Abstract
Lymphatic vessels are known to be derived from veins; however, recent lineage‐tracing experiments propose that specific lymphatic networks may originate from both venous and non‐venous sources. Despite this, direct evidence of a non‐venous lymphatic progenitor is missing. Here, we show that the zebrafish facial lymphatic network is derived from three distinct progenitor populations that add sequentially to the developing facial lymphatic through a relay‐like mechanism. We show that while two facial lymphatic progenitor populations are venous in origin, the third population, termed the ventral aorta lymphangioblast (VA‐L), does not sprout from a vessel; instead, it arises from a migratory angioblast cell near the ventral aorta that initially lacks both venous and lymphatic markers, and contributes to the facial lymphatics and the hypobranchial artery. We propose that sequential addition of venous and non‐venous progenitors allows the facial lymphatics to form in an area that is relatively devoid of veins. Overall, this study provides conclusive, live imaging‐based evidence of a non‐venous lymphatic progenitor and demonstrates that the origin and development of lymphatic vessels is context‐dependent.
Keywords: angioblast, lymphangiogenesis, lymphatic origin, lymphatic specification, Vegfr3 signalling
Subject Categories: Development & Differentiation, Vascular Biology & Angiogenesis
Introduction
Lymphatic vessel development begins through a step‐wise process wherein lymphatic progenitors are specified, migrate and then coalesce to form nascent lymphatic vessels. Prospero‐related homeodomain transcription factor (PROX)1 is the master regulator of lymphatic endothelial cell (LEC) fate and is necessary for lymphatic progenitor specification in mammals 1, 2. Following specification, lymphatic progenitors sprout from the veins by vascular endothelial growth factor receptor (VEGFR)‐3 signalling through its ligand, vascular endothelial growth factor (VEGF)‐C 3, 4, 5. While various models of venous lymphatic sprouting have been proposed, including the “ballooning” mechanism, whereby lymphangioblasts (migratory lymphatic progenitor cells) collectively sprout as several small sacs 6, and the “budding” mechanism where lymphangioblasts bud off as loosely interconnected cells 5, 7, the exact mechanism by which lymphangioblasts coalesce into mature lymphatic vessels remains unclear.
Although veins are currently thought to be the predominant source of lymphatics, historically there have been two opposing models describing the origin of lymphatic progenitors. The first model, proposed by Florence Sabin, suggested that lymphatics sprout from the pre‐existing venous endothelium 8, while the model proposed by Huntington and McClure suggested that perivascular lymphangioblasts in the mesenchyme fuse to form isolated lymphatic vessels that later establish a connection to the venous system 9, 10. Lineage‐tracing experiments, particularly in mice and zebrafish, have largely supported Sabin's model, and until recently, veins were considered the sole contributor of the lymphatic vasculature in fish and mammals 11, 12. However, there is also evidence for a hybrid of these two models, wherein both venous and non‐venous cells provide lymphatic progenitors. This “dual origin” model for lymphatics was first conceived 85 years ago when the anterior lymph sacs of the sea turtle were observed to have both a venous and mesenchymal origin 13, and this model is now supported by contemporary experiments in chick 14, 15 and Xenopus 16. Furthermore, recent lineage‐tracing studies in mice have suggested that part of the cardiac, dermal and mesenteric lymphatic networks are formed by independent lymphangioblast clusters that appear to be non‐venous in origin 17, 18, 19. However, much of the recent evidence for non‐venous lymphatic progenitors comes from genetic lineage tracing using Cre‐LoxP recombination. As such, these results rely on complete Cre‐mediated recombination and lineage‐specific Cre activity 20, 21. Limitations in this technique have resulted in contradictory data regarding the vascular origin of non‐venous progenitors in both the mouse dermal 19, 22 and cardiac lymphatics 17, 20. Taken together, these various studies provide sometimes contradictory evidence that differentiated veins may not be the only progenitor source contributing to lymphatic development and direct evidence of a non‐venous lymphatic progenitor still remains elusive.
The zebrafish has proven to be an invaluable tool for understanding lymphatic development, as their transparent embryos allow for high‐resolution, in vivo live imaging of developing lymphatic vessels 11, 23. Similar to mammals, venous‐derived lymphatic progenitors in zebrafish can be identified through Prox1 expression 24, 25 and lymphatic sprouting is dependent on Vegfr3 (known as Flt4 in zebrafish) signalling 26, 27, 28. There are also some discrepancies between the two animal models; for example, Vegfr3 signalling is required for lymphatic specification, via Prox1 induction, in the zebrafish trunk 25, 29, despite having no effect on initial lymphatic specification in mice 3, 5, 30, 31. However, there still exists a relationship between VEGFR‐3 and PROX1 in mammals, as the two interact via a positive feedback loop in order to maintain the lymphatic identity of LECs 31. Interestingly, a recent study has suggested that, similar to mammals, Vegfr3 activity may be dispensable for facial lymphatic specification in the zebrafish 29. Further work, however, is required to understand why this distinction exists between trunk and facial lymphatic specification.
The complex facial lymphatic network in zebrafish was first described in 2012 32, and initial characterisation of this network showed that while the facial lymphatic sprout (FLS) arises from the common cardinal vein (CCV), it subsequently appears to acquire lymphatic progenitors from two other sources: firstly from another facial vein, called the primary head sinus (PHS), and secondly from a population of highly migratory lyve1b‐positive cells termed the ventral aorta lymphangioblast (VA‐L). The VA‐L was first observed near the ventral aorta, and currently, there is no clear evidence that it is derived from a vein 32. The facial lymphatics are therefore an ideal vascular network to investigate whether a complex lymphatic network can form from distinct venous and non‐venous progenitor populations.
In this study, we identify with single‐cell resolution a definitive, non‐venous lymphatic progenitor in zebrafish. We confirm that the zebrafish facial lymphatic is formed from three distinct Prox1‐positive lymphatic progenitor populations within the CCV, PHS and VA‐L. Lymphangioblast contributions from these populations are tightly regulated at both a spatial and temporal level. The facial lymphatic forms through a relay‐like mechanism, where vessel migration involves the sequential addition of lymphangioblasts to the growing vascular tip; first from the CCV, then the PHS and finally the VA‐L. Finally, we also provide evidence that the VA‐L does not have a venous origin; instead, it arises as an angioblast population that forms near the ventral aorta. This work supports the “dual origin” theory of lymphatic vessel development by providing evidence of a lymphatic progenitor that is not derived from an existing vessel.
Results
Early facial lymphatic development requires lymphangioblast contributions from anatomically distinct sources
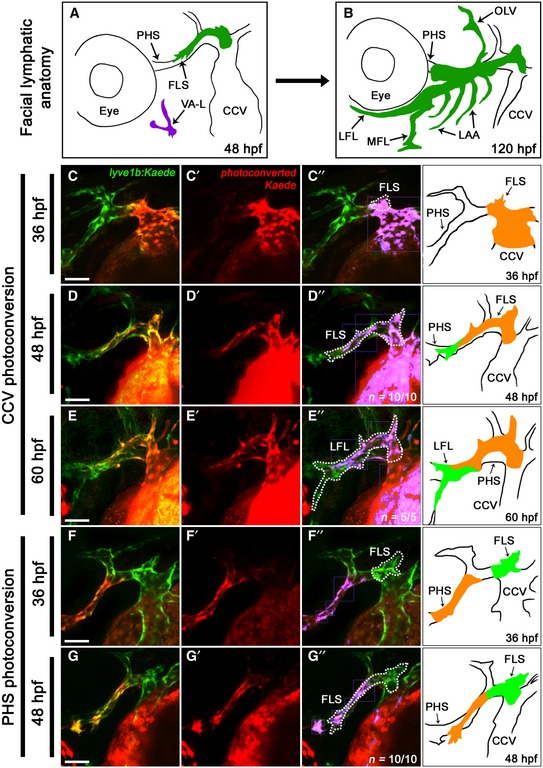
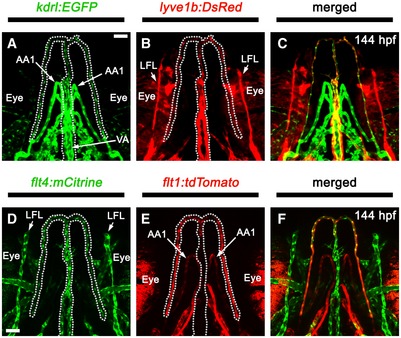
Previous time‐lapse studies suggested that the initial CCV‐derived FLS appeared to recruit lymphangioblasts (defined here as migratory lymphatic progenitor cells) from two other sources: the PHS, between 42 and 48 hpf, and the VA‐L, between 54 and 60 hpf 32 (Fig 1A and B). However, the manner in which these distinct lymphangioblast populations were coalescing to form the facial lymphatic network remained unclear. We generated a photoconvertible lyve1b:Kaede transgenic which recapitulated the expression of previously generated lyve1b:EGFP nz101 transgenic (Appendix FigS1A–C). While lyve1b:Kaede expression is initially weak at 36 hpf, photoconversion at this time allowed us to trace ~96% of lyve1b‐positive cell populations to at least 24 h beyond the initial photoconversion of Kaede (Appendix Fig S2A–C and G). By photoconverting either the CCV or the PHS at 36 hpf, we confirmed that the CCV is the first to contribute lymphangioblasts to the initial facial lymphatic sprout (FLS) at 36 hpf (Fig 1C). By 48 hpf, lymphangioblast contributions from the CCV did not extend to the distal tip of the FLS, comprising only the proximal portion of the FLS, and this is still evident at 60 hpf (Fig 1D and E; Movie EV1). At this time, the remainder of the FLS is derived from the PHS (Fig 1F and G; Movie EV2). Photoconversion of the dorsal half of the VA‐L at 54 hpf confirmed that this cell population provides further lymphangioblasts to the tip of the developing lateral facial lymphatic (LFL) by 72 hpf (Fig 2A and B). However, the ventral portion of the VA‐L does not contribute to facial lymphatic development; instead, this migrates anteromedially and fuses to its contralateral counterpart along the ventral midline to form the anterior end and lateral branches of the hypobranchial artery (HA; Fig 2C and D). This suggests that despite its name, the VA‐L is not entirely comprised of lymphangioblasts as it also contributes to blood vessel development. The HA carries blood from the first aortic arch, through the jaw and back to the sinus venosus 33, 34. Using both venous (lyve1b:DsRed or flt4:mCitrine) and arterial (flt1:tdTomato) enriched transgenes, we observed that the HA displays both arterial and venous identities (Fig EV1A–F). However, the medial portion of the HA, which joins to the sinus venosus, displays higher levels of venous transgene expression (Fig EV1B and D), whereas the arterial‐enriched transgene is more strongly expressed in the bifurcating lateral branches, which join to the aortic arches and are contributed by the ventral VA‐L (Fig EV1E). Overall, we confirmed via lineage tracing that three distinct sources, CCV‐derived lymphangioblasts (CCV‐Ls) at 36 hpf, PHS‐derived lymphangioblasts (PHS‐Ls) by 48 hpf and the VA‐L by 72 hpf, contribute to the developing FLS.
Figure 1. Early facial lymphatic development requires lymphangioblast contributions from anatomically distinct vessels.
-
A, BSchematic diagrams of the zebrafish facial lymphatic and cranial vessel anatomy at 48 hpf (A) and 120 hpf (B).
