Comparative analysis of cold response in Pooideae species shows that cold acclimation is common in Pooideae but that this adaptation has largely evolved independently in different tribes.
Abstract
The grass subfamily Pooideae dominates the grass floras in cold temperate regions and has evolved complex physiological adaptations to cope with extreme environmental conditions like frost, winter, and seasonality. One such adaptation is cold acclimation, wherein plants increase their frost tolerance in response to gradually falling temperatures and shorter days in the autumn. However, understanding how complex traits like cold acclimation evolve remains a major challenge in evolutionary biology. Here, we investigated the evolution of cold acclimation in Pooideae and found that a phylogenetically diverse set of Pooideae species displayed cold acclimation capacity. However, comparing differential gene expression after cold treatment in transcriptomes of five phylogenetically diverse species revealed widespread species-specific responses of genes with conserved sequences. Furthermore, we studied the correlation between gene family size and number of cold-responsive genes as well as between selection pressure on coding sequences of genes and their cold responsiveness. We saw evidence of protein-coding and regulatory sequence evolution as well as the origin of novel genes and functions contributing toward evolution of a cold response in Pooideae. Our results reflect that selection pressure resulting from global cooling must have acted on already diverged lineages. Nevertheless, conservation of cold-induced gene expression of certain genes indicates that the Pooideae ancestor may have possessed some molecular machinery to mitigate cold stress. Evolution of adaptations to seasonally cold climates is regarded as particularly difficult. How Pooideae evolved to transition from tropical to temperate biomes sheds light on how complex traits evolve in the light of climate changes.
Frost is one of the most dramatic abiotic stresses a plant can experience, and exposure to frost is a limiting factor for many species to diversify in temperate and arctic regions. Only a few ancestrally tropical angiosperm lineages have colonized temperate biomes (Judd et al., 1994; Wiens and Donoghue, 2004; Kerkhoff et al., 2014). These lineages represent several independent transitions from tropical to temperate environments and represent a variety of evolutionary adaptations to both unpredictable, short incidents of episodic frost and predictable periods of periodic frosts (i.e. winter). Today’s cold climate is the result of gradual cooling over the last 50 million years, and the temperate biomes originated relatively recently in Earth’s history when the global climate started to cool in the late Eocene (Potts and Behrensmeyer, 1992; Morley, 2000; Zachos et al., 2001; Fine and Ree, 2006; Eldrett et al., 2009; Liu et al., 2009; Strömberg, 2011). Around the Eocene-Oligocene (E-O) transition 34 million years ago, global temperatures fell dramatically (Pound and Salzmann, 2017). Throughout the Oligocene the temperatures continued to drop, and together with increased seasonality (Zachos et al., 2001; Eldrett et al., 2009) this triggered the greater expansion of temperate biomes. The successful colonizers of the emerging temperate climates faced gradually more severe environmental stresses and evolved adaptations to frost, increased temperature seasonality, and short growing seasons (Zachos et al., 2001; Eldrett et al., 2009; Mudelsee et al., 2014). The fact that only a restricted number of plant lineages have transitioned into the temperate region suggests that it is challenging to evolve the coordinated set of physiological changes needed to withstand low temperatures (Donoghue, 2008).
The capacity for surviving environments with subzero temperatures depends on the acquisition of frost tolerance. Most importantly, plants need to maintain the integrity of cell membranes to avoid osmotic stress during prolonged freezing (Thomashow, 1999). To endure predictable, periodic frost, plants must have evolved to integrate both phenology and complex physiological adjustments. Through a process called cold acclimation, freezing tolerance is acquired through a range of physiological changes governed by diverse molecular pathways. These changes result in an increase in the sugar content of cells, change in lipid composition of membranes, and synthesis of antifreeze proteins (Janská et al., 2010; Preston and Sandve, 2013). In addition, low nonfreezing temperatures may affect plant cells by decreasing metabolic turnover rates, inhibiting the photosynthetic machinery, and decreasing stability of biomolecules (e.g. lipid membranes; Sandve et al., 2011; Crosatti et al., 2013). Temperate and arctic plants use the gradually lower temperature and daylength in the autumn as cues to initiate cold acclimation.
In the grass family (Poaceae), the subfamily Pooideae occupies the coldest climate space (Edwards and Smith, 2010). Except for a few hundred species in early diverging Pooideae tribes, most of the approximately 4,200 Pooideae species belong to the species-rich core Pooideae clade (Davis and Soreng, 1993; Soreng and Davis, 1998). The closest sister group to the core Pooideae is the tribe Brachypodieae, which contains the model grass Brachypodium distachyon (Soreng et al., 2015). Because core Pooideae contain economically important species like wheat (Triticum aestivum) and barley (Hordeum vulgare) as well as several forage grasses like ryegrass (Lolium perenne), there is extensive knowledge about the physiological and molecular mechanisms underlying frost tolerance; however, this knowledge is derived from only a limited number of core Pooideae species (Thomashow, 1999; Sandve et al., 2008, 2011; Galiba et al., 2009; Preston and Sandve, 2013; Fjellheim et al., 2014; McKeown et al., 2016, 2017; Woods et al., 2016). It has been shown in numerous studies that several species of the core Pooideae are capable of cold acclimation. Facilitating these responses are, among others, five gene families known to play important roles during cold-stress response and cold acclimation in core Pooideae. These gene families code for C-repeat-binding factors (CBF; Badawi et al., 2007; Li et al., 2012), dehydrins (DHN; Olave-Concha et al., 2004; Rorat, 2006; Kosová et al., 2007, 2014), chloroplast-targeted cold-regulated proteins (ctCOR; Gray et al., 1997; Crosatti et al., 1999, 2013; Tsvetanov et al., 2000), ice recrystallization inhibition proteins (IRIP; Antikainen and Griffith, 1997; Hisano et al., 2004; Kumble et al., 2008; Sandve et al., 2008, 2011; John et al., 2009; Zhang et al., 2010), and fructosyl transferases (FST; Hisano et al., 2004; Tamura et al., 2014).
Although Pooideae species dominate temperate and arctic grass floras (Hartley, 1973; Visser et al., 2014), the ancestors of this group were most likely adapted to tropical or subtropical climates (Bouchenak-Khelladi et al., 2010; Strömberg, 2011). Pooideae originated in the late Cretaceous or early Paleogene period (Bouchenak-Khelladi et al., 2010; Christin et al., 2014; Spriggs et al., 2014; Schubert et al., 2018) during a time where the global climate was generally warm (Zachos et al., 2001; Mudelsee et al., 2014) and seasonality in temperature was relatively low (Archibald et al., 2010). The colonization of temperate biomes by Pooideae was likely facilitated by the evolution of successful adaptations to cold conditions. Although studies have inferred adaptation to cooler environments at the base of the Pooideae phylogeny (Edwards and Smith, 2010; Schubert et al., 2018), it is still not known whether the Pooideae’s most recent common ancestor already was adapted to cold and/or freezing conditions or if adaptations to cold evolved during the diversification of Pooideae along with falling temperatures throughout the Cenozoic and expansion of temperate biomes.
In this study, we investigated the evolution of frost tolerance in response to cold acclimation in the grass subfamily Pooideae. We first conducted classical cold acclimation and freezing test experiments to determine if cold acclimation is a shared trait throughout the Pooideae phylogeny. Next, we took advantage of comparative transcriptomics analyses to investigate if the molecular basis of cold acclimation in Pooideae is shared. Finally, we interpreted our results in a phylogenetic and paleoclimatic context and suggest a model of mostly independent evolution of cold acclimation responses.
RESULTS
Cold Acclimation Exists in Early Diverging Lineages
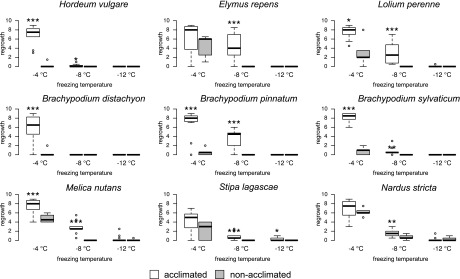
We conducted a classical cold acclimation and freezing test experiment in nine species that represent major, species-rich clades in the Pooideae subfamily or belong to very early diverging lineages (Fig. 1A) and that are distributed in areas where they are regularly exposed to cold, subzero temperatures (Fig. 1B). Freezing tests revealed that cold acclimation (i.e. increased frost tolerance through exposure to cold nonfreezing periods) exists both in core Pooideae and species of early diverging lineages (Fig. 2). All acclimated plants exhibited higher regrowth capacity at −4°C and −8°C compared with that of nonacclimated plants, although the increase in regrowth capacity was not significant at −4°C for Stipa lagascae and Nardus stricta. Nonacclimated plants of early diverging Pooideae species performed better at −4°C than nonacclimated Brachypodium species and H. vulgare and were comparable with the regrowth capacity of nonacclimated plants of the perennial core Pooideae species L. perenne and Elymus repens.
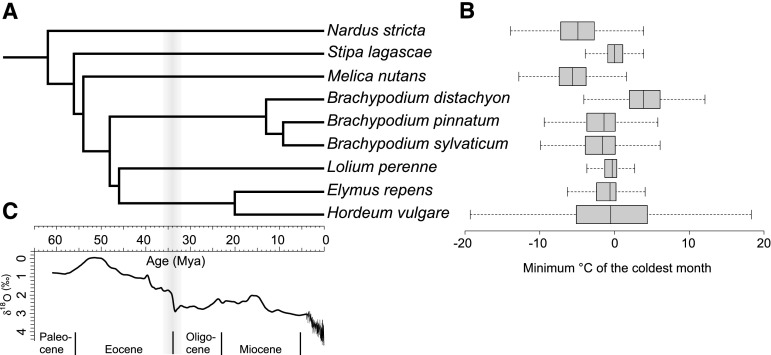
Figure 1.
The Pooideae phylogeny, present temperature data for focal species, and paleotemperature. A, Schematic phylogeny of the study species. The species phylogeny and dates are inferred from Schubert et al. (2018), except for B. sylvaticum, which was not included in the study. Placement and divergence date for B. sylvaticum are thus approximate. B, The range of the minimum temperature of the coldest month (WorldClim v1.4 data set, Bioclim variable 6, 2.5 km2 resolution [Hijmans et al., 2005]) of the species geographical distribution (Global Biodiversity Information Facility, 2018). C, Oxygen isotope ratios as a proxy for historical global temperature (Zachos et al., 2001; Mudelsee et al., 2014). The transition between the Eocene and the Oligocene is shaded. Mya, Million years ago.
Figure 2.
Frost tolerance after cold acclimation. Box plot representation shows the regrowth of nine acclimated and nonacclimated Pooideae species after exposure to three freezing temperatures (−4°C, −8°C, and −12°C). Regrowth is scored on a scale from 0 to 9, where 0 is dead and 9 is undamaged. Significant differences in regrowth between acclimated and nonacclimated plants are indicated by asterisks (***, P ≤ 0.001; **, P ≤ 0.01; and *, P ≤ 0.05).
De Novo Transcriptome Assembly Identified 8,633 High-Confidence Ortholog Groups
To investigate the molecular basis of cold response evolution, we sampled leaf material from five species spanning early to later diverging Pooideae lineages before and after subjecting them to a drop in temperature and shorter days (Supplemental Fig. S1). The transcriptome of each species was assembled de novo from RNA sequencing (RNA-Seq) reads (Supplemental Table S1). Ortholog groups (OGs) were inferred by using protein sequences from the five de novo assemblies and the reference genomes of L. perenne, H. vulgare, B. distachyon, rice (Oryza sativa), sorghum (Sorghum bicolor), and corn (Zea mays). A set of 8,633 high-confidence ortholog groups (HCOGs) was identified after filtering based on gene tree topology and species representation (Supplemental Table S2).
