Abstract
The adverse effects of overdiagnosis and overtreatment observed in men with clinically insignificant prostate cancers after the introduction of prostate-specific antigen-based screening are now being observed in those with thyroid cancer, owing to the introduction of new imaging technologies. Thus, the evolving paradigm of active surveillance in prostate and thyroid cancers might be valuable in informing the development of future active surveillance protocols. The lessons learned from active surveillance and their implications include the need to minimize the use of broad, population-based screening programmes that do not incorporate patient education and the need for individualized or shared decision-making, which can decrease the extent of overtreatment. Furthermore, from the experience in patients with prostate cancer, we have learned that consensus is required regarding the optimal selection of patients for active surveillance, using more-specific evidence-based methods for stratifying patients by risk. In this Review, we describe the epidemiology, pathology, and screening guidelines for the management of patients with prostate and thyroid cancers; the evidence of overdiagnosis and overtreatment; and provide overviews of existing international active surveillance protocols.
Introduction
Many cancers are indolent at the time of diagnosis, meaning they are either stagnant or “grow too slowly to be life threatening in even the longest of lifetimes”1. Prostate and thyroid cancers are both examples of cancers that are often indolent and can be detected early through increased use of screening. Broad population screening of asymptomatic individuals for cancers of the prostate or thyroid has resulted in the detection of many more of these cancers, consequently prompting not only the diagnosis but also the treatment of cancers that might never cause harm during a patient’s lifetime. Such processes are termed overdiagnosis and overtreatment, respectively1.
One strategy for addressing the risk of overdiagnosis and overtreatment resulting from overdetection is to follow screening guidelines that emphasize the use of individualized or shared decision-making considering both the potential benefits and known risk of harms. The US Preventive Services Task Force (USPSTF) recommends, for example, that the decision to undergo screening for prostate cancer should be individualized for asymptomatic men 55–69 years of age2. Active surveillance is another strategy that can be used to circumvent overtreatment of patients with indolent cancers. Active surveillance has emerged over the past two decades as an attractive approach that can enable patients to be spared from receiving definitive treatments, which are not without risks of substantial harms, until their disease has progressed to a stage that warrants such interventions.
Prostate cancer is a prime example of an often indolent cancer, for which active surveillance is recommended to avoid the unnecessary harms associated with overtreatment (such as decreased quality of life owing to bowel, urinary, and/or erectile dysfunction) 3–5. Almost half of all patients with newly diagnosed prostate cancer are eligible for active surveillance owing to a low Gleason score with low serum prostate-specific antigen (PSA) levels on initial presentation6,7. Active surveillance can also be applied to thyroid cancer. The prevalence of papillary thyroid cancer (PTC) has rapidly increased over the past few years, in part following the advent of high-sensitivity imaging techniques8. As a result, an increasing number of patients have been diagnosed with thyroid cancers that would not have been detected using standard clinical examinations alone.
Much of the evidence supporting the use of active surveillance in patients with prostate cancer comes from randomized trials and observational studies comparing the outcomes of patients undergoing active surveillance with those of patients receiving definitive treatments, including prostatectomy and radiotherapy. The findings of these studies suggest that active surveillance is a feasible and promising management strategy that can reduce the risk of overtreatment in selected patient subgroups. Data from two large randomized trials, Prostate Intervention Versus Observation (PIVOT) and Prostate Testing for Cancer and Treatment (ProtecT), have shown that definitive treatment provides no statistically significant improvement in overall survival over active surveillance in selected populations with favourable-risk prostate cancer5,9,10.
The PIVOT Study Group published the initial (2012)11 and follow-up results (2017)5 of a study involving 731 men with localized prostate cancer. The study cohort generally comprised older patients (average age of 67 years, with one-third of patients ≥70 years of age ), a number of whom had comorbidities, such as cardiovascular disease or type II diabetes mellitus. Clinically, the population was generally representative of a low-risk group: 80% of patients had tumours considered to be low risk on the basis of physical examination (or >70% according to tumour Gleason score). Almost half (364; 49.8%) of these men were randomly assigned to undergo radical prostatectomy (RP) and the other half (367) to noncurative intent observation. After a median follow-up duration of 12.7 years, treatment was not associated with a significant reduction in either all-cause or prostate cancer-related mortality, although having surgery did result in a lower frequency of treatment for disease progression. In summary, in a selected group of men with predominantly low-risk localized prostate cancer, undergoing treatment did not offer a survival benefit over active surveillance11–13.
The ProtecT Study Group published the results from a cohort of 1,643 men who were randomly assigned to receive surgery, radiotherapy, or active surveillance. The results, published in 201610, were similar to those of the PIVOT study: no statistically significant difference in prostate cancer-specific mortality was observed among the three groups, although undergoing active treatment was associated with a reduced risk of disease progression and metastasis compared with undergoing active surveillance5,11.
In 2003, investigators at Kuma Hospital, Japan, were the first to report the results of an active surveillance study involving patients with thyroid cancer14. These investigators showed, in a cohort of 162 patients, that lesion size remained unchanged or decreased in >70% of the patients with a mean follow-up duration of 3.88 years. The investigators also reported that larger tumour size at diagnosis was associated with a later finding of tumour enlargement. In their 2014 report14, including data from 1,235 patients with a median follow-up duration of 6.25 years, these investigators observed tumour enlargement in 8% of patients and metastasis in 3.8%. Researchers from Cancer Institute Hospital, Tokyo, Japan, reported even lower percentages: tumour enlargement among 7% of patients and metastasis among 1% of a cohort of 230 patients15–17. None of the patients in this cohort who underwent rescue surgery had clinically significant disease recurrence or died of PTC.
Existing guidelines for active surveillance are useful, although they vary regarding the recommended inclusion criteria, surveillance schedules, and intervention thresholds18. Moving forward, understanding the molecular characteristics of low-grade prostate tumours could enable improved risk stratification and lead to the identification of biomarkers that will enable investigators to differentiate between favorable-risk and unfavourable-risk cancers19.
In this Review, we focus on the development and application of active surveillance protocols in patients with prostate or thyroid cancers, and consider how the paradigm of active surveillance in patients with prostate cancer could inform the development of active surveillance protocols for those with thyroid cancer. We present the epidemiology, pathology and screening guidelines for both cancers, the evidence of overdiagnosis and overtreatment, and describe current international active surveillance protocols.
Prostate cancer
Overview of epidemiology
Prostate cancer is the commonest cancer and the second commonest cause of cancer-related death in men worldwide20. American Cancer Society (ACS) estimates indicate that 164,690 new cases will be diagnosed, and 29,430 deaths from prostate cancer will occur in 2018 in the USA alone20. The probability of a man developing prostate cancer by 79 years of age is estimated to be 1 in 15 in the USA. Risk factors for prostate cancer include ethnicity (with the highest incidence among black men), advanced age, and a family history of prostate cancer21.
Substantial differences in both incidence and prostate cancer-specific mortality have been reported across different geographical regions. According to the International Agency for Research on Cancer, the global incidence of prostate cancer increased from the 1980s until 2010, with the USA having the highest rates, followed by Oceania and Western European countries22. The lowest incidences were reported in Asian and African countries. Only a small number of countries, including the USA, Colombia and some European countries, reported a decreased incidence of prostate cancer after 200523. In the same report, prostate cancer-specific mortality was highest in Scandinavian countries; however, no clear explanation exists for this finding23,24. The declines in prostate cancer-specific mortality seen in the USA and parts of Europe since 2005 have been attributed to the introduction of prostate cancer screening programmes, including the broad use of serum PSA-based screening.
Following the introduction of serum PSA-based screening in the early 1990s, a dramatic increase in the incidence of prostate cancer occurred among men in the USA (Fig. 1)25. The incidence of prostate cancer has since plateaued, likely owing to the 2012 USPSTF recommendation against the use of broad population-based screening. The effects of this recommendation on overdiagnosis and overtreatment are not yet known26,27.
Fig. 1. Incidence and mortality rates associated with prostate and thyroid cancers in the USA, per 100,000 persons, 1980–2015.
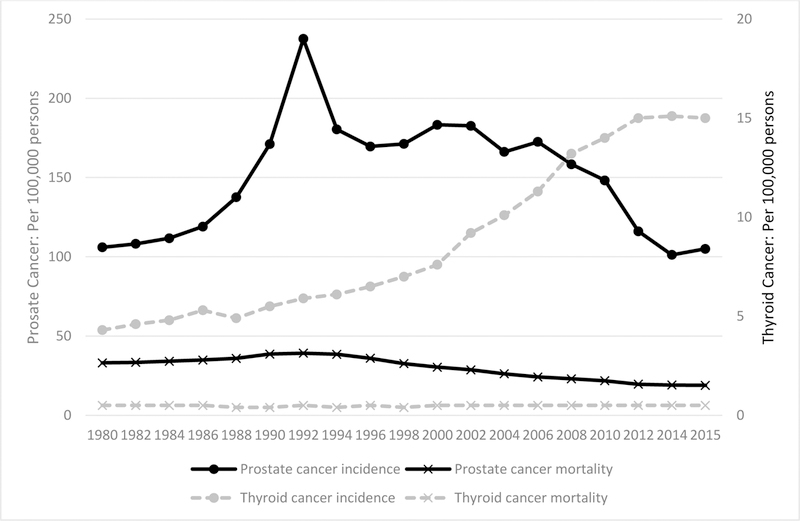
In a plot of the incidence and mortality rates for both prostate and thyroid cancers in the USA, an increase in the incidence of both prostate and thyroid cancers can be observed. However, the mortality rate remained fairly stable from 1980 to 2015. All rates were abstracted from SEER9 estimates (https://seer.cancer.gov/statfacts/)
Guidelines for screening
The advent of screening asymptomatic men for prostate cancer has resulted in many more men being diagnosed; however, no association has been demonstrated between screening and mortality reduction26,27. Owing to this lack of any change in mortality rates during the era of serum PSA-based screening, and the above-mentioned changes in the USPSTF recommendations, the use of serum PSA-based screening of asymptomatic men for prostate cancer remains controversial, and numerous professional organizations do not share a consensus on the use of such protocols (BOX 1). However, the majority of recommendations emphasize the use of shared and/or individualized decision-making.
BOX 1. Current recommendations on use of screening for prostate cancer.
US Preventive Services Task Force (2018)2
Men 55–69 years of age should be informed about the potential benefits and harms of screening; The decision to be screened should be individualized.
Men aged ≥70 should not undergo screening for prostate cancer.
National Comprehensive Cancer Network (2016)32
Men 45–75 years of age should discuss the benefits and harms of prostate cancer screening with a qualified health-care professional.
Serum prostate-specific antigen (PSA) testing should be offered to men who wish to be screened; a baseline digital rectal examination (DRE) could also be considered, particularly for men with elevated serum PSA levels. Subsequent testing would then be based on serum PSA results, from 1- to 2-year intervals to 3- to 4-year intervals.
American Urological Association (2013)30
Men ≤40 years of age should not be screened.
Men 40–54 years of age with an average risk should not undergo routine screening.
Men ≤55 years of age who have a higher risk of prostate cancer (including those with a positive family history or of African-American ethnicity) should make individualized decisions on use of screening.
Men 55–69 years of age should engage in shared decision-making and be informed about the potential benefits and harms of screening. The decision to undergo screening should individualized. To reduce the harms of screening, patients might prefer a routine screening interval of ≥2 years over annual screening.
Men ≥70 years of age or those with a life expectancy <10–15 years should not be screened.
American Cancer Society (2016)28
Men ≥50 years of age with an average risk of prostate cancer should engage in shared decision-making and make an informed decision about serum PSA-based testing with, or without DRE.
Screening should not be offered to men with a life expectancy <10 years.
Men with an initial serum PSA level ≥2.5 ng/ml should undergo annual testing; men with a serum PSA level <2.5 ng/ml can undergo testing every 2 years.
Discussions should be initiated regarding use of screening in men 40–45 years of age with a high risk of developing prostate cancer (such as black men and men with a first-degree relative with prostate cancer diagnosed <65 years of age). Biopsy sampling should be initiated in patients with serum PSA levels >4.0 ng/ml
For men with serum PSA levels of 2.5–4.0 ng/ml, individualized approaches to decision-making and risk assessment are encouraged), which can include age, ethnicity, family history, DRE findings, previous biopsy results, and use of 5α-reductase inhibitors.
