Abstract
Parkin is an ubiquitin‐E3 ligase that acts as a key component of the cellular machinery for mitophagy. We show here that Parkin expression is reciprocally regulated in brown adipose tissue in relation to thermogenic activity. Thermogenic stimuli repress Parkin gene expression via transcriptional mechanisms that are elicited by noradrenergic and PPARα‐mediated pathways that involve intracellular lipolysis in brown adipocytes. Parkin‐KO mice show over‐activated brown adipose tissue thermogenic activity and exhibit improved metabolic parameters, especially when fed a high‐fat diet. Deacclimation, which is the return of a cold‐adapted mouse to a thermoneutral temperature, dramatically induces mitophagy in brown adipocytes, with a concomitant induction of Parkin levels. We further reveal that Parkin‐KO mice exhibit defects in the degradative processing of mitochondrial proteins in brown adipose tissue in response to deacclimation. These results suggest that the transcriptional control of Parkin in brown adipose tissue may contribute to modulating the mitochondrial mass and activity for adaptation to thermogenic requirements.
Keywords: adiposity, autophagy, mitophagy, obesity
Subject Categories: Autophagy & Cell Death, Metabolism
Introduction
Brown adipose tissue (BAT) is the main site of non‐shivering thermogenesis, which is a heat‐producing mechanism that enables an organism to adapt to a cold environment. Brown adipocytes possess large amounts of mitochondria that have a particularly high oxidative capacity and express the uncoupling protein 1 (UCP1) 1. UCP1 gives the mitochondrial inner membrane a unique proton permeability that allows metabolic energy to be used for heat production instead of ATP synthesis, yielding a correspondingly high oxidation rates. The energy expenditure derived from BAT thermogenic activity provides a protective mechanism against excessive body weight and fat accumulation in response to overfeeding 2, 3.
Brown adipose tissue is a highly plastic tissue that is acted upon by hypertrophic and hyperplastic processes. It is enlarged in response to enhanced thermogenic needs, such as a cold environment 4, and becomes atrophied under low thermogenic demands, such as those experienced in warm environments, during underfeeding, or physiological conditions such as pregnancy, lactation, and aging 4. This plasticity is critically governed by regulating the amount of mitochondria and the levels of components of the thermogenic machinery (e.g., UCP1) 5, 6. As an added feature of the plasticity of adipose tissues in adaptive thermogenesis, brown adipocyte‐like (mitochondria‐enriched, UCP1‐containing) beige cells may appear in white adipose tissue (WAT) depots when thermogenic needs are high (the so‐called “browning” process) 7, 8, 9.
Autophagy is a catabolic process that contributes to maintaining cellular homeostasis by removing intracellular components, such as damaged or unnecessary organelles, and targeting them for degradation inside lysosomes 10. Autophagy plays an important role in cellular adaptive changes to physiological and pathological challenges, such as by providing energy substrates during nutrient deprivation 11, 12. The selective degradation of mitochondria (mitophagy) is a quality control mechanism that contributes to maintaining a healthy mitochondrial network by eliminating dysfunctional mitochondria 13. Mitophagy can also play a role in the cellular remodeling events that take place during adaptive processes and differentiation, such as by removing mitochondria from maturing erythrocytes 14. The main stimulus that triggers mitophagy is mitochondrial depolarization, which is sensed by the PTEN‐induced putative kinase (PINK1) and Parkin system. This leads to the recruitment of autophagic receptors (e.g., p62/SQSTM) that serve as bridges between depolarized mitochondria and the autophagic machinery 15. PINK1 is stabilized in the membrane of depolarized mitochondria and recruits the E3‐ubiquitin ligase Parkin 16, 17, 18, which in turn ubiquitylates numerous outer mitochondrial membrane proteins to recruit the autophagic adaptors and initiate mitophagy 15.
Autophagy is involved in the regulation of the differentiation and function of adipocytes. Studies of mice subjected to invalidation of key actors in autophagy (e.g., Atg7) found that browning was induced in these mice as a result of the impairment of autophagy in adipocytes 19, 20, 21. Sustained thermogenic activation impairs autophagy in brown adipocytes 22, although it has been reported that a very short time thermogenic activation elicits lipophagy/autophagy induction 23. In fact, repression of autophagy yields increased thermogenic activity in brown adipocytes 22. Altshuler‐Keylin et al 24 further reported that autophagy contributes to clearance of mitochondria during beige adipocyte inactivation to enable beige‐to‐white transition, and thus acts as a negative regulator of the thermogenic capacity. Here, we identify Parkin as a new regulatory factor of BAT that is reciprocally controlled in relation to BAT thermogenic activity. Parkin acts as a key regulator of adaptive BAT plasticity through its role in the control of mitophagy.
Results
Thermogenic stimuli repress Parkin expression in BAT and beige adipose tissue
We analyzed the expression pattern of autophagy‐related genes using our previously generated RNAseq database 25, which reflects transcript‐level changes in interscapular BAT (iBAT) from mice exposed to cold (4°C) for 24 h versus that from mice maintained at thermoneutral temperature (29°C) (Fig 1A). We observed that genes implicated in advanced stages of autophagosome formation (Atg5, Atg2a, Atg2b, Atg3) or the recycling of autophagic components (Atg4a, Atg4b, Atg4, Atg4d) were mostly upregulated in response to thermogenic activation, whereas those implicated in the initiation complexes (Ulk1, Ulk2, Pik3c3, Atg16l1, Atg14) were downregulated (with the exception of Atg13I). Remarkably, Park2, encoding Parkin, was the most intensely downregulated autophagy‐related gene transcript, followed by Pink1; the products of these genes are known to be involved in the selective autophagic degradation of mitochondria 16, 17, 18. In accordance with the RNAseq data, iBAT from mice exposed to cold for 1 day showed a dramatic decrease (63%) in Parkin transcript levels, as assessed by qRT–PCR (Fig 1B left), and a marked reduction of Parkin protein levels (Fig 1B right). In contrast, the reduction in Pink1 transcript expression was not reflected in a low protein level of PINK1; in fact, PINK1 protein levels were increased in mice exposed to cold for 1 day. This is consistent with previous reports that post‐transcriptional protein stabilization‐mediated mechanism is responsible for regulating the level and activity of PINK1 26, 27. Parkin downregulation was not observed in WAT of mice exposed to cold for 1 day; the levels of Parkin transcripts in subcutaneous WAT (sWAT) showed a significant increase after 1 day of cold, while those in epididymal WAT (eWAT) were unaltered by this treatment (Fig 1C).
Figure 1. Parkin expression is repressed by thermogenic stimuli in brown and beige adipose tissue.
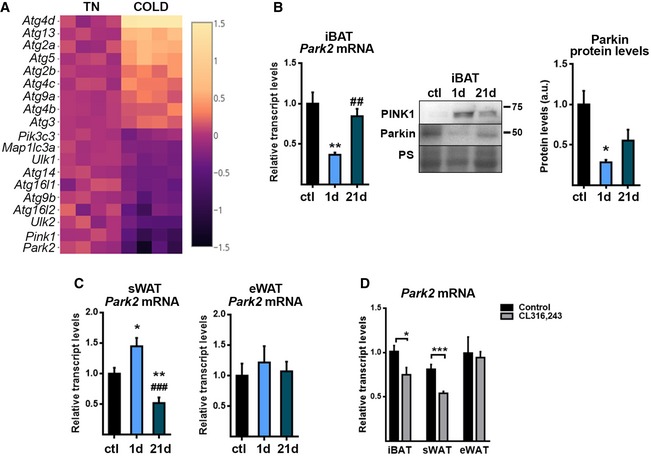
- Profile of transcript levels of autophagy‐related genes in iBAT which are significantly modified in cold‐exposed mice (4°C, 24 h), compared with mice maintained at thermoneutral temperature (29°C) (Fisher's exact test corrected by the Benjamini–Hochberg method, P < 0.05). Heatmap represents log‐fold change of RPKM values of cold versus thermoneutral (TN) sample data obtained from RNAseq analysis. Each square represents replicates (n = 4). The most repressed genes are shown on the bottom of the heatmap.
- Parkin expression in iBAT from mice exposed to cold (4°C) for 1 day (1 d) or 21 days (21 d) compared with control mice (ctl) maintained at 22°C. Left: Relative transcript levels. Right: Representative immunoblot and Parkin protein quantification. Ponceau staining (PS) was used as loading control (n = 6; transcript levels) (n = 3; protein levels). Data are presented as means ± s.e.m. *P < 0.05, **P < 0.01, cold vs. control and ## P < 0.01, 1 d vs. 21 d. One‐way ANOVA with Tukey's post hoc test.
- Relative transcript levels of Park2 in sWAT and eWAT (n = 6). Data are presented as means ± s.e.m. *P < 0.05, **P < 0.01, cold vs. control and ### P < 0.001, 1 d vs. 21 d. One‐way ANOVA with Tukey's post hoc test.
- Relative transcript levels of Parkin in iBAT, sWAT, and eWAT of mice injected with the β3‐AR agonist CL316,243, every day for 1 week (n = 6). Data are presented as means ± s.e.m. *P < 0.05, ***P < 0.001, CL316,243 treatment vs. saline. Two‐tailed unpaired Student's t‐test.
We expanded our analysis to determine the effects of 1‐h cold exposure in mice, in view of a previous report indicating activation of autophagy 23 instead of repression with this very short cold exposure time in BAT. Our results showed that 1‐h exposure to cold caused a minor but significant reduction in Parkin mRNA levels in BAT accompanied by a mild reduction in transcript levels of autophagy‐related genes (Fig EV1A). Assessment of autophagic flux was performed following injection of mice with leupeptin. Blockage of autophagy was evident based on parallel accumulation of LC3B‐II and p62 in BAT of control mice in response to leupeptin (Fig EV1B). Exposure to 1 h of cold led to a similar LC3B‐II and p62 accumulation with no further increases in the presence of leupeptin (Fig EV1B). Accordingly, we conclude that first signs of Parkin mRNA downregulation in response to short‐term cold occur in a context of repressed autophagy. Notably, 1‐h cold exposure was insufficient to induce a significant increase in UCP1 transcript (Fig EV1A) or UCP1 protein (Fig EV1B) levels in BAT, whereas β3‐adrenoreceptor expression was markedly reduced (Fig EV1A), as expected 28, 29, 30 in contrast to the findings by Martinez‐Lopez et al 23. At present, we do not have a plausible explanation for this discrepancy in results on the effects of very short‐term thermogenic stimulation on BAT.
