Arabidopsis BAK1, a co-receptor of multiple receptor-like kinases, undergoes a proteolytic cleavage process that is essential for its functions in plant immunity, growth, and cell death control.
Abstract
Plants have evolved many receptor-like kinases (RLKs) to sense extrinsic and intrinsic cues. The signaling pathways mediated by multiple Leucine-rich repeat (LRR) RLK (LRR–RLK) receptors require ligand-induced receptor-coreceptor heterodimerization and transphosphorylation with BRI1-ASSOCIATED RECEPTOR KINASE1 (BAK1)/SOMATIC EMBRYOGENESIS RECEPTOR KINASES family LRR–RLKs. Here we reveal an additional layer of regulation of BAK1 via a Ca2+-dependent proteolytic cleavage process that is conserved in Arabidopsis (Arabidopsis thaliana), Nicotiana benthamiana, and Saccharomyces cerevisiae. The proteolytic cleavage of BAK1 is intrinsically regulated in response to developmental cues and immune stimulation. The surface-exposed Asp (D287) residue of BAK1 is critical for its proteolytic cleavage and plays an essential role in BAK1-regulated plant immunity, growth hormone brassinosteroid-mediated responses, and cell death containment. BAK1D287A mutation impairs BAK1 phosphorylation on its substrate BOTRYTIS-INDUCED KINASE1 (BIK1), and its plasma membrane localization. Intriguingly, it aggravates BAK1 overexpression–triggered cell death independent of BIK1, suggesting that maintaining homeostasis of BAK1 through a proteolytic process is crucial to control plant growth and immunity. Our data reveal that in addition to layered transphosphorylation in the receptor complexes, the proteolytic cleavage is an important regulatory process for the proper functions of the shared coreceptor BAK1 in diverse cellular signaling pathways.
Plants growing in their natural habitats are at constant risks for various potential attacks, meanwhile maintaining their active growth. To adapt, sessile plants have evolved a large number of cell-surface–resident receptor-like kinases (RLKs) to sense extrinsic and intrinsic cues and elicit distinct biological responses (Shiu and Bleecker, 2003). A typical RLK usually contains a variable extracellular domain that perceives either self- or nonself signals on the cell surface, a single transmembrane domain, and an intracellular kinase domain that is important to relay signaling. A large portion of RLKs contains an extracellular Leucine-rich repeat (LRR) domain with different numbers of LRR repeats. For example, the Arabidopsis (Arabidopsis thaliana) genome encodes more than 200 LRR–RLKs that regulate a wide range of biological processes ranging from plant growth and development, and symbiosis, to immunity (Belkhadir et al., 2014). The LRR–RLK BRASSINOSTEROID INSENSITIVE1 (BRI1) is the receptor of plant hormone brassinosteroids (BRs), important in growth and development (Li and Chory, 1997; Belkhadir et al., 2006). Some LRR-RLKs function as pattern-recognition receptors (PRRs) that recognize microbe-associated molecular patterns (MAMPs) and induce the first line of plant immunity (Macho and Zipfel, 2014; Yu et al., 2017). Arabidopsis PRRs FLAGELLIN-SENSING2 (FLS2) and ELONGATION FACTOR-TU RECEPTOR (EFR) recognize bacterial flagellin and ELONGATION FACTOR-TU, respectively, and initiate the convergent plant immune signaling (Gómez-Gómez and Boller, 2000; Zipfel et al., 2006; Boller and Felix, 2009).
A group of LRR–RLKs, named SOMATIC EMBRYOGENESIS RECEPTOR KINASES (SERKs) with five members in Arabidopsis, function as shared coreceptors of multiple LRR–RLKs (Liebrand et al., 2014; Ma et al., 2016; He et al., 2018). SERK3, also known as BRI1-ASSOCIATED RECEPTOR KINASE1 (BAK1), was originally identified as a BRI1 interacting protein that mediates BR signaling (Li et al., 2002; Nam and Li, 2002). SERK1 and SERK4 were also found to interact with BRI1 and mutation of SERK1, SERK3/BAK1, and SERK4 rendered plants insensitive to BR treatment (Karlova et al., 2006; He et al., 2007a; Gou et al., 2012). SERK3/BAK1 was also shown to dimerize with FLS2 upon flagellin perception and plays a key role in flagellin-activated responses (Chinchilla et al., 2007; Heese et al., 2007). SERK3/BAK1 also heterodimerizes with several other PRRs, including EFR and PEP1 RECEPTOR1 that perceives endogenous danger peptide plant elicitor peptides (Postel et al., 2010; Roux et al., 2011). SERK4, but not SERK1 nor SERK2, performs a function redundant with that of SERK3/BAK1 in mediating PRR signaling (Roux et al., 2011). The crystal structure analysis indicates that BAK1 family RLKs are involved in ligand sensing through contacting the BRI1-BR- or FLS2-flagellin-binding interface respectively, and function as coreceptors (Santiago et al., 2013; Sun et al., 2013a, 2013b).
