Abstract
Despite consistent efforts to protect public health there is still a heavy burden of viral disease, both in the United States and abroad. In addition to conventional medical treatment, there is a need for a holistic approach for early detection and prevention of viral outbreaks at a population level. One-Health is a relatively new integrative approach to the solving of global health challenges. A key component to the One-Health approach is the notion that human health, animal health, and environmental health are all innately interrelated. One-Health interventions, initiated by veterinary doctors, have proven to be effective in controlling outbreaks, but thus far the applications focus on zoonotic viruses transmitted from animals to humans. Environmental engineers and environmental scientists hold a critical role in the further development of One-Health approaches that include water-related transport and transmission of human, animal, and zoonotic viruses. In addition to waterborne viruses, the proposed approach is applicable to a wide range of viruses that are found in human excrement since contaminated water-based surveillance systems may be used for early detection of viral disease. This paper proposes a greater One-Health based framework that involves water-related pathways. The first step in the proposed framework is the identification of critical exposure pathways of viruses in the water environment. Identification of critical pathways informs the second and third steps, which include water-based surveillance systems for early detection at a population level and implementation of intervention approaches to block the critical pathways of exposure.
Keywords: One health, Viruses, Waterborne disease, Wastewater epidemiology
1. One-health and viral disease
The burden of viral disease is a global concern. Due to their unique properties, viruses have a particular relevance when analyzing the interaction among humans, animals, and the environment. Viruses are small compared to other pathogens, facilitating transport in the environment. Moreover, their resistance to disinfection and ability to survive for prolonged periods in water and solids make their transmission from the environment to suitable hosts likely. This is compounded by their low infectious dose, inability to be treated by antibiotics, and their proclivity for adaptive mutation. Additionally, viruses do not replicate outside their host cells, therefore detection in environmental samples can be directly related to the human or animal population that excreted these viruses.
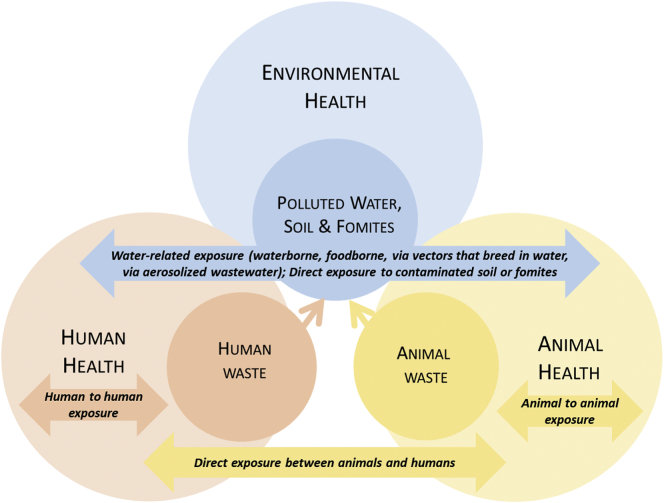
Fig. 1 summarizes viral exposure pathways and the relevance of the One-Health approach. One-Health is a relatively new approach to the solving of global health challenges. Formally put forth by the One Health Commission in 2007, the concept is defined as “the collaborative effort of multiple disciplines – working locally, nationally, and globally – to attain optimal health for people, animals and our environment [1].” Consequently, a key component to the One-Health approach is the notion that human health, animal health, and environmental health are all innately interrelated. The quality and well-being of one group can directly and indirectly impact the quality of the other two groups. By taking all three aspects of health into account, solutions can be generated that not only address the health problems of a specific group but mitigate the source of those problems as well.
Fig. 1.
Schematic representing the relevance of One-Health to viral disease.
Much of the current work using the One-Health approach is focused upon the exposure pathway between humans and animals, while the water-related exposure pathway has not been thoroughly investigated from a One-Health perspective. The purpose of this paper is to explore water-related exposure pathways as they relate to human, animal, and environmental health, to explore the possibility of surveillance of water and wastewater systems as means of identification of endemic disease and potential outbreaks at a population level, and to develop a framework with which to apply the One-Health methodology for early detection and management of water-related viral outbreaks.
2. Burden of viral disease
Communicable disease is one of the leading causes of death worldwide. Lower respiratory infections were responsible for 3.0 million deaths in 2016 according to the World Health Organization (WHO), and diarrheal infections contributed to another 1.4 million deaths in the same year [2]. Viral diseases contribute to these categories; influenza, coronavirus, and adenovirus are all considered lower respiratory infections, and viruses such as rotavirus can cause diarrheal disease. Viral disease outbreaks occur often, with WHO reporting outbreaks of influenza, coronavirus, hepatitis E, yellow fever, Ebola virus, Zika virus, poliovirus, dengue fever, and chikungunya in 2017 alone, located in countries all over the world such as Brazil, Chad, China, France, Italy, Saudi Arabia, and Sri Lanka [3]. Moreover, environmental factors such as water, soil, and zoonotic vectors such as mosquitos and animals have been cited as important contributing factors to viral outbreaks [4].
WHO gathers surveillance statistics for specific viruses and estimates between 290,000 to 650,000 annual deaths from influenza, greater than previous estimates [5]. Data from February 2018 indicated that the disease burden of influenza was highest in north and east Africa, South America, and Europe [6]. Data from the WHO Mortality Database shows over 100,000 deaths from viral hepatitis since 2012 throughout the world [7]. Outbreaks of gastrointestinal disease are also common around the world. Rotavirus, for example, is associated with high rates of pediatric mortality; rotavirus infection was found to be responsible for approximately 453,000 pediatric deaths in 2008 worldwide, accounting for 5% of all deaths in children younger than five years [8]. Viral disease also disproportionately impacts poorer communities around the world. The aforementioned rotavirus study determined that over half of the pediatric rotavirus deaths worldwide occurred in just five developing nations (Democratic Republic of the Congo, Ethiopia, India, Nigeria, and Pakistan) [8]. Academic studies assessing global disease burden also report substantial burden due to viral disease. One study investigating global foodborne disease burden reported approximately 684 million disease cases and 212,000 deaths due to norovirus globally for the year 2010, the largest for any pathogen studied [9]. The same study found hepatitis A virus responsible for approximately 47 million illnesses and 94,000 deaths in 2010 [9].
Beyond diseases arising from direct infection, there are other secondary diseases associated with viruses, such as cervical cancer, which is strongly associated with papillomavirus [10]. Other viruses have also been linked to increased incidences of heart disease [11,12] and kidney disease [13], particularly in immunocompromised patients. Additionally, it is thought that the true impact of viral disease is underestimated. Many disease outbreaks are reported to be caused by agents of unknown etiology, and some of these outbreaks are suspected to be viral in origin [14]. A One-Health approach could assist in discovering the origin of these disease outbreaks.
In the United States, the Centers for Disease Control (CDC) publish surveillance statistics regarding the rate and occurrence of disease for a number of human viruses, including influenza [15], adenovirus [16], hepatitis A virus [17], rotavirus [18], and West Nile virus [19]. Annual summaries of these surveillance statistics are published in various forms from the CDC. The Summary of Notifiable Diseases (SoND) is an annual report containing information on those diseases for which “regular, frequent, and timely information regarding individual cases is considered necessary for the prevention and control of the disease or condition”, a list of which is updated regularly by the CDC. Viruses reported in the SoND include hepatitis A virus, West Nile virus, and Dengue virus [20]. The CDC also maintains the National Outbreak Reporting System (NORS), which includes information on the number of disease cases and outbreaks for a number of infectious agents, including norovirus, rotavirus, and sapovirus. Influenza statistics are reported most frequently by the CDC via published FluView Weekly Influenza Surveillance Reports, documenting the number of cases of influenza and influenza-like illnesses in the United States. Each of these sources includes both monthly and geographic data regarding disease cases. This allows for the analysis of viral disease trends on both a temporal and spatial basis, many of which are unique from one virus to another [[20], [21], [22], [23], [24], [25], [26]].
