Abstract
Nitrate regulation of root stem cell activity is auxin-dependent.
Dear Editor,
Nitrogen is a vital macronutrient, and the main form of inorganic nitrogen that plants take up from well-aerated soil is nitrate (NO3− [N]; O’Brien et al., 2016; Wang et al., 2018). Beyond its role as a nitrogen source, NO3− acts as a signaling molecule during various developmental processes, such as the determination of root architecture, a process directly influenced by root stem cell differentiation (Perilli et al., 2012; Wierzba and Tax, 2013; O’Brien et al., 2016; Wang et al., 2018). However, little is known about the molecular mechanism underlying the adaptive responses of root stem cells to NO3− limitation.
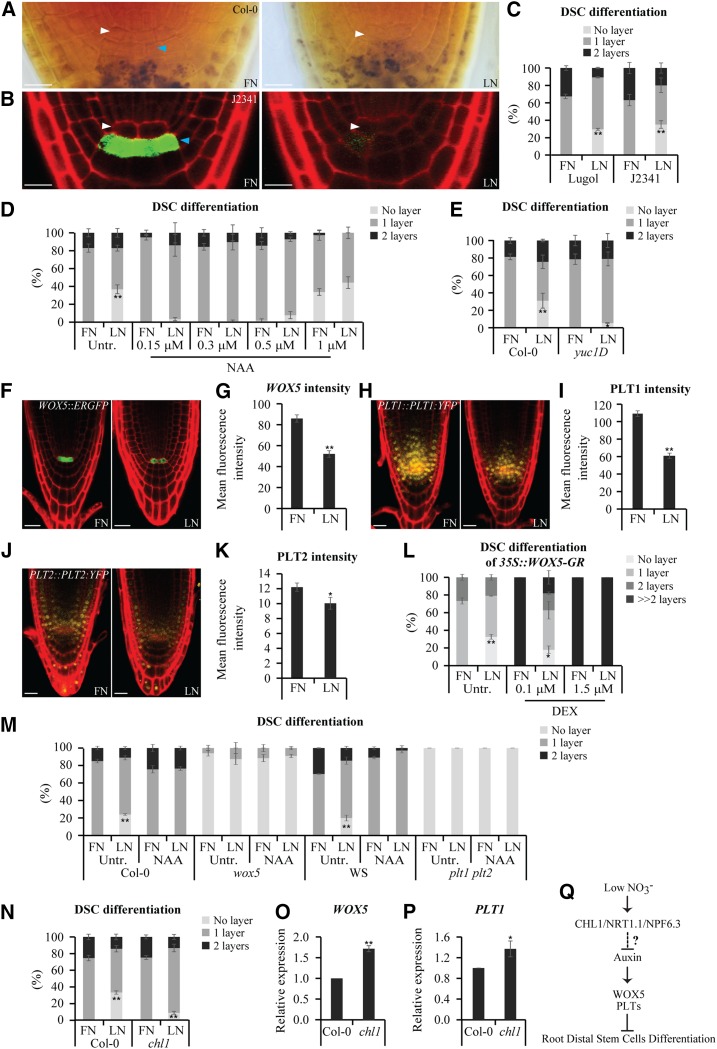
To explore how root stem cells respond to NO3− limitation, we compared the differentiation of distal stem cells (DSC) in roots (Supplemental Methods S1) under normal or low external NO3− levels. In general, most roots (∼67%) of 6-d-old wild-type Arabidopsis (Arabidopsis thaliana) seedlings grown on medium with 10 mM NO3− (full N) contained only a single DSC layer, as indicated by Lugol staining, which stains only differentiated starch-containing columella cells in the roots (Fig. 1, A and C). Germination on medium with 0.05 mM NO3− (low N) promoted DSC differentiation, as evidenced by the absence of DSC in ∼30% of seedlings (Fig. 1, A and C). In addition, low N resulted in the absence of expression of the DSC-specific marker J2341 in ∼35% of seedlings, a feature not observed in the wild-type seedlings grown on full N (Fig. 1, B and C), confirming the results of Lugol staining.
Figure 1.
