Abstract
Background
Combined small cell lung cancer (C‐SCLC) is rare and its clinical features, appropriate treatment, and prognosis are poorly understood. Reports conflict over the prognosis of C‐SCLCs compared to pure small cell lung cancer.
Methods
The records of patients diagnosed with primary SCLC from 1988 to 2014 were extracted from the Surveillance, Epidemiology, and End Results database. The general features of C‐SCLCs were compared to those of SCLCs. T1–2 N0–1 data was extracted and the effects of the histological subtype, treatment modality, and other prognostic factors on lung cancer‐specific survival (CSS) was analyzed in a 3:1 matched dataset. Analysis was performed using the 8th edition tumor node metastasis staging system and previous staging systems adjunctively.
Results
C‐SCLCs comprised 1.5% of all SCLCs (1486/98 667); 184 cases of C‐SCLCs and 2681 cases of non‐combined SCLCs (NC‐SCLCs) were included in this study. C‐SCLCs were more likely to be of a higher grade and to occur in the upper lobe than NC‐SCLCs. Before matching, C‐SCLCs showed better CSS (hazard ratio 0.69; P < 0.001). However, stratified Cox proportional hazards analysis in the matched dataset revealed that only treatment modality and age at diagnosis were associated with CSS; the histological subtype had no effect on survival. Of all treatment modalities, surgery with chemoradiation showed the best CSS in T1–2 N0–1 SCLC.
Conclusion
In early SCLC, surgery with chemoradiation shows the best CSS. C‐SCLC patients might benefit more from multimodal treatments, including surgery, than SCLC patients.
Keywords: Combined small cell lung cancer, SEER, small cell lung cancer, survival analysis
Introduction
Small cell lung cancer (SCLC), a highly aggressive malignancy originating from neuroendocrine cell precursors, has a poor prognosis. In recent decades, improvements in survival have been attributed to concurrent chemoradiation and prophylactic cerebral irradiation.1 Since 1981, World Health Organization (WHO) classifications have regarded combined SCLC (C‐SCLC) as a variant of SCLC. While there have been changes in the classification of lung cancer, C‐SCLC remained a subcategory of SCLC. In the 1981 revision, SCLC was divided into three subtypes: oat cell, intermediate cell, and combined cell.2 In the most recent revision in 2015, WHO classified SCLC as C‐SCLC, a subcategory of SCLC, with both belonging to the neuroendocrine tumor type.3
C‐SCLC is defined by WHO as small cell carcinoma combined with any other non‐SCLC (NSCLC) component, including adenocarcinoma, squamous cell carcinoma, large cell carcinoma, large cell neuroendocrine carcinoma, or another rarer component, such as giant cell carcinoma or a sarcomatoid constituent.3, 4 In the case of a large cell carcinoma component, at least 10% of the large cell carcinoma or large cell neuroendocrine carcinoma component should be present for a diagnosis of C‐SCLC. When another NSCLC histological subtype, such as adenocarcinoma, squamous cell carcinoma, or sarcomatoid carcinoma, coexists with SCLC, C‐SCLC is diagnosed irrespective of cell amounts.4 In addition, more than two components can coexist within C‐SCLC.5
SCLC comprises approximately 13% of all lung cancers.6 The incidence of C‐SCLC varies from 5% to 28% depending on the specimen used.7, 8 Although recent advances in diagnostic techniques have resulted in a reported increase in the incidence of C‐SCLC, the clinicopathologic features, appropriate treatment, and prognosis of C‐SCLC remain poorly elucidated. Several retrospective studies have reported that C‐SCLC has quite characteristic clinical features, including male predominance, peripheral location in half of C‐SCLCs, more limited stages in 70%, and stage I or II in only 3%.8 These characteristics explain the possible benefit of multimodality therapies, including surgery, in patients with C‐SCLC. Treatment for C‐SCLC currently follows SCLC guidelines, but the optimal treatment remains unclear.
Definitive concurrent chemoradiotherapy remains the standard treatment for patients with limited disease. In the 1970s, two significant reports demonstrating poor survival in patients with SCLC after surgery compared to nonsurgical treatment made SCLC the exception for surgery.9, 10 After some decades, several authors began to report the role of surgery as primary treatment in a small number of SCLC patients.11, 12, 13 In 2005, the Japan Clinical Oncology Lung Cancer Study Group reported three‐year survival of 68% for stage I disease after surgery and adjuvant chemotherapy.14
The recently updated National Comprehensive Cancer Network (NCCN) guidelines recommend lobectomy and mediastinal lymph node sampling or dissection and adjuvant therapy according to pathologic N status.15 However, there is limited evidence of the optimal indications for surgical resection and the efficacy of adjuvant chemotherapy. For example, a large series of early‐stage SCLC patients eligible for surgical resection only accounted for 2.4% to 3.4% of resected lung cancer cases.16 With lung cancer screening with low‐dose CT becoming more widespread, the incidence of early‐stage lung cancer, both NSCLC and SCLC, is expected to increase. Therefore, an evidence‐based treatment strategy is necessary.
The objective of our study was to assess the clinical characteristics of C‐SCLC using one of the world's largest databases, the Surveillance, Epidemiology, and End Results (SEER) program. In addition, we compared the effect of the histological subtype on treatment outcomes and prognostic factors of C‐SCLC in a matched cohort of combined versus non‐combined SCLC (NC‐SCLC).
Methods
Patient selection
Data were extracted from the United States (US) National Cancer Institute SEER‐18 database, which consists of patients diagnosed from 1973 to 2014 (submitted in November 2016, http://seer.cancer.gov). SEER is a well‐known database that contains the epidemiological, pathological, and survival data of all cancer cases in 18 areas of the US. Because we used public data that did not identify individual patients, informed consent from the study participants was not required.
SEER*Stat 8.3.4 (http:/www.seer.cancer.gov) was used to extract data via the variable “SEER site recode” with the phrase “lung and bronchus” and the variable “site and morphology behavior recode for analysis” with the term “malignant.” Patients diagnosed before 1988 with incomplete staging data were excluded. The International Classification of Diseases for Oncology‐3 was used to limit the pathology types to “small cell carcinoma, NOS (8041/3),” “oat cell carcinoma (8042/3),” “small cell carcinoma, fusiform cell (8043/3),” “small cell carcinoma, intermediate cell (8044/3),” and “combined small cell carcinoma (8045/3)”. We divided the SCLC cases into two groups: C‐SCLC and NC‐SCLC.
The dataset was then restricted to the first and only primary malignancy in each patient's life. The type of reporting source was restricted to exclude cases with an autopsy report or death certificate alone. The process used for data cleansing is summarized in Figure 1. Three variables regarding surgery “lung surgery to primary site,” “type of cancer‐directed surgery,” and “site specific surgery” were used to exclude unclear cases or patients treated with non‐standard surgery. Following the coding rule of “lung surgery to primary site,” segmentectomy and wedge resection was regarded as “sublobar resection;” bilobectomy or lobectomy was grouped as “lobectomy;” and radical, complete, total, or standard pneumonectomy as “pneumonectomy.” We also excluded unstaged patients or those with unknown stages (n = 46 879), resulting in 51 788 patients with primary SCLC.
Figure 1.