American College of Physicians (2013)31
Men 50–69 should be informed of the limited potential benefits and substantial risk of harms associated with prostate cancer screening.
Men <50 years of age should not be screened.
Men should make an informed decision on use of screening starting at 55 years of age.
Men ≥70 years of age should not be screened.
Men with a life expectancy of ≤10–15 years should not be screened.
ASCO (2012)29
Physicians should discuss with individual men whether serum PSA-based testing is an appropriate step for those with a life expectancy >10 years (a lower age limit is not specified).
General screening for prostate cancer using measurements of total serum PSA level should be discouraged in men with a life expectancy <10 years because the risk of harms seems to outweigh potential benefits in this population.
Information written in language suitable for the lay public should be made available to clinicians and their patients in order to facilitate discussions of the benefits and harms associated with serum PSA-based testing before the routine initiation of a serum PSA-based test.
European Association of Urology (2012)34
Offer individualized risk-adapted early detection strategies, while also covering the potential risks and benefits, to well-informed men with a good performance status and a life-expectancy of ≥10–15 years.
Offer early serum PSA-based testing to men with an elevated risk of prostate cancer, including men >50 years of age, men >45 years of age with a family history of prostate cancer, men of African-American ethnicity >45 years of age; men with a serum PSA level >1 ng/ml at 40 years of age and men with a serum PSA level >2 ng/ml at 60 years of age.
Offer a risk-adapted strategy (based on initial serum PSA level), with follow-up intervals of 2 years for those initially deemed at risk, including men with a serum PSA level >1 ng/ml at 40 years of age and men with a PSA level >2 ng/ml at 60 years of age. Postpone follow-up monitoring to 8 years in those with initial PSA level ≤1 ng/ml..
Decide on the age at which early diagnosis of prostate cancer is not clinically relevant based on life expectancy and performance status: men who have a life expectancy <15 years are unlikely to benefit, based on data from the Prostate Intervention versus Observation and the European Randomized Study of Screening for Prostate Cancer trials11,62.
European Society for Medical Oncology (2012)33
Population-based screening should be rejected in favour of an individualized approach, involving shared decision-making.
Recommendations highlight the inconsistent evidence on the effectiveness of screening men <50 years of age and those 70–75 years of age. Evidence also indicates that the harms of screening outweigh the benefits for men >75 years of age.
In the USA, the recommendations for use of serum PSA-based prostate cancer screening are variable21. The ACS recommends the use of serum PSA-based screening for men with an average risk of prostate cancer from 50 years of age28. ASCO recommends an individualized decision on the use of screening for prostate cancer for all men with a life expectancy >10 years29. The American Urological Association recommends the use of shared decision-making for men 55–69 years of age, and the American College of Physicians similarly recommends that patients make an informed decision on the use of prostate cancer screening from 55 years of age30,31. The latest National Comprehensive Cancer Network guidelines recommend offering serum PSA-based screening and digital rectal examination (DRE) starting at 45 years of age32. The USPSTF 2018 guideline also emphasizes the use of shared and individualized decision making for men 55–69 years of age, which differs from their previous (2012) recommendation against the use of serum PSA-based screening regardless of age, ethnicity, and family history2.
Similar to recommendations from the USA, the European guidelines also emphasize the need for informed decision-making about PSA-based screening for prostate cancer. ESMO recommends that the decision to undergo screening should be individualized and either adopted or rejected following a decision-making process that is shared between the physician and the patient33. The European Association of Urology recommendations are dependent on the patient’s age and risk of prostate cancer. For example, these guidelines recommend the use of screening for men who are >45 years of age and have a family history of prostate cancer34.
Overdiagnosis and overtreatment
Starting in the 1980s, many investigators and societies supported the introduction of broad serum PSA-based screening for prostate cancer for men of at least 45 years of age, which resulted in the overdiagnosis and overtreatment of many men with low-risk prostate cancer27. The risk of overdiagnosis and overtreatment is greater among older men35 because men >60 years of age typically are also more likely to have other comorbidities, such as metabolic syndrome, which increases their 15-year mortality risk36.
A systematic review described estimates of the frequency of overdiagnosis ranging from 1.7% to 67%37. This wide range of estimates is likely the result of differences in the cohorts or populations included in different studies and the methods used in estimating the incidence of overdiagnosis. One method to estimate overdiagnosis uses statistical models that estimate the distribution of the time when a cancer is detected through screening and the time when the cancer would become symptomatic in a population; the probability of the observed survival time exceeding the expected survival time is then estimated38. Another method calculates the difference in disease incidence in a screened population compared with a non-screened population; the excess cases are then categorized as overdiagnosed cases38,39. The USPSTF conducted a review of data from the Prostate, Lung, Colorectal and Ovarian (PLCO) study and from the European Randomized Study of Screening for Prostate Cancer (ERSPC) trial and produced an estimate of overdiagnosis using two methods2,21. When estimating overdiagnosis as a percentage of all diagnosed prostate cancers, an estimated 16.4% and 33.2% of patients diagnosed with prostate cancer in the PLCO and ERSPC studies, respectively, were overdiagnosed. When estimating overdiagnosis as the percentage of cancers detected by screening during the screening period, investigators reported that 20.7% and 50.4% of cancers diagnosed in the PLCO and ERSPC trials, respectively, could be considered overdiagnosis.
Aizer and colleagues40 used the Medicare and Surveillance, Epidemiology and End Results (SEER) combined database for years 2004–2007 to estimate the frequency of overtreatment of men with prostate cancer. The study defined overtreatment as those situations in which patients who had been diagnosed with low-risk prostate cancer and had a life expectancy <10 years, were treated with RP, external beam radiation, cryotherapy, or brachytherapy within 12 months of diagnosis. These investigators identified 3,001 men >66 years of age with low-risk prostate cancer and a life expectancy <10 years. They estimated that 67% of these men were overtreated and estimated that the cumulative annual cost of overtreatment per year in the USA exceeded $58 million.
The overtreatment of indolent prostate cancers is problematic because men are unnecessarily exposed to the adverse effects of treatment. The common treatments of localized prostate cancer include RP and external beam radiation therapy, both of which are associated with considerable risks of adverse events and can negatively affect quality of life, resulting in an increased harm-to-benefit ratio40. The known harms associated with these treatments include reductions of health-related quality of life owing to negative effects on urinary, bowel, and erectile function41.
Current active surveillance protocols
Active surveillance is the preferred management strategy for patients with low-risk prostate cancer42,43. By carefully selecting men with low-risk disease and carefully monitoring them for signs of disease progression, physicians can minimize the risk of overtreatment. A review of clinical guidelines by Bruinsma et al.18 demonstrated that, despite wide variations between active surveillance protocols, eligibility criteria for inclusion are somewhat consistent. Most clinical guidelines apply the following inclusion criteria: stage T1c or T2 tumours, serum PSA levels <10 ng/ml, a Gleason score ≤6, two or fewer cancer-positive core biopsy samples, and/or a maximum of 50% of tumour material per core sample. The recommended tests carried out during active surveillance for most patients include repeat biopsy sampling, measurements of serum PSA levels, and DREs. Additional tests can also be included in active surveillance protocols, such as multiparametric MRI and genomic tests; however, the effectiveness of such tests remains uncertain18.
Since the 1990s, a number of active surveillance cohorts have been created. To our knowledge, 14 active surveillance cohorts currently exist: 6 in the USA, 1 in Canada, 1 in Japan, 2 in Sweden, 1 in UK, and 3 across multiple countries (Table 1). There is detailed information on the protocols, including those of the Prostate Cancer Research International Active Surveillance study44,45; Johns Hopkins Medical Institutions46,47; the University of Toronto48–50; the University of California, San Francisco (UCSF)51–53; the University of Miami54; Memorial Sloan Kettering Cancer Center55; The University of Texas MD Anderson Cancer Center56; one Japanese group57; the Canary Prostate Active Surveillance Study (a USA–Canada team)58,59; the Royal Marsden, UK60; the ERSPC61,62, including a trial in Gӧteborg, Sweden63, and the National Prostate Cancer Register of Sweden64.
Table 1.
Prostate cancer active surveillance cohorts
| Study details | Eligibility criteria | Monitoring Scheme | Disease reclassification criteria | Rate of disease reclassification |
|---|---|---|---|---|
| Prostate Cancer Research International: Active Surveillance44,45; 2006–2021 (ongoing) n = 5,302; median follow-up, 5 years |
Original criteria—2006 • GS ≤3 + 3 • Stage not ≥cT2c • PSA ≤10 ng/ml • PSAD <0.2 ng/ml/cm2 • 1–2 positive cores at repeat biopsy • Fitness for curative treatment Updated criteria—2012 • GS 3 + 4 (≤10% tumour involvement per biopsy core, maximum 2 cores positive) and can be included if ≤70 years of age Updated criteria—2015 • ≥2 cores can be positive (no maximum) if an MRI, including targeted biopsies on positive lesions, is done at inclusion, AND if saturation biopsies are done (≥20 cores), 15% of cores can be positive with a maximum of 4 |
First 2 years: • PSA every 3 months • DRE every 6 months After 2 years: • PSA every 6 months • DRE every 12 months Repeat biopsies: • 1, 4, 7 and 10 years after diagnosis and subsequently every 10 years Annual biopsies only if PSA DT is between 0 and 10 years |
• GS > 3 + 3 • More than 2 positive cores • Stage >cT2 • PSA DT between 0 and 3 years (dropped at the end of 2014, but yearly biopsy is advised as with a PSA DT of 3–10 years. If MRI is available, it can be performed to rule out anterior tumours). • MRI use started in 2015 |
By biopsy 28% (415 of 1,858 repeat biopsies in 1,480 men) |
| Johns Hopkins Medical Institutions46,47; (1995–2014) n = 1,298; median follow-up, 5 years | • GS ≤6 • Stage T1c • PSAD <0.15 ng/ml/cm2 • ≤2 positive biopsy cores • PSA cutoff of 10 ng/ml was not used |
• PSA every 6 months • DRE every 6 months • Repeat biopsy every 12 months |
Biopsy findings no longer meeting the inclusion criteria | 34% at 5 years (440/1,298) 41% at 10 years (527/1,298) 41% at 15 years (537/1,298) |
| University of Toronto48–50 (1995–2013, ongoing) n=993; median follow-up 6.4 years |
Criteria 1995–1999 • GS ≤6 • PSA ≤10 ng/ml Patients >70 years of age with PSA ≤15 ng/ml or GS ≤3 + 4 Criteria after 2000 • GS ≤6 • PSA ≤10 ng/ml • PSA 10–20 ng/ml and/or GS ≤3 + 4 with substantial comorbidities and a life expectancy ≤10 years |
• PSA every 3 months for 2 years and then every 6 months • Repeat biopsy within 12 months after initial sampling and then every 3–4 years • DRE every 6 months |
• PSA DT <3 years (from 1996 to 2008) • Histological upgrade on repeat prostate biopsy • Clinical progression as defined by development of an unequivocal and palpable nodule with histological confirmation |
By PSA/PSA kinetics Short PSA doubling time: 11.7% By biopsy Grade progression: 9.5% Overall 27% |
| University of California, San Francisco51−53 (1990–2013) n = 810; median follow-up 5 years | • GS ≤6 (3 + 3) • Clinical stage T1 or T2 • PSA ≤10 ng/ml • ≤33% positive cores and ≤50% tumour in any single core |