Figure EV1. Effects of one‐hour cold (4°C) exposure of mice on autophagic activity and expression of thermogenesis and autophagy‐related gene transcripts in iBAT .
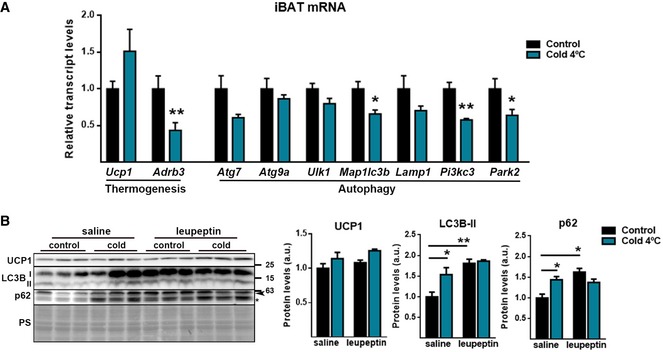
- iBAT relative transcript levels of thermogenic‐ and autophagic‐related genes from mice exposed 1 h to cold (n = 4). Data are presented as means ± s.e.m. *P < 0.05, ** P < 0.01. Two‐tailed unpaired Student's t‐test.
- Immunoblot for the indicated proteins (left) and its quantification (right) in iBAT from mice exposed 1 h to cold. Mice were injected intraperitoneally with saline or leupeptin (40 mg/kg b.w.) 1 h prior to cold exposure. Ponceau staining (PS) was used as loading control (n = 3). Arrowhead indicates p62 specific band and asterisk indicate a non‐specific band. Data are presented as means ± s.e.m. *P < 0.05, **P < 0.01. ANOVA with Tukey's post hoc test.
To analyze the regulation of Parkin in beige adipose tissue, we exposed mice to cold for 21 days (chronic acclimation) to induce browning of WAT. We found that cold‐induced browning reduced the Parkin transcript level in sWAT, whereas eWAT, which is less prone to browning, did not show any change in Parkin mRNA expression even after long‐term (21 day) cold exposure (Fig 1C). Parkin expression levels were partially recovered in iBAT from mice exposed to cold for 21 days (Fig 1B). We also treated mice with the specific β3‐adrenoreceptor (β3‐AR) agonist, CL316,243, for 1 week to mimic the sympathetic thermogenic stimulus that occurs during cold adaptation, and found that Parkin transcript levels were reduced in the brown and beige adipose tissues of CL316,243‐treated mice compared with control mice (Fig 1D). However, single CL316,243 injection also induced a rapid and significant reduction in Parkin mRNA (~20% in iBAT, 3 h after injection, data not shown). Parkin mRNA downregulation observed after chronic CL316,243 treatment may be attributable to both chronic recruitment of BAT and iWAT thermogenic activity and acute effects of CL316,243.
Parkin expression is associated with brown adipocyte differentiation
We found that, similar to UCP1, Parkin is preferentially expressed in mature brown adipocytes rather than in the stromal vascular fraction (SVF) (Fig 2A). In primary cultures of differentiating brown adipocytes, Parkin mRNA levels increased progressively from the first days of differentiation (Fig 2B); this pattern parallels the induction of mitochondrial biogenesis and the progressive acquisition of the thermogenic machinery (e.g., UCP1 expression). Moreover, when brown adipocyte precursor cells were cultured with charcoal‐stripped serum, which allows proliferation but not brown adipocyte differentiation, the level of Parkin mRNA (like that of the UCP1 mRNA) remained low relative to the level seen in differentiated brown adipocytes (Fig 2C).
Figure 2. Parkin expression is repressed by norepinephrine‐induced, cAMP‐mediated, thermogenic activation.
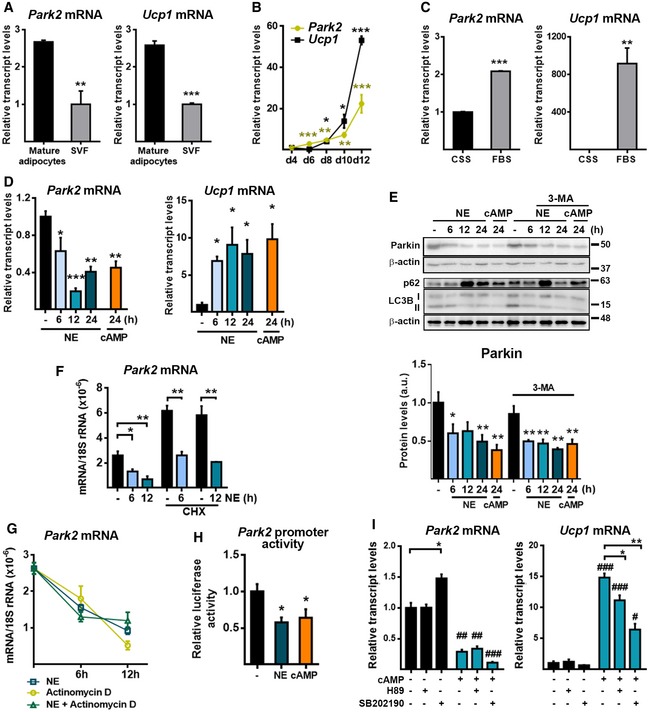
- Relative transcript levels of Park2 and Ucp1 in mature brown adipocytes compared with the stromal vascular fraction (SVF) in iBAT (n = 3). Data are presented as means ± s.e.m. **P < 0.01, ***P < 0.001. Two‐tailed unpaired Student's t‐test.
- mRNA expression profiles of Park2 and Ucp1 during differentiation (n = 3). Data are presented as means ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001 compared to day (d) 4. Two‐tailed unpaired Student's t‐test.
- Relative transcript levels of Park2 and Ucp1 in brown adipocytes in culture with fetal bovine serum (FBS, to trigger in vitro differentiation) or charcoal‐stripped serum (CSS, non‐permissive for differentiation) (n = 3). Data are presented as means ± s.e.m. **P < 0.01, ***P < 0.001. Two‐tailed unpaired Student's t‐test.
- Relative transcript levels of Park2 and Ucp1 in brown adipocytes in culture treated with norepinephrine (NE) or cAMP for indicated times (n = 3). Data are presented as means ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001 compared to non‐treated cells. One‐way ANOVA with Dunnett's post hoc test.
- Parkin protein levels in brown adipocytes treated with NE or cAMP for the indicated times in the presence or absence of 3‐methyladenine (3‐MA). Top: Representative immunoblots. p62 and LC3BII proteins are shown as positive controls of 3‐MA treatment. β‐Actin was used as loading control. Bottom: Parkin protein quantification (performed using three immunoblots processed in parallel due to sample size) (n = 3). Data are presented as means ± s.e.m. *P < 0.05, **P < 0.01 compared to NE/cAMP non‐treated cells. One‐way ANOVA with Dunnett's post hoc test.
- Relative transcript levels of Park2 in brown adipocytes treated with NE in the presence or absence of cycloheximide (CHX) (n = 3). Data are presented as means ± s.e.m. *P < 0.05, **P < 0.01. Two‐tailed unpaired Student's t‐test.
- Relative mRNA expression levels of Parkin in brown adipocytes treated with NE in the presence or absence of actinomycin D (n = 3). Data are presented as means ± s.e.m. One‐way ANOVA with Tukey's post hoc test comparing treatments.
- Park2 gene promoter activity in HIB‐1B cells transfected with a pGL4‐Park2‐Luc reporter construct treated with or without NE or cAMP. Data were normalized using Renilla luciferase activity and are expressed as the relative firefly luciferase activity compared to that in untreated cells (n = 6). Data are presented as means ± s.e.m. *P < 0.05 compared to non‐treated cells. ANOVA with Dunnett's post hoc test
- Relative transcript levels of Park2 and Ucp1 in brown adipocytes treated with cAMP (12 h) plus H89 (a PKA inhibitor) or SB202190 (a p38 MAPK inhibitor) (n = 3). Data are presented as means ± s.e.m. *P < 0.05, **P < 0.01 with vs. without inhibitor and # P < 0.05, and ## P < 0.01, ### P < 0.001 with vs. without cAMP. Two‐tailed unpaired Student's t‐test.
Noradrenergic stimuli repress Parkin expression in brown adipocytes
Norepinephrine (NE) is the major mediator of BAT thermogenic activation; it acts mainly via the β3‐AR and increases the intracellular levels of cyclic AMP (cAMP) 4. To establish whether the effects of cold and β3‐AR activation on Parkin expression observed in vivo occur in a cell‐autonomous manner, we treated in vitro‐differentiated brown adipocytes with NE or with the cell‐permeable cAMP analog dibutyryl‐cAMP. Both thermogenic inducers mimicked the repressive effects of cold on Parkin mRNA and protein levels, but increased the mRNA expression level of UCP1, as expected (Fig 2D and E). Since Parkin can be degraded during the process of mitophagy, we used the autophagic inhibitor, 3‐methyladenine (3‐MA), to evaluate the possible contribution of autophagy to the observed decrease in the Parkin protein level. 3‐MA treatment did not abolish the NE‐ and cAMP‐mediated decreases in the Parkin protein level (Fig 2E). Moreover, NE significantly reduced the mRNA level of Parkin in the presence of the protein synthesis inhibitor, cycloheximide (CHX), indicating that the effect of NE on the Parkin transcript level did not require protein synthesis (Fig 2F). Actinomycin D, an inhibitor of transcription, decreased Parkin mRNA levels to an extent similar to that caused by NE, but there were no additive effects of actinomycin D and NE (Fig 2G). Thus, blunting Parkin gene transcription elicited a degree of Parkin mRNA downregulation resembling that induced by NE. To investigate whether NE and cAMP affect the transcription of Park2, we transfected HIB‐1B brown adipocyte‐derived cells with a plasmid luciferase reporter construct driven by a 920‐bp fragment of the 5’‐flanking region (−800 to +120) of the mouse Parkin gene (pGL4‐Park2‐Luc). Both NE and cAMP were found to cause significant decreases in the promoter activity of the Parkin gene (Fig 2H). We thus conclude that the downregulation of NE‐ and cAMP‐mediated Parkin in brown adipocytes occurs mainly through the transcriptional repression of the Parkin gene, and there is no evidence of significant post‐transcriptional processes. Exposure of brown adipocytes to H89, an inhibitor of protein kinase‐A (PKA), or SB202190, an inhibitor of mitogen‐activated protein kinase p38 (p38‐MAPK), significantly reduced the cAMP‐dependent expression of the UCP1 mRNA but had no effect on the repression of Parkin repression elicited by cAMP (Fig 2I). This indicates that PKA and p38‐MAPK, which are the major intracellular mediators of NE‐cAMP‐dependent thermogenic induction in brown adipocytes, are not involved in the NE‐ or cAMP‐induced repression of Parkin gene expression.