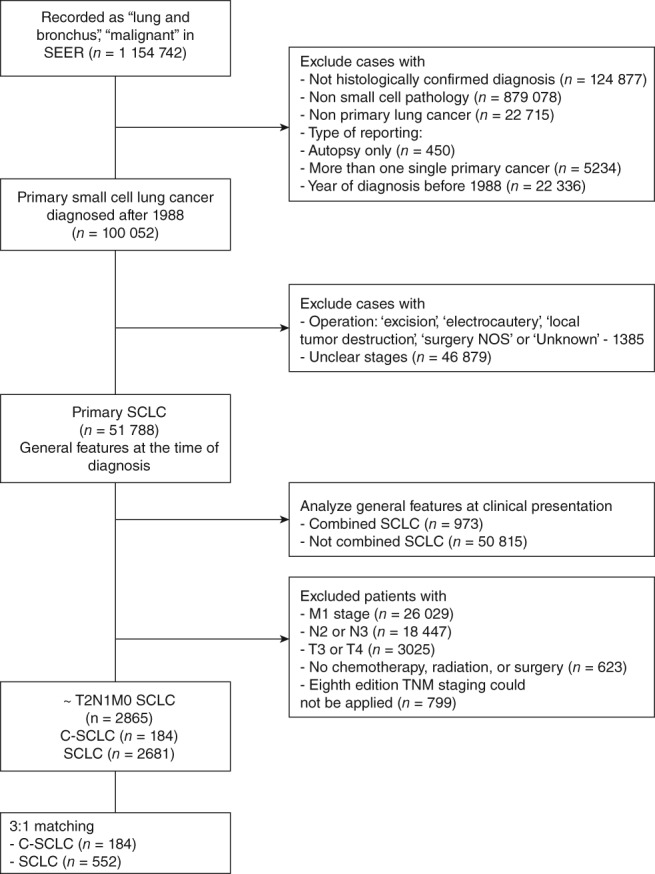
Study flow chart, with patient numbers and reasons for exclusion from the dataset. SCLC, small cell lung cancer; C‐SCLC, combined SCLC. SEER, Surveillance, Epidemiology, and End Results; TNM, tumor node metastasis.
The basic demographics and stage distributions of these 51 788 patients were summarized. We then narrowed the dataset to early T1–2 N0–1 SCLCs to assess the effect of histological subtype and treatment modality on lung cancer‐specific survival (CSS). The dataset included important demographic variables, follow‐up data, survival status, cause of death, and the first course of the treatment (radiation, chemotherapy, or surgery).
Statistical analysis
The primary endpoint of this study was lung CSS. Patients who were alive at the last follow‐up date in the SEER database were right censored.
The basic characteristics were summarized using standard statistical methods, including mean ± standard deviation for continuous variables and frequencies with percentages for categorical variables. Distributions of continuous variables were compared using Student's t or Mann–Whitney U tests depending on the results of the Shapiro–Wilk test of normality. Categorical variables were compared using chi‐square or Fisher's exact tests.
During the period of study, three versions of the American Joint Committee on Cancer (AJCC) staging systems had been used. To respect the original staging system and to reflect the latest 8th edition tumor node metastasis (TNM) staging system, we analyzed the data in two ways. First, we applied the 8th edition TNM system to the data. Although the definition of N0 and N1 has remained the same, a size criterion was introduced in the 7th and 8th editions.17, 18, 19, 20 Using the tumor size variable, we were able to apply the latest T stage to the data. Second, as an adjunctive method, we performed the same matching and analysis based on previous staging systems by considering the editions of stages via matching and stratification.
Given the observational nature of the SEER database and to minimize the effect of stage, we used 8th edition TNM stages and the periods as matching variables. As an adjunctive analysis, we used T, N, and edition of AJCC systems (3rd, 6th, 7th). We used the 3:1 nearest matching method with a caliper of 0.10 using the R package MatchIt. After matching, the balance between the groups was checked via the standardized mean differences, and the values for all variables were < 0.10.21
The Kaplan–Meier method was used to calculate CSS and the log‐rank test was applied to compare the survival curves for C‐SCLCs and NC‐SCLCs. After matching, we used univariate and multivariate Cox proportional hazards models to estimate the predictors for CSS. Cox regression analyses were used to examine possible confounding and prognostic factors with the following variables: demographic factors (including age, gender, race, insurance status, and marital status); tumor characteristics (including tumor location, histologic subtype, and lesion sidedness); and treatment variables (including type of surgery and combinations of treatment). The proportional hazards assumption was checked using statistical tests and graphical diagnostics based on the scaled Schoenfeld residuals. Because the dataset was matched, we used a Cox proportional hazards model by stratifying it with the matched variables. Statistical analyses were performed using R version 3.4.1 (http://www.R-project.org/). Statistical significance was defined as two‐sided P value of < 0.05, and variables with statistical significance in univariate analysis were incorporated into a multivariate model.
This study was approved by the Institutional Review Board of Seoul St. Mary's Hospital (No. KC18ZESI0842).
Results
General features of combined small cell lung cancer (C‐SCLC)
In total, 98 667 patients with SCLC were identified from the database; 1486 (1.5%) had C‐SCLCs. After the exclusion of 46 879 patients with unclear TNM stages, 973 cases of C‐SCLCs and 50 815 cases of NC‐SCLCs were included. C‐SCLCs showed male (57.0% vs. 50.2%; P < 0.001) and upper lobe (56.0% vs. 48.0%; P < 0.001) predominance and were less likely to be located near the main bronchus (7.2% vs. 12.3%; P < 0.001) (Table S1).
For analytical convenience and in consideration of the changes in definitions, we divided the entire study period into three categories according to the corresponding edition of the AJCC staging system used: first (1988–2003), second (2004–2009), and third (2010–2014). Regardless of the period, C‐SCLCs showed earlier T, N, and M stages compared to NC‐SCLCs (Fig S1). For C‐SCLC, 45.4%, 53.3%, and 50.0% of patients were T1 or T2 in the first, second, and third periods, whereas the percentages for SCLCs were 34.6%, 35.2%, and 38.4%, respectively. Throughout the period, C‐SCLCs showed more N1 or N0 compared to NC‐SCLCs (36.7%–41.5% vs. 21.7%–25.5%). Approximately half of the patients with C‐SCLC were M0 at the time of diagnosis, whereas approximately two‐thirds of the patients with NC‐SCLC were M1. Overall, 25% of C‐SCLCs and only 10% of NC‐SCLCs were stage I or II.
All patients in the dataset were actively followed up according to the variable “type of follow‐up expected,” and complete dates of follow‐up were available in 92.5% of cases. Follow‐up was calculated from the date of initial diagnosis and was available through November 2016 at the time of our analysis. The mean follow‐up was 34.31 months for the entire cohort.
Clinical features of early (T1,2/N1) C‐SCLC
In total, 47 501 patients with advanced stages, 623 patients without definite treatment data, and 799 patients with unknown or > 50 mm tumor size were excluded. Thus, the remaining 184 cases of C‐SCLCs and 2681 cases of NC‐SCLCs were included in the analysis. All cases were confirmed microscopically.
