Abstract
Background
Single agent immune checkpoint inhibitors (ICIs) improve survival outcomes compared to chemotherapy for advanced non‐small cell lung cancer (NSCLC), but treatment efficacy widely varies. The combination of ICIs with chemotherapy has shown promising efficacy over chemotherapy alone; however, whether this strategy is superior to single agent ICIs for the treatment of advanced NSCLC remains unknown.
Methods
The records of 109 patients with advanced NSCLC who were administered at least one cycle of ICIs were retrospectively reviewed. Patients were grouped based on the presence or absence of a chemotherapy treatment combination. Efficacy and survival outcomes were analyzed.
Result
Sixty‐nine (58.0%) patients received single agent ICIs (ICI group) and 50 (42.0%) received ICIs and chemotherapy (ICC group). The median (3.2 vs. 3.0 months; P = 0.025) and one‐year (34.5 vs. 9.6%; P = 0.026) progression‐free survival (PFS) rates were significantly better in the ICC than in the ICI group. The superior efficacy of ICC remained in the propensity score matched pairs (median PFS 3.2 vs. 2.6 months, P = 0.032; 1‐year PFS 35.2 vs. 7.6%; P = 0.035). Eastern Cooperative Oncology Group performance status 0–1 (HR 0.37, 95% CI 0.22–0.62; P < 0.001) and the ICC group (HR 0.56, 95% CI 0.34–0.94; P = 0.028) were predictive of PFS. Subgroup‐to‐chemotherapy interaction revealed improved risk reduction for adenocarcinoma and EGFR mutation.
Conclusion
Combing chemotherapy with ICIs improved treatment efficacy over ICIs alone. The additional efficacy of chemotherapy may differ between histological subtypes and EGFR mutation status.
Keywords: Chemotherapy, immune checkpoint inhibitor, NSCLC
Introduction
Immunotherapy targeting the inhibition of immune checkpoint proteins on immune cells has been a revolutionary strategy for the treatment of advanced non‐small cell lung cancer (NSCLC) in recent years.1, 2 Therapeutic strategies largely focus on reawakening immune surveillance that has been inhibited by NSCLC as a result of the engagement between immune checkpoint proteins and their ligands. Currently, inhibition of PD‐1, PD‐L1, and CTLA‐4 via mono or dual blockade, have all demonstrated antitumor activity in some patients.2
In patients administered second‐line NSCLC treatment in clinical trials, immune checkpoint inhibitors (ICIs) alone exhibit variable efficacy compared to chemotherapy, which has been associated with histological subtypes. In the Checkmate 017 trial, nivolumab exhibited better efficacy than docetaxel for squamous NSCLC;3 while this advantage was not observed in the Checkmate 057 trial in which non‐squamous NSCLC dominated the patient population.4 On the other hand, in the Keynote 010 trial characterized by a mixture of squamous and non‐squamous NSCLC, the efficacy of pembrolizumab was similar to that of docetaxel, and the superior performance of pembrolizumab was only noted in the subgroup of patients with PD‐L1 expression ≥ 50%.5 In the context of first‐line treatment settings, in the Checkmate 026 study, the efficacy of nivolumab was only equivalent to a chemotherapy doublet.6 However, in the Keynote 024 trial, pembrolizumab exhibited an efficacy superior to that of the chemotherapy doublet for subjects with PD‐L1 ≥ 50%.7
Recently, accumulating evidence has revealed that the effectiveness of ICIs depends on the presence of pre‐existing immune cell infiltration in the cancer microenvironment, which is recognized as an inflammatory tumor phenotype.8 This finding suggests that immune cells potentially engage with cancer cells; ICI treatment enables the invigoration of immune cells and thereby boosts pre‐existing antitumor immunity. In contrast, immune‐ignorant or immune‐excluded tumor phenotypes, which lack immune cell infiltration or wall off immune cells by the extracellular matrix, render the resistance to ICIs.9, 10
A number of combined treatment approaches with ICIs have been proposed to deal with the issue of resistance.11, 12, 13 Among these, the combination of chemotherapy, the standard of care for advanced NSCLC patients, is one of the most widely used strategies as it is also likely to address the multifaceted mechanisms of tumor immune escape. Earlier studies have shown that chemotherapy can boost tumor immunogenicity for immune recognition by inducing immunogenic cell death and the release of neoantigens.13, 14 Chemotherapy also enhances antigen presentation as a result of the increased expression of MHC class I molecules in murine models,15 thereby boosting the recruitment and proliferation of tumor‐specific effector T cells.16, 17 An immunosuppressive tumor microenvironment can also be downregulated by cisplatin‐based chemotherapy via the inhibition of regulatory T and myeloid‐derived suppressor cells.18, 19
Given this preclinical and clinical evidence, an increasing number of clinical trials have demonstrated the promising efficacy of combining chemotherapy with ICIs. In a recently announced result from the Keynote 189 trial, pembrolizumab plus chemotherapy demonstrated superior efficacy over chemotherapy alone20 while in the IMpower 150 study, atezolizumab plus chemotherapy and bevacizumab performed better than chemotherapy and bevacizumab.21 However, whether there is a difference in performance between ICIs plus chemotherapy and ICIs alone remains unanswered.
