Abstract
Long noncoding RNA (lncRNA) DLEU2 has been shown to be dysregulated in several type of tumor. However, the potential biological roles and molecular mechanisms of DLEU2 in pancreatic cancer (PC) progression are poorly understood. In this study, we found that the DLEU2 level was substantially upregulated in PC tissues and PC cell lines, and significantly associated with poor clinical outcomes in PC patients. Overexpression of DLEU2 significantly induced PC cell proliferation and invasion, whereas knockdown of DLEU2 impaired cell proliferation and invasion in vitro. Furthermore, bioinformatics analysis, luciferase reporter assay, and RNA immunoprecipitation assay revealed that DLEU2 directly bond to microRNA‐455 (miR‐455) and functioned as an endogenous sponge for miR‐455, which could remarkably suppress cell growth and invasion. We also determined that SMAD2 was a direct target of miR‐455, and the restoration of SMAD2 rescued cell growth and invasion that were reduced by DLEU2 knockdown or miR‐455 overexpression. In addition, low miR‐455 expression and high SMAD2 expression was correlated with poor patient survival. These results indicate that DLEU2 is an important promoter of PC development, and targeting the DLEU2/miR‐455/SMAD2 pathway could be a promising therapeutic approach in the treatment of PC.
Keywords: DLEU2, long noncoding RNA, microRNA‐455, pancreatic cancer, SMAD2
Abbreviations
- Ago2
Argonaute2
- lncRNA
long noncoding RNA
- miRNA
microRNA
- PC
pancreatic cancer
- qRT‐PCR
quantitative real‐time PCR
- RIP
RNA immunoprecipitation assay
1. INTRODUCTION
Pancreatic cancer is one of the most lethal malignancies, with a 5‐year survival of 8%.1 There is currently no effective treatment for metastatic PC.1 Investigating the genetic and epigenetic landscape of PC would improve our understanding of the molecular mechanisms underlying its initiation, aggressive progression, and therapy resistance and would lead to better prevention, diagnosis, and treatment of PC.
Long noncoding RNAs refer to noncoding RNAs that are more than 200 nt in length. They have received considerable attention in recent years because of their potential to regulate diverse biological processes such as tumor progression and metastasis.2 According to regulatory mechanisms on the expression of target genes, lncRNAs can work as signals, decoys, scaffolds, guides, and competing endogenous RNAs.3
The lncRNA DLEU2 is transcribed from chromosome 13q14.3;4 it is highly expressed in PC samples compared to normal tissues.5, 6 However, the role and the molecular mechanisms of DLEU2 in PC remain elusive. In this study, we aimed to explore the role and the underlying mechanisms of DLEU2 during PC progression. We showed that DLEU2 was overexpressed in PC, and it promoted cancer cell proliferation and invasion through modulating the miR‐455/SMAD2 pathway.
2. MATERIALS AND METHODS
2.1. Cell lines and transfection
Human PC cell lines AsPC‐1 and PaCa‐2 and human pancreatic duct epithelial cell line HPDE6‐C7 were obtained from ATCC (Rockville, MD, USA). The cells were cultured in RPMI‐1640 or DMEM (Gibco, Waltham, MA, USA) supplemented with 10% FBS. The DLEU2 expression plasmid (pcDNA3.1‐DLEU2) containing DLEU2 (variant uc001vdy.3), and the SMAD2 expression plasmid pcDNA3.1‐SMAD2 were constructed by GenePharma (Shanghai, China). In addition, siRNAs targeting DLEU2, siRNAs targeting SMAD2, control siRNA, miR‐455 mimic, miR‐15a mimic, miR‐16 mimic, control mimic, miR‐455 inhibitor, and control inhibitor were prepared by RiboBio (Guangzhou, China). Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) was used for cell transfection following the manufacturer's instructions.
2.2. RNA extraction and qRT‐PCR assay
Total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. RNA was reverse transcribed using the PrimeScript RT reagent Kit (Takara China, Dalian, China).The qPCR analysis was carried out using SYBR Premix EX Taq (Takara China, Dalian, China). GAPDH was used as an internal control for lncRNA. The primers for DLEU2 and GAPDH have been reported.7 The mirVana qRT‐PCR microRNA Detection Kit (Ambion, Austin, TX, USA) was used for miR‐455 detection according to the manufacturer's instructions. The primers for miR‐455 were obtained from RiboBio. Human U6 RNA was used as an internal control for miRNA. The results were analyzed and expressed relative to threshold cycle values and converted to fold changes. Experiments were repeated at least 3 times.
2.3. Western blot analysis
The entire protein lysates were split by 10% SDS‐PAGE. The split protein was transferred into a PVDF membrane (Millipore, Bedford, MA, USA). The above membranes were incubated with the primary Abs SMAD2 (1:1000 dilution; Abcam, Cambridge, UK) and GAPDH (1:1000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight. The membranes were then treated with secondary Ab conjugated to HRP for 2 hours at room temperature. Detection was undertaken using an ECL western blotting kit (Amersham Biosciences, Little Chalfont, UK). GAPDH was used as the loading control. The intensities of protein bands were quantitated using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
2.4. Proliferation assay
The CCK‐8 assay (Beyotime Institute of Biotechnology, Jiangsu, China) was applied to evaluate cell proliferation. Pancreatic cancer cells (5 × 103 cells per well in 96‐well plates) were transfected and cell proliferation was recorded 72 hours after transfection following the manufacturer's instructions. The absorbance was measured at 450 nm by a microplate reader (Bio‐Rad, Hercules, CA, USA).