The distinctive characteristics of C‐SCLCs were upper lobe predominance, higher grade (grade III or IV), and larger number of lymph nodes pathologically examined (6.5 ± 7.3 vs. 1.9 ± 4.8; P < 0.0001) compared to NC‐SCLCs (Table 1). The difference in the number of lymph nodes examined is attributed to a discrepancy in the proportion of surgery: surgery was performed in 81.5% of C‐SCLCs but in only 31.0% of NC‐SCLCs. Both chemotherapy (55.4% vs. 83.0%; P < 0.001) and radiation (30.4% vs. 60.5%; P < 0.001) were more commonly administered to NC‐SCLC patients, and the most common treatment modality in the NC‐SCLC group was chemoradiation (47.1%). For patients with C‐SCLC, the most common first course of treatment was surgery (37.5%), followed by surgery with chemotherapy (26.6%), and surgery with chemoradiation (15.8%). The mean numbers of positive lymph nodes were similar in the two groups (C‐SCLC 0.4 ± 1.1 vs. SCLC 0.6 ± 1.4; P = 0.169).
Table 1.
Clinical characteristics of C‐SCLC and NC‐SCLC patients before and after matching
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| Characteristics | C‐SCLC (n = 184) | NC‐SCLC (n = 2681) | P | C‐SCLC (n = 184) | NC‐SCLC (n = 552) | P |
| Age (years old) | 67.93 ± 10.04 | 67.74 ± 9.53 | 0.798 | 67.93 ± 10.04 | 68.71 ± 9.34 | 0.333 |
| Gender | 0.149 | 0.201 | ||||
| Female | 88 (47.8%) | 1437 (53.6%) | 88 (47.8%) | 296 (53.6%) | ||
| Male | 96 (52.2%) | 1244 (46.4%) | 96 (52.2%) | 256 (46.4%) | ||
| Race | 0.517 | 0.502 | ||||
| African‐American | 18 (9.8%) | 200 (7.5%) | 18 (9.8%) | 40 (7.2%) | ||
| Other | 8 (4.3%) | 119 (4.4%) | 8 (4.3%) | 21 (3.8%) | ||
| White | 158 (85.9%) | 2362 (88.1%) | 158 (85.9%) | 491 (88.9%) | ||
| Year of diagnosis | 2007 ± 5.74 | 2004 ± 6.86 | < 0.0001 | 2007 ± 5.74 | 2007 ± 6.05 | 0.751 |
| Marital status | 0.256 | 0.430 | ||||
| Married | 101 (54.9%) | 1456 (54.3%) | 101 (54.9%) | 295 (53.4%) | ||
| Separated† | 54 (29.3%) | 889 (33.2%) | 54 (29.3%) | 183 (33.2%) | ||
| Single | 19 (10.3%) | 255 (9.5%) | 19 (10.3%) | 57 (10.3%) | ||
| Unknown | 10 (5.4%) | 81 (3.0%) | 10 (5.4%) | 17 (3.1%) | ||
| Insurance‡ | N = 112 | N = 1147 | 0.215 | N = 112 | N = 335 | 0.037 |
| Insured | 96 (85.7%) | 953 (83.1%) | 96 (85.7%) | 280 (83.6%) | ||
| Medicaid | 11 (9.8%) | 159 (13.9%) | 11 (9.8%) | 49 (14.6%) | ||
| Uninsured | 5 (4.5%) | 24 (2.1%) | 5 (4.5%) | 3 (0.9%) | ||
| Unknown | 0 (0.0%) | 11 (1.0%) | 0 (0.0%) | 3 (0.9%) | ||
| Location | < 0.001 | 0.005 | ||||
| Upper lobe | 125 (67.9%) | 1466 (54.7%) | 125 (67.9%) | 321 (58.2%) | ||
| Middle lobe | 6 (3.3%) | 176 (6.6%) | 6 (3.3%) | 36 (6.5%) | ||
| Lower lobe | 50 (27.2%) | 817(30.5%) | 50 (27.2%) | 153 (27.7%) | ||
| Others | 3 (1.6%) | 222 (8.3%) | 3 (1.6%) | 42 (7.6%) | ||
| Grade | < 0.001 | < 0.001 | ||||
| Grade I/II | 13 (7.1%) | 45 (1.7%) | 13 (7.1%) | 13 (7.1%) | ||
| Grade III/IV | 125 (67.9%) | 1263 (47.1%) | 125 (67.9%) | 242 (43.8%) | ||
| Unknown | 46 (25.0%) | 1373 (51.2%) | 46 (25.0%) | 303 (54.9%) | ||
| Laterality | 0.796 | 0.830 | ||||
| Left | 84 (45.7%) | 1200 (44.8%) | 84 (45.7%) | 247 (44.7%) | ||
| Right | 100 (54.3%) | 1475 (55.0%) | 100 (54.3%) | 304 (55.1%) | ||
| Unilateral | 0 (0.0%) | 6 (0.2%) | 0 (0.0%) | 1 (0.2%) | ||
| Operation | 0.0005 | < 0.001 | ||||
| No surgery | 34 (18.5%) | 1850 (69.0%) | 34 (18.5%) | 402 (72.8%) | ||
| Lobectomy | 112 (60.9%) | 573 (21.4%) | 112 (60.9%) | 95 (17.2%) | ||
| Pneumonectomy | 3 (1.6%) | 25 (0.9%) | 3 (1.6%) | 4 (0.7%) | ||
| Sublobar | 35 (19.0%) | 233 (8.7%) | 35 (19.0%) | 51 (9.2%) | ||
| Chemotherapy | 102 (55.4%) | 2224 (83.0%) | < 0.0001 | 102 (55.4%) | 461 (83.5%) | < 0.001 |
| Radiation | 56 (30.4%) | 1621 (60.5%) | < 0.0001 | 56 (30.4%) | 357 (64.7%) | < 0.001 |
| Treatment | < 0.0001 | < 0.001 | ||||
| CTx | 10 (5.4%) | 443 (16.5%) | 10 (5.4%) | 80 (14.5%) | ||
| CRT | 14 (7.6%) | 1263 (47.1%) | 14 (7.6%) | 288 (52.2%) | ||
| RT | 10 (5.4%) | 144 (5.4%) | 10 (5.4%) | 34 (6.2%) | ||
| Surgery | 69 (37.5%) | 298 (11.1%) | 69 (37.5%) | 55 (10.0%) | ||
| Surgery + CTx | 49 (26.6%) | 319 (11.9%) | 49 (26.6%) | 60 (10.9%) | ||
| Surgery + CRT | 29 (15.8%) | 199 (7.4%) | 29 (15.8%) | 33 (6.0%) | ||
| Surgery + RT | 3 (1.6%) | 15 (0.6%) | 3 (1.6%) | 2 (0.4%) | ||
| T stage (8th edition TNM) | 0.077 | 0.889 | ||||
| T1a | 9 (4.9%) | 125 (4.7%) | 9 (4.9%) | 31 (5.6%) | ||