Low nitrate influences stem cell activity. A to C, DSC differentiation in roots as revealed by Lugol staining (A) and J2341 expression (B). Six-day-old wild-type seedlings germinated on medium with 10 mM NO3− (full N; FN) or 0.05 mM NO3− (low N; LN). Most roots of seedlings grown on full N had typical single-tiered DSC, whereas low N induced complete DSC differentiation in ∼30% of seedlings (A). Lugol staining and J2341 expression to monitor DSC differentiation (A and B) were quantified in C (n ≥ 55 roots). White and blue arrowheads mark the QC and DSC, respectively. Lugol-stained starch indicates differentiated columella cells. Nondifferentiated DSC is present between the QC and starch granules. Scale bars = 10 μm. D and E, Effect of NAA on DSC differentiation in roots. Treatment with NAA largely rescued low-N-enhanced DSC differentiation in a dose-dependent manner (D; n ≥ 50 roots). Note that yuc1D, which contains high levels of endogenous auxin, showed significantly reduced columella differentiation under nitrate-limited conditions (E; n ≥ 50 roots). F to K, Expression analysis of WOX5, PLT1, and PLT2 in the root tips. Low N repressed the expression of WOX5::ERGFP (F), PLT1::PLT1:YFP (H), and PLT2::PLT2:YFP (J). The fluorescence intensity was quantified in G, I, and K. Scale bars = 20 μm. L and M, Role of WOX5 and PLTs in low-N-mediated DSC differentiation. The number of undifferentiated DSC increased in 35S::WOX5-GR roots that were germinated on full N with increasing amounts of DEX. When WOX5 expression was induced by 0.1 μM DEX treatment, we observed less DSC differentiation in the roots grown on low N compared with that of the non-DEX-treated plants. However, low-N-promoted DSC differentiation was not observed when WOX5 expression was strongly induced by 1.5 μM DEX treatment (L). The overexpression of WOX5 severely inhibited root DSC differentiation, as revealed by the presence of multiple tiers of DSC cells (L). The wox5 and plt1 plt2 seedlings grown under both full-N and low-N conditions exhibit more DSC differentiation compared with that of the wild type. DSC differentiation was suppressed in the wild-type roots grown on low-N medium supplemented with 0.3 μM NAA, but not in wox5 or plt1 plt2 roots (M). DSC differentiation was quantified in L and M (n ≥ 50 roots). N, Differentiation status of DSC in chl1-12 mutant roots. chl1-12 DSC was less sensitive to low N as compared with that of the wild-type seedlings (n ≥ 77 roots). O and P, Increased WOX5 and PLT1 transcription in chl1-12 mutant on low N as detected by reverse transcription quantitative PCR analysis. The expression of these genes in the wild type was set to 1. Q, A schematic model of regulation of the root DSC activity in response to low N stress. Low N promotes CHL1/NRT1.1/NPF6.3 protein level and thus potentially represses auxin accumulation, which in turn inhibits WOX5 and PLTs transcription, ultimately stimulating DSC differentiation in the root tips. Activation and repression are represented by arrows and blunt-end line, respectively. Dashed blunt-end line with the question mark represents CHL1/NRT1.1/NPF6.3-mediated potential suppression of auxin accumulation in the primary root tip. Error bars in C–E and L–P represent ±se of three replicates. Error bars in G, I, and K represent ±se from at least 18 seedlings and results are representative of three independent experiments. * P < 0.05; ** P < 0.01 Student’s t test. Representative images are shown.
As reported, auxin signaling regulates DSC differentiation (Ding and Friml, 2010) and provision of NO3− increases the signal from DR5::GUS expression in the root tips (Vidal et al., 2010). Consistent with these observations, we found that low N suppressed auxin accumulation, as revealed by expression analysis of DR5rev::GFP and DII-VENUS in transgenic seedlings that had been transferred from full N to low N and grown for 2 d (Supplemental Fig. S1, A and B). Accordingly, DSC were absent in ∼21% of wild-type seedlings under the same experimental conditions, specifically following 2 d of low-N treatment (Supplemental Fig. S1C). These findings suggest that a gradual reduction in auxin levels is closely related to enhanced DSC differentiation in response to low N.