Shared Cold-Response Genes Included Known Abiotic Stress Genes
To investigate shared expression patterns across species, we constructed a single cross-species gene expression table with HCOGs as rows and samples as columns, which was created by summing the expression values of monophyletic paralogs and setting the expression of missing orthologs to zero (Supplemental Table S3). After removing differences in mean expression levels between species for each gene, clustering reconstructed a tree with replicates, followed by time points, grouping together (Supplemental Fig. S2A). The fact that time points mostly clustered before species indicated a common transcriptional response to cold across species. Exceptions were time points W4 and W9, which tended to cluster together and by species, indicating that responses after 4 and 9 weeks were very similar. We also observed a clear effect of the diurnal rhythm, with time points sampled in the morning (W0, W4, and W9) forming one cluster and time points sampled in the afternoon (D0 and D1) forming another.
Next, we focused on the response to short- and long-term cold treatment by analyzing changes in gene expression from before cold treatment to that following 8 h (short-term) and 4 to 9 weeks (long-term) of cold treatment. For all species pairs, there was a low, but statistically significant, correlation between expression changes after cold treatment of orthologs in HCOGs (Supplemental Fig. S2B). This indicates that orthologs tend to change expression in the same direction after cold treatment, an observation that explains the clustering of samples according to treatment before species (Supplemental Fig. S2A). Finally, we analyzed the statistical significance of expression change after cold treatment by classifying each individual gene as either differentially expressed or not (false discovery rate-adjusted P < 0.05 and fold change > 2 or < 0.5; Supplemental Table S4). Although a considerable number of genes responded to cold in each species (1,000–3,000 genes), the overlap between species was low. Of the 5,577 HCOGs with at least one differentially expressed gene (DEG), 50% contained only one DEG and thus represented orthologs differentially expressed in only one species. Thirty-one percent contained DEGs from two species, 14% contained DEGs from three species, and only 4% contained DEGs from four species. Importantly, this pattern was independent of expression level and did not change for the subsets of HCOGs with medium or high expression (Supplemental Table S4). Sixteen genes shared the same cold response (either short or long term) in the same direction (either up or down) in all five species (Table 1). These genes thus represent strong candidates for responses to cold that might have been conserved throughout the evolution of Pooideae. Nine of these genes belonged to families known to be involved in cold stress or other abiotic stress responses in other plant species. Of the 16 conserved genes, 12 genes displayed short-term up-regulation, indicating that stress response, as opposed to long-term acclimation response, is potentially more conserved.
Table 1. HCOGs with conserved cold responses in all five Pooideae species.
These 16 genes had the same type of cold response (short or long term) with the same manner of expression change (up- or down-regulation) in all five species. L, Responsive to long-term cold treatment; S, responsive to short-term cold treatment; ↗, up-regulated; ↘, down-regulated. Annotations were inferred from the literature using orthologs.
| B. distachyon Ortholog | Description and Relation to Stress Response | Response |
|---|---|---|
| Bradi2g39230 | Hyperosmolality-gated CA2+-permeable channel (OSCA). Stress-activated calcium channels (Yuan et al., 2014) that are highly conserved in eukaryotes (Hou et al., 2014); in O. sativa, OSCA genes are differentially expressed in response to osmotic stress (Li et al., 2015) | S↗ |
| Bradi2g06830 | Calcium-binding EF hand-containing calcium exchange channel (EF-CAX). Calcium ions are important mediators of abiotic stress in plants (Day et al., 2002; Bose et al., 2011); expression of calcium-binding proteins correlates with exposure to cold stress in several plants, such as Arabidopsis (Thomashow, 1999), Musa × paradisiaca (Yang et al., 2015), and H. vulgare (Greenup et al., 2011) | S↗ |
| Bradi2g05226 | GIGANTEA. Promotes flower development in plants (Andrés and Coupland, 2012); in Arabidopsis, this gene is involved in CBF-independent freezing tolerance (Cao et al., 2005; Xie et al., 2015), and it is responsive to cold in Z. mays (Sobkowiak et al., 2014); also part of the circadian clock | S↗ |
| Bradi4g24967 | Arabidopsis Pseudo-Response Regulator3-like (AtPRR3-like). AtPRR3 is a member of the circadian clock quintet AtPRR1/TOC1 (Murakami-Kojima et al., 2002; Murakami et al., 2004); no association with stress response found in the literature; however, AtPRR3-like might be closer related to AtPRR5/9 than to AtPRR3 (see Bradi4g36077, PRR95) | S↗ |
| Bradi2g09060 | Triacylglycerol lipase, α/β-hydrolase superfamily. Studies in Arabidopsis (Wang et al., 2011) and Ipomoea batatas (Liu et al., 2009) suggest that genes with α/β-hydrolase domains respond to osmotic stress; in Triticum monococcum, atriacylglycerol lipase was induced by pathogen stress (Guan et al., 2015) | S↗ |
| Bradi2g07480 | Late-Embryogenesis-Abundant protein14 (LEA-14). Responsive to drought, salt, and cold stress in Arabidopsis (Kimura et al., 2003; Singh et al., 2005), Betula pubescens (Rinne et al., 1998), and B. distachyon (Gagné-Bourque et al., 2015) | S↗ |
| Bradi1g04150 | SNAC1-like/NAC transcription factor67. NAC transcription factors mediate abiotic stress responses; osmotic stress increases the expression of SNAC1 in O. sativa (Nakashima et al., 2012), NAC68 in M. paradisiaca (Negi et al., 2015; Yang et al., 2015), and NAC67 in T. aestivum (Mao et al., 2014) | S↗ |
| Bradi4g36077 | Pseudo-Response Regulator95 (PRR95). Homologous to conserved circadian clock gene AtPRR5/9 (Murakami et al., 2003; Campoli et al., 2012); AtPRR5 gene is cold regulated in Arabidopsis (Lee et al., 2005), and PRR95 is cold responsive in Z. mays (Sobkowiak et al., 2014) | S↗ |
| Bradi2g43040 | DnaJ chaperon protein. DnaJ cochaperons are vital in stress response and have been found to be involved in the maintenance of PSII under chilling stress and to enhance drought tolerance in tomato (Solanum lycopersicum; Kong et al., 2014; Wang et al., 2014) | S+L↗ |
| Bradi3g33080 | Glycogenin Glucuronosyltransferase (GGT). GGT belongs to the GT8 protein family (Yin et al., 2011); in O. sativa, OsGGT transcripts are induced in submerged plants and respond to various abiotic stresses except cold (Qi et al., 2005; Uddin et al., 2012) | L↗ |
| Bradi1g04500 | Major facilitator superfamily transporter. Association with stress response unknown | L↗ |
| Bradi3g14080 | Glycosyl transferase. Association with stress response unknown | L↗ |
| Bradi1g35357 | Uncharacterized membrane protein. Association with stress response unknown | S↗ |
| Bradi2g48850 | Uncharacterized protein. Association with stress response unknown | S↗ |
| Bradi1g33690 | Uncharacterized protein. Association with stress response unknown | S↗ |
| Bradi1g07120 | Putative S-adenosyl-l-Met-dependent methyltransferase. Association with stress response unknown | L↘ |
We compared our DEGs with a compilation of 55 H. vulgare genes shown to be responsive to low temperature in several previous microarray experiments (Greenup et al., 2011). We could map 33 of these genes to unique OGs, of which 11 were HCOGs. We observed significant similarity in cold response between the 33 previously identified cold-response genes and the short-term DEGs in our data (P < 0.05; Supplemental Fig. S3). This similarity was statistically significant for all five species, although noticeably larger in H. vulgare than in the other species. This comparison thus shows that our transcriptome data were consistent with previous findings in H. vulgare and that cold-response genes identified in H. vulgare exhibit some cold response in other Pooideae.
Biological Processes Involved in Cold Response
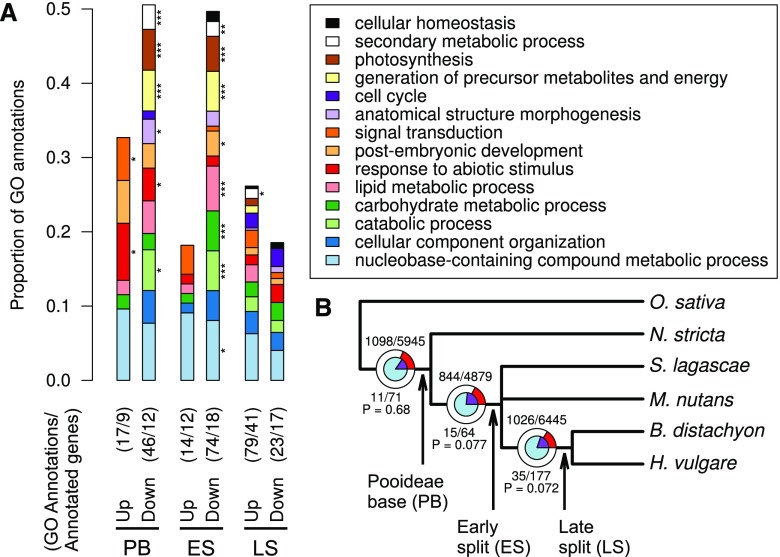
To identify biological processes that evolved regulation at different stages of Pooideae speciation, we targeted gene sets that were exclusively differentially expressed in all species within a clade in the phylogenetic tree (i.e. branch-specific DEGs) and tested these for enrichment of Gene Ontology (GO) biological process annotations (Fig. 3A). For the genes that were differentially expressed in all our species (Pooideae base), we found that up-regulated genes were enriched for GO annotations related to signal transduction (two pseudo-response regulators and diacylglycerol kinase2 [DGK2]) and abiotic stimulus (Gigantea, LEA-14, DnaJ, and DGK2), whereas down-regulated genes were enriched for photosynthesis and metabolism. For the genes that were exclusively differentially expressed in all species except N. stricta (early split), down-regulated genes were again enriched for metabolism and photosynthesis.
Figure 3.
GO enrichment and positive selection in branch-specific cold-responsive genes. A, GO enrichment analysis of HCOGs that were differentially expressed (DEGs) in all species (Pooideae base [PB]), only in species after N. stricta split off (early split [ES]), or only in B. distachyon and H. vulgare (late split [LS]). Significant differences are indicated by asterisks (*, P < 0.05; **, P < 0.01; and ***, P < 0.005, Fisher’s exact test). GO enrichments are shown for up- and down-regulated DEGs, not distinguishing between short- and long-term responses. Both the number of annotated genes and the number of annotations are indicated for each set of branch-specific DEGs. B, Positive selection at different stages in Pooideae evolution. The circles and numbers represent the HCOG gene trees that were tested for positive selection at each split. The inner blue circle and numbers below the branches represent HCOGs with branch-specific differential expression (i.e. genes that were cold responsive exclusively in the species under the respective branch), whereas the outer circles and numbers above the branches represent all other HCOGs. The purple and red pie-chart slices represent the proportions of HCOGs (first number) with positive selection (P < 0.05) among the total number of tested HCOGs (second number). The P values indicate the overrepresentation of positive selection among the branch-specific DEGs (hypergeometric test).
Positive Selection in Cold-Responsive Genes
To resolve the uncertain placement of Meliceae and Stipeae lineages within the Pooideae species tree, we reconstructed gene trees using the 3,914 HCOGs with exactly one sequence from each of the five Pooideae species and O. sativa. In the most common gene tree topology, S. lagascae and Melica nutans formed a monophyletic clade (755 trees), but topologies where either M. nutans or S. lagascae diverged first (434 and 271 trees, respectively) were also common (Supplemental Fig. S4). Averaging the posterior probabilities of the branches leading to the most recent common ancestor of S. lagascae and M. nutans did not produce significant support for any of the four most common topologies. Thus, we displayed the relationships of Meliceae and Stipeae tribes as unresolved trichotomy in Figures 3 and 4, which is congruent with 1,460 of the gene trees.
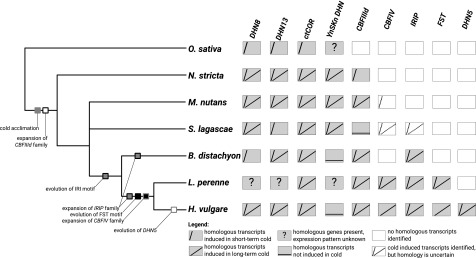
Figure 4.