Involvement of lipolysis and PPARα in the regulation of Parkin expression in brown adipocytes
Activation of lipolysis accompanies thermogenic induction to fuel mitochondrial oxidation, and the intracellular release of fatty acids contributes to the signaling that triggers thermogenic gene transcription in BAT 31. The adipose triglyceride lipase (ATGL) inhibitor, Atglistatin, and the hormone‐sensitive lipase (HSL) inhibitor, CAY10499, decreased cAMP‐induced lipolysis (assessed by the levels of glycerol in the cell culture media), and treatment with both inhibitors completely blocked it (Fig 3A right). Under these conditions, we found that Atglistatin slightly blunted the cAMP‐mediated repression of Parkin, whereas CAY10499 almost completely prevented it (Fig 3A left). We also analyzed the effects of several factors that are known to induce thermogenic activity independent of β‐adrenergic pathways in brown adipocytes and have been proposed to mediate the fatty acid‐derived regulation of transcription. GW7647, an activator of PPARα, had reciprocal effects on the gene expression levels of Parkin (downregulation) and UCP1 (upregulation; Fig 3B). This effect was similar to that elicited by β‐adrenergic pathways, and thus was consistent with the positive PPARα‐dependent transcriptional regulation of the UCP1 gene 31. Conversely, the PPARα inhibitor GW6471 induced the mRNA expression of Parkin (Fig 3B). The very low levels of Parkin mRNA found in the NE‐treated cells were not further decreased by GW7647. Inhibition of PPARα by GW6471 significantly reduced the NE‐mediated downregulation of the Parkin mRNA. PPARα‐dependent transcriptional repression was specific for Parkin and not shared by other components of autophagy machinery (Appendix Table S1) whose transcripts were also downregulated by NE 22. Differentiated brown adipocytes from PPARα‐null mice showed increased Parkin mRNA levels in basal conditions and failed to downregulate Parkin mRNA expression in response to NE (Fig 3C). Moreover, the Parkin gene promoter activity was significantly repressed by co‐transfection with an expression vector for PPARα as well as in response to the PPARα activator GW7647 (Fig 3D), thus indicating the repressive effects of the PPARα pathway on the Parkin gene transcription. Collectively, these results emphasize the importance of the PPARα pathway, which is a positive controller of thermogenic activity (i.e., UCP1 gene expression) in brown adipocytes 31, in the transcriptional repression of Parkin. However, not all inducers of UCP1 gene expression elicited Parkin gene repression: All‐trans‐retinoic acid, which is a thermogenic activator that induces UCP1 expression independent of the PKA‐p38MAPK, fatty acid‐related, and PPARα‐dependent pathways 32, did not reduce Parkin mRNA expression (Fig 3B).
Figure 3. Thermogenic activation represses Parkin through mechanisms involving lipolysis and PPARα pathway.
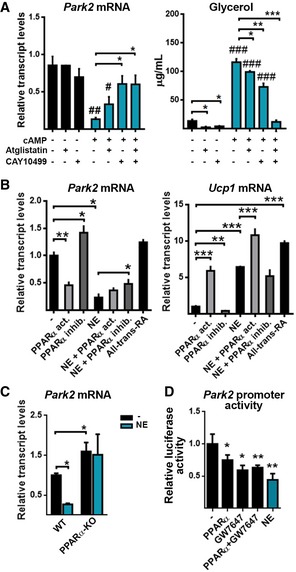
- Relative transcript levels of Park2 and concentration of glycerol in the culture medium of brown adipocytes treated with cAMP (24 h) plus Atglistatin (an adipose triglyceride lipase inhibitor) or CAY10499 (a hormone‐sensitive lipase inhibitor) (n = 3). Data are presented as means ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001 with vs. without inhibitor and # P < 0.05, and ## P < 0.01, ### P < 0.001 with vs. without cAMP. Two‐tailed unpaired Student's t‐test.
- Relative transcript levels of Park2 and Ucp1 in brown adipocytes treated for 24 h with a PPARα activator (GW7647), a PPARα inhibitor (GW6471) with or without norepinephrine (NE), or all‐trans‐retinoic acid (RA) (n = 3). Data are presented as means ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001. Two‐tailed unpaired Student's t‐test.
- Relative transcript levels of Park2 in wild‐type and PPARα‐KO brown adipocytes treated for 24 h with NE (n = 3). Data are presented as means ± s.e.m. *P < 0.05. ANOVA with Tukey's post hoc test.
- Park2 gene promoter activity in HIB‐1B cells transfected with a pGL4‐Park2‐Luc reporter construct and, when indicated, with a pSG5‐Ppara expression vector. Cells were treated with the PPARα activator GW7647 or NE. Data were normalized using Renilla luciferase activity and are expressed as the relative firefly luciferase activity compared to that only transfected with the pGL4‐Park2‐Luc construct (n = 6). Data are presented as means ± s.e.m. *P <0.05, **P < 0.01 compared to non‐treated cells. ANOVA with Dunnett's post hoc test.
Parkin‐KO mice show over‐activated BAT and are protected against high‐fat diet‐induced weight gain and insulin resistance
Considering the strong reciprocal regulation between Parkin levels and thermogenic activity in BAT, we examined how experimental suppression of Parkin in mice affected BAT thermogenic activity and related systemic parameters. Parkin‐KO mice showed a reduced body weight, reduced liver weight, reduced iBAT size, and a tendency for reduced WAT depot sizes (Table EV1) although food intake did not show statistically significant changes. Optical and electron microscopic images revealed that the lipid droplet size was decreased in the brown adipocytes of iBAT from Parkin‐KO mice compared to wild‐type mice (Fig 4A), resulting in a decreased lipid content. Parkin‐KO mice had an increased temperature in their interscapular area (corresponding to the site of iBAT) compared to wild‐type mice (Fig 4B), indicating that the thermogenic activity of their iBAT was increased. Moreover, iBAT from Parkin‐KO mice had increased UCP1 protein levels (Fig 4C) and enhanced mRNA expression of several genes involved in thermogenesis (Ucp1, Ppargc1a, and Dio2) (Fig 3D). Acute cold (24 h, 4°C) induced the expression of thermogenesis‐related genes to a similar extent in wild‐type and Parkin‐KO mice, with the exception of the thermogenesis‐related, secretable factors, FGF21 and IL‐6, which were over‐induced in Parkin‐KO mice acutely exposed to cold (Fig 4D).
Figure 4. Parkin‐KO mice show over‐activation of BAT and are protected against HFD‐induced weight gain and insulin resistance.
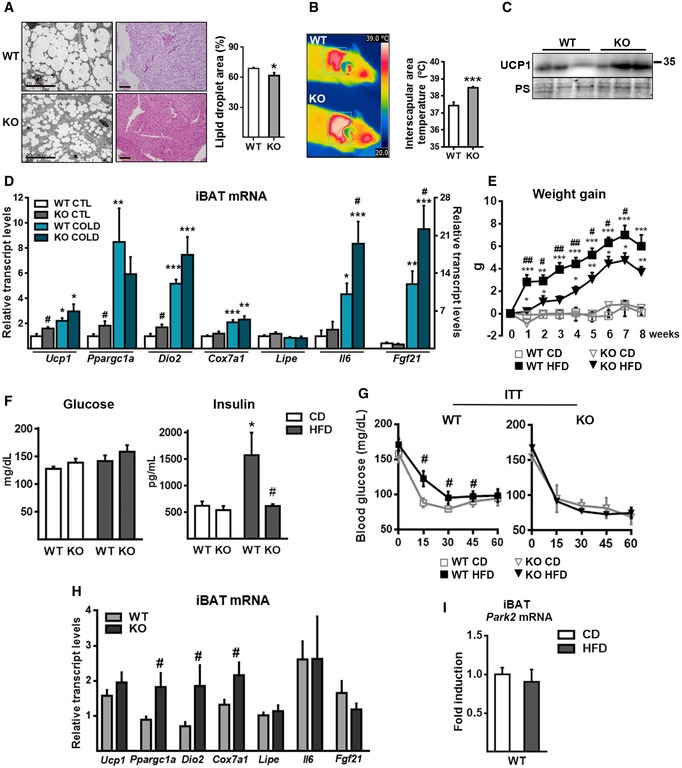
-
ALeft: Representative optical microscopic images of H&E‐stained iBAT (scale bars, 110 μm) and electron microscopic images of iBAT (scale bars, 20 μm) from wild‐type (WT) and Parkin‐KO (KO) mice. Right: Quantification of lipid droplet area from electron microscopic images (10 images per condition were used, n = 3 WT, n = 3 Parkin‐KO). Data are presented as means ± s.e.m. *P < 0.05. Two‐tailed unpaired Student's t‐test.
-
BRepresentative thermal images (left) and quantification of surface temperature (right) for the interscapular region (n = 6). Data are presented as means ± s.e.m. ***P < 0.001. Two‐tailed unpaired Student's t‐test.
-
CRepresentative immunoblot of UCP1 protein and the loading control (Ponceau staining, PS) in iBAT samples.
-
DRelative transcript levels of thermogenesis‐related genes of iBAT from WT and Parkin‐KO mice exposed to acute cold (4°C, 24 h) compared to those maintained at the control temperature (22°C) (n = 8; WT) (n = 6; Parkin‐KO). Data are presented as means ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, CTL vs. cold; # P < 0.05, WT vs. Parkin‐KO. ANOVA with Tukey's post hoc test.
