Two high-CO2/hypoxia responsive transcription factors from persimmon fruit, DkERF24 and DkWRKY1, form a complex and synergistically transactivate the promoter of the hypoxia-responsive gene DkPDC2.
Abstract
Identification and functional characterization of hypoxia-responsive transcription factors is important for understanding plant responses to natural anaerobic environments and during storage and transport of fresh horticultural products. In this study, yeast one-hybrid library screening using the persimmon (Diospyros kaki) pyruvate decarboxylase (DkPDC2) promoter identified three ethylene response factor (ERF) genes (DkERF23/DkERF24/DkERF25) and four WRKY transcription factor genes (DkWRKY/DdkWRKY5/DkWRKY6/DkWRKY7) that were differentially expressed in response to high CO2 (95%, with 4% N2 and 1% oxygen) and high N2 (99% N2 and 1% oxygen). Yeast one-hybrid assays and electrophoretic mobility shift assays indicated that DkERF23, DkERF24, DkERF25, DkWRKY6, and DkWRKY7 could directly bind to the DkPDC2 promoter. Dual-luciferase assays confirmed that these transcription factors were capable of transactivating the DkPDC2 promoter. DkERF24 and DkWRKY1 in combination synergistically transactivated the DkPDC2 promoter, and yeast two-hybrid and bimolecular fluorescence complementation assays confirmed protein–protein interaction between DkERF24 and DkWRKY1. Transient overexpression of DkERF24 and DkWRKY1 separately and in combination in persimmon fruit discs was effective in maintaining insolubilization of tannins, concomitantly with the accumulation of DkPDC2 transcripts. Studies with Arabidopsis (Arabidopsis thaliana) homologs AtERF1 and AtWRKY53 indicated that similar protein–protein interactions and synergistic regulatory effects also occur with the DkPDC2 promoter. We propose that an ERF and WRKY transcription factor complex contributes to responses to hypoxia in both persimmon fruit and Arabidopsis, and the possibility that this is a general plant response requires further investigation.
Transcription factors (TFs) play important roles in plant responses to hypoxia. Our understanding of these responses has been advanced significantly by the characterization of subfamily VII of the ethylene response factors (ERFs). Five ERF genes (HRE1, HRE2, RAP2.2, RAP2.3, and RAP2.12) have been reported as the main plant oxygen-sensing regulators and have been shown to control fermentation-related alcohol dehydrogenase (ADH) and pyruvate decarboxylase (PDC) genes in Arabidopsis (Arabidopsis thaliana; Hinz et al., 2010; Licausi et al., 2010; Yang et al., 2011; Bui et al., 2015; Papdi et al., 2015). ERFs involved in the regulation of hypoxia responses have also been reported in other plants, such as ERFVII in Rumex acetosa, Rumex palustris, Rorippa sylvestris, and Rorippa amphibia (van Veen et al., 2014) and SUBMERGENCE TOLERANCE-RELATED SUBMERGENCE 1 (Sub1) in rice (Oryza sativa; Xu et al., 2006). Control of the stability of hypoxia-responsive ERFs by the N-end rule is thought to be the main mechanism whereby plants sense and respond to low oxygen (Gibbs et al., 2011; Licausi et al., 2011a).
However, there may be other important TFs involved in the hypoxia response that may or may not operate via the N-end rule. A few other hypoxia-related TFs have been reported, such as AtMYB2 in Arabidopsis, which physically interacts with the AtADH1 promoter (Hoeren et al., 1998). Overexpression of AtMYB2 enhanced AtADH1 expression (Abe et al., 2003). In another example, the heat shock factor HsfA2 was shown to be responsive to low-oxygen conditions and to transactivate downstream genes (ADH) to enable plants to acquire anoxia tolerance (Banti et al., 2010). Wheat (Triticum aestivum) TaMYB1 is also responsive to low oxygen (Lee et al., 2007). Moreover, omics-based analyses have shown that additional differentially expressed TFs may also be related to the hypoxia response in different Arabidopsis organs (Branco-Price et al., 2005; Liu et al., 2005; Mustroph et al., 2009; Lee et al., 2011; Licausi et al., 2011b). The interactions between different hypoxia-responsive TFs and their precise roles are still unclear, however, and relationships between different hypoxia-responsive TFs have rarely been reported.
Unlike most plants, fruits frequently experience artificial low-oxygen environments during postharvest storage, where, for example, controlled-atmosphere storage is widely used (Ali et al., 2016; Bekele et al., 2016; Matityahu et al., 2016). Understanding the fruit response to anaerobic environments could lead to improvements in storage technologies and improve fruit quality, and there have been many physiological and biochemical studies on fruit responses to reduced-oxygen environments (Ali et al., 2016). Indeed, it was one such study that led to the discovery of the role of 1-aminocyclopropane-1-carboxylic acid as a precursor in ethylene synthesis (Adams and Yang, 1978). In apple (Malus domestica) fruit, MdRAP2.12 protein has been shown to differentially accumulate in samples held at different oxygen concentrations, indicating that the oxygen-sensing mechanisms described in Arabidopsis are also present in apple fruit (Cukrov et al., 2016).
Persimmon (Diospyros kaki) fruit are ideal material for studies on hypoxia response in fruit. Most cultivated persimmons are of the astringent type and are rich in soluble condensed tannins (Akagi et al., 2009). Mature fruit require postharvest treatments to remove the astringency by insolubilization of soluble condensed tannins (Wang et al., 1997). The mechanism operates by induction of PDC, and to a lesser extent ADH, which leads to acetaldehyde accumulation. This precipitates soluble tannins, removing the astringency (Taira et al., 2001; Salvador et al., 2007; Min et al., 2012). High-CO2 treatment is the most effective and widely used method, with CO2 concentrations usually set at 95% and oxygen reduced to 1%, elevating ADH and PDC activities and triggering acetaldehyde metabolism (Ikegami et al., 2007; Salvador et al., 2007; Min et al., 2012; Yin et al., 2012). Thus, for fruit, the molecular basis of the hypoxia response has been most extensively studied in persimmon. Some TFs have been identified and shown to activate transcription of hypoxia-responsive genes (Hoeren et al., 1998; Abe et al., 2003; Min et al., 2012, 2014; Fang et al., 2016; Zhu et al., 2018), but there are still gaps in our knowledge. Twenty-two high-CO2/hypoxia-responsive DkERF genes have been isolated previously from persimmon, and DkERF9, DkERF10, DkERF19, and DkERF22 were characterized as direct regulators of the alcoholic fermentation genes DkPDC2 and DkADH1 (Min et al., 2012, 2014). However, only DkERF3 and DkERF10 are group VII subfamily members with conserved N-end MC domains (MCGGAII), suggesting that they may be involved in a hypoxia response mechanism in persimmon similar to that described in Arabidopsis (Gibbs et al., 2011; Licausi et al., 2011a). Yet evidence for a cascade involving additional DkERFs (DkERF18, DkERF19, DkERF21, and DkERF22) and DkMYBs (DkMYB6 and DkMYB10) was found that involved transcriptional regulation of DkERF9, DkERF10, and DkERF19 (Zhu et al., 2018). The potential roles of these other TFs in persimmon fruit deastringency, and also in the Arabidopsis anaerobic response, are unclear.
In the current study, yeast one-hybrid (Y1H) library screening was conducted to identify additional persimmon TFs that interact with the DkPDC2 promoter, and interactions between TFs and the DkPDC2 promoter were investigated by dual-luciferase and Y1H assays. Further yeast two-hybrid (Y2H) and bimolecular fluorescence complementation (BiFC) experiments identified a synergistic interaction involving an ERF-WRKY complex that transactivated the PDC2 promoter. Parallel experiments confirmed the ability of Arabidopsis ERF-WRKY homologs to participate in this hypoxia response.