Summary of phylogenetic analyses and gene expression experiments. Emergence of protein motifs and expansion of gene families important for cold acclimation are displayed on a schematic Pooideae species tree. The right-hand section depicts expression profiles of de novo transcripts in response to long- and short-term cold treatment of the respective species in the species tree. Expression patterns are approximated from the literature for L. perenne (CBF [Tamura and Yamada, 2007], WCS19/COR14 [Oishi et al., 2010; Ergon et al., 2016], and FST [Hisano et al., 2008; Paina et al., 2014]) and O. sativa (DHN8 [Lee et al., 2005], DHN13 [Aguan et al., 1991], and ctCOR-like [Maruyama et al., 2014]).
We also tested for positive selection (i.e. selection for beneficial substitutions) in coding sequences at each of the internal branches of the species tree. The tests were only performed on the branches where the respective HCOG gene tree topology was compatible with the species tree topology. Sixteen percent to 18% of the HCOGs showed significant signs of positive selection (P < 0.05) depending on the branch (Fig. 3B). Next, we tested for overrepresentation of positive selection among the branch-specific DEGs. Although not statistically significant, there was a tendency that gain of cold response was associated with positive selection on coding sequence at the early-split and late-split branches (P = 0.077 and P = 0.072, respectively; Fig. 3B). This observation indicates that selection on both regulatory and protein-coding sequences may have played a role in evolving cold responses in Pooideae.
Cold Acclimation Gene Families Have Diverse Evolutionary Histories
Of the 33 previously described cold-responsive H. vulgare genes that were also differentially expressed in our experiment (Supplemental Fig. S3), as many as 22 were not included in the HCOGs, mainly because they belonged to gene families with complex gene duplication histories in lineages leading up to two or more of our studied species. Next, we tested if this was a general trend for all cold-responsive genes. This analysis showed that cold-responsive genes in general are members of larger gene families than genes that do not respond to cold, with the exception of short-term down-regulated genes (Supplemental Fig. S5). Conversely, short-term down-regulated genes were enriched in single-copy gene families, whereas up-regulated genes were less common in these families (Supplemental Fig. S5). We also tested if cold response was associated with the age of the gene family through a phylostratigraphic analysis. The general trend was that cold-responsive genes tended to be underrepresented among the most ancient families (Supplemental Fig. S6).
We conducted detailed analyses of five of the best studied cold acclimation gene families, coding for CBF, DHN, ctCOR, IRIP, and FST. We reconstructed gene trees for these families (Supplemental Figs. S7–S14) using reference sequences and de novo assembled transcripts (Supplemental Table S5) and overlaid information on DEGs responsive to short- and long-term cold treatment. DHN genes are well studied in H. vulgare, with 13 known DHN homologs (termed HvDHN1 to HvDHN13). Structurally, they can be grouped into four distinct types based on the presence of amino acid segments (Y, K, and S): SK3-type (HvDHN8), KS-type (HvDHN13), Kn-type (HvDHN5), and YnSKn-type (the 10 remaining HvDHN homologs) DHN (Kosová et al., 2007). Because the genes for these four groups represent phylogenetically distinct clades (Karami et al., 2013), we reconstructed individual gene trees for each group; however, this was not performed for HvDHN5, because homologs were only found in the H. vulgare transcriptome. Two groups of CBF genes, CBFIIIc/d and CBFIV, are especially important for cold acclimation in Pooideae and are restricted to this subfamily (Badawi et al., 2007; Li et al., 2012). As the CBF gene family is large and highly complex, we restricted our analyses of the CBF gene family to these two groups. Our analyses could not completely resolve the topologies for most of the gene trees, owing to their complex evolutionary history. We identified, however, that DHN8 and DHN13 of the DNH gene family and the ctCOR gene family are likely candidates for conserved cold acclimation genes within the Pooideae (Fig. 4). Also, the expansion of the CBFIIId group likely started in the earliest Pooideae lineage. In contrast, the evolution of the IRIP and FST motifs, the expansion of the CBFIV group, and the evolution of DHN5 (no tree reconstructed) were restricted to later diverging lineages. Furthermore, these detailed analyses revealed a decreasing similarity in transcriptional cold response with increasing phylogenetic distance (Fig. 4). Naturally, this analysis is biased toward H. vulgare, as most of what we know about these gene families is derived from crop species.
DISCUSSION
Cold Acclimation Evolved Independently in Separate Lineages
It has been suggested that the ecological success of the Pooideae subfamily in the northern temperate regions critically relies on adaptations to cold temperatures. Here, we show that cold acclimation increased frost tolerance relative to that in nonacclimated plants in all the major Pooideae lineages (Fig. 2). This demonstrates that the cold acclimation trait is pervasive across Pooideae and an important part of adaptation to a life in temperate and arctic biomes.
To dissect the molecular basis of the cold acclimation responses and determine if the cold acclimation response has a shared molecular basis, we conducted a global comparative, transcriptomic analysis for five phylogenetically diverse species. For each species, 10% to 30% of the highly conserved genes (i.e. genes in the 8,633 HCOGs) responded to cold, which is in line with what we know about cold acclimation programs from other species, such as Arabidopsis (Arabidopsis thaliana; Park et al., 2015). Transcripts involved in photosynthesis and response to abiotic stimuli were significantly enriched among the genes with cold response in all species (Fig. 3A). It is known that down-regulation of the photosynthetic machinery during cold temperatures is one mechanism to prevent photoinhibition (Crosatti et al., 2013) and cellular damage. This protective mechanism might therefore have existed early in the evolution of Pooideae. Enrichment of genes involved in photosynthesis among differentially expressed genes is not specific to cold stress, but it can be observed in response to other stresses, including salt stress (Yamamoto et al., 2015) as well as heat and drought stress (Liu et al., 2015). Interestingly, in our study, nearly half of all cold-responsive genes were species specific in differential expression tests (Supplemental Table S4). This pattern was also supported by low (although significant) correlations in gene expression fold changes (Supplemental Fig. S2B). These results indicate that the evolution of cold acclimation evolved at least partly independently in separate lineages and are in line with recent work on transcriptional responses to vernalization in Pooideae (McKeown et al., 2016; Zhong et al., 2018).
Many genes previously associated with cold acclimation belong to complex gene families (Thomashow, 1999; Sandve and Fjellheim, 2010) and are therefore not included in the global analysis of highly conserved orthologs. We therefore conducted an in-depth analysis of gene families known to be important for cold acclimation (Fig. 4; Supplemental Figs. S7–S14) in well-studied core Pooideae species (Choi et al., 2002; Vágújfalvi et al., 2003; Badawi et al., 2007; Knox et al., 2008, 2010; Livingston et al., 2009; Zhang et al., 2010; Soltész et al., 2013; Jeknić et al., 2014; Todorovska et al., 2014; Marozsán-Tóth et al., 2015). The lack of conserved expression patterns of these genes across the tested Pooideae species (Fig. 4) further supports that cold acclimation is regulated differently in the five Pooideae species.
Three gene families or gene family members known to be involved in cold acclimation in core Pooideae crop species evolved de novo or evolved a new function in some lineages. First, the FST gene family originated at the base of the core Pooideae (Supplemental Fig. S14). Second, the functional domain of the IRIP gene family evolved at the base of core Pooideae/Brachypodium (Supplemental Fig. S13). Lastly, in the DHN gene family, the gene DHN5 evolved in Triticeae (Fig. 4). Together, these three examples provide conclusive evidence that the cold acclimation responses in early diverging lineages at least partly rely on other genes and mechanisms than the core Pooideae and Brachypodium. Some very likely candidates for cold responses specific to early diverging Pooideae lineages are homologs of the YnSKn-type DHN genes in the DHN family (Fig. 4; Supplemental Fig. S11). YnSKn-type DHN genes are neither known to be involved in cold acclimation of Brachypodium and core Pooideae nor have we found any support for that in our data.
Even though our analyses show that the species largely follow independent evolutionary trajectories, the responses to a large extent build on a common genetic basis. The CBF, DHN, and COR gene families are known to be involved in cold stress adaptation across the plant kingdom (Thomashow, 2001; Rorat, 2006), and FST genes has evolved several times from vacuolar invertases independently across the angiosperms (Vijn and Smeekens, 1999). This indicates that some genes code for proteins with biochemical functions suited to be recruited for cold stress mitigation. One gene family whose function in cold acclimation might be conserved throughout the Pooideae is the ctCOR gene family (Fig. 4; Supplemental Fig. S12), rendering this gene family an interesting candidate that could constitute a shared molecular basis of the cold acclimation trait in Pooideae. Interestingly, a ctCOR homolog is up-regulated in response to short-term cold treatment in O. sativa (Maruyama et al., 2014), indicating that the cooption of this gene to the highly specialized cold acclimation pathway in Pooideae builds on a cold stress response that is conserved well beyond Pooideae. A very similar scenario is likely for the two genes DHN8 and DHN13. Homologs of both genes are induced during chilling stress in O. sativa (Aguan et al., 1991; Lee et al., 2005), and there is strong support for both genes having a function in cold acclimation of core Pooideae species (Kosová et al., 2007). Our results suggest that DHN8 and DHN13 are single-copy genes involved in cold acclimation in all but one of the species investigated here.
Although the molecular mechanisms behind adaptive evolution are still an active field of research, it is now indisputably established that novel gene regulation plays a crucial role (Romero et al., 2012). The evolution of gene regulation proceeds by altering noncoding regulatory sequences in the genome, such as cis-regulatory elements (Wittkopp and Kalay, 2011), or by altering the coding sequences of regulatory proteins. The high number of genes with species-specific cold responses observed in this study is consistent with the recruitment of genes with existing cold tolerance functions by means of regulatory evolution. However, previous studies have also pointed to the evolution of coding sequences (Vigeland et al., 2013) as underlying the acquisition of cold tolerance in Pooideae. To investigate possible adaptive evolution in coding regions, we tested for the enrichment of positive selection among branch-specific cold-responsive genes (Fig. 3B). Although not statistically significant (P = 0.07), increased signals for positive selection on coding sequences was observed for cold-responsive genes in a period of gradual cooling preceding the E-O event. In conclusion, we find evidence to support that increased selection for protein-coding sequence, changes in gene regulation, and novel genes and functions play roles in the evolution of cold responses in Pooideae.
In general, it is important to note that lack of shared signals between lineages can also arise for other reasons than biologically meaningful differences and must therefore be interpreted with caution. In our study, limited statistical power and the use of de novo transcriptomes could impact our ability to detect overlap in cold responses between species. To minimize the effect of these putative biases in this study, we only analyzed OGs containing genes that could be assembled across all species in the phylogeny, ensuring that we measured changes in gene expression levels and not changes in gene content. Furthermore, since three of the species lacked reference genomes, we employed a de novo assembly pipeline to reconstruct the transcriptomes. We showed that this pipeline could recover a set of H. vulgare genes previously identified as cold responsive (Greenup et al., 2011) and that most of these genes were also cold responsive in H. vulgare in our experiment (Greenup et al., 2011). Moreover, we conducted analyses to assess if sequencing depth was a significant factor in our ability to identify shared patterns of differential expression (Supplemental Table S4). These results clearly show that sequencing coverage cannot explain the high ratio of lineage-specific cold acclimation responses, indicating that increasing the statistical power will not change the conclusions of this study. Hence, the global trend of low levels of shared cold acclimation responses is unlikely to be a methodological artifact and is consistent with the evolution of cold tolerance mechanisms being shaped mostly independently in the different Pooideae lineages.