2.5. Invasion assay
The invasion assay was carried out using Transwell chambers (24 wells; Corning, Tewksbury, MA, USA) and a previously described protocol.8, 9 Cells (2 × 104) were seeded on the top side of the Transwell filter coated with Matrigel in the chamber. Cells were suspended in the serum‐free medium. The medium supplemented with 10% FBS was added into the lower chamber. After culturing for 24 hours, cells that had invaded the membrane were fixed with 75% methanol and stained with 0.1% crystal violet. The number of invaded cells was observed using an inverted microscope and calculated by counting 5 random views. All experiments were carried out in triplicate.
2.6. Luciferase assay
The fragment of WT DLEU2 (DLEU2‐WT) containing the predicted potential miR‐455 binding site were amplified by PCR. The forward primer was 5′‐CGGCGATCGCCCCCAGAGTTTGCCTGTTT‐3′. The reverse primer was 5′‐ CGGCGGCCGCGCTTTCTCAATTATGGGACAACA‐3′. DLEU2‐WT was then cloned into the luciferase reporter vector pMIR‐REPORT (Promega, Madison, WI, USA) between the SgfI and NotI sites. To produce mutant DLEU2 (DLEU2‐MUT), the mutation was generated using QuikChange II XL Site‐Directed Mutagenesis Kit (Stratagene, San Diego, CA, USA). The WT SMAD2 3′‐UTR (SMAD2‐WT) carrying the complementary site for miR‐455 was amplified by PCR. The forward primer was 5′‐CGGCGATCGCTTGCATTCTTGCATATTCTGGAGTT‐3′. The reverse primer was 5′‐CGGCGGCCGCGCACCGTGTGCAAACATCTC‐3′. SMAD2‐WT was cloned into the luciferase reporter vector pMIR‐REPORT (Promega) between the SgfI and NotI sites. The mutant SMAD2 3′‐UTR (SMAD2‐MUT) was generated using a QuikChange II XL Site‐Directed Mutagenesis Kit (Stratagene). Pancreatic cancer cells were placed in a 24‐well plate and transfected with luciferase plasmids together with miR‐455 mimic, miR‐455 inhibitor, or their respective controls, using Lipofectamine 3000 (Invitrogen). After 48 hours of transfection, firefly and Renilla luciferase activities were detected using the Dual‐Luciferase Reporter Assay System (Promega, Beijing, China). Firefly luciferase activity was normalized to that of Renilla luciferase.
2.7. RNA immunoprecipitation assay
Magna RIP RNA‐Binding Protein Immunoprecipitation Kit (Millipore) was utilized in RIP experiments in accordance with the manufacturer's suggestions. Pancreatic cancer cells were transfected with miR‐455 mimic or control mimic. After 48 hours, PC cells were harvested and lysed in complete RIP lysis buffer. Then the whole cell extract was immunoprecipitated with RIP buffer containing magnetic beads conjugated with anti‐Ago2 Ab (Millipore) or normal mouse IgG (Millipore) as a negative control. Samples were digested with proteinase K, and RNAs were isolated from the immunoprecipitation products. Ultimately, the qRT‐PCR assay was utilized to detect the expression of DLEU2 and GAPDH. GAPDH mRNA was used as the negative control.
2.8. Statistical analysis
We used the SPSS 20.0 statistical software (IBM, Armonk, NY, USA) to statistically analyze the results. Data are presented as the mean ± SD from at least 3 experiments. The significant differences were analyzed using Student's t test or one‐way ANOVA. P‐value < .05 was regarded as significant.
3. RESULTS
3.1. DLEU2 is upregulated in PC cell lines and PC tissues
First, we examined DLEU2 expression in PC tissues and normal pancreatic tissues using the web‐based Oncomine microarray database (https://www.oncomine.org), and found that DLEU2 expression is elevated in PC tissues in comparison to normal pancreatic tissues (Figure 1A). Then the expression levels of DLEU2 were examined in PC tissues and normal pancreatic tissues using The Cancer Genome Atlas data through the web portal of UALCAN (http://ualcan.path.uab.edu/). We found that the DLEU2 expression was significantly higher in PC than corresponding normal tissues (Figure 1B). Then we measured the expression of DLEU2 in PC cell lines (AsPC‐1 and PaCa‐2) compared with normal pancreatic cell line HPDE6‐C7 using qRT‐PCR analysis. Our results indicated that AsPC‐1 and PaCa‐2 cells had higher levels of DLEU2 expression than the normal cell line HPDE6‐C7 (Figure 1C). We also used the UALCAN portal to investigate the relationship between DLEU2 expression and patient survival.10 Kaplan‐Meier analysis revealed that higher DLEU2 expression was associated with poor survival of patients with PC (Figure 1F).
Figure 1.