RESULTS
Identification and Characterization of TFs Targeting the Hypoxia-Responsive DkPDC2 Promoter by Y1H Library Screening
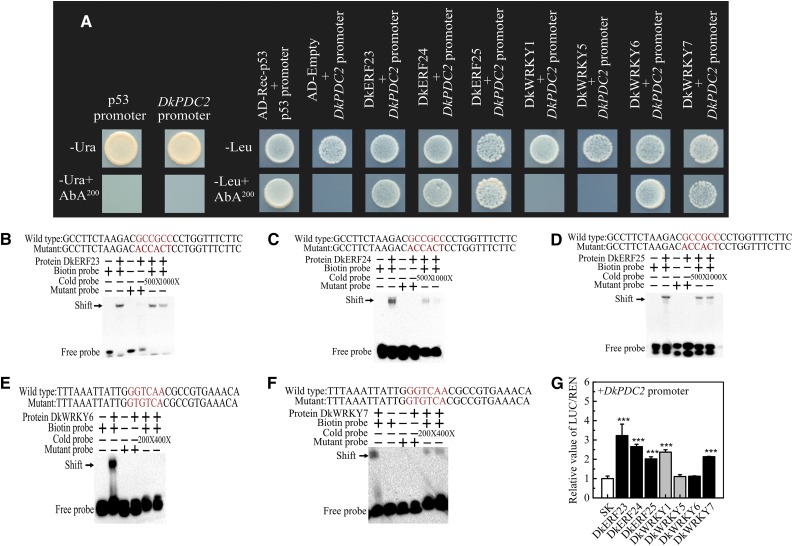
To understand the control of gene expression in response to hypoxia, especially the cross talk between master regulators, the ideal objective would be to identify all hypoxia-responsive TFs that interact with the same promoter. Here, Y1H-based library screening was used with the DkPDC2 promoter as the bait; 95 colonies were sequenced and 45 sequences obtained (Supplemental Table S1). Among them, three DkERF (DkERF23, DkERF24, and DkERF25; MH054905-7) and four DkWRKY (DkWRKY1, KY849608; and DkWRKY5, DkWRKY6, and DkWRKY7; MH054908-10) TF genes were identified and characterized. Individual Y1H assays generated results similar to those from the library screening, except in the case of DkWRKY1 and DkWRKY5 (Fig. 1A). Electrophoretic mobility shift assays were performed in order to verify the interactions and locate more precisely the cis-element involved in binding. A GCC box (GCCGCC) for AP2/ERF TFs and a W-box (GGTCAA; Birkenbihl et al., 2017) for WRKY TFs were found in the DkPDC2 promoter region (Supplemental Fig. S1). Biotinylated probes containing these sequences were able to bind DkERF23, DkERF24, DkERF25, DkWRKY6, and DkWRKY7 proteins, and the addition of a high concentration of cold probe significantly reduced the binding affinity of the biotinylated probe (Fig. 1, B–F), whereas DkWRKY1 and DkWRKY5 could not bind to the DkPDC2 promoter (Supplemental Fig. S2). These results indicated that DkERFs could physically bind to the GCC-box motif, and DkWRKYs targeted the W-box motif of the DkPDC2 promoter. Dual-luciferase assays indicated that DkERF23, DkERF24, DkERF25, DkWRKY1, and DkWRKY7 had significant activation effects on transcription from the DkPDC2 promoter (>2-fold increase), while DkWRKY5 and DkWRKY6 had no significant effects on the DkPDC2 promoter (Fig. 1G). The regulatory effects of these TFs on the DkPDC2 promoter were confirmed by histochemical staining of GUS activity in Nicotiana benthamiana leaves, which showed that transient overexpression (TOX) of DkERF23, DkERF24, DkERF25, DkWRKY1, and DkWRKY7 could significantly up-regulate the DkPDC2 promoter-GUS expression (Supplemental Fig. S3). These TFs had either limited or no effect on promoters of the other deastringency-related persimmon genes, with the exception of DkERF24, which was able to activate the DkERF9 promoter above the 2-fold threshold (Supplemental Fig. S4).
Figure 1.
Action of DkERFs and DkWRKYs on the promoter of DkPDC2. A, Physical interactions between DkERFs, DkWRKYs, and the DkPDC2 promoter, using Y1H analysis. Auto-activation of promoters was tested on SD medium lacking Ura in the presence of AbA (Aureobasidin A; SD/−Ura+AbA). Interaction was determined on SD medium lacking Leu in the presence of AbA (SD/−Leu+AbA). B to F, EMSA of DkERF23, DkERF24, DkERF25, DkWRKY6, and DkWRKY7 binding to the DkPDC2 promoter. Purified TF proteins and biotin-labeled DNA probe were mixed and analyzed on 6% native polyacrylamide gels. The presence (+) or absence (−) of specific probes is indicated. The concentration of the cold probe is shown; the biotinylated probe concentration was 1 nM. G, Regulatory effects of DkERFs and DkWRKYs on the DkPDC2 promoter. The LUC/REN ratio of the empty vector plus promoter was used as calibrator (set as 1). Error bars indicate SEs from five biological replicates (the regulatory effects of various TFs on the DkPDC2 promoter were all compared to the value of the control [empty vector pGreen II 0029 62-SK]; *** P < 0.001).
Expression of TFs in Response to High-CO2 Treatment in Various Persimmon Cultivars
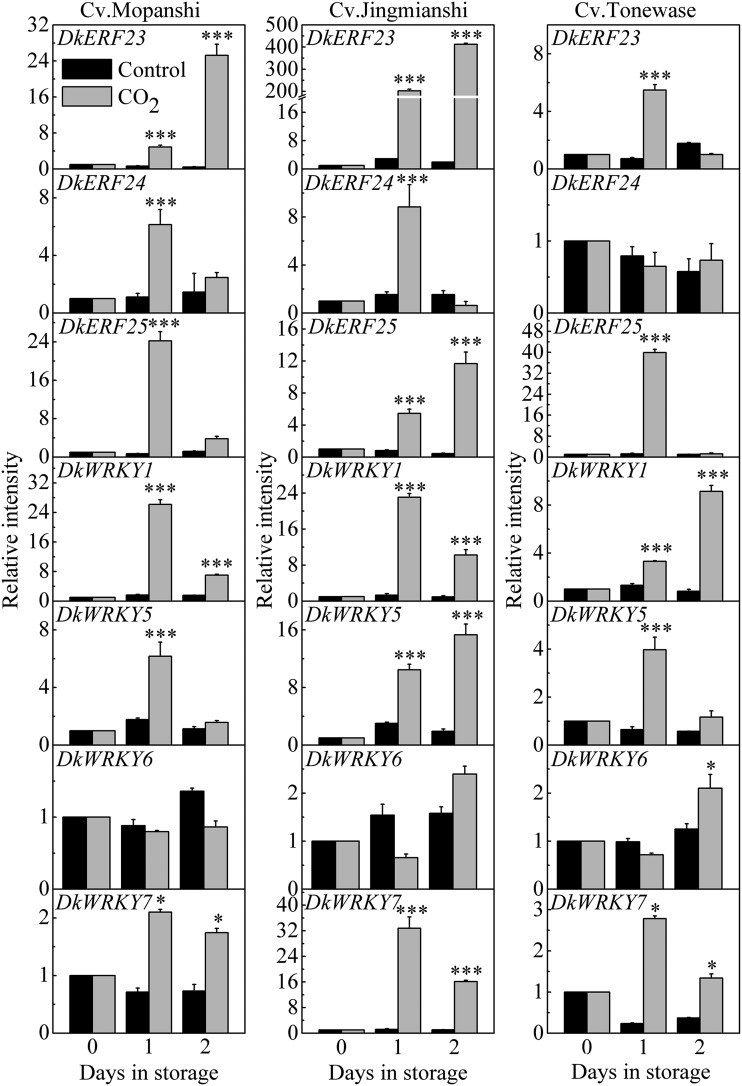
In order to analyze the responses of TFs to deastringency treatment, three different cultivars were selected, ‘Mopanshi’, ‘Jingmianshi’, ‘Tonewase’. Our previous data indicated that CO2 treatment (95%, 1 d) was effective in insolubilizing the soluble tannins in postharvest fruit of all three cultivars (Wang et al., 2017; Zhu et al., 2018). Expression of the seven TFs was analyzed by reverse transcription quantitative PCR (RT-qPCR), and with the exception of DkWRKY6, the TFs were responsive to high-CO2 treatment in all three cultivars (Fig. 2) and were designated as high-CO2/low-oxygen responsive. Some genes showed quantitatively different expression patterns between the cultivars, e.g. DkERF23 showed >200-fold induction in ‘Jingmianshi’ and much less activation in ‘Tonewase’ fruit (∼5-fold) at 1 d; in contrast, DkERF25 showed the highest induction by CO2 treatment in ‘Tonewase’ (∼40-fold at 1 d; Fig. 2).
Figure 2.
Expression of DkERFs and DkWRKYs in response to high CO2 treatment. Persimmon fruit cultivars ‘Mopanshi’, ‘Jingmianshi’, and ‘Tonewase’ were incubated in 95% CO2, 4% N2, and 1% oxygen for 1 d and then in air at 20°C. For determination of relative mRNA abundance, the values at day 0 were set as 1. Values are means (+SE) from three biological replicates (gene expression was compared between high CO2 treated fruit and control fruit at each sampling point; *P < 0.05, ***P < 0.001).
In order to clarify potentially different effects of high CO2 and hypoxia, the hypoxia treatment (99% N2, 1% oxygen) was applied to Gongcheng-shuishi (Zhu et al., 2018). The expression analysis indicated that DkERF25 and DkWRKY5 were up-regulated only by high CO2, not by the hypoxia treatment. DkERF23, DkERF24, and DkWRKY1 were responsive to both high CO2 and hypoxia. Expression of DkWRKY1 was significantly weaker in response to hypoxia treatment compared to high-CO2 treatment. In contrast, DkERF23 showed greater abundance in response to hypoxia treatment (Supplemental Fig. S5). Among the seven TFs, DkWRKY7 was undetectable in Gongcheng-shuishi.