-
C–G’’Lateral images of the cranial vessels in lyve1b:Kaede transgenic embryos with the CCV photoconverted at 36 hpf (C) and followed through to 48 hpf (D) and 60 hpf (E), while in a separate larva, the PHS has been photoconverted at 36 hpf (F) and followed to 48 hpf (G). Unconverted vessels are shown in green (C–G), while photoconverted vessels are shown in red (C’–G’). The extent of lymphangioblast contribution from each source is further clarified by false colouring the overlap between red and green fluorescence (purple), with the FLS demarcated (dotted line) from the adjacent primary veins (C’’–G’’). Schematic included for anatomical reference.
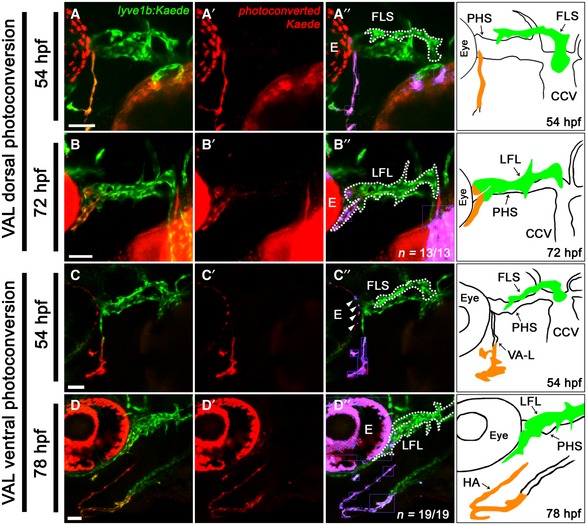
Figure 2. The VA‐L contributes progenitors to both the facial lymphatic and the hypobranchial artery.
-
A–D’’Ventrolateral (A, C, D) and lateral (B) images of the cranial vessels in lyve1b:Kaede transgenic embryos with the dorsal (A) and ventral (C) portions of the VA‐L photoconverted at 54 hpf and followed through to 72 hpf (B) and 78 hpf (D). Unconverted vessels are shown in green (A–D), while photoconverted vessels are shown in red (A’–D’). The extent of lymphatic and arterial contribution from the VA‐L to the LFL (B’’) or HA (D’’) is further clarified by false colouring the overlap between red and green fluorescence (purple), with the FLS (A’’, C’’) and LFL (B’’, D’’) demarcated (dotted line) from the adjacent vessels. Schematic included for anatomical reference. Note, for all images, the overlap of red and green fluorescence in the eye (labelled E) is due to a combination of natural pigmentation and auto‐fluorescence and is not indicative of photoconverted cells (C’’, white arrowheads).
Figure EV1. The HA expresses both venous and arterial markers.
-
A–FVentral images of the pharyngeal vessels at 144 hpf in either a kdrl:EGFP;lyve1b:DsRed compound transgenic (A–C) or a flt:tdTomato;flt4:mCitrine compound transgenic (D–F). The HA (dotted line) expresses kdrl:EGFP (A), lyve1b:DsRed (B), flt4:mCitrine (D) and flt1:tdTomato (E). Stronger expression of the venous‐enriched transgenes lyve1b:DsRed (B; red) and flt4:mCitrine (D; green) is seen in the anterior and medial HA, whereas the arterial‐enriched transgene flt1:tdTomato (E; red) is more strongly expressed in the bifurcating lateral branches.
At 72 hpf, once the FLS has fused to the VA‐L, it is termed the lateral facial lymphatic (LFL). Subsequently, the otolithic lymphatic vessel (OLV) sprouts dorsally from the LFL and develops posterior to the otolith between 60 and 84 hpf, while the medial facial lymphatic (MFL) and lymphatic branchial arches (LAAs) sprout ventromedially from the LFL by 96 hpf (Fig 1B). To determine whether there were any additional lymphangioblast sources contributing to the development of the OLV and MFL, we photoconverted the LFL at either 60 or 72 hpf. This revealed that, up to 120 hpf, the OLV (Fig EV2A and B) and the MFL (Fig EV2C and D) are solely derived from the LFL. In addition, the LFL requires no further lymphangioblasts to complete its extension towards the jaw (Fig EV2E and F). Taken together, late facial lymphatic development appears to rely solely on existing lymphangioblasts within the LFL to form the OLV and MFL, and to complete the growth of the LFL.
Figure EV2. Development of the OLV and MFL, and further extension of the LFL is derived from cells within the LFL .
-
A–F’’Lateral (A, B) and ventrolateral (C–F) images of the cranial vessels in lyve1b:Kaede transgenic embryos with the entire LFL photoconverted at 60 hpf (A) and followed to 84 hpf (B), the LFL tip photoconverted at 72 hpf (C) and followed to 120 hpf (D) and the subocular lymph sac (a lyve1b expressing structure under the eye) photoconverted at 72 hpf (E) and followed to 120 hpf (F). Unconverted tissues are shown in green (A–F), while photoconverted tissues are shown in red (A’–F’). The extent of lymphangioblast contribution from each source is further clarified by false colouring the overlap between red and green fluorescence (purple), with the LFL (A’’, C’’, E’’, F’’), OLV (B”) and MFL (D’’) demarcated (dotted line) from the adjacent vessels. Accompanying schematic included for anatomical reference (A’’–F’’).
Spatiotemporal distribution of facial lymphatic progenitors
Given that distinct lymphangioblast populations contribute to the developing facial lymphatic, we next wanted to investigate how lymphatic progenitor populations are distributed and patterned throughout the head prior to forming the FLS. Using Prox1 immunofluorescence staining in a lyve1b:EGFP transgenic background, we were able to identify Prox1+/lyve1b + lymphatic progenitors. At the onset of facial lymphatic development (36 hpf), lymphatic progenitors are focused to a single point in the dorsolateral region of the CCV (Fig 3A). These then transition into migrating lymphangioblasts by 42 hpf, forming the initial FLS. In addition, Prox1 expression in CCV lymphangioblasts (CCV‐Ls) appears to be polarised, with cells closer to the growing tip displaying a higher level of Prox1 expression than those closer to the CCV (Fig 3B). By contrast, lymphatic progenitors found in the PHS at 36 hpf are initially positioned along the entire length of the vein in an interspersed manner (Fig 3C). Six hours later, these cells form two distinct lymphatic progenitor domains: one at the anterior end of the PHS, the anterior PHS‐derived lymphatic progenitor domain (PHS‐LPA), and one posterior to this, the posterior PHS‐derived lymphatic progenitor domain (PHS‐LPP; Fig 3D). By 48 hpf, PHS‐LPP, the domain closest to the CCV, has sprouted from the PHS and become part of the FLS (Fig 3E). Unlike the CCV‐L and PHS‐Ls, the VA‐L is Prox1‐negative at 36 hpf (Fig 3F), not acquiring lymphangioblast identity until 42 hpf when Prox1 expression becomes evident (Fig 3G). Later at 48 hpf, only the dorsal portion of the VA‐L that contributes to the facial lymphatics contains Prox1‐positive lymphangioblasts, while the ventral portion that contributes to HA formation remains Prox1‐negative (Fig 3H). Altogether, the distinct spatiotemporal pattern of lymphatic progenitors in the CCV, PHS and VA‐L confirms their roles in contributing lymphangioblasts to the growing facial lymphatic.
Figure 3. Spatiotemporal distribution of lymphatic progenitors in the cranial vessels.
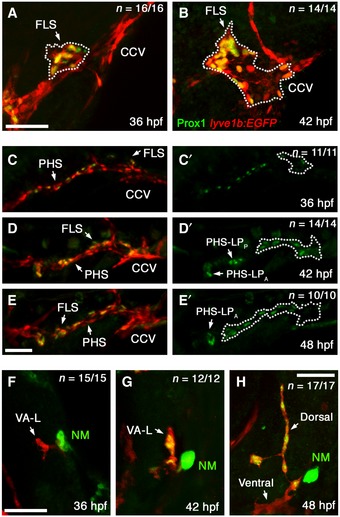
-
A–HLateral images of the CCV fixed at 36 hpf (A) and 42 hpf (B), of the PHS at 36 hpf (C), 42 hpf (D) and 48 hpf (E) and of the VA‐L at 36 hpf (F), 42 hpf (G) and 48 hpf (H) in lyve1b:EGFP fish that have been fluorescently immunostained with anti‐PROX1 (green) and anti‐GFP (red). PHS Prox1 staining shown alone for all timepoints (C’–E’) with the FLS demarcated (dotted line) from the adjacent vessels, and the positions of the PHS‐LPP and PHS‐LPA indicated. Note the Prox1‐positive (green), GFP‐negative structure near to the VA‐L is a prox1‐expressing neuromast (NM).
Vegfr3 signalling is required for lymphatic progenitor domain formation in the PHS but not the CCV or the VA‐L
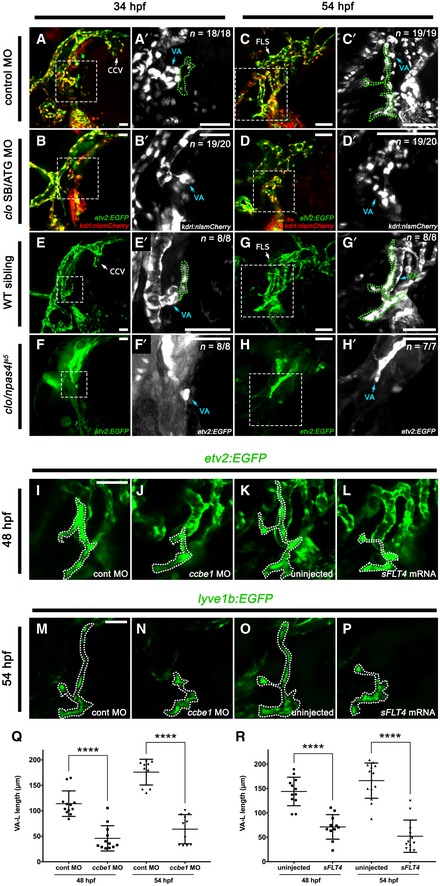
We wanted to compare the role of Vegfr3 signalling on lymphatic specification in the CCV with that of the PHS, including its effect on the formation of the PHS‐LPA and PHS‐LPP domains. In order to disrupt the Vegfr3 signalling axis, we analysed either ccbe1 morphants or embryos injected with a dominant negative inhibitor of Vegfr3 signalling (sFLT4 mRNA) 35, approaches previously shown to inhibit FLS formation 28. We confirmed the efficacy of our knockdowns by quantitating the length of the FLS at 48 hpf and observed robust inhibition of sprouting following injection of either ccbe1 MO or sFLT4 mRNA (Appendix Fig S3A–F). We found that while both ccbe1 morphants and sFLT4 mRNA‐injected embryos displayed a marked reduction in the number of PHS Prox1‐positive lymphatic progenitors at 36 hpf (Fig 4B, F, I and J) and 42 hpf (Fig 4D, H, I and J) compared to controls (Fig 4A, C, E, G, I and J), they nevertheless still had Prox1‐positive cells present in the PHS, indicating that some lymphatic specification had occurred. Importantly, the total numbers of PHS endothelial cells (ECs) in either ccbe1 morpholino or sFLT4‐injected fish were unchanged when compared to control morpholino‐injected and uninjected larvae (Fig 4K), indicating that blood vascular development proceeded normally. Of note, at 42 hpf, ccbe1 morphants and sFLT4‐injected embryos failed to form the distinctive PHS‐LP domains (Fig 4D and H). By contrast, although ccbe1 MO or sFLT4 injections inhibited facial lymphatic sprouting at 36 hpf—evident by the reduced number of cells in the dorsolateral CCV region (Fig 4Q) and inhibition of FLS development (Appendix Fig S3)—it did not reduce the percentage of dorsolateral CCV ECs that are Prox1‐positive lymphatic progenitors compared to controls (Fig 4L–P), which is consistent with what is seen in flt4 mutants 29. In addition, the VA‐L in ccbe1 morphants and sFLT4‐injected embryos also remained Prox1‐positive at 54 hpf (Fig 4R–U), indicating that, like lymphatic progenitors in the CCV, Vegfr3 signalling is not required for the initial induction and maintenance of Prox1 expression in the VA‐L. Taken together, these results suggest that Vegfr3 signalling is required for the formation of lymphatic progenitor domains within the PHS, but not for the initial specification of facial lymphatic progenitors.