-
E–GWeight gain, blood glucose levels and plasma insulin levels, and insulin tolerance test (ITT) from WT and Parkin‐KO mice fed with chow (CD) or high‐fat diet (HFD) (n = 8; WT) (n = 6; Parkin‐KO). Data are presented as means ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, CTL vs. cold; # P < 0.05, ## P < 0.01 WT vs. Parkin‐KO. (E and G) were analyzed using the two‐tailed unpaired Student's t‐test. (F) was analyzed using ANOVA with Tukey's post hoc test.
-
HRelative transcript levels of thermogenesis‐related genes in iBAT from WT and Parkin‐KO mice given HFD (n = 8; WT) (n = 6; Parkin‐KO). Data are presented as means ± s.e.m. # P < 0.05. Two‐tailed unpaired Student's t‐test.
-
IRelative transcript levels of Park2 in iBAT from WT mice fed CD or HFD (n = 8; WT) (n = 6; Parkin‐KO). Data are presented as means ± s.e.m. Two‐tailed unpaired Student's t‐test.
Overall, these data are consistent with the notion that iBAT is generally over‐activated in mice lacking Parkin. To analyze whether the enhanced thermogenic activity observed in Parkin‐KO mice could have protective effects against obesity, we fed wild‐type and Parkin‐KO mice a high‐fat diet (HFD) for 8 weeks. We found that Parkin‐KO mice were resistant to HFD‐induced weight gain (Fig 4E). The expansion of adipose tissues in response to HFD was reduced in Parkin‐KO mice, and, accordingly, their circulating leptin levels were lower (Table EV1). Parkin‐KO mice appeared to be protected against the metabolic complications associated with obesity, as they did not develop the HFD‐induced hyperinsulinemia (Fig 4F) or loss of insulin sensitivity (Fig 4G) seen in wild‐type mice. Moreover, under HFD conditions, the expression levels of genes involved in thermogenic activity were enhanced (Ppargc1a, Dio2, Cox7a1) or tended to be higher (Ucp1) in Parkin‐KO mice relative to wild‐type mice (Fig 4H). We did not find any significant HFD‐induced change in the Parkin transcript level of wild‐type mice (Fig 4I). The relative mRNA expression levels of genes related to energy metabolism exhibited minor changes in the livers of wild‐type compared to Parkin‐KO mice either fed CD or HFD. Multivariate ANOVA indicated that HFD significantly increased the hepatic expression levels of Ppara, Mcad, and Fgf21 (P < 0.05) but genotype did not have any significant effect (Appendix Table S2). Similarly, no significant genotype‐related difference was detected in terms of plasma ketone bodies, non‐esterified fatty acids, or the level of circulating FGF21 (Table EV1).
Deacclimation to cold induces Parkin expression and mitophagy in BAT
To explore the potential involvement of Parkin in BAT mitophagy, we used cold deacclimation as a model of BAT inactivation involving mitochondrial protein loss. We analyzed the impact of deacclimation by exposing cold‐acclimated (21 days, 4°C) mice to thermoneutrality (29°C) for 1 or 7 days. We observed that mice lost 25% and 50% of their iBAT protein content after 1 and 7 days, respectively, of deacclimation (Fig 5A top). This was accompanied by a progressive accumulation of fat, which replaced iBAT protein content (Fig 5A bottom), such that there was no major change in iBAT weight. Cold deacclimation inactivated the thermogenic program of gene expression, as exhibited by a dramatic and sustained decrease in the relative mRNA expression levels of Ucp1, Ppargc1a, Fgf21, and Bmp8b (Fig 5B) and the protein level of UCP1 (Fig 5C). Conversely, the mRNA expression of Parkin was induced after 1 day of deacclimation and remained high in mice deacclimated for 7 days at thermoneutrality (Fig 5B). The protein levels of PINK1 and Parkin were progressively induced by deacclimation (Fig 5C). Deacclimation increased the protein level of LC3B‐II but decreased that of p62 (Fig 5C). Moreover, the extent of Parkin phosphorylation, which was reduced due to cold, was increased during deacclimation (Fig 5D). Collectively, these results indicate that increased mitophagy/autophagy occurs in response to the deacclimation‐induced blockage of iBAT thermogenic activity.
Figure 5. Deacclimation to cold induces Parkin expression and autophagic degradation of mitochondria.
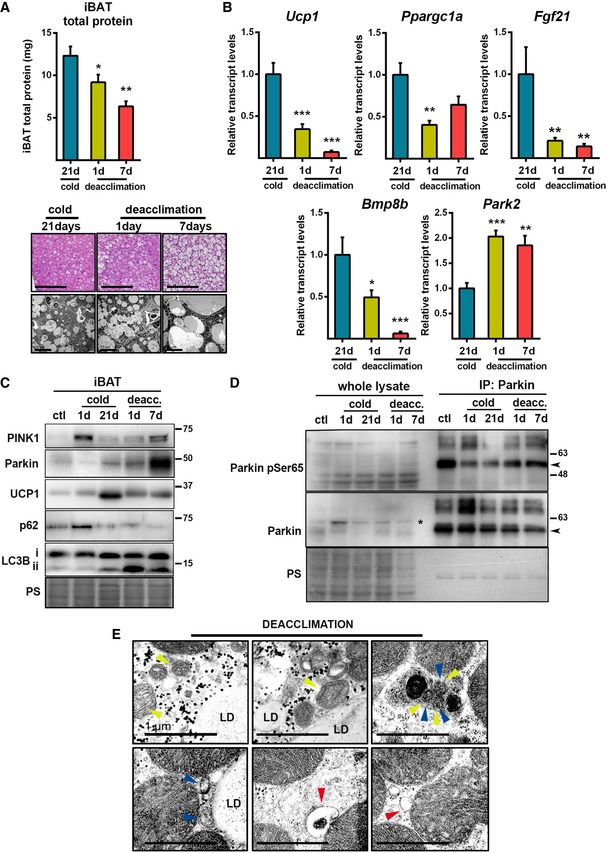
- Top: Total protein content in iBAT during cold deacclimation (n = 6). Data are presented as means ± s.e.m. *P < 0.05, **P < 0.01 compared to 21 d. ANOVA with Dunnett's post hoc test. Bottom: Representative optical microscopic images of H&E‐stained iBAT (scale bars, 100 μm) and electron microscopic images of iBAT (scale bars, 10 μm).
- Relative transcript levels of thermogenesis‐related genes and Park2 in iBAT (n = 6). Data are presented as means ± s.e.m. *P < 0.05, **P < 0.01, ***P <0.001 compared to 21 d. ANOVA with Dunnett's post hoc test.
- Representative immunoblot of the indicated thermogenic and autophagic proteins in iBAT during cold acclimation and deacclimation. PS: Ponceau staining.
- Representative immunoblot of Parkin phosphoSer65 and total Parkin in whole lysates of iBAT and after Parkin immunoprecipitation. Arrowheads indicate Parkin 52 kDa band, and asterisk indicates a non‐specific band.
- Representative electron microscopic images of autophagic and mitophagic events in iBAT after 1 day of cold deacclimation. Yellow arrowheads indicate mitochondrial cristae, blue arrowheads indicate double‐membrane autophagosomes, and red arrowheads indicate structures compatible with mitochondria‐derived vesicles. LD: lipid droplet. Scale bars, 1 μm.
Electron microscopic images of iBAT from 1‐day cold‐deacclimated mice revealed the presence of mitochondria that contained few cristae, and had multiple double‐membrane structures consistent with autophagosomes, some of which contained fragments of mitochondrial cristae (Fig 5E). This accumulation of autophagosomes is consistent with the increase of LC3B‐II protein detected in iBAT from 1‐day deacclimated mice. Electron microscopy of iBAT from mice deacclimated to cold (1 day) also revealed the presence of membrane vesicles emerging from mitochondria (Fig 5E). Their appearance was consistent with that of mitochondria‐derived vesicles (MDVs), which convey a recently described selective mechanism for degrading mitochondrial substrates associated with PINK1 and Parkin activity 33, 34, 35.
Adaptive deacclimation to cold is compromised in Parkin‐KO mice
We used Parkin‐KO mice to analyze the involvement of Parkin in the responsiveness of iBAT to cold deacclimation (24 h). Transfer of cold‐adapted wild‐type mice to a thermoneutral environment for 24 h did not significantly alter glycemia but strongly increased insulin levels (Appendix Table S3), which is consistently with the reciprocal relationship between systemic insulin resistance and BAT activity 36, 37. However, deacclimation of Parkin‐KO mice did not significantly increase insulin levels (Appendix Table S3). We next examined the role of Parkin in the autophagic processes of iBAT in response to deacclimation, and found that the level of LC3B‐II in iBAT increased similarly in wild‐type and Parkin‐KO mice subjected to 1 day of deacclimation (Fig 6A). However, the significant deacclimation‐induced downregulation of p62 did not occur in Parkin‐KO mice (Fig 6B). These findings suggest that Parkin is particularly involved in the cold deacclimation‐associated processing of mitochondrial equipment in brown adipocytes. Moreover, p62 has been reported to have an important role in BAT activity, probably through transcriptional mechanisms unrelated to mitophagy 38. Thus, it cannot be ruled out that altered p62 levels in Parkin‐KO mice may affect BAT function beyond its role in autophagy/mitophagy processes. We additionally determined changes in the levels of optineurin (OPTN), a protein recently shown to intervene in mitophagy processes through mechanisms largely independent of Parkin 39. We observed a trend of reduction in OPTN levels due to cold deacclimation (P < 0.05 in two‐way ANOVA for the factor “deacclimation”), while OPTN levels were unaffected in Parkin‐KO mice (Fig 6B).
Figure 6. Responsiveness of Parkin‐KO mice to cold deacclimation.