Subcellular Localization Analysis of TFs
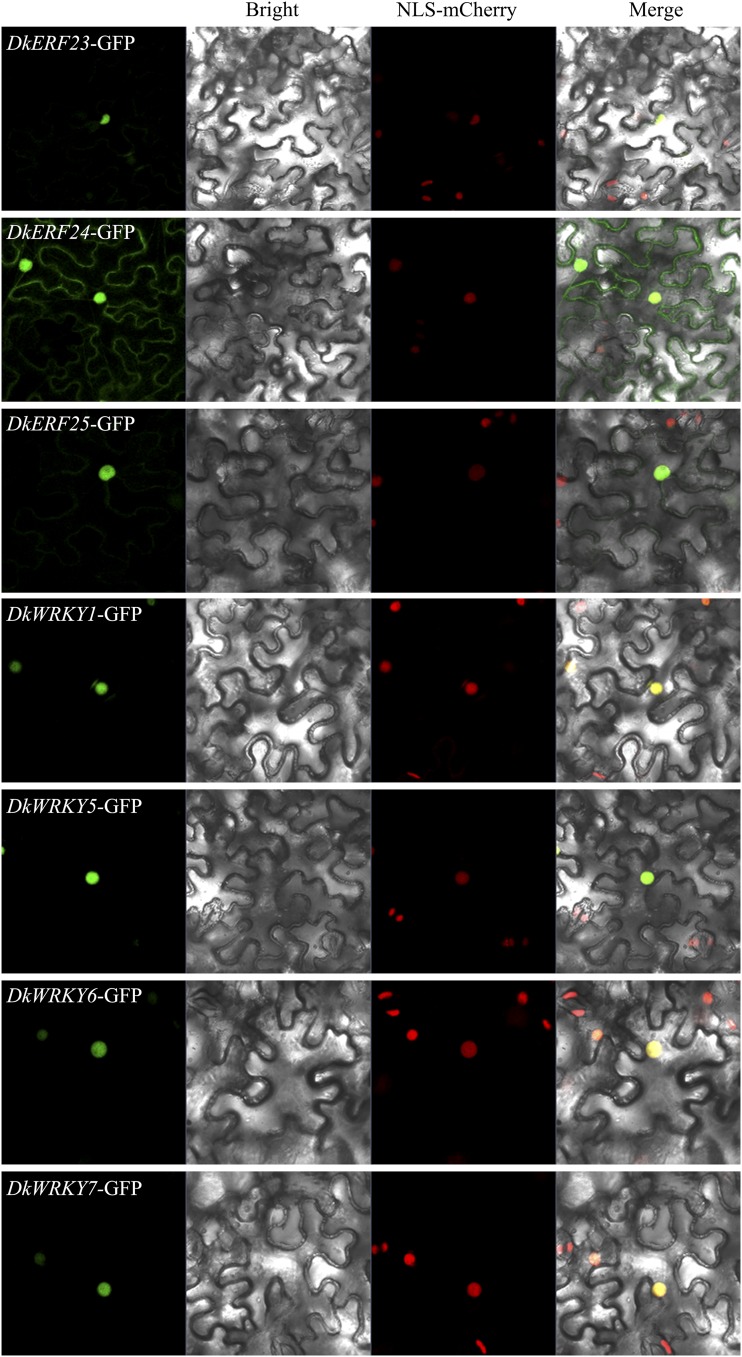
Subcellular localization assays were performed in N. benthamiana leaves stably transformed with a nuclear marker in order to visualize the subcellular locations of the seven TFs. DkERF23, DkERF25, DkWRKY1, DkWRKY5, DkWRKY6, and DkWRKY7 all gave strong signals in the nucleus, while DkERF24 showed signals in both the nucleus and the cell membrane (Fig. 3).
Figure 3.
Subcellular localization of DkERFs-GFP and DkWRKYs-GFP in N. benthamiana leaves stably transformed with a red nuclear localization marker and agroinfiltrated with GFP DkERFs-GFP and DkWRKYs-GFP. The fluorescence was measured at 488 nm with a laser scanning microscope 780 microscope and photographed. Bars = 25 μm.
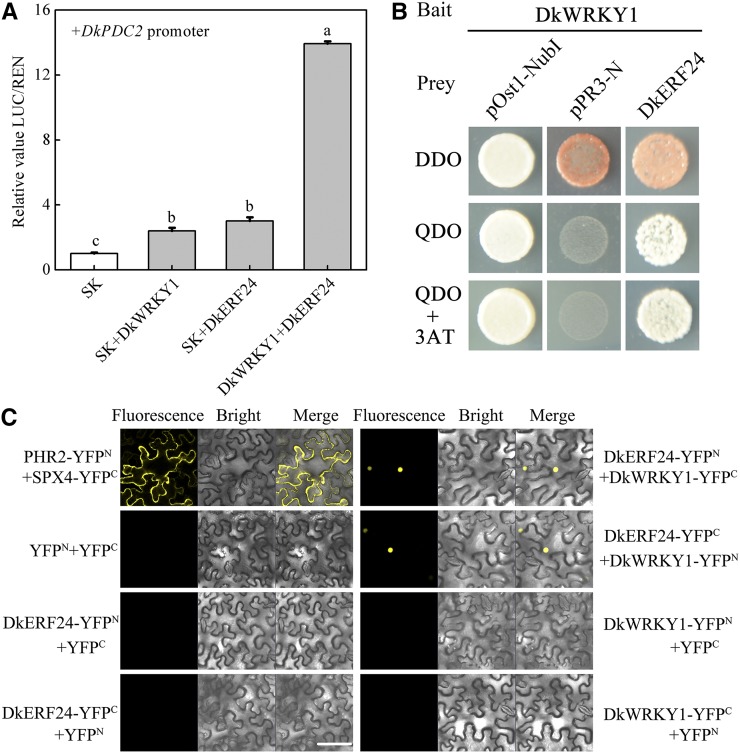
Synergistic Effects of DkERF24 and DkWRKY1 on Transcription from the DkPDC2 Promoter and Analysis of Protein–Protein Interactions
The effects of different combinations of TFs on the DkPDC2 promoter were analyzed by the dual-luciferase assay. A few combinatorial effects were found between various TFs (Supplemental Fig. S6A), the most notable being the interaction between DkERF24 and DkWRKY1. This showed a 13-fold synergistic activation of DkPDC2 promoter compared to the <4-fold activation of either TF separately (Fig. 4A). The Y2H assay indicated that the interaction between DkERF24 and DkWRKY1 (Fig. 4B) was the only direct protein–protein interaction (Supplemental Fig. S6B). The interaction between DkERF24 and DkWRKY1 was also verified by the BiFC assay (Fig. 4C). The BiFC results showed that negative combinations, such as those containing yellow fluorescent protein (YFP)—YFPN/DkWRKY1-YFPC, YFPC/DkWRKY1-YFPN, YFPN/DkERF24-YFPC, YFPC/DkERF24-YFPN, and YFPN/YFPC—did not produce any detectable fluorescence signals, while the positive combination of PHR2-YFPN/SPX4-YFPC, and the coexpression of DkERF24-YFPN/DkWRKY1-YFPC with DkERF24-YFPC/DkWRKY1-YFPN, gave strong signals located in the nuclei (Fig. 4C).
Figure 4.
Effects of DkERF24 and DkWRKY1 separately and in combination on transcription from the DkPDC2 promoter and analysis of their protein–protein interactions. A, Effect of the combination of DkERF24 and DkWRKY1 on the DkPDC2 promoter. The LUC/REN ratio of the empty vector (pGreen II 0029 62-SK [SK]) plus promoter was used as calibrator (set as 1). Error bars indicate SEs from five biological replicates. Different letters above the columns represent significant differences (the combination effects were compared to two individual effects; P < 0.05). B, Interaction between DkERF24 and DkWRKY1 in Y2H assays. Liquid cultures of double transformants were plated at OD600 = 0.01 dilutions on synthetic dropout selective media: (1) SD medium lacking Trp and Leu (DDO); (2) SD medium lacking Trp, Leu, His, and Ade (QDO); and (3) SD medium lacking Trp, Leu, His, and Ade and supplemented with 5 mm 3-amino-1,2,4-triazole (QDO+3AT). Protein–protein interactions were determined on QDO and QDO+3AT. pOst1-NubI, positive control; pPR3-N, negative control. C, In vivo interaction between DkERF24 and DkWRKY1 was determined using BiFC. N- and C-terminal fragments of YFP (indicated in the figure as YFPN and YFPC) were fused to the C and N termini of DkERF24 and DkWRKY1, respectively. Combinations of YFPN and YFPC with the corresponding DkERF24 and DkWRKY1 constructs were used as negative controls. Fluorescence of YFP represents protein–protein interaction. Bars = 50 μm.
TOX Analysis in Persimmon Fruit Discs
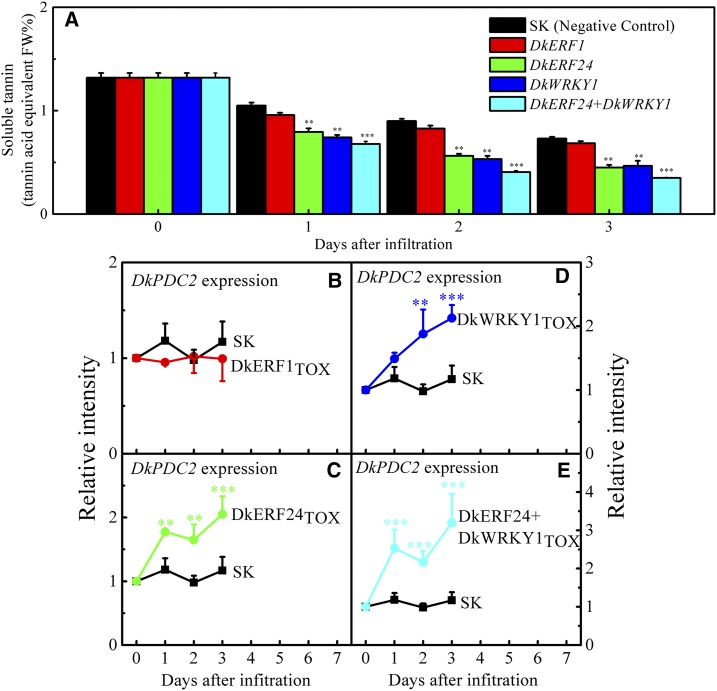
TOX analyses were performed with fruit discs to verify the functions of TFs involved in persimmon fruit deastringency. DkERF1, which had no transactivation effect on the DkPDC2 promoter (Min et al., 2012), was chosen as a negative control. The content of soluble tannins in the discs treated with both the empty vector (pGreen II 0029 62-SK, the second negative control) and TFs declined during incubation (Fig. 5A). The combination of DkERF24 and DkWRKY1, as well as the individual TFs in that combination, resulted in significantly lower content of soluble tannins compared with the controls (pGreen II 0029 62-SK and DkERF1; Fig. 5A). Interactions between TFs and DkPDC2 were also analyzed, and the results indicated that TOX of these TFs could significantly up-regulate the endogenous DkPDC2 transcript in persimmon fruit discs, supporting the evidence for interactions of hypoxia-responsive TFs with the DkPDC2 promoter (Fig. 5, C–E). In particular, the combination of DkERF24 and DkWRKY1 showed a more obvious up-regulation in DkPDC2 transcripts than did either TF alone (Fig. 5, C–E).