• PSA every 3 months for the first 2 years and then every 6 months • Transrectal ultrasonography • Repeat biopsy within 12 months of diagnostic biopsy and every 12–24 months after |
• Patient anxiety • Clinical stage change guided by the UCSF Cancer of the Prostate Risk Assessment score15 |
Overall 43% |
| University of Miami54 n=230; median follow-up 2.7 years | • GS ≤6 • Clinical stage T1a–T2 PSA ≤10 ng/ml • ≤2 biopsy cores with ≤20% tumour present in each core |
PSA and DRE at 3–4 months for the first 2 years and then every 6 months | • Gleason grade >3 on rebiopsy • Increase in tumour volume measured by percentage of tumour in each core • Increase in the number of positive cores • Personal preference |
Overall 14% |
| Memorial Sloan Kettering Cancer Center55 (1997–2009) n=238; median follow-up 1.8 years | • No GS 4 or 5 histology • Clinical stage T1–T2a • PSA ≤10 ng/ml • ≤3 biopsy cores and no biopsy samples containing ≥50% cancer involvement |
• DRE every 6 months • PSA every 6 months • Repeat biopsy within 12–18 months of starting AS and subsequently every 2–3 years |
Treatment was recommended when the patient no longer met study eligibility criteria | NR |
| The University of Texas MD Anderson Cancer Center56 (2006–2012) n=191; median follow-up 3 years (Group I only) |
Group I (favourable risk) • Biopsy of ≥10 cores, showing either GS 3+3 in one core (tumour focus <3.0 mm) or a GS 3+4 in one core (tumour focus <2.0 mm) PSA ≤4 ng/ml Group II (patient’s choice) • Patients with GS 3 + 3 or 3 + 4/4 + 3 not meeting the criteria for Group I. Group III (therapy prevented by comorbidities) |
• DRE, PSA, testosterone every 6 months • Biopsies were repeated every 1–2 years; if biopsy samples were negative, the following year’s biopsy was omitted, unless requested by the patient |
• Detection of an increase in tumour length in a positive core beyond the study entry criteria • >1 positive core at biopsy • Upgrade to a dominant GS 4 or any GS 5 component. • PSA >30% increase, confirmed within 1 month of laboratory results or 3 months after biopsy |
By biopsy 16.8% |
| Japanese Active Surveillance Trial57 (2002–2006) n=118; median follow-up NR | • Stage T1cN0M0 • Age 50–80 years • PSA ≤20 ng/ml • 1 or 2 positive • GS ≤ 6 • Cancer involvement in positive core ≤50% |
• DRE every 6 months • PSA every 2 months for the first 6 months and every 3 months thereafter • CXR, CT scan or MRI for abdominal /pelvic cavity and bone scintigraphy were performed at least once ever 2 years to rule out the presence of metastasis |
• PSA DT of ≤2 years • Pathological progression at rebiopsy |
PSA DT ≤2 years progressors (27%) 33% at 1-year rebiopsy 3-year pathological progression: (25%) |
| Canary Prostate Active Surveillance Study58,59 (United States–Canada) (2008–2013); n = 905 Median follow-up, 2.3 years | • Histologically confirmed prostate adenocarcinoma • cT1–2 disease • No previous treatment for prostate cancer • Willingness to undergo serial monitoring |
• PSA every 3 months • DRE every 6 months • Repeat prostate biopsy 6–12, 24, 48 and 72 months after diagnosis with at least 10-core regimens |
• Any GS increase (primary or secondary) on repeat biopsy and/or • Increase in biopsy tumour volume (cutoff of 34%) |
24% of patients (216/905) reclassified by biopsy results, GS, or tumour volume |
| European Randomized Study of Screening for Prostate Cancer61,62 (1993–2007); n = 509 Median follow-up, NR |
Low risk • Clinical stage T1c/T2 • PSA ≤10 ng/ml • PSAD <0.2 ng/ml • GS ≤6 • Maximum 2 positive biopsy cores Intermediate risk • PSA 10–20 ng/ml and/or • GS 7 and/or 3 positive biopsy cores |
Treating physician’s discretion | Treating physician’s discretion | NR |
| European Randomized Study of Screening for Prostate Cancer Göteborg trial63 (1995–2010); n = 439 Median follow-up, NR |
Very low risk • T1c, not N1 or M1 • GS ≤6 • PSAD <0.15 ng/ml; <3 cores with cancer • ≤50% cancer in any core Low risk • T1, not N1 or M1 • GS ≤6 • PSA <10 ng/ml but not meeting the very low-risk criteria Intermediate risk • T1–2, not N1 or M1 • GS ≤7 and/or • PSA <20 ng/ml and not meeting the very low risk or low-risk criteria High risk • T1–4, not N1 or M1 • GS ≤8 and/or • PSA <100 ng/ml and not meeting the other risk groups’ criteria • Advanced-stage disease N1 or M1 or PSA ≥100 ng/ml |
• PSA and clinical follow-up every 3–6 months • Patients with diagnostic biopsy containing <2 mm of cancer were recommended for early rebiopsy • Imaging (bone scan, MRI, CT) was performed only if a patient had symptoms or clinical features (PSA >20 ng/ml or Gleason score >7) indicating a risk of metastasized disease |
NR | PSA increase in 45 men (27.7%); increase in GS at repeat biopsy in 77 men (47.5%) |
| National Prostate Cancer Register of Sweden64 (Included patients diagnosed between 2003 and 2007: complete follow-up until December 2013); n=1729; follow-up 5 years | • Clinical stage T1c • GS ≤6 |
NR | • PSA progression • Biopsy progression (upgrading or a larger extent of cancer on biopsy) • Patient preference • Other reasons |
• 52% of progressors had a PSA increase • 24% of progressors had progression on biopsy sampling • 46% of patients overall had progressive disease after 5 years |
| Royal Marsden, UK60 (2002–2011); n=471; median follow-up, 5.7 years | • Clinical stage T1/T2 • PSA <15 ng/ml • GS ≤3 + 3 (GS 3 + 4 for patients aged >65 years) • Percentage of positive cores ≤50% |
• Clinical assessment, DRE, and PSA every 3 months for the first year, every 4 months for the second year, and every 6 months thereafter • Transrectal ultrasound-guided prostate biopsy at 18–24 months and every 2 years |
• PSA velocity of >1 ng/ml per year • GS ≥4 + 3 • Presence of cancer in >50% of the total number of cores |
By biopsy 11.5% |
| University of California, Los Angeles (NCT00949819). Recruiting phase | • Histologically confirmed prostate adenocarcinoma • Clinically localized prostate cancer: T1–2, NX or N0, MX or M0 |
NR | NR | NR |
AS, active surveillance; CXR, chest x-ray; DT, doubling time; GS, Gleason score; NR, not reported; PSA, prostate-specific antigen; PSAD, prostate-specific antigen density; US, ultrasonography.
Consensus is crucially needed regarding several aspects of active surveillance, including specific patient inclusion criteria, an optimal surveillance schedule, the most appropriate surveillance tests, and a threshold for definitive treatment18,65,66. Most protocols included biopsy sampling at baseline and obtaining a confirmatory biopsy sample at 6–12 months. DREs were typically performed every 6 months and serum PSA testing was performed every 3–6 months (Fig. 2). The UCSF active surveillance cohort used the UCSF Cancer of the Prostate Risk Assessment score to determine whether or not the cancer had progressed53.
Fig. 2. Follow-up monitoring schedules from ongoing prostate cancer active surveillance programs.
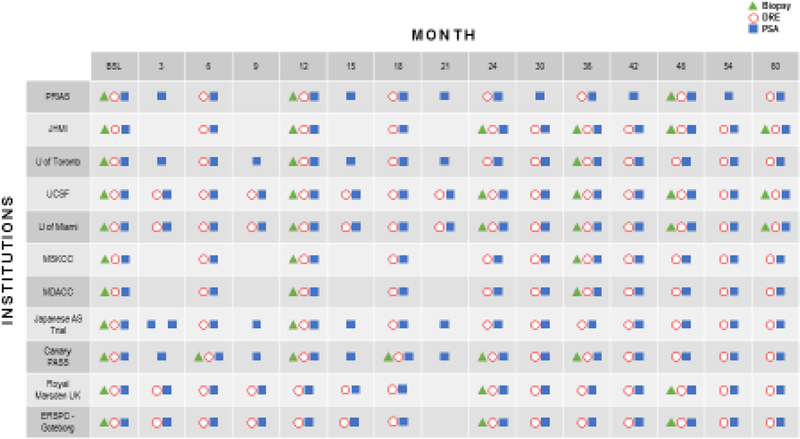
All the active surveillance programmes involve an initial biopsy, PSA test, and a digital rectal exam. Many of the active surveillance programmes include a PSA and a digital rectal exam every 3–6 months and a yearly biopsy; however, variations exists between the frequency of testing among active surveillance programmes. AS, active surveillance; BSL, baseline; DRE, digital rectal examination; ERSPC, European Randomized Study of Screening for Prostate Cancer; JHMI, Johns Hopkins Medical Institutions; MDACC, The University of Texas MD Anderson Cancer; MSKCC, Memorial Sloan Kettering Cancer Center; PASS, Prostate Active Surveillance Study; PRIAS, Prostate Cancer Research International Active Surveillance; PSA, prostate-specific antigen; UCSF, University of California, San Francisco; UK, United Kingdom.
The Movember Foundation has started the Global Action Plan Prostate Cancer Active Surveillance (GAP3) initiative to develop a global database of active surveillance cohorts to help develop a global consensus on the selection criteria and active surveillance protocol.67 Inoue and colleagues have attempted to provide some guidance regarding the optimal frequency of biopsy sampling68. Using data from the Canary Prostate Active Surveillance Study and studies conducted at Johns Hopkins, UCSF, and the University of Toronto, these researchers modelled the trade-offs associated with different biopsy sampling schedules used to detect disease progression. After controlling for variable surveillance intervals and the effects of competing treatments, all cohorts were found to have a delay of 3–5 months in the detection of disease progression using biennial biopsy sampling after the first confirmatory biopsy versus the use of annual biopsy sampling. The delay did not appear to have a significant consequence because it was associated with only a minor 3% increase in prostate cancer-related deaths. The repeat biopsy sampling might be enabling the detection of an increased tumour volume and not tumour progression; therefore, continued efforts to more accurately stratify patients on active surveillance are needed.
Evolution of the Gleason grading system
Over time, the classification of prostate cancer has evolved owing to improvements in pathology-based risk stratification, including two updates to the original Gleason prostate pathology grading system (Table 2). The original grading system was based on the detection of microscopic tumour architectural patterns in biopsy samples. This system included five patterns, starting from the most nonmalignant and benign glandular architecture to the most malignant69. In 1974 and 1977, Gleason and Mellinger revised and described the characteristics of each pattern with a greater level of detail, providing an improved understanding of the relationship between tumour histology and patient prognosis70,71.
Table 2.
Evolution of the Gleason grading scale
| Pattern | Original Gleason five69 | 2005 ISUP modified Gleason system73 | Histological definition of 2014 ISUP modified Gleason system75 |
|---|---|---|---|
| 1 | Very well differentiated, small, closely packed, uniform glands in essentially circumscribed masses | Circumscribed, closely packed nodules with separate, uniform, rounded to oval, medium-sized acini (larger glands than pattern 3) | Only individual, discrete, well-formed glands (Gleason score ≤6) |
| 2 | Similar to pattern 1, but with moderate variations in size and shape of glands and more atypia in histological appearance in individual cells; cribriform pattern might be present, still essentially circumscribed, but more loosely arranged | Like pattern 1, fairly circumscribed, yet at the edge of the tumour nodule there might be minimal levels of infiltration; glands are more loosely arranged and not quite as uniform as in Gleason pattern 1 | Predominantly well-formed glands with a lesser component of poorly formed/fused/cribriform glands (Gleason score 3 + 4 = 7) |
| 3 | Similar to pattern 2, but with marked irregularity in size and shape of glands, with tiny glands or individual cells invading the stroma away from circumscribed masses, or solid cords and masses with easily identifiable differentiation within most glands | Discrete glandular units; typically smaller glands than seen in Gleason pattern 1 or 2; infiltrates in and amongst non-neoplastic prostate acini; marked variations in size and shape; smoothly circumscribed, small cribriform tumour nodules | Predominantly poorly formed/fused/cribriform glands with a lesser component of well-formed glandsa (Gleason score 4 + 3 = 7) |
| 4 | Large clear cells growing in a diffuse pattern resembling hypernephroma; might show signs of gland formation | Fused microacinar glands. Ill-defined glands with a poorly formed glandular lumina. Large cribriform glands, with an irregular border and a hypernephromatoid histological pattern | Only poorly formed/fused/cribriform glands or predominantly well-formed glands and a lesser component lacking in glandsb or predominantly lacking in glands and with a lesser component of well-formed glandsb (Gleason score 4 + 4 = 8; 3 + 5 = 8; or 5 + 3 = 8) |
| 5 | Very poorly differentiated tumours; usually solid masses or diffuse areas of growth with little or no differentiation into glands | Essentially no glandular differentiation, composed of solid sheets, cords, or single cells; comedocarcinoma of the prostate with central necrosis surrounded by papillary, cribriform, or solid masses | Lack of gland formation (or with necrosis) with or without poorly formed/fused/cribriform glands (Gleason scores 9–10) |
ISUP, International Society of Urological Pathology.
