Abstract
The Ras/Raf/ERK pathway is one of the most frequently dysregulated signaling pathways in various cancers. In some such cancers, Ras and Raf are hotspots for mutations, which cause continuous activation of this pathway. However, in some other cancers, it is known that negative regulators of the Ras/Raf/ERK pathway are responsible for uncontrolled activation. The Sprouty/Spred family is broadly recognized as important negative regulators of the Ras/Raf/ERK pathway, and its expression is downregulated in many malignancies, leading to hyperactivation of the Ras/Raf/ERK pathway. After the discovery of this family, intensive research investigated the mechanism by which it suppresses the Ras/Raf/ERK pathway and its roles in developmental and pathophysiological processes. In this review, we discuss the complicated roles of the Sprouty/Spred family in tumor initiation, promotion, and progression and its future therapeutic potential.
Keywords: cancer, oncogene, Ras/Raf/ERK, Sprouty/Spred, tumor suppressor
Abbreviations
- AML
acute myeloid leukemia
- CRC
colorectal cancer
- CRD
cysteine‐rich domain
- DSS
dextran sulfate sodium
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- EMT
epithelial–mesenchymal transition
- EVH
Ena/vasodilator‐stimulated phosphoprotein homology
- FGF
fibroblast growth factor
- FGFR
fibroblast growth factor receptor
- FRS2
fibroblast growth factor receptor substrate 2
- GBM
glioblastoma
- GDNF
glial cell line‐derived neurotrophic factor
- HCC
hepatocellular carcinoma
- KBD
c‐Kit‐binding domain
- lncRNA
long noncoding RNA
- MAPK
mitogen‐activated protein kinase
- miR
micro‐RNA
- NF1
neurofibromatosis type 1
- NSCLC
non‐small‐cell lung carcinoma
- PP2A
protein phosphatase 2A
- Spred
Sprouty‐related protein with EVH‐1 domain
- SPRY4‐IT1
Sprouty4 intronic transcript 1
- TGF
transforming growth factor
- VEGF
vascular endothelial growth factor
1. INTRODUCTION
The Sprouty/Spred family functions as crucial negative regulators of Ras/Raf/ERK signaling and is evolutionarily conserved from Drosophila to mammals.1, 2, 3, 4 Drosophila sprouty was discovered as an antagonist of FGF signaling in 1998.1, 2 In mammals, there are four Sprouty homologs (Sprouty1‐4). Later, Sproutys were shown to suppress ERK activation induced by various growth factors, such as FGF, platelet‐derived growth factor, VEGF‐A, nerve growth factor, and GDNF in a cell type‐ and growth factor‐specific manner. To suppress the Ras/Raf/ERK pathway, Sprouty proteins contain several conserved amino acid sequences, such as the c‐Cbl‐binding domain at the N‐terminus, which includes a conserved tyrosine residue, the serine‐rich motif, and the CRD at the C‐terminus.
In 2001, Spred1 and Spred2 were first described as Sprouty‐related proteins by Yoshimura's group, who revealed that both Spred1 and Spred2 function as negative regulators of the Ras/Raf/ERK pathway through binding to Ras and suppression of Raf activation.3, 4 These Spred proteins have 3 domains of EVH‐1 in the N‐terminus, KBD, and Sprouty‐related CRD in the C‐terminus. Spred3, which lacks a functional KBD, was also cloned by the same group. Spreds are expressed in various organs such as lung, heart, kidney, brain, testis, thymus, uterus, and ovary, but the expression pattern differs among Spreds. Germline loss‐of‐function mutations in Spred1 causes Legius syndrome, which shows a similar phenotype to NF1 with café‐au‐lait spots and axillary freckling, but without cutaneous neurofibromas, or any detectable NF1 mutation.5 After this report, it was shown that Spred1 binds to neurofibromin (encoded by NF1) by its EVH‐1 domain and recruits neurofibromin to the plasma membrane, which explains the overlapping features of the two human diseases.6 Spreds are also known to regulate inflammatory signaling in various organs. Spred1 negatively modulates airway eosinophilia and hyperresponsiveness without affecting T‐cell differentiation and inhibits interleukin‐5‐induced ERK activation.4 Spred2 deficiency increases the severity of lipopolysaccharide‐induced acute lung inflammation7 and liver injury8 in mice, whereas Spred2−/− mice showed resistance to DSS‐induced acute colitis.9
The Ras/Raf/ERK pathway is dysregulated by many factors in most cancers. There are numerous negative regulators for fine‐tuning of the Ras/Raf/ERK pathway, including Ras GTPase‐activating proteins, MAPK phosphatases, and the Sprouty/Spred family.2 As the Sprouty/Spred family is one of the most important suppressors in the Ras/Raf/ERK pathway, many researchers have intensively investigated the roles of Sproutys and Spreds as tumor suppressors during tumorigenesis and metastasis. In this review, we discuss the elucidated roles of the Sprouty/Spred family in cancer.
2. MODE OF ACTION OF SPROUTY/SPRED IN REGULATION OF THe Ras/Raf/ERK PATHWAY
Many studies have revealed a variety of binding partners of the Sprouty/Spred family, including Grb2, SHP‐2, c‐Cbl, Raf, FRS2, and neurofibromin,1, 2, 3, 4 and the complicated mechanisms by which the Sprouty/Spred family inhibits the Ras/Raf/ERK pathway and tumorigenesis, but promotes tumorigenesis in certain types of cancer. The inhibitory mechanisms of the pathway appear to differ between Sproutys and Spreds (Figures 1 and 2). Sprouty2 is localized in organelles in a steady state and is recruited to the plasma membrane after growth factor stimulation.2 In contrast, Spreds are localized in the plasma membrane both before and after stimulation.3 More confusingly, Sprouty isoforms have slightly distinct suppressive mechanisms. For example, the conserved tyrosine residue of Sprouty1 and Sprouty2 at the N‐terminus, whose phosphorylation is important for their suppressive function, is phosphorylated by growth factor stimulation, whereas that of Sprouty4 is not phosphorylated by the same stimulation.2
Figure 1.
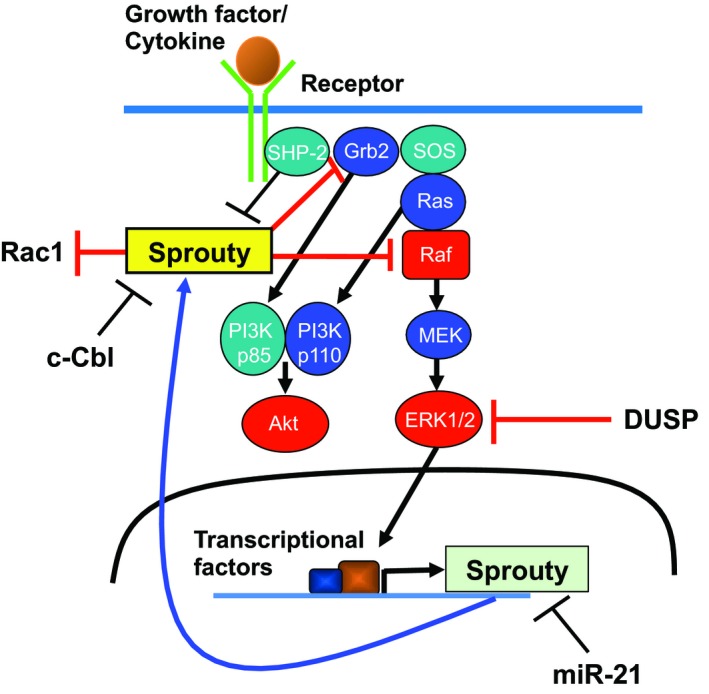
Inhibitory mechanisms of Sproutys on the Ras/Raf/ERK pathway. DUSP, dual‐specificity phosphatase; miR‐21, microRNA‐21; SHP‐2, Src homology 2 domain‐containing protein tyrosine phosphatase‐2; SOS, Son of Sevenless
Figure 2.