3. Viruses of concern
Viruses that infect humans can be both specific to humans and zoonotic in nature. Human viruses are categorized as those that exclusively infect humans and are transmissible from the environment to humans or from human to human. Zoonotic viruses, meanwhile, are defined as viruses “which are naturally transmitted between vertebrate animals and man” [27]. Zoonotic viruses can also be further split into direct and indirect categories. Direct zoonoses involve infection via direct contact between humans and animals, such as skin contact, a bite, or ingestion of tissue. Indirect zoonoses, meanwhile, require a vector or vehicle for transmission of the virus between humans and animals [27].
Viruses can also be divided to categories based on their water-related transmission potential. This classification was put forth by Bradley (1977), splitting water-related infections into four main categories: water-washed infections (diseases arising from poor hygiene) and water-based infections (infections from worm parasites that spend their life cycle in an aquatic environment), as well as waterborne infections and infections with water-related insect vectors, the latter two designations being most relevant when discussing water-related viruses [[27], [28], [29]]. The foremost category is that of waterborne viruses, in which a virus is present in water and infection occurs via ingestion of the contaminated water source. Waterborne viruses will often enter the water source due to fecal contamination, making waste and wastewater management a critical pathway for tracking the spread of viral waterborne disease. The second important category of water-related viruses are those with water-related insect vectors [27]. This includes viruses transmitted by insects that breed in water, such as mosquitos, which carry numerous significant human viruses, such as Zika virus and West Nile virus. In areas where primary water sources may be infested with these insect vectors, this is critical pathway for the spread of viral disease. Finally, another potential transmission pathway for water-related disease is the aerosolization of contaminated water [27,30], in which viruses capable of respiratory transmission are inhaled following aerosolization.
With these categories of zoonotic and water-related viruses in place, there is potential for crossover among them; some viruses may be both zoonotic and water-related. In a report on waterborne zoonoses in 2004, WHO put forth criteria for determining if a pathogen meets these qualifications: the pathogen must spend part of its life cycle within animal species, it is probable the pathogen will have a life stage that will enter water, and transmission of the pathogen between humans and animals must be through a water-related route. If a virus meets all of these, it can be classified as a zoonotic water-related virus.
Table 1 lists several human viruses of concern (including all viruses included in SoND) and classifies them according to the aforementioned categories. As mentioned above, a primary exposure pathway to viral disease for humans is wastewater. The ability to detect viruses in wastewater is therefore critical for investigation via One-Health, and this information is also summarized in Table 1. As noted in the table, several of these viruses fall under multiple categories, being both water-related and zoonotic. For instance, enteroviruses, hepatitis E virus, and rotaviruses are all classified as waterborne viruses, and each of them has been reported to have potential zoonotic properties as well, as cases of these viruses have been observed in animals [27]. Additionally, a number of viruses are zoonotic due to their transmission between mosquitos and humans, such as West Nile virus, Zika virus, and Dengue virus [[31], [32], [33]]. Zoonotic diseases comprise approximately 64% of all human pathogens, with viruses accounting for 5% of pathogens [34]. Zoonoses are also responsible for 26% of the disease burden in low-income countries, whereas they only account for 0.7% in wealthier nations [35].
Table 1.
Categorization of notifiable, waterborne, water-related, and potentially water-related human viruses of concern [27,[31], [32], [33],[36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52]].
| Virus | Transmission route |
Detected in wastewater or human excrement | |||
|---|---|---|---|---|---|
| Human-to-human transmission | Zoonotic | Waterborne | Water-related | ||
| Adenoviruses | ✓ | ✓ | ✓ | ||
| Astroviruses | ✓ | ✓ | ✓ | ||
| Enteroviruses | ✓ | ✓ | ✓ | ✓ | |
| Hepatitis A virus | ✓ | ✓ | ✓ | ||
| Hepatitis E virus | ✓ | ✓ | ✓ | ✓ | |
| Noroviruses | ✓ | ✓ | ✓ | ||
| Rotaviruses | ✓ | ✓ | ✓ | ✓ | |
| Aichi virus | ✓ | ✓ | ✓ | ||
| Polyomaviruses | ✓ | ✓ | ✓ | ||
| Salivirus | ✓ | ✓ | ✓ | ||
| Sapovirus | ✓ | ✓ | ✓ | ||
| Torque Teno virus | ✓ | ✓ | ✓ | ||
| Dengue virus | ✓ | ✓ | ✓ | ||
| West Nile virus | ✓ | ✓ | ✓ | ||
| Zika virus | ✓ | ✓ | ✓ | ||
| Yellow fever virus | ✓ | ✓ | ✓ | ||
| Chikungunya virus | ✓ | ✓ | |||
| Rift Valley fever virus | ✓ | ✓ | |||
| Coronaviruses | ✓ | ✓ | ✓ | ||
| Ebola virus | ✓ | ✓ | ✓ | ||
| Influenza | ✓ | ✓ | ✓ | ||
| Herpesvirus | ✓ | ✓ | |||
| Papillomavirus | ✓ | ✓ | |||
| Parechovirus | ✓ | ✓ | |||
| Arboviral diseasesa | ✓ | ||||
| Hepatitis B virus | ✓ | ||||
| Hepatitis C virus | ✓ | ||||
| HIV | ✓ | ||||
| Rabies | ✓ | ||||
| Rubella | ✓ | ||||
| Smallpox | ✓ | ||||
| Varicella | ✓ | ||||
| Crimean-Congo hemorrhagic fever virus | ✓ | ✓ | |||
| Marburg virus | ✓ | ✓ | |||
| Arenavirusesb | ✓ | ✓ | |||
Including California serogroup, eastern equine encephalitis, Powassan, St. Louis encephalitis, and western equine encephalitis viruses.
Including Lassa, Lujo, Guanarito, Junin, Machupo, and Sabia viruses.
Moreover, many of the viruses listed in Table 1 have been reported as detected in wastewater or human excrement. This is important as it signifies that these viruses are present or potentially present in aquatic pathways. This is true even for viruses that are not typically designated as waterborne or water-related, such as influenza, herpesvirus, and papillomavirus.
Regarding animal viruses of concern, the U.S. Department of Agriculture (USDA) issues annual reports of the domestic status of reportable diseases put forth by the World Organization for Animal Health (OIE) [53]. Table 2 summarizes livestock viral diseases that were reported as present in the United States by the USDA. Many of the same viral families that affect humans are represented in this list, including Coronaviridae, Flaviviridae, Herpesviridae, Orthomyxoviridae, and Reoviridae. A few of the reportable viral animal diseases are also considered zoonotic, which are of even greater significance to human health.
Table 2.