Recent studies have revealed additional functions of SERKs as coreceptors of different LRR–RLK receptors in regulating plant development and growth, including PHYTOSULFOKINE (PSK) RECEPTOR1 (PSKR1)-recognizing peptide hormone PSK for root growth (Wang et al., 2015); the ERECTA family sensing EPIDERMAL PATTERNING FACTORS for cell fate specification in stomatal patterning (Meng et al., 2015); the HAESA family perceiving INFLORESCENCE DEFICIENT IN ABSCISSION for floral organ abscission (Meng et al., 2016; Santiago et al., 2016); ROOT MERISTEM GROWTH FACTOR receptors perceiving peptide hormones, ROOT MERISTEM GROWTH FACTOR for regulating root meristem development (Ou et al., 2016; Song et al., 2016); and EXCESS MICROSPOROCYTES1 perceiving TAPETUM DETERMINANT1 in controlling anther cell fate determination (Li et al., 2017). In addition, SERK3/BAK1 and SERK4 (also named BAK1-LIKE1) negatively regulate cell death in a BR-independent manner, and the Arabidopsis bak1serk4 mutant exhibits a seedling lethality and constitutive defense responses (He et al., 2007a; Kemmerling et al., 2007). Recent studies suggest the involvement of protein n-glycosylation and nucleocytoplasmic trafficking in the cell death control regulated by BAK1 and SERK4 (de Oliveira et al., 2016; Du et al., 2016). Interestingly, excessive expression of BAK1 or its ectodomain could also trigger cell death in Arabidopsis with constitutive activation of defense (Domínguez-Ferreras et al., 2015). Thus, the homeostasis of BAK1 is important for its functions in plant growth and immunity.
A common theme for BAK1-associated RLK complex activation is rapid heterodimerization and transphosphorylation upon the cognate ligand perception (Wang et al., 2008; Perraki et al., 2018). Members of receptor-like cytoplasmic kinases, including BOTRYTIS-INDUCED KINASE1 (BIK1), associate with multiple RLKs and can be phosphorylated by BAK1 (Lu et al., 2010; Zhang et al., 2010). It has been reported that members of mammalian receptor Tyr kinases (RTKs) involved in growth regulation, and Toll-like receptors (TLRs) involved in innate immunity, require proteolytic cleavage for activation (Park et al., 2008; Ancot et al., 2009; Chen and Hung, 2015). Recently, several plant RLKs, including rice LRR-RLK XANTHOMONAS RESISTANCE21 (XA21; Park and Ronald, 2012), Arabidopsis lysine motif domain containing RLK CHITIN ELICITOR RECEPTOR KINASE1 (CERK1; Petutschnig et al., 2014), and legume RLK SYMBIOSIS RECEPTOR-LIKE KINASE (Antolín-Llovera et al., 2014) are shown to undergo proteolytic cleavage. In this study, we report that BAK1 and other SERKs undergo Ca2+-dependent proteolytic cleavage by a conserved protease(s) present in both plants and yeast. Bacteria or MAMP treatment promotes the accumulation of cleaved BAK1, which occurs between the transmembrane domain and ectodomain. Through an extensive mutagenesis screen, we identified the Asp residue (D287) of BAK1 as an important site for its proteolytic cleavage. The BAK1D287A mutation is impaired in FLS2-mediated immunity, BRI1-mediated BR signaling, and cell death control. Our data suggest the proteolytic cleavage of plasma membrane-resident RLKs as a common mechanism in the regulation of intracellular signaling.