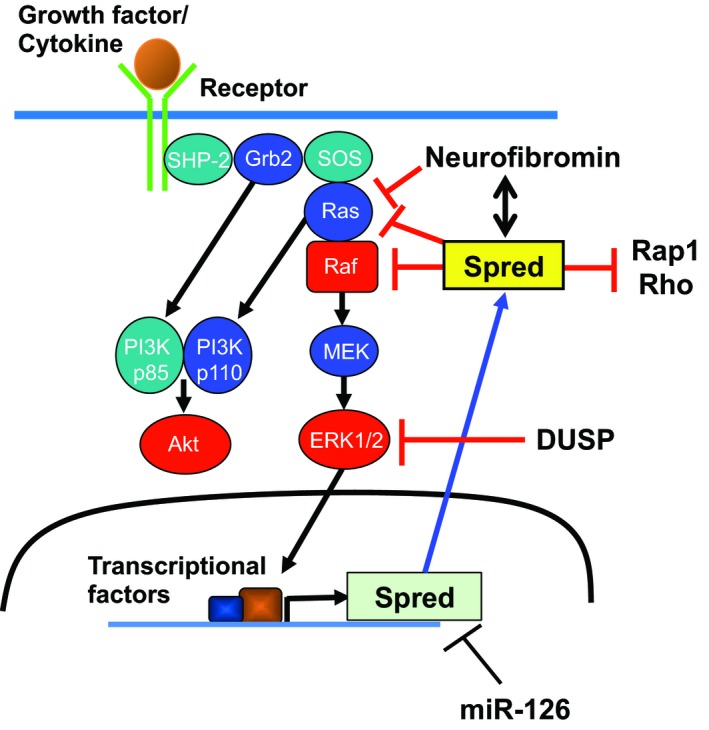
Inhibitory mechanisms of Spreds on the Ras/Raf/ERK pathway. DUSP, dual‐specificity phosphatase; miR‐126, microRNA‐126; SHP‐2, Src homology 2 domain‐containing protein tyrosine phosphatase‐2; SOS, Son of Sevenless
Sproutys are believed to have several mechanisms by which they can suppress the Ras/Raf/ERK pathway. Sproutys are generally considered to function upstream of Ras as they cannot suppress constitutively active Ras mutants.1 However, Sprouty4 inhibits VEGF‐A‐induced ERK activation by direct binding to c‐Raf.1 Interestingly, in mammals, Sproutys do not suppress EGF signaling; rather, they activate the signaling more by binding to c‐Cbl, an E3 ubiquitin‐protein ligase for EGFR.2 Sprouty2 can also suppress Rac1 activation and cell migration through protein tyrosine phosphatase 1B.2 Furthermore, Sprouty1 and Sprouty2 are reported to be negative regulators of TGF‐β‐Smad signaling.10
Spreds inhibit ERK activation by associating with Ras and neurofibromin and suppressing the phosphorylation and activation of Raf.3, 6 Spreds can also modulate the activation of the small GTPases, Ras, Rap1, and Rho.3 In Spred1, the inhibition of the Ras/Raf/ERK pathway was recognized when its 2 tyrosine (Y) residues 377/420 were phosphorylated.11 Three other tyrosine residues (Y303/343/353) within the CRD are necessary for regulating Spred2 activity.12
3. REGULATION OF SPROUTY/SPRED EXPRESSION
The Sprouty/Spred family is induced by many receptor tyrosine kinase signals and functions as negative feedback regulators of Ras/Raf/ERK signaling.1, 2, 3, 4 The mRNA and protein levels of members of this family are tightly regulated by several mechanisms, including epigenetic and posttranslational modifications. For example, Sprouty2 transcription is inhibited by DNA methyltransferase 1‐mediated methylation of the proximal promoter of Sprouty2,13 and the aberrant methylation of the Spred1 promoter regions is associated with low expression level of Spred1 mRNA in AML.14
An oncomiR, miR‐21, targets both Sprouty1 and Sprouty2.2 In multiple myeloma cells, the reduction of Sprouty2 induced by miR‐21 results in the enhancement of proliferation and invasion and the inhibition of apoptosis.15 In ovarian cancer cells, lncRNA GAS5 suppresses cell proliferation by decreasing miR‐21, thereby increasing Sprouty2 expression.16 Interestingly, lncRNA SPRY4‐IT1 is overexpressed in many cancers, including gastric cancer, CRC, and HCC and functions as an oncogene in contrast to Sprouty4.17 However, in testicular germ cell tumors, both Sprouty4 and SPRY4‐IT were found to function as oncogenes through activation of the PI3K‐Akt pathway.18 Moreover, miR‐122, miR‐27a, and miR‐330‐5p target Sprouty2, leading to enhanced cell proliferation.19, 20, 21 MicroRNA‐31 targets several negative regulators of the Ras/Raf/ERK pathway, including Sprouty1/3/4 and Spred1/2.22 The stability of Sprouty2 protein is regulated by several E3 ubiquitin‐protein ligases, such as c‐Cbl, Siah2, Nedd4‐1, and pVHL,2 although it is unknown how the stability of other Sprouty proteins is regulated.
Concerning the upstream signal of Spreds, miR‐126 is the most intensively studied component upstream of Spred1. MicroRNA‐126 promotes angiogenesis by suppressing the expression of Spred1, which normally inhibits the VEGF‐A signaling pathway.23, 24 In miR‐126−/− mice, the expression of Spred1 was increased and the intracellular angiogenic signal from VEGF‐A and FGF was suppressed.
4. PATHOPHYSIOLOGICAL ROLES OF SPROUTY/SPRED
Genetic studies with genetically modified mice have revealed the developmental and pathophysiological roles of the Sprouty/Spred family proteins. Sprouty1−/− mice die after birth due to defects in kidney development caused by enhanced GDNF/RET signaling.1 Half of Sprouty2−/− mice die after birth, and the remainder show growth retardation due to enteric neuronal hyperplasia, caused by hyperactivation of GDNF/RET signaling, resulting in esophageal achalasia.1 Sprouty2−/− mice also have severe hearing impairment.1 Moreover, Sprouty4−/− mice show polysyndactyly; most Sprouty4−/− mice die shortly after birth due to mandible defects, and the remainder show growth retardation.25 Finally, Sprouty2−/−/Sprouty4−/− mice show severe defects in craniofacial, limb, and lung morphogenesis, as well as angiogenesis, and embryonic lethality, suggesting redundant and nonredundant roles of Sprouty2 and Sprouty4.25, 26
Spred1−/− mice have a shortened face, melanin deposits in the spleen, and enhanced functions of hematopoietic stem cells.5, 27 Overexpression of Spred2 reduced the number of hematopoietic stem cells in the aorta‐gonad‐mesonephros culture, and hematopoietic stem cells from Spred2−/− mice were upregulated.4 We generated Spred1−/−/Spred2−/− mouse embryos, which showed embryonic lethality due to subcutaneous hemorrhage, edema, and dilated lymphatic vessels filled with erythrocytes.28 Spred1 and Spred2 are necessary for lymphatic vessel separation from the parental vein, and Spreds have an important role by negatively controlling VEGF‐C/VEGF receptor‐3 signaling in the development of lymphatic vessels of mice.28
The Sprouty/Spred family negatively regulates VEGF‐A and VEGF‐C signaling pathways as well as angiogenesis and lymphangiogenesis, both of which are important processes for tumor development.4 These findings suggest that expression of the Sprouty/Spred family in the tumor microenvironment also indirectly affects tumorigenesis and metastasis. Actually, we showed that s.c. tumors in Sprouty4−/− mice grow much faster than those in WT mice through enhanced neovascularization.29
It has recently been reported that the loss of Sprouty1 and Sprouty2 promotes the survival of effector CD8+ T cells, resulting in the formation of more protective memory CD8+ T cells.30 Another study has shown that Spred1 is upregulated in CD8+ tumor‐infiltrating lymphocytes in a TGF‐β‐dependent manner.31 These findings implicate the Sprouty/Spred family as the new potential targets in cancer immunotherapy.
