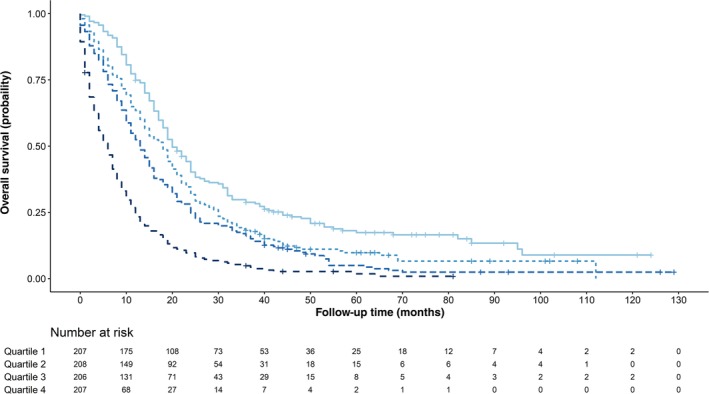
Overall survival of patients who underwent surgery based on the quartiles of the nomogram predicted score.  Quartile 1,
Quartile 1,  Quartile 2,
Quartile 2,  Quartile 3,
Quartile 3,  Quartile 4.
Quartile 4.
Model performance and validation in the training cohort
In the training cohort, the Harrell's C‐index for the established nomogram to predict OS (0.715, 95% CI 0.698–0.736) was significantly higher than that of the TNM staging system (0.564, 95% CI 0.539–0.589; P < 0.01). A calibration plot showed high consistency between predicted and actual survival in the training cohort at one and three years (Fig 5a).
Figure 5.
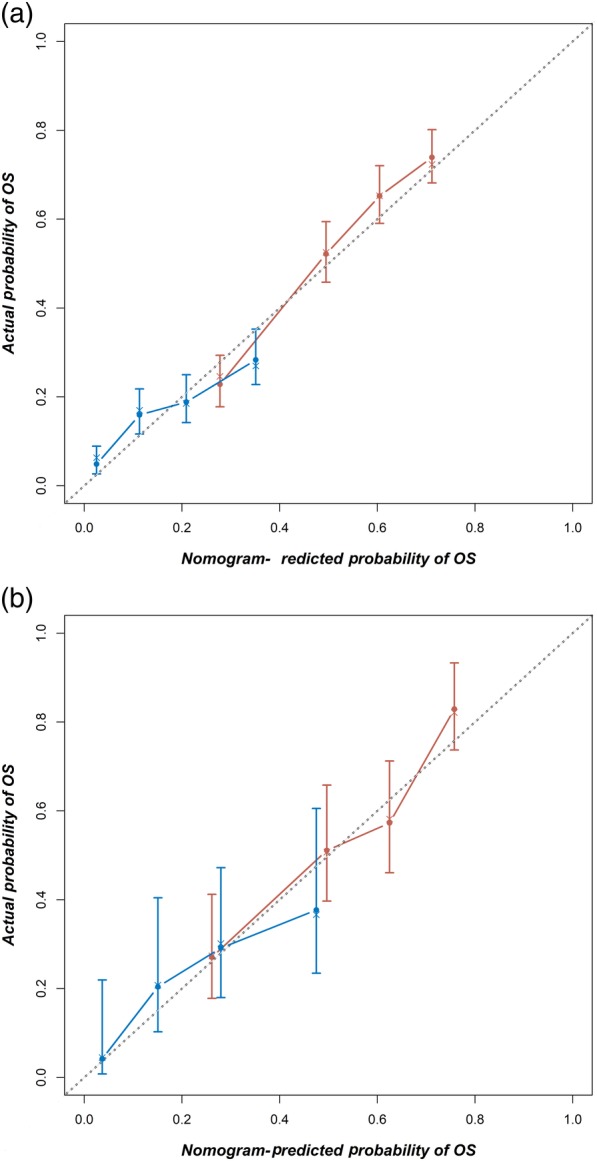
Calibration curves to predict overall survival (OS) in the (a) training,  1‐year OS,
1‐year OS,  3‐year OS and (b) validation cohorts,
3‐year OS and (b) validation cohorts,  1‐year OS,
1‐year OS,  3‐year OS. OS predicted by the nomogram is plotted on the x‐axis, while the actual probability of OS is on the y‐axis. A 45‐degree curve (dotted line) would mean that the model was perfectly calibrated such that the predicted probabilities and actual outcomes were identical.