Figure 5.
TOX of TFs in persimmon fruit discs. The TOX experiments were conducted with two negative controls (empty vector pGreen II 002962-SK [SK] and DkERF1) and target TFs (DkERF24, DkWRKY1, and DkERF24+DkWRKY1) using persimmon fruit discs. Tissues from each of the infiltrated discs were taken to measure the soluble tannin content (A) and relative gene expression levels of related downstream genes compared with the SK (B–E) at daily intervals during the 3-d incubation. Soluble tannin content was measured with Folin-Ciocalteu reagent and quantitated by reference to standard tannin acid. Error bars indicate SEs from three biological replicates. The soluble tannin content and gene expression were compared between geneTOX and the control (empty vector) at each sampling point; **P < 0.01, ***P < 0.001.
Arabidopsis AtERF1 and AtWRKY53 Also Transactivate the DkPDC2 Promoter
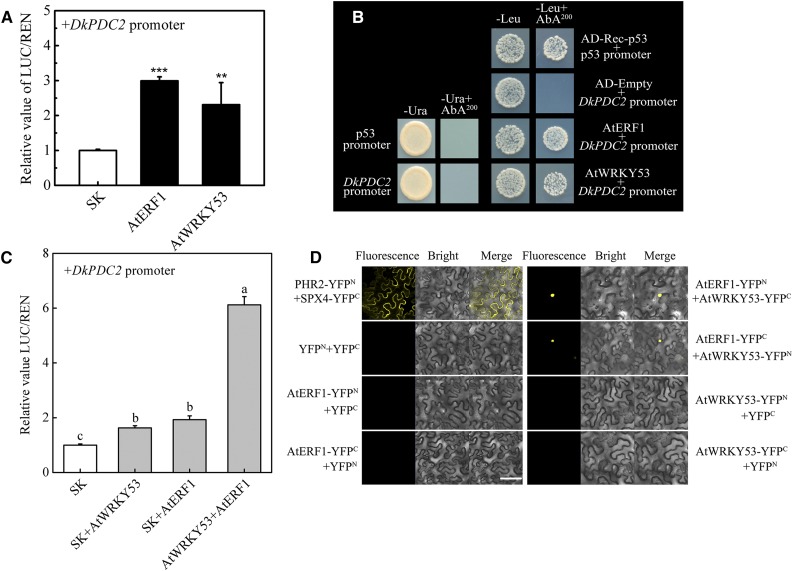
Based on the phylogenetic trees (Supplemental Figs. S7 and S8), AtERF1, AtWRKY41, and AtWRKY53, are genes homologous to DkERF24 and DkWRKY1 in Arabidopsis. AtWRKY53 has been shown to be responsive to hypoxia, while AtERF1 showed a slight negative response (Mustroph et al., 2009). The possibility of a conserved mechanism for the hypoxia response involving ERF and WRKY action in both Arabidopsis and persimmon was investigated. Using dual-luciferase assays, AtERF1 and AtWRKY53 showed significant transcriptional activation of the DkPDC2 promoter (∼3.0- and 2.3-fold, respectively; Fig. 6A), while AtWRKY41 had no effect on the DkPDC2 promoter (Supplemental Fig. S9A). Histochemical GUS staining using the DkPDC2 promoter fused to the GUS reporter gene gave more intensive blue color with AtERF1 and AtWRKY53 than did the empty vector (pGreen II 0029 62-SK). The gus (formerly uidA) transcripts in AtERF1TOX and AtWRKY53TOX were higher than the empty vector (Supplemental Fig. S10). Furthermore, using the Y1H assay, it was found that both AtERF1 and AtWRKY53 could physically bind to the DkPDC2 promoter (Fig. 6B).
Figure 6.
Regulatory roles of AtERF1 and AtWRKY53 on the DkPDC2 promoter. A, Regulatory effects of AtERF1 and AtWRKY53 on the promoter of DkPDC2. The LUC/REN ratio of the empty vector plus promoter was used as calibrator (set as 1). Error bars indicate SEs from five biological replicates (the regulatory effects of various TFs on the DkPDC2 promoter were all compared to the value of the control [empty vector pGreen II 0029 62-SK (SK)]; **P < 0.01, ***P < 0.001). B, Physical interactions between AtERF1, AtWRKY53 and DkPDC2 promoter, using Y1H analysis. Auto-activation of promoters was tested on SD medium lacking Ura in the presence of AbA (SD/−Ura+AbA). Interaction was determined on SD medium lacking Leu in the presence of AbA (SD/−Leu+AbA). C, Effect of the combination of AtERF1 and AtWRKY53 on the DkPDC2 promoter. The LUC/REN ratio of the empty vector (SK) plus promoter was used as calibrator (set as 1). Error bars indicate SEs from five biological replicates. Different letters above the columns represent significant differences (the combination effects were compared to two individual effects; P < 0.05). D, In vivo interaction between AtERF1 and AtWRKY53, determined using BiFC. AtERF1 and AtWRKY53 proteins were fused to the N and C termini of YFP (YFPN and YFPC), respectively. PHR2-YFPN and SPX4-YFPC were used as positive controls, while combinations of YFPN and YFPC with the corresponding AtERF1 and AtWRKY53 constructs were used as negative controls. Fluorescence of YFP represents protein–protein interaction. Bars = 50 μm.
The combination of AtERF1 and AtWRKY53 also showed higher activation of the DkPDC2 promoter (LUC/REN = 6.12) than did either of them singly (1.92-fold and 1.63-fold; Fig. 6C), the combination of AtERF1 and AtWRKY41 showing no additive effect on the DkPDC2 promoter (Supplemental Fig. S9B). BiFC analysis showed that the coexpression of AtERF1-YFPN/AtWRKY53-YFPC and AtERF1-YFPC/AtWRKY53-YFPN also gave strong signals in the nucleus (Fig. 6D), confirming a protein–protein interaction between AtERF1 and AtWRKY53.
DISCUSSION
Multiple TFs Involved in Anaerobic Metabolism/Hypoxia Response May Target the Same Promoter
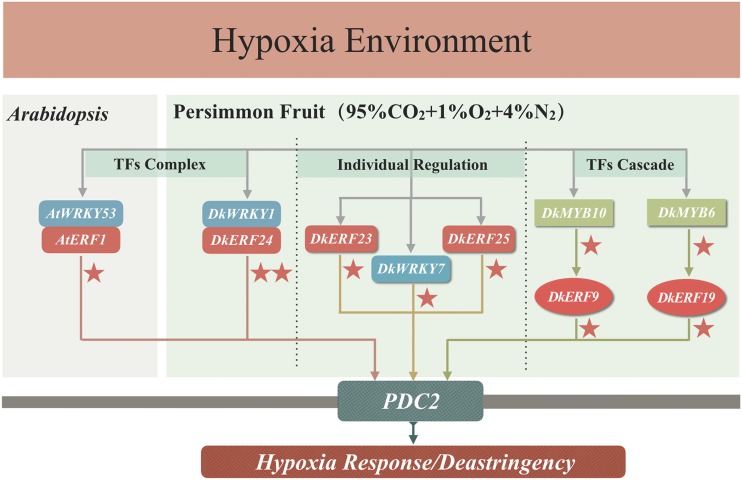
In our previous studies, at least two DkERFs (DkERF9 and DkERF19; Min et al., 2012, 2014) and DkMYB6 (Fang et al., 2016) were shown to be direct activators of the DkPDC2 promoter. Several types of TFs, in addition to ERFs, have been suggested to be hypoxia responsive in both persimmon (e.g. TGA [Zhu et al., 2016] and MYB [Zhu et al., 2018]) and Arabidopsis. Omics-based approaches in Arabidopsis indicated that hundreds of TFs are regulated by low-oxygen environments (e.g. submergence; Liu et al., 2005; Licausi et al., 2011b). It is possible, therefore, that several different TFs may contribute to plant hypoxia tolerance, but there is very little information about such interactions. In the present study, six additional TFs (DkERF23, DkERF24, DkERF25, DkWRKY1, DkWRKY6, and DkWRKY7) were shown to have either binding affinity and/or transactivation ability for the DkPDC2 promoter. Y1H-based complementary DNA (cDNA) library screening and dual-luciferase assay demonstrated that some of theseTFs (ERF and WRKY) could bind to and/or transactivate the DkPDC2 promoter (Fig. 1). Taken together, there appear to be at least eight TFs that can participate in regulating the DkPDC2 promoter (Fig. 7), providing new candidates for unraveling the regulatory complexities of the response to anaerobic or, more particularly, hypoxic conditions.