For cases with >95% poorly formed/fused/ cribriform glands or lack of glands on a core or at radical prostatectomy, the component of <5% well-formed glands is not factored into the grade.
Poorly-formed/fused/cribriform glands can be a more minor component.
In 2005, the International Society of Urological Pathology (ISUP) convened a conference in San Antonio, Texas, to create a common base of interpretation of Gleason scoring and introduced the 2005 ISUP Modified Gleason System72–74. The most clinically important changes included the limitation of the Gleason pattern 3 definition and the expansion of the Gleason pattern 4 definition, which resulted in a greater number of patients being classified as having a Gleason score of 7. In other words, the ‘old’ Gleason score of 6 was transitioned to the ‘new’ Gleason score of 7. This change in scoring has had important clinical implications, particularly in selecting patients to receive treatment or active surveillance.
In 2014, experts in prostate cancer gathered to update the 2005 ISUP Modified Gleason System. The most important outcome of the meeting was the proposal of a new prognostic grade grouping system: Gleason score 6 or less, group I; Gleason score 3 + 4 = 7, group II; Gleason score 4 + 3 = 7, group III; Gleason score 4 + 4 = 8, group IV; and Gleason score 9–10, group V. The purpose of this change was to reduce the risk of overtreatment and use information from tumour pathology more efficiently in terms of diagnosis, prognosis, and treatment75,76. This new grade group system was incorporated into the new edition of the World Health Organization classification of prostate tumors77 and its impact on treatment decisions is yet to be determined.
Molecular evolution
The future role of risk stratification in the management of patients with prostate cancer likely rests on incorporating molecular assessments of the underlying tumour biology and potential for progression. Identifying tumours that are most likely to progress, and over what timeframe, is crucial. Specifically, the ability to identify tumours with metastatic potential — and therefore the population of patients who have the highest risk of cancer-related mortality — is the advance that will benefit patient care the most. Prostate cancer is biologically heterogeneous, as demonstrated by the spectrum of aggressiveness from indolent, low-grade tumours to lethal, high-grade prostate cancers.
Nelson, De Marzo, and Isaacs78 proposed a pathological model of prostate cancer progression involving activation of the androgen receptor (AR) by circulating androgens, genomic heterogeneity, and germline mutations. Among patients who underwent RP, those with serum testosterone levels <3 ng/ml before surgery were more likely to have disease upgrading between examinations of the initial prostate biopsy sample and subsequent RP specimen79. Similarly, low serum testosterone levels have been associated with higher levels of AR expression, increased capillary vessel density within the tumour, and a higher Gleason score in men with newly diagnosed and untreated prostate cancer80. Higher AR expression correlates with a greater level of differentiation and thus a lower Gleason score81–88. In a cohort of 67 patients with clinically localized prostate cancer who had undergone retropubic RP, the frequency of AR mutations was 16%89. A decreased Gleason score has been associated with the presence of AR mutations (P = 0.004)85,86.
In a multicentre study88, a 12% incidence of germline mutations in genes encoding proteins involved in the DNA damage response (DDR) was observed among patients with metastatic prostate cancer compared to 4.6% in localized prostate cancer as observed in the TCGA studyThe presence of such mutations was found to be associated with more aggressive disease. This frequency of DDR mutations is almost six-fold higher than the incidence of BRCA2 mutations in patients with localized prostate cancer. Gallagher et al.90 reported that carriers of germline BRCA2 mutations had an increased risk of prostate cancer and a higher histological grade at diagnosis than did patients who lacked detectable BRCA2 mutations. They also noted that BRCA1 mutation carriers seem to have similar levels of risk.
Tumour heterogeneity is a major challenge in identifying and utilizing molecular markers for risk stratification91,92. The body of literature on the genomic evolution and progression of focal, low-grade prostate cancer to high-grade castration-resistant prostate cancer is growing. Analysis of whole-genome sequencing (WGS) data from 10 patients with multiple prostate cancer metastases has provided data that support the seed-and-soil hypothesis, which stipulates that multiple potentially metastatic subclones are able to develop within the primary tumour and that the ability to metastasize is a unique characteristic of each subclone and not of the primary tumour. Metastatic seeding happens not only from the primary tumour to distant locations but also from metastatic lesions to other organs93. The findings of a study of inter-individual and intra-individual genomic heterogeneity in tumours from patients with metastatic prostate cancer using WGS, array comparative genomic hybridization, and RNA transcript profiling suggest that evaluation of a single metastatic lesion can provide adequate assessment of the most important oncogenic driver alterations in metastases within an individual patient94. Multiple attempts to use a number of genomic-based tests to stratify patients by risk has been made, although none have been prospectively validated as being able to improve outcomes in patients enrolled in active surveillance19, and a detailed discussion of these efforts is outside the scope of this Review. Specific DDR mutations might be useful prognostic biomarkers of early stage prostate cancer, for example, in identifying patients for inclusion or exclusion in active surveillance. However, data from additional experimental and population-based studies are needed in order to better inform this application of genomic testing.
Genomic testing might ultimately provide the optimal risk assessment test for patients who are eligible for active surveillance. However, minimally invasive blood-based assays are gaining traction due to limitations of tissue-based genomic testing.In this vein, selected core molecules and pathways with biological implications in early stage prostate cancer growth might be useful prognostic biomarkers. For example, caveolin-1 (Cav-1) is a cholesterol-binding protein with complex, cell-specific scaffolding functions within the context of caveolae, which are bulb-shaped surface pits located within the lipid raft microdomains of the plasma membrane19. Cav-1 is upregulated in primary tumour focal areas95, and men with prostate cancer have greater levels of serum Cav-1 than those with benign prostatic hyperplasia96. Moreover, elevations in serum Cav-1 levels are associated with tumour burden in patients with regionally advanced disease97, and might also predict disease recurrence following RP98. We have shown that baseline plasma Cav-1 level is an independent predictor of disease reclassification in men with early prostate cancer undergoing active surveillance99,100. Interestingly, in association with MYC, Cav-1 has been shown to be involved in the development of high-risk prostate cancer precursor lesions96,101. Overall, investigations of Cav-1 and/or MYC-based prognostic biomarkers might lead to new and more effective methods of refining active surveillance and intervention; nonetheless, all potential molecular markers require clinical validation before regular use in clinical practice can be justified. Unfortunately, blood-borne markers of biological relevance that may aid in the patient selection and treatment/management decision-making process in active surveillance in prostate cancer remains a critical unmet need102.
Thyroid cancer
Trends in the diagnosis, treatment and management of patients with thyroid cancer similar to those observed in patients with prostate cancer are beginning to emerge. Data from ecological and cross-sectional studies show that a notable increase in the incidence of thyroid cancer has occurred without a comparable decline in mortality (Fig. 1), suggesting that the harms of overdiagnosis and overtreatment are probably secondary to screening for thyroid cancer in asymptomatic individuals103.
Overview of epidemiology
The thyroid gland is a butterfly shaped endocrine organ located in the central neck, anatomically anterior to the trachea. The majority of carcinomas of the thyroid (>95%) arise from follicular cells103. More specifically, >85% of thyroid cancers derived from these cells are classified as PTC. PTCs are considered essentially well-differentiated, low-risk cancers, and only rare subtypes, such as medullary thyroid carcinoma, have warranted a designation of higher risk. Rarer nonpapillary subtypes of thyroid carcinoma can occur, such as follicular carcinoma and Hürthle cell carcinoma, although they are often biologically distinct and are not the focus of the overdiagnosis problem. Therefore, this discussion of overdiagnosis and overtreatment of thyroid cancer is focused specifically on PTC.
The vast majority of thyroid tumours diagnosed worldwide are PTCs, therefore, global statistics, including those revealing an increase in incidence, essentially reflect this form of thyroid cancer. In 2012, almost 300,000 thyroid cancers were diagnosed worldwide, with a two-fold higher incidence in economically developed countries compared with in less economically developed countries104. The incidence of thyroid cancer, or essentially PTC, has increased in most countries over the past few years104. In the United States, this incidence has increased from 4.9 cases per 100,000 persons in 1975 to 15.3 cases per 100,000 persons in 2013; meanwhile, thyroid cancer-related mortality has remained somewhat stable, at 0.4–0.6 deaths per 100,000 persons105,106. If the incidence of thyroid cancer in the USA continues to rise at the current rate, thyroid cancer could be the fourth most commonly diagnosed cancer by 2030. Much of the increased incidence of PTCs can be attributed to advances in technology, which have enabled more-sensitive detection of small, subclinical lesions107. Similarly, the widespread use of fine-needle aspiration guided by ultrasonography has enabled the pathological evaluation and diagnosis of these subclinical lesions. Such lesions typically fall into the category of microcarcinomas, a term indicating a diameter of ≤1 cm8,108–110. A diagnosis of thyroid cancer based upon the presence of these small tumours could be considered overdiagnosis, as the majority of these tumours would likely not result in death if left untreated. However, patients with thyroid cancers typically have a much broader age range than those with prostate cancer, with a median age at diagnosis of 51 years8. Individuals diagnosed with thyroid cancer might, therefore, require decades of follow-up monitoring. Similarly, active surveillance studies would need outcome data from several decades in order to fully address the potential rate of disease progression, and consequences for survival, in patients with thyroid microcarcinomas.
The low-risk nature of thyroid cancers is highlighted by SEER statistics, which show a disease-specific survival of 99.9% at 5 years when the tumour is localized to the thyroid and 98.0% at 5 years even when regionally advanced disease (lymph-node metastasis) is present109,111. The main life-threatening risk factor in patients with thyroid cancer is the presence of distant metastatic spread.
Guidelines for screening
An estimated 16 million individuals in the USA have palpable thyroid nodules, thus creating a challenge to accurate screening for thyroid cancer in the general population. Imaging data indicate that >60% of adults have thyroid nodules, of which 90% are benign. Unlike prostate cancer, in which serum PSA tests can be used to identify patients who might be at risk, PTC currently has no blood test designed to identify patients who might have an increased risk. Accordingly, screening for thyroid cancer is either not mentioned or not recommended by professional organizations. Recommendations from the ACS, American Thyroid Association (ATA), the American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi make no mention of screening for patients with thyroid cancer112–114. Screening for thyroid cancer is also not included in the Canadian Task Force on the Periodic Health Examination 2015 Preventive Care Checklist Form115. The American Academy of Family Physicians recommends against thyroid cancer screening, either using neck palpation or ultrasonography in asymptomatic individuals in their 2017 recommendations116. The USPSTF, in the 2017 update of its 1996 recommendation on screening for thyroid cancer with neck palpation and ultrasonoography105, recommends against the use of screening for thyroid cancer in asymptomatic individuals. In the judgement of the USPSTF, the overall harms outweighed the potential benefits of screening. Furthermore, the available evidence for calculating the risks of overdiagnosis and overtreatment from a population-based screening protocol was found to be inadequate.
Although screening is not recommended, a substantial proportion of thyroid nodules and subsequent microcarcinomas are detected incidentally on imaging performed for other indications, such as trauma or car accident, sinus symptoms, headaches, or voice changes. ‘Incidental’ thyroid nodules are seen in 20–67% of ultrasoundscans117, in up to 25% of CT scans118 and in about 1–2% of PET scans119, with notable variations in the frequency of subsequent malignancies detected in incidentally identified nodules on ultrasonography (~2%), CT or MRI (up to 11%), and F-fluorodeoxyglucose–PET (33–35%). These observations then create the potential for overscreening, overdiagnosis, and potential harm in patients with thyroid cancer and/or thyroid nodules. To minimize this known risk created by imaging studies detecting incidental thyroid nodules, the American College of Radiology published a white paper containing specific recommendations regarding which thyroid nodules are most likely to warrant evaluation for clinically relevant disease120. Algorithms for each imaging modality were also developed, incorporating patient age and nodule size as components of risk stratification120.
At the next step, when a patient undergoes a dedicated thyroid ultrasound scan for an incidentally detected thyroid nodule, recommendations have now been established regarding which nodules warrant fine-needle aspiration biopsy sampling for possibly clinically relevant disease. In an effort to further minimize the overdiagnosis of patients with microcarcinomas, the ATA guidance stipulates that fine-needle aspiration biopsy sampling should be limited to patients with nodules ≥1 cm in diameter121. The effects of these recommendations, which were introduced in 2015, in reducing the incidence of microcarcinomas of the thyroid have yet to be determined.