3‐year OS. OS predicted by the nomogram is plotted on the x‐axis, while the actual probability of OS is on the y‐axis. A 45‐degree curve (dotted line) would mean that the model was perfectly calibrated such that the predicted probabilities and actual outcomes were identical.
We further tested the nomogram with a validation cohort (n = 312). The C‐index was significantly higher in the nomogram (0.656, 95% CI 0.645–0.667) than in the TNM system (0.543, 95% CI 0.532–0.554; P < 0.01). A calibration plot for the nomogram for predicting one‐year and three‐year survival indicated that the nomogram has high predictive accuracy in the validation cohort (Fig 5b).
Discussion
To our knowledge, this was the first large‐scale study to analyze the prognosis of MPM patients who underwent surgery using a nomogram based on data from the SEER database. In a training cohort of 828 cases, independent prognostic factors were identified, including age, gender, histology type, differentiation, N stage, chemotherapy, type of surgery, and lymph node dissection. We developed a nomogram that could enable us to visually evaluate patient survival. An advantage of this study was the use of a validation cohort that consisted of 312 patients to evaluate the performance of the nomogram, which makes our conclusions more convincing. The nomogram showed good discriminative ability and was well calibrated. These results improve our understanding of resected MPM lesions and provide a reliable tool for predicting patient OS.
There have been several studies of survival involving a SEER dataset of MPM patients.8, 11, 18, 19, 20, 21, 22 Taioli et al. reported median survival of 6.5 months in patients who were not administered radiotherapy or surgery, 14.5 months in the surgical group, and 13 months in patients administered both radiation and surgery.22 These data are similar to our finding of 14 months median OS, thus supporting our opinion that the prognosis of surgically treated MPM patients should be analyzed separately from non‐surgery patients. In a study by Yang et al. based on a SEER dataset, the survival rate in surgically treated patients was higher than that in our data (1‐year survival 63% vs. 53.9%; 3‐year survival 21% vs. 17.1%).11 This may be because Yang et al. excluded patients with sarcomatoid histology and stage IV disease. In general, the survival data in our study are comparable to data presented in earlier reports.
Similar to our findings, prior studies have shown that female gender, younger age, and early stage are independent predictors of longer survival in multivariate analysis.8, 22 It is interesting that several studies have found that female MPM patients have a better prognosis than male patients21, 22 this would appear to be worth investigating further. In contrast to the study by Wang et al.,8 the diagnoses in our patient cohorts were based on resected surgical specimens while cases involving only a small biopsy were excluded, which enhances the accuracy of our diagnoses and the reliability of pathological staging compared to clinical staging in survival analysis.
In accordance with previous reports, we also found that advanced age was a significant factor associated with poor survival.3, 11, 19 Furthermore, we found that the age at diagnosis had nonlinear effects on the log of the hazard ratio of OS, which has not previously been reported in MPM. In a Cox proportional hazards survival model constructed by Yang et al., surgery was associated with improved survival in patients aged ≥ 70 years but not in those aged ≥ 80.11 Consistent with this result, our nomogram also showed that the mortality risk rises sharply with the increase in age in patients aged > 70. These data suggest that we must be very cautious in deciding whether or not to perform surgery on patients aged > 70 years, as it may not translate into a survival benefit.
We also found that survival is significantly affected by tumor histology. The biphasic subtype showed poor prognosis compared to the epithelioid subtype, whereas the sarcomatoid subtype was even poorer. This finding is consistent with prior studies.19, 23, 24, 25, 26, 27 Meyerhoff et al. reported that epithelioid histology is related to better survival compared to sarcomatoid and biphasic histology.19 Surgery significantly improves survival for epithelioid MPM patients, but sarcomatoid and biphasic patients have poor prognoses and may not benefit from surgery. The specific histologic type should be considered during the surgical decision‐making process. In addition, our data showed that tumor differentiation is associated with survival in MPM patients. Well or moderately differentiated subtypes resulted in better prognoses than poorly differentiated or undifferentiated subtypes. However, in our cohort, most MPM tumors were listed as NOS, indicating the importance of determining the differentiation status in order to make a pathological diagnosis of MPM.
It has been reported that TNM staging is related to MPM prognosis;28, 29, 30, 31 however, we found no significant association between OS and TNM staging. Similarly, Meyerhoff et al. observed no survival difference between different clinical stages within the epithelioid histotype.19 In the IASLC‐IMIG study, Rusch et al. also reported no significant differences in survival among the patients of different stages that underwent surgery.12 One possible reason for this is that most patients in this study (70.4%) underwent palliative rather than curative surgery. More studies are needed to resolve this issue.