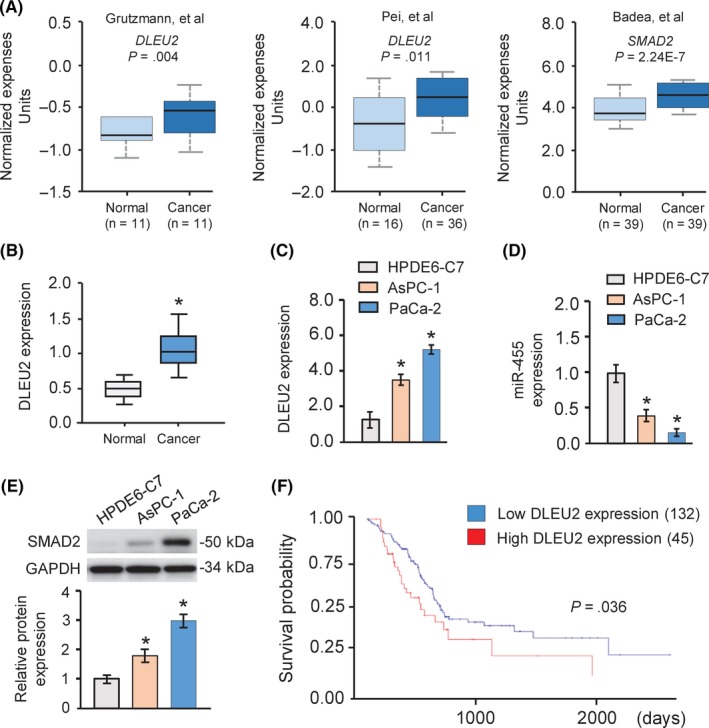
DLEU2 was upregulated in pancreatic cancer (PC) cell lines and tissues. A, Comparison of DLEU2 (left and middle panel) and SMAD2 (right panel) expression in normal pancreatic tissues and PC tissues based on Oncomine datasets. B, Expression of DLEU2 in human PC samples and normal pancreatic tissues. The Cancer Genome Atlas datasets were obtained from the UALCAN web server (http://ualcan.path.uab.edu/). C,D, Expression levels of DLEU2 (C) and microRNA‐455 (miR‐455) (D) in PC cell lines and normal pancreatic epithelial cell line HPDE6‐C7 were examined using quantitative real‐time PCR analysis. E, Images and quantification of SMAD2 protein expression levels in PC cell lines and normal cell line HPDE6‐C7 by western blot analysis. GAPDH was used as a normalization control. F, Survival curve for patients with high or low DLEU2 expression using the online tool UALCAN. *P < .05
3.2. DLEU2 promotes PC cell proliferation and invasion in vitro
We analyzed the expression of DLEU2 transcript variants in 178 patients with PC using RNA sequencing data available from the ISOexpresso database.11 We found that 9 DLEU2 transcript variants and a short variant of DLEU2 (uc001vdy.3, hg19; chr13:50,618,048‐50,656,139) are predominantly expressed in PC tissues (Figure 2A). These results suggested that uc001vdy.3 is the major variant among all variants in PC samples, and we focused on this variant in this study.
Figure 2.
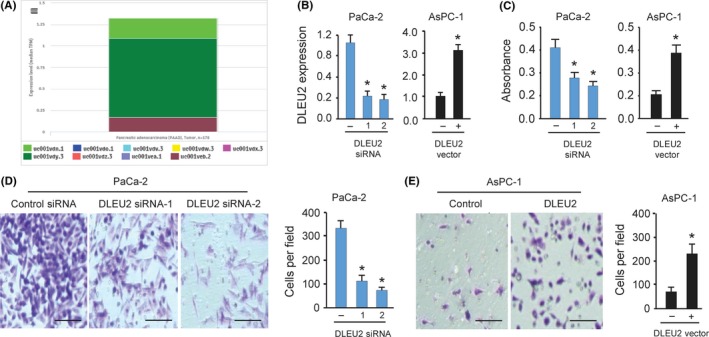
DLEU2 promotes proliferation and invasion of pancreatic cancer (PC) cells in vitro. A, Median expression levels in transcripts per million reads for all DLEU2 variants across 178 PC patient samples were obtained from the ISOexpresso platform. B, Quantitative real‐time PCR analysis of DLEU2 expression in PaCa‐2 cells after DLEU2 knockdown and in AsPC‐1 cells after DLEU2 overexpression. C, PC cells were transfected with DLEU2 siRNAs or control siRNA, DLEU2 vector or control vector, and cell proliferation was measured using a CCK‐8 assay. D, Invasion of PaCa‐2 cells was investigated after DLEU2 knockdown. Scale bar = 100 μm. E, Invasion of AsPC‐1 cells was measured after overexpression of DLEU2. Scale bar = 100 μm. *P < .05
To elucidate the biological functions of DLEU2 in PC tumorigenesis and progression, we silenced DLEU2 expression by transfection with 2 specific siRNAs targeting DLEU2 in PaCa‐2 cells that expresses the highest level of DLEU2. Upon transfection, DLEU2 expression decreased in the PaCa‐2 cells as measured by qRT‐PCR analysis (Figure 2B). Next, we transiently overexpressed DLEU2 in another PC line AsPC‐1 and confirmed that DLEU2 expression was significantly elevated in AsPC‐1 cells transfected with DLEU2 expression vector compared to control cells transfected with control vector (Figure 2B). Data from CCK‐8 assays and invasion assays showed that, compared to the control cells transfected with control siRNA, knockdown of DLEU2 significantly suppressed cell proliferation and invasion of PaCa‐2 cells (Figure 2C,D). Conversely, ectopic expression of DLEU2 accelerated the proliferative and invasive capacity of AsPC‐1 cells (Figure 2C,E). Together, these findings show that DLEU2 exerts an oncogenic role in stimulating PC cell proliferation and invasion.
MicroRNA‐15a and miR‐16 are encoded within the intronic region of DLEU2.12 The expression of miR‐15a and miR‐16 were reduced in PC samples and they were shown to act as tumor suppressors in PC.13, 14, 15 We explored the roles of miR‐15a and miR‐16 in PC cell lines used in this study. The levels of miR‐15a and miR‐16 were measured in PC cells compared to normal pancreatic cell line HPDE6‐C7. Our qRT‐PCR analysis suggested that both of them were downregulated in PC cells (Figure 3A). Moreover, cell proliferation and invasion assays showed that transient overexpression of miR‐15a and miR‐16 significantly inhibited cell proliferation and invasion of AsPC‐1 and PaCa‐2 cells (Figure 3B,C).
Figure 3.