A Shared Adaptive Potential for Evolution of Cold Adaptation
Our differential expression analysis revealed 16 genes sharing cold-associated gene expression shifts across all species (Table 1). An overwhelming majority (12 of 16) of the conserved genes was responsive to short-term cold treatment (Table 1), and this observation suggests that existing stress genes have been the first to be coopted into the cold-response program. Ten of these genes have previously been shown to be regulated by other abiotic and biotic stressors (Table 1). Nine of these conserved cold-response genes are involved in response to abiotic stresses in other plants, such as osmotic stress and drought. The SNAC1-like/NAC transcription factor67 is one example, with homologs induced by osmotic stress in the three monocots M. paradisiaca (Negi et al., 2015; Yang et al., 2015), O. sativa (Nakashima et al., 2012), and T. aestivum (Mao et al., 2014). Cooption of such genes into a cold-responsive pathway might have been the key to acquire cold tolerance. Some responses may be even more ancient than the most recent common ancestor of the Pooideae, as some of the genes with cold response in all five species are also expressed in response to cold in other species (Table 1), among them O. sativa, which is a related tropical species with some tolerance to chilling stress (Wang et al., 2016). Shaar-Moshe et al. (2017) recently identified B. distachyon genes induced by drought, high salinity, or heat as well as by their combinatorial effects. All genes from Table 1 were differentially expressed in at least one of these stress conditions. Noticeably, however, none of those genes were responsive to drought stress alone. We also found that cold-responsive genes are underrepresented among the most ancient gene families (Supplemental Fig. S6). Taken together, it seems that transcriptional cold responses that are shared across the Pooideae phylogeny represent deeply conserved ancestral responses to mitigate general cellular stress rather than specific adaptations to prepare for periodic frost.
Pooideae Lineages Evolved Specific Cold Adaptation by Expanding Gene Families
Gene family expansion has previously been suggested to play a role in cold adaptation in Pooideae (Sandve and Fjellheim, 2010; Li et al., 2012). As previously discussed, the conservative filtering of OGs employed in this study removed complex gene families containing duplication events shared by two or more species. A majority of the previously described H. vulgare cold-responsive genes (Greenup et al., 2011) belonged to gene families with complex gene duplication histories. Moreover, an analysis of all cold-responsive genes confirmed a positive association between gene family size and cold response in our data, with the exception of those genes down-regulated in response to short-term cold treatment, which were instead enriched among single-copy gene families (Supplemental Fig. S5). These results thus confirm previous findings that stress-related genes are enriched in fast-evolving gene families (Panchy et al., 2016). The gene trees of the gene families targeted for phylogenetic analyses (Fig. 4; Supplemental Figs. S7–S14) displayed several duplication events and gene family expansions. Based on our results, we propose that gene family expansion was an important mode of cold adaptation in the Pooideae subfamily. Our results corroborate findings from Sandve and Fjellheim (2010), who identified an increase of gene copy number in the CBFIV group and the FST and IRIP gene families as an evolutionary force of cold climate adaptation of core Pooideae and Brachypodium species. Although we lack sufficient genomic data for early diverging Pooideae species, we found evidence for Pooideae-specific expansions in the CBFIIId group, the ctCOR gene family, and in YnSKn-type DHN genes. Expansion of gene families may have led to functional specialization or novel functions of the various gene copies. Other studies have shown that stress-related gene families tend to expand via tandem duplications (Hanada et al., 2008), which may lead to lineage-specific expansion of the gene family (Lespinet et al., 2002). Although de novo assembly of transcriptomes from short-read RNA-Seq data are a powerful tool that has vastly expanded the number of target species for conducting transcriptomic analysis, the approach has limited power to distinguish highly similar transcripts such as paralogs. Homologous genes might exist in early diverging Pooideae species but may not be expressed. Furthermore, coding sequences may contain insufficient informative substitutions to reconstruct the true species topology. Further insight into the role of gene duplication events in the evolution of the Pooideae cold adaptation would therefore benefit immensely from additional reference genomes.
Evolution of Cold Adaptation in a Paleoclimatic Context
At face value, the most striking result from our study is the presence of cold acclimation capacity in all our study species and at the same time a generally low similarity between the species in transcriptomic responses to cold acclimation as well as the presence of well-described cold acclimation genes, such as FSTs, DHN5, and functional IRIP, in only some of the species. However, in a phylogenetic and paleoclimatic context, these contrasting patterns can easily be reconciled. A recent study by Schubert et al. (2018) places the divergence of all study species well before the E-O transition, when the global climate was warm, seasonality was low, and temperate biomes had yet to expand. Thus, the most likely scenario is that our study species have experienced more than 45 million years of independent evolution and that adaptations to periodic frost have independent evolutionary origins.
Responding to episodic and periodic frost are two fundamentally different physiological processes (Körner, 2016). Episodic frost induces a stress response, and such existing responses could be exploited to tackle frost when first experienced by the plants in the cooling climate during the Eocene. On the other hand, responses to periodic frost require controlled changes in cellular components initiated by plants upon sensing shortening photoperiod and lower temperatures in the autumn. This complex response must have evolved over a long period of evolutionary time, during which the plants were exposed to increasingly more severe winters, exerting selection pressure for the cold acclimation response. Ancestral state reconstructions of climatic niches of Pooideae species suggest that severe winter was not encountered by the Pooideae ancestor but was first experienced by Pooideae species more recently in their evolutionary history (Schubert et al., 2018). Based on phylogenetic and paleoclimatic data, we would therefore expect that selection pressure for adaptation to winter seasons and frost spells across the Pooideae phylogeny would result in the independent evolution of cold acclimation capacity, involving many lineage- and species-specific gene expression responses, as shown by our analyses.
Nevertheless, ancestral state reconstruction of the climatic niche of the ancestor shows that it likely experienced episodic incidents of frost and that Pooideae possibly originated in a temperate microniche in emerging alpine orogenies (Schubert et al., 2018). The conserved set of stress-response genes identified in our comparative transcriptome analysis may have represented a preliminary toolkit to cope with frost stress. This may have represented a fitness advantage for the Pooideae ancestor in the newly emerging environment with incidents of mild frost, allowing time to evolve the more complex physiological adaptations required to endure the temperate climate with strong seasonality and cold winters that emerged following the E-O transition (Eldrett et al., 2009). Thus, preadaptations to temperate climate may have given Pooideae species the advantage to expand and diversify with the expanding temperate biomes after the E-O transition while gradually building more complex adaptations to cold. This is in line with the results of Schubert et al. (2018) that show that diversification rates in Pooideae are inversely correlated with temperature.
MATERIALS AND METHODS
Plant Material
Seeds from nine Pooideae species were acquired from germplasm collections or collected in nature. The selected species represent major, species-rich clades in the Pooideae subfamily or belong to very early diverging lineages (Soreng et al., 2015). All species are distributed in areas where they are regularly exposed to cold, subzero temperatures. Furthermore, to ensure that the accessions of the wild species that were used in the experiment are adapted to cold conditions, accessions were selected from localities with cold winters (mean temperature of the coldest quarter is below 0°C, according to data extracted from the WorldClim v.1.4 data set [Hijmans et al., 2005]; Supplemental Fig. S15A).
Nardus stricta (2n = 2x = 26) is a perennial species, distributed in Europe, western parts of Asia, and North Africa and introduced to New Zealand and North America (Tutin, 1980; Hultén and Fries, 1986; Clayton et al., 2006). Seeds were collected in July 2012 in Romania (46.69098 N, 22.58302 E). The locality of the collected accession experiences subzero temperatures in November to March, and the mean temperature of the coldest quarter is −3°C (Supplemental Fig. S15B).
Stipa lagascae (2n = 2x = 22) is a perennial species that is distributed in temperate regions around the Mediterranean Sea and parts of temperate west Asia (Tutin, 1980; Hultén and Fries, 1986; Clayton et al., 2006). Seeds from accession PI 250751 were acquired from the U.S. National Plant Germplasm System (U.S.-NPGS) via the Germplasm Resources Information Network (GRIN). The accession was collected 49 km northwest of Tabriz, Iran, in 1958. The locality of the collected accession experiences subzero temperatures in December to March, and the mean temperature of the coldest quarter is –0.2°C (Supplemental Fig. S15B).
Melica nutans (2n = 2x = 18) is a perennial species distributed in temperate parts of Eurasia (Tutin, 1980; Hultén and Fries, 1986; Clayton et al., 2006). Seeds were collected in Germany (50.70708 N, 11.23838 E) in June 2012. The locality of the collected accession experiences subzero temperatures in December to March, and the mean temperature of the coldest quarter is –0.9°C (Supplemental Fig. S15B).
Brachypodium distachyon (2n = 2x = 10) is an annual species natively distributed in Europe, east Africa, and temperate parts of west Asia (Tutin, 1980; Hultén and Fries, 1986; Clayton et al., 2006). Seeds for accession Bd1-1 (W6 46201) were acquired from U.S.-NPGS via GRIN. This accession is a single-seed-descent inbred line of the accession PI170218 (U.S.-NPGS, GRIN) collected in Soma, Manisa, Turkey, in 1948. The line is classified as a winter variety (i.e. Bd1-1 requires vernalization to flower and is freeze tolerant; Colton-Gagnon et al., 2014).
Brachypodium pinnatum (2n = 2x = 18) is a perennial species distributed in temperate parts of Eurasia (Tutin, 1980; Hultén and Fries, 1986; Clayton et al., 2006). Seeds were collected in October 2015 in Norway (59.71861 N, 10.59333 E). The locality of the collected material experiences subzero temperatures in November to March, and the mean temperature of the coldest quarter is –3°C (Supplemental Fig. S15B).
Brachypodium sylvaticum (2n = 2x = 18) is a perennial species distributed in temperate parts of Eurasia (Tutin, 1980; Hultén and Fries, 1986; Clayton et al., 2006). Seeds were collected in October 2015 in Norway (59.68697 N, 10.61012 E). The locality of the collected material experiences subzero temperatures in November to March, and the mean temperature of the coldest quarter is –3.2°C (Supplemental Fig. S15B).
Hordeum vulgare (2n = 2x = 14) seeds for cv Igri were provided by Åsmund Bjørnstad (Department of Plant Sciences, Norwegian University of Life Sciences). The cv Igri is a two-row winter barley developed in Germany in 1976 and is much used across western Europe (Fischbeck, 2003). Igri requires vernalization to flower and is tolerant to frost (Kosová et al., 2010). The cv Sonja is a two-row winter barley developed in Germany in 1974 (Fischbeck, 2003).
Lolium perenne seed for cv Fagerlin were provided by Mallikarjuna Rao Kovi (Department of Plant Sciences, Norwegian University of Life Sciences). The cv Fagerlin is a winter-hardy variety developed in Norway in 2008.
Elymus repens (2n = 4x, 6x = 28, 42) is a perennial species distributed in Europe and northern parts of Asia and is introduced to North America (Tutin, 1980; Hultén and Fries, 1986; Clayton et al., 2006). Seeds were collected in October 2015 in Norway (59.66111 N, 10.89194 E). The locality of the collected material experiences subzero temperatures between November and March, and the mean temperature of the coldest quarter is −3.1°C (Supplemental Fig. S15B).
Freezing Tests
Seeds from all nine species (for H. vulgare, cv Sonja was used) were germinated and plants were grown in a greenhouse at 20°C under natural daylight. Each individual was divided into four clones, one for each treatment and control. The plants were acclimated at 4°C and short days (8 h) for 3 weeks. Control conditions were short days and 20°C. The light intensity was 50 µmol m−2 s−1. At the end of the cold acclimation period, plants were subjected to freezing at three different temperatures (−4°C, −8°C, and −12°C) following Alm et al. (2011). For each temperature, we used 15 acclimated and 15 nonacclimated individuals per species. After freezing, plants were cut down to approximately 3 cm and grown at 20°C under long days in a greenhouse with natural light conditions. Two and 3 weeks after the plants were moved into 20°C and long days, they were assessed for regeneration ability and scored from 0 (dead) to 9 (growth without damage). Differences between acclimated and nonacclimated individuals within each species were tested with a one-tailed Students t test in R (R Core Team, 2016) using the stats package.