RESULTS
BAK1 Undergoes Ca2+-Dependent Proteolytic Cleavage
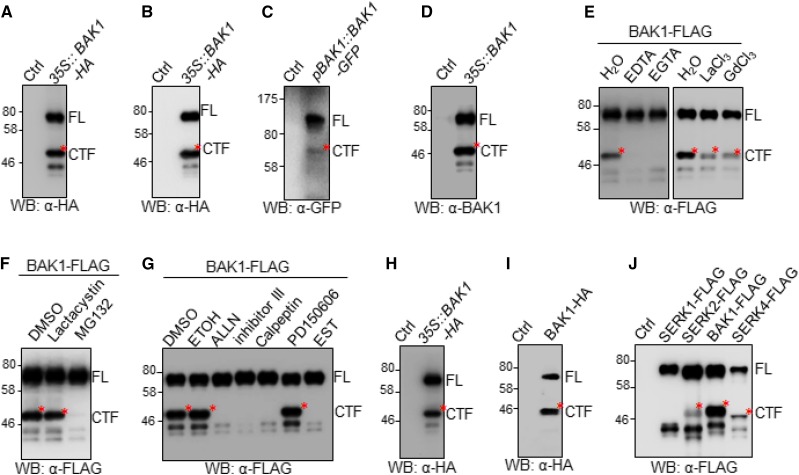
When the C-terminal hemagglutinin (HA)-tagged BAK1 under the control of the 35S promoter was expressed in Arabidopsis Col-0 protoplasts, we observed a major protein band with a molecular mass of ∼75 kD corresponding to the full-length (FL) glycosylated BAK1-HA proteins, along with at least three polypeptide bands with molecular mass ranging from 42 to 50 kD in an immunoblot (Fig. 1A). The truncated BAK1 fragments were also observed when BAK1 was tagged with FLAG (Supplemental Fig. S1A) or green fluorescent protein (GFP; Supplemental Fig. S1B) at its C terminus and expressed in protoplasts, and when BAK1 was expressed in transgenic Arabidopsis plants under the control of either the 35S promoter (35S::BAK1-HA; Fig. 1B) or its native BAK1 promoter (pBAK1::BAK1-GFP; Fig. 1C). To eliminate the potential complication from the C-terminal epitope tag (Ntoukakis et al., 2011), we expressed BAK1 without any tag in Arabidopsis protoplasts and also observed the truncated fragments of BAK1 detected by anti (α)-BAK1 polyclonal antibodies directing against the C-terminal peptide (DSTSQIENEYPSGPR) in an immunoblot (Fig. 1D). Notably, among the truncated BAK1 polypeptide bands, the one with the molecular mass of ∼48 kD (∼50 kD for HA- or FLAG-tagged BAK1 and ∼75 kD for GFP-tagged BAK1) was the most abundant in different expression systems and named as C-terminal fragment (CTF) of BAK1.
Figure 1.
Proteolytic processing of BAK1 in plants by a conserved protease. A, Expression of BAK1-HA in Arabidopsis protoplasts. Protein extracts from Arabidopsis protoplasts transfected with 35S::BAK1-HA or a control vector (Ctrl) were analyzed by western blot (WB) with an α-HA antibody. The upper band corresponding to the FL BAK1 protein was indicated by FL, and the lower band corresponding to the CTF of BAK1 was indicated by CTF, and labeled with a red star. B and C, BAK1CTF is produced in Arabidopsis transgenic plants. Protein extracts from 35S::BAK1-HA (B) and pBAK1::BAK1-GFP (C) transgenic plants were analyzed by WB with respective α-HA and α-GFP antibodies. Wild-type Col-0 Arabidopsis was used as Ctrl. D, Nontagged BAK1CTF is produced in Arabidopsis protoplasts. Protein extracts from Arabidopsis protoplasts transfected with 35S::BAK1 were analyzed by WB with an α-BAK1 antibody. E and F, Effects of chemical inhibitors on the production of BAK1CTF. 1 mm of EDTA, 1 mm of EGTA, 1 mm of lanthanum chloride, 0.5 of mm gadolinium chloride, 2 μm of MG132, or 2 μm of Lactacystin was added immediately after protoplast transfection with 35S::BAK1-FLAG. Protein extracts were analyzed by WB with an α-FLAG antibody. Lactacystin and MG132 were stored in DMSO, and other chemicals were stored in H2O. G, Effects of Calpain inhibitors on the production of BAK1CTF. ALLN, Calpain inhibitor III, Calpeptin, PD150606, or EST (cat. no. 208733; Calbiochem) at a final concentration of 20 μm was added immediately after protoplast transfection with 35S::BAK1-FLAG. Protein extracts were analyzed by western blot with an α-FLAG antibody. EST was stored in ethanol (ETOH), and other inhibitors were stored in DMSO. H and I, BAK1CTF is produced in N. benthamiana and S. cerevisiae. Protein extracts from N. benthamiana transiently expressing 35S::BAK1-HA (H) and S. cerevisiae expressing pGAL1::BAK1-HA (I) were analyzed by WB with α-HA antibodies. J, Proteolytic cleavage is conserved in SERK family members. Protein extracts from Arabidopsis protoplasts expressing FLAG-tagged SERK1, SERK2, SERK3 (BAK1), or SERK4 were analyzed by western blot with α-FLAG antibodies. The above experiments were repeated three times with similar results.