Regarding the heterogeneity within our patient population, optimal management suggests surgery within multimodality therapy to suitable patients, for example, young patients with early‐stage epithelioid histology. As the most common site of recurrence after surgery is the ipsilateral hemithorax, involvement of the N1 and N2 stations poses an increased risk,10, 13 thus future postoperative treatment should consider improved radiotherapy techniques, as well as new target and immunotherapy strategies.32, 33
This study has several limitations. First, because it is retrospective, large randomized controlled trials are needed to confirm our findings. Second, many other factors can influence the outcome of surgery, including cardiopulmonary conditions and comorbidities. However, this information is not available in the SEER database. Furthermore, detailed information of the chemotherapy regimens and radiation doses was not available in the SEER database; therefore, we could not further analyze the association between OS and these factors.
More research is needed to improve the nomogram so that it will better predict patient survival. Identification of the key factors associated with survival enables us to plan individualized treatments that will provide the most benefit to patients.
Disclosure
No authors report any conflict of interest.
Acknowledgment
This work was supported by the Science Foundation of Peking University Cancer Hospital (No. 18‐02).
References
- 1. Chailleux E, Dabouis G, Pioche D et al. Prognostic factors in diffuse malignant pleural mesothelioma. A study of 167 patients. Chest 1988; 93 (1): 159–62. [DOI] [PubMed] [Google Scholar]
- 2. Ruffie P, Feld R, Minkin S et al. Diffuse malignant mesothelioma of the pleura in Ontario and Quebec: A retrospective study of 332 patients. J Clin Oncol 1989; 7 (8): 1157–68. [DOI] [PubMed] [Google Scholar]
- 3. Ettinger DS, Akerley W, Borghaei H et al. Malignant pleural mesothelioma. J Natl Compr Canc Netw 2012; 10 (1): 26–41. [DOI] [PubMed] [Google Scholar]
- 4. Ai J, Stevenson JP. Current issues in malignant pleural mesothelioma evaluation and management. Oncologist 2014; 19 (9): 975–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaufman AJ, Flores RM. Surgical treatment of malignant pleural mesothelioma. Curr Treat Options Oncol 2011; 12 (2): 201–16. [DOI] [PubMed] [Google Scholar]
- 6. Rena O, Casadio C. Lack of evidence in malignant pleural mesothelioma surgery. Interact Cardiovasc Thorac Surg 2011; 12 (3): 347–8. [DOI] [PubMed] [Google Scholar]
- 7. Treasure T, Internullo E, Fiorentino F et al. A survey of opinions and beliefs concerning surgery for malignant pleural mesothelioma amongst 802 members of the European Association for Cardio‐Thoracic Surgery (EACTS), the European Society of Thoracic Surgeons (ESTS) and the Society of Thoracic Surgeons (STS). Interact Cardiovasc Thorac Surg 2011; 12 (3): 341–6. [DOI] [PubMed] [Google Scholar]
- 8. Wang S, Ma K, Chen Z et al. A nomogram to predict prognosis in malignant pleural mesothelioma. World J Surg 2018; 42 (7): 2134–42. [DOI] [PubMed] [Google Scholar]
- 9. Kindler HL, Ismaila N, Armato SG 3rd et al. Treatment of malignant pleural mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018; 36: 1343–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baldini EH, Richards WG, Gill RR et al. Updated patterns of failure after multimodality therapy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2015; 149 (5): 1374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang CJ, Yan BW, Meyerhoff RR et al. Impact of age on long‐term outcomes of surgery for malignant pleural mesothelioma. Clin Lung Cancer 2016; 17 (5): 419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rusch VW, Giroux D, Kennedy C et al. Initial analysis of the International Association for the Study of Lung Cancer mesothelioma database. J Thorac Oncol 2012; 7 (11): 1631–9. [DOI] [PubMed] [Google Scholar]