Figure 4. Vegfr3 signalling is not required for facial lymphatic progenitor formation.
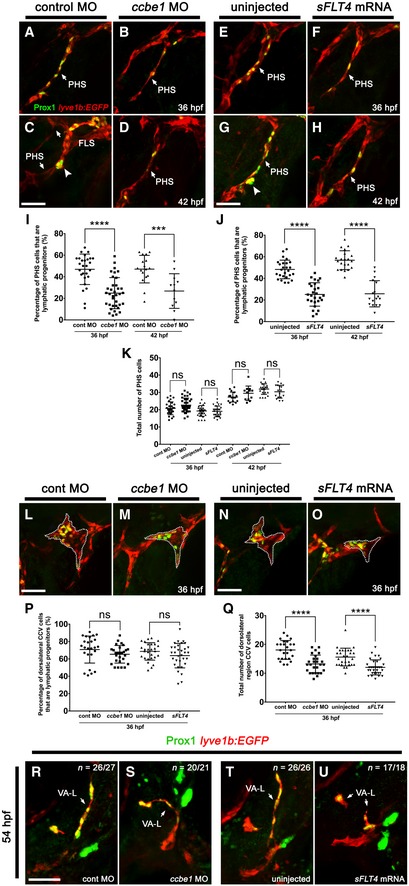
-
A–HLateral images of the PHS fixed at 36 hpf (A, B, E, F) and 42 hpf (C, D, G, H) in lyve1b:EGFP fish that have been fluorescently immunostained with anti‐PROX1 (green) and anti‐GFP (red). These show a decrease in the number of Prox1‐positive PHS ECs in the ccbe1 morpholino‐injected (B, D) and sFLT4 mRNA‐injected embryos (F, H) compared with control morpholino‐injected (A, C) and uninjected embryos (E, G). At 42 hpf, ccbe1 morpholino‐injected embryos (D) and sFLT4 mRNA‐injected embryos (H) lack the PHS‐LP domains present in the control embryos (C, G; large arrow heads).
-
I, JQuantitation of the percentage of PHS ECs that are lymphatic progenitors relative to the total number of PHS ECs in control morpholino‐injected embryos at 36 hpf (n = 27) and 42 hpf (n = 17) and ccbe1 morpholino‐injected embryos at 36 hpf (n = 36) and 42 hpf (n = 12) (I) or in uninjected embryos at 36 hpf (n = 27) and 42 hpf (n = 21) and sFLT4 mRNA‐injected embryos at 36 hpf (n = 26) and 42 hpf (n = 17) (J).
-
KQuantitation of the total number of PHS ECs in control morpholino‐injected embryos at 36 hpf (n = 27) and 42 hpf (n = 17) and ccbe1 morpholino‐injected embryos at 36 hpf (n = 36) and 42 hpf (n = 12) or in uninjected embryos at 36 hpf (n = 27) and 42 hpf (n = 21) and sFLT4 mRNA‐injected embryos at 36 hpf (n = 26) and 42 hpf (n = 17).
-
L–OLateral images of the FLS (L, N; dotted line) or dorsolateral CCV region from where the FLS normally sprouts (M, O; dotted line) fixed at 36 hpf in lyve1b:EGFP embryos that have been fluorescently immunostained with anti‐PROX1 (green) and anti‐GFP (red). These show no change in the number of Prox1‐positive lymphatic progenitors in the ccbe1 morpholino‐injected (M) and sFLT4 mRNA‐injected (O) embryos compared with control morpholino‐injected (L) and uninjected (N) embryos.
-
PQuantitation of the percentage of dorsolateral CCV ECs that are lymphatic progenitors relative to the total number of dorsolateral CCV ECs in control morpholino‐injected (n = 26), ccbe1 morpholino‐injected (n = 27), uninjected (n = 28) and sFLT4 mRNA‐injected embryos (n = 32).
-
QQuantitation of the total number of dorsolateral region CCV ECs in 36 hpf embryos that were uninjected or injected with control morpholino (n = 26), ccbe1 morpholino (n = 27) or sFLT4 mRNA (n = 32).
-
R–UDorsolateral images of the VA‐L fixed at 54 hpf in lyve1b:EGFP larva that have been fluorescently immunostained with anti‐PROX1 (green) and anti‐GFP (red). These show that lymphatic progenitor formation still occurs within the VA‐L in ccbe1 morpholino‐injected (S) and sFLT4 mRNA‐injected embryos (U), with Prox1 expression resembling that of controls (R, T).
The early facial lymphatic forms through the sequential addition of lymphangioblasts to the growing tip
Given that lymphangioblast populations from the CCV, PHS and the VA‐L come together to form the FLS, we wished to examine the process in real time to better understand the migratory behaviour of specific lymphangioblast populations. Using time‐lapse imaging of lyve1b:EGFP;kdrl:nlsmCherry compound transgenics, we were able to track the events leading to, and after, fusion of the FLS tip—initially comprised of CCV‐Ls—to the PHS‐Ls sprouting out of the PHS‐LPP (Movie EV3), and from the FLS tip—now comprised of PHS‐Ls—to the VA‐L (Movie EV4). We also quantitated the migratory behaviour of the lymphangioblasts involved by tracking the leading cell of the CCV‐L‐derived FLS tip, the PHS‐L‐derived FLS tip and the VA‐L 3–4 h before and 3–4 h following fusion to the subsequent lymphangioblast populations. Early in FLS development, we found that the CCV‐Ls idle for some time and only migrate rapidly towards the PHS‐LPP and PHS‐LPA domains at around 42 hpf (Fig 5A; Movie EV3). As the CCV‐L‐derived FLS approaches, cells within the PHS‐LPP increase in number by undergoing 1–2 rounds of cell division before sprouting to fuse with the arriving facial lymphatic tip at 48 hpf (Fig 5B). At this juncture, the initial CCV‐Ls that were leading the FLS essentially cease migration (Fig 5C and D), and the PHS‐Ls (derived initially from the PHS‐LPP) form the distal portion of the FLS and migrate towards the VA‐L (Fig 5E and F; Movie EV4). Meanwhile, a secondary sprout from the distal end of the FLS—comprised of PHS‐LPP cells—fuses to the PHS‐LPA domain by 52 hpf (Fig 5E; Movie EV4). Similar to the initial CCV‐Ls, the VA‐L remains idle during the 12‐h period from the onset of facial lymphatic development to the FLS fusing to the PHS‐Ls. Subsequent to this fusion event, the VA‐L begins migrating towards the PHS‐L‐derived FLS tip, with the two populations fusing together by 55 hpf (Fig 5E; Movie EV4). Upon fusion, the PHS‐L‐derived FLS tip and the VA‐L stall migration (Fig 5C, G and H) as several rounds of cell division occur (Fig 5F; Movie EV4), before the now LFL resumes migration along the ventral wall of the eye and towards the jaw. Further evidence supporting the timing of fusion events comes from total facial lymphatic cell count over time, which shows that although cell division plays a role in contributing to the increase in facial LECs, the greatest increase in cell number comes from the PHS‐Ls and VA‐L fusing to the FLS (Appendix Fig S4). Altogether, facial lymphatic development involves the sequential addition of lymphangioblasts, first from the CCV, then the PHS and finally the VA‐L, to the growing tip, creating a “relay‐like” mechanism which drives the formation of the facial lymphatics.
Figure 5. The early facial lymphatic forms through the sequential addition of lymphangioblasts to the growing tip.
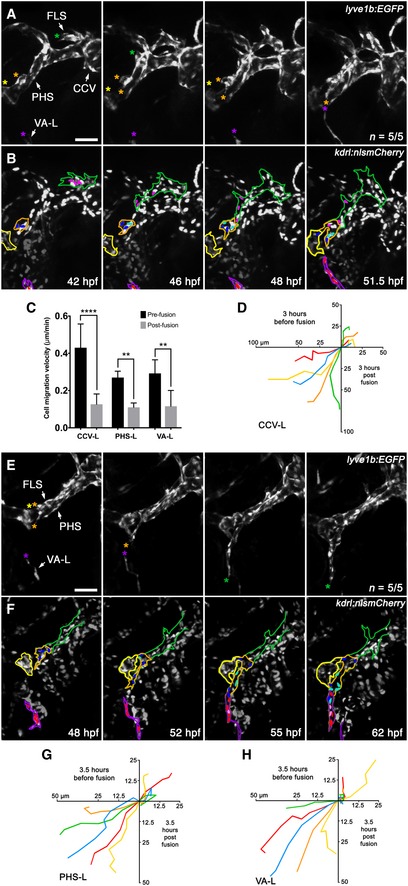
-
A, BStill images from Movie EV3 of early facial lymphatic development in a lyve1b:EGFP (A), kdrl:nlsmCherry (B) compound transgenic from 42 to 51.5 hpf, with the CCV‐L‐derived leading tip cell (green asterisk), the PHS‐LP domains (orange and yellow asterisk) and the distal tip of the VA‐L (purple asterisk) highlighted (A). Between 42 and 46 hpf, the PHS‐LPP (orange outline) cells divide (dark blue parent cells, light blue daughter cells) before sprouting as lymphangioblasts from the PHS and fusing with the tip (pink cells) of the CCV‐derived FLS (green outline) at 48 hpf (B). Cells (red) within the VA‐L (purple outline) can also be seen migrating towards the now PHS‐L‐derived FLS tip by 48 hpf. The PHS‐LPA is also shown (yellow outline).
-
CQuantitation of leading lymphangioblast velocity (μm/min) before and after fusion with another lymphangioblast [CCV‐L‐derived FLS tip (pink cells) to PHS‐L (B, blue cells; n = 5/5) and PHS‐L‐derived FLS tip (blue cells) to VA‐L (F, red cells; n = 5/5)] or by the VA‐L to the PHS‐L‐derived FLS tip (B, n = 5/5).
-
DCell migration tracks where each coloured track depicts the distance (μm) travelled over 3 h by the leading tip cell in a separate animal before and after fusion with the CCV‐L‐derived FLS tip (pink cells) and the PHS‐Ls (B, blue cells; n = 5/5).
-
E, FStill images from Movie EV4 of facial lymphatic development in a lyve1b:EGFP (C), kdrl:nlsmCherry (D) compound transgenic from 48 to 62 hpf showing that after the CCV‐derived FLS fuses to the PHS‐L, these take over as the leading tip cells (orange asterisks), with one PHS‐L migrating anteriorly to fuse with PHS‐LPA (yellow asterisk), and others migrating ventrally to fuse with the VA‐L (purple asterisk) (C). After the PHS‐derived portion (orange outline) of the FLS (green outline) fuses to the VA‐L (purple outline), migration of the entire facial lymphatic pauses to allow for lymphangioblast proliferation (dark blue and red parent cells, light blue and pink daughter cells) before resuming migration anteriorly along the ventral base of the eye (D).