Overdiagnosis and overtreatment
According to ecological data on the incidence and mortality associated with thyroid cancer, autopsy data, and data on natural history provided by a single study122, overdiagnosis and overtreatment and their associated adverse effects seem likely to have arisen from screening for thyroid cancer103. This suggestion is supported by data from a number of studies that have documented the increased incidence of thyroid cancer, with no notable change in mortality103. The findings of studies conducted in Japan in the 1990s highlight the increased rates of thyroid cancer detection that emerged from widespread use of screening123, but the authors of these reports also recognized that the incidence of thyroid cancer was similar to that of incidental thyroid cancer detected in autopsy studies.
Data from South Korea clearly demonstrate the likely overdiagnosis and overtreatment of thyroid cancer124. South Korea has had an organized national cancer screening program since 1999 for most major cancers, and although not part of the official screening programme, additional thyroid cancer screening has been offered by physicians for a small fee. As a result, the incidence of thyroid cancer increased from 5 cases per 100,000 persons in 1993 to 70 cases per 100,000 persons in 2011124.
Data from autopsy studies and a few natural history studies have provided additional evidence of the existence of potentially indolent thyroid cancer. A systematic review of 15 studies published between 1969 and 2005 found that 11.5% (989/8,619) of thyroid glands harboured PTC125. In none of these patients was thyroid cancer related to the patient’s death, thus highlighting a relatively high prevalence of thyroid cancer that is largely without clinical consequences. Directly equating autopsy-detected thyroid nodules with microcarcinomas is a tempting conclusion to make, although Lee et al. noted that, in fact, incidental microcarcinomas in patients seem to be distinctly different from the disease described in historical autopsy studies125. Specifically, a higher rate of thyroid microcarcinomas is detected through screening in women than in men, although no gender-specific differences in this incidence have emerged in autopsy studies. Similarly, the frequency of clinically detectable lymph-node metastases is higher during screening than at autopsy for those with nodules of a similar size.
The increased use of imaging and ease of evaluating the thyroid gland using ultrasonography are both key contributing factors to the increasing incidence of thyroid cancer in the USA. From 1988 to 1989, 25% of new thyroid cancers were <1 cm in diameter at diagnosis. Whereas, by 2008–2009, 39% of new thyroid cancer diagnoses were microcarcinomas8. This increase in the number of patients being diagnosed has resulted in more patients receiving surgical treatment, with the associated risks of adverse effects of such procedures.
Surgery, the primary treatment of thyroid cancer, can include removing only the side of the thyroid with detectable disease (hemithyroidectomy) or removing the entire gland (total thyroidectomy). The risk of complications increases with the extent of surgery, and undergoing central lymph-node dissection raises the potential risk of comorbidities. The national trend over the past few decades for PTC treatment has been a total thyroidectomy, even for patients with microcarcinomas. The rationale for this practice was that: PTC is often multifocal (in 25%–30% of patients); lymph node metastases might be present even if the primary tumour is <1 cm in diameter (10%–33%); and if lymph-node metastases are present, adjuvant radioactive iodine therapy might be warranted after total thyroidectomy.
The sequela of a total thyroidectomy for all patients is hypothyroidism, requiring daily, lifelong oral replacement of thyroid hormones. Thyroid hormones (thyroxine and tri-iodothyronine) regulate numerous endocrine functions, including basal metabolic rate, and a substantial portion of patients never ‘feel right’, despite having replacement hormone levels in the same range as those of individuals with an intact thyroid gland. Other complications include the risk of damage to the recurrent laryngeal nerve, leading to permanent hoarseness and damage to the parathyroid glands, which can be either temporary or permanent126,127. Thus, the requirement of lifelong correction of hypoparathyroidism is a major quality-of-life issue, marked by the necessity of daily oral and/or injectable medications and reliance on medical follow-up monitoring for biochemical regulation, often accompanied by alterations in sense of personal wellbeing.
McMullen et al.128 retrospectively reviewed the outcomes of a series of patients who had undergone thyroidectomy and concluded that the risk of hypoparathyroidism was as high as 4% despite hormone replacement. The risk of hypocalcaemia increased with use of more extensive surgery, ranging from 5% to 10%. Injury of the laryngeal nerves presents as postoperative hoarseness or even breathiness in <5%129. If the injury is bilateral, the clinical manifestations of stridor (wheezing) and respiratory distress will appear after the patient is extubated. In the long term, patients might have an increased risk of aspiration and have difficulties with speech and swallowing.
Current active surveillance protocols
The apparent overdiagnosis and overtreatment of patients with low-risk papillary thyroid microcarcinomas led to the initiation of observation and active surveillance studies at multiple institutions in Japan14–17. As a consequence, patient selection for active surveillance has been informed primarily by the outcomes of trials conducted in Japan. These studies helped to identify contraindications to enrolment such as clinically detectable tumour-positive lymph nodes or other metastatic disease, or disease in other high-risk locations, including near a recurrent laryngeal nerve, abutting the trachea, and/or abutting areas of high-risk cytology. Data have also suggested that age at diagnosis is associated with risk of disease progression. In 1,235 patients diagnosed with papillary thyroid microcarcinoma between 1993 and 2011, Ito et al. observed progression to clinical disease in 8.9% of patients <40 years of age, in 3.5% of patients 40–59 years of age, and in 1.6% of patients >60 years of age14,130. In their 2018 paper, which included data from 1,211 patients on active surveillance, these investigators confirmed their prior finding that age at diagnosis is associated with risk of disease progression.131 The rate of disease progression at 10 years after diagnosis was 36.9%, 13.5%, 14.5%, 5.6%, 6.6%, and 3.5% for patients in their 20s, 30s, 40s, 50s, 60s, and 70s, respectively. Similarly, the lifetime probability of progression for those diagnosed during these different decades of life was 60.3%, 37.1%, 27.3%, 14.9%, 9.9%, and 3.5%, respectively.
Studies exploring the effectiveness of active surveillance in patients with thyroid cancer began in Japan in the 1990s, although only after 2014 have similar studies been initiated in the USA. A small number of active surveillance cohort studies involving patients with thyroid carcinoma are currently ongoing. As of February 2017, four studies were registered on ClinicalTrials.gov (Table 2). Two of these cohorts are from South Korea (Seoul National University Hospital and Seoul St. Mary’s Hospital) and two are from the USA (Memorial Sloan Kettering Cancer Center, New York, NY, and Cedars-Sinai Medical Center, Los Angeles, CA). All four trials have similar inclusion criteria. The investigators estimate that they will enrol 200 to 400 patients >18 years of age with histologically confirmed PTC. Some small variations in inclusion criteria exist regarding the maximum nodule size enabling eligibility (range, <1 cm to 2 cm). The cohorts also differ regarding disease stage and other tumour pathology characteristics, such as invasion of surrounding structures. The primary outcome for all the cohorts is rate of disease progression, with follow-up durations ranging from 4 to 10 years.
Molecular pathways
The molecular biology of papillary thyroid microcarcinomas mirrors that of larger PTCs, in that approximately 50% of microcarcinomas harbour the BRAFV600E mutation. Agrawal et al.132 genomically characterized approximately 500 PTCs as part of The Cancer Genome Atlas consortium and demonstrated that MAPK pathway-driven PTCs have two main, mutually exclusive drivers (BRAF and RAS mutations). The authors also hypothesized that other gene networks, such as PIK3CA, could overlap with BRAFV600E and lead to tumour progression by activation of other pathways related to PTC, such as the PI3K pathway.
Modern molecular assessment techniques have been adapted for the analysis of small amounts of tissue, including fine-needle aspiration samples from thyroid nodules, and are included in the adjuvant evaluation of thyroid nodules not readily diagnosed on cytology review121,133; however, the identification of a molecular marker that indicates disease progression has, thus far, eluded researchers. Yabuta and colleagues134 probed for BRAFV600E and TERT-promoter mutations in samples from patients who had been on active surveillance for 10 years. The patients were divided then into groups based on either the absence of disease progression (n = 11), presence of an increase in microcarcinoma diameter (n = 10), and the presence of metastases (n = 5). At least 64% of patients in each group had BRAFV600E mutations (64% of those without disease progression; 70% of patients with an increase in tumour diameter; and 80% of those with lymph node metastases); however, neither the TERT C228T nor C250T point mutations were present in any patients in any categories.
Cost-effectiveness considerations
Active surveillance of indolent malignancies of the prostate and thyroid gland could be instrumental in reducing exposure of patients to unnecessary clinical interventions and costs and, most importantly, could improve the standards of personalized patient care. The use of active surveillance for prostate cancer has, for 20 years, served patients while continuing to evolve; however, much remains to be done to optimize the various patient selection, risk stratification, and monitoring methodologies18,66. By contrast, active surveillance of thyroid cancer is a relatively new approach to the management of patients in the USA. Lessons learned from active surveillance for prostate cancer and in trials conducted in Japan involving patients with thyroid cancer might be useful in developing active surveillance protocols for use in patients with thyroid cancer in the USA. However, patients with prostate cancer are typically older (65–74 years of age) 20 whereas patients with thyroid cancer can be younger, (45–54 years of age)135.
Comparing the cost-effectiveness of active surveillance with that of immediate treatment is often challenging: for patients with prostate cancer, findings are mixed, and for those with thyroid cancer, they are more nuanced. Lao et al. found, using Markov modelling, that active surveillance of prostate cancer was not more cost-effective than RP for men 45–55 years of age but was more cost-effective than RP for men 60–70 years of age136. Becerra et al. conducted a systematic review of economic valuation for studies conducted using data from European health-care systems and found that RP was more cost-effective than watchful waiting or active surveillance in terms of quality-adjusted life years137. However, in a claims-based study using data from Germany, investigators found that active surveillance was more effective than RP138. Investigators in several studies have modelled the cost-effectiveness of active surveillance against that of hemithyroidectomy, on the basis on numerous assumptions, and found that the cost-effectiveness of hemithyroidectomy reflects decreased quality of life and life expectancy for patients on active surveillance139,140. However, these models did not account for the increased probability of disease progression in patients younger than 40 years.
Conclusions
The historical mantra of detecting all cancers regardless of tumour size and malignant potential is being silenced. The overdiagnosis and overtreatment of both prostate cancer and thyroid cancer have caused patients to suffer avoidable deteriorations in quality of life and placed unnecessary burdens on health-care systems. Furthermore, the recognition that potential harms secondary to diagnosis and subsequent treatments might not be balanced with overall patient benefit is growing, following the observation that treatment has not increased survival. The multifactorial and complex nature of overdiagnosis and overtreatment in both patients with prostate cancers and in those with thyroid cancers highlight several similarities and differences for future consideration.
The primary lesson to be learned is that broad, population-based screening can result in the detection, and increased prevalence of cancers that are unlikely to be life threatening. Following the introduction of broad, population-based screening, we have seen an increase in the incidence of prostate and thyroid cancers without comparable decreases in mortality. Following this increase in incidence, current guidelines for prostate or thyroid cancers do not strongly endorse the broad use of population-based screening. The screening guidelines for prostate cancer emphasize individualized decision-making along with a discussion of the potential beneficial and harmful effects of screening2,28–30,32,34. By contrast, the guidelines for thyroid cancer either do not mention or advise against the use of screening owing to the risks of overdetection and/or overdiagnosis of thyroid nodules105,112,114,116.
Screening tests for both cancers are nonspecific, resulting in the detection of cancers that might never cause harm. The serum PSA test, either with or without a DRE is widely used for prostate cancer screening. However, conditions other than prostate cancer, such as benign prostatic hyperplasia and/or prostatitis, could lead to elevated serum PSA levels21. The false-positive rate could also change depending on the serum PSA threshold used to indicate a need for diagnostic biopsy sampling and analysis, which can range between 2.5 ng/ml and 10.0 ng/ml.
Neck palpation and ultrasonography are both used for thyroid cancer screening, although few high-quality studies have examined the accuracy of these screening tests103. The sensitivity of neck palpation for the differentiation of malignant from benign goiterous nodules is low (11.6%) but improves with the addition of ultrasonography (to 27.8%). The sensitivity of ultrasonography across several studies is high (94.3–94.8%), but many cancerous nodules that are detected are very small, are likely to be slow growing, and are unlikely to cause harm.