Cold Treatment: Sampling, RNA Extraction, and Sequencing
Seeds from N. stricta, M. nutans, S. lagascae, B. distachyon, and H. vulgare (cv Igri) were germinated and initially grown in a greenhouse at a neutral daylength (12 h of light), 17°C, and a minimum artificial light intensity of 150 µmol m−2 s−1. To ensure that individual plants were at comparable developmental stages at sampling, the onset of treatment for different species was based on developmental stage rather than absolute time. Most importantly, none of the plants had transitioned from vegetative to generative phase, as this could have affected the cold response. Plants were grown until three to four leaves had emerged for M. nutans, S. lagascae, B. distachyon, and H. vulgare or six to seven leaves for N. stricta (which is a cushion-forming grass that produces many small leaves compared with its overall plant size). Depending on the species, this process took 1 week (H. vulgare), 3 weeks (B. distachyon and S. lagascae), 6 weeks (M. nutans), or 8 weeks (N. stricta) from the time of sowing. Subsequently, plants from all species were randomized and distributed to two cold chambers with short days (8 h of light), constant 6°C, and a light intensity of 50 µmol m−2 s−1. Plants were kept in cold treatment for the duration of the experiment. Leaf material for RNA isolation was collected (1) in the afternoon (at zeitgeber time [ZT] 8) on the day before cold treatment (D0) and in the afternoon (ZT 8) on the first day of cold treatment (8 h after initiation of cold treatment; D1) and (2) in the morning (ZT 0) before cold treatment (W0), 4 weeks of cold treatment (W4), and 9 weeks of cold treatment (W9; Supplemental Fig. S1). The sampling time points were chosen to be able to separate chilling stress responses (first day of treatment) and responses to long-term cold treatment that represent acclimation to freezing temperatures (4 and 9 weeks of treatment). Flash-frozen leaves were individually homogenized using a TissueLyser (Qiagen Retsch), and total RNA was isolated (from each leaf) using an RNeasy Plant Mini Kit (Qiagen) following the manufacturer’s instructions. The purity and integrity of total RNA extracts was determined using a NanoDrop 8000 UV-Vis Spectrophotometer (Thermo Scientific) and 2100 Bioanalyzer (Agilent), respectively. For each time point, RNA extracts from five leaves sampled from five different plants were pooled and sequenced as a single sample. In addition, replicates from single individual leaves were sequenced for selected time points (see Supplemental Table S1 and “Differential Expression” below). Two time points lacked expression values: W9 in B. distachyon (RNA integrity was insufficient for RNA-Seq) and W0 in S. lagascae (insufficient supply of plant material). Samples were sent to the Norwegian Sequencing Centre, where strand-specific cDNA libraries were prepared and sequenced (paired end) on an Illumina HiSeq 2000 system.
Transcriptome Assembly and Ortholog Inference
Using Trimmomatic v0.32 (Bolger et al., 2014), all reads were trimmed to a length of 120 bp, Illumina TruSeq adapters were removed from the raw reads, low-quality bases were trimmed using a sliding window of 40 bp and an average quality cutoff of 15, and reads below a minimum length of 36 bp were discarded. Read quality was checked using fastqc v0.11.2. For each species, transcripts were assembled de novo with Trinity v2.0.6 (Grabherr et al., 2011; strand-specific option, otherwise default parameters) using reads from all samples. We assessed the completeness of the transcriptomes using Benchmarking of Universal Single-Copy Orthologs v3.0.2 (Waterhouse et al., 2018) with the provided Embryophyta database, based on OrthoDB version 9 (Zdobnov et al., 2017; Supplemental Table S6). Coding sequences (CDS) were identified using TransDecoder rel16JAN2014 (Haas et al., 2013). Where Trinity reported multiple isoforms, only the longest CDS was retained. OGs were constructed from the five de novo transcriptomes and public reference transcriptomes of H. vulgare (barley_HighConf_genes_MIPS_23Mar12), B. distachyon (brachypodium v1.2), Oryza sativa (rap2), Zea mays (ZmB73_5a_WGS), Sorghum bicolor (sorghum 1.4), and L. perenne (GenBank TSA accession GAYX01000000) using OrthoMCL v2.0.9 (Li et al., 2003). All reference sequences except L. perenne were downloaded from http://pgsb.helmholtz-muenchen.de/plant/plantsdb.jsp.
HCOGs
To compare gene expression across Pooideae, we required OGs to contain one gene from each species that all descended from a single gene in the Pooideae ancestor. As the OGs inferred using orthoMCL sometimes cluster more distantly related homologs as well as include both paraphyletic and monophyletic paralogs, we further refined the OGs by phylogenetic analysis. Several approaches to phylogenetic refinement have been proposed previously (Yang and Smith, 2014). Here, we first aligned protein sequences within each OG using mafft v7.130 (Katoh and Standley, 2013) and then converted these protein alignments to codon alignments using pal2nal v14 (Suyama et al., 2006). Gene trees were then constructed from the codon alignments using Phangorn v1.99.14 (Schliep et al., 2011; maximum likelihood GTR+I+G). Trees with apparent duplication events before the most recent common ancestor of the included species were split into several trees. This was accomplished by identifying in-group (Pooideae) and out-group (Z. mays, S. bicolor, and O. sativa) clades in each tree and then splitting the trees so that each resulting subtree contained a single out-group and a single in-group clade. Finally, we only retained the trees where all species in the tree formed one clade each (i.e. only monophyletic paralogs), B. distachyon and H. vulgare formed a clade, and at least three of the five studied species were included. These trees constituted the HCOGs.
Differential Expression
Reads were mapped to the de novo transcriptomes using bowtie v1.1.2 (Langmead et al., 2009), and read counts were calculated with RSEM v1.2.9 (Li and Dewey, 2011). We observed higher numbers of monophyletic species-specific paralogs in the de novo assembled transcriptome than in the reference genomes of H. vulgare and B. distachyon. Since the de novo assembly procedure thus seemed to overestimate the number of paralogs (most likely because alleles or alternative transcript isoforms were assembled into separate contigs), we chose to represent each species in each HCOG by a single read-count value equal to the sum of the expression of all assembled paralogs (analogous to so-called monophyly masking [Smith et al., 2011]). By additionally setting counts for missing orthologs to zero, we created a single cross-species expression matrix with HCOGs as rows and samples as columns (Supplemental Table S3).
To identify conserved and diverged cold response across species, DEGs were identified using DESeq2 v1.6.3 (Love et al., 2014) with a linear regression model that combined the species factor and the time point factor (with time points W4/9 as a single level). Pooled samples provided robust estimates of the mean expression in each time point. To also obtain robust estimates of the variance, the model assumed common variance across all time points and species within each HCOG, thus taking advantage of both biological replicates available for individual time points within species and the replication provided by analyzing several species. For each species, we tested the expression difference between D0 and D1 (response to short-term cold treatment) and the difference between W0 and W4/9 (response to long-term cold treatment; Supplemental Fig. S1). B. distachyon lacked the W9 sample, and response to long-term cold treatment was therefore based on W4 only. S. lagascae lacked the W0 sample, and response to long-term cold treatment was therefore calculated based on D0. Due to the observed diurnal effect (Supplemental Fig. S2A), this might have resulted in more unreliable estimates of the response to long-term cold treatment in S. lagascae. Genes with a false discovery rate-adjusted P < 0.05 and a fold change > 2 or < 0.5 were classified as differentially expressed.
Sample Clustering
Sample clustering was based on read counts normalized using the variance-stabilizing transformation (VST) implemented in DESeq2. The purpose of the VST transformation is to create expression values where the variability is not related to the mean. For large counts, these VST values are equal to log2-transformed values. HCOGs that lacked orthologs from any of the five species, or that contained orthologs with low expression (VST < 3), were removed, resulting in 4,981 HCOGs used for the clustering. To highlight the effect of the cold treatment over the effect of expression level differences between species, the expression values were normalized per gene and species. First, one expression value was obtained per time point per gene by taking the mean of the replicates. Then, these expression values were centered by subtracting the mean expression of all time points. Distances between all pairs of samples were calculated as the sum of absolute expression differences between orthologs in the 4,981 HCOGs (i.e. Manhattan distance). The sample clustering tree (Supplemental Fig. S2A) was generated using neighbor joining (Saitou and Nei, 1987).
Comparison with Known Cold-Responsive Genes
Genes identified as responsive to short-term cold treatment in H. vulgare were acquired from Supplemental Table S10 of Greenup et al. (2011). These genes were found to be responsive to cold in three independent experiments with Plexdb accessions BB64 (Svensson et al., 2006), BB81 (no publication), and BB94 (Greenup et al., 2011). The probe sets of the Affymetrix Barley1 GeneChip microarray used in these studies were BLASTed (BLASTX) against all protein sequences in our OGs. Each probe was assigned to the OG with the best match in the H. vulgare reference. If several probes were assigned to the same OG, only the probe with the best hit was retained. Correspondingly, if a probe matched several paralogs within the same OG, only the best match was retained. DESeq2 was used to identify DEGs responsive to short-term cold treatment for all transcripts in all OGs (i.e. this analysis was not restricted to the HCOGs), and these were compared with DEGs from Greenup et al. (2011). The statistical significance of the overlap between our results and those reported by Greenup et al. (2011) was assessed for each species by counting the number of genes that had the same response (up- or down-regulated DEGs) and comparing that with a null distribution. The null distribution was obtained from equivalent counts obtained from 100,000 trials where genes were randomly selected from all expressed genes (mean read count > 10) with an ortholog in H. vulgare.
GO Enrichment Tests
GO annotations for B. distachyon were downloaded from Ensembl Plants Biomart and assigned to the HCOGs. The TopGO v2.18.0 R package (Alexa and Rahnenfuhrer, 2018) was used to calculate statistically significant enrichments (Fisher’s exact test, P < 0.05) of GO biological process annotations in each set of branch-specific DEGs using all annotated HCOGs as the background. The enrichment analysis was restricted to a subset of GO terms relevant to plants (i.e. plant GO plant). Branch-specific DEGs were those genes that were exclusively differentially expressed in all species within a clade in the phylogenetic tree.
Positive Selection Tests
To test for positive selection, we first identified a species tree topology. HCOGs with a single ortholog from each of the five Pooideae species and O. sativa were used to infer gene trees. BEAST v1.7.5 (Drummond and Rambaut, 2007) was run with an HKY + Γ nucleotide substitution model using an uncorrelated lognormal relaxed clock model. A Yule process (birth only) was used as prior for the tree and monophyly of the Pooideae was constrained. Markov chain Monte Carlo analyses were run for 10 million generations, and parameters were sampled every 10,000 generations. For each gene tree analysis, the first 10% of the estimated trees were discarded and the remaining trees were summarized to a maximum clade credibility tree using TreeAnnotator v1.7.5. The four most supported gene tree topologies are shown in Supplemental Figure S4. The placement of the lineages Meliceae and Stipeae within the Pooideae species tree is uncertain. The species tree topology used in the positive selection tests was equal to the most common topologies among the 3,914 maximum clade credibility trees.
Each of the HCOG gene trees was tested for positive selection using the branch-site model in codeml, which is part of PAML v4.7 (Yang, 2007). We only tested branches for positive selection in HCOGs meeting the following criteria. (1) The tested branch had to be an internal branch also in the gene tree (i.e. there were at least two species below the branch). (2) The species below and above the tested branch in the gene tree had to be the same as in the species tree or a subset thereof. (3) The first species to split off under the branch had to be the same as in the species tree (for the early split, either S. lagascae or M. nutans was allowed). We then used the hypergeometric test to identify statistically significant overrepresentation of positive selection among branch-specific DEGs (see “GO Enrichment Tests”) at the Pooideae base, the early-split, and the late-split branches.
Gene Family Size and Age
The gene family size was estimated as the number of genes in the OG divided by the number of species (i.e. the average copy number per species in an OG). Family size was then plotted for each category of DEGs (Supplemental Fig. S5). Error bars were computed as 95% confidence intervals of the mean.
Gene trees were downloaded from the Ensembl plants compara database (Herrero et al., 2016) and used to assign OGs to phylostrata through matching B. distachyon gene identifiers. The age of the root of each ensembl gene tree was used as the phylostratum for the corresponding OG. We used log odds ratio to visualize the enrichment of cold-response genes within each phylostratum (Supplemental Fig. S6).