In this study, we investigated a cohort of advanced and metastatic NSCLC patients administered ICI treatment and grouped them based on the presence or absence of a chemotherapy treatment combination. The survival outcomes of the overall population and the subgroups were analyzed.
Methods
Patients
From September 2015 to March 2018, the records of 137 patients with advanced or metastatic lung cancer who were administered at least one cycle of ICI treatment at Chang Gung Memorial Hospital were retrospectively reviewed. Patients were excluded for the following reasons: a short follow‐up duration (< 4 weeks, 7 patients), a diagnosis of small cell carcinoma (5 patients), a diagnosis of mesothelioma (1 patient), and concurrent administration of two ICI agents (7 patients). The remaining 119 patients were eligible for the analysis (Fig 1a).
Figure 1.
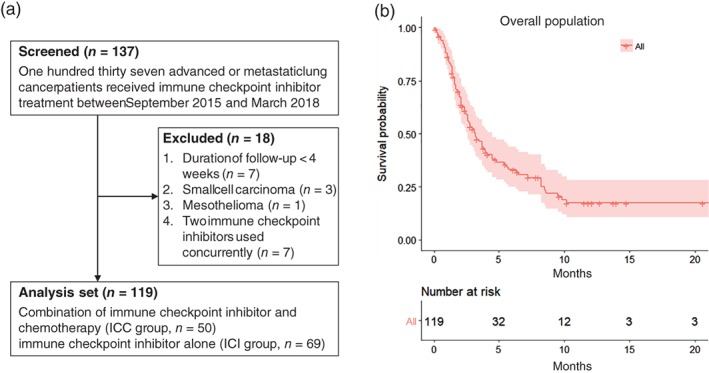
(a) Flow chart of the study population. (b) Kaplan–Meier curve (with a 95% confidence interval, red shade) of progression‐free survival (PFS) of the study population. ( ) All.
) All.
Sixty‐nine (58.0%) patients received single agent ICIs (ICI group) and 50 (42.0%) received ICIs and chemotherapy (ICC group). Patients were administered one of four ICI agents: pembrolizumab (2 mg/kg IV every 3 weeks) in 53 patients, nivolumab (3 mg/kg IV every 2 weeks) in 36 patients, atezolizumab (1200 mg IV every 3 weeks) in 25 patients, and durvalumab (1500 mg IV every 4 weeks) in five patients. The toxicities noted during ICI treatment were systemically reviewed.
Progression‐free survival (PFS) was defined as the interval between the start of ICI treatment and the date of either death or radiologically documented progression; overall survival (OS) was defined as the interval between the start of ICI treatment and the date of either death or the last follow‐up. The treatment response, defined as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD), was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria, version 3.0. PD‐L1 levels were assessed using a Dako PharmaDx 22C3 immunohistochemistry assay (Glostrup, Denmark) and the tumor proportion score (TPS) was calculated and reported as previously described.22 The Ethics Committee of Chang Gung Memorial Hospital approved the study.
Statistical analysis
The Mann–Whitney U test was used to determine the statistical significance between two groups of continuous variables, while Fisher's exact tests were used for categorical variables. The Kaplan–Meier survival curve was analyzed using the R package survival, and the hazard ratio (HR) was analyzed using the Cox regression model. For propensity score matching analysis, the ICC versus the ICI group served as the dependent variable; the covariates used included age, gender, smoking history, Eastern Cooperative Oncology Group performance status (ECOG PS), histology, first‐line treatment, EGFR and ALK status, and brain metastasis. The coefficient for each covariate was determined by logistic regression analysis, and the propensity score of each individual was calculated as the sum of the product of each coefficient and the value of each covariate. Pairs of ICC and ICI patients with equivalent propensity scores were selected in a 1:1 manner. All calculations were performed using the R package MatchIt. All of the reported P values are two sided, and P < 0.05 was considered statistically significant. All of the data were also analyzed using SPSS version 25.