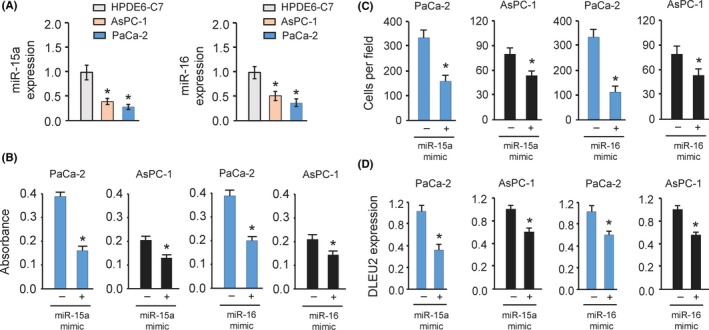
MicroRNA‐15a (miR‐15a) and miR‐16 inhibit the proliferation and invasion of pancreatic cancer (PC) cells. A, The expression of miR‐15a (left) and miR‐16 (right) in PC cell lines and normal pancreatic epithelial cell line HPDE6‐C7 was measured by quantitative real‐time PCR analysis. B,C, Proliferation (B) and invasion (C) of PaCa‐2 and AsPC‐1 cells was measured after overexpression of miR‐15a or miR‐16. D, Quantitative real‐time PCR analysis of DLEU2 expression in PaCa‐2 and AsPC‐1 cells after miR‐15a or miR‐16 overexpression. *P < .05
Recent studies have proposed that the expression and function of some intronic miRNAs could be the opposite to that of their host genes.16 Intronic miRNAs can potentially influence the expression and function of their host genes.17 Therefore, we investigated whether miR‐15a and miR‐16 could influence the expression of DLEU2 in PC cells. The qRT‐PCR results suggested that overexpression of miR‐15a and miR‐16 resulted in significant reduction of DLEU2 levels (Figure 3D). Collectively, these data indicated that miR‐15a and miR‐16 inhibit cell proliferation and invasion and might regulate the expression of their host gene DLEU2 in PC cells.
3.3. DLEU2 promotes PC cell proliferation and invasion by acting as a sponge of miR‐455
Many lncRNAs are known to contain motifs with sequences complementary to miRNAs and can inhibit miRNA expression and activity.18 To determine whether DLEU2 affects the behavior of PC cells by functioning as a molecular sponge of miRNA, we used the public prediction algorithm StarBase V2.0 to predict the potential miRNA binding sites in DLEU2.19 We identified a miR‐455‐binding site in DLEU2 (Figure 4A). The expression of miR‐455 was examined in AsPC‐1 and PaCa‐2 cells and normal pancreatic cell line HPDE6‐C7 using qRT‐PCR analysis. AsPC‐1 and PaCa‐2 cells had lower levels of miR‐455 expression than the normal pancreatic cell line HPDE6‐C7 (Figure 1D), suggesting a negative correlation between miR‐455 and DLEU2 expression.
Figure 4.
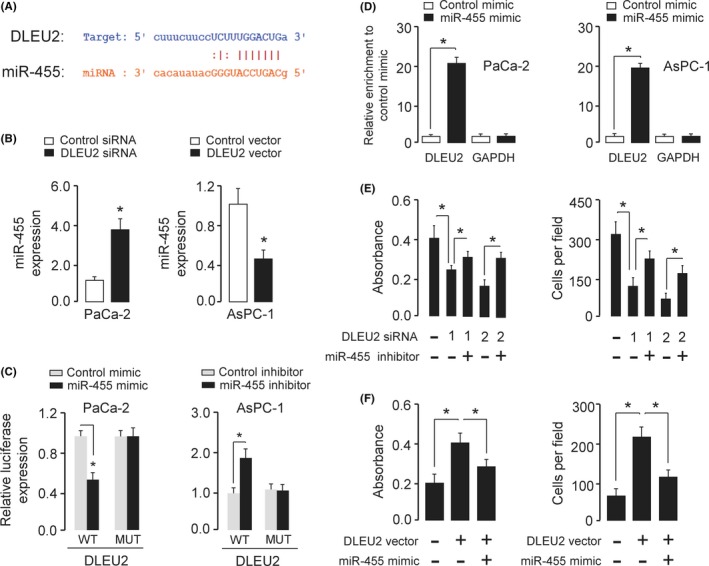
DLEU2 acts as a molecular sponge for microRNA‐455 (miR‐455). A, The putative miR‐455‐binding site in the PDLEU2 sequence is shown. B, Quantitative real‐time PCR analysis of miR‐455 in PaCa‐2 cells after DLEU2 knockdown and in AsPC‐1 cells after DLEU2 overexpression. C, Luciferase reporter gene assays were used to evaluate the interaction between miR‐455 and DLEU2 in PaCa‐2 cells (left) and AsPc‐1 cells (right). MUT, mutant. D, RNA immunoprecipitation assay was carried out in PaCa‐2 cells (left) and AsPc‐1 cells (right) transiently overexpressing miR‐455, followed by quantitative real‐time PCR to detect DLEU2 or GAPDH expression associated with AGO2. RNA levels are presented as fold enrichment of Ago2 relative to IgG immunoprecipitates. E, DLEU2 siRNA or control siRNA was transfected into PaCa‐2 cells, together with (or without) miR‐455 inhibitor. The cells were assayed for cell proliferation (left) and invasion (right). F, DLEU2 vector or control vector was transfected into AsPC‐1 cells, together with (or without) miR‐455 mimic. The cells were assayed for cell proliferation (left) and invasion (right). *P < .05
To examine the impact of DLEU2 on miR‐455 expression, we undertook the qRT‐PCR analysis and found that miR‐455 expression was upregulated in PaCa‐2 cells with DLEU2 knockdown (Figure 4B). Conversely, overexpression of DLEU2 decreased miR‐455 expression in AsPC‐1 cells (Figure 4B).