Summary of viral OIE reportable diseases (2016) for livestock [[53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63]].
| Disease | Virus | Viral family | Animals affected | Zoonotic | Water-related | Detected in animal waste |
|---|---|---|---|---|---|---|
| Aujeszky's disease | Suid herpesvirus 1 | Herpesviridae | Swine | ✓ | ✓ | |
| Avian infectious bronchitis | Avian IB virus | Coronaviridae | Birds | ✓ | ||
| Avian infectious laryngotracheitis | Gallid herpesvirus 1 | Herpesviridae | Birds | ✓ | ||
| Avian influenza | Influenza A virus | Orthomyxoviridae | Birds, Mammals | ✓ | ✓ | |
| Bluetongue | Bluetongue virus | Reoviridae | Ruminants | ✓ | ||
| Bovine viral diarrhea | BVD virus 1 | Flaviviridae | Cattle | ✓ | ||
| Caprine arthritis/encephalitis | CAE virus | Retroviridae | Goats | |||
| Eastern equine encephalitis | EEE virus | Togaviridae | Equines | ✓ | ||
| Epizootic hemorrhagic disease | EHD virus | Reoviridae | Ruminants | ✓ | ||
| Equine herpesvirus 1 | EHV-1 | Herpesviridae | Equines | ✓ | ||
| Equine infectious anemia | EIA virus | Retroviridae | Equines | |||
| Equine influenza | Influenza A virus | Orthomyxoviridae | Equines | ✓ | ||
| Equine viral arteritis | Equine arteritis virus | Arteriviridae | Equines | |||
| Infectious bovine rhinotracheitis | Bovine herpesvirus 1 | Herpesviridae | Cattle | ✓ | ||
| Infectious bursal disease | IBD virus | Birnaviridae | Birds | |||
| Maedi-visna | Visna virus | Retroviridae | Sheep | ✓ | ||
| Myxomatosis | Myxoma virus | Poxviridae | Rabbits | |||
| Newcastle disease | Avian avulavirus 1 | Paramyxoviridae | Birds | ✓ | ✓ | |
| Porcine reproductive and respiratory syndrome | PRRS virus | Arteriviridae | Swine | |||
| Rabies | Lyssaviruses | Rhabdoviridae | Mammals | ✓ | ||
| Transmissible gastroenteritis | TGE coronavirus | Coronaviridae | Swine | ✓ | ||
| Turkey rhinotracheitis | Avian metapneumovirus | Paramyxoviridae | Birds | |||
| West Nile fever | West Nile virus | Flaviviridae | Mammals, birds | ✓ | ✓ |
The USDA also collects and maintains disease data for domesticated, agricultural, and wild animals, primarily via the National Animal Health Surveillance System (NAHSS). A number of viruses are investigated via NAHSS for various animals, including influenza A virus in swine [64], and herpesvirus and West Nile virus in horses [65,66]. Annual reports regarding cases of equine West Nile virus in domesticated horses are published via NAHSS, making them a useful comparison to reported human cases of West Nile virus [66]. In addition to domesticated animals, wild animals are also an important consideration, as wildlife has been shown to be a source of disease to both livestock and humans [62].
4. One-health methodology: current status
The One-Health approach has recently been applied to combat zoonotic viral disease, spearheaded by the veterinary community. Viral infection in humans can be prevented with the use of vaccinations, a common practice for a number of viruses for which vaccines have been developed, including influenza [67], poliovirus [68], and rotavirus [18,69]. Vaccines are known to lessen the disease burden in a population both by preventing infection and promoting herd immunity [70].
In cases for which the pathway to human infection has an intermediary animal vector between the host organism and humans, the vaccination of the intermediary animal could also prove vital. This was performed in one of the most successful One-Health implementations to date, for a Hendra virus outbreak in Australia. Hendra virus is one of several zoonotic viruses, including Nipah virus, Tioman virus, and lyssavirus, that originate in bats. It was determined that Hendra virus is first transmitted from bats to horses before being transmitted to humans, and no direct infection between bats and humans was observed. Therefore, a Hendra virus vaccine was developed for horses in order to eliminate the transmission route to humans [71].
On different occasions the strategy is to eliminate or lessen the disease vector responsible for transmission of the disease to humans; for example, mosquito control strategies can be utilized to lessen the burden of West Nile virus, Zika virus, and other viruses for which mosquitos are the primary transmission vector. Chemical methods of mosquito control (such as insecticides, insect growth regulators, and sprays) are commonly used, though these methods could also have an adverse effect on the health of humans, animals, and the surrounding environment, which are of important consideration to the One-Health approach. More “eco-friendly” options are also recently in use, such as sterile insect techniques and plant-based non-harmful mosquitocidals [72]. A One-Health approach has been shown in the past to be effective at reducing costs and improving efficiency in the mitigation of Rift Valley fever virus to improve public health [73].
Smaller-scale policy changes that focus on particular viruses could also help to curb the spread of viral disease. For example, after it was determined that bats were the host species and civets the transmittance vector species for SARS coronavirus, affected areas (such as China) enacted bans on civet trading and the mixing of bats with other species in local markets [71]. This shows that policies can be created to require the use of interventions to block exposure pathways for particular viruses. Public education is also a useful approach, utilized with outbreaks of Nipah virus in Malaysia and Bangladesh, in which people were encouraged to avoid direct contact with bats and taking preventative measures to minimize the chances of viral transmission [71].
5. One-health methodology: proposed approach for water-related viruses
Still, these One- Health success stories have focused on zoonotic viruses, concentrating on how animal health relates to human health. Because viruses can be transmitted in a variety of exposure pathways, environmental health is also of vital importance. While environmental considerations have begun to be considered on a more local scale [74], these aspects have still not been as thoroughly investigated using the One-Health approach. Environmental engineers and scientists therefore have a critical role in the application of the One-Health methodology.
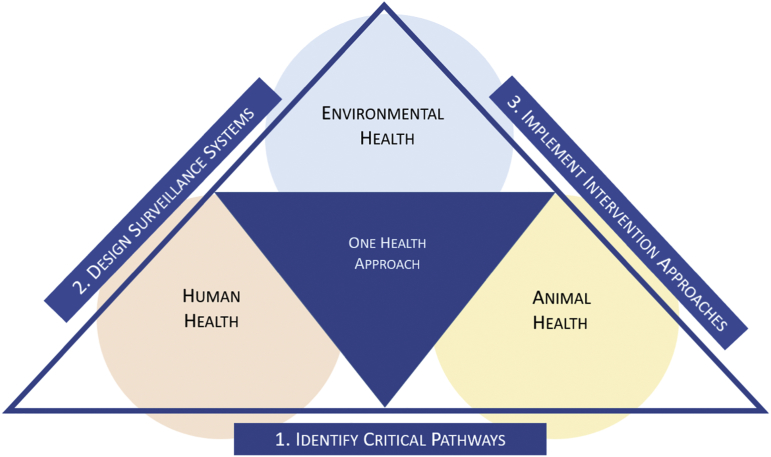
In the application of the One-Health concept to combat water-related viral disease, a three-tiered approach is appropriate (Fig. 2). The first step is to identify critical pathways of exposure. The goal of this step is to identify and prioritize environmental virus reservoirs and critical exposure pathways that facilitate transmission and transport of viral disease among humans and animals. The second step is to design surveillance of the critical environmental reservoirs and pathways. The goal of this step is to identify critical times and critical locations for the onset of water-related viral outbreaks. The final step is to design intervention approaches. The goal of this step is design barriers to interrupt critical pathways at critical times and locations.
Fig. 2.
Concept map of the proposed One Health framework.