Results
Baseline patient characteristics
Among the 119 patients, 87 (73.1%) were male, 73 (61.3%) were smokers or ex‐smokers, and 92 (77.3%) had an ECOG PS of 0–1 when they received immunotherapy treatment (Table 1). Seventy‐six (63.9%) had adenocarcinoma, 33 (27.7%) had squamous cell carcinoma, and 10 (8.4%) had poorly differentiated NSCLC (NSCLC‐PD). EGFR and ALK driver mutations were observed in 21 (17.6%) and 6 (5.0%) patients, respectively. Sixty‐nine (58.0%) patients were administered ICIs alone (ICI group) and 50 (42.0%) were administered a combination of ICIs and chemotherapy (ICC group). Platinum‐based doublets were administered in 20 (16.8%) patients: pemetrexed plus cisplatin/carboplatin in 14 (11.8%), docetaxel plus cisplatin/carboplatin in 4 (3.4%), and gemcitabine plus cisplatin/carboplatin in 2 (1.7%). Non‐platinum monotherapy was administered to 30 (25.2%) patients: pemetrexed in 14 (11.8%), docetaxel in 6 (5.0%), gemcitabine in 5 (4.2%), and vinorelbine in 5 (4.2%). The median PFS of the overall population was 3.2 months (Fig 1b).
Table 1.
Baseline demographics and clinical characteristics of the study population
| Variable, N (%) | Total (n = 119) | ICI plus chemotherapy (n = 50) | ICI alone (n = 69) | P |
|---|---|---|---|---|
| Age, median (range), year | 59 (53–65) | 57 (50–65) | 59 (54–67) | 0.990 |
| Gender (male) | 87 (73.1) | 35 (70.0) | 52 (75.4) | 0.659 |
| Smoker/ex‐smoker | 73 (61.3) | 29 (58.0) | 44 (63.8) | 0.655 |
| ECOG PS (0,1) | 92 (77.3) | 38 (76.0) | 54 (78.3) | 0.945 |
| Stage | ||||
| III | 16 (13.4) | 7 (14.0) | 9 (13.0) | 1.000 |
| IV | 103 (86.6) | 43 (86.0) | 60 (87.0) | |
| Histology | ||||
| Adenocarcinoma | 76 (63.9) | 37 (74.0) | 39 (56.5) | 0.147 |
| Squamous cell carcinoma | 33 (27.7) | 10 (20.0) | 23 (33.3) | |
| NSCLC‐PD | 10 (8.4) | 3 (6.0) | 7 (10.2) | |
| EFGR mutation | 21 (17.6) | 5 (10.0) | 16 (23.2) | 0.105 |
| ALK mutation | 6 (5.0) | 2 (4.0) | 4 (5.8) | 0.986 |
| Brain metastasis | 25 (21.0) | 10 (20.0) | 15 (21.7) | 0.966 |
| First‐line treatment | 36 (30.3) | 26 (52.0) | 10 (14.5) | <0.001 |
| PD‐L1 TPS, median (range) | 30 (2–75) | 35 (2–75) | 25 (5–70) | 0.805 |
| Immunotherapy agent | ||||
| Pembrolizumab | 53 (44.5) | 23 (46.0) | 30 (43.5) | 0.931 |
| Non‐pembrolizumab | 66 (55.5) | 27 (54.0) | 39 (56.5) | — |
ECOG PS, Eastern Cooperative Oncology Group performance status; ICI, immune checkpoint inhibitor; NSCLC‐PD, poorly differentiated non‐small cell lung cancer; TPS, tumor proportion score.
Most of the demographic and clinical characteristics, including age, gender, smoking history, and ECOG PS, were well balanced between groups (Table 1). The frequency of ICC as a first‐line treatment was higher than ICI alone (52.0% vs. 14.5%; P < 0.001). In addition, the frequency of EGFR mutations was lower in the ICC compared to the ICI group (10.0% vs. 23.2%; P = 0.105). Patients in the ICC group were more likely to have adenocarcinoma (74.0 vs. 56.5%) than squamous cell carcinoma (20.0 vs. 33.3%) compared to patients in the ICI group. The rate of NSCLC‐PD tended to be equivalent (6.0 vs. 10.2%; P = 0.147) between the groups.