Identification of Candidate de Novo Transcripts and Gene Tree Reconstruction
For five cold acclimation gene families (CBFIII and CBFIV, DHN, ctCOR, IRIP, and FST), we downloaded previously identified H. vulgare DNA-coding (CDS) and amino acid sequences (Supplemental Table S5) from GenBank. The amino acid reference sequences were used in protein BLAST searches against translated de novo transcripts. Potential candidate transcripts were identified by discarding protein BLAST results with a maximum bitscore < 90 and e-value > 1E-21. When at least one transcript met those criteria, all transcripts of the respective HCOG were defined as candidate transcripts. The HCOGs also contained reference CDS from the Pooideae species H. vulgare, L. perenne, and B. distachyon as well as from the outgroup species O. sativa, S. bicolor, and Z. mays. De novo transcripts not part of an HCOG were included when they met a maximum bitscore > 110 and e-value < 1E-31. MUSCLE v.3.8.31 (Edgar, 2004) was used to create initial multiple sequence alignments (MSAs) by aligning all de novo candidate transcripts and reference CDSs with H. vulgare GenBank CDSs and best BLAST hits for Triticum spp., B. distachyon, and non-Pooideae CDS. To reduce redundancy, reference CDSs were merged with GenBank CDSs when these were identical. To ensure proper tree root resolution, additional outgroups were included where necessary.
After reconstructing an initial gene tree using PhyML (Guindon et al., 2010) assuming an HKY + Γ nucleotide substitution model, we removed sequences that were not part of the clade defined by GenBank CDS and outgroup sequences. Subsequently, we used the program CD-HIT-EST (available from http://weizhongli-lab.org/cd-hit/) to merge de novo transcripts from each species with a sequence identity greater than 90%. Remaining intronic and noncoding regions were removed from the MSAs with exonerate v2.2.0 (Slater and Birney, 2005). Additionally, we excluded fragmented de novo transcripts that were shorter than one-third of the GenBank CDS length. We produced a final MSA with MACSE v1.2 (Ranwez et al., 2011) setting de novo transcripts as less reliable sequences and generated gene trees with PhyML applying a GTR + Γ model.
Accession Number
The raw reads are available in the ArrayExpress database (www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-5300. Major genes identified by our analyses are listed in Table 1.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Experimental design.
Supplemental Figure S2. Comparison of cold response across the Pooideae.
Supplemental Figure S3. Comparison of cold response with previous studies.
Supplemental Figure S4. Gene tree topologies.
Supplemental Figure S5. Association between cold response and gene family size.
Supplemental Figure S6. Phylostratigraphic analysis.
Supplemental Figure S7. Gene tree for the CBFIIIc/d group of the CBF gene family.
Supplemental Figure S8. Gene tree for the CBFIV group of the CBF gene family.
Supplemental Figure S9. Gene tree for the DHN8 gene.
Supplemental Figure S10. Gene tree for the DHN13 gene.
Supplemental Figure S11. Gene tree for the YnSKn-type DHN genes.
Supplemental Figure S12. Gene tree for the ctCOR gene family.
Supplemental Figure S13. Gene tree for the IRIP gene family.
Supplemental Figure S14. Gene tree for the FST gene family.
Supplemental Figure S15. Climatic data.
Supplemental Table S1. Summary statistics for the sampling, transcriptome assembly, coding sequence detection, and OG inference.
Supplemental Table S2. HCOGs.
Supplemental Table S3.. A cross-species expression matrix.
Supplemental Table S4.. Differential expression results for the HCOGs.
Supplemental Table S5. Candidate genes.
Supplemental Table S6. Results for Benchmarking of Universal Single-Copy Orthologs analyses.
Acknowledgments
We thank Åsmund Bjørnstad, Ben Trevaskis, and Mallikarjuna Rao Kovi for providing seeds of cv H. vulgare Igri and Sonja and L. perenne Fagerlin, respectively. We thank U.S.-NPGS GRIN for providing seeds of B. distachyon and S. lagascae. For technical assistance handling plants during growth experiments, we thank Øyvind Jørgensen and Camilla Lorange Lindberg. We thank Erica Leder, Thomas Marcussen, Ursula Brandes, Martin Paliocha, and Camilla Lorange Lindberg for helpful comments on earlier versions of the article.
Footnotes
This work was supported by grants from the Nansen Foundation to S.F. and the TVERRforsk program at the Norwegian University of Life Sciences (NMBU) to S.F., T.R.H., and S.R.S. This work was part of the Ph.D. projects of M.S. and L.G. funded by NMBU.
Articles can be viewed without a subscription.
References
- Aguan K, Sugawara K, Suzuki N, Kusano T (1991) Isolation of genes for low-temperature-induced proteins in rice by a simple subtractive method. Plant Cell Physiol 32: 1285–1289 [Google Scholar]
- Alexa A, Rahnenfuhrer J (2018) topGO: Enrichment analysis for gene ontology. R package version 2.32.0. https://rdrr.io/bioc/topGO/. [Google Scholar]
- Alm V, Busso CS, Ergon A, Rudi H, Larsen A, Humphreys MW, Rognli OA (2011) QTL analyses and comparative genetic mapping of frost tolerance, winter survival and drought tolerance in meadow fescue (Festuca pratensis Huds.). Theor Appl Genet 123: 369–382 [DOI] [PubMed] [Google Scholar]
- Andrés F, Coupland G (2012) The genetic basis of flowering responses to seasonal cues. Nat Rev Genet 13: 627–639 [DOI] [PubMed] [Google Scholar]
- Antikainen M, Griffith M (1997) Antifreeze protein accumulation in freezing-tolerant cereals. Physiol Plant 99: 423–432 [Google Scholar]
- Archibald SB, Bossert WH, Greenwood DR, Farrell BD (2010) Seasonality, the latitudinal gradient of diversity, and Eocene insects. Paleobiology 36: 374–398 [Google Scholar]
- Badawi M, Danyluk J, Boucho B, Houde M, Sarhan F (2007) The CBF gene family in hexaploid wheat and its relationship to the phylogenetic complexity of cereal CBFs. Mol Genet Genomics 277: 533–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30: 2114–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose J, Pottosin II, Shabala SS, Palmgren MG, Shabala S (2011) Calcium efflux systems in stress signaling and adaptation in plants. Front Plant Sci 2: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchenak-Khelladi Y, Verboom AG, Savolainen V, Hodkinson TR (2010) Biogeography of the grasses (Poaceae): A phylogenetic approach to reveal evolutionary history in geographical space and geological time. Bot J Linn Soc 162: 543–557 [Google Scholar]
- Campoli C, Shtaya M, Davis SJ, von Korff M (2012) Expression conservation within the circadian clock of a monocot: Natural variation at barley Ppd-H1 affects circadian expression of flowering time genes, but not clock orthologs. BMC Plant Biol 12: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Ye M, Jiang S (2005) Involvement of GIGANTEA gene in the regulation of the cold stress response in Arabidopsis. Plant Cell Rep 24: 683–690 [DOI] [PubMed] [Google Scholar]
- Choi DW, Rodriguez EM, Close TJ (2002) Barley Cbf3 gene identification, expression pattern, and map location. Plant Physiol 129: 1781–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin PA, Spriggs E, Osborne CP, Strömberg CAE, Salamin N, Edwards EJ (2014) Molecular dating, evolutionary rates, and the age of the grasses. Syst Biol 63: 153–165 [DOI] [PubMed] [Google Scholar]
- Clayton WD, Vorontsova MS, Harman KT, Williamson H (2006) GrassBase: The Online World Grass Flora. Kew Royal Botanical Gardens. https://www.kew.org/data/grassbase/.(January 2018)
- Colton-Gagnon K, Ali-Benali MA, Mayer BF, Dionne R, Bertrand A, Do Carmo S, Charron JB (2014) Comparative analysis of the cold acclimation and freezing tolerance capacities of seven diploid Brachypodium distachyon accessions. Ann Bot 113: 681–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosatti C, Polverino de Laureto P, Bassi R, Cattivelli L (1999) The interaction between cold and light controls the expression of the cold-regulated barley gene cor14b and the accumulation of the corresponding protein. Plant Physiol 119: 671–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosatti C, Rizza F, Badeck FW, Mazzucotelli E, Cattivelli L (2013) Harden the chloroplast to protect the plant. Physiol Plant 147: 55–63 [DOI] [PubMed] [Google Scholar]
- Davis JI, Soreng RJ (1993) Phylogenetic structure in the grass family (Poaceae) as inferred from chloroplast DNA restriction site variation. Am J Bot 80: 1444–1454 [Google Scholar]
- Day IS, Reddy VS, Shad Ali G, Reddy ASN (2002) Analysis of EF-hand-containing proteins in Arabidopsis. Genome Biol 3: RESEARCH0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue MJ. (2008) Colloquium paper: A phylogenetic perspective on the distribution of plant diversity. Proc Natl Acad Sci USA (Suppl 1) 105: 11549–11555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond AJ, Rambaut A (2007) BEAST: Bayesian Evolutionary Analysis by Sampling Trees. BMC Evol Biol 7: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. (2004) MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32: 1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards EJ, Smith SA (2010) Phylogenetic analyses reveal the shady history of C4 grasses. Proc Natl Acad Sci USA 107: 2532–2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldrett JS, Greenwood DR, Harding IC, Huber M (2009) Increased seasonality through the Eocene to Oligocene transition in northern high latitudes. Nature 459: 969–973 [DOI] [PubMed] [Google Scholar]
- Ergon Å, Melby TI, Höglind M, Rognli OA (2016) Vernalization requirement and the chromosomal VRN1-region can affect freezing tolerance and expression of cold-regulated genes in Festuca pratensis. Front Plant Sci 7: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine PVA, Ree RH (2006) Evidence for a time-integrated species-area effect on the latitudinal gradient in tree diversity. Am Nat 168: 796–804 [DOI] [PubMed] [Google Scholar]
- Fischbeck G. (2003) Diversification through breeding. In von Bothmer R, van Hintum T, Knüpffer H, Sato K, eds, Diversity in Barley (Hordeum vulgare). Elsevier, Amsterdam, pp 29–52 [Google Scholar]
- Fjellheim S, Boden S, Trevaskis B (2014) The role of seasonal flowering responses in adaptation of grasses to temperate climates. Front Plant Sci 5: 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagné-Bourque F, Mayer BF, Charron JB, Vali H, Bertrand A, Jabaji S (2015) Accelerated growth rate and increased drought stress resilience of the model grass Brachypodium distachyon colonized by Bacillus subtilis B26. PLoS ONE 10: e0130456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiba G, Vágújfalvi A, Li C, Soltész A, Dubcovsky J (2009) Regulatory genes involved in the determination of frost tolerance in temperate cereals. Plant Sci 176: 12–19 [Google Scholar]
- Global Biodiversity Information Facility (2018) GBIF Occurrence Download. 10.15468/dl.du05xj [DOI] [Google Scholar]
- Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, et al. (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29: 644–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray GR, Chauvin LP, Sarhan F, Huner N (1997) Cold acclimation and freezing tolerance: A complex interaction of light and temperature. Plant Physiol 114: 467–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenup AG, Sasani S, Oliver SN, Walford SA, Millar AA, Trevaskis B (2011) Transcriptome analysis of the vernalization response in barley (Hordeum vulgare) seedlings. PLoS ONE 6: e17900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W, Ferry N, Edwards MG, Bell HA, Othman H, Gatehouse JA, Gatehouse AMR (2015) Proteomic analysis shows that stress response proteins are significantly up-regulated in resistant diploid wheat (Triticum monococcum) in response to attack by the grain aphid (Sitobion avenae). Mol Breed 35: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst Biol 59: 307–321 [DOI] [PubMed] [Google Scholar]
- Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, Couger MB, Eccles D, Li B, Lieber M, et al. (2013) De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc 8: 1494–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K, Zou C, Lehti-Shiu MD, Shinozaki K, Shiu SH (2008) Importance of lineage-specific expansion of plant tandem duplicates in the adaptive response to environmental stimuli. Plant Physiol 148: 993–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley W. (1973) Studies on origin, evolution, and distribution of Gramineae. 5. The subfamily Festucoideae. Aust J Bot 21: 201–234 [Google Scholar]
- Herrero J, Muffato M, Beal K, Fitzgerald S, Gordon L, Pignatelli M, Vilella AJ, Searle SM, Amode R, Brent S, et al. (2016) Ensembl comparative genomics resources. Database (Oxford) 2016: bav096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25: 1965–1978 [Google Scholar]
- Hisano H, Kanazawa A, Kawakami A, Yoshida M, Shimamoto Y, Yamada T (2004) Transgenic perennial ryegrass plants expressing wheat fructosyltransferase genes accumulate increased amounts of fructan and acquire increased tolerance on a cellular level to freezing. Plant Sci 167: 861–868 [Google Scholar]
- Hisano H, Kanazawa A, Yoshida M, Humphreys MO, Iizuka M, Kitamura K, Yamada T (2008) Coordinated expression of functionally diverse fructosyltransferase genes is associated with fructan accumulation in response to low temperature in perennial ryegrass. New Phytol 178: 766–780 [DOI] [PubMed] [Google Scholar]
- Hou C, Tian W, Kleist T, He K, Garcia V, Bai F, Hao Y, Luan S, Li L (2014) DUF221 proteins are a family of osmosensitive calcium-permeable cation channels conserved across eukaryotes. Cell Res 24: 632–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultén E, Fries M (1986) Atlas of North European Vascular Plants (North of the Tropic of Cancer). Koeltz Scientific Books, Königstein, Germany [Google Scholar]
- Janská A, Marsík P, Zelenková S, Ovesná J (2010) Cold stress and acclimation: What is important for metabolic adjustment? Plant Biol (Stuttg) 12: 395–405 [DOI] [PubMed] [Google Scholar]
- Jeknić Z, Pillman KA, Dhillon T, Skinner JS, Veisz O, Cuesta-Marcos A, Hayes PM, Jacobs AK, Chen THH, Stockinger EJ (2014) Hv-CBF2A overexpression in barley accelerates COR gene transcript accumulation and acquisition of freezing tolerance during cold acclimation. Plant Mol Biol 84: 67–82 [DOI] [PubMed] [Google Scholar]
- John UP, Polotnianka RM, Sivakumaran KA, Chew O, Mackin L, Kuiper MJ, Talbot JP, Nugent GD, Mautord J, Schrauf GE, et al. (2009) Ice recrystallization inhibition proteins (IRIPs) and freeze tolerance in the cryophilic Antarctic hair grass Deschampsia antarctica E. Desv. Plant Cell Environ 32: 336–348 [DOI] [PubMed] [Google Scholar]
- Judd WS, Sanders RW, Donoghue MJ (1994) Angiosperm family pairs: Preliminary phylogenetic analyses. Harvard Papers in Botany 1: 1–51 [Google Scholar]
- Karami A, Shahbazi M, Niknam V, Shobbar ZS, Tafreshi RS, Abedini R, Mabood HE (2013) Expression analysis of dehydrin multigene family across tolerant and susceptible barley (Hordeum vulgare L.) genotypes in response to terminal drought stress. Acta Physiol Plant 35: 2289–2297 [Google Scholar]
- Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol Biol Evol 30: 772–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhoff AJ, Moriarty PE, Weiser MD (2014) The latitudinal species richness gradient in New World woody angiosperms is consistent with the tropical conservatism hypothesis. Proc Natl Acad Sci USA 111: 8125–8130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Yamamoto YY, Seki M, Sakurai T, Sato M, Abe T, Yoshida S, Manabe K, Shinozaki K, Matsui M (2003) Identification of Arabidopsis genes regulated by high light-stress using cDNA microarray. Photochem Photobiol 77: 226–233 [DOI] [PubMed] [Google Scholar]
- Knox AK, Li C, Vágújfalvi A, Galiba G, Stockinger EJ, Dubcovsky J (2008) Identification of candidate CBF genes for the frost tolerance locus Fr-Am2 in Triticum monococcum. Plant Mol Biol 67: 257–270 [DOI] [PubMed] [Google Scholar]
- Knox AK, Dhillon T, Cheng H, Tondelli A, Pecchioni N, Stockinger EJ (2010) CBF gene copy number variation at Frost Resistance-2 is associated with levels of freezing tolerance in temperate-climate cereals. Theor Appl Genet 121: 21–35 [DOI] [PubMed] [Google Scholar]
- Kong F, Deng Y, Zhou B, Wang G, Wang Y, Meng Q (2014) A chloroplast-targeted DnaJ protein contributes to maintenance of photosystem II under chilling stress. J Exp Bot 65: 143–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner C. (2016) Plant adaptation to cold climates. F1000Research 5: F1000 Faculty Rev-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosová K, Vítámvás P, Prášil IT (2007) The role of dehydrins in plant response to cold. Biol Plant 51: 601–617 [Google Scholar]
- Kosová K, Prásil IT, Prásilová P, Vítámvás P, Chrpová J (2010) The development of frost tolerance and DHN5 protein accumulation in barley (Hordeum vulgare) doubled haploid lines derived from Atlas 68 × Igri cross during cold acclimation. J Plant Physiol 167: 343–350 [DOI] [PubMed] [Google Scholar]
- Kosová K, Vítámvás P, Prášil IT (2014) Wheat and barley dehydrins under cold, drought, and salinity: What can LEA-II proteins tell us about plant stress response? Front Plant Sci 5: 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumble KD, Demmer J, Fish S, Hall C, Corrales S, DeAth A, Elton C, Prestidge R, Luxmanan S, Marshall CJ, et al. (2008) Characterization of a family of ice-active proteins from the ryegrass, Lolium perenne. Cryobiology 57: 263–268 [DOI] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Henderson DA, Zhu JK (2005) The Arabidopsis cold-responsive transcriptome and its regulation by ICE1. Plant Cell 17: 3155–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lespinet O, Wolf YI, Koonin EV, Aravind L (2002) The role of lineage-specific gene family expansion in the evolution of eukaryotes. Genome Res 12: 1048–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Dewey CN (2011) RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12: 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Rudi H, Stockinger EJ, Cheng H, Cao M, Fox SE, Mockler TC, Westereng B, Fjellheim S, Rognli OA, et al. (2012) Comparative analyses reveal potential uses of Brachypodium distachyon as a model for cold stress responses in temperate grasses. BMC Plant Biol 12: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Stoeckert CJ Jr, Roos DS (2003) OrthoMCL: Identification of ortholog groups for eukaryotic genomes. Genome Res 13: 2178–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Yuan F, Wen Z, Li Y, Wang F, Zhu T, Zhuo W, Jin X, Wang Y, Zhao H, et al. (2015) Genome-wide survey and expression analysis of the OSCA gene family in rice. BMC Plant Biol 15: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Pagani M, Zinniker D, Deconto R, Huber M, Brinkhuis H, Shah SR, Leckie RM, Pearson A (2009) Global cooling during the Eocene-Oligocene climate transition. Science 323: 1187–1190 [DOI] [PubMed] [Google Scholar]
- Liu Z, Xin M, Qin J, Peng H, Ni Z, Yao Y, Sun Q (2015) Temporal transcriptome profiling reveals expression partitioning of homeologous genes contributing to heat and drought acclimation in wheat (Triticum aestivum L.). BMC Plant Biol 15: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston DP III, Hincha DK, Heyer AG (2009) Fructan and its relationship to abiotic stress tolerance in plants. Cell Mol Life Sci 66: 2007–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Chen S, Li A, Zhai C, Jing R (2014) Novel NAC transcription factor TaNAC67 confers enhanced multi-abiotic stress tolerances in Arabidopsis. PLoS ONE 9: e84359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marozsán-Tóth Z, Vashegyi I, Galiba G, Tóth B (2015) The cold response of CBF genes in barley is regulated by distinct signaling mechanisms. J Plant Physiol 181: 42–49 [DOI] [PubMed] [Google Scholar]
- Maruyama K, Urano K, Yoshiwara K, Morishita Y, Sakurai N, Suzuki H, Kojima M, Sakakibara H, Shibata D, Saito K, et al. (2014) Integrated analysis of the effects of cold and dehydration on rice metabolites, phytohormones, and gene transcripts. Plant Physiol 164: 1759–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown M, Schubert M, Marcussen T, Fjellheim S, Preston JC (2016) Evidence for an early origin of vernalization responsiveness in temperate Pooideae grasses. Plant Physiol 172: 416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown M, Schubert M, Preston JC, Fjellheim S (2017) Evolution of the miR5200-FLOWERING LOCUS T flowering time regulon in the temperate grass subfamily Pooideae. Mol Phylogenet Evol 114: 111–121 [DOI] [PubMed] [Google Scholar]
- Morley RJ. (2000) Origin and Evolution of Tropical Rain Forests. John Wiley & Sons, Chichester, UK [Google Scholar]
- Mudelsee M, Bickert T, Lear CH, Lohmann G (2014) Cenozoic climate changes: A review based on time series analysis of marine benthic δ18O records. Rev Geophys 52: 333–374 [Google Scholar]
- Murakami M, Ashikari M, Miura K, Yamashino T, Mizuno T (2003) The evolutionarily conserved OsPRR quintet: Rice pseudo-response regulators implicated in circadian rhythm. Plant Cell Physiol 44: 1229–1236 [DOI] [PubMed] [Google Scholar]
- Murakami M, Yamashino T, Mizuno T (2004) Characterization of circadian-associated APRR3 pseudo-response regulator belonging to the APRR1/TOC1 quintet in Arabidopsis thaliana. Plant Cell Physiol 45: 645–650 [DOI] [PubMed] [Google Scholar]
- Murakami-Kojima M, Nakamichi N, Yamashino T, Mizuno T (2002) The APRR3 component of the clock-associated APRR1/TOC1 quintet is phosphorylated by a novel protein kinase belonging to the WNK family, the gene for which is also transcribed rhythmically in Arabidopsis thaliana. Plant Cell Physiol 43: 675–683 [DOI] [PubMed] [Google Scholar]
- Nakashima K, Takasaki H, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K (2012) NAC transcription factors in plant abiotic stress responses. Biochim Biophys Acta 1819: 97–103 [DOI] [PubMed] [Google Scholar]
- Negi S, Tak H, Ganapathi TR (2015) Expression analysis of MusaNAC68 transcription factor and its functional analysis by overexpression in transgenic banana plants. Plant Cell Tissue Organ Cult 125: 59–70 [Google Scholar]
- Oishi H, Takahashi W, Ebina M, Takamizo T (2010) Expression and gene structure of the cold-stimulated gene Lcs19 of Italian ryegrass (Lolium multiflorum Lam.). Breed Sci 60: 330–335 [Google Scholar]
- Olave-Concha N, Ruiz-Lara S, Muñoz X, Bravo LA, Corcuera LJ (2004) Accumulation of dehydrin transcripts and proteins in response to abiotic stresses in Deschampsia antarctica. Antarct Sci 16: 175–184 [Google Scholar]
- Paina C, Byrne SL, Domnisoru C, Asp T (2014) Vernalization mediated changes in the Lolium perenne transcriptome. PLoS ONE 9: e107365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchy N, Lehti-Shiu M, Shiu SH (2016) Evolution of gene duplication in plants. Plant Physiol 171: 2294–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Lee CM, Doherty CJ, Gilmour SJ, Kim Y, Thomashow MF (2015) Regulation of the Arabidopsis CBF regulon by a complex low-temperature regulatory network. Plant J 82: 193–207 [DOI] [PubMed] [Google Scholar]
- Potts R, Behrensmeyer A (1992) Late Cenozoic terrestrial ecosystems. In Behrensmeyer AK, Damuth JD, DiMichele WA, Potts R, Sues HD, Wing SL, eds, Terrestrial Ecosystems through Time: Evolutionary Paleoecology of Terrestrial Plants and Animals. University of Chicago Press, Chicago, pp 419–451 [Google Scholar]