5.1. Identification of critical pathways
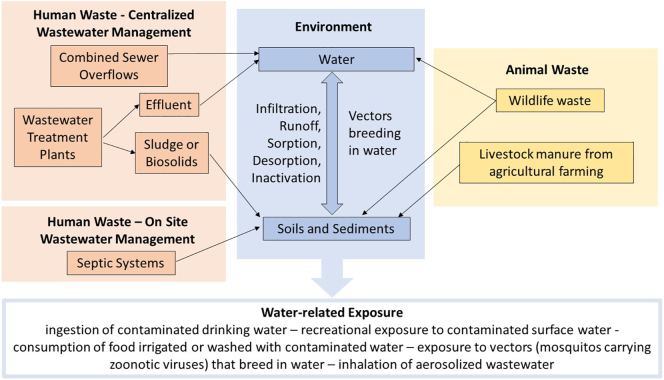
To determine critical pathways of exposure the following should be identified: 1) potential sources/reservoirs of viruses in environmental, human and animal systems; 2) natural processes that affect transport within and between systems; 3) human behaviors that affect exposure to viruses, such as management practices for water, wastewater, agricultural waste, human disease determinants, and animal disease determinants. The goal of this step is to prioritize the most critical reservoirs and exposure pathways for viruses. Fig. 3 presents an example of water-related pathways that include urban and rural areas in the US.
Fig. 3.
Example of water-related pathways for viruses in the United States.
There are several potential pathways by which humans are exposed to waterborne or water-related viruses. The foremost among them is the ingestion of contaminated water. Surface water, treated or untreated, may be used as a drinking water source, and there are a number of pathways for viral contamination of surface water. In urban areas, combined sewer overflows during high rainfall events can introduce untreated wastewater into surface water bodies. In impoverished areas, people often dispose untreated wastewater into surface water bodies that are used elsewhere for drinking water, for example downstream of a river. Even treated wastewater effluent, which is often released into surface water, can contain detectable concentrations of human viruses [75]. Treated or untreated livestock waste and wildlife waste is also washed off the land during precipitation events and can be carried into surface water bodies via runoff, and animal wastes have been shown to be a potential exposure pathway of disease to humans [76]. In addition to ingestion of water, recreational exposure to contaminated surface water, for example in public swimming pools or on public beaches, can be a pathway of viral infection via surface water [77,78]. Groundwater and/or aquifers are also used as drinking water sources around the world, and various pathways exist for the contamination of these sources. For example, in rural areas which dispose of wastewater in private septic systems, the leakage of these septic tanks may allow for the leaching of contaminated wastewater into a groundwater source [79,80]. Another pathway for exposure to waterborne disease is the consumption of food that has been contaminated during the agricultural process. This could be due to irrigation using contaminated water as well as uptake from contaminated soil or sediment. Biosolids (treated wastewater sludge) are often used as an agricultural soil amendment, and viruses are known to have been detected in these biosolids [14,81]. Manure or livestock is a possible source of contamination, whether it is used as a fertilizer or transported in the agricultural environment [82]. Wildlife waste could also contaminate soils and sediments, just as it can contaminate surface water [83].
Data collection is crucial to attain preliminary information for the identification of critical pathways for viral transmission. Numerous governmental agencies publish data regarding clinical cases of disease both spatially and temporally. Animal disease data can also be collected and analyzed in this manner, but there is a need for an integrated human-animal disease surveillance to assess zoonotic disease occurrence [84]. Additionally, appropriate data that may indicate correlations with spatial and temporal patters may be compiled and analyzed. Numerous factors may contribute to the likelihood of infectious disease in certain areas or time periods, including but not limited to hydrological patterns (e.g. precipitation) [85], land use [86], human/livestock/wildlife population density [87], and others. Potential pathways can be prioritized to determine which are most relevant to the region being studied. A system of weight factors could be developed to perform prioritization quantitatively with the use of a statistical model. For example, the degree of regulation of wastewater, storm-water, and livestock waste could impact the importance placed upon the potential pathways associated to the impact of waste disposal systems.
5.2. Design of surveillance systems
The second step in the framework is to design surveillance systems of the critical environmental reservoirs and pathways that will allow for early detection of outbreaks. The objective is to identify critical times and critical locations for the onset of viral outbreaks. This can be achieved by monitoring viral disease indicators (such as concentrations of viruses or other indicators) in critical reservoirs identified in the previous step. The approach includes environmental sampling (such as polluted wastewater from a particular population), as well as clinical samples from infected people, livestock and wildlife. Regular monitoring of critical reservoirs will identify peaks in viral concentrations or indicators that in turn can be related to early signals of disease outbreaks.
The central premise of the proposed surveillance approach is that community fecal pollution represents a snapshot of the status of public health or livestock health. Traditional human and livestock disease detection and management systems are based on diagnostic analyses of clinical samples. However, these systems fail to detect early warnings of public health threats at a wide population level and fail to predict outbreaks in a timely manner. Wastewater analysis, manure analysis, or polluted water analysis is equivalent to obtaining and analyzing a community-based urine and fecal sample of the representative sub-watershed. Monitoring temporal changes in pathogen concentration and diversity excreted in a sub-watershed allows early detection of outbreaks (critical moments for the onset of an outbreak). In addition, carefully designed spatial sampling will allow detection of locations where an outbreak may begin to develop and spread (critical locations for the onset of an outbreak). Modeling the fate of pathogens, including shedding rates, transport, growth and inactivation processes in the environmental, are critical for the effectiveness of the proposed method.
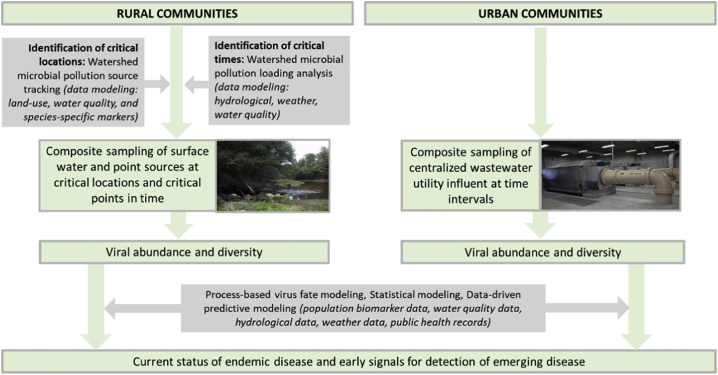
Fig. 4 presents an example of potential surveillance systems that could be implemented. Urban communities offer a convenient point of sampling at the influent of a wastewater treatment plant. Untreated wastewater can be considered as a population sample for the serviced community. Wastewater can therefore be used as an epidemiological tool to help identify potential viral outbreaks.
Fig. 4.
Proposed surveillance system. Notes: sampling and characterizing community wastewater, livestock manure, and wildlife waste represents a snapshot of the status of community human and animal health.
The goal of wastewater-based epidemiology is to sample community wastewater, or polluted water, to identify spikes in concentrations of excreted viruses before clinical cases are reported. Already employed in Europe to quantify illicit drug use in a population [88,89], wastewater-based epidemiology can also be applied to quantify the approximate concentrations of viruses in the population serviced by a wastewater treatment plant. Urban wastewater treatment plants that serve metropolitan areas sometimes have several interceptors at which wastewater is collected. Sampling at each interceptor and mapping each interceptor to the specific neighborhoods it serves can facilitate virus occurrence data collection representative of each serviced area of the city. Should viral concentrations be observed to be higher in one interceptor than the rest, the corresponding serviced area would therefore be of greater concern for a potential viral outbreak. Sampling in rural areas is more complex and necessitates the determination of where in the environment to sample, which can be based upon watershed modeling and microbial source tracking.
Several factors are of importance to attain reliable data when using wastewater-based epidemiology. Normalization of population is vital to ensure that a significant increase in viral concentration in a wastewater sample does not correspond to an increase in population in the serviced area. This can be performed with the quantification of biomarkers in the wastewater sample, substances that are natural excreted by humans in constant quantities. Other factors that need to be considered include shedding rate (the number of viruses excreted by infected humans) and natural degradation (the rate at which viruses degrade in the wastewater environment). Comparison and correlation with clinical data in the surrounding community is also a valuable tool to confirm the credibility of the methodology.