Efficacy of immune checkpoint inhibitor (ICI) treatment alone versus chemotherapy combination
The efficacy between the ICC and ICI groups was analyzed using the Kaplan–Meier estimator; the median PFS of the ICC group (3.2 vs. 3.0 months; P = 0.025) (Fig 2a) as well as the one‐year PFS (34.5 vs. 9.6%, P = 0.026) were significantly better than those of the ICI group. To address confounding factors that can potentially affect the therapeutic efficacy between the groups (i.e. mainly the frequency of first‐line treatment, histological subtypes, and EGFR mutation status), we performed propensity score matching analysis. After 1:1 matching according to an individual's propensity score, pairs of patients (one from each group) with balanced clinical profiles were selected (Table S1). Among the propensity score matched subpopulation, the median PFS (3.2 vs. 2.6 months; P = 0.032) (Fig 2b) and the one‐year PFS (35.2 vs. 7.6%, P = 0.035) of the ICC group remained superior to that of patients in the ICI group. Objective response rates of PR, SD, and PD were 32.0%, 28.0%, and 40.0% for the ICC group and 21.4%, 17.4%, and 60.9% for the ICI group, respectively; a significantly better disease control rate was observed in patients in the ICC group (60.0 vs. 39.1%; P = 0.039) (Table 2).
Figure 2.

Kaplan–Meier curve of progression‐free survival (PFS) of (a) the overall population and (b) the propensity‐score‐matched pairs between the immune checkpoint inhibitor plus chemotherapy (ICC) and immune checkpoint inhibitor (ICI) alone groups. ( ) No. (
) No. ( ) Yes.
) Yes.
Table 2.
Objective response of the study population
| Treatment response N (%) | Total (n = 119) | ICI plus chemotherapy (n = 50) | ICI alone (n = 69) | P |
|---|---|---|---|---|
| PR | 31 (26.0) | 16 (32.0) | 15 (21.7) | 0.039* |
| SD | 26 (21.8) | 14 (28.0) | 12 (17.4) | — |
| PD | 62 (52.2) | 20 (40.0) | 42 (60.9) | — |
P value for disease control (partial response [PR] and stable disease [SD]) versus progressive disease (PD). ICI, immune checkpoint inhibitor.
Predictors of treatment efficacy
The Cox regression model was used to determine the predictors of PFS. In univariate analysis, ECOG PS 0–1 (HR 0.38, 95% confidence interval [CI] 0.23–0.61; P < 0.001), the combination of chemotherapy (HR 0.58, 95% CI 0.36–0.93; P = 0.024), and pembrolizumab use (HR 0.64, 95% CI 0.40–0.98; P = 0.042) were positive predictors of PFS, while brain metastasis was a negative predictor (HR 1.83, 95% CI 1.11–3.01; P = 0.018) (Table 3). According to multivariate analysis, ECOG PS 0–1 (HR 0.37, 95% CI 0.22–0.62; P < 0.001) and the combination treatment with chemotherapy (HR 0.56, 95% CI 0.34–0.94, P = 0.028) remained positive predictors of PFS.
Table 3.
Cox regression analysis of progression‐free survival
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age | 1.01 (0.98–1.02) | 0.661 | — | — |
| Gender (male) | 0.81 (0.50–1.31) | 0.388 | — | — |
| Smoker/ex‐smoker | 0.76 (0.49–1.17) | 0.212 | — | — |
| ECOG PS (0,1) | 0.38 (0.23–0.61) | < 0.001 | 0.37 (0.22–0.62) | < 0.001 |
| Histology* | ||||
| Squamous cell carcinoma | 0.92 (0.56–1.51) | 0.734 | — | — |
| NSCLC‐PD | 1.73 (0.85–3.52) | 0.130 | — | — |
| EGFR mutation | 1.28 (0.72–2.25) | 0.399 | — | — |
| Brain metastasis | 1.83 (1.11–3.01) | 0.018 | 1.49 (0.89–2.52) | 0.132 |
| Chemotherapy combination | 0.58 (0.36–0.93) | 0.024 | 0.56 (0.34–0.94) | 0.028 |
| First‐line treatment | 0.60 (0.35–1.01) | 0.055 | 0.92 (0.51–1.66) | 0.771 |
| PD‐L1 TPS, median (range) | 0.99 (0.99–1.01) | 0.980 | — | — |
| Pembrolizumab treatment | 0.64 (0.40–0.98) | 0.042 | 0.67 (0.41–1.07) | 0.095 |
Adenocarcinoma as reference.
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; NSCLC‐PD, poorly differentiated non‐small cell lung cancer; TPS, tumor proportion score.