Figure 7.
Regulatory roles of TFs involved in the regulation of the DkPDC2 promoter as part of the hypoxia response/deastringency process in persimmon fruit. DkERF9 and DkERF19 are two direct activators of the DkPDC2 promoter (Min et al., 2012, 2014). In addition, two MYB TFs, DkMYB6 and DkMYB10, were characterized as the upstream activators via binding to DkERF promoters (Zhu et al., 2018). In this study, an ERF/WRKY TF complex responsive to hypoxia and which transactivated the PDC2 promoter was found with both persimmon fruit (DkERF24 and DkWRKY1) and Arabidopsis (AtERF1 and AtWRKY53) homologs. Meanwhile, three individual DkWRKY/DkERF TFs (DkERF23, DkERF25, and DkWRKY7) also directly recognized and activated the PDC2 promoter. Arrows indicate direct interactions. The red stars indicate the transactivation effects (one star, 2- to 5-fold; two stars, >10-fold).
Not all these persimmon ERFs belong to the well-characterized Group VII subfamily involved in plant hypoxia responses (Hinz et al., 2010; Licausi et al., 2010, 2011b; Yang et al., 2011; Gasch et al., 2016). In persimmon only DkERF3 and DkERF10 belong to the Group VII subfamily, but DkERF3 (Supplemental Fig. S11) and DkERF10 have no effects on the DkPDC2 promoter, and only DkERF10 had a transactivation effect on the DkADH1 promoter (Min et al., 2012). At least three of the TFs (DkERF9, DkERF19, and DkERF22) involved in persimmon fruit hypoxic responses and the deastringency process (Min et al., 2012, 2014) do not belong to Group VII, but to different subfamilies (DkERF9 to Group IV, DkERF19 to Group IX, and DkERF22 to the RAV subfamily; Min et al., 2012, 2014; Zhu et al., 2018). Here, all the newly identified ERF genes, DkERF23, DkERF24, and DkERF25, belong to Group IX (Supplemental Fig. S7), which indicates that multiple subfamilies of ERFs, not just the Group VII family, may contribute to fruit anaerobic metabolism. Moreover, there have been a few studies, both in model plants and fruit, indicating that Group IX ERFs are involved in stress responses, such as Arabidopsis ERF6 for oxidative stress (Wang et al., 2013), ESE1 for salt response (Zhang et al., 2011), and tomato Pti4 for plant defense (Gu et al., 2000). MdERF2, a Group IX ERF, mediated regulatory effects of jasmonate on ethylene biosynthesis and ripening in apple fruit (Li et al., 2017a). Also, our previous results indicated that DkERF19, a Group IX ERF, is involved in persimmon fruit deastringency (Wang et al., 2017). Thus, the multiple Group IX ERFs (DkERF19, DkERF23, DkERF24, and DkERF25) shown to be responsive to high CO2/low oxygen are also likely to be involved in high-CO2/low-oxygen-driven persimmon fruit deastringency and ripening.
In Arabidopsis, many WRKYs play important roles in plant stress tolerance (Chen et al., 2017), although there is no report on WRKY involvement in plant hypoxia tolerance or persimmon fruit deastringency, and the identification of DkWRKY genes (Fig. 1) provides new targets in researching responses to anaerobic treatments.
Interactions between Persimmon DkERF24 and DkWRKY1 Regulate Expression of the Hypoxia-Responsive DkPDC2 Promoter
To analyze the relations between different hypoxia-responsive TFs from persimmon fruit, experiments with random paired combinations of these TFs were carried out. The combination of DkERF24 and DkWRKY1 showed significantly higher activation (13-fold) of the DkPDC2 promoter than did either of them acting individually, and the results of the Y2H and BiFC assays confirmed protein–protein interaction between DkERF24 and DkWRKY1 (Fig. 4). Taken together, these results suggest that high-CO2/low-oxygen treatment could trigger both DkERF24 and DkWRKY1 accumulation and that the induced TFs may form a complex that synergistically stimulates substantially higher activation of the DkPDC2 target gene and accelerates persimmon fruit deastringency. Another well-known protein–protein interaction related to the response to aerobic environments occurs between the hypoxia-responsive RAP2.12 (an ERF) and acyl-coA binding protein, which dissociates under an anaerobic environment, whereupon RAP2.12 moves into the nucleus to transcriptionally regulate anaerobic related genes (Licausi et al., 2011a). Acyl-coA binding protein is not a TF, however, and the interactions between the hypoxia-responsive ERF-WRKY TFs described here reveal the role of a different TF complex in the hypoxia response.
Arabidopsis ERF and WRKY Also Regulate the DkPDC2 Promoter
Parallel analysis with Arabidopsis homologs indicated that AtERF1 and AtWRKY53 could interact with each other in Y2H and BiFC assays. The combination of AtERF1 and AtWRKY53 also strongly activated the DkPDC2 promoter (Fig. 6). These results suggest that the protein–protein interaction between hypoxia-responsive ERFs and WRKY may be conserved in different plants. In Arabidopsis, the homologous gene AtERF1 is a key integrator of jasmonate and ethylene signals in the regulation of ethylene/jasmonate-dependent defense in response to different plant pathogens (Solano et al., 1998; Berrocal-Lobo et al., 2002; Lorenzo et al., 2003). Overexpression of AtWRKY53 in Arabidopsis could accelerate leaf senescence, coordinated by the interaction of salicylic acid and jasmonate signaling pathways (Miao et al., 2004), although their involvement in the regulation of the plant hypoxia response has not been comprehensively analyzed. At the transcriptomic level, AtWRKY53 could be significantly induced by low oxygen conditions and is among the most sensitive of the WRKY family members (Mustroph et al., 2009). Based on the present analysis, it could be proposed that hypoxia-responsive AtWRKY53 could regulate alcoholic fermentation-related genes via interaction with AtERF1.
In Vivo Interactions of DkERF24 and DkWRKY1 with Persimmon Fruit DkPDC2 Measured by Insolubilizing Soluble Tannins
Due to the difficulty of stable transformation of perennial fruit, a TOX system was employed to analyze persimmon gene function and the regulation of endogenous genes by their transcriptional regulators. In the persimmon system, the reduction in soluble tannin content can be used as a measurement of the induction of PDC and the anaerobic response. Overexpression of DkMYB4 in kiwifruit calluses could significantly enhance tannin biosynthesis (Akagi et al., 2009), and expression of DkPDC2 and DkADH1 in persimmon leaves decreased soluble tannin content (Min et al., 2012; Mo et al., 2016). Overexpression of DkERF18, DkERF19, DkMYB6, and DkMYB10 caused a rapid decrease in the content of soluble tannins in persimmon fruit discs (Zhu et al., 2018) and up-regulated the expression of the corresponding genes (DkERF9 and DkERF19). Here, a slight modification was introduced by adding a negative control, DkERF1, which was shown not to activate the DkPDC2 promoter (Min et al., 2012). This additional control increased the reliability of the TOX system, and the results indicated that DkERF1TOX produced no significant change in expression of DkPDC2 (Fig. 5). DkERF24TOX, DkWRKY1TOX, and DkERF24+DkWRKY1TOX, however, significantly accelerated insolubilization of tannins in persimmon fruit discs, indicating that they participate in persimmon fruit deastringency. The synergistic effects of DkERF24 and DkWRKY1 on transactivation of the expression of DkPDC2 were also confirmed. These results not only support the regulatory roles and involvement in a transcriptional complex of DkERF24 and DkWRKY1, but also confirm the role of these TFs in persimmon anaerobic response and fruit deastringency.
In conclusion, Y1H library screening and further investigations with the dual-luciferase assay, the electrophoretic mobility shift assay, Y2H, and BiFC, identified multiple novel hypoxia-responsive TFs. TOX analysis confirmed the role of this complex in persimmon fruit deastringency, which is critical for the persimmon industry. Furthermore, the fact that there is a similar synergistic effect of DkERF24 and DkWRKY1 homologs from Arabidopsis on the DkPDC2 promoter suggests that this may be a conserved feature of the anaerobic response in plants.
MATERIALS AND METHODS
Plant Material and Treatments
The persimmon (Diospyros kaki) fruit material was from the same batch as those used in our previous studies (Wang et al., 2017; Zhu et al., 2018). In brief, three astringent-type persimmon fruit (two Chinese cultivars [‘Mopanshi’ and ‘Jingmianshi’] and one Japanese cultivar [‘Tonewase’]) were collected at the mature stage from an orchard in Qingdao (Shandong, China) in 2014. The fruit were treated for 1 d with air (as control) or CO2 (95%, with 4% N2 and 1% oxygen) to accelerate insolubilization of soluble tannins (deastringency). The mature persimmon fruit ‘Gongcheng-shuishi’, harvested from Guilin (Guangxi, China), was treated with air (control), CO2 (95%, with 4% N2 and 1% oxygen), and N2 (99% N2 and 1% oxygen). The physiological data and sampling information are described in Wang et al. (2017) and Zhu et al. (2018).