While wastewater-based epidemiology can primarily be utilized for the examination of waterborne viruses, it has the potential to be applied to non-waterborne viruses as well, provided those viruses can be detected in wastewater or human waste. As shown in Table 1, numerous viruses not typically classified as waterborne meet these criteria, such as influenza, coronavirus, herpesvirus, dengue virus, and Zika virus. In addition to the surveillance of wastewater, it is also prudent to perform surveillance of agricultural livestock and other domesticated animals. Other critical pathways identified in step one (see Fig. 3) could fall under this methodology as well.
5.3. Intervention approaches
The third and final step is to design intervention approaches. The goal is to design barriers to interrupt critical pathways at critical times and locations. These interventions may include: (1) sustainable engineering technologies for human and animal water/wastewater/waste management, (2) medical and veterinary interventions to manage infections, and (3) education of local communities and governance to modify human behavior, current practices and policy based on relationships between environmental health, human health and animal health.
The foremost intervention for waterborne viruses is that of drinking water and wastewater treatment facilities. Both types of treatment plants provide the most immediate barrier between a drinking water source and consumption and utilize several unit processes, such as filtration and disinfection, to ensure the removal of pathogens, including viruses, from water. Both types of treatment plants have been shown to be effective at reducing the concentrations of human viruses from influent to effluent, but it has also been shown that wastewater treatment plants may release viruses in effluent [14,75,[90], [91], [92], [93], [94], [95]]. There also exist interventions to prevent non-point-source pollution of water sources. One such intervention is that of watershed protection plans set by the states. Similarly, stormwater management is implemented in several states based on multiple strategies [[96], [97], [98], [99]].
Numerous policy measures in the United States and abroad can be considered examples of the permanent implementation of interventions. Since the Safe Drinking Water Act in 1974, a number of additional policies have been put into effect to strengthen water quality and prevent disease. The EPA sets Maximum Contaminant Levels (MCLs) for several contaminants, including viruses; drinking water treatment facilities are required to attain a 4-log reduction in viral concentration to meet the MCL for viruses [100]. Another example, the Groundwater Rule, was put into effect in 2006 and requires the regular surveillance of groundwater sources that are used for drinking water to ensure that MCLs for pathogens are met [101]. Internationally, the Guidelines for Drinking-water Quality put forth by WHO is used as a basis for the setting of regulations [102]. There still exists a need, however, for the regulation of animal waste products, especially in rural areas in which animal waste is determined to be a critical pathway for viral transport.
The modification of human behavior is also imperative to minimize the transmittance of viral disease along pathways in which interventions cannot be performed for reasons of cost, capability, or convenience. The primary method of altering behavior is education. This applies to the education of medical professionals, both doctors and veterinarians, and environmental professionals in One-Health approaches. It is also critical to educate the public to prevent situations in which people are leaving themselves vulnerable to transmission of disease. Especially in impoverished, high-risk areas, robust measures should be taken to educate the public on the concept of the critical pathways of transmission of viral disease.
6. Conclusions
Viral transmission involves complex systems that include interactions between humans, animals and the environment. Understanding the interactions between the involved human, animal and environmental systems, and the processes within each of the systems, is critical for efficient prevention and minimization of viral outbreaks. These systems vary in both spatial and temporal scales. For example, urban systems are different than rural systems, and wet weather is different than dry weather with regards to the potential for viral transmission. The most important step in the process of understanding water-related transmission is the identification of critical viral reservoirs and critical transport pathways in a certain environment at a certain time. Much of the One-Health based approaches to manage viral disease that have been utilized thus far have been responsive in order to control existing zoonotic outbreaks. This paper presents a three-step framework utilizing the One-Health approach to mitigate viral disease. First, identification of critical pathways is proposed, to determine the most important water-related pathways with which viruses are transported and transmitted throughout the environment. Next, surveillance systems are designed to monitor these critical pathways to identify the times and locations where viral outbreaks are occurring or likely to occur. Finally, water-related and other intervention approaches are implemented to mitigate the critical pathways and prevent the spread of viral disease. Identification and surveillance of critical pathways of potential exposure can allow early detection of outbreaks at a population level, which is a critical first step for prevention. While it involves the interconnectivity of human, animal, and environmental health, One-Health has still only primarily been embraced by the veterinary community. More deliberate efforts should be made to encourage environmental professionals to analyze the issues of viral disease through a One-Health lens. Only through the extensive participation of all related field stakeholders can One-Health truly reach its potential to mitigate viral disease.
Acknowledgments
Acknowledgments
We thank Dr. Reza Nassiri, Dr. Thomas Voice, Dr. David Long, and Dr. John Kaneene, Professors at Michigan State University, for their guidance and for inspiring and insightful discussions.
Competing interests
The authors declare that they have no competing interests.
References
- 1.One Health Commission What is One Health? 2018. https://www.onehealthcommission.org/en/why_one_health/what_is_one_health/
- 2.WHO . World Health Organization. 2018. The top 10 causes of death.http://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death [Google Scholar]
- 3.WHO . WHO. 2018. Disease outbreak news | 2017.http://www.who.int/csr/don/archive/year/2017/en/ [Google Scholar]
- 4.Nassiri R. vol. 3. 2019. Viruses without Borders: Deadly Outbreaks of the 21st Century, Archives of Infectious Diseases & Therapy; p. 4. [Google Scholar]