Analysis of subgroup‐to‐chemotherapy effect interaction and progression‐free survival
Given the improved treatment efficacy associated with the combination of chemotherapy and ICIs, we subsequently explored whether the chemotherapy effect between subgroups was consistent. Two sets of potential subgroup‐to‐chemotherapy interactions were evaluated using a forest plot: adenocarcinoma versus NSCLC‐PD (interaction P value = 0.057) and EGFR mutation versus EGFR wild type (interaction P value = 0.091) (Fig 3). Kaplan–Meier analysis revealed that compared to ICI, ICC improved treatment efficacy in adenocarcinoma (median PFS: 5.4 vs. 2.8 months; P = 0.020) (Fig 4a, blue and red curves), while in NSCLC‐PD the efficacies were equivalent (median PFS: 1.6 vs. 2.7 months; P = 0.400) (Fig 4a, purple and green curve). ICC also exhibited improved efficacy over ICI in patients with EGFR mutations (median PFS: not reached vs. 2.3 months; P = 0.030) (Fig 4b, blue and red curves), whereas the efficacy between them was similar for patients with wild‐type EGFR (median PFS: 3.2 vs. 3.0 months; P = 0.200) (Fig 4b, purple and green curve). The efficacy and objective response between the ICI and ICC groups in the subpopulation with the first‐line treatment setting (Fig S1A, Table S2) also exhibited a similar trend, whereas the efficacy and objective response were equivalent between the two groups in the subpopulation of patients with high PD‐L1 expression (TPS ≥ 50%) (Fig S1B, Table S3).
Figure 3.

Forest plot of treatment for subgroup analysis. CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; ICI, immune checkpoint inhibitor; NSCLC‐PD, poorly differentiated non‐small cell lung cancer TPS, tumor proportion score.
Figure 4.
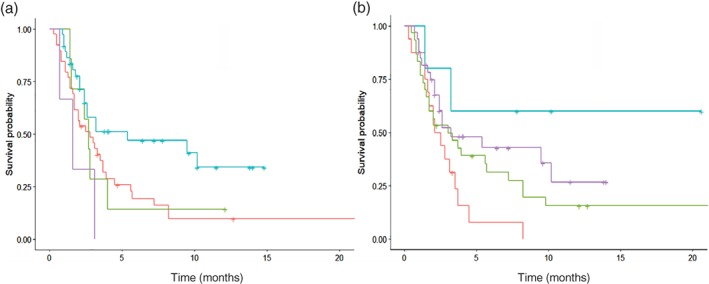
Kaplan–Meier survival curve of progression‐free survival (PFS) stratified by (a) adenocarcinoma or poorly differentiated non‐small cell lung cancer (NSCLC‐PD) ( ) Adenocarcinoma, immune checkpoint inhibitor (ICI) alone, (
) Adenocarcinoma, immune checkpoint inhibitor (ICI) alone, ( ) NSCLC‐PD, ICI alone, (
) NSCLC‐PD, ICI alone, ( ) Adenocarcinoma, ICI plus chemo, (
) Adenocarcinoma, ICI plus chemo, ( ) NSCLC‐PD, ICI plus chemotherapy (chemo) and (b) EGFR mutation or wild‐type between the immune checkpoint inhibitor plus chemotherapy (ICC) and ICI alone (ICI) groups. (
) NSCLC‐PD, ICI plus chemotherapy (chemo) and (b) EGFR mutation or wild‐type between the immune checkpoint inhibitor plus chemotherapy (ICC) and ICI alone (ICI) groups. ( ) Mutation, ICI alone, (
) Mutation, ICI alone, ( ) Wild type, ICI alone, (
) Wild type, ICI alone, ( ) Mutation, ICI plus chemo, (
) Mutation, ICI plus chemo, ( ) Wild type, ICI plus chemo.
) Wild type, ICI plus chemo.
Adverse events profile
The most commonly observed adverse events in patients in the ICC group, predominantly grade 1 or 2 in severity, included leukopenia (14 patients, 28.0%), skin rash (10 patients, 20.0%), and nausea/poor appetite (nine patients, 18.0%). In the ICI group, the most commonly observed adverse events included skin rash (15 patients, 21.7%) and thyroid function disorder (five patients, 7.2%) (Table 4). Serious adverse events above grade 3 were observed in 7 (14.0%) patients in the ICC group and 14 (0.5%) patients in the ICI group. The most frequent serious adverse event was skin rash, which was observed in 3 (6.0%) patients in the ICC group and 7 (10.1%) patients in the ICI group.
Table 4.