To determine if BAK1CTF was derived from proteolytic cleavage or nonspecific protein degradation, we applied different chemical inhibitors, including protease inhibitors, to explore the biochemical requirements for the formation of BAK1CTF. The calcium (Ca2+) chelating agents EDTA and ethylene glycol tetra-acetic acid (EGTA) blocked the production of BAK1CTF (Fig. 1E). In addition, the Ca2+ channel blockers lanthanum chloride and gadolinium chloride markedly compromised the production of BAK1CTF, suggesting the involvement of Ca2+ in the formation of BAK1CTF (Fig. 1E). Carbobenzoxy-Leu-Leu-leucinal (MG132), a commonly used cell-permeable proteasome inhibitor, also blocked the production of BAK1CTF (Fig. 1F). However, lactacystin, a specific 26S proteasome inhibitor that binds and inhibits catalytic subunits of the proteasome (Fenteany et al., 1995), did not affect the production of BAK1CTF (Fig. 1F), suggesting the effect of MG132 on BAK1 cleavage may not be caused by its inhibition on the 26S proteasome. Notably, lactacystin has no reported effect on Ser or Cys proteases, whereas MG132 could also inhibit the activity of certain proteases, including calpains (Tsubuki et al., 1996), a family of cytosolic calcium-dependent Cys proteases that regulate a wide variety of cellular processes (Zatz and Starling, 2005; Ono and Sorimachi, 2012). The Arabidopsis genome encodes one calpain gene DEFECTIVE KERNEL1 (DEK1), the mutant of which is embryonic-lethal (Lid et al., 2005; Johnson et al., 2008), making it difficult to genetically assess the role of calpain in BAK1 cleavage. However, by using a wide collection of calpain inhibitors, we could show that pretreatment of certain calpain inhibitors, such as N-Acetyl-L-leucyl-L-leucyl-L-norleucinal (ALLN), calpain inhibitor III, calpeptin, or ethyl(+)-(2S,3S)-3-[(S)-3-methyl-1-(3-methylbutylcarbamoyl)butylcarbamoyl]-2-oxirane carboxylate (EST) but not PD150606 nor the solvent dimethyl sulfoxide (DMSO) and ethanol, controls blocked the production of BAK1CTF (Fig. 1G). The data suggest that BAK1 undergoes Ca2+-dependent cleavage in Arabidopsis, and calpain is a potential candidate that mediates BAK1 proteolytic cleavage. The protease that cleaves BAK1 is conserved in plants and yeast, because BAK1CTF was observed when it was expressed in Nicotiana benthamiana (Fig. 1H) or Saccharomyces cerevisiae (Fig. 1I; Supplemental Fig. S1C). Other SERK members, including SERK1, SERK2, and SERK4, also produced cleaved products when they were expressed in Arabidopsis protoplasts (Fig. 1J). BAK1 bears strong kinase activity. Pretreatment of the kinase inhibitor K252a did not affect the formation of BAK1CTF (Supplemental Fig. S1D). Consistently, HA-tagged BAK1 kinase inactive mutant (BAK1KM-HA) produced BAK1CTF, similar to the wild-type BAK1-HA (Supplemental Fig. S1E), suggesting that the kinase activity may not be required for the BAK1 cleavage.