- 5.Iuliano A.D., Roguski K.M., Chang H.H., Muscatello D.J., Palekar R., Tempia S., Cohen C., Gran J.M., Schanzer D., Cowling B.J., Wu P., Kyncl J., Ang L.W., Park M., Redlberger-Fritz M., Yu H., Espenhain L., Krishnan A., Emukule G., van Asten L., Pereira da Silva S., Aungkulanon S., Buchholz U., Widdowson M.-A., Bresee J.S., Azziz-Baumgartner E., Cheng P.-Y., Dawood F., Foppa I., Olsen S., Haber M., Jeffers C., MacIntyre C.R., Newall A.T., Wood J.G., Kundi M., Popow-Kraupp T., Ahmed M., Rahman M., Marinho F., Sotomayor Proschle C.V., Vergara Mallegas N., Luzhao F., Sa L., Barbosa-Ramírez J., Sanchez D.M., Gomez L.A., Vargas X.B., Herrera aBetsy Acosta, Llanés M.J., Fischer T.K., Krause T.G., Mølbak K., Nielsen J., Trebbien R., Bruno A., Ojeda J., Ramos H., an der Heiden M., del Carmen Castillo Signor L., Serrano C.E., Bhardwaj R., Chadha M., Narayan V., Kosen S., Bromberg M., Glatman-Freedman A., Kaufman Z., Arima Y., Oishi K., Chaves S., Nyawanda B., Al-Jarallah R.A., Kuri-Morales P.A., Matus C.R., Corona M.E.J., Burmaa A., Darmaa O., Obtel M., Cherkaoui I., van den Wijngaard C.C., van der Hoek W., Baker M., Bandaranayake D., Bissielo A., Huang S., Lopez L., Newbern C., Flem E., Grøneng G.M., Hauge S., de Cosío F.G., de Moltó Y., Castillo L.M., Cabello M.A., von Horoch M., Medina Osis J., Machado A., Nunes B., Rodrigues A.P., Rodrigues E., Calomfirescu C., Lupulescu E., Popescu R., Popovici O., Bogdanovic D., Kostic M., Lazarevic K., Milosevic Z., Tiodorovic B., Chen M., Cutter J., Lee V., Lin R., Ma S., Cohen A.L., Treurnicht F., Kim W.J., Delgado-Sanz C., de mateo Ontañón S., Larrauri A., León I.L., Vallejo F., Born R., Junker C., Koch D., Chuang J.-H., Huang W.-T., Kuo H.-W., Tsai Y.-C., Bundhamcharoen K., Chittaganpitch M., Green H.K., Pebody R., Goñi N., Chiparelli H., Brammer L., Mustaquim D. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391:1285–1300. doi: 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO . WHO; 2018. WHO | Influenza update - 309.http://www.who.int/influenza/surveillance_monitoring/updates/latest_update_GIP_surveillance/en/ [Google Scholar]
- 7.WHO . WHO; 2018. WHO Mortality Database.http://www.who.int/healthinfo/mortality_data/en/ [Google Scholar]
- 8.Tate J.E., Burton A.H., Boschi-Pinto C., Steele A.D., Duque J., Parashar U.D. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect. Dis. 2012;12:136–141. doi: 10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- 9.Kirk M.D., Pires S.M., Black R.E., Caipo M., Crump J.A., Devleesschauwer B., Döpfer D., Fazil A., Fischer-Walker C.L., Hald T., Hall A.J., Keddy K.H., Lake R.J., Lanata C.F., Torgerson P.R., Havelaar A.H., Angulo F.J. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosch F.X., Manos M.M., Muñoz N., Sherman M., Jansen A.M., Peto J., Schiffman M.H., Moreno V., Kurman R., Shan K.V. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J. Natl. Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 11.Danesh J., Collins R., Peto R. Chronic infections and coronary heart disease: is there a link? Lancet. 1997;350:430–436. doi: 10.1016/S0140-6736(97)03079-1. [DOI] [PubMed] [Google Scholar]
- 12.Savès M., Chêne G., Ducimetière P., Leport C., Le Moal G., Amouyel P., Arveiler D., Ruidavets J.-B., Reynes J., Bingham A., Raffi F. Risk factors for coronary heart disease in patients treated for human immunodeficiency virus infection compared with the general population. Clin. Infect. Dis. 2003;37:292–298. doi: 10.1086/375844. [DOI] [PubMed] [Google Scholar]
- 13.Fabrizi F., Plaisier E., Saadoun D., Martin P., Messa P., Cacoub P. Hepatitis C virus infection, mixed Cryoglobulinemia, and kidney disease. Am. J. Kidney Dis. 2013;61:623–637. doi: 10.1053/j.ajkd.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 14.Xagoraraki I., Yin Z., Svambayev Z. Fate of viruses in water systems. J. Environ. Eng.-ASCE. 2014;140 [Google Scholar]
- 15.CDC Weekly U.S. Influenza Surveillance Report | Seasonal Influenza (Flu) | CDC. 2018. https://www.cdc.gov/flu/weekly/index.htm
- 16.Binder A.M. Human adenovirus surveillance — United States, 2003–2016. MMWR Morb. Mortal. Wkly Rep. 2017;66 doi: 10.15585/mmwr.mm6639a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CDC U.S. 2014 Surveillance Data for Viral Hepatitis | Statistics & Surveillance | Division of Viral Hepatitis | CDC. 2017. https://www.cdc.gov/hepatitis/statistics/2015surveillance/index.htm
- 18.Aliabadi N., Tate J.E., Haynes A.K., Parashar U.D. Sustained Decrease in Laboratory Detection of Rotavirus after Implementation of Routine Vaccination—United States, 2000–2014. MMWR Morb. Mortal. Wkly Rep. 2015;64:337–342. [PMC free article] [PubMed] [Google Scholar]
- 19.CDC Preliminary Maps & Data for 2017 | West Nile Virus | CDC. 2018. https://www.cdc.gov/westnile/statsmaps/preliminarymapsdata2017/index.html
- 20.Adams D.A. Summary of Notifiable infectious diseases and conditions — United States, 2015. MMWR Morb. Mortal. Wkly Rep. 2017;64 doi: 10.15585/mmwr.mm6453a1. [DOI] [PubMed] [Google Scholar]
- 21.Adams D.A., Jajosky R.A., Ajani U., Kriseman J., Sharp P., Onwen D.H., Schley A.W., Anderson W.J., Grigoryan A., Aranas A.E. Summary of notifiable diseases–United States, 2012. MMWR Morb. Mortal. Wkly Rep. 2014;61:1–121. [PubMed] [Google Scholar]
- 22.Adams D., Fullerton K., Jajosky R., Sharp P., Onweh D., Schley A., Anderson W., Faulkner A., Kugeler K. Summary of notifiable infectious diseases and conditions—United States, 2013. MMWR Morb. Mortal. Wkly Rep. 2015;62:1–122. doi: 10.15585/mmwr.mm6253a1. [DOI] [PubMed] [Google Scholar]
- 23.Adams D.A. Summary of notifiable infectious diseases and conditions—United States, 2014. MMWR Morb. Mortal. Wkly Rep. 2016;63 doi: 10.15585/mmwr.mm6354a1. [DOI] [PubMed] [Google Scholar]
- 24.Annual Statistics from the National Notifiable Diseases Surveillance System (NNDSS) 2018. https://wonder.cdc.gov/nndss/nndss_annual_tables_menu.asp
- 25.CDC National Outbreak Reporting System (NORS) Dashboard. 2018. https://wwwn.cdc.gov/norsdashboard/
- 26.CDC GIS FluView. 2018. https://gis.cdc.gov/grasp/fluview/fluportaldashboard.html
- 27.Cotruvo J.A., Dufour A., Rees G., Bartram J., Carr R., Cliver D.O., Craun G.F., Fayer R., Gannon V.P. Iwa Publishing; 2004. Waterborne Zoonoses. [Google Scholar]
- 28.Feachem R., McGarry M., Mara D. John Wiley & Sons Ltd. Baffins Lane; Chichester, Sussex: 1977. Water, wastes and health in hot climates. [Google Scholar]
- 29.Bradley D. 1977. Health Aspects of Water Supplies in Tropical Countries, Water, Wastes and Health in Hot Climates; pp. 3–17. [Google Scholar]
- 30.Lin K., Marr L.C. Aerosolization of Ebola virus surrogates in wastewater systems. Environ. Sci. Technol. 2017;51:2669–2675. doi: 10.1021/acs.est.6b04846. [DOI] [PubMed] [Google Scholar]
- 31.CDC Dengue. 2018. https://www.cdc.gov/dengue/index.html
- 32.CDC West Nile virus. 2018. https://www.cdc.gov/westnile/index.html
- 33.CDC Zika Virus, CDC. 2014. https://www.cdc.gov/zika/index.html
- 34.Heeney J.L. Zoonotic viral diseases and the frontier of early diagnosis, control and prevention. J. Intern. Med. 2006;260:399–408. doi: 10.1111/j.1365-2796.2006.01711.x. [DOI] [PubMed] [Google Scholar]