5. ROLES OF SPROUTY/SPRED DURING TUMORIGENESIS AND METASTASIS
Aberrant activation of the Ras/Raf/ERK pathway induces excessive cell proliferation and tumorigenic behavior.2 Therefore, negative regulators of this signaling pathway have been observed to protect against tumorigenesis. The Sprouty/Spred family is one of the most important negative regulators of ERK signaling, and thus, evidence of its antitumorigenic efficacy has accumulated (Tables 1 and 2).1‐4,19,32‐60
Table 1.
Expression of Sproutys in various human cancer types
| Tissue | Type | Sample size | Country | Target (method) | Result | Reference |
|---|---|---|---|---|---|---|
| Kidney | Renal cell carcinoma | 40 | China | miR‐122, Sprouty2 (western blot, real‐time PCR) | Expression of Sprouty2 is downregulated compared to adjacent normal tissue, whereas miR‐122 was upregulated compared to adjacent normal tissue | Wang et al19 |
| Liver | Hepatocellular carcinoma | 75 | USA | Sprouty2 (microarray, real‐time PCR) | Expression of Sprouty2 is decreased compared to normal liver tissues | Fong et al32 |
| Liver | Hepatocellular carcinoma | 31 | Thailand | Sprouty1‐4 (real‐time PCR) | Expression of Sprouty1 is increased but the expression of Sprouty2 and Sprouty4 is decreased compared to normal liver tissues | Sirivatanauksorn et al33 |
| Liver | Hepatocellular carcinoma | 240 | China | Sprouty4 (immunohistochemistry) | Expression of Sprouty2 is decreased in 86.3% of patients. Patients negative for Sprouty2 show poorer survival and increased recurrence | Song et al34 |
| Liver | Intrahepatic cholangiocarcinoma | 108 | China | Sprouty1‐4 (real‐time PCR, immunohistochemistry) | High expression of Sprouty2 is correlated with favorable prognosis | Xu et al35 |
| Colon | Colorectal cancer | 70 | China | Sprouty4 (real‐time PCR) | Patients with lower expression of Sprouty4 indicated poorer prognosis | Zhou et al36 |
| Colon | Colon cancer | 67 | Taiwan | Sprouty2 (real‐time PCR) | Sprouty2 expression is downregulated in colon cancer and associated with clinical stage | Feng et al37 |
| Colon | Colon adenocarcinoma | 10 | USA | Sprouty2 (western blot, real‐time PCR) | Expression of Sprouty2 is upregulated compared to adjacent normal mucosa | Holgren et al38 |
| Colon | Colorectal cancer | 24 | USA | Sprouty1, Sprouty2 (real‐time PCR) | Expression of Sprouty1 and Sprouty2 is upregulated compared to adjacent normal samples | Zhang et al39 |
| Stomach | Gastric cancer | 104 | China | Sprouty1‐4 (immunohistochemistry) | High expression of Sprouty2 correlates with favorable prognosis | Xu et al40 |
| Stomach | Gastric cancer | 70 | China | Sprouty2 (real‐time PCR) | Expression of Sprouty2 is downregulated compared to adjacent normal samples | He et al41 |
| Prostate | Prostate cancer | 20 | USA | Sprouty1 (real‐time PCR, immunohistochemistry) | Expression of Sprouty1 is downregulated compared to normal prostatic peripheral zone | Kwabi‐Addo et al42 |
| Prostate | Prostate cancer | 49 | Germany | Sprouty1, Sprouty2 (real‐time PCR) | Expression of Sprouty2 is downregulated compared to normal tissue | Fritzsche et al43 |
| Prostate | Prostate cancer | 25 | USA | Sprouty4 (real‐time PCR) | Expression of Sprouty4 is downregulated compared to normal tissue | Wang et al44 |
| Breast | Breast cancer | 19 | Singapore | Sprouty1, Sprouty2 (real‐time PCR) | Expression of Sprouty1 and Sprouty2 is downregulated compared to paired normal tissue | Lo et al45 |
| Breast | Breast cancer | 1107 | UK | Sprouty1, Sprouty2 (microarray, immunohistochemistry) | Low gene expression of Sprouty2 is a significant poorer prognostic marker | Faratian et al46 |
| Uterus | Endometrioid carcinoma | 157 | Spain | Sprouty2 (immunohistochemistry) | Expression of Sprouty2 is increased compared to normal proliferative endometrial tissue | Velasco et al47 |
| Ovary | Epithelial ovarian cancer | 100 | Australia | Sprouty1 (immunohistochemistry) | Expression of Sprouty1 is lower compared to normal tissue. High expression of Sprouty1 indicates better prognosis | Masoumi‐Moghaddam et al48 |
| Ovary | Epithelial ovarian cancer | 99 | Australia | Sprouty2, Sprouty4 (immunohistochemistry) | Expression of Sprouty2 and Sprouty4 is downregulated compared to normal epithelial cells. High expression of Sprouty2 but not Sprouty4 correlates with better prognosis | Masoumi‐Moghaddam et al49 |
| Lung | Non‐small‐cell lung carcinoma | 25 | Australia | Sprouty1, Sprouty2 (real‐time PCR, immunohistochemistry) | Expression of Sprouty2 but not Sprouty1 is downregulated compared to adjacent normal tissue | Sutterluty et al50 |
| Ewing sarcoma | Ewing sarcoma | 162 | USA | Sprouty1 (real‐time PCR) | Low expression of Sprouty1 correlates with poorer prognosis | Cidre‐Aranaz et al51 |
| Peripheral blood | Chronic lymphocytic leukemia | 15 | USA | Sprouty2 (western blot, real‐time PCR) | Sprouty2 is downregulated in chronic lymphocytic leukemia cells from patients with poor prognosis | Shukla et al52 |
| Bone marrow | Acute myeloid leukemia | 275 | Germany | Sprouty4 (real‐time PCR) | Higher Sprouty4 expression levels show a favorable prognosis | Kayser et al53 |
miR, microRNA.
Table 2.
Expression of Spreds in various human cancer types
| Tissue | Type | Sample size | Country | Target (method) | Result | Reference |
|---|---|---|---|---|---|---|
| Liver | Hepatocellular carcinoma | 32 | Japan | Spred1, Spred2 (western blot, real‐time PCR, immunohistochemistry) |
Expression level of Spred1 and Spred2 is decreased compared to adjacent nontumoral lesion. Expression of Spred1 and Spred2 is inversely correlated with the incidence of tumor invasion and metastasis |
Yoshida et al54 |
| Liver | Hepatocellular carcinoma | 140 | USA | miR‐126, Spred1 (real‐time PCR) | mRNA level of Spred1 is inversely correlated with that of miR‐126. Expression level of Spred1 in patients with TACE + operation is lower than those with operation | Ji et al55 |
| Oral cavity | Squamous cell carcinoma | 10 | China | Spred1 (western blot, real‐time PCR) | Expression of Spred1 is downregulated in tumor tissues | Wang et al56 |
| Esophagus | Squamous cell carcinoma and adenocarcinoma | 43 | India | Spred1 (real‐time PCR) | Spred1 is downregulated in 69% of esophageal cancer patients | Sharma et al57 |
| Prostate | Prostate cancer | 15 | UK | Spred1, Spred2 (real‐time PCR) | Spred2 is downregulated in tumors compared to benign glands, whereas Spred1 expression remains unchanged | Kachroo et al58 |
| Breast | Breast cancer | 46 | China | Spred1 (real‐time PCR) | Expression of Spred1 is negatively correlated to estrogen receptor status | Jiang et al59 |
| Bone marrow | Acute myeloid leukemia | 58 | France | Spred1 (real‐time PCR) | Expression of Spred1 is lower compared to normal bone marrow cells | Pasmant et al60 |
miR, microRNA; TACE, transcatheter arterial chemoembolization.