BAK1 Cleavage Is MAMP-Induced
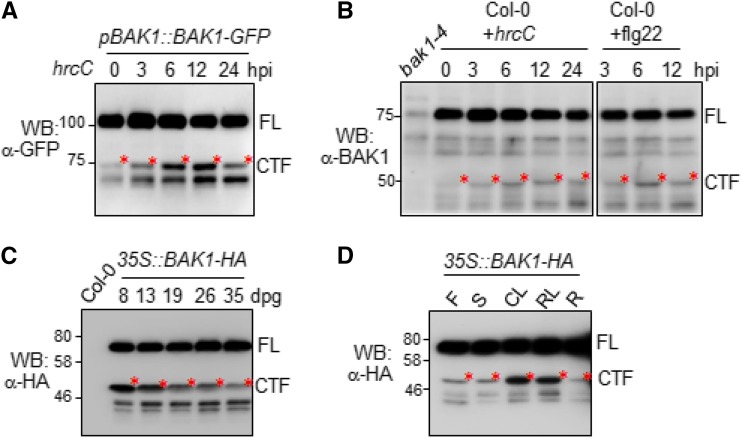
As a coreceptor, BAK1 plays an important role in multiple cellular processes, including MAMP-mediated immunity and BR-mediated growth and development (Ma et al., 2016). We examined whether BAK1 cleavage is regulated upon the cognate ligand perception and signaling activation. When we treated the pBAK1::BAK1-GFP plants with the nonpathogenic bacterium Pseudomonas syringae pv Tomato (Pst) DC3000 hrcC, the abundance of BAK1CTF was increased at 3, 6, and 12 h postinoculation (Fig. 2A). Pst hrcC is a type-III secretion mutant strain of Pst DC3000, which is defective in secretion of type-III effectors, but possesses a variety of MAMPs. Furthermore, using the α-BAK1 antibody, we were able to detect both the endogenous BAK1FL proteins and BAK1CTF in wild-type Col-0 plants (Fig. 2B). Notably, Pst hrcC treatment enhanced the abundance of the endogenous BAK1CTF proteins (Fig. 2B). Treatment of purified MAMP flg22, the synthesized 22-amino acid peptide derived from bacterial flagellin, also induced the production of BAK1CTF in Col-0 plants (Fig. 2B). Only a small portion of endogenous BAK1 is cleaved in the cells (Fig. 2B). The abundance of BAK1CTF was gradually reduced during the plant maturation process from the seedling to adult stages (Fig. 2C). BAK1CTF was mostly abundant at 8–13 d post germination, indicating a potential endogenous regulatory mechanism during plant growth and development underlying the BAK1 cleavage (Fig. 2C). Additionally, we observed that BAK1CTF was mostly observed in rosette and cauline leaves, but it was less abundant in stems, flowers, and roots of 4-week-old 35S::BAK1-HA transgenic plants (Fig. 2D). Taken together, the data indicate that BAK1 cleavage is a temporospatial-regulated process, and is also regulated upon pathogen recognition.
Figure 2.
Regulation of BAK1CTF production. A, Production of BAK1CTF in pBAK1::BAK-GFP transgenic plants upon Pst hrcC infection. Four-week-old soil-grown Arabidopsis pBAK1::BAK-GFP transgenic plants were hand-inoculated with Pst hrcC at 5 × 105 cfu. Total proteins from inoculated leaf extracts were analyzed by western blot (WB) with an α-GFP antibody. B, Production of BAK1CTF in wild-type plants upon infections. Four-week-old soil-grown Arabidopsis Col-0 plants were hand-inoculated with Pst hrcC at 5 × 105 cfu or 1 μM flg22. Total proteins from inoculated leaf extracts were analyzed by WB with an α-BAK1 antibody. C, Developmental regulation of BAK1CTF production. Total proteins extracted from Arabidopsis 35S::BAK1-HA transgenic plants at different growth stages from 8 through 35 d past germination (dpg) on 1/2 MS plates were analyzed by WB with an α-HA antibody. D, BAK1CTF production in different tissues. Total proteins extracted from different tissues of 2-month-old soil-grown 35S::BAK1-HA transgenic plants were analyzed by WB with an α-HA antibody. The above experiments were repeated three times with similar results. Hpi, hours post inoculation; F, flowers, S, stem; CL, cauline leaves; RL, rosette leaves; R, roots.