-
G, HCell migration tracks where each coloured track depicts the distance (μm) travelled over 3.5 h by the leading tip cell in a separate animal before and after fusion with another lymphangioblast [PHS‐L‐derived FLS tip (blue cells) to VA‐L (F, red cells; n = 5/5)] or by the VA‐L to the PHS‐L‐derived FLS tip (F, n = 5/5).
The VA‐L does not arise from a vein
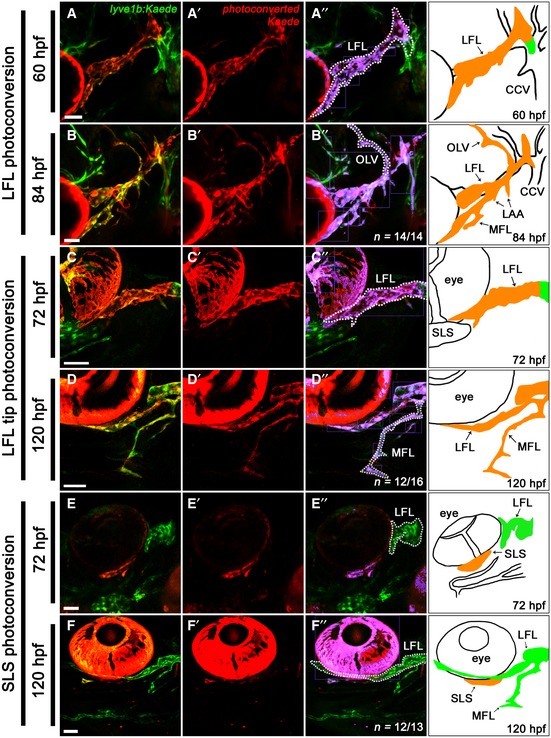
While the VA‐L provides lymphangioblasts to the developing facial lymphatic, the origin of this cell population remains unknown. As the VA‐L is lyve1b‐positive from 36 hpf onwards 32, we initially surmised that its origin could be a previously uncharacterised vein located within the vicinity of the VA. To explore this possibility, we developed a kdrl:nlsKaede transgenic to lineage trace any kdrl‐expressing structure near the VA that could give rise to the VA‐L. Our kdrl:nlsKaede transgenic recapitulated the expression of the previously published kdrl:nlsmCherrynz49 transgenic (Appendix Fig S1D–F), and photoconversion at 24 hpf allowed us to detect at least 90% of photoconverted cells 24–48 h beyond the initial photoconversion of Kaede (Appendix Fig S2D–G). We photoconverted various kdrl‐expressing tissues at 24 hpf and traced the photoconverted Kaede to 54 hpf, a timepoint when the VA‐L is clearly visible. However, with this photoconversion regimen, we were unable to trace the VA‐L progenitor from either the CCV, PHS, optic veins, endocardium, lateral dorsal aorta or pharyngeal endoderm (Fig EV3A–F and H–M). Furthermore, even when photoconverting the entire kdrl‐expressing population within the head at 24 hpf, we did not see any photoconverted cells within the VA‐L (Fig EV3G and N), suggesting that the VA‐L acquires kdrl:nlsKaede expression after this timepoint. However, we noted that at 34 hpf, there was a small cluster of 4–5 kdrl‐positive cells closely associated with the ventral region of the VA, and by photoconverting these cells at 34 hpf, we were able to identify this as the earliest visible kdrl‐positive form of the VA‐L (Fig 6A and B). We then performed time‐lapse imaging in this area between 31 and 35.8 hpf in kdrl:EGFP;kdrl:nlsmCherry compound transgenics and found that the VA‐L begins expressing kdrl:nlsmCherry between 32 and 33 hpf in a manner that was independent of any neighbouring kdrl‐expressing tissues (Fig 6C; Movie EV5); despite closely associating with the VA, the VA‐L was never seen to form a connection with the VA (Fig 6D; Movie EV6), supporting previous data that showed that the VA‐L does not express an arterially enriched transgene (flt1:YFP hu4624) 23, 32. We also investigated the possibility that myeloid cells could give rise to the VA‐L, or that they may play an indirect role in mediating its formation. We performed double morpholino knockdown of pu.1 and csfr3, a combination known to broadly inhibit myelopoiesis 36, and saw that the VA‐L forms normally after myeloid cells were depleted (Appendix Fig S5A–D). Overall, our data suggest that the VA‐L does not originate from any existing kdrl‐positive vessels or myeloid cells in this region; instead, it begins to independently express kdrl as an isolated cell population.
Figure EV3. The VA‐L does not arise from any known kdrl‐positive tissue or vessel in the head.
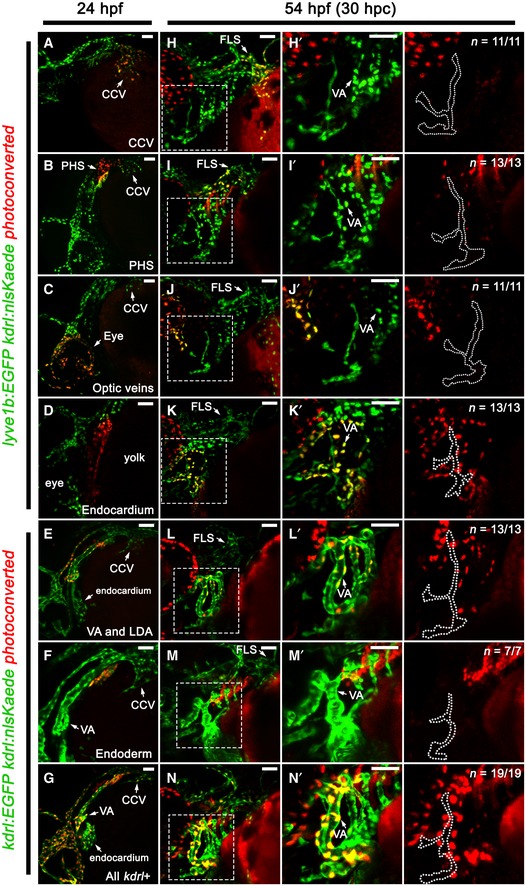
-
A–N’Lateral (A–G) and ventrolateral (H–N) images of the facial vessels in kdrl:nlsKaede;lyve1b:EGFP (A–D, H–K) or kdrl:nlsKaede;kdrl:EGFP (E–G, L–N) embryos. The CCV (A), PHS (B), optic veins (C), endocardium (D), VA, LDA (E), pharyngeal endoderm (F) and all kdrl‐positive tissues in the head (G) were photoconverted red at 24 hpf, while unconverted Kaede, and kdrl:EGFP or lyve1b:EGFP for clarity, is shown in green. All photoconverted embryos were traced to 54 hpf (H–N). Panels (H’–N’) are higher magnification images of the dotted boxes in (H–N) and are accompanied by a red channel only (traced photoconverted cells) image with the VA‐L (dotted line) demarcated from the adjacent VA. No photoconverted kaede‐positive cells seen in the VA‐L in all cases.
Figure 6. The VA‐L is not derived from a vein; instead, it arises independently from a single angioblast cell.
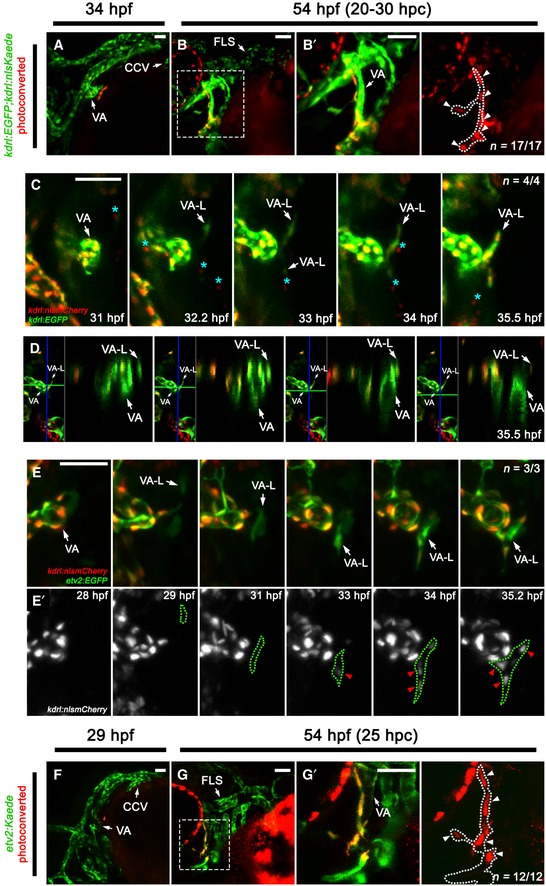
-
A–B’Lateral (A) and ventrolateral (B, B’) images of the cranial vessels in kdrl:nlsKaede;kdrl:EGFP embryos, with unconverted tissue shown in green (A, B) while photoconverted tissue is shown in red (B’). The early kdrl‐positive VA‐L was photoconverted in 34 hpf embryos (A) and followed through to 54 hpf (B, B’). (B’) is a higher magnification image of the box in (B) and is accompanied by a red channel only (traced photoconverted cells) image with the VA‐L (dotted line) demarcated from the adjacent VA. The VA‐L photoconverted cells are indicated by arrowheads.
-
CStill images from Movie EV5 of VA‐L development in a kdrl:EGFP;kdrl:nlsmCherry compound transgenic from 31 to 35.5 hpf. This movie shows that the VA‐L begins expressing kdrl just after 32 hpf, in a manner that is isolated and independent of any neighbouring kdrl‐positive tissue or vessel. Putative kdrl‐positive primitive myeloid cells (light blue asterisks) can also be seen migrating along the surface of the yolk in the immediate vicinity of the VA and VA‐L.
- D
-
E, E’Still images from Movie EV7 of VA‐L development in an etv2:EGFP (E), kdrl:nlsmCherry (E’) compound transgenic from 28 to 35.2 hpf. This movie shows that the VA‐L begins expressing etv2 (E) at 29 hpf and is independent of any neighbouring vessel. kdrl expression (red arrowheads) occurs 4 h later in the VA‐L (demarcated by the green dotted line) (E’).
-
F–G’Lateral images of the cranial vessels in etv2:Kaede embryos, with unconverted tissue shown in green (F, G) while photoconverted tissue is shown in red (G’). The earliest visible form of the VA‐L was photoconverted at 29 hpf (F), then followed through to 54 hpf (G). (G’) is a higher magnification image of the box in (G) and is accompanied by a red channel only (traced photoconverted cells) image with the VA‐L (dotted line) demarcated from the adjacent VA, and VA‐L photoconverted cells indicated (arrowheads).