5.1. Sprouty/Spred and HCC
It is thought that deregulation of the Ras/Raf/ERK pathway in HCC is due to downregulation of the negative regulators, including Sproutys, Spreds, and dual‐specificity phosphatases. In HCC, the expression of Sprouty1 is increased, whereas the expression of Sprouty2 and Sprouty4 is decreased, compared with the levels in corresponding nontumor tissues.32, 33, 34 Sprouty2 is suggested to be a candidate tumor suppressor in HCC and is an independent factor associated with postoperative recurrence in HCC.34 Sprouty2‐Y55F, a dominant negative mutant form of Sprouty2, promotes Akt‐induced HCC development through the activation of ERK1/2 and pyruvate kinase M2 and cooperates with activated β‐catenin to induce HCC in mice.2
In human HCC tissues, the expression of Spred1 and Spred2 was found to be lower than that of adjacent nontumoral tissue and the expression levels of Spred1 and Spred2 were negatively correlated with the rates of tumor invasion and metastasis.54 Spred1 overexpression in human HCC cell lines inhibited increased cell proliferation and negatively regulated the secretion of MMP‐2/9.54 Another group reported that, in a human HCC cell line, Spred2 overexpression inhibited cell proliferation and migration and induced apoptosis and Spred2 knockdown promoted tumor growth in vivo.61 Silibinin, a natural polyphenolic flavonoid, plays an antitumorigenic role in HCC through inhibition of the ERK1/2 cascade through the upregulation of Spreds.62
Taking these findings together, the Sprouty/Spred family inhibits the proliferation and migration of human HCC.
5.2. Sprouty and pancreatic cancer
Only a few papers have been published about the role of Sproutys and Spreds in pancreatic cancer. Dual‐specificity tyrosine phosphorylation‐regulated kinase 1A, which is highly expressed in pancreatic ductal adenocarcinoma, stabilizes c‐Met through Sprouty2 phosphorylation, thereby resulting in the promotion of tumor growth.63 In pancreatic cancer cells, miR‐21 targets Sprouty2 for promoting EGF‐induced cell proliferation.64 Sprouty4 does not affect β‐cell carcinogenesis in mice, although it regulates endocrine pancreas development.2
5.3. Sprouty/Spred and CRC
The role of Sproutys, especially Sprouty2, in CRC is still controversial. The expression of Sprouty2 is reported to be reduced in human CRC and is inversely correlated with the expression of miR‐21.37 The expression level of Sprouty4 is also reduced in human CRC due to DNA methylation and serves as an independent predictor of overall survival.36 The expression of Sprouty2, which promotes the cytotoxic effect of 5‐fluorouracil and metformin, is downregulated by miR‐21 in a human colon cancer cell line.65 Sprouty2 also increases the sensitivity to gefitinib, an EGFR inhibitor, by increasing the expression of phosphorylated and total EGFR in human CRC cell lines.2 Moreover, in colon cancer cells, Sprouty2 induces PTEN expression and suppresses the PI3K‐Akt pathway in addition to the Ras/Raf/ERK pathway.37 However, in contrast, Holgren's group reported that Sprouty2 is upregulated in human CRC samples and functions as an oncogene by inducing the expression of c‐Met.38 The same group showed that the downregulation of Sprouty2 induces E‐cadherin and p21 expression and inhibits EMT and cell proliferation.39 Sprouty2 also increases ZEB1 expression through Akt and Src activation and induces EMT in colon cancer cells.66
A study about Spreds and CRC was carried out using an azoxymethane/DSS‐induced colon tumor model.9 The number and size of colon tumors in Spred2−/− mice were lower and smaller than those in WT mice.9 Knockdown of Spred2 in a human colon cancer cell line modestly increased the cell proliferation and migration.9 To elucidate the role of Spreds in CRC, further investigation is necessary.
5.4. Sprouty/Spred and upper digestive tract cancer
In gastric cancer, low expression of Sprouty2 is associated with poor prognosis via the upregulation of FGFR2‐induced ERK activation, positive lymphatic invasion, and metastasis.40 MicroRNA‐23a, which is considered as an oncogene, is upregulated in gastric cancer samples and promotes cell proliferation and metastasis by targeting Sprouty2.67 MicroRNA‐592 also enhances the proliferation and invasion of gastric cancer cells by targeting Sprouty2.41 In esophageal squamous cell carcinoma, lncRNA CCAT1 is upregulated and induces the histone methylation of the promoter of Sprouty4, resulting in the suppression of Sprouty4 expression.68
MicroRNA‐182 was found to be upregulated in malignant oral carcinoma tissues and overexpression of miR‐182 sustained Ras/Raf/ERK signaling activation and promoted cell proliferation through the inhibition of Spred1, whereas the knockdown of miR‐182 revealed the inverse effect using a human tongue squamous cell carcinoma cell line.56 The expression of Spred1 in esophageal cancer including both squamous cell carcinoma and adenocarcinoma was analyzed.57 Spred1 was downregulated compared with that in nonmalignant tissue in two‐thirds of esophageal cancer patients. An inverse correlation of Spred1 and miR‐144, whose upregulation was seen in dysplastic esophageal cancer cells, was also observed.57
5.5. Sprouty/Spred and melanoma
Sprouty2 directly binds to and suppresses WT BRAF and is downregulated in WT BRAF melanoma cells.2 However, it does not bind to or inhibit BRAF V600E and is instead upregulated in BRAF‐mutant melanoma cells.2 Sprouty2 is also related to the induction of resistance to BRAF inhibitor in BRAF‐mutant melanoma cells.69 Salmonella typhimurium strain VNP20009 harboring Sprouty2‐expressing plasmid suppresses the s.c. growth of B16F10, a murine melanoma cell line, in vivo.70
The knockdown of Spred1 and Spred2 shows an effect similar to that of an MEK inhibitor and protects against apoptosis of BRAF V600E‐positive melanoma cell lines.71 The inhibition of the ERK pathway can even worsen the tumorigenesis of melanoma under certain conditions. Recently, biallelic inactivation mutations of Spred1 have been reported in patients with mucosal melanoma.72
5.6. Sprouty/Spred and prostate cancer
The expression of Sprouty1 and Sprouty2 is reduced in human prostate cancer, and Sprouty2 expression is mainly suppressed by epigenetic inactivation.2, 42, 43 Sprouty4 expression is also reduced by methylation of the Sprouty4 promoter region in human prostate cancer.44 Only loss of Sprouty2 induces growth arrest and suppresses prostate tumorigenesis through PP2A‐mediated nuclear accumulation of PTEN.73 However, the loss of Sprouty2, followed by the inactivation of PP2A or PTEN, accelerates prostate tumor progression.73 Concomitant suppression of Sprouty isoforms and Spred isoforms has been reported in prostate cancer, suggesting a dose effect of negative regulators.43, 74 Loss of both Sprouty1 and Sprouty2 in prostate epithelium results in ductal hyperplasia and low‐grade prostatic intraepithelial neoplasia in mice.2
The expression of Spred2, but not Spred1, was elucidated to be downregulated in human prostate cancer.58, 74 Spred2 overexpression in a human prostate cancer cell line reduced ERK activation and decreased cell proliferation and migration, and Spred2 knockdown indicated the inverse effect.58 Thus, Spred2 rather than Spred1 appears to be involved in prostate cancer as a tumor suppressor.