| T1b | 49 (26.6%) | 652 (24.3%) | 49 (26.6%) | 139 (25.2%) | ||
| T1c | 44 (23.9%) | 655 (24.4%) | 44 (23.9%) | 138 (25.0%) | ||
| T2a | 68 (37.0%) | 847 (31.6%) | 68 (37.0%) | 191 (34.6%) | ||
| T2b | 14 (7.6%) | 402 (15.0%) | 14 (7.6%) | 53 (9.6%) | ||
| T stage (2010–2014) | N = 69 | N = 729 | 0.999 | N = 69 | N = 105 | 0.807 |
| T1 | 40 (58.0%) | 422 (57.6%) | 40 (58.0%) | 129 (55.4%) | ||
| T2 | 29 (42.0%) | 309 (42.4%) | 29 (42.0%) | 104 (44.6%) | ||
| T stage (2005–2009) | N = 80 | N = 886 | 0.802 | N = 80 | N = 214 | 0.712 |
| T1 | 40 (50.0%) | 462 (52.1%) | 40 (50.0%) | 114 (53.3%) | ||
| T2 | 40 (50.0%) | 424 (47.9%) | 40 (50.0%) | 100 (46.7%) | ||
| T stage (1988–2004) | N = 35 | N = 1066 | 0.254 | N = 35 | N = 105 | 0.999 |
| T1 | 22 (62.9%) | 550 (51.6%) | 22 (62.9%) | 65 (61.9%) | ||
| T2 | 13 (37.1%) | 516 (48.5%) | 13 (37.1%) | 40 (38.1%) | ||
| N stage | 0.092 | 0.999 | ||||
| N0 | 144 (78.3%) | 1937 (72.2%) | 144 (78.3%) | 432 (78.3%) | ||
| N1 | 40 (21.7%) | 744 (27.8%) | 40 (21.7%) | 120 (21.7%) | ||
| Stage | 0.117 | 0.996 | ||||
| IA1 | 9 (4.9%) | 90 (3.4%) | 9 (4.9%) | 27 (4.9%) | ||
| IA2 | 40 (21.7%) | 486 (18.1%) | 40 (21.7%) | 110 (19.9%) | ||
| IA3 | 34 (18.5%) | 464 (17.3%) | 34 (18.5%) | 102 (18.5%) | ||
| IB | 49 (26.6%) | 610 (22.8%) | 49 (26.6%) | 157 (28.4%) | ||
| IIA | 12 (6.5%) | 287 (10.7%) | 12 (6.5%) | 36 (6.5%) | ||
| IIB | 40 (21.7%) | 744 (27.8%) | 40 (21.7%) | 120 (21.7%) | ||
| Stage (2010–2014) | N = 69 | N = 729 | N = 69 | N = 233 | ||
| I | 59 (85.5%) | 495 (67.9%) | 59 (85.5%) | 179 (76.8%) | ||
| II | 10 (14.5%) | 234 (32.1%) | 0.004 | 10 (14.5%) | 54 (23.2%) | 0.167 |
| Stage (2004–2009) | N = 80 | N = 886 | 0.999 | N = 80 | N = 214 | 0.250 |
| I | 57 (71.3%) | 629 (71.0%) | 57 (71.3%) | 168 (78.5%) | ||
| II | 23 (28.7%) | 257 (29.0%) | 23 (28.7%) | 46 (21.5%) | ||
| Stage (1988–2004) | N = 35 | N = 1066 | 0.748 | N = 35 | N = 105 | 0.999 |
| I | 28 (80.0%) | 812 (76.2%) | 28 (80.0%) | 84 (80.0%) | ||
| II | 7 (20.0%) | 254 (23.8%) | 7 (20.0%) | 21 (20.0%) | ||
Separated” includes divorce or widowed.
Data available from the 2007 or later. CTx, chemotherapy; CRT, chemoradiation; RT, radiotherapy; TNM, tumor node metastasis.
Under the 8th edition TNM staging, there was no difference in the distribution of T, N, or stages between the groups. The same findings were demonstrated in each period under the corresponding staging system. More than 70% of the patients in the study cohort were N0. The mean survival durations were 39.4 ± 42.3 months for C‐SCLCs and 34.0 ± 41.5 months for NC‐SCLCs.
We matched the study cohort to balance the stage and period. The absolute and standardized mean differences were < 0.10 in all matched variables (Table S2, Fig S2), which is indicative of well‐balanced matching. The basic demographic features and tumor characteristics remained the same after matching (Table 1).
Effect of histological subtype and predictors of lung cancer‐specific survival
Lung CSS decreased as the stage progressed in the entire pre‐matched dataset (Fig 2a). The three‐year CSS rates were 52.7%, 49.7%, 43.4%, 39.4%, 35.2%, and 35.2% for stage I1A, IA2, IA3, IB, IIA, and IIB, respectively. C‐SCLCs showed better CSS than NC‐SCLCs before matching (Fig 2b), but this difference was only evident in the N0 subset, not in the N1 subset (Fig 3).
Figure 2.
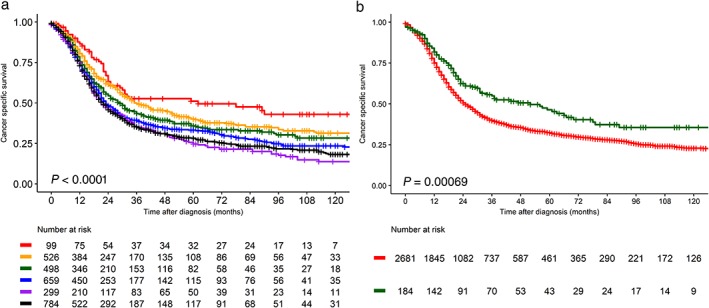
Lung cancer‐specific survival curves for the entire small cell lung cancer group at T1–2 N0–1 M0 stage (a) according to the 8th edition tumor node metastasis (TNM) system, Stages ( ) IA1, (
) IA1, ( ) IA2, (
) IA2, ( ) IA3, (
) IA3, ( ) IB, (
) IB, ( ) IIA and (
) IIA and ( ) IIB, and (b) combined SCLC and SCLC before matching. Histology (
) IIB, and (b) combined SCLC and SCLC before matching. Histology ( ) combined variant SCC and (
) combined variant SCC and ( ) SCLC. The P value was obtained with the log‐rank test.
) SCLC. The P value was obtained with the log‐rank test.
Figure 3.
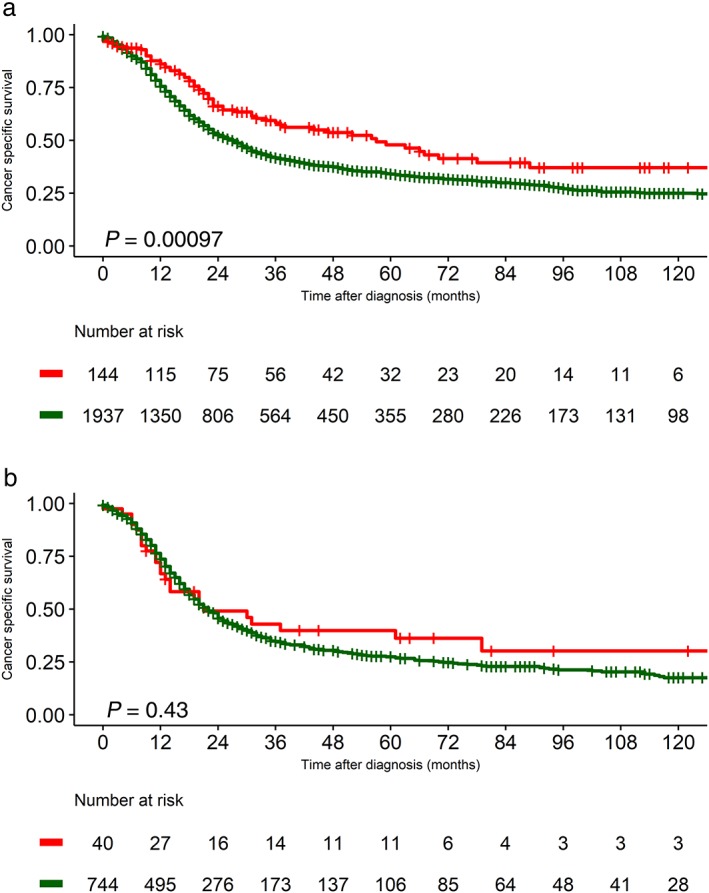
Lung cancer‐specific survival of combined small cell lung cancer (C‐SCLC) versus SCLC before matching for (a) T1–2 N0 and (b) T1–2 N1 stages. The P value was obtained with the log‐rank test. Histology ( ) Combined variant SCLC and (
) Combined variant SCLC and ( ) SCLC.