The VA‐L originates from a single late‐forming angioblast
As the VA‐L appears to turn on kdrl expression later than the established vessels in the head, we sought to use a vascular progenitor marker that precedes the expression of kdrl, such as etv2, to identify what, if any, angioblast population it may be affiliated with. Time‐lapse imaging the angioblast‐marking etv2:EGFP transgenic 37 crossed with kdrl:nlsmCherry, revealed that the VA‐L begins expressing etv2:EGFP as a single isolated cell as early as 29 hpf in the avascular perivitelline space above the anterodorsal yolk sac region (Fig 6E; Movie EV7). This migrates ventrally towards the VA, and once adjacent to the VA, begins to express kdrl:nlsmCherry at 33 hpf while undergoing rounds of cell division (Fig 6E’; Movie EV7). To further confirm that the VA‐L arises from this etv2‐expressing angioblast, we photoconverted it at 29 hpf using an etv2:Kaede transgenic (Fig 6F) 38, which—despite mosaic expression of this transgene (Appendix Fig S2H–J)—was subsequently traced to the VA‐L at 54 hpf (Fig 6G). We used cell ablation to further confirm our hypothesis that the VA‐L arises from this angioblast cell and that it then fuses with the FLS to contribute to facial lymphatic formation by 60 hpf. We used a 405 nm SIM scanning laser to ablate the angioblast cell on the left lateral side of the embryo at 30 hpf (Fig 7A and B). We found that the VA‐L was not present at 54 hpf on the ablated side, but was still seen on the contralateral unablated side (Fig 7C and D), which was used as an internal control. By 78 hpf, both the LFL and the HA were significantly affected by VA‐L ablation; the LFL was not extending towards the eye (Fig 7E and F), and the left lateral branch of the HA, which connects to the first aortic arch, was missing on the ablated side compared to the unablated contralateral side (Fig 7G). In addition, both the length of the LFL and the number of cells within the LFL were significantly decreased on the ablated side compared to the contralateral side (Fig 7H and I). Overall these ablation studies confirm that this isolated etv2‐expressing angioblast population gives rise to the VA‐L, which subsequently contributes to both LFL and HA formation.
Figure 7. Ablating the etv2‐expressing angioblast population on the anterodorsal yolk sac surface leads to loss of VA‐L formation and defects in LFL and HA development.
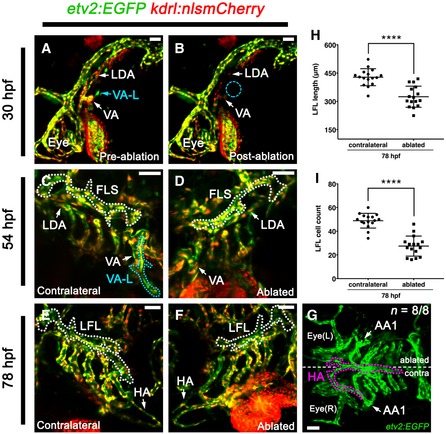
-
A–GLateral (A, B), ventrolateral (C–F) and ventral (G) images of the cranial vessels in an etv2:EGFP;kdrl:nlsmCherry compound transgenic embryo, which has had the etv2‐expressing angioblast population (early VA‐L) on the left lateral side of the yolk sac ablated. Images were taken at 30 hpf before (A) and after (B) ablation of the early VA‐L, with the site of ablation indicated (B; blue dotted circle). Note that the LDA and VA adjacent to the VA‐L become photobleached during the ablation process (B), however, these were not ablated as they can be seen developing normally at 54 hpf (D). Follow‐up images were taken of both the contralateral (C, E) and ablated (D, F) sides of the embryo's head at 54 hpf (C, D) and 78 hpf (E–G). Although the VA‐L (light blue dotted line) is seen migrating towards the FLS (white dotted lined) on the contralateral side at 54 hpf (C), it is missing from the ablated side (D). Subsequently, the LFL (white dotted line) of the ablated side is short (F) compared to that of the contralateral side (E). In addition, the HA (pink dotted line) is underdeveloped on the ablated side compared to the contralateral side, as it no longer extends towards nor connects to the left AA1 (G).
-
HQuantitation of the length of the LFL (μm) on the contralateral (control) side and on the ablated side at 78 hpf (n = 16).
-
IQuantitation of the total number of cells within the LFL on the contralateral (control) side and from the ablated side at 78 hpf (n = 16).
To further characterise the genes involved in forming this VA‐L‐contributing angioblast population, we also looked at a second angioblast marker, fli1. Via time‐lapse imaging, we observed fli1a:nEGFP turning on in the isolated VA‐L cell population at approximately 30–31 hpf, followed shortly after by kdrl:mCherry (Movie EV8). The VA‐L angioblast population therefore displays delayed induction of etv2, fli1 and kdrl, with expression of these transgenes in this population occurring 15–20 h after they are first observed in the developing embryo 37, 39. Given that this angioblast population is forming well after other angioblasts involved in cranial vasculogenesis, we wondered whether formation of the VA‐L is still regulated by clo/npas4l, the master regulator of endothelial and haematopoietic cell fate 40, 41. We injected a combination of ATG and splice‐blocking clo/npas4l morpholinos 41 into etv2:EGFP;kdrl:nlsmCherry embryos and found that VA‐L formation was sensitive to npas4l knockdown; although morphants formed some semblance of the cranial vessels, the VA‐L progenitor was not present at 34 hpf (Fig EV4A and B), and the VA‐L completely failed to form by 54 hpf (Fig EV4C and D). Furthermore, the morphant phenotype was found to be recapitulated in the mutant, with the VA‐L absent in clo/npas4l s5 mutants at 34 (Fig EV4E and F) and 54 hpf (Fig EV4G and H). Overall, the VA‐L does not sprout from an existing vein; instead, it arises from a single isolated etv2/fli1/kdrl‐expressing angioblast that is dependent on clo/npas4l activity and its formation occurs well after the major cranial vessels have already been established.
Figure EV4. clo/npas4l is required for initial formation of the VA‐L, whereas Vegfr3 signalling is only required for the dorsal migration of the VA‐L.
-
A–D’Lateral (A–B’) and ventrolateral (C–D’) images of the cranial vessels in etv2:EGFP (green), kdrl:nlsmCherry (red) compound transgenics injected with either control morpholino (A, C) or a combination of the ATG and SB clo/npas4l morpholinos (B, D), and imaged at 34 hpf (A–B’) and 54 hpf (C–D’). Panels (A’–D’) are higher magnification images of the dotted boxes in (A–D), and portray only the kdrl expression channel, with the VA‐L (green dotted line) demarcated from the adjacent VA.
-
E–H’Lateral (E–F’) and ventrolateral (G–H’) images of the cranial vessels in clo/npas4l s5 ;etv2:EGFP mutants (F, H) and wild‐type siblings (E, G) at 34 hpf (E, F) and 54 hpf (G, H). (E’–H’) are higher magnification images of the dotted boxes in (E–H), with the VA‐L (green dotted line) demarcated from the adjacent VA in wild‐type siblings (E, G). The VA‐L is not present at either 34 or 54 hpf in clo/npas4l morphants or mutants.
-
I–PVentrolateral images of etv2:EGFP (I–L) or lyve1b:EGFP (M–P) embryos comparing the effects of ccbe1 MO (J, N) or sFLT4 mRNA (L, P) injection on VA‐L formation at 48 hpf (J, L) and 54 hpf (N, P) compared to controls (I, K, M, O).
-
QQuantitation of the length of the VA‐L (μm) in control morpholino‐injected embryos at 48 hpf (n = 12) and 54 hpf (n = 10) and ccbe1 morpholino‐injected embryos at 48 hpf (n = 12) and 54 hpf (n = 11).
-
RQuantitation of the length of the VA‐L (μm) in uninjected embryos at 48 hpf (n = 12) and 54 hpf (n = 14) and sFLT4‐injected embryos at 48 hpf (n = 11) and 54 hpf (n = 12).
Vegfr3 signalling is not necessary for VA‐L formation but is required for VA‐L migration
Given that lymphatic progenitor formation in the CCV and the PHS displayed differing sensitivity to knockdown of Vegfr3 signalling, we decided to test the role of this pathway in the formation of the VA‐L. In order to abrogate Vegfr3 signalling, we separately injected either ccbe1 morpholino or sFLT4 mRNA into etv2:EGFP or lyve1b:EGFP fish. We found that in ccbe1 morphants and sFLT4‐injected fish, the VA‐L was present but significantly shorter in length than that of controls from 48 hpf onwards (Fig EV4I–R), indicating that the VA‐L fails to migrate dorsally in the absence of Vegfr3 signalling which is consistent with previous findings 32. However, in both ccbe1 morphants and sFLT4‐injected fish, the initial formation and development of the VA‐L until 48 hpf progresses normally, with the timing of the VA‐L migration defect being concomitant with the expression of Prox1 in the dorsal region of the VA‐L (Fig 3F–H). Overall, while Vegfr3 signalling is required for dorsal migration and expansion of the VA‐L towards the developing FLS, it is not necessary for the formation of the VA‐L or the initial induction and maintenance of Prox1 expression within the dorsal VA‐L.
Discussion
Recent work in both mouse and zebrafish has given rise to the hypothesis that the origin and formation of the lymphatic vasculature is heterogenous between distinct vascular beds 42, 43. In this study, we show that the zebrafish facial lymphatic network is formed via mechanisms that are distinct from that of the zebrafish trunk lymphatic network. Using lineage tracing and live imaging techniques, we confirm that the initial facial lymphatic is formed by the relay‐like coalescence of separate lymphangioblast populations arising from the CCV, PHS and VA‐L (Fig 8). Finally, we demonstrate that the VA‐L originates from a unique clo/npas4l‐dependant angioblast population that arises late in development and does not sprout from an existing blood vessel, providing the first definitive evidence of a non‐venous lymphatic progenitor in zebrafish. Throughout this study, we have named this non‐venous lymphangioblast population the “ventral aorta lymphangioblast” (VA‐L); however, it does not meet the definition of a lymphangioblast until it acquires Prox1 expression in the dorsal population at 42 hpf. Therefore, we propose to rename the initial etv2/fli1/kdrl‐positive cell(s) that arise at 29 hpf in the anterodorsal perivitelline space the “ventral aorta angioblast” (VA‐A), as this cell population is migratory, Prox1‐negative and is able to give rise to both blood vascular and lymphatic progenitors. We also propose to limit the term “VA‐L” to the dorsal, Prox1‐positive portion of the VA‐A that later contributes to the facial lymphatic (Fig 8).
Figure 8. Development of the VA‐L and the facial lymphatics.
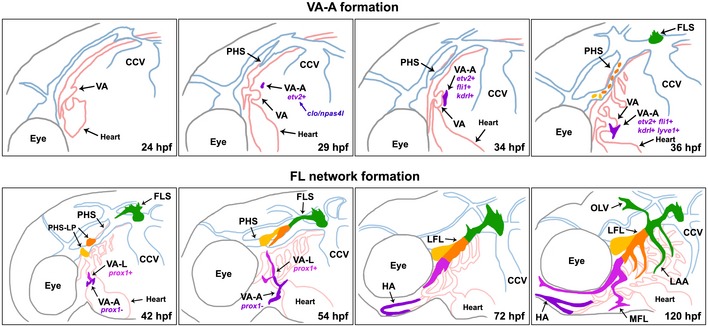
The VA‐A (purple) appears as an isolated, etv2‐expressing, clo/npas4l‐dependent angioblast in the anterodorsal yolk sac region at 29 hpf, before migrating ventrally towards the VA and expressing fli1 and kdrl by 34 hpf, and lyve1b at 36 hpf. Around this time, facial lymphatic development in the head begins as the FLS (green) arises from the dorsolateral region of the CCV, while lymphatic progenitors become specified in the PHS (orange and yellow cells). At 42 hpf, the dorsal portion of the VA‐A differentiates into the VA‐L (pink) as it acquires a lymphatic cell fate by turning on Prox1 expression, while the ventral portion of the VA‐A remains Prox1‐negative (purple). Both the VA‐L and the FLS begin migrating dorsally and anteriorly, respectively, towards the PHS, which at this time contains the PHS‐LP (orange and yellow) domains. By 54 hpf, the VA‐L has reached the FLS tip, which now consists of the PHS‐Ls (orange and yellow) that have sprouted from the PHS and fused to the FLS. By 72 hpf, the VA‐L has fused to the FLS to form the LFL, while the ventral Prox1‐negative VA‐A (purple) fuses to its contralateral counterpart and to the first aortic arch to form the HA. At 120 hpf, all components of the facial lymphatic network are present, with the OLV, MFL and LAAs sprouting from the existing LFL. CCV, common cardinal vein; FLS, facial lymphatic sprout; HA, hypobranchial artery; hpf, hours post‐fertilisation; LAA, lymphatic branchial arches; LFL, lateral facial lymphatic; MFL, medial facial lymphatic; OLV, otolithic lymphatic vessel; PHS, primary head sinus; VA, ventral aorta; VA‐A, ventral aorta angioblast; VA‐L, ventral aorta lymphangioblast.