To further explore the relationship between DLEU2 and miR‐455, dual‐luciferase assays were carried out. As shown in Figure 4C, the transfection with miR‐455 mimic reduced the luciferase activity of WT DLEU2 in PaCa‐2 cells, but not that of mutant DLEU2. Consistent with these findings, the transfection with miR‐455 inhibitor resulted in a significant increase in the luciferase activity of WT DLEU2 in AsPC‐1 cells, whereas miR‐455 did not influence the luciferase activity of mutant DLEU2 (Figure 4C), suggesting that DLEU2 interacts with miR‐455 in PC cells.
To test whether DLEU2 associates with miR‐455, RIP experiments were undertaken in PaCa‐2 and AsPC‐1 cells transiently overexpressing miR‐455. We found that, compared with the control mimic‐transfected cells, DLEU2, but not GAPDH, was specifically enriched in Ago2 pellets of AsPC‐1 and PaCa‐2 cells overexpressing miR‐455 (Figure 4D). Taken together, these results suggested that DLEU2 is a target of miR‐455.
To determine the biological function of miR‐455, cell proliferation, and invasion were measured in PaCa‐2 cells transfected with DLEU2 siRNAs in combination with miR‐455 inhibitor, or in AsPC‐1 cells transfected with DLEU2 vector in combination with miR‐455 mimic. We observed that knockdown of DLEU2 significantly impeded cell proliferation and invasion of PaCa‐2 cells, and the inhibition of miR‐455 rescued the effects of DLEU2 on PaCa‐2 cell proliferation and invasion (Fig. 4E). We also found that overexpression of miR‐455 overcame the effects of DLEU2 overexpression in AsPC‐1 cells (Figure 4F). The above results suggested that DLEU2 could sponge miR‐455, which serves as a tumor suppressor in PC cells.
3.4. MicroRNA‐455 directly targets SMAD2 in PC cells
To further explore the mechanism through which miR‐455 exerts its tumor suppressive role in PC, we predicted the potential targets of miR‐455 using multiple algorisms (including TargetScan, http://www.targetscan.org/vert_72/; DIANA‐TarBase, http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=tarbase/index; and microRNA.org, http://www.microrna.org/microrna/home.do), and narrowed the predicted targets of miR‐455 down to 23 common genes, including ADD3, MYLIP, DYNC1LI2, PPP1R12A, CCDC174, and SMAD2 (Figure 5A). Among these, SMAD2 has the binding sequence of miR‐455 in its 3′‐UTR region (Figure 5B).
Figure 5.
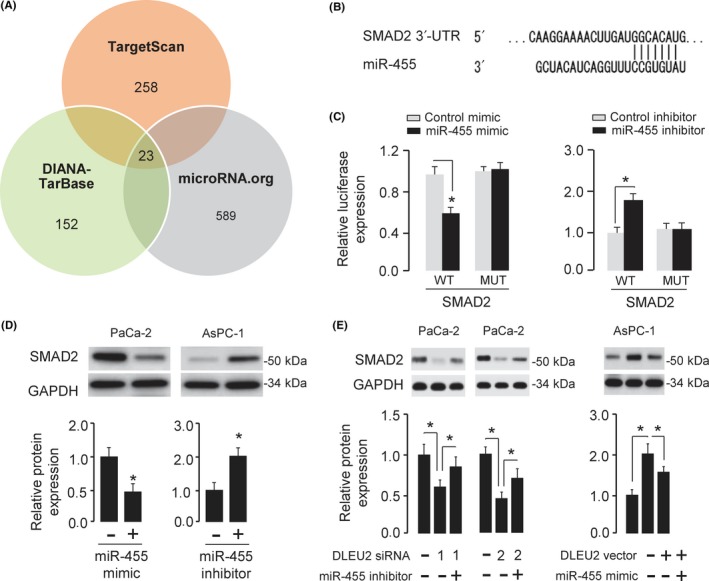
MicroRNA‐455 (miR‐455) directly targets SMAD2 and represses its expression in pancreatic cancer (PC) cells. A, Venn diagram depicting 23 genes (including SMAD2) that were predicted potential targets of miR‐455. B, Schematic illustration of the predicted miR‐455 binding site in the 3′‐UTR of SMAD2. C, Luciferase assays were used to verify the direct interaction between miR‐455 and SMAD2 in PaCa‐2 cells (left) and AsPc‐1 cells (right). MUT, mutant. D, Western blot analysis of SMAD2 expression in PaCa‐2 cells transfected with miR‐455 mimic or control mimic, and in AsPC‐1 cells transfected with miR‐455 inhibitor or control inhibitor. The intensities of protein bands were quantitated using ImageJ software. GAPDH was used as a normalization control. E, Western blot analysis of SMAD2 expression in PaCa‐2 cells transfected with DLEU2 siRNAs or control siRNA, together with (or without) miR‐455 inhibitor, and in AsPC‐1 cells transfected with DLEU2 vector or control vector, together with (or without) miR‐455 mimic. The intensities of protein bands were quantitated using ImageJ software. GAPDH was used as a normalization control. *P < .05
Considering the relationship between DLEU2 and miR‐455, and miR‐455 and SMAD2, we speculated that DLEU2 can regulate SMAD2 expression via miR‐455. Our western blot analysis demonstrated that knockdown of DLEU2 reduced the protein level of SMAD2 in PaCa‐2 cells, while the introduction of miR‐455 inhibitor partly abolished this effect (Fig. 5E). The induction of SMAD2 expression caused by DLEU2 overexpression could be largely reversed by the transfection of miR‐455 mimic in AsPC‐1 cells (Fig. 5E). Altogether, these results suggested that DLEU2 could promote the expression of oncogene SMAD2 by competitively binding to tumor suppressor miR‐455.