) SCLC.
The effect of the treatment modality on the different histological subtypes was analyzed using the Kaplan–Meier method. In the entire N0 SCLC cohort, treatments including surgery showed better survival and surgery with chemoradiation showed the best CSS (Fig 4a). When the stage increased to N1, the survival benefits of the different treatment strategies were similar (Fig 4b), although therapeutic strategies involving surgery showed better CSS than nonsurgical therapies in N1 SCLC (P = 0.002) (Fig S3a).
Figure 4.
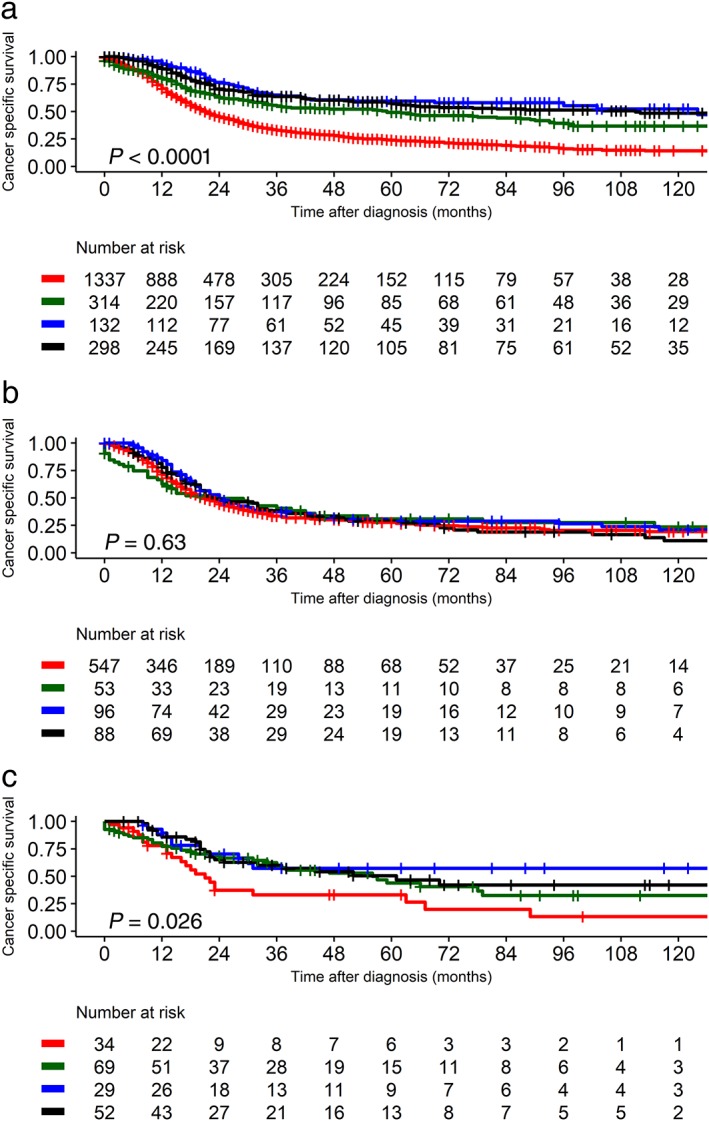
Lung cancer‐specific survival according to treatment (chemotherapy, radiation, and/or surgery) in (a) all T1–2 N0 small cell lung cancer (SCLC) patients before matching, (b) T1–2 N0 combined SCLC patients, and (c) all T1–2 N1 SCLC patients before matching. The P value was obtained with the log‐rank test. C/RT, chemotherapy with or without radiation; S, surgery; S + CRT, surgery with chemoradiation; S and/or CRT, surgery with either chemotherapy or radiation. Treatment ( ) C/RT, (
) C/RT, ( ) S, (
) S, ( ) S + CRT, (
) S + CRT, ( ) S and/or C/RT
) S and/or C/RT
In T1–2 N0–1 C‐SCLCs, surgical therapy was superior to chemotherapy or radiation (Fig 4c). For the C‐SCLC N0 group, chemotherapy did not directly affect CSS (P = 0.26) (Fig S3b). In addition, either lobectomy or sublobar resection showed better CSS than nonsurgical treatment (P = 0.01) (Fig S3c), although the number of sublobar resections was low.
To assess the prognostic factors, univariate Cox proportional hazards analysis was performed. After matching, C‐SCLC histology; age at diagnosis; lobectomy or sublobar resection rather than no surgery; two or more combined modalities rather than chemotherapy alone, especially treatment involving surgery; and earlier stages were associated with significantly better CSS (Table 2).
Table 2.