- 35.Grace D., Gilbert J., Randolph T., Kang'ethe E. The multiple burdens of zoonotic disease and an ecohealth approach to their assessment. Trop. Anim. Health Prod. 2012;44:67–73. doi: 10.1007/s11250-012-0209-y. [DOI] [PubMed] [Google Scholar]
- 36.CDC Adenovirus | Home. 2017. https://www.cdc.gov/adenovirus/index.html
- 37.CDC Hepatitis A Information | Division of Viral Hepatitis. 2016. http://www.cdc.gov/hepatitis/hav/
- 38.CDC Hepatitis E Information | Division of Viral Hepatitis. 2016. http://www.cdc.gov/hepatitis/hev/
- 39.CDC Norovirus | Home. 2018. https://www.cdc.gov/norovirus/index.html
- 40.CDC Rotavirus | Home | Gastroenteritis. 2016. http://www.cdc.gov/rotavirus/
- 41.CDC Yellow Fever. 2016. https://www.cdc.gov/yellowfever/index.html
- 42.CDC Ebola Hemorrhagic Fever. 2018. https://www.cdc.gov/vhf/ebola/index.html
- 43.CDC Chikungunya virus. 2017. https://www.cdc.gov/chikungunya/index.html
- 44.CDC Rift Valley Fever. 2018. https://www.cdc.gov/vhf/rvf/index.html
- 45.CDC Hantavirus. 2018. https://www.cdc.gov/hantavirus/index.html
- 46.CDC Hendra Virus Disease. 2018. https://www.cdc.gov/vhf/hendra/index.html
- 47.CDC Nipah Virus (NiV) 2018. https://www.cdc.gov/vhf/nipah/index.html
- 48.Ambert-Balay K., Lorrot M., Bon F., Giraudon H., Kaplon J., Wolfer M., Lebon P., Gendrel D., Pothier P. Prevalence and genetic diversity of Aichi virus strains in stool samples from community and hospitalized patients. J. Clin. Microbiol. 2008;46:1252–1258. doi: 10.1128/JCM.02140-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haramoto E., Kitajima M., Otagiri M. Development of a reverse transcription-quantitative PCR assay for detection of Salivirus/Klassevirus. Appl. Environ. Microbiol. 2013;79:3529–3532. doi: 10.1128/AEM.00132-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oka T., Katayama K., Hansman G.S., Kageyama T., Ogawa S., Wu F., White P.A., Takeda N. Detection of human sapovirus by real-time reverse transcription-polymerase chain reaction. J. Med. Virol. 2006;78:1347–1353. doi: 10.1002/jmv.20699. [DOI] [PubMed] [Google Scholar]
- 51.Siebrasse E.A., Reyes A., Lim E.S., Zhao G., Mkakosya R.S., Manary M.J., Gordon J.I., Wang D. Identification of MW Polyomavirus, a novel Polyomavirus in human stool. J. Virol. 2012;86:10321–10326. doi: 10.1128/JVI.01210-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hino S., Miyata H. Torque teno virus (TTV): current status. Rev. Med. Virol. 2006;17:45–57. doi: 10.1002/rmv.524. [DOI] [PubMed] [Google Scholar]
- 53.USDA APHIS | Status of Reportable Diseases in the United States. 2018. https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/monitoring-and-surveillance/sa_nahss/status-reportable-disease-us
- 54.Tischer B.K., Osterrieder N. Herpesviruses—a zoonotic threat? Vet. Microbiol. 2010;140:266–270. doi: 10.1016/j.vetmic.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xie T., Anderson B., Daramragchaa U., Chuluunbaatar M., Gray G. A review of evidence that equine influenza viruses are zoonotic. Pathogens. 2016;5:50. doi: 10.3390/pathogens5030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haas B., Bohm R., Strauch D. Inactivation of viruses in liquid manure. Rev. Sci. Tech. 1995;14:435–445. doi: 10.20506/rst.14.2.844. [DOI] [PubMed] [Google Scholar]
- 57.Whitley R.J., Gnann J.W. Viral encephalitis: familiar infections and emerging pathogens. Lancet. 2002;359:507–513. doi: 10.1016/S0140-6736(02)07681-X. [DOI] [PubMed] [Google Scholar]
- 58.Lu H., Castro A.E., Pennick K., Liu J., Yang Q., Dunn P., Weinstock D., Henzler D. Survival of avian influenza virus H7N2 in SPF chickens and their environments. Avian Dis. 2003;47:1015–1021. doi: 10.1637/0005-2086-47.s3.1015. [DOI] [PubMed] [Google Scholar]
- 59.Guan J., Chan M., Grenier C., Wilkie D.C., Brooks B.W., Spencer J.L. Survival of avian influenza and Newcastle disease viruses in compost and at ambient temperatures based on virus isolation and real-time reverse transcriptase PCR. Avian Dis. 2009;53:26–33. doi: 10.1637/8381-062008-Reg.1. [DOI] [PubMed] [Google Scholar]
- 60.Strauch D. Survival of pathogenic micro-organisms and parasites in excreta, manure and sewage sludge. Rev. Sci. Tech. 1991;10:813–846. doi: 10.20506/rst.10.3.565. [DOI] [PubMed] [Google Scholar]
- 61.Swayne D.E., King D.J. vol. 222. 2003. Avian Influenza and Newcastle Disease; p. 7. [DOI] [PubMed] [Google Scholar]
- 62.Chomel B.B., Belotto A., Meslin F.-X. Wildlife, exotic pets, and emerging Zoonoses. Emerg. Infect. Dis. 2007;13:6–11. doi: 10.3201/eid1301.060480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Short K.R., Richard M., Verhagen J.H., van Riel D., Schrauwen E.J.A., van den Brand J.M.A., Mänz B., Bodewes R., Herfst S. One health, multiple challenges: the inter-species transmission of influenza a virus. One Health. 2015;1:1–13. doi: 10.1016/j.onehlt.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.USDA APHIS | Swine Health Monitoring Surveillance. 2018. https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/animal-disease-information/swine-disease-information/ct_swine_health_monitoring_surveillance
- 65.USDA APHIS | CT_Equine_Herpes_Virus_type_1. 2018. https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/animal-disease-information/horse-disease-information/sa_herpes_virus/ct_equine_herpes_virus_type_1
- 66.USDA APHIS | Equine West Nile Virus Case Reporting and Surveillance Information. 2018. https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/animal-disease-information/horse-disease-information/sa_west_nile_virus/ct_wnv_index
- 67.WHO . World Health Organization. 2009. Pandemic influenza preparedness and response: a WHO guidance document. [PubMed] [Google Scholar]
- 68.Lago P.M., Gary H.E., Pérez L.S., Cáceres V., Olivera J.B., Puentes R.P., Corredor M.B., Jímenez P., Pallansch M.A., Cruz R.G. Poliovirus detection in wastewater and stools following an immunization campaign in Havana, Cuba. Int. J. Epidemiol. 2003;32:772–777. doi: 10.1093/ije/dyg185. [DOI] [PubMed] [Google Scholar]
- 69.Fumian T.M., Gagliardi Leite J.P., Rose T.L., Prado T., Miagostovich M.P. One year environmental surveillance of rotavirus specie a (RVA) genotypes in circulation after the introduction of the Rotarix® vaccine in Rio de Janeiro, Brazil. Water Res. 2011;45:5755–5763. doi: 10.1016/j.watres.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 70.Kim T.H., Johnstone J., Loeb M. Vaccine herd effect. Scand. J. Infect. Dis. 2011;43:683–689. doi: 10.3109/00365548.2011.582247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang L.-F., Crameri G. Emerging zoonotic viral diseases. Rev. Sci. Tech. Off. Int. Epizoot. 2014;33:569–581. doi: 10.20506/rst.33.2.2311. [DOI] [PubMed] [Google Scholar]
- 72.Benelli G. Research in mosquito control: current challenges for a brighter future. Parasitol. Res. 2015;114:2801–2805. doi: 10.1007/s00436-015-4586-9. [DOI] [PubMed] [Google Scholar]