Construction of cDNA Library and Y1H Library Screening
Total RNA was extracted from ‘Mopanshi’ persimmon fruit flesh and used for cDNA library construction according to the Matchmaker Gold Yeast One-Hybrid Library Screening System Kit user manual (Clontech). The DkPDC2 promoter was constructed in a pAbAi vector as in Min et al. (2014). The screening was according to the protocol of the Yeastmaker Yeast Transformation System 2 User Manual (Clontech), performed on synthetic dextrose (SD)/−Leu+AbA250 plates in a 30°C incubator for 4 d. Single colonies were selected and amplified by PCR. The DNA sequences were determined and colonies encoding TFs were used as candidates for further investigation.
Gene Isolation and Sequence Analysis
After BLAST analysis, the partial coding sequences were amplified with the primers (Supplemental Table S2) and a SMART RACE cDNA amplification Kit (Clontech) to obtain the complete coding sequences. The sequences of full-length TF candidates were confirmed and amplified with primers spanning the start and stop codons (Supplemental Table S3) and translated with the ExPASy software (http://web.expasy.org/translate). These newly isolated TFs were named according to their homologs in GenBank and the previously reported TFs in persimmon.
Y1H Assay
The Y1H assay, to identify individual interactions between a single TF and the target gene promoter, was used to verify the interactions indicated by library screening, using the Matchmaker Gold Yeast One-Hybrid Library Screening System (Clontech). The full-length TF candidate sequences were subcloned into the pGADT7 AD vector (primers are listed in Supplemental Table S4) and the interaction analyses were conducted according to the manufacturer’s protocol.
Dual-Luciferase Assay and GUS Histochemical Staining
Dual-luciferase assays have been widely used for investigations on the transregulation of target promoters by TFs (Zeng et al., 2015; Wang et al., 2017). Full-length TFs were cloned into the pGreen II 002962-SK vector (primers are listed in Supplemental Table S5) and tested with the promoters of DkADH1, DkPDC2, DkERF9, DkERF10, and DkERF19, originally constructed in the pGreen II 0800-LUC vector (LUC) by Min et al. (2012, 2014). All constructs were electroporated into Agrobacterium tumefaciens GV1301. The constructed pGreen II 002962-SK vector and LUC plasmids were transiently expressed in Nicotiana benthamiana leaves as described by Min et al. (2012). The Agrobacterium were suspended in infiltration buffer (10 mm MES, 10 mm MgCl2, 150 mm acetosyringone, pH 5.6) to an OD600 of ∼0.75. TFs and promoter were combined in a v/v ratio of 10:1 and infiltrated into N. benthamiana leaves by needleless syringes. A dual-luciferase assay kit (Promega) was used to analyze the transient expression in N. benthamiana leaves after 3 d of infiltration. Absolute LUC and REN were measured in a GLOMAX 96 Microplate Luminometer (Promega).
GUS histochemical assays were performed according to Xiao et al. (2018), but with some minor revisions, and the GUS gene was amplified by PCR using forward primer 5′-CGCCCATGGTACGTCCTGTAGAAACCC-3′ and reverse primer 5′-GATTCTAGATCATTGTTTGCCTCCCTGCTG-3′. The PCR product was inserted into the pGreen II 0800-LUC vector by replacing the LUC region to generate the construct containing the GUS-coding region under the control of the DkPDC2 promoter. The constructs were electroporated into A. tumefaciens GV1301 and then the cultured Agrobacterium were suspended in infiltration buffer to an OD600 of ∼0.75. TFs and promoter were combined in a v/v ratio of 5:1 and infiltrated into N. benthamiana leaves by needleless syringes. The photos were taken after 5 d of infiltration. The staining buffer was 0.1 m sodium phosphate buffer (pH 7.0), 10 mm EDTA, 1 mm ferricyanide, 1 mm ferrocyanide, 0.5% (v/v) Triton X-100, and 1 mm 5-bromo-4-chloro-3-indolyl-β-d-glucuronide. The infiltrated leaves of N. benthamiana were immersed in the staining buffer under vacuum for 30 min and then incubated at 37°C for 24 h. The leaves were decolorized in 75% ethanol for 2 h for proper visualization. The gus (uidA) transcripts were detected by RT-qPCR using N. benthamiana leaves collected at 1-d intervals after injections. The RT-qPCR primers for gus are forward primer 5′-CGGGTGAAGGTTATCTCTAT-3′ and reverse primer 5′-TTCGGTCATTTCATCTTGCC-3′. RT-qPCR was carried out on a Bio-Rad CFX96 Real-Time PCR System using the SsoFastTM EvaGreen Supermix (Bio-Rad) according to the manufacturer’s instructions. The housekeeping gene NtACT (GenBank No. JQ256516; Zhang et al., 2017) was chosen as the internal control and the 2−ΔCt method was used to calculate the relative expression (Livak and Schmittgen, 2001).
EMSA
The intact open reading frames of DkERF23/24/25 and DkWRKY1/5/6/7 were inserted into pET-32a (Clontech; primers are listed in Supplemental Table S6) to generate a TF-His fusion protein. The reconstructed plasmids were transformed into Escherichia coli strain BL21. The transformed cells were treated with 0.5 mm isopropyl β-d-1-thiogalactopyranoside (IPTG) followed by incubation at 16°C for 20 h. HisTALON Gravity Columns (Clontech) were used to purify proteins.
EMSA experiments were performed according to the Lightshift Chemiluminescent EMSA kit (Thermo Fisher Scientific) manufacturer’s instructions. Oligonucleotide probes were synthesized and labeled with biotin (HuaGen Biotech). The 3′ biotin end-labeled dsDNA probes were prepared by annealing complementary oligonucleotides. They were then heated at 95°C for 5 min, after which the temperature was gradually decreased to 25°C at the rate of 0.1°C s−1. The probes used for EMSA are listed in Supplemental Table S6. EMSA was performed as previously described in Ge et al. (2017).
RNA Extraction and cDNA Synthesis
Total RNA was extracted from frozen persimmon fruit flesh (2.0 g for each) using the method described by Chang et al. (1993). Contaminating genomic DNA in the total RNA was removed using a TURBO DNA-free kit (Ambion). After quantification by Nanophotometer Pearl (Implen), 1.0 μg DNA-free RNA was initiated for cDNA synthesis with an iScript cDNA Synthesis Kit (Bio-Rad). For each sampling point, three biological replicates were used for RNA extraction and the subsequent cDNA synthesis.
RT-qPCR Analysis
Gene-specific oligonucleotide primers were designed for RT-qPCR and are described in Supplemental Table S7. The quality and specificity of each pair of primers were checked by melting curves and product resequencing. RT-qPCR was carried out on a Bio-Rad CFX96 Real-Time PCR System using the SsoFast EvaGreen Supermix (Bio-Rad) following the manufacturer’s instructions. The housekeeping gene DkACT (GenBank No. AB473616; Akagi et al., 2009) was chosen as the internal control and the 2−ΔΔCt method was used to calculate the relative expression (Livak and Schmittgen, 2001).
Subcellular Localization Analysis
Full-length TFs were cloned into pCAMBIA1300-sGFP (Lv et al., 2014) using the primers described in Supplemental Table S8. All constructs were electroporated into A. tumefaciens GV1301. These TF clones were transiently expressed in transgenic N. benthamiana leaves (stably transformed with the nuclear location signal NLS-mCherry; Li et al., 2017b) using needleless syringes with the infiltration buffer. Fluorescence from GFP transiently expressed in N. benthamiana leaves was imaged 2 d after infiltration using a Zeiss LSM780NLO confocal laser scanning microscope.
Y2H Assay
Protein-protein interactions were investigated in yeast with the DUAL hunter system (Dual-Systems Biotech). Full-length coding sequences of DkWRKY1 were cloned into the pDHB1 vector as bait, and the full-length DkERF24 was cloned into pPR3-N vector as prey. The primers used for vector construction are described in Supplemental Table S9. All constructs were transformed into the yeast strain NMY51. The assays were performed with different media: (1) SD medium lacking Trp and Leu; (2) QDO (SD medium lacking Trp, Leu, His, and Ade); and (3) QDO+3AT (QDO with 5 mm 3-amino-1,2,4-triazole). Auto-activations were tested with empty pPR3-N vectors and target genes with pDHB1, which were cotransformed into NMY51 and plated on QDO. Auto-activations were indicated by the presence of colonies. Protein–protein interaction assays were performed with cotransformation of DkWRKY1 in pDHB1 and DkERF24 in pPR3-N. The presence of colonies in QDO and QDO+3AT indicated protein–protein interaction.
BiFC Assay
BiFC was used to confirm the results of Y2H analysis. Full-length DkERF24 and DkWRKY1 were cloned into both C-terminal and N-terminal fragments of YFP vectors, and BiFC assays were performed according to the protocols of Sainsbury et al. (2009). Primers used are listed in Supplemental Table S10. All constructs were transiently expressed in N. benthamiana leaves by Agrobacterium-mediated infiltration (GV3101) based on previous reports (Li et al., 2017b). The YFP fluorescence of N. benthamiana leaves was imaged 2 d after infiltration using a Zeiss LSM780NLO confocal laser scanning microscope.
TOX in Persimmon Fruit Discs
In order to verify the potential roles of these TFs in the persimmon fruit anaerobic/deastringency response, TOX was performed with persimmon fruit (‘Gongcheng-shuishi’) discs, using the same protocol as in our previous report (Zhu et al., 2018). Discs of 1 cm diameter and 0.3 cm thickness were prepared and divided into eight batches. The discs were incubated with Agrobacterium-carrying constructs for 1 h in the same buffer used for the dual-luciferase assay. The discs were then transferred to filter papers (wetted by Murashige and Skoog medium) in tissue-culture dishes and placed in an incubator at 25°C for 3 d. All of the experiments were performed with three biological replicates. Each day, samples of the discs were dried on filter papers and then frozen in liquid nitrogen and stored at −80°C for further use.