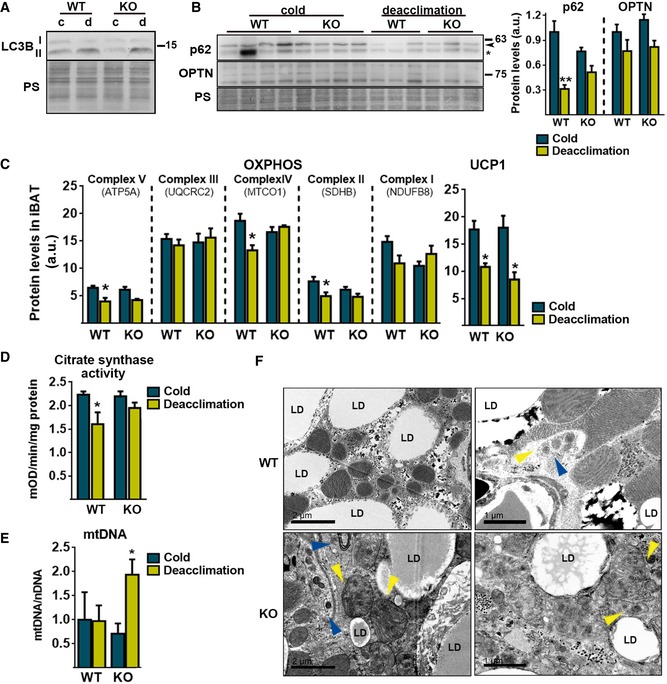
- Representative immunoblot of LC3B in iBAT, and the loading control (Ponceau staining, PS).
- Immunoblot for p62 and OPTN (left) and its quantification (right) in iBAT. PS was used as the loading control. Arrowhead indicates p62 band, and asterisk indicates a non‐specific band (n = 4; cold) (n = 3; deacclimation). Data are presented as means ± s.e.m. **P < 0.01. ANOVA with Tukey's post hoc test.
- Quantification of the total protein amounts of the indicated OXPHOS protein subunits and UCP1 in iBAT (n = 4; cold) (n = 3; deacclimation). Data are presented as means ± s.e.m. *P < 0.05. ANOVA with Tukey's post hoc test.
- Citrate synthase activity in iBAT protein extracts (n = 6; WT) (n = 8; Parkin‐KO). Data are presented as means ± s.e.m. *P < 0.05. ANOVA with Tukey's post hoc test.
- Mitochondrial DNA (mtDNA) content in iBAT relative to nuclear DNA (nDNA) (n=6; WT) (n = 8; Parkin‐KO). Data are presented as means ± s.e.m. *P < 0.05. ANOVA with Tukey's post hoc test.
- Representative electron microscopic images of mitochondrial integrity and autophagic/mitophagic events in iBAT after 1 day of cold deacclimation. Yellow arrowheads indicate mitochondria cristae, and blue arrowheads indicate double‐membrane autophagosomes. Left panels scale bars, 2 μm. Right panels scale bars, 1 μm.
The deacclimation of Parkin‐KO mice to cold did not alter the downregulation of mRNA transcripts encoding components of BAT thermogenic function (e.g., Ucp1, Pgc1, and Fgf21; Fig EV2A). Parkin deletion did not affect the deacclimation‐induced loss of total tissue protein in iBAT (Fig EV2B). Assessment of mitochondrial components revealed that some OXPHOS subunits showed significant decreases (subunit 5A of Complex V, subunit I of Complex IV, SDHB in Complex II) or tended to decrease (subunit NDUF8B of Complex I) in response to deacclimation in wild‐type mice but not in Parkin‐KO mice (Fig 6C). Citrate synthase activity, which is a marker of cellular mitochondrial content, decreased significantly upon cold deacclimation in wild‐type but not in Parkin‐KO mice (Fig 6D). However, the deacclimation‐induced reduction in UCP1 protein levels was not affected by the lack of Parkin (Fig 6C). Despite no significant changes in mitochondrial DNA (mtDNA) levels due to deacclimation in wild‐type mice, mtDNA abundance was higher in cold‐deacclimated Parkin‐KO relative to deacclimated wild‐type mice (Fig 6E). The lack of parallel accumulation of mtDNA‐encoded proteins (e.g., subunit I of Complex I) highlights the non‐functional nature of the mtDNA species accumulating in BAT from Parkin‐KO mice. Electron microscopic examination of brown adipocytes did not reveal any major differences between wild‐type and Parkin‐KO mice after chronic cold exposure (Fig EV2C). However, profound morphological alterations were found in brown adipocytes from deacclimated Parkin‐KO mice, relative to deacclimated wild‐type mice. The lipid droplet size and distribution did not appear to differ (Fig EV2C), but massive amounts of unstructured membrane bodies were present in the cytoplasm of brown adipocytes from deacclimated Parkin‐KO mice (Fig 6F). This accumulation was so great that it precluded the assessment of specific intracellular structures such as autophagosomes or MDVs. In such cells, mitochondria appeared strongly altered and many had only few and unstructured cristae.
Figure EV2. Effects of 21‐day cold (4°C) and subsequent deacclimation at thermoneutrality (29°C) for 24 h on iBAT in Parkin‐KO and wild‐type mice.
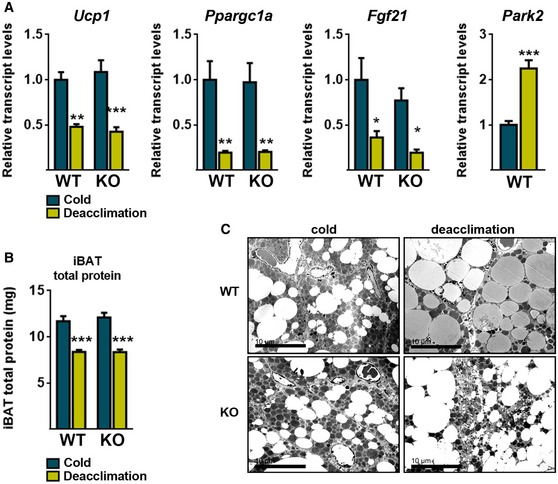
- Relative transcript levels of thermogenesis‐related genes and Park2 (n = 6; WT) (n = 8; Parkin‐KO). Data are presented as means ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001. ANOVA with Tukey's post hoc test.
- Total protein content in iBAT (n = 6; WT) (n = 8; Parkin‐KO). Data are presented as means ± s.e.m. ***P < 0.001. ANOVA with Tukey's post hoc test.
- Representative electron microscopic images of iBAT (scale bars, 10 μm).
Effects of withdrawal from chronic CL316,243 treatment on BAT in Parkin‐KO mice
We extended our study to analyze a second model of impairment of BAT activity using mice one and two weeks after withdrawal from chronic treatment with the β3‐adrenergic agonist CL316,243. In contrast to cold deacclimation, 7 days or 15 days of withdrawal from CL316,243 treatment did not induce alterations in Parkin mRNA or Parkin protein levels (Fig EV3A and C). Moreover, chronic CL316,243 treatment did not result in significant differences in UCP1 protein (Fig EV3A) and thermogenesis/mitochondrial function‐related transcript (Fig EV3C) levels in BAT from wild‐type and Parkin‐KO mice, similar to that observed for chronic cold exposure. Parkin‐KO and wild‐type mice showed a similar decline in UCP1 protein and iBAT total protein levels after 7 or 15 days of withdrawal (Fig EV3A and B). After 7 days of withdrawal from CL316,243, a trend of increased thermogenesis and mitochondrial activity‐related transcript expressions was observed in BAT from Parkin‐KO mice, which was significant for Ppargc1a and mt‐Nd1 (Fig EV3C). Moreover, mtDNA levels in BAT were significantly higher in Parkin‐KO relative to wild‐type mice after 7 days of CL316,243 withdrawal. However, we observed no differences due to genotype at day 15 of withdrawal. These data indicate minor signs of altered BAT plasticity due to lack of Parkin in the adaptation to suppression of β3‐adrenergic‐mediated stimulus.
Figure EV3. Responsiveness of Parkin‐KO mice to β3‐adrenergic stimuli withdrawal.
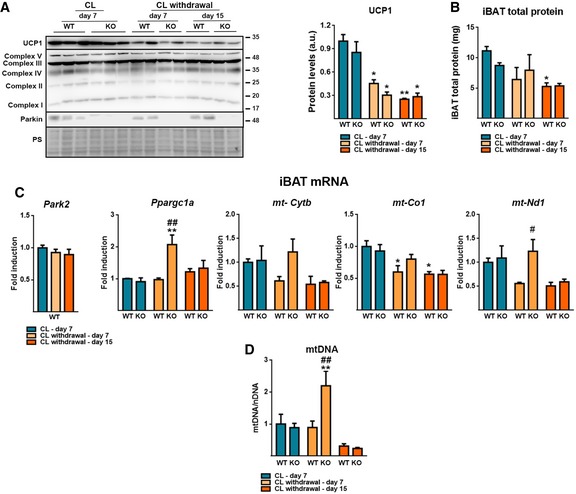
- Left: Representative immunoblot for UCP1, OXPHOS proteins subunits (Complex V: ATP5A, Complex III: UQCRC2, Complex IV: MTCO1, Complex II: SDHB, Complex I: NDUFB8), and Parkin. Right: UCP1 protein levels. Ponceau staining (PS) was used as loading control (n = 5). Data are presented as means ± s.e.m. *P < 0.05, **P < 0.01. ANOVA with Tukey's post hoc test.
- Total protein content in iBAT (n = 5). Data are presented as means ± s.e.m. *P < 0.05. ANOVA with Tukey's post hoc test.
- Relative transcript levels of Park2 and mitochondrial function‐related genes (n = 5). Data are presented as means ± s.e.m. *P < 0.05, **P < 0.01 compared to CL‐day 7; # P < 0.05, ## P < 0.01 WT vs. Parkin‐KO. ANOVA with Tukey's post hoc test.
- Mitochondrial DNA (mtDNA) content in iBAT relative to nuclear DNA (nDNA) (n = 5). Data are presented as means ± s.e.m. **P < 0.01 compared to CL‐day 7; ## P < 0.01 WT vs. Parkin‐KO. ANOVA with Tukey's post hoc test.
Discussion
In the present study, we identify Parkin as a component of BAT function and reveal that its levels are reciprocally associated with thermogenic activity, exhibiting strong repression when BAT is activated and induction when BAT thermogenesis is blunted. We further show that the thermogenesis‐associated downregulation of Parkin occurs through repression of Parkin gene transcription, involves the noradrenergic/cAMP‐mediated and PPARα‐dependent mechanisms associated with enhanced thermogenesis, and requires active lipolysis in brown adipocytes. Conversely, the transcript and protein levels of Parkin are strongly induced when thermogenic pathways are suppressed, such as during the deacclimation to cold. The existence of transcriptional mechanisms of regulating the autophagic system (e.g., via the nuclear receptors PPARα and FXR) has recently been highlighted in liver 40, 41. Our data further support the idea that transcriptional regulation is a key mechanism for controlling components of the mitophagic machinery, such as Parkin.