Univariate Cox proportional hazards analysis of the risk factors for lung cancer‐specific survival in surgically resected early SCLC (TN and period matching)
| Variable | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Pathology | < 0.001 | |||||
| NC‐SCLC | 1 | 1 | ||||
| C‐SCLC | 0.693 | 0.560–0.857 | 0.729 | 0.575–0.924 | 0.009 | |
| Age | 1.025 | 1.02–1.031 | < 0.001 | 1.026 | 1.015–1.037 | < 0.001 |
| Gender: male | 1.149 | 1.045–1.264 | 0.0042 | 1.111 | 0.910–1.357 | 0.301 |
| Race | ||||||
| African‐American | 1 | 1 | ||||
| Other | 1.135 | 0.857–1.504 | 0.376 | 1.192 | 0.603–2.354 | 0.614 |
| White | 0.991 | 0.825–1.190 | 0.921 | 1.244 | 0.816–1.898 | 0.310 |
| Year | 0.985 | 0.978–0.992 | < 0.001 | 0.992 | 0.975–1.009 | 0.354 |
| Marital status | ||||||
| Married | 1 | 1 | ||||
| Separated | 1.057 | 0.951–1.174 | 0.304 | 1.028 | 0.825–1.282 | 0.807 |
| Single | 0.819 | 0.684–0.980 | 0.029 | 0.809 | 0.557–1.173 | 0.263 |
| Unknown | 1.306 | 1.002–1.703 | 0.049 | 1.376 | 0.839–2.257 | 0.205 |
| Insurance | ||||||
| Insured | 1 | 1 | ||||
| Medicaid | 1.186 | 0.929–1.515 | 0.171 | 1.022 | 0.671–1.556 | 0.919 |
| Uninsured | 1.004 | 0.564–1.775 | 0.999 | 0.678 | 0.216–2129 | 0.505 |
| Unknown | 0.802 | 0.300–2.147 | 0.661 | 0.431 | 0.060–3.080 | 0.401 |
| Location | ||||||
| Lower | 1 | 1 | ||||
| Middle | 0.992 | 0.808–1.219 | 0.940 | 0.869 | 0.539–1.399 | 0.563 |
| Others | 1.171 | 0.975–1.407 | 0.092 | 1.269 | 0.843–1.911 | 0.254 |
| Upper | 0.905 | 0.813–1.007 | 0.068 | 0.943 | 0.751–1.185 | 0.614 |
| Laterality | ||||||
| Left | 1 | 1 | ||||
| Right | 1.024 | 0.931–1.128 | 0.623 | 0.969 | 0.792–1.185 | 0756 |
| Other | 2.622 | 1.088–6.321 | 0.032 | 0.796 | 0.111–5.689 | 0.819 |
| Operation | ||||||
| No surgery | 1 | 1 | ||||
| Lobectomy | 0.488 | 0.431–0.553 | < 0.001 | 0.510 | 0.398–0.652 | < 0.001 |
| Pneumonectomy | 0.918 | 0.590–1.428 | 0.704 | 0.513 | 0.191–1.377 | 0.185 |
| Sublobar | 0.673 | 0.566–0.799 | < 0.00 | 0.565 | 0.400–0.780 | 0.001 |
| CTx | 1.106 | 0.975–1.255 | 0.116 | 1.178 | 0.922–1.505 | 0191 |
| RT | 0.981 | 0.891–1.081 | 0.700 | 1.07 | 0.874–1.309 | 0.515 |
| Treatment | ||||||
| CTx | 1 | 1 | ||||
| CRT | 0.504 | 0.444–0.573 | < 0.001 | 0.479 | 0.360–0.637 | < 0.001 |
| RT | 0.732 | 0.583–0.919 | 0.008 | 0.527 | 0.325–0.854 | 0.009 |
| Surgery | 0.370 | 0.309–0.443 | < 0.001 | 0.332 | 0.232–0.476 | < 0.001 |
| Surgery + CTx | 0.293 | 0.244–0.352 | < 0.001 | 0.258 | 0.176–0.380 | < 0.001 |
| Surgery + CRT | 0.311 | 0.250–0.386 | < 0.001 | 0.257 | 0.161–0.411 | < 0.001 |
| Surgery + RT | 0.538 | 0.309–0.936 | 0.028 | 0.557 | 0.203–1529 | 0.256 |
| TNM stage | ||||||
| IA1 | 1 | 1 | ||||
| IA2 | 1.318 | 0.959–1.813 | 0.089 | 1.072 | 0.609–1.890 | 0.809 |
| IA3 | 1.514 | 1.101–2.083 | 0.011 | 1.641 | 0.943–2.855 | 0.080 |
| IB | 1.736 | 1.272–2.371 | 0.001 | 1.466 | 0.852–2.523 | 0.167 |
| IIA | 1.991 | 1.439–2.756 | < 0.001 | 2.069 | 1.132–3.783 | 0.018 |
| IIB | 1.893 | 1.390–2.577 | < 0.001 | 1.669 | 0.967–2.880 | 0.066 |
CI, confidence interval; C‐SCLC, combined small cell lung cancer; CTx, chemotherapy; CRT, chemoradiation; HR, hazard ratio; NC‐SCLC, non‐combined SCLC; RT, radiotherapy; TNM, tumor node metastasis.
We performed two types of multivariate Cox proportional hazards analysis: a multivariate model stratified by matching variables, and a multivariate model adjusted with matching variables. After model fitting, the final result indicated that the histological subtype was not associated with CSS (Table 3) in either model. Age and treatment modality were significantly associated with CSS. When the model was constructed with the combinations of treatments, surgery with chemoradiation (hazard ratio [HR] 0.31, 95% confidence interval [CI] 0.19–0.52; P ≤ 0.001) most significantly reduced the risk of CSS. In addition, when the model was fitted with the individual treatment options, lobectomy (HR 0.40, 95% CI 0.29–0.56; P < 0.001) showed better CSS than pneumonectomy or sublobar resection. The adjusted model also revealed that histological subtype was not related to CSS but a younger age and lobectomy was associated with better CSS.
Table 3.
Multivariate Cox proportional hazards analysis after matching
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Stratified model | |||
| C‐SCLC | 0.953 | 0.718–1.264 | 0.736 |
| Age | 1.022 | 1.010–1.034 | <0.001 |
| Treatment | |||
| CTx | 1.000 | ||
| CRT | 0.505 | 0.372–0.687 | < 0.001 |
| RT | 0.507 | 0.303–0.851 | 0.010 |
| Surgery | 0.378 | 0.257–0.559 | < 0.001 |
| Surgery + CTx | 0.302 | 0.200–0.460 | < 0.001 |
| Surgery + CRT | 0.313 | 0.189–0.517 | < 0.001 |
| Surgery + RT | 0.668 | 0.228–1.963 | 0.463 |
| Stratified model 2 | |||
| C‐SCLC | 0.993 | 0.750–1.315 | 0.961 |
| Age | 1.022 | 1.010–1.034 | < 0.001 |
| Operation | |||
| Lobectomy | 0.400 | 0.285–0.561 | < 0.001 |
| Pneumonectomy | 0.375 | 0.129–1.092 | 0.072 |
| Sublobar resection | 0.496 | 0.324–0.759 | 0.001 |
| CTx | 0.962 | 0.721–1.282 | 0.790 |
| RT | 0.625 | 0.479–0.814 | < 0.001 |
| Adjusted model | |||
| C‐SCLC | 1.005 | 0.763–1.324 | 0.972 |
| Age | 1.020 | 1.001–1.032 | < 0.001 |
| Gender: Male | 1.057 | 0.863–1.294 | 0.592 |
| Operation | |||
| Lobectomy | 0.389 | 0.279–0.543 | < 0.001 |
| Pneumonectomy | 0.379 | 0.138–1.044 | 0.061 |
| Sublobar | 0.442 | 0.296–0.660 | < 0.001 |
| CTx | 1.013 | 0.767–1.338 | 0.926 |
| RT | 0.616 | 0.477–0.795 | < 0.001 |
CI, confidence interval; C‐SCLC, combined small cell lung cancer; CTx, chemotherapy; CRT, chemoradiation; HR, hazard ratio; RT, radiotherapy.
To test the robustness of the results, we fitted a multivariate Cox proportional hazards model based on the previous staging systems, and the results were the same. The results are presented in the supplementary data (Table S3, S4).
Discussion
C‐SCLCs are an uncommon type of SCLC, with a reported incidence of 1–3%. The true frequency might actually be higher because small or crushed samples hinder the diagnosis of C‐SCLC. The present study included patients with microscopically confirmed diagnosis, and the incidence was 6.4% of T1–2 N0–1 stages (184/2681) under the 8th edition TNM staging system. This incidence coincides with findings by Babakoohi et al. of 5.1% (22 C‐SCLCs and 428 SCLCs).7 However, accurate preoperative diagnosis of C‐SCLCs is difficult, with the sample type potentially affecting diagnosis. Previously, the frequency of C‐SCLCs was higher in autopsy specimens (14.3%) than in biopsy or cytologic specimens (8.6%, P < 0.05).22 With the development of diagnostic technologies and the subsequent increase in early diagnosis of lung cancer, the diagnosis of C‐SCLC before treatment planning is expected to increase.