We investigated the mRNA expression of SMAD2 between PC and normal tissues using the Oncomine database. This analysis revealed that SMAD2 was up‐regulated in PC samples (Figure 1A, right panel). Consistent with this result, our qRT‐PCR analysis supported that AsPC‐1 and PaCa‐2 cells expressed higher levels of SMAD2 protein compared to the normal pancreatic cell line HPDE6‐C7 (Figure 1E). We also examined the levels of miR‐455 in PC samples using the dbDEMC 2.0 database (http://www.picb.ac.cn/dbDEMC/). As expected, miR‐455 was downregulated in PC tissues compared to normal tissues, suggesting that SMAD2 expression was positively correlated with DLEU2 levels and negatively correlated with miR‐455 expression in PC cells.
To find out whether SMAD2 is a real target of miR‐455, WT SMAD2 3′‐UTR fragment containing the miR‐455‐binding site and the mutant SMAD2 3′‐UTR fragment were cloned downstream of the pMIR‐REPORT luciferase reporter vector. Dual‐luciferase assays showed that miR‐455 mimic reduced the luciferase activities of WT SMAD2 3′‐UTR in PaCa‐2 cells, whereas miR‐455 knockdown increased the luciferase activities of WT SMAD2 3′‐UTR in AsPC‐1 cells (Figure 5C). However, miR‐455 did not change the luciferase activities of mutant SMAD2 3′‐UTR (Figure 5C). Moreover, western blot analysis showed that miR‐455 overexpression inhibited the protein level of SMAD2 in PC cells, whereas miR‐455 knockdown increased it (Figure 5D).
We examined the association of miR‐455 and SMAD2 expression and overall survival rate in PC patients using the online survival analysis tool KM plotter (http://kmplot.com/analysis/). Lower miR‐455 expression or higher SMAD2 expression correlates with reduced overall survival in PC patients (Figure 6). These data showed that SMAD2 was a downstream target of miR‐455.
Figure 6.
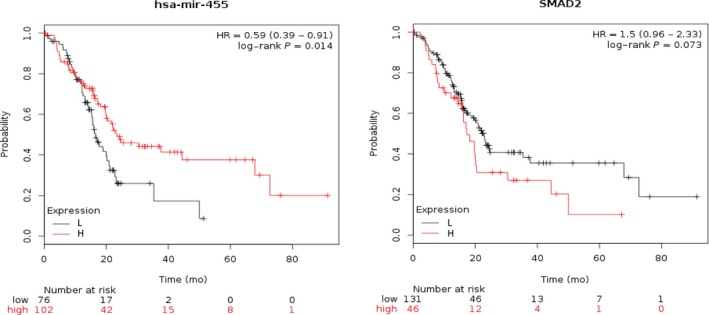
Low expression of microRNA‐455 (miR‐455) and high expression of SMAD2 correlates with poorer survival in pancreatic cancer patients. HR, hazard ratio
3.5. Restoration of SMAD2 reversed the effects of DLEU2 knockdown and miR‐455 overexpression
To further confirm that the effects of DLEU2 and miR‐455 on PC cells were dependent on the expression of SMAD2, we restored SMAD2 expression in PaCa‐2 cells transfected with DLEU2 siRNA or in PaCa‐2 cells transfected with miR‐455 mimic. Both CCK‐8 and invasion assays suggested that knockdown of DLEU2 or miR‐455 overexpression significantly suppressed PaCa‐2 cell proliferation and invasion, whereas restoration of SMAD2 expression could partially eliminate the inhibitory effects of DLEU2 knockdown or miR‐455 overexpression (Figure 7A‐D). Conversely, the overexpression of DLEU2 or miR‐455 inhibition significantly induced proliferation and invasion in AsPC‐1 cells, whereas the silencing of SMAD2 could significantly reverse the effects of DLEU2 overexpression or miR‐455 inhibition (Figure 7E‐H). These data revealed that DLEU2 enhances PC cell growth and invasion by enhancing SMAD2 expression by competitively interacting with miR‐455.
Figure 7.
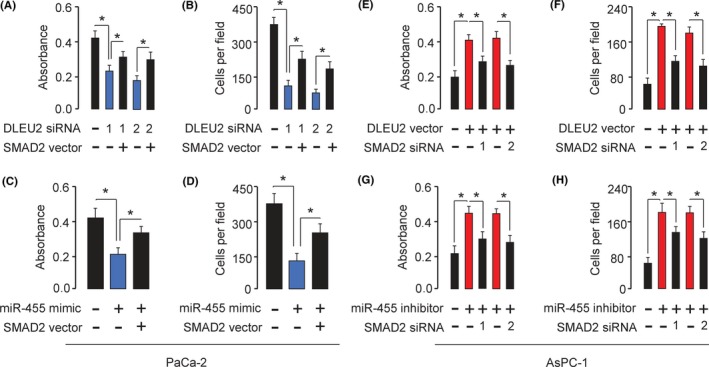
Restoration of SMAD2 reverses the effects of DLEU2 knockdown or microRNA‐455 (miR‐455) overexpression on pancreatic cancer cells. A,B, DLEU2 siRNA or control siRNA was transfected into PaCa‐2 cells, together with (or without) SMAD2 vector. The cells were assayed for cell proliferation (A) and invasion (B). C,D, miR‐455 mimic or control mimic was transfected into PaCa‐2 cells, together with (or without) SMAD2 vector. The cells were assayed for cell proliferation (C) and invasion (D). E,F, DLEU2 vector or control vector was transfected into AsPC‐1 cells, together with (or without) SMAD2 siRNAs. The cells were assayed for cell proliferation (E) and invasion (F). G,H, miR‐455 inhibitor or control inhibitor was transfected into AsPC‐1 cells, together with (or without) SMAD2 siRNAs. The cells were assayed for cell proliferation (G) and invasion (H). *P < .05
4. DISCUSSION
Long noncoding RNA DLEU2 is downregulated in leukemia12 and laryngeal carcinoma.20 However, it was found to be elevated in renal cell carcinoma cells, and higher expression of DLEU2 was associated with a poor prognosis in patients with renal cell carcinoma.21 These previous results indicated that DLEU2 might have distinct functions in different tumors but did not provide enough information about the biological activity and mechanism by which DLEU2 regulates carcinogenesis and progression of human tumors (including PC). In this study, our findings showed that upregulation of DLEU2 was a frequent occurrence in PC tissues, and DLEU2 dramatically promoted the proliferation and invasion of PC cells in vitro. These results suggest that DLEU2 plays a crucial role in the growth and invasiveness potential of PC and could act as a therapeutic target for PC patients.