Soluble Condensed Tannins
Soluble condensed tannins are the main source of astringency for persimmon fruit. The decrease in soluble condensed tannins in high-CO2 treatment is one of the main responses of persimmon fruit to anaerobic environments. The content of soluble condensed tannins in persimmon fruit discs was measured from 1 g frozen samples with Folin-Ciocalteu reagent, with three biological replicates, according to the method described by Yin et al. (2012). The results were calculated using a standard curve of tannic acid equivalents.
Arabidopsis Gene Isolation and Analysis
Based on the phylogenetic trees (Supplemental Figs. S7 and S8), AtERF1 (AT3G23240), AtWRKY41 (AT4G11070), and AtWRKY53 (AT4G23810) are the potential homologs of DkERF24 and DkWRKY1 in Arabidopsis (Arabidopsis thaliana). The three Arabidopsis genes were cloned into the vectors pGreen II 002962-SK, and AtERF1 and AtWRKY53 were also cloned into pGADT7 AD and N-terminal and C-terminal fragments of YFP. The primers used for gene isolation and vector construction are listed in Supplemental Table S11. The protocol for the dual-luciferase assay, the Y1H assay, GUS histochemical staining, gene expression, and the BiFC assay were as described above.
Statistical Analysis
The statistical significance of differences was determined using Student’s t test (a t test was performed if two values were compared, as indicated in the paper by asterisks) or an ANOVA test for significance analysis and lsd for multiple comparisons by DPS 2.05 (Zhejiang University). Figures were drawn using Origin 8.0 (Microcal Software).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers MH054905 to MH054910 and KY849608.
SUPPLEMENTAL DATA
The following supplemental materials are available.
Supplemental Figure S1. Promoter of DkPDC2 from persimmon with marked motifs.
Supplemental Figure S2. EMSA of DkWRKY1, DkWRKY5, and DkWRKY6 binding to the DkDPC2 promoter.
Supplemental Figure S3.GUS histochemical assays of effects of TFs on the DkPDC2 promoter. Error bars indicate SEs from three biological replicates (*P < 0.05, **P < 0.01, ***P < 0.001).
Supplemental Figure S4. Regulatory effects of DkERF23, DkERF24, DkERF25, DkWRKY1, DkWRKY5, DkWRKY6, and DkWRKY7 on the promoters of DkADH1, DkERF9, DkERF10, and DkERF19. Error bars indicate SEs from five biological replicates (***P < 0.001).
Supplemental Figure S5. Expression of TFs responsive to CO2 and N2 treatments in ‘Gongcheng-shuishi’ persimmon fruit.
Supplemental Figure S6. The combinatorial effects of various TFs on activation of the DkPDC2 promoter.
Supplemental Figure S7. Phylogenetic tree analysis of DkERFs in persimmon and AtERFs in Arabidopsis.
Supplemental Figure S8. Phylogenetic tree of DkWRKYs in persimmon and AtWRKYs in Arabidopsis.
Supplemental Figure S9. Regulatory effect of AtWRKY41 on the DkPDC2 promoter. Error bars indicate SEs from five biological replicates. Letters above the columns represent no differences (P < 0.05) between different constructs.
Supplemental Figure S10. GUS histochemical assays of effects of AtERF1 and AtWRKY53 on the DkPDC2 promoter. Error bars indicate SEs from three biological replicates (*P < 0.05, **P < 0.01, ***P < 0.001).
Supplemental Figure S11. Regulatory effect of DkERF3 on the DkPDC2 promoter.
Supplemental Table S1. Y1H library screening with DkDPC2 promoter.
Supplemental Table S2. Primer sequences used for RACE.
Supplemental Table S3. Primer sequences used for full-length amplification.
Supplemental Table S4. Primer sequences used for Y1H assay.
Supplemental Table S5. Primer sequences for gene vector construction used for dual-luciferase assays.
Supplemental Table S6. Primer sequences for EMSA experiments.
Supplemental Table S7. Primer sequences for RT-qPCR.
Supplemental Table S8. Primer sequences for subcellular localization.
Supplemental Table S9. Primer sequences for the Y2H assay.
Supplemental Table S10. Primer sequences for BiFC.
Supplemental Table S11. Primer sequences for SK, AD, and BiFC of genes from Arabidopsis.
Acknowledgments
The authors thank Dr. Ian Ferguson (Plant and Food Research) for critical reading of the article.
Footnotes
This research was supported by the National Key Research and Development Program (2016YFD0400102), the National Natural Science Foundation of China (31672204, 31722042), the Natural Science Foundation of Zhejiang Province, China (LR16C150001), the Fok Ying Tung Education Foundation (161028), Fundamental Research Funds for the Central Universities (2018XZZX002-03), and the 111 Project (B17039).
References
- Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 15: 63–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DO, Yang SF (1978) Effect of anaerobiosis on ethylene production and metabolism by apple tissue. Plant Physiol 61: 90 [Google Scholar]
- Akagi T, Ikegami A, Tsujimoto T, Kobayashi S, Sato A, Kono A, Yonemori K (2009) DkMyb4 is a Myb transcription factor involved in proanthocyanidin biosynthesis in persimmon fruit. Plant Physiol 151: 2028–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Khan AS, Malik AU, Shahid M (2016) Effect of controlled atmosphere storage on pericarp browning, bioactive compounds and antioxidant enzymes of litchi fruits. Food Chem 206: 18–29 [DOI] [PubMed] [Google Scholar]
- Banti V, Mafessoni F, Loreti E, Alpi A, Perata P (2010) The heat-inducible transcription factor HsfA2 enhances anoxia tolerance in Arabidopsis. Plant Physiol 152: 1471–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekele EA, Alis ARR, Hertog MLATM, Nicolai BM, Geeraerd AH (2016) Dynamics of metabolic adaptation during initiation of controlled atmosphere storage of ‘Jonagold’ apple: Effects of storage gas concentrations and conditioning. Postharvest Biol Technol 117: 9–20 [Google Scholar]
- Berrocal-Lobo M, Molina A, Solano R (2002) Constitutive expression of ETHYLENE-RESPONSE-FACTOR1 in Arabidopsis confers resistance to several necrotrophic fungi. Plant J 29: 23–32 [DOI] [PubMed] [Google Scholar]
- Birkenbihl RP, Kracher B, Roccaro M, Somssich IE (2017) Induced genome-wide binding of three Arabidopsis WRKY transcription factors during early MAMP-triggered immunity. Plant Cell 29: 20–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco-Price C, Kawaguchi R, Ferreira RB, Bailey-Serres J (2005) Genome-wide analysis of transcript abundance and translation in Arabidopsis seedlings subjected to oxygen deprivation. Ann Bot 96: 647–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui LT, Giuntoli B, Kosmacz M, Parlanti S, Licausi F (2015) Constitutively expressed ERF-VII transcription factors redundantly activate the core anaerobic response in Arabidopsis thaliana. Plant Sci 236: 37–43 [DOI] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney J (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Report 11: 113–116 [Google Scholar]
- Chen F, Hu Y, Vannozzi A, Wu KC, Cai HY, Qin Y, Mullis A, Lin ZG, Zhang LS (2017) The WRKY transcription factor family in model plants and crops. Crit Rev Plant Sci 36: 311–335 [Google Scholar]
- Cukrov D, Zermiani M, Brizzolara S, Cestaro A, Licausi F, Luchinat C, Santucci C, Tenori L, Van Veen H, Zuccolo A, et al. (2016) Extreme hypoxic conditions induce selective molecular responses and metabolic reset in detached apple fruit. Front Plant Sci 7: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F, Wang MM, Zhu QG, Min T, Grierson D, Yin XR, Chen KS (2016) DkMYB6 is involved in persimmon fruit deastringency, via transcriptional activation on both DkPDC and DkERF. Postharvest Biol Technol 111: 161–167 [Google Scholar]
- Gasch P, Fundinger M, Müller JT, Lee T, Bailey-Serres J, Mustroph A (2016) Redundant ERF-VII transcription factors bind to an evolutionarily conserved cis-motif to regulate hypoxia-responsive gene expression in Arabidopsis. Plant Cell 28: 160–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge H, Zhang J, Zhang YJ, Li X, Yin XR, Grierson D, Chen KS (2017) EjNAC3 transcriptionally regulates chilling-induced lignification of loquat fruit via physical interaction with an atypical CAD-like gene. J Exp Bot 68: 5129–5136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs DJ, Lee SC, Isa NM, Gramuglia S, Fukao T, Bassel GW, Correia CS, Corbineau F, Theodoulou FL, Bailey-Serres J, et al. (2011) Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 479: 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu YQ, Yang C, Thara VK, Zhou J, Martin GB (2000) Pti4 is induced by ethylene and salicylic acid, and its product is phosphorylated by the Pto kinase. Plant Cell 12: 771–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz M, Wilson IW, Yang J, Buerstenbinder K, Llewellyn D, Dennis ES, Sauter M, Dolferus R (2010) Arabidopsis RAP2.2: an ethylene response transcription factor that is important for hypoxia survival. Plant Physiol 153: 757–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeren FU, Dolferus R, Wu Y, Peacock WJ, Dennis ES (1998) Evidence for a role for AtMYB2 in the induction of the Arabidopsis alcohol dehydrogenase gene (ADH1) by low oxygen. Genetics 149: 479–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami A, Eguchi S, Kitajima A, Inoue K, Yonemori K (2007) Identification of genes involved in proanthocyanidin biosynthesis of persimmon (Diospyros kaki) fruit. Plant Sci 172: 1037–1047 [Google Scholar]