BAK1D287 Is Required for the Production of BAK1CTF
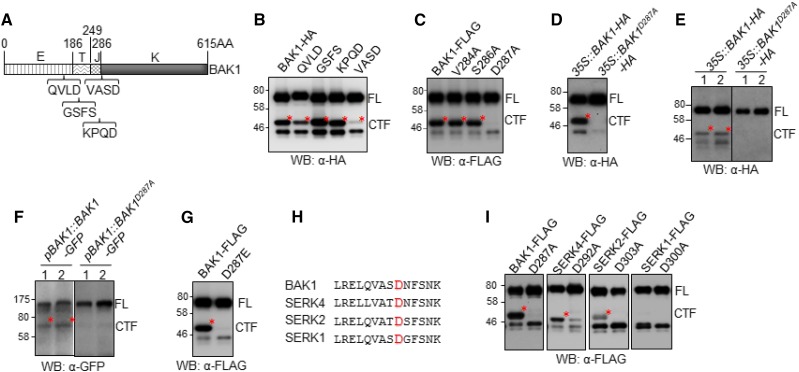
BAK1 possesses an extracellular LRR domain (E), a transmembrane domain (T), a juxtamembrane domain (J), and an intracellular kinase domain (K; Fig. 3A). To map the BAK1 cleavage region, we first compared the expression pattern of BAK1FL proteins with different BAK1 truncations, including the BAK1 kinase domain (BAK1K), the juxtamembrane and kinase domains (BAK1JK), and the juxtamembrane, transmembrane, and kinase domains (BAK1TJK; Supplemental Fig. S2A). BAK1TJK exhibited a migration pattern similar to that of BAK1CTF in an immunoblot detected by an α-BAK1 antibody (Supplemental Fig. S2B), indicating that the BAK1 proteolytic cleavage may occur close to the transmembrane domain. We then performed an extensive Ala substitution mutagenesis screen to systemically mutate 35 residues in this region with each construct carrying three- or four-amino acid substitutions to Ala (A; Supplemental Fig. S2C). However, none of these mutations blocked the BAK1 cleavage (Supplemental Fig. S2D). We extended our mutagenesis screen to the neighboring regions—in particular, the motifs showing certain homology to the cleavage sites of mammalian RTKs by proteases (Fig. 3A). Notably, substitution of a four-amino acid sequence (valine-alanine-serine-aspartic acid [VASD]) at the residues 284–287 (BAK1VASD) to Ala blocked the production of BAK1CTF (Fig. 3, A and B). Subsequent single-amino acid substitution among these residues indicated that the Asp-to-Ala mutation at the residue 287 (BAK1D287A) was sufficient to block the production of BAK1CTF (Fig. 3C). When transiently expressed in N. benthamiana, BAK1D287A also blocked the production of BAK1CTF (Fig. 3D). We further generated Arabidopsis transgenic plants stably expressing HA-tagged BAK1D287A under the control of the 35S promoter (35S::BAK1D287A-HA; Fig. 3E) and GFP-tagged BAK1D287A under the control of its native BAK1 promoter (pBAK1::BAK1D287A-GFP; Fig. 3F). BAK1CTF was no longer detected in either 35S::BAK1D287A-HA or pBAK1::BAK1D287A-GFP transgenic plants (Fig. 3, E and F). Asp is a negatively charged amino acid. We also substituted Asp to the negatively charged Glu. Similarly, Asp-to-Glu (BAK1D287E) substitution blocked the production of BAK1CTF (Fig. 3G), suggesting that blocking of the cleavage in BAK1D287A was not due to the loss of negative charge in residue 287. The Asp-287 residue is located at the beginning of the BAK1 kinase domain and is a surface-exposed residue, according to the published BAK1 kinase domain structure (Yan et al., 2012). BAK1D287 is also conserved among SERKs (Fig. 3H). To determine if this site is required for proteolytic cleavage of other SERKs, we substituted the cognate Asp residue to Ala in SERK1, SERK2, and SERK4, and found that the corresponding cleaved fragment in SERK4 and SERK2 was reduced (Fig. 3I). The data indicate that the conserved Asp residue is required for the proteolytic cleavage of BAK1 and probably SERK4 and SERK2.
Figure 3.