Treatment‐related adverse events
| Frequency N (%) | ICI plus chemotherapy (n = 50) | ICI alone (n = 69) | ||
|---|---|---|---|---|
| All | Grade ≥ 3 | All | Grade ≥ 3 | |
| Cutaneous tissue disorder | ||||
| Skin rash | 10 (20.0) | 3 (6.0) | 15 (21.7) | 7 (10.1) |
| Hair loss | 4 (8.0) | 0 | 0 | 0 |
| Endocrine disorder | ||||
| Thyroid function disorder | 3 (6.0) | 0 | 5 (7.2) | 1 (1.4) |
| Hypophysitis | 0 | 0 | 1 (1.4) | 0 |
| Gastrointestinal disorder | ||||
| Nausea/poor appetite | 9 (18.0) | 0 | 1 (1.4) | 0 |
| Diarrhea | 2 (4.0) | 0 | 2 (2.9) | 1 (1.4) |
| Hepatitis | 3 (6.0) | 1 (2.0) | 4 (5.8) | 0 |
| Respiratory disorder | ||||
| Pneumonitis | 2 (4.0) | 1 (2.0) | 4 (5.8) | 1 (1.4) |
| Pleuritis | 1 (1.4) | 0 | 1 (1.4) | 0 |
| TB reactivation | — | 1 (2.0) | — | — |
| Cardiovascular disorder | ||||
| Arrhythmia | 0 | 0 | 1 (1.4) | 0 |
| Hematologic disorder | ||||
| Leukopenia | 14 (28.0) | 1 (2.0) | 0 | 0 |
| Anemia | 5 (10.0) | 0 | 1 (1.4) | 0 |
| Thrombocytopenia | 7 (14.0) | 1 (2.0) | 0 | 0 |
ICI, immune checkpoint inhibitor; TB, tuberculosis.
Overall survival
OS after ICI was also analyzed: the median OS of patients in the ICC group was longer than that of patients in the ICI group (16.6 vs. 7.5 months; P = 0.200) (Fig 5a). In the propensity score matched pairs, a similar trend of median OS between the ICC and ICI groups was noted (12.8 vs. 4.4 months, P = 0.200) (Fig 5b). OS between the ICI and ICC groups in the subpopulations of first‐line treatment setting (Fig S2A) and high PD‐L1 expression (TPS ≥ 50%) was equivalent (Fig S2B).
Figure 5.
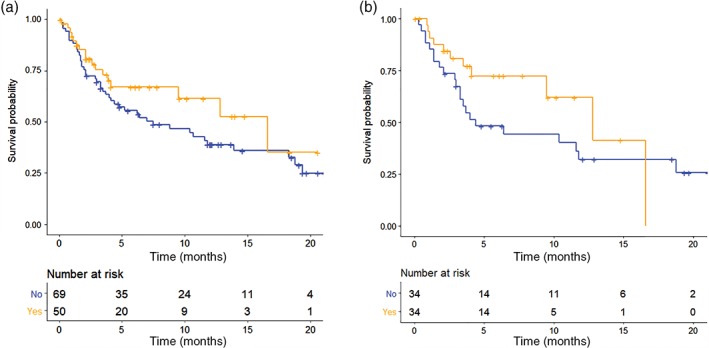
Kaplan–Meier survival curve of overall survival of (a) the overall population and (b) propensity‐score‐matched pairs between the immune checkpoint inhibitor plus chemotherapy (ICC) and immune checkpoint inhibitor (ICI) alone groups. ( ) No, (
) No, ( ) Yes.
) Yes.
Discussion
This study focused on the therapeutic strategy of combining chemotherapy with ICIs and found that the combination yielded significantly better treatment efficacy, as well as a trend toward better OS. Treatment‐to‐subgroup analysis further indicated that histological subtype and EGFR mutation status may be associated with a benefit from the addition of chemotherapy. Although more hematologic toxicities were observed in patients treated with the combination approach, most were tolerable and manageable.
Although previous studies have shown a survival advantage of single agent ICIs over chemotherapy for the treatment of advanced NSCLC, efficacy varies widely between subgroups and also between studies. These variations can be partly attributed to the expression level of PD‐L1. In a recent Keynote 042 trial comparing single agent pembrolizumab and a platinum‐doublet, the survival benefit of pembrolizumab in the overall population was mainly attributed to a subgroup of patients characterized with high PD‐L1 expression; pembrolizumab was not superior to platinum‐doublet in the subgroup of patients with weak PD‐L1 expression.23 However, variation not associated with the expression of PD‐L1 was also noted. An earlier study focusing on patients with high PD‐L1 expression showed that pembrolizumab improved treatment efficacy over a platinum‐doublet,5 whereas this effect was not fully reproduced in the equivalent backdrop of high PD‐L1 expression in the Keynote 042 study.23 Similar variations were also noted in a nivolumab study, in which nivolumab monotherapy showed higher efficacy over a platinum‐doublet only in a subgroup of patients with a high tumor mutation burden (TMB)24 The differential efficacy associated with histology has been noted previously.3, 4 Overall, these findings suggest that a durable effect of single agent ICIs did occur in a small subset of patients who can only be partially identified by PD‐L1 and TMB biomarkers.