Lessons learned from active surveillance of patients with prostate cancer can inform the development of active surveillance protocols for those with thyroid cancer, particularly in enabling the careful identification of the most appropriate patients for participation in active surveillance. Eligibility criteria for the selection of such patients have evolved over time in the large cohort studies and in the guidelines, but some variability still exists18,43,48. Similar trends are emerging in the eligibility of patients with PTC for active surveillance, in that the ATA does not recommend cytology-based assessments for patients with low-risk nodules16,39,46, although Ito and colleagues104 do endorse this form of assessment. With these variations in mind, professional communities must come to a consensus regarding the most appropriate tests and testing schedules for active surveillance. Such a consensus has currently not been reached. The ATA guidelines provide no recommendations regarding the specific protocol for active surveillance in patients with thyroid cancer. Furthermore, a universally accepted threshold for disease progression, indicating a need to initiate definitive treatment, is needed in order to avoid overtreatment in patients with prostate cancer and in those with thyroid cancer. In both diseases, the future of patient selection will likely be augmented by the availability of molecular assessments that enable clinicians to predict which tumours are more likely to be indolent and to commence immediate interventions in patients with tumours that are potentially aggressive. As an additional cost to health-care systems, the value of any additional tool or test that is introduced will need to be weighed up against the magnitude of benefit to the population at risk.
Table 3.
Thyroid cancer active surveillance cohorts
| Institution | Estimated enrollment | Inclusion criteria | Exclusion criteria | Outcomes |
|---|---|---|---|---|
| Kuma Hospital, Kuma, Japan131 | n = 1,211 | • Diagnosed using ultrasonography -guided fine- needle aspiration • Absence of distant or nodal metastasis, substantial extrathyroidal extension, aggressive cytology |
• Presence of tumours attached to the trachea or located on the course of the laryngeal nerve | • Timeframe: 10 years • Rate of progression of thyroid papillary microcarcinoma |
| Cancer Instititue Hospital, Tokyo, Japan123 | n = 230 | • Diagnosed using ultrasonography-guided fine- needle aspiration | • Presence of clinically apparent distant metastases, lymphadenopathy (≥1 cm), or extrathyroidal invasion | • Timeframe: 13 years • Rate of progression of thyroid papillary microcarcinoma |
| Seoul National University Hospital, Seoul, South Korea (NCT02938702) | n = 400 | • Diagnosed using fine-needle aspiration or needle biopsy of Bethesda category V or VI • Thyroid cancer <1 cm in diameter |
• Cannot routinely follow up according to the study schedule • Hyperthyroidism that needs treatment |
• Timeframe: 5 years • Rate of progression of thyroid papillary microcarcinoma* • Comparison of rate of disease progression between active surveillance group and surgery group |
| Seoul St. Mary’s Hospital, Seoul, South Korea (NCT02952612) | n = 300 | • <1 cm solitary nodule • Surrounded by ≥2 mm nonmalignant thyroid parenchyma • Subcapsular locations not adjacent to surrounding structures, recurrent laryngeal nerve, trachea, or carotid artery • No evidence of invasion to surrounding structures, recurrent laryngeal nerve, trachea, or carotid artery • No extrathyroidal extension • N0 and M0 stage disease • Combined severe comorbidities or inoperable condition (optional) • No wish to undergo surgery and willingness to accept active surveillance • No history of radiotherapy to the head and neck area |
• ≥1 cm nodule and/or multifocal nodules, regardless of size • PTC variants associated with poor prognosis (tall cell variant, columnar variant, hobnail variant, or other types of thyroid cancer) • Subcapsular locations adjacent to surrounding structures, such as the recurrent laryngeal nerve, trachea, or carotid artery • Evidence of invasion of surrounding structures, recurrent laryngeal nerve, trachea, or carotid artery • Extrathyroidal extension • N1 and/or M1 disease • Documented increase in tumour size, according to ATA guidelines in a previously confirmed papillary thyroid microcarcinoma • Age <18 years |
• Timeframe: 5 years • Progression of papillary thyroid microcarcinoma |
| Memorial Sloan Kettering Cancer Center, New York, NY, USA (NCT02363595) | n = 300 | • Biopsy-proven PTC (or case suspected of being PTC) confirmed by MSKCC cytopathologist • Being followed with active surveillance at MSKCC • Biopsied index nodule ≤2 cm in maximum dimension • Thyroid and neck ultrasonography performed and interpreted by a MSKCC radiologist <6 months prior to study entry |
• Biopsied index nodule >2 cm in any dimension • Age <18 years |
• Time frame: 4 years • Estimate the rate of disease progression |
| Cedars-Sinai Medical Center, Los Angeles, CA, USA (NCT02609685) | n = 216 | • Pathologically confirmed Bethesda V or VI thyroid nodules with papillary thyroid carcinoma • ≤1.5 cm nodules by ultrasonographic criteria • Ability to understand and the willingness to sign a written informed consent and Health Insurance Portability and Accountability Act authorization form |
• High-grade or poorly differentiated PTC variants • Central or lateral neck lymphadenopathy suspected of being PTC • Unfavorable nodule location (such as near dorsal surface [by recurrent laryngeal nerve]); adjacent to the trachea (risk of cartilage invasion) • History of radiation to neck |
• Time frame: from time of diagnosis up to 10-years of follow up • Rate of disease progression over a 3-year, 5-year, and 10-year period • Percentage of patients electing to undergo surgery despite absence of clinical progression • Impact of thyroid-stimulating hormone suppression on thyroid nodule growth (in cm) as measured using ultrasonography over 5 years • Identify the clinicopathological features associated with disease progression in papillary thyroid microcarcinoma followed with active surveillance by 5 years • Identify the genetic factors associated with an increased risk of disease progression at 5 years • Quality of life score as measured using the City of Hope Quality of Life Scale • Anxiety score as measured using the Memorial Anxiety Scale at 5 years |
ATA, American Thyroid Association; MSKCC, Memorial Sloan Kettering Cancer Center; PTC, papillary thyroid cancer.
Progression defined as an increase in tumour size >3 mm in diameter, or a tumour that involves new lymph nodes, or leads to the development of metastases.
Progression defined as any change in the size and/or shape of papillary thyroid microcarcinoma detected using thyroid sonography.
Key points.
The harms of overdiagnosis and overtreatment that were observed in patients with prostate cancer following the introduction of prostate-specific antigen (PSA)-based screening are now being observed in those with thyroid cancer.
Broad population-based screening can lead to the overdiagnosis and overtreatment of indolent cancers, as originally seen in prostate cancer and now also seen in thyroid cancer.
Data from clinical trials demonstrate that active surveillance is an appropriate management strategy for patients with low-risk prostate cancer, and the results from similar trials in those with thyroid cancer are promising.
Consensus is needed regarding both the optimal approach to patient selection for active surveillance and the most appropriate active surveillance scheduling and monitoring strategies
More specific methods of stratifying patients by risk, prior to consideration for active surveillance, are needed.
Acknowledgements
The work from the authors is supported in part by a Cancer Center Support Grant (CA16672) from the National Cancer Institute, and National Institutes of Health.
Footnotes
Competing interests
At the time of this work, J.K. was a Professor at the University of Texas MD Anderson Cancer Center. She is now a full-time employee of Merck. L.M.L, S.P.B, M.D.W, P.T., J.R.G., and T.C.T have no conflicts of interest to report.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
REFERENCES
- 1.Carter SM et al. The challenge of overdiagnosis begins with its definition. BMJ 350, h869, 10.1136/bmj.h869 (2015). [DOI] [PubMed] [Google Scholar]
- 2.U. S. Preventive Services Task Force et al. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. JAMA 319, 1901–1913, 10.1001/jama.2018.3710 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Resnick MJ et al. Long-term functional outcomes after treatment for localized prostate cancer. N. Engl. J. Med 368, 436–445, 10.1056/NEJMoa1209978 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donovan JL et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N. Engl. J. Med 375, 1425–1437, 10.1056/NEJMoa1606221 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilt TJ, Andriole GL & Brawer MK Prostatectomy versus observation for early prostate cancer. N. Engl. J. Med 377, 1302–1303, 10.1056/NEJMc1710384 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Cooperberg MR & Carroll PR Trends in management for patients with localized prostate cancer, 1990–2013. JAMA 314, 80–82, 10.1001/jama.2015.6036 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Barocas DA, Cowan JE, Smith JA Jr., Carroll PR & Ca PI What percentage of patients with newly diagnosed carcinoma of the prostate are candidates for surveillance? An analysis of the CaPSURE database. J. Urol 180, 1330–1334; 10.1016/j.juro.2008.06.019 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Davies L & Welch HG Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 140, 317–322, 10.1001/jamaoto.2014.1 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Sun F et al. Therapies for clinicially localized prostate cancer: update of a 2008 systematic review. Comparative Effectiveness Review No. 146. (Prepared by the ECRI Institute-Penn Medicine Evidence-based Practice Center under Contract No. 290–2007-10063.) AHRQ Publication No. 15-EHC004-EF (Agency for Healthcare Research and Quality, Rockville, MD, 2014). [PubMed] [Google Scholar]
- 10.Hamdy FC et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N. Engl. J. Med 375, 1415–1424, 10.1056/NEJMoa1606220 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Wilt TJ et al. Radical prostatectomy versus observation for localized prostate cancer. N. Engl. J. Med 367, 203–213, 10.1056/NEJMoa1113162 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilt TJ Management of low risk and low PSA prostate cancer: long term results from the prostate cancer intervention versus observation trial. Recent Results Cancer Res 202, 149–169, 10.1007/978-3-642-45195-9_18 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Wilt TJ The Prostate Cancer Intervention Versus Observation Trial:VA/NCI/AHRQ Cooperative Studies Program #407 (PIVOT): Design and Baseline Results of a Randomized Controlled Trial Comparing Radical Prostatectomy With Watchful Waiting for Men With Clinically Localized Prostate Cancer. JNCI Monographs 2012, 184–190, 10.1093/jncimonographs/lgs041 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito Y et al. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid 24, 27–34, 10.1089/thy.2013.0367 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyauchi A Clinical trials of active surveillance of papillary microcarcinoma of the thyroid. World J. Surg 40, 516–522, 10.1007/s00268-015-3392-y (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugitani I & Fujimoto Y Management of low-risk papillary thyroid carcinoma: unique conventional policy in Japan and our efforts to improve the level of evidence. Surg. Today 40, 199–215, 10.1007/s00595-009-4034-5 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Sugitani I et al. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J. Surg 34, 1222–1231, 10.1007/s00268-009-0359-x (2010). [DOI] [PubMed] [Google Scholar]
- 18.Bruinsma SM et al. Active surveillance for prostate cancer: a narrative review of clinical guidelines. Nat Rev Urol 13, 151–167, 10.1038/nrurol.2015.313 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Loeb S et al. Active surveillance for prostate cancer: a systematic review of clinicopathologic variables and biomarkers for risk stratification. Eur. Urol 67, 619–626, 10.1016/j.eururo.2014.10.010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cancer Stat Facts: Prostate Cancer, <https://seer.cancer.gov/statfacts/html/prost.html> (2018).
- 21.Fenton JJ et al. Prostate-specific antigen--based screening for prostate cancer: a systematic evidence review for the U.S. Preventive Services Task Force. Evidence Synthesis No. 154. AHRQ Publication No. 17–05229-EF-1 (Agency for Healthcare Research and Quality, Rockville, MD, 2017). [PubMed] [Google Scholar]
- 22.Global Burden of Disease Cancer Collaboration et al. The global burden of cancer 2013. JAMA Oncol 1, 505–527, 10.1001/jamaoncol.2015.0735 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haas GP, Delongchamps N, Brawley OW, Wang CY & de la Roza G The worldwide epidemiology of prostate cancer: perspectives from autopsy studies. Can J Urol 15, 3866–3871 (2008). [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer MS et al. Homogeneous prostate cancer mortality in the Nordic countries over four decades. Eur. Urol 58, 427–432, 10.1016/j.eururo.2010.05.040 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jemal A, Siegel R, Xu J & Ward E Cancer statistics, 2010. CA Cancer J. Clin 60, 277–300, 10.3322/caac.20073 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Moyer VA & Preventive US Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med 157, 120–134, 10.7326/0003-4819-157-2-201207170-00459 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Fleshner K, Carlsson SV & Roobol MJ The effect of the USPSTF PSA screening recommendation on prostate cancer incidence patterns in the USA. Nature Reviews Urology 14, 26, 10.1038/nrurol.2016.251 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Cancer Society. Prostate cancer prevention and early detection, <https://www.cancer.org/cancer/prostate-cancer/early-detection/acs-recommendations.html> (2016).