The facial lymphatic has a non‐venous progenitor
In the early 20th century, Sabin proposed the “venous” model of lymphatic origin 8, which is supported by contemporary lineage‐tracing evidence showing that lymphatic progenitors are specified within and sprout from the veins of mice and zebrafish 11, 12. More recently, the “dual origin” theory, wherein a combination of venous and non‐venous lymphatic progenitors form the lymphatic vasculature 13, 14, 16, has been gaining popularity following a series of Cre‐LoxP lineage‐tracing experiments in mice 17, 18, 19. However, many of these studies have been contradictory 17, 19, 20, 22, in part due to technical limitations in the genetic lineage‐tracing techniques, and direct evidence of a non‐venous lymphatic progenitor has remained elusive.
In this study, we have used a complementary approach involving high‐resolution live cell imaging and cell ablation to show definitive evidence of a non‐venous lymphatic progenitor. We show that the VA‐L, which contributes lymphangioblasts to the facial lymphatic, does not sprout from any blood vessels in the head. Instead, it is derived from a unique clo/npas4l‐dependant angioblast population we called the VA‐A, which arises at 29 hpf and later contributes to both lymphatic and blood vascular development by 72 hpf. Previously, a specialised angioblast niche has been described in the ventral wall of the PCV that contributes progenitors to the zebrafish trunk lymphatics, subintestinal veins and supraintestinal arteries 24. While this study demonstrated that lymphatic progenitors can differentiate directly from “angioblast cells” within a vein, these lymphatic progenitors are nevertheless still derived from PCV cells, making them by definition venous in origin. In support of this, previous live imaging studies have confirmed that the trunk lymphatics are derived solely from cells within the PCV 11, 44.
The anterior lateral plate mesoderm (ALPM) gives rise to the angioblasts that form the cranial and facial vessels, heart endocardial cells and the primitive myeloid progenitors 37. During zebrafish development, Cloche/Npas4l functions at the top of the mesodermal‐to‐angioblast transcriptional cascade 41. Cloche/Npas4l induces etv2, one of the earliest known endothelial and haemangioblast‐specific genes, with expression beginning from the 1‐somite stage (~10.5 hpf), followed shortly after by fli1 at the 3‐somite stage (~12 h) and subsequently kdrl by the 7‐somite stage (13–14 hpf) 37, 41, 45, 46, 47. Similar to early ALPM angioblasts, the VA‐A is dependent on clo/npas4l activity, but by contrast, the VA‐A begins expressing the angioblast‐marking transgenes etv2:EGFP, fli1a:nEGFP and kdrl:mCherry approximately 20 h later than the early angioblasts of the head. In addition, lyve1b:EGFP expression in the VA‐A is observed 10 h later than in the primary veins 32, while the dorsal portion of the VA‐A only begins expressing Prox1—indicating differentiation into the VA‐L—at least 6 h after lymphatic progenitors are first observed in the CCV and PHS.
Altogether we have four observations supporting the idea that the VA‐A is a distinct angioblast population: (i) it expresses angioblast markers (etv2/fli1/kdrl), (ii) it is dependent on clo/npas4l, (iii) it is able to directly contribute to both the blood and lymphatic vascular networks, and (iv) it migrates as a string of cells rather than sprouting from a lumenised vessel. We propose that by retaining or developing a migratory angioblast‐like phenotype, it allows the VA‐A the ability to position itself spatiotemporally to contribute towards both facial lymphatic and HA development in an area of the head that is relatively devoid of lymphatic‐forming veins. It is possible that other non‐venous, lymphatic progenitors in mice may serve the same role; by retaining a migratory phenotype, they facilitate the formation of lymphatics at sites distant from venous‐derived lymph sacs. In support of this, putative non‐venous progenitors in the mesentery and dermis arise in areas distal to their respective venous‐derived lymph sacs 18, 19, 22. Of interest, the zebrafish intestinal lymphatic network develops distinct from the posterior cardinal vein 32, and therefore, it is also possible that non‐venous progenitors are also involved in the formation of this lymphatic network.
The mechanisms of facial lymphatic formation are distinct
In this study, we used lineage tracing, cell ablation and live cell imaging to confirm that anatomically distinct veins, the CCV and the PHS, as well as the VA‐L, individually contribute lymphangioblasts to the developing facial lymphatic. The distinction between how the zebrafish facial lymphatic develops—involving multiple lymphangioblast sources—compared to the trunk lymphatic network—involving a single source—may be explained by their differing vascular environments. The PCV is a large axial vein that spans the length of the trunk, which allows lymphatic development to occur simultaneously in both the anterior and the posterior of the trunk 23, 44. This is in contrast to the head, where the CCV is found only at its posterior end, with the majority of CCV ECs found in sheets overlaying the yolk sac, rather than within the cranial tissue stroma 33, 48. Thus, rather than relying on multiple sprouts from a single vein as seen in the trunk, the facial lymphatic network forms from the CCV in a polarised manner, growing from the posterior to anterior head and therefore requires additional lymphangioblast populations from both the PHS and the VA‐L. Our findings appear to be outwardly similar to two recent descriptions of mammalian lymphatic growth: the dorso‐cervical dermal lymphatics, which initially form from the jugular lymph sac but subsequently acquire progenitors from the immature dermal vasculature 22 and the ventral primordial thoracic duct, which arises from the cardinal vein and the superficial venous plexus 5. We show that these lymphangioblast populations form the facial lymphatic network through sequential cell fusion events in a “relay” mechanism, where the initial CCV‐Ls lead FLS migration until they fuse with the PHS‐Ls, which then drive lymphatic migration until fusion to cells from the VA‐L. Evidence for this mechanism comes from live imaging lymphangioblast migration before and after fusion and is supported by the fact we see little intermingling of cells between photoconverted and non‐photoconverted progenitor populations. We also confirmed through cell ablation experiments that the addition of the VA‐L is necessary for proper LFL extension under the eye. It remains to be determined what the mechanisms are that coordinate the formation and fusion of these lymphangioblast populations and whether this “relay” mechanism is observed in other examples of polarised lymphatic vessel growth.
The role of Vegfr3 signalling in facial lymphatic progenitor formation
In mice, PROX1 directly targets and binds the regulatory regions upstream of Vegfr3 to induce its expression, while Prox1 transcription and translation are dependent on Vegfr3 levels, thus forming a positive feedback loop to maintain lymphatic identity within mice LECs 31. In the zebrafish trunk, loss of vegfc and vegfr3 leads to a pronounced reduction in Prox1 expression in the PCV, while vegfc overexpression is sufficient for inducing ectopic expression of Prox1 in venous endothelium 25. A recent study in zebrafish found that although Erk signalling downstream of Vegfr3 activation is required for trunk lymphatic differentiation and sprouting, it appears dispensable for facial lymphatic specification 29, raising the idea that the role of Vegfr3‐signalling in lymphatic specification is context‐dependent.
To confirm the role of Vegfr3 signalling in facial lymphatic specification, we used either ccbe1 morpholino knockdown, which phenocopies the ccbe1 mutant 28, 44 or dominant negative flt4 (sFLT4) mRNA 28, 35. We chose these methods as both approaches are known to completely block facial lymphatic sprouting 28. Similar to previous studies 29, we found that while either approach blocked facial lymphatic sprouting, it had no effect on lymphatic specification in the CCV. Similarly, the VA‐L requires Vegfr3 signalling for migration towards the PHS, yet it is not necessary for initial Prox1 induction in the dorsal VA‐L. Finally, we demonstrate that lymphatic PHS‐LP domain formation in the PHS is dependent on Vegfr3 signalling; however, it is unclear if this phenotype is the result of a migration/proliferation defect in the PHS‐derived lymphatic progenitors or a true inhibition of lymphatic specification. Previous studies have shown that both vegfc knockdown and vegfd knockdown are required to perturb facial lymphatic development whereas in the trunk, lymphatic development only involves vegfc 28, 49. It is possible that the differences in lymphatic specification between the trunk and head are related to this; for example, lymphatic specification in the head may also involve Vegfd‐mediated signalling.
In summary, this study reveals the unique mechanisms by which the zebrafish facial lymphatic network is formed. This involves the “relay‐like” coalescence of distinct lymphangioblast populations: starting from that of the CCV, followed by the PHS and finally fusing to the VA‐L to form the LFL. We show that the VA‐L does not sprout from a vein, or indeed any blood vessel, instead arising from a single isolated angioblast cell on the avascular perivitelline space of the anterodorsal yolk sac. We propose that the migratory nature of this progenitor population allows it to contribute to lymphatic development in an area of the head that is relatively devoid of veins, and it provides direct, live imaging‐based evidence to support the “dual origin” theory of lymphatic vessel development. Overall, our study further supports the notion that the origin and development of lymphatic vessels is heterogenous between distinct vascular beds 42, 43, which has implications for our understanding of both developmental and pathological lymphangiogenesis in organ‐specific lymphatic vessels.
Materials and Methods
Zebrafish lines and transgenesis
All zebrafish strains were maintained under standard husbandry conditions and followed protocols approved by the Animal Ethics Committee of the University of Auckland. Adult zebrafish (Danio rerio) were maintained in a Tecniplast zebrafish housing facility, controlled by an automated 14–h day/10‐h night light cycle with water treated with carbon filters and UV light, and water conditions maintained between 25.5 and 29.5°C, pH 7.2–7.6 and 250–500 μS conductivity. Juvenile and adult fish (> 30 dpf) were fed three times daily on weekdays and once daily on weekends. Larval stage zebrafish (< 30 dpf) were fed on the same schedule. Health checks were done daily to remove sick or dead fish from tanks. All zebrafish used in this study were randomised, and the researchers were not blinded when conducting experiments.