5.7. Sprouty/Spred and breast cancer
The expression of Sprouty1 and Sprouty2 is reported to be downregulated in human breast cancer samples, and overexpression of the dominant negative form of human Sprouty2 in breast cancer cells promotes cell proliferation and anchorage‐independent growth.45 Another paper reported that the expression of Sprouty2 is inversely correlated with human epidermal growth factor receptor 2 (HER2) expression and is an independent prognostic marker in breast cancer.46 However, it has also been reported that Sprouty1 knockdown in a human breast cancer cell line suppressed cell proliferation, migration, and colony formation.75 Stromal Sprouty1 expression regulates mammary branching morphogenesis by modulating EGFR‐dependent paracrine signaling and ECM remodeling.76 This mechanism might be related to mammary tumorigenesis. Sprouty4 also appears to function as a tumor suppressor in human breast cancer cells.2 MicroRNA‐196a is known to be involved in estrogen‐induced breast cancer development and directly inhibits Spred1 and the tumorigenic activity of miR‐196a is due to the suppression of Spred1.59
5.8. Sprouty and gynecological cancer
In human endometrial carcinoma, Sprouty2 is silenced because of promoter hypermethylation.2 In human high‐grade serous ovarian carcinoma, the expression of Sprouty2, but not of Sprouty1 and Sprouty4, is decreased, and Sprouty2 loss enhances EGF‐ or amphiregulin‐mediated E‐cadherin downregulation and cell invasion.77 The suppression of Sprouty1 promotes the proliferation and invasion of human ovarian cancer cell lines.48 Moreover, low expression of Sprouty1 and Sprouty2, but not of Sprouty4, is associated with poor prognosis in patients with human epithelial ovarian cancer.48, 49 Finally, decreased expression of Sprouty2 is associated with posttreatment development of ascites in carbotaxol‐treated patients with epithelial ovarian cancer.78
5.9. Sprouty and lung cancer
Sprouty2, but not Sprouty1, is downregulated in human NSCLC.50 In a urethane‐induced mouse lung tumor model, overexpression of Sprouty2 inhibited tumor initiation and growth.2 Consistent with this report, Sprouty2 was shown to regulate lung tumorigenesis in a K‐rasG12D‐mediated lung tumor model.2 In human NSCLC lines, Sprouty4 suppresses cell proliferation, invasion, and EMT.2 Trametinib, an MEK inhibitor, suppresses Sprouty4 expression, leading to FGFR1‐FRS2 activation, in mesenchymal‐like K‐ras‐mutant NSCLC.79 Downregulation of Sprouty4 by an EGFR inhibitor also contributes to the emergence of tolerant lung cancer cells.80 The role of Spreds in lung cancer has not yet been investigated.
5.10. Sprouty and brain tumors
Sprouty2 expression is upregulated and is associated with poor prognosis in GBM patients.81 Sprouty2 promotes ERK activation, proliferation, and drug resistance in GBM.81, 82 Moreover, Sprouty1 is induced by insulin‐like growth factor binding protein‐2 through the nuclear factor‐κB (NF‐κB) pathway, which might play an important role in maintenance of the mesenchymal phenotypes of GBM cells.83
5.11. Sprouty/Spred and bone and soft tissue tumors
In Ewing sarcoma, EWS‐FLI1, a fusion gene driving this disease, suppresses Sprouty1 expression, leading to basic FGF‐induced cell proliferation.51 Sprouty2, but not Sprouty4, inhibits the proliferation and migration of osteosarcoma cells.2 In rhabdomyosarcoma, Sprouty2 binds and stabilizes c‐Met.84 Therefore, depletion of Sprouty2 leads to c‐Met degradation and reduction of ERK activation.
By using a highly metastatic human osteosarcoma cell line, Miyoshi et al85 indicated that Spred1 inhibits their metastasis in nude mice as Spred1 and Spred2 suppress the activation of RhoA‐induced stress fiber formation and migration. Spreds are assumed to function as tumor suppressors in bone and soft tissue tumors, but further evidence in support of this is needed.
5.12. Sprouty/Spred and hematological malignancies
In B‐cell lymphoma, Sprouty2 is epigenetically silenced and promotes cell proliferation.2 Sprouty2 is also downregulated in chronic lymphocytic leukemia and is an important negative regulator of B‐cell receptor‐mediated ERK activation.52 In multiple myeloma, Sprouty2 is downregulated by miR‐21 expression.15, 86 High expression of Sprouty4 is associated with a favorable prognosis in patients with AML.53
From a large series of 230 pediatric acute lymphoblastic and myeloblastic leukemias, Spred1 mutations were found.60 In 1 of the 4 patients with Legius syndrome, a loss‐of‐function frameshift Spred1 mutation was detected. In this patient, the karyotype of blast cells showed Spred1 loss of heterozygosity. Moreover, Spred1 was significantly decreased in the bone marrow of patients with AML compared with normal bone marrow.60 Conditional double knockout of Spred1 and Spred2 induced the aberrant self‐renewal of hematopoietic stem cells, whereas the knockout of Spred1 did not show aberrant proliferation of hematopoietic stem cells.27 Both Spred1 and Spred2 appear to function as tumor suppressors in hematopoietic malignancies.27
6. CONCLUDING REMARKS
Sproutys and Spreds are believed to be tumor suppressors as they are downregulated in most human cancers and are important negative regulators of the Ras/Raf/ERK pathway, which is usually activated in cancer and promotes tumorigenesis and metastasis. As shown in Tables 1 and 2, the expression level of the Sprouty/Spred family could be used as a prognostic marker in some types of cancer. The downregulation of the Sprouty/Spred family is due to aberrant promoter hypermethylation or mRNA degradation by specific miRNAs, such as miR‐21 and miR‐126, in some cases; therefore, treatment using epigenetic modifiers, such as 5‐aza‐2′‐deoxycytidine and trichostatin A, or miRNA‐targeted therapy should be tested to restore the expression, although these therapies are nonspecific to the Sprouty/Spred family. Further studies are required for specifically restoring the expression of the Sprouty/Spred family. However, as mentioned above, Sprouty1, Sprouty2, and Sprouty4 function as oncogenes in some types of cancer. The roles of the Sprouty/Spred family, especially Sproutys, in cancer, that is, as tumor suppressors or oncogenes, appear to be cell‐type and tumor‐type dependent. Furthermore, the importance of Sprouty expression appears to be dependent on oncogenic Ras mutations in certain tumors.2 In addition, the expression of the Sprouty/Spred family in CD8+ T cells might contribute to the suppression of antitumor immunity.30, 31 Therefore, when the Sprouty/Spred family is targeted in cancer therapy, these should be taken into consideration to avoid unexpected results of the targeted therapy.
DISCLOSURE
The authors have no conflict of interest.