We next examined the effects of various concentrations of auxin on seedlings and found that increased DSC differentiation in response to low N was largely suppressed in seedlings grown on medium supplemented with 0.15, 0.3, or 0.5 μM 1-naphthalene acetic acid (NAA; Fig. 1D). In line with a previous report, Ding and Friml (2010), 1 μM NAA promoted DSC differentiation on full N (Fig. 1D), indicating that auxin restored normal DSC differentiation under low-N conditions in a dose-dependent manner. Similarly, treatment with the auxin biosynthesis inhibitor L-kynurenine (Kyn; He et al., 2011; Supplemental Fig. S1, D and E) in low-N medium enhanced DSC differentiation (Supplemental Fig. S1H). The auxin precursor L-tryptophan (Trp; Jing et al., 2009; Supplemental Fig. S1, F and G) diminished DSC differentiation under low-N conditions (Supplemental Fig. S1I). In addition, root tips with decreased auxin activity mediated by Kyn treatment showed more undifferentiated DSC on full N (Ding and Friml, 2010; Supplemental Fig. S1H). As expected, the increased DSC differentiation in response to low N was also largely rescued in yuc1D (Fig. 1E), a dominant mutant with excess endogenous auxin (Cheng et al., 2006). These findings suggest that enhanced DSC differentiation in plants on low N is caused by reduced auxin levels in the root tips.
The homeodomain transcription factor WUSCHEL-RELATED HOMEOBOX 5 (WOX5) and the APETALA2-type PLETHORA (PLT) transcription factors are involved in maintaining root stem cell activity (Aida et al., 2004; Sarkar et al., 2007; Ding and Friml, 2010; Wierzba and Tax, 2013). WOX5::ERGFP, PLT1::PLT1:YFP, and PLT2::PLT2:YFP signals were strongly reduced in the roots under low-N conditions (Fig. 1, F to K), suggesting that WOX5 and PLTs might be involved in the effects of low N on promoting DSC differentiation. WOX5, PLT1, and PLT2 were up-regulated after the addition of NAA to low-N medium (Supplemental Fig. S1, J to L). We then examined DSC differentiation in the roots of a dexamethasone (DEX)-inducible 35S::WOX5-GR line. Under treatment with a low level of DEX (0.1 μM), DSC differentiation was completely delayed in 35S::WOX5-GR seedlings grown on full N and the roots grown on low N showed less DSC differentiation compared with that in non-DEX-treated controls (Fig. 1L). However, as WOX5 expression gradually increased in response to a higher level of DEX (1.5 μM), low-N treatment no longer influenced DSC differentiation (Fig. 1L). These observations indicate that WOX5 helps control low-N-mediated DSC differentiation. Moreover, under low-N conditions, the strong promotion of DSC differentiation resulting from the loss-of-function mutations wox5 and plt1 plt2 could not be rescued by exogenous auxin application (Fig. 1M). Instead, auxin treatment effectively suppressed the increased DSC differentiation in the wild-type roots grown on low N (Fig. 1M). In summary, our expression and genetic analyses suggest that WOX5 and the PLTs act downstream of auxin in the pathway that inhibit DSC differentiation in response to low N.
Next, we investigated the molecular mechanism by which low N regulates DSC activity. The known nitrate signaling pathway involves the CHL1/NRT1.1/NPF6.3 transceptor (transporter/receptor; O’Brien et al., 2016; Wang et al., 2018). Nitrate provision represses CHL1/NRT1.1/NPF6.3 protein expression and the CHL1/NRT1.1/NPF6.3 transceptor inhibits auxin accumulation in the absence of nitrate in plant roots (Krouk et al., 2010; Bouguyon et al., 2016). Notably, the chl1-12 mutant, defective in nitrate responsiveness (Medici et al., 2015), was less sensitive to low N than the wild type in terms of DSC differentiation (Fig. 1N). Furthermore, as detected by reverse transcription quantitative PCR analysis (Supplemental Methods S1), the lack of the CHL1/NRT1.1/NPF6.3 gene promoted the transcription of WOX5 and PLT1 under low-N conditions in the chl1-12 mutant (Fig. 1, O and P). These observations indicate that CHL1/NRT1.1/NPF6.3 is an important mediator of low-N-promoted DSC differentiation.
Our work provides insights into the mechanism underlying how low-N stress mediates root stem cell activity. Low N restricts auxin distribution in the root tips via the CHL1/NRT1.1/NPF6.3-mediated nitrate signaling pathway, which subsequently represses the expression of WOX5 and PLTs, ultimately promoting DSC differentiation in the primary root tip (Fig. 1Q). Auxin has been shown to promote DSC differentiation by repressing WOX5 and PLT activity under normal growth conditions (Ding and Friml, 2010). Our study highlights an antagonistic regulatory mechanism of DSC maintenance or differentiation in response to low-N stress, which improves the ability of the root to adapt to NO3− deficiency, thereby underlying a plant survival strategy.