- 73.Rostal M.K., Ross N., Machalaba C., Cordel C., Paweska J.T., Karesh W.B. Benefits of a one health approach: an example using Rift Valley fever. One Health. 2018;5:34–36. doi: 10.1016/j.onehlt.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Musoke D., Ndejjo R., Atusingwize E., Halage A.A. The role of environmental health in one health: a Uganda perspective. One Health. 2016;2:157–160. doi: 10.1016/j.onehlt.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Simmons F.J., Xagoraraki I. Release of infectious human enteric viruses by full-scale wastewater utilities. Water Res. 2011;45:3590–3598. doi: 10.1016/j.watres.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 76.Penakalapati G., Swarthout J., Delahoy M.J., McAliley L., Wodnik B., Levy K., Freeman M.C. Exposure to animal Feces and human health: a systematic review and proposed research priorities. Environ. Sci. Technol. 2017;51:11537–11552. doi: 10.1021/acs.est.7b02811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sinclair R.G., Jones E.L., Gerba C.P. Viruses in recreational water-borne disease outbreaks: a review. J. Appl. Microbiol. 2009;107:1769–1780. doi: 10.1111/j.1365-2672.2009.04367.x. [DOI] [PubMed] [Google Scholar]
- 78.Fong T.-T., Mansfield L.S., Wilson D.L., Schwab D.J., Molloy S.L., Rose J.B. Massive microbiological groundwater contamination associated with a waterborne outbreak in Lake Erie, south Bass Island, Ohio. Environ. Health Perspect. 2007;115:856–864. doi: 10.1289/ehp.9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morens DavidM., Zweighaft RonaldM., Vernon Thomas M., Gary G.W., Eslien Jack J., Wood Bruce T., Holman Robert C., Dolin R. A waterborne outbreak of gastroenteritis with secondary person-to-person spread: association with a viral agent. Lancet. 1979;313:964–966. doi: 10.1016/s0140-6736(79)91734-3. [DOI] [PubMed] [Google Scholar]
- 80.Borchardt M.A., Bradbury K.R., Alexander E.C., Kolberg R.J., Alexander S.C., Archer J.R., Braatz L.A., Forest B.M., Green J.A., Spencer S.K. Norovirus outbreak caused by a new septic system in a dolomite aquifer. Groundwater. 2011;49:85–97. doi: 10.1111/j.1745-6584.2010.00686.x. [DOI] [PubMed] [Google Scholar]
- 81.Wong K., Onan B.M., Xagoraraki I. Quantification of enteric viruses, pathogen indicators, and Salmonella bacteria in class B anaerobically digested biosolids by culture and molecular methods. Appl. Environ. Microbiol. 2010;76:6441–6448. doi: 10.1128/AEM.02685-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Venglovsky J., Martinez J., Placha I. Hygienic and ecological risks connected with utilization of animal manures and biosolids in agriculture. Livest. Sci. 2006;102:197–203. doi: 10.1016/j.livsci.2006.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ferguson C.M., Charles K., Deere D.A. Quantification of microbial sources in drinking-water catchments. Crit. Rev. Environ. Sci. Technol. 2008;39:1–40. [Google Scholar]
- 84.Mauer W.A., Kaneene J.B. Integrated human-animal disease surveillance. Emerg. Infect. Dis. 2005;11:1490–1491. doi: 10.3201/eid1109.050180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Curriero F.C., Patz J.A., Rose J.B., Lele S. The association between extreme precipitation and waterborne disease outbreaks in the United States, 1948–1994. Am. J. Public Health. 2001;91:1194–1199. doi: 10.2105/ajph.91.8.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gottdenker N.L., Streicker D.G., Faust C.L., Carroll C.R. Anthropogenic land use change and infectious diseases: a review of the evidence. EcoHealth. 2014;11:619–632. doi: 10.1007/s10393-014-0941-z. [DOI] [PubMed] [Google Scholar]
- 87.Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L., Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Daughton C.G., Jones-Lepp T.L. American Chemical Society; Washington, DC: 2001. Pharmaceuticals and Personal Care Products in the Environment: Scientific and Regulatory Issues.http://library.wur.nl/WebQuery/clc/1726161 [Google Scholar]
- 89.Zuccato E., Chiabrando C., Castiglioni S., Bagnati R., Fanelli R. Estimating community drug abuse by wastewater analysis. Environ. Health Perspect. 2008;116:1027–1032. doi: 10.1289/ehp.11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haramoto E., Katayama H., Oguma K., Ohgaki S. Quantitative analysis of human enteric adenoviruses in aquatic environments. J. Appl. Microbiol. 2007;103:2153–2159. doi: 10.1111/j.1365-2672.2007.03453.x. [DOI] [PubMed] [Google Scholar]
- 91.Hewitt J., Leonard M., Greening G.E., Lewis G.D. Influence of wastewater treatment process and the population size on human virus profiles in wastewater. Water Res. 2011;45:6267–6276. doi: 10.1016/j.watres.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 92.Katayama H., Haramoto E., Oguma K., Yamashita H., Tajima A., Nakajima H., Ohgaki S. One-year monthly quantitative survey of noroviruses, enteroviruses, and adenoviruses in wastewater collected from six plants in Japan. Water Res. 2008;42:1441–1448. doi: 10.1016/j.watres.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 93.Kuo D.H.-W., Simmons F.J., Blair S., Hart E., Rose J.B., Xagoraraki I. Assessment of human adenovirus removal in a full-scale membrane bioreactor treating municipal wastewater. Water Res. 2010;44:1520–1530. doi: 10.1016/j.watres.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 94.Simmons F.J., Kuo D.H.-W., Xagoraraki I. Removal of human enteric viruses by a full-scale membrane bioreactor during municipal wastewater processing. Water Res. 2011;45:2739–2750. doi: 10.1016/j.watres.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 95.O'Brien E., Nakyazze J., Wu H., Kiwanuka N., Cunningham W., Kaneene J.B., Xagoraraki I. Viral diversity and abundance in polluted waters in Kampala, Uganda. Water Res. 2017;127:41–49. doi: 10.1016/j.watres.2017.09.063. [DOI] [PubMed] [Google Scholar]
- 96.Barbosa A.E., Fernandes J.N., David L.M. Key issues for sustainable urban stormwater management. Water Res. 2012;46:6787–6798. doi: 10.1016/j.watres.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 97.US EPA National Menu of Best Management Practices (BMPs) for Stormwater, US EPA. 2015. https://www.epa.gov/npdes/national-menu-best-management-practices-bmps-stormwater
- 98.Roy A.H., Wenger S.J., Fletcher T.D., Walsh C.J., Ladson A.R., Shuster W.D., Thurston H.W., Brown R.R. Impediments and solutions to sustainable, watershed-scale urban Stormwater management: lessons from Australia and the United States. Environ. Manag. 2008;42:344–359. doi: 10.1007/s00267-008-9119-1. [DOI] [PubMed] [Google Scholar]
- 99.Burns M.J., Fletcher T.D., Walsh C.J., Ladson A.R., Hatt B.E. Hydrologic shortcomings of conventional urban stormwater management and opportunities for reform. Landsc. Urban Plan. 2012;105:230–240. [Google Scholar]
- 100.US EPA National Primary Drinking Water Regulations, US EPA. 2015. https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations
- 101.US EPA Ground Water Rule, US EPA. 2015. https://www.epa.gov/dwreginfo/ground-water-rule
- 102.World Health Organization . 2017. Guidelines for Drinking-Water Quality. [Google Scholar]