In this subset of patients, a theory of ingenuity known as the cancer‐immunity cycle may be helpful to predict responsiveness to single agent ICIs.25, 26 It is plausible that effective antitumor immunity has been successfully mounted yet frozen in this cohort, mainly as a result of mechanisms involving the secondary activation of multiple checkpoint pathways. Therefore, a straightforward approach of checkpoint blockade untethers antitumor effector cells and leads to a significant tumor response. However, in the majority of patients, antitumor immunity may not be perfectly generated to allow this straightforward approach to be effective. In such circumstances, the addition of chemotherapy, as demonstrated by this study, may improve drug resistance to single agent ICIs.
Imperfectly generated antitumor immunity may be associated with certain tumor characteristics of particular molecular and histological traits. These characteristics include tumors with EGFR mutation, which exhibit weaker immunogenicity, less abundant immune cell infiltration in the tumor microenvironment27 and lower responsiveness to ICI monotherapy.28 This finding echoes recent results from the IMpower 130 trial in which the population with EGFR and ALK driver mutations did not receive a boost in treatment efficacy from the addition of ICIs;24 the IMpower 150 trial showed that only the administration of bevacizumab significantly improved the treatment efficacy of ICIs in such patients.21 Consistent with these findings, our results also showed that patients in the EGFR mutation subgroup who received single agent ICI treatment exhibited the shortest PFS (Fig 4b). On the other hand, the histological subtype of lung adenocarcinoma, compared to squamous cell or poorly differentiated NSCLC, frequently showed a lower TMB level.29, 30 This characteristic is associated with a lower frequency of tumor neoantigens, weaker immunogenicity, and thereby weaker antitumor immunity.23 The addition of chemotherapy in this context has been shown to play an important role in damaging the DNA mismatch repair mechanism and dramatically increasing tumor immunogenicity.13 Consistent with these results, the present study also showed that the boost in PFS from the addition of chemotherapy in the adenocarcinoma subgroups was higher than in the NSCLC‐PD groups (Fig 4a).
A limitation of this study is the inherent bias as a result of the retrospective nature and the imbalance of some of the clinical characteristics between the groups. However, this imbalance was addressed by analyzing a subpopulation of propensity score matched pairs, where the recapitulation of the finding in the overall population was confirmed. Multivariate regression analysis also revealed that the addition of chemotherapy remained an independent predictor of PFS in the overall population.
In conclusion, combining chemotherapy with ICIs improved treatment efficacy over ICIs alone for advanced NSCLC patients. However, the additional benefit of chemotherapy may differ as a function of histological subtype and EGFR mutation status.
Disclosure
No authors report any conflict of interest.
Supporting information
Table S1. Propensity score matched subpopulation.
Table S2. Objective response of subpopulation in first‐line treatment setting.
Table S3. Objective response of subpopulation with high PD‐L1 expression (tumor proportion score [TPS] ≥ 50%).
Figure S1. Kaplan–Meier survival curve of progression‐free survival (PFS) of the subpopulation in (a) a first‐line treatment setting and (b) patients with high PD‐L1 expression between the IOC and the ICI groups. TPS, tumor proportion score.
Figure S2. Kaplan–Meier survival curve of overall survival (OS) of the subpopulation in (a) a first‐line treatment setting and (b) patients with high PD‐L1 expression between the ICC and the ICI groups. TPS, tumor proportion score.
Acknowledgments
This study is supported through the grant CMRPG5E0103 and CMRPG3G1672 of the Chang‐Gung Medical Foundation.
References
- 1. Sundar R, Soong R, Cho BC, Brahmer JR, Soo RA. Immunotherapy in the treatment of non‐small cell lung cancer. Lung Cancer 2014; 85 (2): 101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hendriks L, Besse B. New windows open for immunotherapy in lung cancer. Nature 2018; 558 (7710): 376–7. [DOI] [PubMed] [Google Scholar]
- 3. Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015; 373 (2): 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015; 373 (17): 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Herbst RS, Baas P, Kim DW et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016; 387 (10027): 1540–50. [DOI] [PubMed] [Google Scholar]
- 6. Carbone DP, Reck M, Paz‐Ares L et al. First‐line nivolumab in stage IV or recurrent non‐small‐cell lung cancer. N Engl J Med 2017; 376 (25): 2415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reck M, Rodríguez‐Abreu D, Robinson AG et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016; 375 (19): 1823–33. [DOI] [PubMed] [Google Scholar]