Supplemental Material
The following supplemental materials are available.
Supplemental Figure S1. Low nitrate influences stem cell activity.
Supplemental Methods S1. Detailed information on experimental procedures.
Acknowledgments
We thank Dr. Zhaojun Ding (Shandong University, China) and Hongwei Guo (Southern University of Science and Technology, China) for sharing research materials. We are grateful to Wei Xuan (Nanjing Agricultural University) and Yan Guo (China Agricultural University, China) for helpful discussion.
Footnotes
This work was supported by a grant from the National Natural Science Foundation of China (31370309), the 1000-Talents Plan from China for Young Researchers, the Fundamental Research Funds for the Central Universities, and partly supported by the open funds of the State Key Laboratory of Crop Genetics and Germplasm Enhancement (ZW201804).
Articles can be viewed without a subscription.
References
- Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, Nussaume L, Noh YS, Amasino R, Scheres B (2004) The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 119: 109–120 [DOI] [PubMed] [Google Scholar]
- Bouguyon E, Perrine-Walker F, Pervent M, Rochette J, Cuesta C, Benkova E, Martinière A, Bach L, Krouk G, Gojon A, et al. (2016) Nitrate controls root development through posttranscriptional regulation of the NRT1.1/NPF6.3 transporter/sensor. Plant Physiol 172: 1237–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Dai X, Zhao Y (2006) Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev 20: 1790–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Friml J (2010) Auxin regulates distal stem cell differentiation in Arabidopsis roots. Proc Natl Acad Sci USA 107: 12046–12051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Brumos J, Li H, Ji Y, Ke M, Gong X, Zeng Q, Li W, Zhang X, An F, et al. (2011) A small-molecule screen identifies L-kynurenine as a competitive inhibitor of TAA1/TAR activity in ethylene-directed auxin biosynthesis and root growth in Arabidopsis. Plant Cell 23: 3944–3960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y, Cui D, Bao F, Hu Z, Qin Z, Hu Y (2009) Tryptophan deficiency affects organ growth by retarding cell expansion in Arabidopsis. Plant J 57: 511–521 [DOI] [PubMed] [Google Scholar]
- Krouk G, Lacombe B, Bielach A, Perrine-Walker F, Malinska K, Mounier E, Hoyerova K, Tillard P, Leon S, Ljung K, et al. (2010) Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev Cell 18: 927–937 [DOI] [PubMed] [Google Scholar]
- Medici A, Marshall-Colon A, Ronzier E, Szponarski W, Wang R, Gojon A, Crawford NM, Ruffel S, Coruzzi GM, Krouk G (2015) AtNIGT1/HRS1 integrates nitrate and phosphate signals at the Arabidopsis root tip. Nat Commun 6: 6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JA, Vega A, Bouguyon E, Krouk G, Gojon A, Coruzzi G, Gutiérrez RA (2016) Nitrate transport, sensing, and responses in plants. Mol Plant 9: 837–856 [DOI] [PubMed] [Google Scholar]
- Perilli S, Di Mambro R, Sabatini S (2012) Growth and development of the root apical meristem. Curr Opin Plant Biol 15: 17–23 [DOI] [PubMed] [Google Scholar]
- Sarkar AK, Luijten M, Miyashima S, Lenhard M, Hashimoto T, Nakajima K, Scheres B, Heidstra R, Laux T (2007) Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 446: 811–814 [DOI] [PubMed] [Google Scholar]
- Vidal EA, Araus V, Lu C, Parry G, Green PJ, Coruzzi GM, Gutiérrez RA (2010) Nitrate-responsive miR393/AFB3 regulatory module controls root system architecture in Arabidopsis thaliana. Proc Natl Acad Sci USA 107: 4477–4482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YY, Cheng YH, Chen KE, Tsay YF (2018) Nitrate transport, signaling, and use efficiency. Annu Rev Plant Biol 69: 85–122 [DOI] [PubMed] [Google Scholar]
- Wierzba MP, Tax FE (2013) Notes from the underground: Receptor-like kinases in Arabidopsis root development. J Integr Plant Biol 55: 1224–1237 [DOI] [PubMed] [Google Scholar]