The repression of Parkin expression during acute thermogenic activation may be viewed as a protective mechanism that acts against excessive mitophagy when maximal mitochondrial activity is required, and physiological uncoupling of mitochondrial should not yield potentially harmful over‐activation of mitophagy processes. We found that PINK1 is stabilized in thermogenically activated BAT, likely due to the known reduced mitochondrial membrane potential in thermogenically active brown adipocytes 42. This could also put brown adipocytes at risk for over‐activation of mitophagy unless Parkin levels are reduced. On the other hand, paradoxical re‐induction of Parkin expression and phosphorylation in response to cold deacclimation, when thermogenesis is blunted and mitochondrial polarization is likely to recover, suggests that intracellular events other than membrane potential play a role in activation of the PINK1/Parkin system in BAT.
The recognition here of a mechanism of preventing Parkin activity in brown adipocytes based on the downregulation of Parkin levels through transcriptional repression converges with a recent report describing a PKA‐mediated impairment of the recruitment of Parkin to mitochondria in beige cells 43. The two processes are likely to represent complementary mechanisms for downregulating mitophagy in association with thermogenic activation. In any case, although our data support that the regulation of Parkin and PINK1 in BAT is related to the control of mitophagy, further experiments are required to confirm a direct link.
Our analysis of Parkin‐KO mice provides evidence that Parkin downregulation is linked to enhanced BAT thermogenesis (as evidenced by enhanced BAT activity and subsequent metabolic improvement) under conditions of mild thermogenic activity, when enhanced mitophagy is not required. A previous study reported impairment of white adipogenesis and hepatic fatty acid transport in Parkin‐KO mice 44. Although BAT activity was not specifically addressed in this analysis, some of the data reported were indicative of increased energy expenditure, metabolic protection against high‐fat diet, and enhanced BAT activation due to Parkin invalidation. However, when high rates of mitophagy occur, such as during deacclimation to cold, Parkin appears to act as a key component of the capacity of brown adipocytes to process and degrade mitochondria. Moreover, our observations made in BAT from cold‐deacclimated Parkin‐KO mice are consistent with previous observations in Drosophila where Parkin was found to be particularly relevant for the appropriate processing of specific components of the mitochondrial OXPHOS system through the formation of MDVs 33, 34, 35.
The importance of Parkin for regulating mitophagy with respect to BAT activity may be expanded to the thermogenically competent beige adipose tissue in iWAT. Mitophagy is reportedly involved in the reacquisition of the white phenotype by beige adipose tissue 24. During the preparation of the current manuscript, Lu et al reported that Parkin plays a role in this process 43. The authors found that Parkin had a more minor involvement in BAT than in iWAT in their system, based on long‐term (15 days) withdrawal from pharmacological treatment with β3‐adrenergic agonists in mice as an experimental model. Analogous experiments performed by our group confirmed the majority of the findings by Lu and co‐workers 43 on BAT, but also indicate that BAT plasticity in response to β3‐adrenergic stimulus withdrawal is mildly affected by Parkin deletion, especially within times shorter than a 15‐day withdrawal end‐point. In addition to experiment‐driven variability, it is possible that long‐term adaptations to suppress thermogenic activity are sufficient to cope with the absence of Parkin in BAT. Moreover, as shown in brown adipocyte cultures, the possibility that mechanisms other than β3‐adrenergic pathways are additionally relevant in the involvement of Parkin in BAT plasticity following thermogenic challenges elicited by ambient temperature cannot be ruled out.
In conclusion, we herein show for the first time that Parkin appears to be a key component of BAT plasticity in response to thermogenic requirements, either positive or negative, and the corresponding adaptive control of mitophagy. Reciprocal transcriptional regulation of Parkin in relation to thermogenic activation in BAT appears to be a mechanism for controlling mitochondrial homeostasis in brown adipocytes. The identification of Parkin as a key player in the adaptation of BAT to reduced thermogenic activity may be relevant for understanding the processes underlying the inactivation of BAT under physiopathological conditions, such as obesity, aging, or the reproductive cycle (pregnancy/lactation). The role of Parkin in adipose tissue plasticity may also underlie the recently reported association between a polymorphic form of the Parkin gene and obesity, which was identified in genome‐wide association studies 45, 46.
Materials and Methods
Animal experiments
Experiments using wild‐type animals were conducted on 2‐ to 3‐month‐old C57BL/6J male mice (Harlan Laboratories). Animals were maintained at the standard animal facility temperature (22°C), at thermoneutrality (29°C), or under cold exposure (4°C), as stated in each experiment. Unless otherwise specified, all animals were maintained under a 12‐h dark/light cycle with ad libitum access to food and water. For experiments involving cold acclimation, mice were single‐caged and maintained at 4°C for 1 h (very short time cold exposure), 1 day (acute), or 21 days (chronic). When indicated, mice were injected intraperitoneally with leupeptin (40 mg/kg b.w.) (Sigma‐Aldrich) or saline. For experiments involving cold deacclimation (the return to thermoneutrality after cold acclimation), mice were acclimated to cold for 21 days and then divided into three experimental groups (matched by age and weight): cold‐acclimated (used as controls), deacclimated at thermoneutrality for 1 day, and deacclimated at thermoneutrality for 7 days. When indicated, mice were injected intraperitoneally 1 mg/kg of CL316,243 (17499, Cayman Chemical) or saline once per day for 1 week. Mice were sacrificed by decapitation, and blood and tissues were collected. In the CL316,243 chronic treatment experiments, mice were sacrificed 5 h after the last injection. For the experiments addressing withdrawal from chronic CL316,243 treatment, mice were sacrificed 7 days and 15 days after the last CL316,243 injection. In chronic CL316,243 treatment and withdrawal experiments, mice were maintained at thermoneutral (29°C) temperature, as in Ref. 43 Tissues were dissected, weighed, and frozen for further mRNA and protein analyses, or they were fixed and processed for microscopic analyses (see below).
All experiments were performed in accordance with European Community Council Directive 86/609/EEC, and the experiments and numbers of animals to be used were approved by the Institutional Animal Care and Use Committee of the University of Barcelona.
Parkin‐KO mice studies
Parkin‐KO (B6.129S4‐Park2tm1Shn/J) mice were obtained from Jackson Laboratories, and wild‐type littermates were used as controls. For diet‐induced obesity studies, 3‐month‐old mice were fed a control diet (2018; Teklad Diet, Harlan Laboratories) or a high‐fat diet (D12451; Research Diets Inc.) for 2 months. The insulin tolerance test (ITT) was performed in the evening after a short fast (2 h); mice were injected intraperitoneally with 0.75 IU/kg insulin (Actrapid, Novo Nordisk), and glucose levels were measured (see below) using blood samples collected from the tail vein. For analysis of responsiveness to cold, Parkin‐KO mice and wild‐type littermates were single‐caged and exposed to 4°C for 24 h. Experiments addressing cold deacclimation and CL316,243 withdrawal were performed as described above.
Serum biochemistry
Glucose and triglyceride levels were measured using Accutrend Technology (Roche Diagnostics). FGF21 levels were quantified with ELISA (RD291108200R, BioVendor). Insulin, adipokines, and cytokines were quantified using a Multiplex system (MADKMAG‐HK, Millipore), and NEFA and 3‐hydroxybutyrate plasma levels were quantified using Wako HR series NEFA‐HR(2) and Wako Autokit Total Ketone Bodies, respectively (Wako Chemicals).
Thermography imaging
Surface temperatures were recorded using a T335 infrared digital thermography camera (FLIR Systems), which features a thermal sensitivity of 0.1°C and an image resolution of 640 × 480 pixels. Environmental parameters (relative humidity, room temperature, and reflected apparent temperature) were measured in situ and set in the camera as parametrical inputs for each experiment. Mice were shaved and allowed to recover for 2 days, and then, infrared pictures of non‐anesthetized animals were taken from a distance of 30 cm. The images were analyzed using the FLIR QuickReport software (FLIR Systems), which allowed us to normalize temperature ranges and quantify temperature values arising from the isotherms. Maximal temperature values obtained from the interscapular area were compared between genotypes.
Optical and electron microscopy
For hematoxylin and eosin (H&E) staining, tissue samples were fixed overnight in 4% formalin, paraffin‐embedded, processed according to the standard procedures, and observed under an optical microscope. For electron microscopic analysis, tissue samples were fixed in 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) and post‐fixed in 1% osmium tetroxide and 0.8% FeCNK in phosphate buffer. After dehydration in a graded acetone series, tissue samples were embedded in Spurr resin. Ultrathin sections were stained with uranyl acetate and lead citrate and examined with a Jeol 1010 transmission electron microscope (Izasa Scientific).
RNAseq and RNAseq data analysis
For analysis of cold‐induced genes, we used an RNAseq‐based database (GSE77534, GEO) that was obtained by comparing BAT of mice exposed at 4°C for 24 h versus mice kept at thermoneutrality (29°C). Retrieval of cold‐induced transcripts was performed as reported previously 25. To identify genes that were upregulated or downregulated by cold, the fold change was calculated as the proportion between the sum of the RPKM (reads per kilobase per million mapped reads) for all gene transcripts under the cold condition versus the control condition. Significance was tested using Fisher's exact test (comparing the number of reads mapped to a given gene and the number of reads mapped to all other genes in the cold condition versus the control condition) and corrected by the Benjamini–Hochberg method (taking the four samples as independent tests for each gene). A difference in gene expression was considered significant if the corrected P‐value was < 0.05. As additional criteria, a gene was considered to be “modified by cold” only if its expression changed significantly in the same direction (i.e., “up” or “down”) in at least three of the four samples per group and no significant change in the opposite direction was observed.