The D287A mutation of BAK1 blocks its cleavage. A, Scheme of BAK1 protein domains and sites for mutagenesis. B, VASD residues are required for BAK1 cleavage. Arabidopsis protoplasts expressing HA-tagged BAK1 mutants, in which the indicated four amino acids were mutagenized to Ala, were analyzed by western blot (WB)with an α-HA antibody. C, D287 residue is required for BAK1 cleavage in Arabidopsis protoplasts. D, D287 residue is required for BAK1 cleavage in N. benthamiana. The N. benthamiana leaves transiently expressing HA-tagged BAK1 or BAK1D287A mutant were analyzed by WB with α-HA antibody. E, D287 residue is required for BAK1 cleavage in Arabidopsis 35S::BAK1-HA transgenic plants. F, D287 residue is required for BAK1 cleavage in Arabidopsis pBAK1::BAK1-GFP transgenic plants. G, D287E mutation blocks BAK1 cleavage in Arabidopsis protoplasts. H, D287 residue of BAK1 is conserved among Arabidopsis SERKs. I, BAK1 D287 corresponding residue in other SERK members is required for cleavage in Arabidopsis protoplasts. The above experiments were repeated three times with similar results.
BAK1D287 Is Required for PTI Signaling and Responses
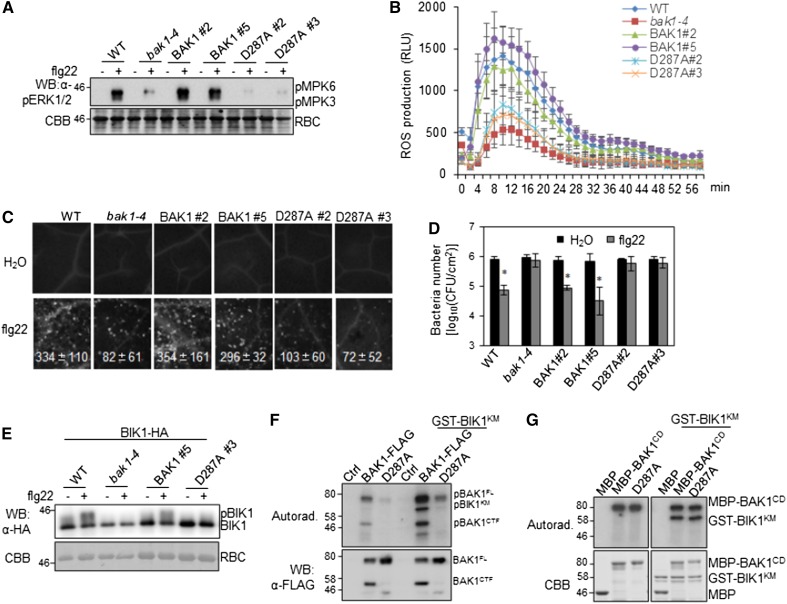
BAK1 plays an important role in pathogen-associated molecular pattern-triggered immunity (PTI) signaling and the bak1-4 mutant shows compromised immune responses (Chinchilla et al., 2007; Roux et al., 2011). To determine whether the cleavage is required for BAK1 function in PTI signaling, we generated the transgenic plants carrying the wild-type BAK1 or BAK1D287A under the control of the native BAK1 promoter in the bak1-4 mutant (pBAK1::BAK1/bak1 or pBAK1::BAK1D287A/bak1). Multiple transgenic lines were obtained and two representative lines for each construct with a comparable BAK1 expression level between wild-type BAK1 and BAK1D287A were used to systematically characterize PTI responses (Supplemental Fig. S3). As shown in Figure 4, pBAK1::BAK1/bak1, but not pBAK1::BAK1D287A/bak1 transgenic plants, were able to complement the deficiency of the flg22-induced MAPK activation (Fig. 4A), reactive oxygen species (ROS) production (Fig. 4B), and callose deposition (Fig. 4C) in the bak1-4 mutant. Pretreatment of flg22 protected the wild-type Col-0 and pBAK1::BAK1/bak1 transgenic plants from the infection of the virulent bacterium Pst DC3000 as indicated by the reduced in planta bacterial multiplication (Fig. 4D). However, similar to the bak1-4 mutant, the pBAK1::BAK1D287A/bak1 transgenic plants lost the flg22-mediated resistance against Pst DC3000 infection. Taken together, the data indicate that BAK1D287-mediated cleavage is critical for BAK1-dependent PTI responses.