- Lee SC, Mustroph A, Sasidharan R, Vashisht D, Pedersen O, Oosumi T, Voesenek LACJ, Bailey-Serres J (2011) Molecular characterization of the submergence response of the Arabidopsis thaliana ecotype Columbia. New Phytol 190: 457–471 [DOI] [PubMed] [Google Scholar]
- Lee TG, Jang CS, Kim JY, Kim DS, Park JH, Kim DY, Seo YW (2007) A Myb transcription factor (TaMyb1) from wheat roots is expressed during hypoxia: Roles in response to the oxygen concentration in root environment and abiotic stresses. Physiol Plant 129: 375–385 [Google Scholar]
- Li SJ, Yin XR, Wang WL, Liu XF, Zhang B, Chen KS (2017a) Citrus CitNAC62 cooperates with CitWRKY1 to participate in citric acid degradation via up-regulation of CitAco3. J Exp Bot 68: 3419–3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Xu Y, Zhang L, Ji Y, Tan D, Yuan H, Wang A (2017b) The jasmonate-activated transcription factor MdMYC2 regulates ETHYLENE RESPONSE FACTOR and ethylene biosynthetic genes to promote ethylene biosynthesis during apple fruit ripening. Plant Cell 29: 1316–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licausi F, van Dongen JT, Giuntoli B, Novi G, Santaniello A, Geigenberger P, Perata P (2010) HRE1 and HRE2, two hypoxia-inducible ethylene response factors, affect anaerobic responses in Arabidopsis thaliana. Plant J 62: 302–315 [DOI] [PubMed] [Google Scholar]
- Licausi F, Kosmacz M, Weits DA, Giuntoli B, Giorgi FM, Voesenek LA, Perata P, van Dongen JT (2011a) Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 479: 419–422 [DOI] [PubMed] [Google Scholar]
- Licausi F, Weits DA, Pant BD, Scheible WR, Geigenberger P, van Dongen JT (2011b) Hypoxia responsive gene expression is mediated by various subsets of transcription factors and miRNAs that are determined by the actual oxygen availability. New Phytol 190: 442–456 [DOI] [PubMed] [Google Scholar]
- Liu F, Vantoai T, Moy LP, Bock G, Linford LD, Quackenbush J (2005) Global transcription profiling reveals comprehensive insights into hypoxic response in Arabidopsis. Plant Physiol 137: 1115–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Lorenzo O, Piqueras R, Sánchez-Serrano JJ, Solano R (2003) ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell 15: 165–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Q, Zhong Y, Wang Y, Wang Z, Zhang L, Shi J, Wu Z, Liu Y, Mao C, Yi K, et al. (2014) SPX4 negatively regulates phosphate signaling and homeostasis through its interaction with PHR2 in rice. Plant Cell 26: 1586–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matityahu I, Marciano P, Holland D, Ben-Arie R, Amir R (2016) Differential effects of regular and controlled atmosphere storage on the quality of three cultivars of pomegranate (Punica granatum L.). Postharvest Biol Technol 115: 132–141 [Google Scholar]
- Miao Y, Laun T, Zimmermann P, Zentgraf U (2004) Targets of the WRKY53 transcription factor and its role during leaf senescence in Arabidopsis. Plant Mol Biol 55: 853–867 [DOI] [PubMed] [Google Scholar]
- Min T, Yin XR, Shi YN, Luo ZR, Yao YC, Grierson D, Ferguson IB, Chen KS (2012) Ethylene-responsive transcription factors interact with promoters of ADH and PDC involved in persimmon (Diospyros kaki) fruit de-astringency. J Exp Bot 63: 6393–6405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min T, Fang F, Ge H, Shi YN, Luo ZR, Yao YC, Grierson D, Yin XR, Chen KS (2014) Two novel anoxia-induced ethylene response factors that interact with promoters of deastringency-related genes from persimmon. PLoS One 9: e97043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo RL, Yang SC, Huang YM, Chen WX, Zhang QL, Luo ZR (2016) ADH and PDC genes involved in tannins coagulation leading to natural de-astringency in Chinese pollination constant and non-astringency persimmon (Diospyros kaki Thunb.). Tree Genet Genomes 12: 17 [Google Scholar]
- Mustroph A, Zanetti ME, Jang CJH, Holtan HE, Repetti PP, Galbraith DW, Girke T, Bailey-Serres J (2009) Profiling translatomes of discrete cell populations resolves altered cellular priorities during hypoxia in Arabidopsis. Proc Natl Acad Sci USA 106: 18843–18848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papdi C, Pérez-Salamó I, Joseph MP, Giuntoli B, Bögre L, Koncz C, Szabados L (2015) The low oxygen, oxidative and osmotic stress responses synergistically act through the ethylene response factor VII genes RAP2.12, RAP2.2 and RAP2.3. Plant J 82: 772–784 [DOI] [PubMed] [Google Scholar]
- Sainsbury F, Thuenemann EC, Lomonossoff GP (2009) pEAQ: Versatile expression vectors for easy and quick transient expression of heterologous proteins in plants. Plant Biotechnol J 7: 682–693 [DOI] [PubMed] [Google Scholar]
- Salvador A, Arnal L, Besada C, Larrea V, Quiles A, Pérez-Munuera I (2007) Physiological and structural changes during ripening and deastringency treatment of persimmon fruit cv. ‘Rojo Brillante’. Postharvest Biol Technol 46: 181–188 [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR (1998) Nuclear events in ethylene signaling: A transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12: 3703–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira S, Ikeda K, Ohkawa K (2001) Comparison of insolubility of tannins induced by acetaldehyde vapor in fruit of three types of astringent persimmon. J Jpn Soc Hortic Sci 48: 684–687 [Google Scholar]
- van Veen H, Akman M, Jamar DC, Vreugdenhil D, Kooiker M, van Tienderen P, Voesenek LA, Schranz ME, Sasidharan R (2014) Group VII ethylene response factor diversification and regulation in four species from flood-prone environments. Plant Cell Environ 37: 2421–2432 [DOI] [PubMed] [Google Scholar]
- Wang MM, Zhu QG, Deng CL, Luo ZR, Sun NJ, Grierson D, Yin XR, Chen KS (2017) Hypoxia-responsive ERFs involved in postdeastringency softening of persimmon fruit. Plant Biotechnol J 15: 1409–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Du Y, Zhao X, Miao Y, Song CP (2013) The MPK6-ERF6-ROS-responsive cis-acting Element7/GCC box complex modulates oxidative gene transcription and the oxidative response in Arabidopsis. Plant Physiol 161: 1392–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RZ, Yang Y, Li GC (1997) Chinese persimmon germplasm resources. Acta Hortic 436: 43–50 [Google Scholar]
- Xiao YY, Kuang JF, Qi XN, Ye YJ, Wu ZX, Chen JY, Lu WJ (2018) A comprehensive investigation of starch degradation process and identification of a transcriptional activator MabHLH6 during banana fruit ripening. Plant Biotechnol J 16: 151–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Xu X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ (2006) Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 442: 705–708 [DOI] [PubMed] [Google Scholar]
- Yang CY, Hsu FC, Li JP, Wang NN, Shih MC (2011) The AP2/ERF transcription factor AtERF73/HRE1 modulates ethylene responses during hypoxia in Arabidopsis. Plant Physiol 156: 202–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin XR, Shi YN, Min T, Luo ZR, Yao YC, Xu Q, Ferguson I, Chen KS (2012) Expression of ethylene response genes during persimmon fruit astringency removal. Planta 235: 895–906 [DOI] [PubMed] [Google Scholar]
- Zeng JK, Li X, Xu Q, Chen JY, Yin XR, Ferguson IB, Chen KS (2015) EjAP2-1, an AP2/ERF gene, is a novel regulator of fruit lignification induced by chilling injury, via interaction with EjMYB transcription factors. Plant Biotechnol J 13: 1325–1334 [DOI] [PubMed] [Google Scholar]
- Zhang L, Li Z, Quan R, Li G, Wang R, Huang R (2011) An AP2 domain-containing gene, ESE1, targeted by the ethylene signaling component EIN3 is important for the salt response in Arabidopsis. Plant Physiol 157: 854–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zhang GQ, Kang HH, Zhou SM, Wang W (2017) TaPUB1, a putative E3 ligase gene from wheat, enhances salt stress tolerance in transgenic Nicotiana benthamiana. Plant Cell Physiol 58: 1673–1688 [DOI] [PubMed] [Google Scholar]
- Zhu QG, Wang MM, Gong ZY, Fang F, Sun NJ, Li X, Grierson D, Yin XR, Chen KS (2016) Involvement of DkTGA1 transcription factor in anaerobic response leading to persimmon fruit postharvest de-astringency. PLoS One 11: e0155916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu QG, Gong ZY, Wang MM, Li X, Grierson D, Yin XR, Chen KS (2018) A transcription factor network responsive to high CO2/hypoxia is involved in deastringency in persimmon fruit. J Exp Bot 69: 2061–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]