Cell culture and reagents
Cell culture reagents were from Sigma‐Aldrich unless otherwise indicated. Primary cultures of brown adipocytes were obtained by first isolating preadipocytes from interscapular, cervical, and axillary BAT depots from 3‐week‐old Swiss ICR (CD‐1) mice (Harlan Laboratories), or wild‐type and PPARα‐KO mice (B6.129S4‐Pparatm1Gonz/J; Jackson Laboratory). The isolated precursor cells were plated and grown in Dulbecco's modified Eagle's medium (DMEM)/Ham's F12 medium (1:1) supplemented with 10% fetal bovine serum (FBS) or charcoal‐stripped serum (CSS), 1% Fungizone, 20 nM insulin, 2 nM T3, and 100 μM ascorbate. Experiments were performed on day 10 of culture, when more than 90% of cells were differentiated (as assessed by lipid accumulation and brown adipocyte morphology). When indicated, cells were treated with 0.5 μM NE, 1 mM dibutyryl‐cAMP, 5 μg/ml actinomycin D, 20 μg/ml cycloheximide, 10 mM 3‐methyladenine (3‐MA), 10 μM H89,10 μM SB202190, 10 μM Atglistatin, 10 μM CAY10499 (Cayman Chemical), 1 μM GW7647, 10 μM GW6471, 1 μM all‐trans‐retinoic acid. Glycerol concentration in the media was determined with spectrophotometric methods.
Mitochondrial DNA quantification
DNA was prepared from iBAT using a proteinase K plus phenol/chloroform‐based method. Relative mtDNA was quantified using quantitative PCR and employing TaqMan (Applied Biosystems) probes for mt‐Cytb (Appendix Table S4). Data were referred to nuclear DNA abundance as estimated by quantitative PCR of the Cebpa gene, as already reported 47.
RNA isolation and real‐time quantitative PCR
RNA was extracted from tissues and cells using a NucleoSpin RNA Kit (Macherey‐Nagel), and the mRNA levels were determined by quantitative reverse transcription–polymerase chain reaction (qRT–PCR), using the appropriate TaqMan probes (Appendix Table S4). The mRNA level of each gene of interest was normalized to that of a housekeeping reference gene (18S rRNA) using the comparative (2−ΔCt) method.
Western blotting
Cell extracts were prepared by homogenization in a buffer consisting of 20 mM Tris–HCl (pH 7.4), 1 mM EDTA, 1 mM EGTA, 1% Triton X‐100, a protease inhibitor cocktail (Roche Diagnostics), 2 mM sodium orthovanadate, and 10 mM β‐glycerophosphate. Interscapular BAT extracts were prepared by homogenization in a buffer consisting of 50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1% NP‐40, 0.5% sodium deoxycholate, 0.1% SDS, 0.1 mM EDTA, 0.1 mM EGTA, a protease inhibitor cocktail, 2 mM sodium orthovanadate, and 10 mM β‐glycerophosphate. The total protein content was measured using a Pierce™ BCA Protein Assay Kit (Fisher Scientific). The proteins (40 μg/lane) were resolved by 12 or 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and electrotransferred to Immobilon‐P polyvinylidene difluoride (PVDF) membranes (GE Healthcare). The membranes were incubated with primary antibodies specific for Parkin (2132, Cell Signaling Technology), PINK1 (BC100‐494, Novus Biologicals), UCP1 (ab10983, Abcam), LC3B (2775S, Cell Signaling), p62/SQSTM1 (ab91526, Abcam), total OXPHOS rodent WB Antibody Cocktail (ab110413, Abcam), OPTN (10837‐1‐AP, Proteintech), β‐actin (A5441, Sigma‐Aldrich), and then with horseradish peroxidase (HRP)‐conjugated anti‐mouse IgG (170‐6516, Bio‐Rad) or anti‐rabbit IgG (sc‐2004, Santa Cruz), as appropriate. PINK1 detection was validated using tissue extracts from PINK1‐KO mice 48, kindly provided by Dr. J. Bolaños (Salamanca, Spain) (see Appendix Fig S1). Signals were detected using a chemiluminescence‐HRP substrate (EMD Millipore). Densitometric analyses of digitalized images were performed using the Multi Gauge V3.0 software (Fujifilm). Images were processed using Adobe Photoshop CS6 (Adobe Systems); brightness and contrast adjustments were applied uniformly across the entire image.
Parkin immunoprecipitation
iBAT samples were homogenized in a buffer consisting of 50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1% NP‐40, 10 mM EDTA, a protease inhibitor cocktail, 2 mM sodium orthovanadate, 10 mM β‐glycerophosphate, 5 mM sodium fluoride, and 5 μg/ml aprotinin. 500 μg of total lysate protein was incubated overnight with a mouse monoclonal antibody against Parkin (sc‐32282, Santa Cruz). Protein A/G PLUS‐agarose (sc‐2003, Santa Cruz) was used for immunoprecipitation following the manufacturer's instruction (using the protease and phosphatase inhibitors listed above in the PBS washing buffer). Western blot analysis was performed using a sheep polyclonal antibody against Parkin pSer65 (68‐0056‐100, Ubiquigent) with 10 μg of the non‐phosphorylated peptide immunogen (68‐1010‐001, Ubiquigent) per 1 μg of polyclonal antibody. Membranes were stripped, checked for no remaining signals, and incubated with a rabbit polyclonal antibody against total Parkin (2132, Cell Signaling Technology). The secondary antibodies used were as follows: donkey anti‐sheep (sc‐2473, Santa Cruz) and goat anti‐rabbit (ab6721, Abcam), respectively. All the antibodies were selected to profit species diversity to avoid non‐specific IgG detection and signal cross‐reactivity.
Parkin promoter activity assay
HIB‐1B cells (kindly supplied by B.M. Spiegelman, Dana‐Farber Cancer Institute, Boston, MA, USA) were cultured in DMEM and transiently transfected with the pGL4‐Park2‐Luc plasmid vector containing the 5’‐flanking region (−800 to +120) of the mouse Parkin gene 49, following previously described procedures 50. Empty pGL4 vector was used as a control for the basal luciferase activity, and the pRL‐CMV expression plasmid for sea pansy (Renilla reniformis) luciferase was used as a control for transfection efficiency (Promega). At 24 h post‐transfection, the cells were treated with 0.5 μM NE, 1 mM dibutyryl‐cAMP. When indicated, cells were co‐transfected with 0.1 μg of the PPARα expression vector pSG5‐PPARα 31 or pSG5 control vector, and treated with 1 μM GW7647 or 0.5 μM NE after 24 h. After a further 24 h, firefly and Renilla luciferase activities were measured using a Dual‐Luciferase Reporter Assay system (Promega) and a Turner Designs luminometer (TD 20/20). Firefly luciferase activity of transfected cells was normalized using Renilla luciferase activity.
Citrate synthase enzymatic activity
Brown adipose tissue was homogenized in SETH buffer (0.25 M sucrose, 2.0 mM EDTA, 10 mM Tris–HCl, 50 U/ml heparin). The enzymatic activity of citrate synthase in the presence of 1 mM DTNB (5,5′‐dithiobis(2‐nitrobenzoic acid)), Acetyl‐CoA, and oxaloacetic acid was assessed by spectrophotometric measurement.
Statistical analysis
Statistical significance was assessed using the two‐tailed unpaired Student's t‐test or one‐way ANOVA followed by the Dunnett's or Tukey's post hoc tests, or by two‐way ANOVA, all which were applied with the GraphPad statistical software (GraphPad Software). Analysis of discrepancies among standard deviation in experimental groups was assessed with the Brown–Forsythe test or F‐test as appropriate. Welch's correction was applied whenever unequal variances were detected. Exact numbers of replicates are shown at each figure legend. Outliers were detected and removed prior to significance analyses using Grubbs’ test. Statistical significance was set with an α‐value of P < 0.05, and the underlying assumptions for validity were assessed for all tests. Data are expressed as means ± standard error of the mean (s.e.m.).
Author contributions
The experiments were conceived and designed by MC, JV, MG, and FV. RNAseq data were obtained and analyzed by RC and MC. Experiments with mice were performed by MC, JV, AD‐A, and AG‐N. Cell culture experiments were performed by MC, LC, and TQ‐L. Overall data were analyzed by MC, JV, MG, and FV. The manuscript was written by JV and FV and revised/approved by all contributors.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Table EV1
Review Process File
Acknowledgements
We thank Dr. M.G. Tansey (Emory University, USA) for supplying the plasmid reporter construct driven by the Parkin promoter, Dr. Bolaños (Institute of Functional Biology and Genomics, University of Salamanca‐CSIC, Spain) for supplying PINK1‐KO mouse samples, and A. Peró and M. Morales for technical support. This work was supported by grants from the Ministerio de Economia y Competitividad (MINECO), Spain (SAF2017‐85722R and PI17/00420), co‐financed by the European Regional Development Fund (ERDF). M.C., R.C., and L.C. were supported by PhD scholarships from MINECO and University of Barcelona. T.Q.‐L. was supported by a CONACyT (National Council for Science and Technology in Mexico) Ph. D. scholarship. J.V. is a “Juan de la Cierva” post‐doctoral researcher by MINECO.
EMBO Reports (2019) 20: e46832
Contributor Information
Joan Villarroya, Email: joanvillarroya@gmail.com.
Francesc Villarroya, Email: fvillarroya@ub.edu.