ACKNOWLEDGMENTS
This work is supported by the Japan Society for the Promotion of Science KAKENHI (JP15K21775), the Japan Agency for Medical Research and Development (PRIME; Grant Nos. JP18gm6210008 and JP19gm6210008), the “Kibou Projects” Startup Support for Young Researchers in Immunology, the Astellas Foundation for Research on Metabolic Disorders, the SENSHIN Medical Research Foundation, grants from Bristol‐Myers Squibb, the SGH Foundation, the MSD Life Science Foundation, the Ichiro Kanehara Foundation for the Promotion of Medical Sciences and Medical Care, the Yasuda Medical Foundation, the Suzuken Memorial Foundation, the Pancreas Research Foundation of Japan, the Waksman Foundation of Japan, the Japanese Foundation for Multidisciplinary Treatment of Cancer, Project Mirai Cancer Research Grants from the Japan Cancer Society, the Okinaka Memorial Institute for Medical Research, the Asahi Glass Foundation, the Foundation for Promotion of Cancer Research, the Kobayashi Foundation for Cancer Research, the Toray Science Foundation, the Vehicle Racing Commemorative Foundation, the JSR‐Keio University Medical and Chemical Innovation Center (JKiC), a Research Grant of the Princess Takamatsu Cancer Research Fund, the Tokyo Biomedical Research Foundation, the Daiichi Sankyo Foundation of Life Science, the Mochida Memorial Foundation for Medical and Pharmaceutical Research, the Medical Research Encouragement Prize of the Japan Medical Association, the Terumo Foundation for Life Sciences and Arts, the Life Science Foundation of Japan, the NOVARTIS Foundation (Japan) for the Promotion of Science, the Yakult‐Bioscience Foundation and the Takeda Science Foundation (all to K.T.).
Kawazoe T, Taniguchi K. The Sprouty/Spred family as tumor suppressors: Coming of age. Cancer Sci. 2019;110:1525–1535. 10.1111/cas.13999
REFERENCES
- 1. Mason JM, Morrison DJ, Basson MA, Licht JD. Sprouty proteins: multifaceted negative‐feedback regulators of receptor tyrosine kinase signaling. Trends Cell Biol. 2006;16:45‐54. [DOI] [PubMed] [Google Scholar]
- 2. Masoumi‐Moghaddam S, Amini A, Morris DL. The developing story of Sprouty and cancer. Cancer Metastasis Rev. 2014;33:695‐720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bundschu K, Walter U, Schuh K. Getting a first clue about SPRED functions. BioEssays. 2007;29:897‐907. [DOI] [PubMed] [Google Scholar]
- 4. Yoshimura A. Regulation of cytokine signaling by the SOCS and Spred family proteins. Keio J Med. 2009;58:73‐83. [DOI] [PubMed] [Google Scholar]
- 5. Brems H, Chmara M, Sahbatou M, et al. Germline loss‐of‐function mutations in SPRED1 cause a neurofibromatosis 1‐like phenotype. Nat Genet. 2007;39:1120‐1126. [DOI] [PubMed] [Google Scholar]
- 6. Stowe IB, Mercado EL, Stowe TR, et al. A shared molecular mechanism underlies the human rasopathies Legius syndrome and Neurofibromatosis‐1. Genes Dev. 2012;26:1421‐1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu Y, Ito T, Fushimi S, et al. Spred‐2 deficiency exacerbates lipopolysaccharide‐induced acute lung inflammation in mice. PLoS ONE. 2014;9:e108914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang X, Fujisawa M, Yoshimura T, et al. Spred2 deficiency exacerbates D‐Galactosamine/Lipopolysaccharide ‐induced acute liver injury in mice via increased production of TNFalpha. Sci Rep. 2018;8:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takahashi S, Yoshimura T, Ohkura T, et al. A novel role of Spred2 in the colonic epithelial cell homeostasis and inflammation. Sci Rep. 2016;6:37531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shin EH, Basson MA, Robinson ML, McAvoy JW, Lovicu FJ. Sprouty is a negative regulator of transforming growth factor beta‐induced epithelial‐to‐mesenchymal transition and cataract. Mol Med. 2012;18:861‐873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quintanar‐Audelo M, Yusoff P, Sinniah S, Chandramouli S, Guy GR. Sprouty‐related Ena/vasodilator‐stimulated phosphoprotein homology 1‐domain‐containing protein (SPRED1), a tyrosine‐protein phosphatase non‐receptor type 11 (SHP2) substrate in the Ras/extracellular signal‐regulated kinase (ERK) pathway. J Biol Chem. 2011;286:23102‐23112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meng S, Zhang M, Pan W, et al. Tyrosines 303/343/353 within the Sprouty‐related domain of Spred2 are essential for its interaction with p85 and inhibitory effect on Ras/ERK activation. Int J Biochem Cell Biol. 2012;44:748‐758. [DOI] [PubMed] [Google Scholar]
- 13. Gao X, Hicks KC, Neumann P, Patel TB. Hypoxia inducible factors regulate the transcription of the sprouty2 gene and expression of the sprouty2 protein. PLoS ONE. 2017;12:e0171616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sun J, Zhang J, Wang Y, Li Y, Zhang R. A pilot study of aberrant CpG island hypermethylation of SPRED1 in acute myeloid leukemia. Int J Med Sci. 2019;16:324‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang JH, Zhou WW, Cheng ST, Liu BX, Liu FR, Song JQ. Downregulation of Sprouty homolog 2 by microRNA‐21 inhibits proliferation, metastasis and invasion, however promotes the apoptosis of multiple myeloma cells. Mol Med Rep. 2015;12:1810‐1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ma N, Li S, Zhang Q, Wang H, Qin H, Wang S. Long non‐coding RNA GAS5 inhibits ovarian cancer cell proliferation via the control of microRNA‐21 and SPRY2 expression. Exp Ther Med. 2018;16:73‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li J, Chen Y, Chen Z, et al. SPRY4‐IT1: a novel oncogenic long non‐coding RNA in human cancers. Tumour Biol. 2017;39:1010428317711406. [DOI] [PubMed] [Google Scholar]
- 18. Das MK, Furu K, Evensen HF, Haugen OP, Haugen TB. Knockdown of SPRY4 and SPRY4‐IT1 inhibits cell growth and phosphorylation of Akt in human testicular germ cell tumours. Sci Rep. 2018;8:2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Z, Qin C, Zhang J, et al. MiR‐122 promotes renal cancer cell proliferation by targeting Sprouty2. Tumour Biol. 2017;39:1010428317691184. [DOI] [PubMed] [Google Scholar]
- 20. Gao W, Hong Z, Huang H, et al. miR‐27a in serum acts as biomarker for prostate cancer detection and promotes cell proliferation by targeting Sprouty2. Oncol Lett. 2018;16:5291‐5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xiao S, Yang M, Yang H, Chang R, Fang F, Yang L. miR‐330‐5p targets SPRY2 to promote hepatocellular carcinoma progression via MAPK/ERK signaling. Oncogenesis. 2018;7:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Edmonds MD, Boyd KL, Moyo T, et al. MicroRNA‐31 initiates lung tumorigenesis and promotes mutant KRAS‐driven lung cancer. J Clin Invest. 2016;126:349‐364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nicoli S, Standley C, Walker P, Hurlstone A, Fogarty KE, Lawson ND. MicroRNA‐mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature. 2010;464:1196‐1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang S, Aurora AB, Johnson BA, et al. The endothelial‐specific microRNA miR‐126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taniguchi K, Ayada T, Ichiyama K, et al. Sprouty2 and Sprouty4 are essential for embryonic morphogenesis and regulation of FGF signaling. Biochem Biophys Res Commun. 2007;352:896‐902. [DOI] [PubMed] [Google Scholar]