As mentioned, the standard treatments for SCLC are chemotherapy and radiotherapy. Since the International Association for the Study of Lung Cancer staging project showed that TNM staging provides more accurate prognosis and treatment options, most recommended guidelines for SCLC now include TNM staging.23 However, several prominent guidelines use combined approaches for staging and treatment. For example, staging and planning treatment requires the two‐stage method of the Veterans Administration Lung Study Group (VALG), whereas the choice of surgery and specific treatment requires the TNM system. There are also discrepancies among guidelines in the terms of the two‐stage system. The NCCN and Chinese Society of Clinical Oncology (CSCO) guidelines adopt the terms “limited” and “extensive” stage, whereas the ESMO uses “localized” and “metastatic” disease.15, 24, 25
Most differences refer to the benefit and role of surgery in SCLC. The NCCN guidelines state that after staging work‐up and confirmation of pathologically negative N2 nodes, the patients most likely to benefit from surgery have clinical stage I–IIA (T1–2 N0 M0) SCLC. This corresponds with the CSCO guidelines, while the ESMO guidelines broaden the indication of surgery to T1–2 N0–1 M0 SCLC and emphasize that surgery in this group can be justified after mediastinal lymph node involvement is ruled out via computed tomography, positron emission tomography‐computed tomography, endobronchial ultrasound, and/or mediastinoscopy.26 As an alternative to surgery, the NCCN recommends stereotactic radioablation or concurrent chemoradiation, while the ESMO clearly states that concurrent chemoradiation is the standard of care.
There are two basic questions over C‐SCLC: (i) Should C‐SCLC be treated differently from SCLC? and (ii) What is the prognosis of C‐SCLC? To address the first question, there are no specific recommendations, and C‐SCLCs are currently treated based on SCLC guidelines. The benefit of surgery in early resectable SCLC has recently been demonstrated; however, the role of surgery or outcome in C‐SCLC is inconclusive because of the lack of large studies. Our results show that the treatment strategy depends on the histological subtype. Surgery was performed 2.6 times more frequently in the C‐SCLC group, and 37.5% of C‐SCLCs were treated with surgery alone. Surgery with additional chemotherapy or radiation showed better CSS. Takei et al. reviewed the surgical results of 243 patients with SCLC from the Japanese Lung Cancer Registry.27 The most common mode of surgery was lobectomy/bilobectomy (n = 174, 71.6%), and sublobar resection was performed in 21.0%, which was similar to our results. The five‐year overall survival (OS) was 52.6%, and 68% of patients were stage I or II. Because of its rarity, there are few studies of C‐SCLC.
Babakoohi et al. compared 22 cases of C‐SCLC and 406 SCLC and reported that C‐SCLC patients were more likely to undergo surgery than SCLC patients (45% vs. 3%; P < 0.001) and that all surgical patients received adjuvant chemotherapy while the nonsurgical group received standard SCLC treatment.7 In the surgical subset, OS was 2.5 times worse in pure SCLC than C‐SCLC, although the difference was statistically insignificant because of the sample size. Men et al. reported the treatment outcomes of 114 cases of C‐SCLC in a single institution; 70.2% of patients received ≥ 2 treatment modalities, and the five‐year OS was better in the surgical than in the nonsurgical group (48.9% vs. 36.6%). Although small in number, these reports suggest that the role of surgery is more important in patients with C‐SCLC.28
When surgery is considered, the current guidelines recommend standard lobectomy with mediastinal lymph node dissection. In this study, the main type of surgery was lobectomy (among surgical cases: C‐SCLCs, 74.6%; SCLCs, 69.0%). Considering the aggressiveness of the SCLC category and the rarity of C‐SCLC, the current evidence and our findings suggest that lobectomy should be performed as the surgery of choice. Surgery with or without chemotherapy or radiation is possibly the best choice for C‐SCLC in T1–2 N0 stages, as well as in SCLC of the same stage. This study includes the largest number of early‐stage C‐SCLC patients investigated; however, the choice, timing, and sequence of chemotherapy with or without radiation urgently require further study. In the SEER dataset, no information on the sequence of chemotherapy or prophylactic cranial irradiation is available.
There are conflicting reports over the prognosis of C‐SCLC. Zhao et al. reported poorer OS in C‐SCLC patients and concluded that there were no significant differences in clinicopathologic features between pure SCLC and C‐SCLC.5 Others studies reported better OS in patients with C‐SCLC.7, 28 However, the multivariate stratified and adjunctively fitted adjusted models revealed no statistically significant survival benefit of C‐SCLC over SCLC. Age at diagnosis and treatment strategies were more important. C‐SCLC patients may have shown better CSS for this variable before matching as such patients might benefit more from surgery than SCLC patients (81.5% of the C‐SCLC study cohort underwent surgery).
This study has several limitations. First, this is a retrospective study and there might be several confounding variables that could not be included in the analysis. The SEER database does not contain major comorbidity profiles, which could affect the choice of treatment. Second, no detailed data on chemotherapy was available, which is of paramount importance in SCLC treatment. The main regimens have changed over time, and the sequence of chemotherapy and radiation is unknown. Thus, to maximally decrease the bias, we matched the TN stage and the AJCC system edition used, and applied the 8th edition TNM system. The AJCC system used reflects the year of diagnosis, which is important because most chemotherapy regimens administered would thus be similar. Notwithstanding these limitations, the SEER database is well managed and the quality of the data has been verified. The results of this study lead to further questions: Which should be first, concurrent chemoradiation or surgery? Is there a possible role of surgery for treating N1 disease? Should we consider the amount of the NSCLC component in the treatment process? Further study is required to address these questions.
In conclusion, analysis of a population‐based dataset revealed that surgery with adjunctive chemotherapy or radiation is associated with better survival than chemoradiation alone for T1–2 N0 SCLC and C‐SCLC. This study did not determine any differences between treatment strategies in T1–2 N1 disease; however, in selected patients, treatment including surgery may lead to favorable long‐term survival. Well‐planned and individualized multimodality treatment will improve patient outcomes. Finally, the histological subtype was not associated with CSS.
Disclosure
No authors report any conflict of interest.
Supporting information
Figure S1. Distributions of T, N, M, and stages in primary small cell lung cancer (SCLC). C‐SCLC, combined SCLC; NC‐SCLC, non‐combined SCLC.
Figure S2. Absolute standardized differences before and after matching.
Figure S3. (a) Lung cancer‐specific survival (CSS) curves according to surgery in all N1 small cell lung cancer (SCLC) patients before matching. Sixteen cases of pneumonectomy are not shown in this graph. (b) CSS curves of patients according to whether chemotherapy was performed as part of the treatment or not in the N0 combined SCLC (C‐SCLC) patients before matching. (c) CSS curves according to surgery type in the N0 C‐SCLC patients before matching. One pneumonectomy patient at C‐SCLC T1–2 N0 stage is not included in this graph.
Table S1. General features of combined small cell lung cancer (C‐SCLC) and non‐combined SCLC (NC‐SCLC) at the time of diagnosis
Table S2. Balance measured by standardized mean differences before and after matching
Table S3. Multivariate Cox proportional hazards analysis after matching with the previous staging system
Table S4. Balance measured by standardized mean differences before and after matching with the previous matching system
Acknowledgments
We would like to give special thanks to the biostatistical consulting team of our institute. We also acknowledge the efforts of the SEER tumor registry team.