The lines used in this study were Tg(lyve1b:EGFP) nz150, Tg(lyve1b:DsRed2) nz101 32, Tg(kdrl:EGFP) s843 50 , Tg(kdrl:nlsmCherry) nz49 51, TgBAC(flt4:mCitrine) hu7135 52, Tg(−0.8flt1:tdTomato) hu5333 23, Tg(lyz:DsRed) nz50 53, TgBAC(etv2:EGFP) ci1 37, TgBAC(etv2:Kaede) ci6 38, Tg(fli1a:nEGFP) y7 54 and Tg(kdrl:Hsa.HRAS‐mCherry) s916 (referred to as kdrl:mCherry) 44 and clo/npas4l s5 40. Lines generated in this study are Tg(lyve1b:Kaede) nz102 and Tg(kdrl:nlsKaede) nz70. To make the lyve1b:Kaede line, EGFP was excised from the original pt2k/lyve1b:EGFP Tol2 construct 32 using BamHI and ClaI. Kaede was then PCR amplified using cDNA from Tg(mpeg1:Gal4) gl24 ;Tg(UAS:Kaede) s1999t embryos 55, using primers flanked by BamHI (5′) and ClaI (3′) restriction enzyme sites, and directionally cloned into the EGFP site of the pT2KXIGΔin vector containing the lyve1b promoter. To make the kdrl:nlsKaede line, the approximately 6.5 kb kdrl promoter fragment was liberated from pCRII‐TOPO:kdrl (a gift from Didier Stainier) 50, using BamHI and EcoRV, and directionally cloned into BamHI/SacI(blunted)‐linearised p5E‐MCS 56 to generate p5E:‐6.5kdrl. Using Gateway technology (Invitrogen), a two‐fragment LR recombination reaction was performed using the p5E:‐6.5kdrl, pME:nlsKaede (kindly provided by P. Currie and P. Nguyen) and pTolDestR4‐R2pA 56 fragments to make the kdrl:nlsKaede Tol2 construct. Stable transgenic lines were generated as previously described 53.
Photoconversion
lyve1b:Kaede (36–72 hpf), kdrl:nlsKaede (24–33 hpf) and etv2:Kaede (24–33 hpf) embryos were mounted as previously described 57 and photoconverted using an Olympus FV1000 confocal microscope equipped with a diode‐pumped 405 nm laser. Specific Kaede‐positive structures or populations were converted by highlighting a region of interest using FluoView 3.0 (Olympus) software and exposing them to 405 nm laser light using a SIM Scanner for 5–10 s. Embryos were imaged immediately after photoconversion with a Nikon D‐Eclipse C1 confocal microscope, followed by rescue from the agarose and then individual sorting into numbered wells of tissue culture plates for later identification. Each larva was subsequently imaged again 12–48 h post‐conversion. During post‐processing, representative images were falsely coloured in purple using Volocity 6.3 software (Improvision/PerkinElmer Life and Analytical Sciences) to highlight the overlap between (Kaede) green and (photoconverted) red pixels.
Morpholino and mRNA injections
Morpholino injections were performed on lyve1b:EGFP, etv2:EGFP or etv2:EGFP;kdrl:nlsmCherry embryos as previously described 58. Embryos between the 1 and 4‐cell stage were injected either with 5 ng standard control morpholino (Gene Tools), 10 ng ccbe1 morpholino 44, 2 ng csf3r and 5 ng pu.1 morpholino 36 or 6.4 ng ATG and 1.8 ng splice‐blocking clo/npas4l morpholinos 41. The ccbe1 morpholino has previously been shown to phenocopy the fof mutant 44, the combination of csf3r and pu.1 morpholinos has previously been shown to broadly inhibit myelopoiesis 36, and the combination of two distinct clo/npas4l morpholinos, each at a suboptimal dose, has previously been shown to phenocopy the cloche mutant 41. The pCS2+/sFLT4 construct 27 and protocol for synthesis and injection of 200 pg sFLT4 mRNA into single‐cell embryos have been previously described 28.
Ablations
Cell ablations were performed on etv2:EGFP;kdrl:nlsmCherry embryos at 30 hpf using an Olympus FV1000 confocal microscope equipped with a diode‐pumped 405 nm laser. The etv2‐expressing VA‐A on the left lateral side of the zebrafish head was highlighted using FluoView 3.0 (Olympus) software and exposed to 405 nm laser light at maximum intensity using a SIM Scanner and with an open pinhole for 10–15 min. Embryos were also imaged immediately before and after ablation using the Olympus FV1000 confocal microscope. Ablated embryos were reimaged 3 h following ablation to ensure the cells had been fully ablated. Each larva was subsequently imaged again at 48 and 78 hpf using a Nikon D‐Eclipse C1 confocal microscope.
Immunohistochemistry
Prox1 immunofluorescence staining was performed as described 25, with DAPI (1:400, Invitrogen) added during the secondary antibody incubation step for cell count quantitation. Antibodies used in this study were chicken anti‐GFP (1:400, Abcam, #ab13970), rabbit anti‐PROX1 (1:500, AngioBio Co, #11‐002P) and anti‐rabbit IgG‐HRP (1:1,000, Cell Signaling, #7074). Superficial, non‐specific, background staining in Prox1 immunofluorescence images was removed during post‐processing using Photoshop CC 2015 (Adobe).
Confocal imaging
Live and fixed embryos were mounted laterally or ventrolaterally and imaged as previously described 57 with a Nikon D‐Eclipse C1 confocal microscope and/or an Olympus FV1000 confocal microscope for either still or time‐lapse microscopy. Z‐stacks of still images were acquired at 5 μm increments with a 20× objective or 4 μm increments with a 60× objective. For time‐lapse microscopy, z‐stacks were taken at 10‐min intervals. Images were processed using Fiji 59, Photoshop CC 2015 (Adobe) and Volocity 6.3 image analysis software (Improvision/PerkinElmer Life and Analytical Sciences).
Image analysis and statistics
All quantitative analyses were performed using Volocity 6.3 software. Endothelial nuclei were identified using DAPI and anti‐GFP in fixed Tg(lyve1b:EGFP) larvae. Lymphatic precursors or lymphangioblasts were identified using DAPI, anti‐GFP and anti‐Prox1 in fixed Tg(lyve1b:EGFP) larvae. Lymphatic precursors that co‐expressed DAPI, GFP and Prox1 within the FLS, PHS and VA‐L were manually counted through the complete confocal z‐stack at 36 and 42 hpf. In morpholino and mRNA injection studies, this was expressed as a percentage of the total number of GFP and DAPI‐positive endothelial nuclei within each vessel. Using time‐lapse confocal images, the total number of lymphangioblast nuclei [expressing Tg(lyve1b:EGFP) and Tg(kdrl:nlsmCherry)] within the developing FLS was also manually counted across the complete confocal z‐stack at 1.5‐h intervals from 43.5 to 58.5 hpf. Lymphangioblast velocity was calculated by tracking and measuring the total distance (in μm) a single tip cell travelled over 3–3.5 h (180–210 min) prior to fusing with the next lymphangioblast population, followed by the length travelled by the same cell 3–3.5 h post‐fusion. Statistical analyses were performed using Prism 7.0 software (GraphPad Software). Significance was determined by unpaired Student's t‐test or one‐way ANOVA, and normality of the data sets was confirmed using the Shapiro–Wilk normality test.
Author contributions
Conceptualisation: TCYE, KSO, JWA; Methodology: TCYE, KSO, CJH, BMH, JWA; Investigation: TCYE, JPM, KSO, YP, WC, JWA; Writing and Review: TCYE, JWA; Funding Acquisition: KEC, PSC, SS‐M, JWA.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Movie EV1
Movie EV2
Movie EV3
Movie EV4
Movie EV5
Movie EV6
Movie EV7
Movie EV8
Review Process File
Acknowledgements
We thank Mr Alhad Mahagaonkar for managing the fish facility and the Biomedical Imaging Research Unit, University of Auckland, for assistance in microscopy and cell ablation. We also thank the Sumanas laboratory for providing the etv2:EGFP and etv2:Kaede lines. Work in this study was supported by a Health Research Council of New Zealand project grant (14/105) awarded to P.S.C and J.W.A and a Royal Society of New Zealand Marsden Project Grant (UOA1602) awarded to J.W.A. T.C.Y.E was supported by the University of Auckland Doctoral Scholarship and the School of Medical Sciences Performance‐based Research Fund Publication Assistance Bursary awarded by the University of Auckland. Y.P. and S.S.‐M. were supported by the CiM Cluster of Excellence (EXC 1003‐CiM), and CRC 1348 and SCHU1228/3‐1 from the DFG.
EMBO Reports (2019) 20: e47079
References
- 1. Hong Y, Harvey N, Noh Y, Schacht V, Hirakawa S, Detmar M, Oliver G (2002) Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn 225: 351–357 [DOI] [PubMed] [Google Scholar]
- 2. Wigle JT, Oliver G (1999) Prox1 function is required for the development of the murine lymphatic system. Cell 98: 769–778 [DOI] [PubMed] [Google Scholar]
- 3. Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H (2004) Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol 5: 74–80 [DOI] [PubMed] [Google Scholar]
- 4. Zhang L, Zhou F, Han W, Shen B, Luo J, Shibuya M, He Y (2010) VEGFR‐3 ligand‐binding and kinase activity are required for lymphangiogenesis but not for angiogenesis. Cell Res 20: 1319–1331 [DOI] [PubMed] [Google Scholar]
- 5. Hägerling R, Pollmann C, Andreas M, Schmidt C, Nurmi H, Adams RH, Alitalo K, Andresen V, Schulte‐Merker S, Kiefer F (2013) A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy. EMBO J 32: 629–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. François M, Short K, Secker GA, Combes A, Schwarz Q, Davidson T, Smyth I, Hong Y, Harvey NL, Koopman P (2012) Segmental territories along the cardinal veins generate lymph sacs via a ballooning mechanism during embryonic lymphangiogenesis in mice. Dev Biol 364: 89–98 [DOI] [PubMed] [Google Scholar]
- 7. Yang Y, García‐Verdugo JM, Soriano‐Navarro M, Srinivasan RS, Scallan JP, Singh MK, Epstein JA, Oliver G (2012) Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood 120: 2340–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sabin FR (1902) On the origin of the lymphatic system from the veins and the development of the lymph hearts and thoracic duct in the pig. Am J Anat 1: 367–389 [Google Scholar]
- 9. Huntington GS (1908) Symposium on the development and structure of the lymphatic system. II. The genetic in erpretation of the development of the mammalian lymphatic system. Anat Rec 2: 19–45 [Google Scholar]
- 10. Huntington GS, McClure CF (1910) The anatomy and development of the jugular lymph sacs in the domestic cat (Felis domestica). Am J Anat 10: 177–312 [Google Scholar]
- 11. Yaniv K, Isogai S, Castranova D, Dye L, Hitomi J, Weinstein BM (2006) Live imaging of lymphatic development in the zebrafish. Nat Med 12: 711–716 [DOI] [PubMed] [Google Scholar]
- 12. Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhvalov IM, Oliver G (2007) Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev 21: 2422–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. der Jagt Van, Raymond Ervin (1932) Memoirs: the origin and development of the anterior lymph‐sacs in the sea‐turtle (Thalassochelys caretta). J Cell Sci 2: 151–163 [Google Scholar]
- 14. Wilting J, Aref Y, Huang R, Tomarev SI, Schweigerer L, Christ B, Valasek P, Papoutsi M (2006) Dual origin of avian lymphatics. Dev Biol 292: 165–173 [DOI] [PubMed] [Google Scholar]