- 26. Taniguchi K, Sasaki K, Watari K, et al. Suppression of Sproutys has a therapeutic effect for a mouse model of ischemia by enhancing angiogenesis. PLoS ONE. 2009;4:e5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tadokoro Y, Hoshii T, Yamazaki S, et al. Spred1 safeguards hematopoietic homeostasis against diet‐induced systemic stress. Cell Stem Cell. 2018;22:713‐725.e8. [DOI] [PubMed] [Google Scholar]
- 28. Taniguchi K, Kohno R, Ayada T, et al. Spreds are essential for embryonic lymphangiogenesis by regulating vascular endothelial growth factor receptor 3 signaling. Mol Cell Biol. 2007;27:4541‐4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taniguchi K, Ishizaki T, Ayada T, et al. Sprouty4 deficiency potentiates Ras‐independent angiogenic signals and tumor growth. Cancer Sci. 2009;100:1648‐1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shehata HM, Khan S, Chen E, Fields PE, Flavell RA, Sanjabi S. Lack of Sprouty 1 and 2 enhances survival of effector CD8(+) T cells and yields more protective memory cells. Proc Natl Acad Sci USA. 2018;115:E8939‐E8947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. di Bari MG, Lutsiak ME, Takai S, et al. TGF‐beta modulates the functionality of tumor‐infiltrating CD8 + T cells through effects on TCR signaling and Spred1 expression. Cancer Immunol Immunother. 2009;58:1809‐1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fong CW, Chua MS, McKie AB, et al. Sprouty 2, an inhibitor of mitogen‐activated protein kinase signaling, is down‐regulated in hepatocellular carcinoma. Cancer Res. 2006;66:2048‐2058. [DOI] [PubMed] [Google Scholar]
- 33. Sirivatanauksorn Y, Sirivatanauksorn V, Srisawat C, Khongmanee A, Tongkham C. Differential expression of sprouty genes in hepatocellular carcinoma. J Surg Oncol. 2012;105:273‐276. [DOI] [PubMed] [Google Scholar]
- 34. Song K, Gao Q, Zhou J, et al. Prognostic significance and clinical relevance of Sprouty 2 protein expression in human hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2012;11:177‐184. [DOI] [PubMed] [Google Scholar]
- 35. Xu YF, Liu HD, Liu ZL, et al. Sprouty2 suppresses progression and correlates to favourable prognosis of intrahepatic cholangiocarcinoma via antagonizing FGFR2 signalling. J Cell Mol Med. 2018;22:5596‐5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhou X, Xie S, Yuan C, et al. Lower expression of SPRY4 predicts a poor prognosis and regulates cell proliferation in colorectal cancer. Cell Physiol Biochem. 2016;40:1433‐1442. [DOI] [PubMed] [Google Scholar]
- 37. Feng YH, Wu CL, Tsao CJ, et al. Deregulated expression of sprouty2 and microRNA‐21 in human colon cancer: correlation with the clinical stage of the disease. Cancer Biol Ther. 2011;11:111‐121. [DOI] [PubMed] [Google Scholar]
- 38. Holgren C, Dougherty U, Edwin F, et al. Sprouty‐2 controls c‐Met expression and metastatic potential of colon cancer cells: sprouty/c‐Met upregulation in human colonic adenocarcinomas. Oncogene. 2010;29:5241‐5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang Q, Wei T, Shim K, et al. Atypical role of sprouty in colorectal cancer: sprouty repression inhibits epithelial‐mesenchymal transition. Oncogene. 2016;35:3151‐3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu Y, Yang X, Li Z, et al. Sprouty2 correlates with favorable prognosis of gastric adenocarcinoma via suppressing FGFR2‐induced ERK phosphorylation and cancer progression. Oncotarget. 2017;8:4888‐4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. He Y, Ge Y, Jiang M, et al. MiR‐592 promotes gastric cancer proliferation, migration, and invasion through the PI3K/AKT and MAPK/ERK signaling pathways by targeting Spry2. Cell Physiol Biochem. 2018;47:1465‐1481. [DOI] [PubMed] [Google Scholar]
- 42. Kwabi‐Addo B, Wang J, Erdem H, et al. The expression of Sprouty1, an inhibitor of fibroblast growth factor signal transduction, is decreased in human prostate cancer. Cancer Res. 2004;64:4728‐4735. [DOI] [PubMed] [Google Scholar]
- 43. Fritzsche S, Kenzelmann M, Hoffmann MJ, et al. Concomitant down‐regulation of SPRY1 and SPRY2 in prostate carcinoma. Endocr Relat Cancer. 2006;13:839‐849. [DOI] [PubMed] [Google Scholar]
- 44. Wang J, Thompson B, Ren C, Ittmann M, Kwabi‐Addo B. Sprouty4, a suppressor of tumor cell motility, is down regulated by DNA methylation in human prostate cancer. Prostate. 2006;66:613‐624. [DOI] [PubMed] [Google Scholar]
- 45. Lo TL, Yusoff P, Fong CW, et al. The ras/mitogen‐activated protein kinase pathway inhibitor and likely tumor suppressor proteins, sprouty 1 and sprouty 2 are deregulated in breast cancer. Cancer Res. 2004;64:6127‐6136. [DOI] [PubMed] [Google Scholar]
- 46. Faratian D, Sims AH, Mullen P, et al. Sprouty 2 is an independent prognostic factor in breast cancer and may be useful in stratifying patients for trastuzumab therapy. PLoS ONE. 2011;6:e23772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Velasco A, Pallares J, Santacana M, et al. Promoter hypermethylation and expression of sprouty 2 in endometrial carcinoma. Hum Pathol. 2011;42:185‐193. [DOI] [PubMed] [Google Scholar]
- 48. Masoumi‐Moghaddam S, Amini A, Ehteda A, Wei AQ, Morris DL. The expression of the Sprouty 1 protein inversely correlates with growth, proliferation, migration and invasion of ovarian cancer cells. J Ovarian Res. 2014;7:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Masoumi‐Moghaddam S, Amini A, Wei AQ, Robertson G, Morris DL. Sprouty 2 protein, but not Sprouty 4, is an independent prognostic biomarker for human epithelial ovarian cancer. Int J Cancer. 2015;137:560‐570. [DOI] [PubMed] [Google Scholar]
- 50. Sutterluty H, Mayer CE, Setinek U, et al. Down‐regulation of Sprouty2 in non‐small cell lung cancer contributes to tumor malignancy via extracellular signal‐regulated kinase pathway‐dependent and ‐independent mechanisms. Mol Cancer Res. 2007;5:509‐520. [DOI] [PubMed] [Google Scholar]
- 51. Cidre‐Aranaz F, Grunewald TG, Surdez D, et al. EWS‐FLI1‐mediated suppression of the RAS‐antagonist Sprouty 1 (SPRY1) confers aggressiveness to Ewing sarcoma. Oncogene. 2017;36:766‐776. [DOI] [PubMed] [Google Scholar]
- 52. Shukla A, Rai K, Shukla V, et al. Sprouty 2: a novel attenuator of B‐cell receptor and MAPK‐Erk signaling in CLL. Blood. 2016;127:2310‐2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kayser S, Feszler M, Krzykalla J, et al. Clinical impact of KMT2C and SPRY4 expression levels in intensively treated younger adult acute myeloid leukemia patients. Eur J Haematol. 2017;99:544‐552. [DOI] [PubMed] [Google Scholar]
- 54. Yoshida T, Hisamoto T, Akiba J, et al. Spreds, inhibitors of the Ras/ERK signal transduction, are dysregulated in human hepatocellular carcinoma and linked to the malignant phenotype of tumors. Oncogene. 2006;25:6056‐6066. [DOI] [PubMed] [Google Scholar]