Previous studies showed that intronic miRNAs, such as miR‐15a and miR‐16, could be regulated without any association with their host gene, and the functions of intronic miRNAs might be the opposite to that of their host genes.22, 23 Our results indicated that miR‐15a and miR‐16 were downregulated and they acted as tumor suppressors in PC cells, suggesting that miR‐15a and miR‐16 might be regulated through mechanisms that are independent of their host gene DLEU2, and miR‐15a/16 and DLEU2 have opposite functions in the regulation of PC cell proliferation and invasion.
Several previous reports described the possibility that some intronic miRNAs can potentially influence the expression and function of their host genes through regulating the expression of the host gene, or by modulating the expression of other genes that are involved in the regulation of the host gene.17 In this study, we reported that miR‐15a and miR‐16 could suppress the expression of DLEU2 in PC cells, although the detailed mechanisms by which miR‐15a and miR‐16 participate in the regulation of DLEU2 are still unclear.
Recent studies have revealed that there is an interplay and cross‐regulation between lncRNAs and miRNAs.24 Long noncoding RNAs might function as molecular sponges in modulating miRNAs.24 Importantly, DLEU2 could act as an endogenous sponge by directly binding to miR‐30a in renal cell carcinoma.21 Here, we confirmed the existence of an interaction between DLEU2 and miR‐455 in PC cells. We showed that DLEU2 directly binds to miR‐455, resulting in an induction of the mRNA targets of miR‐455 (such as SMAD2) and facilitating the proliferation and invasion of PC cells.
Numerous studies have reported a tumor suppressive role for miR‐455 in many tumors, including melanoma,25 colorectal cancer,26 lung cancer,27 gastric cancer28 and prostate cancer.29 MicroRNA‐455 was reported to be downregulated in PC tissues and cell lines and inhibits the proliferation of PC cells.30 In the present study, we reported that miR‐455 could significantly suppress PC cell growth and invasion. Furthermore, we showed that miR‐455 overexpression abrogated DLEU2‐mediated PC cell proliferation and invasion, suggesting that miR‐455 could be an important downstream regulator of DLEU2 function.
SMADs can integrate multiple signaling pathways and regulate the expression of target genes in transforming growth factor‐β‐activated cells.31 In PC cells, SMAD2 overexpression could increase proliferation and invasion.32 Identification of the upstream regulators of SMAD2 will help elucidate its critical role in tumorigenesis. Our present study showed that miR‐455 interacts with SMAD2 to repress its expression in PC cells. Thus, interactions between DLEU2, miR‐455, and SMAD2 might be biologically significant in the network regulating PC aggressiveness.
In conclusion, we show for the first time that upregulation of DLEU2 acts as an oncogenic driver in PC through modulating the miR‐455/SMAD2 axis. Therefore, our results provide the basis for a novel therapeutic strategy to target the DLEU2/miR‐455/SMAD2 axis in PC.
CONFLICT OF INTEREST
The authors have no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by a grant from Linyi People's Hospital.
Xu B, Gong X, Zi L, et al. Silencing of DLEU2 suppresses pancreatic cancer cell proliferation and invasion by upregulating microRNA‐455. Cancer Sci. 2019;110:1676–1685. 10.1111/cas.13987
Funding information
Linyi People's Hospital
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359‐E386. [DOI] [PubMed] [Google Scholar]
- 2. Dong P, Xiong Y, Yue J, et al. Long non‐coding RNA NEAT1: a novel target for diagnosis and therapy in human tumors. Front Genet. 2018;9:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rashid F, Shah A, Shan G. Long non‐coding RNAs in the cytoplasm. Genom Proteom Bioinf. 2016;14(2):73‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dowd AA, Homeida S, Elkarem HA. Detection of chromosome 13 (13q14) deletion among Sudanese patients with multiple myeloma using a molecular genetics fluorescent in situ hybridization technique (FISH). Malays J Pathol. 2015;37(2):95‐100. [PubMed] [Google Scholar]
- 5. Giulietti M, Righetti A, Principato G, et al. LncRNA co‐expression network analysis reveals novel biomarkers for pancreatic cancer. Carcinogenesis 2018. 10.1093/carcin/bgy069. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6. Gadaleta E, Cutts RJ, Kelly GP, et al. A global insight into a cancer transcriptional space using pancreatic data: importance, findings and flaws. Nucleic Acids Res. 2011;39(18):7900‐7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lerner M, Harada M, Lovén J, et al. DLEU2, frequently deleted in malignancy, functions as a critical host gene of the cell cycle inhibitory microRNAs miR‐15a and miR‐16‐1. Exp Cell Res. 2009;315(17):2941‐2952. [DOI] [PubMed] [Google Scholar]