References
- 1. Ricquier D, Bouillaud F (2000) Mitochondrial uncoupling proteins: from mitochondria to the regulation of energy balance. J Physiol 529(Pt 1): 3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feldmann HM, Golozoubova V, Cannon B, Nedergaard J (2009) UCP1 ablation induces obesity and abolishes diet‐induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 9: 203–209 [DOI] [PubMed] [Google Scholar]
- 3. Lowell BB, S‐Susulic V, Hamann A, Lawitts JA, Himms‐Hagen J, Boyer BB, Kozak LP, Flier JS (1993) Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 366: 740–742 [DOI] [PubMed] [Google Scholar]
- 4. Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359 [DOI] [PubMed] [Google Scholar]
- 5. Golozoubova V, Hohtola E, Matthias A, Jacobsson A, Cannon B, Nedergaard J (2001) Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J 15: 2048–2050 [DOI] [PubMed] [Google Scholar]
- 6. Gospodarska E, Nowialis P, Kozak LP (2015) Mitochondrial turnover: a phenotype distinguishing brown adipocytes from interscapular brown adipose tissue and white adipose tissue. J Biol Chem 290: 8243–8255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giralt M, Villarroya F (2013) White, brown, beige/brite: different adipose cells for different functions? Endocrinology 154: 2992–3000 [DOI] [PubMed] [Google Scholar]
- 8. Harms M, Seale P (2013) Brown and beige fat: development, function and therapeutic potential. Nat Med 19: 1252–1263 [DOI] [PubMed] [Google Scholar]
- 9. Shabalina IG, Petrovic N, de Jong JM, Kalinovich AV, Cannon B, Nedergaard J (2013) UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep 5: 1196–1203 [DOI] [PubMed] [Google Scholar]
- 10. Bento CF, Renna M, Ghislat G, Puri C, Ashkenazi A, Vicinanza M, Menzies FM, Rubinsztein DC (2016) Mammalian autophagy: how does it work? Annu Rev Biochem 85: 685–713 [DOI] [PubMed] [Google Scholar]
- 11. Kaur J, Debnath J (2015) Autophagy at the crossroads of catabolism and anabolism. Nat Rev Mol Cell Biol 16: 461–472 [DOI] [PubMed] [Google Scholar]
- 12. Singh R, Cuervo AM (2011) Autophagy in the cellular energetic balance. Cell Metab 13: 495–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim I, Rodriguez‐Enriquez S, Lemasters JJ (2007) Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys 462: 245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ashrafi G, Schwarz TL (2013) The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ 20: 31–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bingol B, Sheng M (2016) Mechanisms of mitophagy: PINK1, Parkin, USP30 and beyond. Free Radic Biol Med 100: 210–222 [DOI] [PubMed] [Google Scholar]
- 16. Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, et al (2010) PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol 189: 211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Narendra D, Tanaka A, Suen DF, Youle RJ (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183: 795–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ (2010) PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol 8: e1000298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martinez‐Lopez N, Athonvarangkul D, Sahu S, Coletto L, Zong H, Bastie CC, Pessin JE, Schwartz GJ, Singh R (2013) Autophagy in Myf5+ progenitors regulates energy and glucose homeostasis through control of brown fat and skeletal muscle development. EMBO Rep 14: 795–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singh R, Xiang Y, Wang Y, Baikati K, Cuervo AM, Luu YK, Tang Y, Pessin JE, Schwartz GJ, Czaja MJ (2009) Autophagy regulates adipose mass and differentiation in mice. J Clin Invest 119: 3329–3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Y, Goldman S, Baerga R, Zhao Y, Komatsu M, Jin S (2009) Adipose‐specific deletion of autophagy‐related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc Natl Acad Sci USA 106: 19860–19865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cairo M, Villarroya J, Cereijo R, Campderros L, Giralt M, Villarroya F (2016) Thermogenic activation represses autophagy in brown adipose tissue. Int J Obes (Lond) 40: 1591–1599 [DOI] [PubMed] [Google Scholar]
- 23. Martinez‐Lopez N, Garcia‐Macia M, Sahu S, Athonvarangkul D, Liebling E, Merlo P, Cecconi F, Schwartz GJ, Singh R (2016) Autophagy in the CNS and periphery coordinate lipophagy and lipolysis in the brown adipose tissue and liver. Cell Metab 23: 113–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Altshuler‐Keylin S, Shinoda K, Hasegawa Y, Ikeda K, Hong H, Kang Q, Yang Y, Perera RM, Debnath J, Kajimura S (2016) Beige adipocyte maintenance is regulated by autophagy‐induced mitochondrial clearance. Cell Metab 24: 402–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quesada‐Lopez T, Cereijo R, Turatsinze JV, Planavila A, Cairo M, Gavalda‐Navarro A, Peyrou M, Moure R, Iglesias R, Giralt M, et al (2016) The lipid sensor GPR120 promotes brown fat activation and FGF21 release from adipocytes. Nat Commun 7: 13479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Greene AW, Grenier K, Aguileta MA, Muise S, Farazifard R, Haque ME, McBride HM, Park DS, Fon EA (2012) Mitochondrial processing peptidase regulates PINK1 processing, import and Parkin recruitment. EMBO Rep 13: 378–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ (2010) Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol 191: 933–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bengtsson T, Redegren K, Strosberg AD, Nedergaard J, Cannon B (1996) Down‐regulation of beta3 adrenoreceptor gene expression in brown fat cells is transient and recovery is dependent upon a short‐lived protein factor. J Biol Chem 271: 33366–33375 [DOI] [PubMed] [Google Scholar]
- 29. Jacobsson A, Muhleisen M, Cannon B, Nedergaard J (1994) The uncoupling protein thermogenin during acclimation: indications for pretranslational control. Am J Physiol 267: R999–R1007 [DOI] [PubMed] [Google Scholar]
- 30. Milner RE, Trayhurn P (1988) Evidence that the acute unmasking of GDP‐binding sites in brown adipose tissue mitochondria is not dependent on mitochondrial swelling. Biochem Cell Biol 66: 1226–1230 [DOI] [PubMed] [Google Scholar]
- 31. Barbera MJ, Schluter A, Pedraza N, Iglesias R, Villarroya F, Giralt M (2001) Peroxisome proliferator‐activated receptor alpha activates transcription of the brown fat uncoupling protein‐1 gene. A link between regulation of the thermogenic and lipid oxidation pathways in the brown fat cell. J Biol Chem 276: 1486–1493 [DOI] [PubMed] [Google Scholar]
- 32. Alvarez R, de Andres J, Yubero P, Vinas O, Mampel T, Iglesias R, Giralt M, Villarroya F (1995) A novel regulatory pathway of brown fat thermogenesis. Retinoic acid is a transcriptional activator of the mitochondrial uncoupling protein gene. J Biol Chem 270: 5666–5673 [DOI] [PubMed] [Google Scholar]
- 33. McLelland GL, Soubannier V, Chen CX, McBride HM, Fon EA (2014) Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J 33: 282–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Soubannier V, McLelland GL, Zunino R, Braschi E, Rippstein P, Fon EA, McBride HM (2012) A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr Biol 22: 135–141 [DOI] [PubMed] [Google Scholar]
- 35. Vincow ES, Merrihew G, Thomas RE, Shulman NJ, Beyer RP, MacCoss MJ, Pallanck LJ (2013) The PINK1‐Parkin pathway promotes both mitophagy and selective respiratory chain turnover in vivo . Proc Natl Acad Sci USA 110: 6400–6405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, et al (2013) Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 123: 215–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Villarroya F, Cereijo R, Villarroya J, Giralt M (2017) Brown adipose tissue as a secretory organ. Nat Rev Endocrinol 13: 26–35 [DOI] [PubMed] [Google Scholar]
- 38. Muller TD, Lee SJ, Jastroch M, Kabra D, Stemmer K, Aichler M, Abplanalp B, Ananthakrishnan G, Bhardwaj N, Collins S, et al (2013) p62 links beta‐adrenergic input to mitochondrial function and thermogenesis. J Clin Invest 123: 469–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, Sideris DP, Fogel AI, Youle RJ (2015) The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524: 309–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee JM, Wagner M, Xiao R, Kim KH, Feng D, Lazar MA, Moore DD (2014) Nutrient‐sensing nuclear receptors coordinate autophagy. Nature 516: 112–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seok S, Fu T, Choi SE, Li Y, Zhu R, Kumar S, Sun X, Yoon G, Kang Y, Zhong W, et al (2014) Transcriptional regulation of autophagy by an FXR‐CREB axis. Nature 516: 108–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wikstrom JD, Mahdaviani K, Liesa M, Sereda SB, Si Y, Las G, Twig G, Petrovic N, Zingaretti C, Graham A, et al (2014) Hormone‐induced mitochondrial fission is utilized by brown adipocytes as an amplification pathway for energy expenditure. EMBO J 33: 418–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lu X, Altshuler‐Keylin S, Wang Q, Chen Y, Henrique Sponton C, Ikeda K, Maretich P, Yoneshiro T, Kajimura S (2018) Mitophagy controls beige adipocyte maintenance through a Parkin‐dependent and UCP1‐independent mechanism. Sci Signal 11: eaap8526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim KY, Stevens MV, Akter MH, Rusk SE, Huang RJ, Cohen A, Noguchi A, Springer D, Bocharov AV, Eggerman TL, et al (2011) Parkin is a lipid‐responsive regulator of fat uptake in mice and mutant human cells. J Clin Invest 121: 3701–3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ahmad S, Poveda A, Shungin D, Barroso I, Hallmans G, Renstrom F, Franks PW (2016) Established BMI‐associated genetic variants and their prospective associations with BMI and other cardiometabolic traits: the GLACIER Study. Int J Obes (Lond) 40: 1346–1352 [DOI] [PubMed] [Google Scholar]
- 46. Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, et al (2015) Genetic studies of body mass index yield new insights for obesity biology. Nature 518: 197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Villarroya J, Diaz‐Delfin J, Hyink D, Domingo P, Giralt M, Klotman PE, Villarroya F (2010) HIV type‐1 transgene expression in mice alters adipose tissue and adipokine levels: towards a rodent model of HIV type‐1 lipodystrophy. Antivir Ther 15: 1021–1028 [DOI] [PubMed] [Google Scholar]
- 48. Requejo‐Aguilar R, Lopez‐Fabuel I, Fernandez E, Martins LM, Almeida A, Bolanos JP (2014) PINK1 deficiency sustains cell proliferation by reprogramming glucose metabolism through HIF1. Nat Commun 5: 4514 [DOI] [PubMed] [Google Scholar]
- 49. Tran TA, Nguyen AD, Chang J, Goldberg MS, Lee JK, Tansey MG (2011) Lipopolysaccharide and tumor necrosis factor regulate Parkin expression via nuclear factor‐kappa B. PLoS One 6: e23660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rosell M, Hondares E, Iwamoto S, Gonzalez FJ, Wabitsch M, Staels B, Olmos Y, Monsalve M, Giralt M, Iglesias R, et al (2012) Peroxisome proliferator‐activated receptors‐alpha and ‐gamma, and cAMP‐mediated pathways, control retinol‐binding protein‐4 gene expression in brown adipose tissue. Endocrinology 153: 1162–1173 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Table EV1
Review Process File