- 55. Ji JS, Xu M, Song JJ, et al. Inhibition of microRNA‐126 promotes the expression of Spred1 to inhibit angiogenesis in hepatocellular carcinoma after transcatheter arterial chemoembolization: in vivo study. Onco Targets Ther. 2016;9:4357‐4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang J, Wang W, Li J, Wu L, Song M, Meng Q. miR182 activates the Ras‐MEK‐ERK pathway in human oral cavity squamous cell carcinoma by suppressing RASA1 and SPRED1. Onco Targets Ther. 2017;10:667‐679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sharma P, Saraya A, Sharma R. Potential diagnostic implications of miR‐144 overexpression in human oesophageal cancer. Indian J Med Res. 2016;143:S91‐S103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kachroo N, Valencia T, Warren AY, Gnanapragasam VJ. Evidence for downregulation of the negative regulator SPRED2 in clinical prostate cancer. Br J Cancer. 2013;108:597‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jiang CF, Shi ZM, Li DM, et al. Estrogen‐induced miR‐196a elevation promotes tumor growth and metastasis via targeting SPRED1 in breast cancer. Mol Cancer. 2018;17:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pasmant E, Gilbert‐Dussardier B, Petit A, et al. SPRED1, a RAS MAPK pathway inhibitor that causes Legius syndrome, is a tumour suppressor downregulated in paediatric acute myeloblastic leukaemia. Oncogene. 2015;34:631‐638. [DOI] [PubMed] [Google Scholar]
- 61. Ma XN, Liu XY, Yang YF, et al. Regulation of human hepatocellular carcinoma cells by Spred2 and correlative studies on its mechanism. Biochem Biophys Res Comm. 2011;410:803‐808. [DOI] [PubMed] [Google Scholar]
- 62. Momeny M, Khorramizadeh MR, Ghaffari SH, et al. Effects of silibinin on cell growth and invasive properties of a human hepatocellular carcinoma cell line, HepG‐2, through inhibition of extracellular signal‐regulated kinase 1/2 phosphorylation. Eur J Pharmacol. 2008;591:13‐20. [DOI] [PubMed] [Google Scholar]
- 63. Luna J, Boni J, Cuatrecasas M, et al. DYRK1A modulates c‐MET in pancreatic ductal adenocarcinoma to drive tumour growth. Gut. 2018. In press. [DOI] [PubMed] [Google Scholar]
- 64. Zhao Q, Chen S, Zhu Z, et al. miR‐21 promotes EGF‐induced pancreatic cancer cell proliferation by targeting Spry2. Cell Death Dis. 2018;9:1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Feng YH, Wu CL, Shiau AL, et al. MicroRNA‐21‐mediated regulation of Sprouty2 protein expression enhances the cytotoxic effect of 5‐fluorouracil and metformin in colon cancer cells. Int J Mol Med. 2012;29:920‐926. [DOI] [PubMed] [Google Scholar]
- 66. Barbachano A, Fernandez‐Barral A, Pereira F, et al. SPROUTY‐2 represses the epithelial phenotype of colon carcinoma cells via upregulation of ZEB1 mediated by ETS1 and miR‐200/miR‐150. Oncogene. 2016;35:2991‐3003. [DOI] [PubMed] [Google Scholar]
- 67. Li Y, Chen H, She P, et al. microRNA‐23a promotes cell growth and metastasis in gastric cancer via targeting SPRY2‐mediated ERK signaling. Oncol Lett. 2018;15:8433‐8441. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68. Zhang E, Han L, Yin D, et al. H3K27 acetylation activated‐long non‐coding RNA CCAT1 affects cell proliferation and migration by regulating SPRY4 and HOXB13 expression in esophageal squamous cell carcinoma. Nucleic Acids Res. 2017;45:3086‐3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ahn JH, Han BI, Lee M. Induction of resistance to BRAF inhibitor is associated with the inability of Spry2 to inhibit BRAF‐V600E activity in BRAF mutant cells. Biomol Ther (Seoul). 2015;23:320‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liu Z, Liu X, Cao W, Hua ZC. Tumor‐specifically hypoxia‐induced therapy of SPRY1/2 displayed differential therapeutic efficacy for melanoma. Am J Cancer Res. 2015;5:792‐801. [PMC free article] [PubMed] [Google Scholar]
- 71. Haydn JM, Hufnagel A, Grimm J, Maurus K, Schartl M, Meierjohann S. The MAPK pathway as an apoptosis enhancer in melanoma. Oncotarget. 2014;5:5040‐5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ablain J, Xu M, Rothschild H, et al. Human tumor genomics and zebrafish modeling identify SPRED1 loss as a driver of mucosal melanoma. Science. 2018;362:1055‐1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Patel R, Gao M, Ahmad I, et al. Sprouty2, PTEN, and PP2A interact to regulate prostate cancer progression. J Clin Invest. 2013;123:1157‐1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Assinder SJ, Beniamen D, Lovicu FJ. Cosuppression of Sprouty and Sprouty‐related negative regulators of FGF signalling in prostate cancer: a working hypothesis. Biomed Res Int. 2015;2015:827462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. He Q, Jing H, Liaw L, et al. Suppression of Spry1 inhibits triple‐negative breast cancer malignancy by decreasing EGF/EGFR mediated mesenchymal phenotype. Sci Rep. 2016;6:23216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Koledova Z, Zhang X, Streuli C, et al. SPRY1 regulates mammary epithelial morphogenesis by modulating EGFR‐dependent stromal paracrine signaling and ECM remodeling. Proc Natl Acad Sci USA. 2016;113:E5731‐E5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. So WK, Cheng JC, Fan Q, et al. Loss of Sprouty2 in human high‐grade serous ovarian carcinomas promotes EGF‐induced E‐cadherin down‐regulation and cell invasion. FEBS Lett. 2015;589:302‐309. [DOI] [PubMed] [Google Scholar]
- 78. Masoumi‐Moghaddam S, Amini A, Wei AQ, Robertson G, Morris DL. Sprouty2 protein in prediction of post‐treatment ascites in epithelial ovarian cancer treated with adjuvant carbotaxol chemotherapy. Am J Cancer Res. 2015;5:2498‐2507. [PMC free article] [PubMed] [Google Scholar]
- 79. Kitai H, Ebi H, Tomida S, et al. Epithelial‐to‐mesenchymal transition defines feedback activation of receptor tyrosine kinase signaling induced by MEK inhibition in KRAS‐mutant lung cancer. Cancer Discov. 2016;6:754‐769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Taniguchi H, Yamada T, Wang R, et al. AXL confers intrinsic resistance to osimertinib and advances the emergence of tolerant cells. Nat Commun. 2019;10:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Park JW, Wollmann G, Urbiola C, et al. Sprouty2 enhances the tumorigenic potential of glioblastoma cells. Neuro Oncol. 2018;20:1044‐1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Walsh AM, Kapoor GS, Buonato JM, et al. Sprouty2 drives drug resistance and proliferation in glioblastoma. Mol Cancer Res. 2015;13:1227‐1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yuan ZS, Cao Y, Li ZY. IGFBP2 induces SPRY1 expression via NF‐kappaB signaling pathway in glioblastoma multiforme (GBM). Eur Rev Med Pharmacol Sci. 2017;21:5072‐5080. [DOI] [PubMed] [Google Scholar]
- 84. Saini M, Verma A, Mathew SJ. SPRY2 is a novel MET interactor that regulates metastatic potential and differentiation in rhabdomyosarcoma. Cell Death Dis. 2018;9:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Miyoshi K, Wakioka T, Nishinakamura H, et al. The Sprouty‐related protein, Spred, inhibits cell motility, metastasis, and Rho‐mediated actin reorganization. Oncogene. 2004;23:5567‐5576. [DOI] [PubMed] [Google Scholar]
- 86. Wang JH, Zheng WW, Cheng ST, Liu BX, Liu FR, Song JQ. Correlation between microRNA21 and sprouty homolog 2 gene expression in multiple myeloma. Mol Med Rep. 2015;11:4220‐4224. [DOI] [PMC free article] [PubMed] [Google Scholar]