- 8. Dong P, Xiong Y, Hanley SJB, et al. Musashi‐2, a novel oncoprotein promoting cervical cancer cell growth and invasion, is negatively regulated by p53‐induced miR‐143 and miR‐107 activation. J Exp Clin Cancer Res. 2017;36(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dong P, Xiong Y, Yue J, et al. miR‐34a, miR‐424 and miR‐513 inhibit MMSET expression to repress endometrial cancer cell invasion and sphere formation. Oncotarget. 2018;9(33):23253‐23263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chandrashekar DS, Bashel B, Balasubramanya SAH, et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19(8):649‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang IS, Son H, Kim S, et al. ISOexpresso: a web‐based platform for isoform‐level expression analysis in human cancer. BMC Genom 2016;17(1):631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klein U, Lia M, Crespo M, et al. The DLEU2/miR‐15a/16‐1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell. 2010;17(1):28‐40. [DOI] [PubMed] [Google Scholar]
- 13. Guo S, Xu X, Tang Y, et al. miR‐15a inhibits cell proliferation and epithelial to mesenchymal transition in pancreatic ductal adenocarcinoma by down‐regulating Bmi‐1 expression. Cancer Lett. 2014;344(1):40‐46. [DOI] [PubMed] [Google Scholar]
- 14. Zhang XJ, Ye H, Zeng CW, et al. Dysregulation of miR‐15a and miR‐214 in human pancreatic cancer. J Hematol Oncol. 2010;3:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiao LR, Frampton AE, Jacob J, et al. MicroRNAs targeting oncogenes are down‐regulated in pancreatic malignant transformation from benign tumors. PLoS ONE. 2012;7(2):e32068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gao X, Qiao Y, Han D, et al. Enemy or partner: relationship between intronic micrornas and their host genes. IUBMB Life. 2012;64(10):835‐840. [DOI] [PubMed] [Google Scholar]
- 17. Steiman‐Shimony A, Shtrikman O, Margalit H. Assessing the functional association of intronic miRNAs with their host genes. RNA. 2018;24(8):991‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xiao H, Tang K, Liu P, et al. LncRNA MALAT1 functions as a competing endogenous RNA to regulate ZEB2 expression by sponging miR‐200s in clear cell kidney carcinoma. Oncotarget. 2015;6(35):38005‐38015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li JH, Liu S, Zhou H, et al. starBase v2.0: decoding miRNA‐ceRNA, miRNA‐ncRNA and protein‐RNA interaction networks from large‐scale CLIP‐Seq data. Nucleic Acids Res. 2014;42(Database issue):D92‐D97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xie ZZ, Xiao ZC, Song YX, et al. Long non‐coding RNA Dleu2 affects proliferation, migration and invasion ability of laryngeal carcinoma cells through triggering miR‐16‐1 pathway. Eur Rev Med Pharmacol Sci. 2018;22(7):1963‐1970. [DOI] [PubMed] [Google Scholar]
- 21. Chen Z, Zhang J, Zhang Z, et al. The putative tumor suppressor microRNA‐30a‐5p modulates clear cell renal cell carcinoma aggressiveness through repression of ZEB2. Cell Death Dis. 2017;8(6):e2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morenos L, Chatterton Z, Ng JL, et al. Hypermethylation and down‐regulation of DLEU2 in paediatric acute myeloid leukaemia independent of embedded tumour suppressor miR‐15a/16‐1. Mol Cancer. 2014;13:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Veronese A, Pepe F, Chiacchia J, et al. Allele‐specific loss and transcription of the miR‐15a/16‐1 cluster in chronic lymphocytic leukemia. Leukemia. 2015;29:86‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang C, Wu D, Gao L, et al. Competing endogenous RNA networks in human cancer: hypothesis, validation, and perspectives. Oncotarget. 2016;7(12):13479‐13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chai L, Kang XJ, Sun ZZ, et al. MiR‐497‐5p, miR‐195‐5p and miR‐455‐3p function as tumor suppressors by targeting hTERT in melanoma A375 cells. Cancer Manag Res. 2018;10:989‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yunqi H, Fangrui Y, Yongyan Y, et al. MiR‐455 functions as a tumor suppressor through targeting GATA6 in colorectal cancer. Oncol Res 2019;27(3):311‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gao X, Zhao H, Diao C, et al. miR‐455‐3p serves as prognostic factor and regulates the proliferation and migration of non‐small cell lung cancer through targeting HOXB5. Biochem Biophys Res Commun. 2018;495(1):1074‐1080. [DOI] [PubMed] [Google Scholar]
- 28. Ning T, Peng Z, Li S, et al. miR‐455 inhibits cell proliferation and migration via negative regulation of EGFR in human gastric cancer. Oncol Rep. 2017;38(1):175‐182. [DOI] [PubMed] [Google Scholar]
- 29. Zhao Y, Yan M, Yun Y, et al. MicroRNA‐455‐3p functions as a tumor suppressor by targeting eIF4E in prostate cancer. Oncol Rep. 2017;37(4):2449‐2458. [DOI] [PubMed] [Google Scholar]
- 30. Zhan T, Huang X, Tian X, et al. Downregulation of MicroRNA‐455‐3p links to proliferation and drug resistance of pancreatic cancer cells via targeting TAZ. Mol Ther Nucleic Acids. 2018;10:215‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu S, Chen S, Zeng J. TGF‐β signaling: a complex role in tumorigenesis (Review). Mol Med Rep. 2018;17(1):699‐704. [DOI] [PubMed] [Google Scholar]
- 32. Wang C, Liu P, Wu H, et al. MicroRNA‐323‐3p inhibits cell invasion and metastasis in pancreatic ductal adenocarcinoma via direct suppression of SMAD2 and SMAD3. Oncotarget. 2016;7(12):14912‐14924. [DOI] [PMC free article] [PubMed] [Google Scholar]