- 13. Sugarbaker DJ, Richards WG, Bueno R. Extrapleural pneumonectomy in the treatment of epithelioid malignant pleural mesothelioma: Novel prognostic implications of combined N1 and N2 nodal involvement based on experience in 529 patients. Ann Surg 2014; 260 (4): 577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hess KR. Assessing time‐by‐covariate interactions in proportional hazards regression models using cubic spline functions. Stat Med 1994; 13 (10): 1045–62. [DOI] [PubMed] [Google Scholar]
- 15. Harrell F. Regression Modeling Strategies with Application to Linear Models, Logistic Regression, and Survival Analysis. Springer, New York, NY: 2001. [Google Scholar]
- 16. Wen J, Liu D, Xu X et al. Nomograms for predicting survival outcomes in patients with primary tracheal tumors: A large population‐based analysis. Cancer Manag Res 2018; 10: 6843–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996; 15 (4): 361–87. [DOI] [PubMed] [Google Scholar]
- 18. Baumann F, Ambrosi JP, Carbone M. Asbestos is not just asbestos: An unrecognised health hazard. Lancet Oncol 2013; 14 (7): 576–8. [DOI] [PubMed] [Google Scholar]
- 19. Meyerhoff RR, Yang CF, Speicher PJ et al. Impact of mesothelioma histologic subtype on outcomes in the Surveillance, Epidemiology, and End Results database. J Surg Res 2015; 196 (1): 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spirtas R, Connelly RR, Tucker MA. Survival patterns for malignant mesothelioma: The SEER experience. Int J Cancer 1988; 41 (4): 525–30. [DOI] [PubMed] [Google Scholar]
- 21. Taioli E, Wolf AS, Camacho‐Rivera M, Flores RM. Women with malignant pleural mesothelioma have a threefold better survival rate than men. Ann Thorac Surg 2014; 98 (3): 1020–4. [DOI] [PubMed] [Google Scholar]
- 22. Taioli E, Wolf AS, Camacho‐Rivera M et al. Determinants of survival in malignant pleural mesothelioma: A Surveillance, Epidemiology, and End Results (SEER) study of 14,228 patients. PLoS ONE 2015; 10 (12): e0145039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Galateau‐Salle F, Churg A, Roggli V, Travis WD. The 2015 World Health Organization Classification of Tumors of the Pleura: Advances since the 2004 classification. J Thorac Oncol 2016; 11 (2): 142–54. [DOI] [PubMed] [Google Scholar]
- 24. British Thoracic Society Standards of Care Committee . BTS statement on malignant mesothelioma in the UK, 2007. Thorax 2007; 62 (Suppl 2): ii1–ii19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scherpereel A, Astoul P, Baas P et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010; 35 (3): 479–95. [DOI] [PubMed] [Google Scholar]
- 26. Beckett P, Edwards J, Fennell D, Hubbard R, Woolhouse I, Peake MD. Demographics, management and survival of patients with malignant pleural mesothelioma in the National Lung Cancer Audit in England and Wales. Lung Cancer 2015; 88 (3): 344–8. [DOI] [PubMed] [Google Scholar]
- 27. Bovolato P, Casadio C, Bille A et al. Does surgery improve survival of patients with malignant pleural mesothelioma?: A multicenter retrospective analysis of 1365 consecutive patients. J Thorac Oncol 2014; 9 (3): 390–6. [DOI] [PubMed] [Google Scholar]
- 28. Helland A, Solberg S, Brustugun OT. Incidence and survival of malignant pleural mesothelioma in Norway: A population‐based study of 1686 cases. J Thorac Oncol 2012; 7 (12): 1858–61. [DOI] [PubMed] [Google Scholar]
- 29. Leuzzi G, Rea F, Spaggiari L et al. Prognostic score of long‐term survival after surgery for malignant pleural mesothelioma: A multicenter analysis. Ann Thorac Surg 2015; 100 (3): 890–7. [DOI] [PubMed] [Google Scholar]
- 30. Nakas A, Waller D. Predictors of long‐term survival following radical surgery for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2014; 46 (3): 380–5. [DOI] [PubMed] [Google Scholar]
- 31. van der Bij S, Koffijberg H, Burgers JA et al. Prognosis and prognostic factors of patients with mesothelioma: A population‐based study. Br J Cancer 2012; 107 (1): 161–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alley EW, Lopez J, Santoro A et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE‐028): Preliminary results from a non‐randomised, open‐label, phase 1b trial. Lancet Oncol 2017; 18 (5): 623–30. [DOI] [PubMed] [Google Scholar]
- 33. Rossini M, Rizzo P, Bononi I et al. New perspectives on diagnosis and therapy of malignant pleural mesothelioma. Front Oncol 2018; 8: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]