References
- 1. Alvarado‐Luna G, Morales‐Epinosa D. Treatment for small cell lung cancer: Where are we now? A review. Transl Lung Cancer Res 2016; 5: 26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. David L. Histological classification of lung cancer. Thorax 1984; 39: 161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Travis WD, Brambilla E, Nicholson AG et al The 2015 World Health Organization Classification of Lung Tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015; 10: 1243–60. [DOI] [PubMed] [Google Scholar]
- 4. Nicholson SA, Beasley MB, Brambilla E et al Small cell lung carcinoma (SCLC): A clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol 2002; 26: 1184–97. [DOI] [PubMed] [Google Scholar]
- 5. Zhao X, McCutcheon JN, Kallakury B et al Combined small cell carcinoma of the lung: Is it a single entity? J Thorac Oncol 2018; 13: 237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Govindan R, Page N, Morgensztern D et al Changing epidemiology of small‐cell lung cancer in the United States over the last 30 years: Analysis of the Surveillance, Epidemiologic, and End Results database. J Clin Oncol 2006; 24: 4539–44. [DOI] [PubMed] [Google Scholar]
- 7. Babakoohi S, Fu P, Yang M, Linden PA, Dowlati A. Combined SCLC clinical and pathologic characteristics. Clin Lung Cancer 2013; 14: 113–9. [DOI] [PubMed] [Google Scholar]
- 8. Zhang C, Yang H, Zhao H et al Clinical outcomes of surgically resected combined small cell lung cancer: A two‐institutional experience. J Thorac Dis 2017; 9: 151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fox W, Scadding JG. Medical Research Council comparative trial of surgery and radiotherapy for primary treatment of small‐celled or oat‐celled carcinoma of bronchus. Ten‐year follow‐up. Lancet 1973; 14: 63–5. [DOI] [PubMed] [Google Scholar]
- 10. Mountain CF. Clinical biology of small cell carcinoma: Relationship to surgical therapy. Semin Oncol 1978; 5: 272–9. [PubMed] [Google Scholar]
- 11. Combs SE, Hancock JG, Boffa DJ, Decker RH, Detterbeck FC, Kim AW. Bolstering the case for lobectomy in stages I, II, and IIIA small‐cell lung cancer using the National Cancer Data Base. J Thorac Oncol 2015; 10: 316–23. [DOI] [PubMed] [Google Scholar]
- 12. Paximadis P, Beebe‐Dimmer JL, George J, Schwartz AG, Wozniak A, Gadgeel S. Comparing treatment strategies for stage I small‐cell lung cancer. Clin Lung Cancer 2018; 19: e559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takenaka T, Takenoyama M, Inamasu E et al Role of surgical resection for patients with limited disease‐small cell lung cancer. Lung Cancer 2015; 88: 52–6. [DOI] [PubMed] [Google Scholar]
- 14. Tsuchiya R, Suzuki K, Ichinose Y et al Phase II trial of postoperative adjuvant cisplatin and etoposide in patients with completely resected stage I‐IIIa small cell lung cancer: The Japan Clinical Oncology Lung Cancer Study Group Trial (JCOG9101). J Thorac Cardiovasc Surg 2005; 129: 977–83. [DOI] [PubMed] [Google Scholar]
- 15. National Comprehensive Cancer Network . NCCN Guidelines ‐ Small Cell Lung Cancer (Version 1.2019). 2018. [Cited 10 October 2018.] Available from URL: https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf.
- 16. Sawabata N, Miyaoka E, Asamura H et al, Japanese Joint Committee for Lung Cancer Registration. Japanese lung cancer registry study of 11,663 surgical cases in 2004: Demographic and prognosis changes over decade. J Thorac Oncol 2011; 6: 1229–35. [DOI] [PubMed] [Google Scholar]
- 17. Edge SB, Compton C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2018; 17: 1471–4. [DOI] [PubMed] [Google Scholar]
- 18. Greene FL, Page D, Fleming ID et al Lung, In: AJCC cancer staging manual, 6th edn. Springer, New York: 2002; 167–78. [Google Scholar]
- 19. Beahrs OH, Henson DE, Hutter RVP, Myers MH. Lung, In: Manual for staging of cancer, 3rd edn. J.B. Lippincott Company, Philadelphia: 1988; 115–22. [Google Scholar]
- 20. Goldstraw P, Chansky K, Rami‐Porta R et al The IASLC Lung Cancer Staging Project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2015; 11: 39–51. [DOI] [PubMed] [Google Scholar]
- 21. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med 2009; 28: 3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fushimi H, Kikui M, Morino H et al, International Association for the Study of Lung Cancer International Staging Committee. Histologic changes in small cell lung carcinoma after treatment. Cancer 1996; 77: 278–83. [DOI] [PubMed] [Google Scholar]
- 23. Giroux DJ, Rami‐Porta R, Chansky K et al The IASLC Lung Cancer Staging Project: Data elements for the prospective project. J Thorac Oncol 2009; 4: 679–83. [DOI] [PubMed] [Google Scholar]
- 24. Zhao H, Ren D, Liu H, Chen J. Comparison and discussion of the treatment guidelines for small cell lung cancer. Thorac Cancer 2018; 9: 769–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhi XY, Yu JM, Shi YK. Chinese guidelines on the diagnosis and treatment of primary lung cancer (2015 version). Cancer 2015; 121 (Suppl 17): 3165–81. [DOI] [PubMed] [Google Scholar]
- 26. Früh M, De Ruysscher D, Popat S et al, ESMO Guidelines Working Group. Small‐cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2013; 24: vi99–105. [DOI] [PubMed] [Google Scholar]
- 27. Takei H, Kondo H, Miyaoka E et al, Japanese Joint Committee of Lung Cancer Registry. Surgery for small cell lung cancer: A retrospective analysis of 243 patients from Japanese Lung Cancer Registry in 2004. J Thorac Oncol 2014; 9: 1140–5. [DOI] [PubMed] [Google Scholar]
- 28. Men Y, Hui Z, Liang J et al Further understanding of an uncommon disease of combined small cell lung cancer: Clinical features and prognostic factors of 114 cases. Clin J Cancer Res 2016; 28: 486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Distributions of T, N, M, and stages in primary small cell lung cancer (SCLC). C‐SCLC, combined SCLC; NC‐SCLC, non‐combined SCLC.
Figure S2. Absolute standardized differences before and after matching.
Figure S3. (a) Lung cancer‐specific survival (CSS) curves according to surgery in all N1 small cell lung cancer (SCLC) patients before matching. Sixteen cases of pneumonectomy are not shown in this graph. (b) CSS curves of patients according to whether chemotherapy was performed as part of the treatment or not in the N0 combined SCLC (C‐SCLC) patients before matching. (c) CSS curves according to surgery type in the N0 C‐SCLC patients before matching. One pneumonectomy patient at C‐SCLC T1–2 N0 stage is not included in this graph.
Table S1. General features of combined small cell lung cancer (C‐SCLC) and non‐combined SCLC (NC‐SCLC) at the time of diagnosis
Table S2. Balance measured by standardized mean differences before and after matching
Table S3. Multivariate Cox proportional hazards analysis after matching with the previous staging system
Table S4. Balance measured by standardized mean differences before and after matching with the previous matching system