- Pound MJ, Salzmann U (2017) Heterogeneity in global vegetation and terrestrial climate change during the late Eocene to early Oligocene transition. Sci Rep 7: 43386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston JC, Sandve SR (2013) Adaptation to seasonality and the winter freeze. Front Plant Sci 4: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Kawano N, Yamauchi Y, Ling J, Li D, Tanaka K (2005) Identification and cloning of a submergence-induced gene OsGGT (glycogenin glucosyltransferase) from rice (Oryza sativa L.) by suppression subtractive hybridization. Planta 221: 437–445 [DOI] [PubMed] [Google Scholar]
- Ranwez V, Harispe S, Delsuc F, Douzery EJP (2011) MACSE: Multiple Alignment of Coding SEquences accounting for frameshifts and stop codons. PLoS ONE 6: e22594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2016) R: A Language and Environment for Statistical Computing. The R Foundation. https://www.r-project.org/
- Rinne P, Welling A, Kaikuranta P (1998) Onset of freezing tolerance in birch (Betula pubescens Ehrh.) involves LEA proteins and osmoregulation and is impaired in an ABA-deficient genotype. Plant Cell Environ 21: 601–611 [Google Scholar]
- Romero IG, Ruvinsky I, Gilad Y (2012) Comparative studies of gene expression and the evolution of gene regulation. Nat Rev Genet 13: 505–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorat T. (2006) Plant dehydrins: Tissue location, structure and function. Cell Mol Biol Lett 11: 536–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M (1987) The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol 4: 406–425 [DOI] [PubMed] [Google Scholar]
- Sandve SR, Fjellheim S (2010) Did gene family expansions during the Eocene-Oligocene boundary climate cooling play a role in Pooideae adaptation to cool climates? Mol Ecol 19: 2075–2088 [DOI] [PubMed] [Google Scholar]
- Sandve SR, Rudi H, Asp T, Rognli OA (2008) Tracking the evolution of a cold stress associated gene family in cold tolerant grasses. BMC Evol Biol 8: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandve SR, Kosmala A, Rudi H, Fjellheim S, Rapacz M, Yamada T, Rognli OA (2011) Molecular mechanisms underlying frost tolerance in perennial grasses adapted to cold climates. Plant Sci 180: 69–77 [DOI] [PubMed] [Google Scholar]
- Schliep K, Lopez P, Lapointe FJ, Bapteste E (2011) Harvesting evolutionary signals in a forest of prokaryotic gene trees. Mol Biol Evol 28: 1393–1405 [DOI] [PubMed] [Google Scholar]
- Schubert M, Marcussen T, Meseguer AS, Fjellheim S (2018) The grass subfamily Pooideae: Late Cretaceous origin and climate-driven Cenozoic diversification. bioRxiv 462440 [Google Scholar]
- Shaar-Moshe L, Blumwald E, Peleg Z (2017) Unique physiological and transcriptional shifts under combinations of salinity, drought, and heat. Plant Physiol 174: 421–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Cornilescu CC, Tyler RC, Cornilescu G, Tonelli M, Lee MS, Markley JL (2005) Solution structure of a late embryogenesis abundant protein (LEA14) from Arabidopsis thaliana, a cellular stress-related protein. Protein Sci 14: 2601–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater GSC, Birney E (2005) Automated generation of heuristics for biological sequence comparison. BMC Bioinformatics 6: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Wilson NG, Goetz FE, Feehery C, Andrade SCS, Rouse GW, Giribet G, Dunn CW (2011) Resolving the evolutionary relationships of molluscs with phylogenomic tools. Nature 480: 364–367 [DOI] [PubMed] [Google Scholar]
- Sobkowiak A, Jończyk M, Jarochowska E, Biecek P, Trzcinska-Danielewicz J, Leipner J, Fronk J, Sowiński P (2014) Genome-wide transcriptomic analysis of response to low temperature reveals candidate genes determining divergent cold-sensitivity of maize inbred lines. Plant Mol Biol 85: 317–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltész A, Smedley M, Vashegyi I, Galiba G, Harwood W, Vágújfalvi A (2013) Transgenic barley lines prove the involvement of TaCBF14 and TaCBF15 in the cold acclimation process and in frost tolerance. J Exp Bot 64: 1849–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreng RJ, Davis JI (1998) Phylogenetics and character evolution in the grass family (Poaceae): Simultaneous analysis of morphological and chloroplast DNA restriction site character sets. Bot Rev 64: 1–85 [Google Scholar]
- Soreng RJ, Peterson PM, Romaschenko K, Davidse G, Zuloaga FO, Judziewicz EJ, Filgueiras TS, Davis JI, Morrone O (2015) A worldwide phylogenetic classification of the Poaceae (Gramineae). J Syst Evol 53: 117–137 [Google Scholar]
- Spriggs EL, Christin PA, Edwards EJ (2014) C4 photosynthesis promoted species diversification during the Miocene grassland expansion. PLoS ONE 9: e97722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strömberg CAE. (2011) Evolution of grasses and grassland ecosystems. Annu Rev Earth Planet Sci 39: 517–544 [Google Scholar]
- Suyama M, Torrents D, Bork P (2006) PAL2NAL: Robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res 34: W609–W612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson JT, Crosatti C, Campoli C, Bassi R, Stanca AM, Close TJ, Cattivelli L (2006) Transcriptome analysis of cold acclimation in barley albina and xantha mutants. Plant Physiol 141: 257–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Yamada T (2007) A perennial ryegrass CBF gene cluster is located in a region predicted by conserved synteny between Poaceae species. Theor Appl Genet 114: 273–283 [DOI] [PubMed] [Google Scholar]
- Tamura K, Sanada Y, Tase K, Kawakami A, Yoshida M, Yamada T (2014) Comparative study of transgenic Brachypodium distachyon expressing sucrose:fructan 6-fructosyltransferases from wheat and timothy grass with different enzymatic properties. Planta 239: 783–792 [DOI] [PubMed] [Google Scholar]
- Thomashow MF. (1999) Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol 50: 571–599 [DOI] [PubMed] [Google Scholar]
- Thomashow MF. (2001) So what’s new in the field of plant cold acclimation? Lots! Plant Physiol 125: 89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovska EG, Kolev S, Christov NK, Balint A, Kocsy G, Vágújfalvi A, Galiba G (2014) The expression of CBF genes at Fr-2 locus is associated with the level of frost tolerance in Bulgarian winter wheat cultivars. Biotechnol Biotechnol Equip 28: 392–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanov S, Atanassov A, Nakamura C (2000) Gold responsive gene/protein families and cold/freezing tolerance in cereals. Biotechnol Biotechnol Equip 14: 3–11 [Google Scholar]
- Tutin TG. (1980) Flora Europaea. Cambridge University Press, Cambridge, UK [Google Scholar]
- Uddin MI, Kihara M, Yin L, Perveen MF, Tanaka K (2012) Expression and subcellular localization of antiporter regulating protein OsARP in rice induced by submergence, salt and drought stresses. Afr J Biotechnol 11: 12849–12855 [Google Scholar]
- Vágújfalvi A, Galiba G, Cattivelli L, Dubcovsky J (2003) The cold-regulated transcriptional activator Cbf3 is linked to the frost-tolerance locus Fr-2A on wheat chromosome 5A. Mol Genet Genomics 269: 60–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigeland MD, Spannagl M, Asp T, Paina C, Rudi H, Rognli OA, Fjellheim S, Sandve SR (2013) Evidence for adaptive evolution of low-temperature stress response genes in a Pooideae grass ancestor. New Phytol 199: 1060–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijn I, Smeekens S (1999) Fructan: More than a reserve carbohydrate? Plant Physiol 120: 351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser V, Clayton WD, Simpson DA, Freckleton RP, Osborne CP (2014) Mechanisms driving an unusual latitudinal diversity gradient for grasses. Glob Ecol Biogeogr 23: 61–75 [Google Scholar]
- Wang D, Liu J, Li C, Kang H, Wang Y, Tan X, Liu M, Deng Y, Wang Z, Liu Y, et al. (2016) Genome-wide association mapping of cold tolerance genes at the seedling stage in rice. Rice (N Y) 9: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Cai G, Kong F, Deng Y, Ma N, Meng Q (2014) Overexpression of tomato chloroplast-targeted DnaJ protein enhances tolerance to drought stress and resistance to Pseudomonas solanacearum in transgenic tobacco. Plant Physiol Biochem 82: 95–104 [DOI] [PubMed] [Google Scholar]
- Wang ZY, Xiong L, Li W, Zhu JK, Zhu J (2011) The plant cuticle is required for osmotic stress regulation of abscisic acid biosynthesis and osmotic stress tolerance in Arabidopsis. Plant Cell 23: 1971–1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse RM, Seppey M, Simão FA, Manni M, Ioannidis P, Klioutchnikov G, Kriventseva EV, Zdobnov EM (2018) BUSCO applications from quality assessments to gene prediction and phylogenomics. Mol Biol Evol 35: 543–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens JJ, Donoghue MJ (2004) Historical biogeography, ecology and species richness. Trends Ecol Evol 19: 639–644 [DOI] [PubMed] [Google Scholar]
- Wittkopp PJ, Kalay G (2011) Cis-regulatory elements: Molecular mechanisms and evolutionary processes underlying divergence. Nat Rev Genet 13: 59–69 [DOI] [PubMed] [Google Scholar]
- Woods DP, McKeown MA, Dong Y, Preston JC, Amasino RM (2016) Evolution of VRN2/Ghd7-like genes in vernalization-mediated repression of grass flowering. Plant Physiol 170: 2124–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Lou P, Hermand V, Aman R, Park HJ, Yun DJ, Kim WY, Salmela MJ, Ewers BE, Weinig C, et al. (2015) Allelic polymorphism of GIGANTEA is responsible for naturally occurring variation in circadian period in Brassica rapa. Proc Natl Acad Sci USA 112: 3829–3834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Takano T, Tanaka K, Ishige T, Terashima S, Endo C, Kurusu T, Yajima S, Yano K, Tada Y (2015) Comprehensive analysis of transcriptome response to salinity stress in the halophytic turf grass Sporobolus virginicus. Front Plant Sci 6: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. (2007) PAML 4: Phylogenetic analysis by maximum likelihood. Mol Biol Evol 24: 1586–1591 [DOI] [PubMed] [Google Scholar]
- Yang Y, Smith SA (2014) Orthology inference in nonmodel organisms using transcriptomes and low-coverage genomes: Improving accuracy and matrix occupancy for phylogenomics. Mol Biol Evol 31: 3081–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang QS, Gao J, He WD, Dou TX, Ding LJ, Wu JH, Li CY, Peng XX, Zhang S, Yi GJ (2015) Comparative transcriptomics analysis reveals difference of key gene expression between banana and plantain in response to cold stress. BMC Genomics 16: 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Mohnen D, Gelineo-Albersheim I, Xu Y, Hahn MG (2011) Glycosyltransferases of the GT8 family. In Ulvskov P, ed, Annual Plant Reviews: Plant Polysaccharides, Biosynthesis and Bioengineering, Vol 41 Wiley-Blackwell, pp 167–211 [Google Scholar]
- Yuan F, Yang H, Xue Y, Kong D, Ye R, Li C, Zhang J, Theprungsirikul L, Shrift T, Krichilsky B, et al. (2014) OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 514: 367–371 [DOI] [PubMed] [Google Scholar]
- Zachos J, Pagani M, Sloan L, Thomas E, Billups K (2001) Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292: 686–693 [DOI] [PubMed] [Google Scholar]
- Zdobnov EM, Tegenfeldt F, Kuznetsov D, Waterhouse RM, Simão FA, Ioannidis P, Seppey M, Loetscher A, Kriventseva EV (2017) OrthoDB v9.1: Cataloging evolutionary and functional annotations for animal, fungal, plant, archaeal, bacterial and viral orthologs. Nucleic Acids Res 45: D744–D749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Fei SZ, Arora R, Hannapel DJ (2010) Ice recrystallization inhibition proteins of perennial ryegrass enhance freezing tolerance. Planta 232: 155–164 [DOI] [PubMed] [Google Scholar]
- Zhong J, Robbett M, Poire A, Preston JC (2018) Successive evolutionary steps drove Pooideae grasses from tropical to temperate regions. New Phytol 217: 925–938 [DOI] [PubMed] [Google Scholar]