- 29.Basch E et al. Screening for prostate cancer with prostate-specific antigen testing: American Society of Clinical Oncology provisional clinical opinion. J. Clin. Oncol 30, 3020–3025, 10.1200/jco.2012.43.3441 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carter HB et al. Early detection of prostate cancer: AUA Guideline. J. Urol 190, 419–426, 10.1016/j.juro.2013.04.119 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qaseem A et al. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann. Intern. Med 158, 761–769, 10.7326/0003-4819-158-10-201305210-00633 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Carroll PR et al. NCCN guidelines insights: prostate cancer early detection, version 2.2016. J. Natl. Compr. Canc. Netw 14, 509–519 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horwich A et al. Prostate cancer: ESMO consensus conference guidelines 2012. Ann. Oncol 24, 1141–1162, 10.1093/annonc/mds624 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Heidenreich A et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur. Urol 65, 124–137, 10.1016/j.eururo.2013.09.046 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Pashayan N et al. Mean sojourn time, overdiagnosis, and reduction in advanced stage prostate cancer due to screening with PSA: implications of sojourn time on screening. Br. J. Cancer 100, 1198–1204, 10.1038/sj.bjc.6604973 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fouad MN et al. Comorbidity independently predicted death in older prostate cancer patients, more of whom died with than from their disease. J. Clin. Epidemiol 57, 721–729, 10.1016/j.jclinepi.2003.11.009 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Loeb S et al. Overdiagnosis and overtreatment of prostate cancer. Eur. Urol 65, 1046–1055, 10.1016/j.eururo.2013.12.062 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Etzioni R, Gulati R, Mallinger L & Mandelblatt J Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann. Intern. Med 158, 831–838, 10.7326/0003-4819-158-11-201306040-00008 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Gelder R et al. Interpreting overdiagnosis estimates in population-based mammography screening. Epidemiol. Rev 33, 111–121, 10.1093/epirev/mxr009 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aizer AA et al. Cost implications and complications of overtreatment of low-risk prostate cancer in the United States. J. Natl. Compr. Canc. Netw 13, 61–68 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Heijnsdijk EA et al. Quality-of-life effects of prostate-specific antigen screening. N. Engl. J. Med 367, 595–605, 10.1056/NEJMoa1201637 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen RC et al. Active surveillance for the management of localized prostate cancer (Cancer Care Ontario Guideline): American Society of Clinical Oncology clinical practice guideline endorsement. J. Clin. Oncol 34, 2182–2190, 10.1200/JCO.2015.65.7759 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Sanda MG et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline (2017).
- 44.Bokhorst LP et al. A decade of active surveillance in the PRIAS study: An update and evaluation of the criteria used to recommend a switch to active treatment. Eur. Urol 70, 954–960, 10.1016/j.eururo.2016.06.007 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Bul M et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur. Urol 63, 597–603, 10.1016/j.eururo.2012.11.005 (2013). [DOI] [PubMed] [Google Scholar]
- 46.Tosoian JJ et al. Intermediate and longer-term outcomes from a prospective active-surveillance program for favorable-risk prostate cancer. J. Clin. Oncol 33, 3379–3385, 10.1200/JCO.2015.62.5764 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tosoian JJ et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J. Clin. Oncol 29, 2185–2190, 10.1200/JCO.2010.32.8112 (2011). [DOI] [PubMed] [Google Scholar]
- 48.Klotz L Active surveillance for prostate cancer: for whom? J. Clin. Oncol 23, 8165–8169, 10.1200/JCO.2005.03.3134 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Klotz L et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J. Clin. Oncol 33, 272–277, 10.1200/JCO.2014.55.1192 (2015). [DOI] [PubMed] [Google Scholar]
- 50.Klotz L et al. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J. Clin. Oncol 28, 126–131, 10.1200/JCO.2009.24.2180 (2010). [DOI] [PubMed] [Google Scholar]
- 51.Whitson JM et al. The relationship between prostate specific antigen change and biopsy progression in patients on active surveillance for prostate cancer. J. Urol 185, 1656–1660, 10.1016/j.juro.2010.12.042 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Welty CJ et al. Extended followup and risk factors for disease reclassification in a large active surveillance cohort for localized prostate cancer. J. Urol 193, 807–811, 10.1016/j.juro.2014.09.094 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Cooperberg MR, Broering JM & Carroll PR Risk assessment for prostate cancer metastasis and mortality at the time of diagnosis. J. Natl. Cancer Inst 101, 878–887, 10.1093/jnci/djp122 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soloway MS et al. Careful selection and close monitoring of low-risk prostate cancer patients on active surveillance minimizes the need for treatment. Eur. Urol 58, 831–835, 10.1016/j.eururo.2010.08.027 (2010). [DOI] [PubMed] [Google Scholar]
- 55.Adamy A et al. Role of prostate specific antigen and immediate confirmatory biopsy in predicting progression during active surveillance for low risk prostate cancer. J. Urol 185, 477–482, 10.1016/j.juro.2010.09.095 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davis JW et al. Disease reclassification risk with stringent criteria and frequent monitoring in men with favourable-risk prostate cancer undergoing active surveillance. BJU Int 118, 68–76, 10.1111/bju.13193 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kakehi Y et al. Prospective evaluation of selection criteria for active surveillance in Japanese patients with stage T1cN0M0 prostate cancer. Jpn. J. Clin. Oncol 38, 122–128, 10.1093/jjco/hym161 (2008). [DOI] [PubMed] [Google Scholar]
- 58.Lin DW et al. Urinary TMPRSS2:ERG and PCA3 in an active surveillance cohort: results from a baseline analysis in the Canary Prostate Active Surveillance Study. Clin. Cancer Res 19, 2442–2450, 10.1158/1078-0432.CCR-12-3283 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Newcomb LF et al. Outcomes of active surveillance for clinically localized prostate cancer in the prospective, multi-institutional Canary PASS cohort. J. Urol 195, 313–320, 10.1016/j.juro.2015.08.087 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Selvadurai ED et al. Medium-term outcomes of active surveillance for localised prostate cancer. Eur. Urol 64, 981–987, 10.1016/j.eururo.2013.02.020 (2013). [DOI] [PubMed] [Google Scholar]
- 61.van den Bergh RC et al. Outcomes of men with screen-detected prostate cancer eligible for active surveillance who were managed expectantly. Eur. Urol 55, 1–8, 10.1016/j.eururo.2008.09.007 (2009). [DOI] [PubMed] [Google Scholar]
- 62.Schroder FH et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 384, 2027–2035, doi:10.1016/S0140-6736(14)60525-0 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Godtman RA et al. Long-term results of active surveillance in the Goteborg randomized, population-based prostate cancer screening trial. Eur. Urol 70, 760–766, 10.1016/j.eururo.2016.03.048 (2016). [DOI] [PubMed] [Google Scholar]
- 64.Loeb S et al. Five-year nationwide follow-up study of active surveillance for prostate cancer. Eur. Urol 67, 233–238, 10.1016/j.eururo.2014.06.010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tosoian JJ, Carter HB, Lepor A & Loeb S Active surveillance for prostate cancer: current evidence and contemporary state of practice. Nat Rev Urol 13, 205–215, 10.1038/nrurol.2016.45 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kinsella N et al. Active surveillance for prostate cancer: a systematic review of contemporary worldwide practices. Transl Androl Urol 7, 83–97, 10.21037/tau.2017.12.24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bruinsma SM et al. The Movember Foundation’s GAP3 cohort: a profile of the largest global prostate cancer active surveillance database to date. BJU Int 121, 737–744, 10.1111/bju.14106 (2018). [DOI] [PubMed] [Google Scholar]
- 68.Inoue LYT et al. Comparative analysis of biopsy upgrading in four prostate cancer active surveillance cohorts. Ann. Intern. Med, 10.7326/M17-0548 (2017). [DOI] [PMC free article] [PubMed]
- 69.Gleason DF Classification of prostatic carcinomas. Cancer Chemother. Rep 50, 125–128 (1966). [PubMed] [Google Scholar]
- 70.Gleason DF & Mellinger GT Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J. Urol 111, 58–64 (1974). [DOI] [PubMed] [Google Scholar]
- 71.Mellinger GT Prognosis of prostatic carcinoma. Recent Results Cancer Res, 61–72 (1977). [DOI] [PubMed]
- 72.Gleason DF, Mellinger GT & Veterans Administration Cooperative Urological Research Group. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. 1974. J. Urol 167, 953–958; discussion 959 (2002). [PubMed] [Google Scholar]
- 73.Montironi R et al. Original Gleason system versus 2005 ISUP modified Gleason system: the importance of indicating which system is used in the patient’s pathology and clinical reports. Eur. Urol 58, 369–373, 10.1016/j.eururo.2010.04.028 (2010). [DOI] [PubMed] [Google Scholar]
- 74.Epstein JI, Allsbrook WC Jr., Amin MB, Egevad LL & Isup Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason grading of prostatic carcinoma. Am. J. Surg. Pathol 29, 1228–1242 (2005). [DOI] [PubMed] [Google Scholar]
- 75.Epstein JI et al. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am. J. Surg. Pathol 40, 244–252, 10.1097/PAS.0000000000000530 (2016). [DOI] [PubMed] [Google Scholar]
- 76.Epstein JI et al. A contemporary prostate cancer grading system: a validated alternative to the Gleason score. Eur. Urol 69, 428–435, 10.1016/j.eururo.2015.06.046 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Humphrey PA, Moch H, Cubilla AL, Ulbright TM & Reuter VE The 2016 WHO classification of tumours of the urinary system and male genital organs-part B: prostate and bladder tumours. Eur. Urol 70, 106–119, 10.1016/j.eururo.2016.02.028 (2016). [DOI] [PubMed] [Google Scholar]
- 78.Nelson WG, De Marzo AM & Isaacs WB Prostate cancer. N. Engl. J. Med 349, 366–381, 10.1056/NEJMra021562 (2003). [DOI] [PubMed] [Google Scholar]
- 79.Pichon A et al. Preoperative low serum testosterone is associated with high-grade prostate cancer and an increased Gleason score upgrading. Prostate Cancer Prostatic Dis, 10.1038/pcan.2015.44 (2015). [DOI] [PubMed]
- 80.Schatzl G et al. Association of polymorphisms within androgen receptor, 5alpha-reductase, and PSA genes with prostate volume, clinical parameters, and endocrine status in elderly men. Prostate 52, 130–138, 10.1002/pros.10101 (2002). [DOI] [PubMed] [Google Scholar]
- 81.Chodak GW et al. Nuclear localization of androgen receptor in heterogeneous samples of normal, hyperplastic and neoplastic human prostate. J. Urol 147, 798–803 (1992). [DOI] [PubMed] [Google Scholar]
- 82.Lee DK & Chang C Endocrine mechanisms of disease: expression and degradation of androgen receptor: mechanism and clinical implication. J. Clin. Endocrinol. Metab 88, 4043–4054, 10.1210/jc.2003-030261 (2003). [DOI] [PubMed] [Google Scholar]
- 83.Takeda H et al. Androgen receptor content of prostate carcinoma cells estimated by immunohistochemistry is related to prognosis of patients with stage D2 prostate carcinoma. Cancer 77, 934–940 (1996). [PubMed] [Google Scholar]
- 84.Khera M, Crawford D, Morales A, Salonia A & Morgentaler A A new era of testosterone and prostate cancer: from physiology to clinical implications. Eur. Urol 65, 115–123, 10.1016/j.eururo.2013.08.015 (2014). [DOI] [PubMed] [Google Scholar]