- 8. Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013; 14 (10): 1014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Teng MW et al. Classifying cancers based on T‐cell infiltration and PD‐L1. Cancer Res 2015; 75 (11): 2139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015; 348 (6230): 74–80. [DOI] [PubMed] [Google Scholar]
- 11. Hellmann MD, Ciuleanu TE, Pluzanski A et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018; 378 (22): 2093–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levy A, Massard C, Soria JC, Deutsch E. Concurrent irradiation with the anti‐programmed cell death ligand‐1 immune checkpoint blocker durvalumab: Single centre subset analysis from a phase 1/2 trial. Eur J Cancer 2016; 68: 156–62. [DOI] [PubMed] [Google Scholar]
- 13. Germano G et al. Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature 2017; 552 (7683): 116–20. [DOI] [PubMed] [Google Scholar]
- 14. Bezu L, Gomes‐de‐Silva LC, Dewitte H et al. Combinatorial strategies for the induction of immunogenic cell death. Front Immunol 2015; 6: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gameiro SR, Caballero JA, Hodge JW. Defining the molecular signature of chemotherapy‐mediated lung tumor phenotype modulation and increased susceptibility to T‐cell killing. Cancer Biother Radiopharm 2012; 27 (1): 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen J, Huang X, Huang G, Chen Y, Chen L, Song H. Preconditioning chemotherapy with cisplatin enhances the antitumor activity of cytokine‐induced killer cells in a murine melanoma model. Cancer Biother Radiopharm 2012; 27 (3): 210–20. [DOI] [PubMed] [Google Scholar]
- 17. Chang CL, Hsu YT, Wu CC et al. Dose‐dense chemotherapy improves mechanisms of antitumor immune response. Cancer Res 2013; 73 (1): 119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roselli M, Cereda V, di Bari MG et al. Effects of conventional therapeutic interventions on the number and function of regulatory T cells. Oncoimmunology 2013; 2 (10): e27025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tseng CW, Hung CF, Alvarez RD et al. Pretreatment with cisplatin enhances E7‐specific CD8+ T‐cell‐mediated antitumor immunity induced by DNA vaccination. Clin Cancer Res 2008; 14 (10): 3185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gandhi L, Rodríguez‐Abreu D, Gadgeel S et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med 2018; 378 (22): 2078–92. [DOI] [PubMed] [Google Scholar]
- 21. Mok TS, Socinski MA, Reck M et al. IMpower 150: An exploratory analysis of efficacy outcomes in patients with EGFR mutations. Ann Oncol 2018; 29 (suppl_9): ix173–8. [Google Scholar]
- 22. Roach C, Zhang N, Corigliano E et al. Development of a companion diagnostic PD‐L1 immunohistochemistry assay for pembrolizumab therapy in non‐small‐cell lung cancer. Appl Immunohistochem Mol Morphol 2016; 24 (6): 392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goodman AM, Kato S, Bazhenova L et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther 2017; 16 (11): 2598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cappuzzo F, McCleod M, Hussein M et al. IMpower130: Progression‐free survival (PFS) and safety analysis from a randomised phase III study of carboplatin + nab‐paclitaxel (CnP) with or without atezolizumab (atezo) as first‐line (1L) therapy in advanced non‐squamous NSCLC. Ann Oncol 2018; 29 (suppl_8): mdy424.065. [Google Scholar]
- 25. Chen DS, Mellman I. Oncology meets immunology: The cancer‐immunity cycle. Immunity 2013; 39 (1): 1–10. [DOI] [PubMed] [Google Scholar]
- 26. Chen DS, Mellman I. Elements of cancer immunity and the cancer‐immune set point. Nature 2017; 541 (7637): 321–30. [DOI] [PubMed] [Google Scholar]
- 27. Chalmers ZR, Connelly CF, Fabrizio D et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017; 9 (1): 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dong ZY, Zhang JT, Liu SY et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD‐1 blockade in non‐small cell lung cancer. Oncoimmunology 2017; 6 (11): e1356145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee CK, Man J, Lord S et al. Checkpoint inhibitors in metastatic EGFR‐mutated non‐small cell lung cancer: A meta‐analysis. J Thorac Oncol 2017; 12 (2): 403–7. [DOI] [PubMed] [Google Scholar]
- 30. Nagahashi M, Sato S, Yuza K et al. Common driver mutations and smoking history affect tumor mutation burden in lung adenocarcinoma. J Surg Res 2018; 230: 181–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Propensity score matched subpopulation.
Table S2. Objective response of subpopulation in first‐line treatment setting.
Table S3. Objective response of subpopulation with high PD‐L1 expression (tumor proportion score [TPS] ≥ 50%).
Figure S1. Kaplan–Meier survival curve of progression‐free survival (PFS) of the subpopulation in (a) a first‐line treatment setting and (b) patients with high PD‐L1 expression between the IOC and the ICI groups. TPS, tumor proportion score.
Figure S2. Kaplan–Meier survival curve of overall survival (OS) of the subpopulation in (a) a first‐line treatment setting and (b) patients with high PD‐L1 expression between the ICC and the ICI groups. TPS, tumor proportion score.