- 15. Wilting J, Papoutsi M, Othman‐Hassan K, Rodriguez‐Niedenführ M, Pröls F, Tomarev SI, Eichmann A (2001) Development of the avian lymphatic system. Microsc Res Tech 55: 81–91 [DOI] [PubMed] [Google Scholar]
- 16. Ny A, Koch M, Schneider M, Neven E, Tong RT, Maity S, Fischer C, Plaisance S, Lambrechts D, Héligon C (2005) A genetic Xenopus laevis tadpole model to study lymphangiogenesis. Nat Med 11: 998–1004 [DOI] [PubMed] [Google Scholar]
- 17. Klotz L, Norman S, Vieira JM, Masters M, Rohling M, Dubé KN, Bollini S, Matsuzaki F, Carr CA, Riley PR (2015) Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature 522: 62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stanczuk L, Martinez‐Corral I, Ulvmar MH, Zhang Y, Laviña B, Fruttiger M, Adams RH, Saur D, Betsholtz C, Ortega S (2015) cKit lineage hemogenic endothelium‐derived cells contribute to mesenteric lymphatic vessels. Cell Rep 10: 1708–1721 [DOI] [PubMed] [Google Scholar]
- 19. Martinez‐Corral I, Ulvmar M, Stanczuk L, Tatin F, Kizhatil K, John SW, Alitalo K, Ortega S, Makinen T (2015) Non‐venous origin of dermal lymphatic vasculature. Circ Res 116: 1649–1654 [DOI] [PubMed] [Google Scholar]
- 20. Ulvmar MH, Martinez‐Corral I, Stanczuk L, Mäkinen T (2016) Pdgfrb‐Cre targets lymphatic endothelial cells of both venous and non‐venous origins. Genesis 54: 350–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heffner CS, Pratt CH, Babiuk RP, Sharma Y, Rockwood SF, Donahue LR, Eppig JT, Murray SA (2012) Supporting conditional mouse mutagenesis with a comprehensive cre characterization resource. Nat Commun 3: 1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pichol‐Thievend C, Betterman KL, Liu X, Ma W, Skoczylas R, Lesieur E, Bos FL, Schulte D, Schulte‐Merker S, Hogan BM et al (2018) A blood capillary plexus‐derived population of progenitor cells contributes to genesis of the dermal lymphatic vasculature during embryonic development. Development 145: dev160184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bussmann J, Bos FL, Urasaki A, Kawakami K, Duckers HJ, Schulte‐Merker S (2010) Arteries provide essential guidance cues for lymphatic endothelial cells in the zebrafish trunk. Development 137: 2653–2657 [DOI] [PubMed] [Google Scholar]
- 24. Nicenboim J, Malkinson G, Lupo T, Asaf L, Sela Y, Mayseless O, Gibbs‐Bar L, Senderovich N, Hashimshony T, Shin M (2015) Lymphatic vessels arise from specialized angioblasts within a venous niche. Nature 522: 56–61 [DOI] [PubMed] [Google Scholar]
- 25. Koltowska K, Lagendijk AK, Pichol‐Thievend C, Fischer JC, Francois M, Ober EA, Yap AS, Hogan BM (2015) Vegfc regulates bipotential precursor division and Prox1 expression to promote lymphatic identity in zebrafish. Cell Rep 13: 1828–1841 [DOI] [PubMed] [Google Scholar]
- 26. Küchler AM, Gjini E, Peterson‐Maduro J, Cancilla B, Wolburg H, Schulte‐Merker S (2006) Development of the zebrafish lymphatic system requires vegfc signaling. Curr Biol 16: 1244–1248 [DOI] [PubMed] [Google Scholar]
- 27. Hogan BM, Herpers R, Witte M, Helotera H, Alitalo K, Duckers HJ, Schulte‐Merker S (2009) Vegfc/Flt4 signalling is suppressed by Dll4 in developing zebrafish intersegmental arteries. Development 136: 4001–4009 [DOI] [PubMed] [Google Scholar]
- 28. Astin JW, Haggerty MJ, Okuda KS, Le Guen L, Misa JP, Tromp A, Hogan BM, Crosier KE, Crosier PS (2014) Vegfd can compensate for loss of Vegfc in zebrafish facial lymphatic sprouting. Development 141: 2680–2690 [DOI] [PubMed] [Google Scholar]
- 29. Shin M, Male I, Beane TJ, Villefranc JA, Kok FO, Zhu LJ, Lawson ND (2016) Vegfc acts through ERK to induce sprouting and differentiation of trunk lymphatic progenitors. Development 143: 3785–3795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bowles J, Secker G, Nguyen C, Kazenwadel J, Truong V, Frampton E, Curtis C, Skoczylas R, Davidson T, Miura N (2014) Control of retinoid levels by CYP26B1 is important for lymphatic vascular development in the mouse embryo. Dev Biol 386: 25–33 [DOI] [PubMed] [Google Scholar]
- 31. Srinivasan RS, Escobedo N, Yang Y, Interiano A, Dillard ME, Finkelstein D, Mukatira S, Gil HJ, Nurmi H, Alitalo K et al (2014) The Prox1‐Vegfr3 feedback loop maintains the identity and the number of lymphatic endothelial cell progenitors. Genes Dev 28: 2175–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Okuda KS, Astin JW, Misa JP, Flores MV, Crosier KE, Crosier PS (2012) Lyve1 expression reveals novel lymphatic vessels and new mechanisms for lymphatic vessel development in zebrafish. Development 139: 2381–2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Isogai S, Horiguchi M, Weinstein BM (2001) The vascular anatomy of the developing zebrafish: an atlas of embryonic and early larval development. Dev Biol 230: 278–301 [DOI] [PubMed] [Google Scholar]
- 34. Crucke J, Huysseune A (2013) Unravelling the blood supply to the zebrafish pharyngeal jaws and teeth. J Anat 223: 399–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ober EA, Olofsson B, Makinen T, Jin SW, Shoji W, Koh GY, Alitalo K, Stainier DY (2004) Vegfc is required for vascular development and endoderm morphogenesis in zebrafish. EMBO Rep 5: 78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pase L, Layton JE, Wittmann C, Ellett F, Nowell CJ, Reyes‐Aldasoro CC, Varma S, Rogers KL, Hall CJ, Keightley MC (2012) Neutrophil‐delivered myeloperoxidase dampens the hydrogen peroxide burst after tissue wounding in zebrafish. Curr Biol 22: 1818–1824 [DOI] [PubMed] [Google Scholar]
- 37. Proulx K, Lu A, Sumanas S (2010) Cranial vasculature in zebrafish forms by angioblast cluster‐derived angiogenesis. Dev Biol 348: 34–46 [DOI] [PubMed] [Google Scholar]
- 38. Kohli V, Schumacher JA, Desai SP, Rehn K, Sumanas S (2013) Arterial and venous progenitors of the major axial vessels originate at distinct locations. Dev Cell 25: 196–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pham VN, Lawson ND, Mugford JW, Dye L, Castranova D, Lo B, Weinstein BM (2007) Combinatorial function of ETS transcription factors in the developing vasculature. Dev Biol 303: 772–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stainier DY, Weinstein BM, Detrich HW III, Zon LI, Fishman MC (1995) Cloche, an early acting zebrafish gene, is required by both the endothelial and hematopoietic lineages. Development 121: 3141–3150 [DOI] [PubMed] [Google Scholar]
- 41. Reischauer S, Stone OA, Villasenor A, Chi N, Jin S, Martin M, Lee MT, Fukuda N, Marass M, Witty A (2016) Cloche is a bHLH‐PAS transcription factor that drives haemato‐vascular specification. Nature 535: 294 [DOI] [PubMed] [Google Scholar]
- 42. Potente M, Mäkinen T (2017) Vascular heterogeneity and specialization in development and disease. Nat Rev Mol Cell Biol 18: 477 [DOI] [PubMed] [Google Scholar]
- 43. Wong B, Zecchin A, García‐Caballero M, Carmeliet P (2018) Emerging concepts in organ‐specific lymphatic vessels and metabolic regulation of lymphatic development. Dev Cell 45: 289–301 [DOI] [PubMed] [Google Scholar]
- 44. Hogan BM, Bos FL, Bussmann J, Witte M, Chi NC, Duckers HJ, Schulte‐Merker S (2009) ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat Genet 41: 396–398 [DOI] [PubMed] [Google Scholar]
- 45. Thompson MA, Ransom DG, Pratt SJ, MacLennan H, Kieran MW, Detrich HW III, Vail B, Huber TL, Paw B, Brownlie AJ (1998) The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Dev Biol 197: 248–269 [DOI] [PubMed] [Google Scholar]
- 46. Liao W, Bisgrove BW, Sawyer H, Hug B, Bell B, Peters K, Grunwald DJ, Stainier DY (1997) The zebrafish gene cloche acts upstream of a flk‐1 homologue to regulate endothelial cell differentiation. Development 124: 381–389 [DOI] [PubMed] [Google Scholar]
- 47. Sumanas S, Gomez G, Zhao Y, Park C, Choi K, Lin S (2008) Interplay among Etsrp/ER71, Scl, and Alk8 signaling controls endothelial and myeloid cell formation. Blood 111: 4500–4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Helker CS, Schuermann A, Karpanen T, Zeuschner D, Belting HG, Affolter M, Schulte‐Merker S, Herzog W (2013) The zebrafish common cardinal veins develop by a novel mechanism: lumen ensheathment. Development 140: 2776–2786 [DOI] [PubMed] [Google Scholar]
- 49. Bower NI, Vogrin AJ, Le Guen L, Chen H, Stacker SA, Achen MG, Hogan BM (2017) Vegfd modulates both angiogenesis and lymphangiogenesis during zebrafish embryonic development. Development 144: 507–518 [DOI] [PubMed] [Google Scholar]
- 50. Jin SW, Beis D, Mitchell T, Chen JN, Stainier DY (2005) Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development 132: 5199–5209 [DOI] [PubMed] [Google Scholar]
- 51. Lam EY, Hall CJ, Crosier PS, Crosier KE, Flores MV (2010) Live imaging of Runx1 expression in the dorsal aorta tracks the emergence of blood progenitors from endothelial cells. Blood 116: 909–914 [DOI] [PubMed] [Google Scholar]
- 52. van Impel A, Zhao Z, Hermkens DM, Roukens MG, Fischer JC, Peterson‐Maduro J, Duckers H, Ober EA, Ingham PW, Schulte‐Merker S (2014) Divergence of zebrafish and mouse lymphatic cell fate specification pathways. Development 141: 1228–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hall CJ, Flores MV, Storm T, Crosier KE, Crosier PS (2007) The zebrafish lysozyme C promoter drives myeloid‐specific expression in transgenic fish. BMC Dev Biol 7: 42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Roman BL, Pham VN, Lawson ND, Kulik M, Childs S, Lekven AC, Garrity DM, Moon RT, Fishman MC, Lechleider RJ et al (2002) Disruption of acvrl1 increases endothelial cell number in zebrafish cranial vessels. Development 129: 3009–3019 [DOI] [PubMed] [Google Scholar]
- 55. Hall CJ, Boyle RH, Astin JW, Flores MV, Oehlers SH, Sanderson LE, Ellett F, Lieschke GJ, Crosier KE, Crosier PS (2013) Immunoresponsive gene 1 augments bactericidal activity of macrophage‐lineage cells by regulating β‐oxidation‐dependent mitochondrial ROS production. Cell Metab 18: 265–278 [DOI] [PubMed] [Google Scholar]
- 56. Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien C (2007) The Tol2kit: a multisite gateway‐based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn 236: 3088–3099 [DOI] [PubMed] [Google Scholar]
- 57. Hall CJ, Flores MV, Crosier KE, Crosier PS (2009) Live cell imaging of zebrafish leukocytes. Methods Mol Biol 546: 255–271 [DOI] [PubMed] [Google Scholar]
- 58. Nasevicius A, Ekker SC (2000) Effective targeted gene ‘knockdown'in zebrafish. Nat Genet 26: 216 [DOI] [PubMed] [Google Scholar]
- 59. Schindelin J, Arganda‐Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B (2012) Fiji: an open‐source platform for biological‐image analysis. Nat Methods 9: 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Movie EV1
Movie EV2
Movie EV3
Movie EV4
Movie EV5
Movie EV6
Movie EV7
Movie EV8
Review Process File