- 85.Weischenfeldt J et al. Integrative genomic analyses reveal an androgen-driven somatic alteration landscape in early-onset prostate cancer. Cancer Cell 23, 159–170, 10.1016/j.ccr.2013.01.002 (2013). [DOI] [PubMed] [Google Scholar]
- 86.Demichelis F, Garraway LA & Rubin MA Molecular archeology: unearthing androgen-induced structural rearrangements in prostate cancer genomes. Cancer Cell 23, 133–135, 10.1016/j.ccr.2013.01.019 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sowalsky AG, Ye H, Bubley GJ & Balk SP Clonal progression of prostate cancers from Gleason grade 3 to grade 4. Cancer Res 73, 1050–1055, 10.1158/0008-5472.CAN-12-2799 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pritchard CC et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N. Engl. J. Med 375, 443–453, 10.1056/NEJMoa1603144 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sanchez D et al. Androgen receptor mutations are associated with Gleason score in localized prostate cancer. BJU Int 98, 1320–1325, 10.1111/j.1464-410X.2006.06438.x (2006). [DOI] [PubMed] [Google Scholar]
- 90.Gallagher DJ et al. Germline BRCA mutations denote a clinicopathologic subset of prostate cancer. Clin. Cancer Res 16, 2115–2121, 10.1158/1078-0432.CCR-09-2871 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boutros PC et al. Spatial genomic heterogeneity within localized, multifocal prostate cancer. Nat. Genet 47, 736–745, 10.1038/ng.3315 (2015). [DOI] [PubMed] [Google Scholar]
- 92.Agoulnik IU et al. Role of SRC-1 in the promotion of prostate cancer cell growth and tumor progression. Cancer Res 65, 7959–7967, 10.1158/0008-5472.CAN-04-3541 (2005). [DOI] [PubMed] [Google Scholar]
- 93.Gundem G et al. The evolutionary history of lethal metastatic prostate cancer. Nature 520, 353–357, 10.1038/nature14347 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kumar A et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat. Med 22, 369–378, 10.1038/nm.4053 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Parton RG & del Pozo MA Caveolae as plasma membrane sensors, protectors and organizers. Nat. Rev. Mol. Cell Biol 14, 98–112, 10.1038/nrm3512 (2013). [DOI] [PubMed] [Google Scholar]
- 96.Yang G, Truong LD, Wheeler TM & Thompson TC Caveolin-1 expression in clinically confined human prostate cancer: a novel prognostic marker. Cancer Res 59, 5719–5723 (1999). [PubMed] [Google Scholar]
- 97.Tahir SA et al. Development of an immunoassay for serum caveolin-1: a novel biomarker for prostate cancer. Clin. Cancer Res 9, 3653–3659 (2003). [PubMed] [Google Scholar]
- 98.Gumulec J et al. Caveolin-1 as a potential high-risk prostate cancer biomarker. Oncol. Rep 27, 831–841, 10.3892/or.2011.1587 (2012). [DOI] [PubMed] [Google Scholar]
- 99.Tahir SA et al. Preoperative serum caveolin-1 as a prognostic marker for recurrence in a radical prostatectomy cohort. Clin. Cancer Res 12, 4872–4875, 10.1158/1078-0432.CCR-06-0417 (2006). [DOI] [PubMed] [Google Scholar]
- 100.Basourakos SP et al. Baseline and longitudinal plasma caveolin-1 level as a biomarker in active surveillance for early-stage prostate cancer. BJU Int 121, 69–76, 10.1111/bju.13963 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang G et al. Caveolin-1 upregulation contributes to c-Myc-induced high-grade prostatic intraepithelial neoplasia and prostate cancer. Mol. Cancer Res 10, 218–229, 10.1158/1541-7786.MCR-11-0451 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kretschmer A & Tilki D Biomarkers in prostate cancer - Current clinical utility and future perspectives. Crit. Rev. Oncol. Hematol 120, 180–193, 10.1016/j.critrevonc.2017.11.007 (2017). [DOI] [PubMed] [Google Scholar]
- 103.Lin JS, Bowles EJA, Williams SB & Morrison CC Screening for thyroid cancer updated evidence report and systematic review for the US Preventive Services Task Force. Jama-Journal of the American Medical Association 317, 1888–1903, 10.1001/jama.2017.0562 (2017). [DOI] [PubMed] [Google Scholar]
- 104.Jemal A et al. Global cancer statistics. CA Cancer J. Clin 61, 69–90, 10.3322/caac.20107 (2011). [DOI] [PubMed] [Google Scholar]
- 105.U.S. Preventive Services Task Force et al. Screening for thyroid cancer: U.S. Preventive Services Task Force recommendation statement. JAMA 317, 1882–1887, 10.1001/jama.2017.4011 (2017). [DOI] [PubMed] [Google Scholar]
- 106.Siegel RL, Miller KD & Jemal A Cancer statistics, 2015. CA Cancer J. Clin 65, 5–29, 10.3322/caac.21254 (2015). [DOI] [PubMed] [Google Scholar]
- 107.La Vecchia C et al. Author’s reply to thyroid cancer: an epidemic of disease or an epidemic of diagnosis? Int. J. Cancer 136, 2740, 10.1002/ijc.29310 (2015). [DOI] [PubMed] [Google Scholar]
- 108.Hughes DT, Haymart MR, Miller BS, Gauger PG & Doherty GM The most commonly occurring papillary thyroid cancer in the United States is now a microcarcinoma in a patient older than 45 years. Thyroid 21, 231–236, 10.1089/thy.2010.0137 (2011). [DOI] [PubMed] [Google Scholar]
- 109.Davies L & Welch HG Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295, 2164–2167, 10.1001/jama.295.18.2164 (2006). [DOI] [PubMed] [Google Scholar]
- 110.Horn-Ross PL et al. Continued rapid increase in thyroid cancer incidence in california: trends by patient, tumor, and neighborhood characteristics. Cancer Epidemiol. Biomarkers Prev 23, 1067–1079, 10.1158/1055-9965.EPI-13-1089 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Malandrino P et al. Papillary thyroid microcarcinomas: a comparative study of the characteristics and risk factors at presentation in two cancer registries. J. Clin. Endocrinol. Metab 98, 1427–1434, 10.1210/jc.2012-3728 (2013). [DOI] [PubMed] [Google Scholar]
- 112.American Cancer Society. Can thyroid cancer be found early?, <https://www.cancer.org/cancer/thyroid-cancer/detection-diagnosis-staging/detection.html> (2016).
- 113.Haugen BR et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid 26, 1–133, 10.1089/thy.2015.0020 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gharib H et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for Clinical practice for the diagnosis and management of thyroid nodules−−2016 update. Endocr. Pract 22, 622–639, 10.4158/EP161208.GL (2016). [DOI] [PubMed] [Google Scholar]
- 115.Ridley J, Ischayek A, Dubey V & Iglar K Adult health checkup: Update on the Preventive Care Checklist Form(c). Can. Fam. Physician 62, 307–313 (2016). [PMC free article] [PubMed] [Google Scholar]
- 116.American Academy of Family Physicians. Clinical preventive service recommendation: Thyroid cancer, <http://www.aafp.org/patient-care/clinical-recommendations/all/thyroid.html> (2017).
- 117.Rad MP, Zakavi SR, Layegh P, Khooei A & Bahadori A Incidental Thyroid Abnormalities on Carotid Color Doppler Ultrasound: Frequency and Clinical Significance. J. Med. Ultrasound 23, 25–28, 10.1016/j.jmu.2014.04.005 (2015). [DOI] [Google Scholar]
- 118.Nguyen XV et al. Incidental thyroid nodules on CT: evaluation of 2 risk-categorization methods for work-up of nodules. AJNR Am. J. Neuroradiol 34, 1812–1817, 10.3174/ajnr.A3487 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Soelberg KK, Bonnema SJ, Brix TH & Hegedus L Risk of malignancy in thyroid incidentalomas detected by 18F-fluorodeoxyglucose positron emission tomography: a systematic review. Thyroid 22, 918–925, 10.1089/thy.2012.0005 (2012). [DOI] [PubMed] [Google Scholar]
- 120.Hoang JK et al. Managing incidental thyroid nodules detected on imaging: white paper of the ACR Incidental Thyroid Findings Committee. J Am Coll Radiol 12, 143–150, 10.1016/j.jacr.2014.09.038 (2015). [DOI] [PubMed] [Google Scholar]
- 121.Haugen BR et al. American Thyroid Association Guidelines on the Management of Thyroid Nodules and Differentiated Thyroid Cancer Task Force Review and Recommendation on the Proposed Renaming of Encapsulated Follicular Variant Papillary Thyroid Carcinoma Without Invasion to Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features. Thyroid 27, 481–483, 10.1089/thy.2016.0628 (2017). [DOI] [PubMed] [Google Scholar]
- 122.Durante C et al. The natural history of benign thyroid nodules. JAMA 313, 926–935, 10.1001/jama.2015.0956 (2015). [DOI] [PubMed] [Google Scholar]
- 123.Takebe K, Date M & Yamamoto Y Mass screening for thyroid cancer with ultrasonography. Karkinos 7, 309–317 (1994). [Google Scholar]
- 124.Ahn HS et al. Thyroid cancer screening in South Korea increases detection of papillary cancers with no impact on other subtypes or thyroid cancer mortality. Thyroid 26, 1535–1540, 10.1089/thy.2016.0075 (2016). [DOI] [PubMed] [Google Scholar]
- 125.Lee YS, Lim H, Chang HS & Park CS Papillary thyroid microcarcinomas are different from latent papillary thyroid carcinomas at autopsy. J. Korean Med. Sci 29, 676–679, 10.3346/jkms.2014.29.5.676 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Flynn MB, Lyons KJ, Tarter JW & Ragsdale TL Local complications after surgical resection for thyroid carcinoma. Am. J. Surg 168, 404–407 (1994). [DOI] [PubMed] [Google Scholar]
- 127.Hundahl SA et al. Initial results from a prospective cohort study of 5583 cases of thyroid carcinoma treated in the united states during 1996. U.S. and German Thyroid Cancer Study Group. An American College of Surgeons Commission on Cancer Patient Care Evaluation study. Cancer 89, 202–217 (2000). [DOI] [PubMed] [Google Scholar]
- 128.McMullen C, Rocke D & Freeman J Complications of bilateral neck dissection in thyroid cancer from a single high-volume center. JAMA Otolaryngol Head Neck Surg 143, 376–381, 10.1001/jamaoto.2016.3670 (2017). [DOI] [PubMed] [Google Scholar]
- 129.Bergenfelz A et al. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch. Surg 393, 667–673, 10.1007/s00423-008-0366-7 (2008). [DOI] [PubMed] [Google Scholar]
- 130.Ito Y & Miyauchi A A therapeutic strategy for incidentally detected papillary microcarcinoma of the thyroid. Nat. Clin. Pract. Endocrinol. Metab 3, 240–248, 10.1038/ncpendmet0428 (2007). [DOI] [PubMed] [Google Scholar]
- 131.Miyauchi A et al. Estimation of the lifetime probability of disease progression of papillary microcarcinoma of the thyroid during active surveillance. Surgery 163, 48–52, 10.1016/j.surg.2017.03.028 (2018). [DOI] [PubMed] [Google Scholar]
- 132.Agrawal N et al. Integrated genomic characterization of papillary thyroid carcinoma. Cell 159, 676–690, 10.1016/j.cell.2014.09.050 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Haugen BR 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: What is new and what has changed? Cancer 123, 372–381, 10.1002/cncr.30360 (2017). [DOI] [PubMed] [Google Scholar]
- 134.Yabuta T et al. TERT Promoter Mutations Were Not Found in Papillary Thyroid Microcarcinomas That Showed Disease Progression on Active Surveillance. Thyroid 27, 1206–1207, 10.1089/thy.2016.0645 (2017). [DOI] [PubMed] [Google Scholar]
- 135.Cancer Stat Facts: Thyroid Cancer, <https://seer.cancer.gov/statfacts/html/thyro.html> (2018).
- 136.Lao C et al. The cost-effectiveness of active surveillance compared to watchful waiting and radical prostatectomy for low risk localised prostate cancer. BMC Cancer 17, 529, 10.1186/s12885-017-3522-z (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Becerra V et al. Economic evaluation of treatments for patients with localized prostate cancer in Europe: a systematic review. BMC Health Serv. Res 16, 541, 10.1186/s12913-016-1781-z (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brandes A et al. Cost-effectiveness of radical prostatectomy, radiation therapy and active surveillance for the treatment of localized prostate cancer - a claims data analysis. Value Health 17, A636–637, 10.1016/j.jval.2014.08.2287 (2014). [DOI] [PubMed] [Google Scholar]
- 139.Lang BH & Wong CK A cost-effectiveness comparison between early surgery and non-surgical approach for incidental papillary thyroid microcarcinoma. Eur. J. Endocrinol 173, 367–375, 10.1530/EJE-15-0454 (2015). [DOI] [PubMed] [Google Scholar]
- 140.Venkatesh S et al. Cost-effectiveness of active surveillance versus hemithyroidectomy for micropapillary thyroid cancer. Surgery 161, 116–126, 10.1016/j.surg.2016.06.076 (2017). [DOI] [PubMed] [Google Scholar]