Figure 4.
Compromised immune responses in pBAK1::BAK1D287A/bak1 transgenic plants. A, flg22-induced MAPK activation. Two-week-old seedlings of wild-type Col-0, bak1-4, pBAK1::BAK1/bak1, and pBAK1::BAK1D287A/bak1 were treated with 100 nm of flg22 for 15 min. Phosphorylated MPK3 (pMPK3) and MPK6 (pMPK6) were detected by western blot (WB) with an α-pERK antibody (top). Protein loading is shown by Coomassie Brilliant Blue (CBB) staining for Rubisco (RBC; bottom). B, flg22-induced ROS production. Leaf discs from four leaves (technical repeats) of each of six 5-week-old plants (biological repeats) of indicated genotypes were treated with 100 nm of flg22, and ROS production was detected at the indicated time points. The data are shown as the mean ± sd from six biological repeats. C, flg22-induced callose deposition. Leaves of 4-week-old plants were collected for aniline blue staining 12 h after inoculation with 500 nm of flg22. Callose deposits were counted using the software ImageJ (1.43U). The data are shown as the mean ± sd from six biological repeats. D, flg22-mediated resistance to bacterial infection. Four-week-old plants were pretreated with 200 nm of flg22 or water and then infected with Pst DC3000 at 5 × 105 cfu/mL. The bacterial growth assays were performed 2 d after infection. The data are shown as the mean ± sd from three biological repeats. The asterisks indicate statistical significance compared to H2O pretreatment by using Student’s t test (P < 0.05). E, flg22-induced BIK1 mobility shift. Arabidopsis protoplasts isolated from Col-0, bak1-4 mutant, pBAK1::BAK1/bak1, and pBAK1::BAK1D287A/bak1 transgenic plants were used to express BIK1-HA and treated with 100 nm of flg22 for 15 min. Mobility shift of BIK1 was detected by WB with an α-HA antibody (top). Equal loading of protein was indicated by CBB staining toward RBC (bottom). F, The kinase activity of BAK1 and BAK1D287A. FL BAK1-FLAG or BAK1D287A-FLAG were expressed in Arabidopsis Col-0 protoplasts and precipitated with an α-FLAG antibody. Kinase activity of the precipitated proteins were detected using GST-BIK1KM (kinase mutant) as a substrate. Phosphorylation was detected by autoradiography (top), and the protein loading is shown by WB with an α-FLAG antibody (bottom). G, The in vitro kinase activity of the cytosolic domain of BAK1 and BAK1D287A. GST-BIK1KM protein was used as a substrate and maltose-binding protein-BAK1CD (cytosolic domain) or its D287A mutant was used as the kinase in an in vitro kinase assay. Phosphorylation was detected by autoradiography (top), and the protein loading is shown by CBB staining (bottom). The above experiments were repeated three times with similar results. WT, wild type; pBIK1, phosphorylated BIK1.
BAK1 directly transphosphorylates BIK1 to relay PTI signaling (Lin et al., 2014). The pBAK1::BAK1/bak1, but not pBAK1::BAK1D287A/bak1, transgenic plants restored the deficiency of flg22-induced BIK1 phosphorylation in the bak1-4 mutant evidenced by phosphoprotein mobility shift in immunoblots (Fig. 4E). When BAK1 was expressed in Arabidopsis bak1-4 protoplasts and immunoprecipitated for a kinase assay, the in vivo expressed BAK1D287A showed reduced autophosphorylation compared to wild-type BAK1 (Fig. 4F). In addition, in vivo expressed BAK1D287A showed reduced transphosphorylation activities toward BIK1KM (Fig. 4F), consistent with the reduced BIK1 phosphorylation upon flg22 treatment (Fig. 4E). Notably, the cleaved BAK1CTF also possessed autophosphorylation activity (Fig. 4F). Interestingly, an in vitro kinase assay showed that the cytosolic domain of BAK1 (BAK1CD) and BAK1CD D287A fused with maltose-binding protein purified from Escherichia coli possessed similar autophosphorylation and transphosphorylation activities toward BIK1 (Fig. 4G), suggesting that BAK1D287A is a functionally competent kinase, and BAK1D287 mainly regulates its activity in vivo.
BAK1D287 Is Required for BAK1 Function in BR Signaling and Cell Death Control
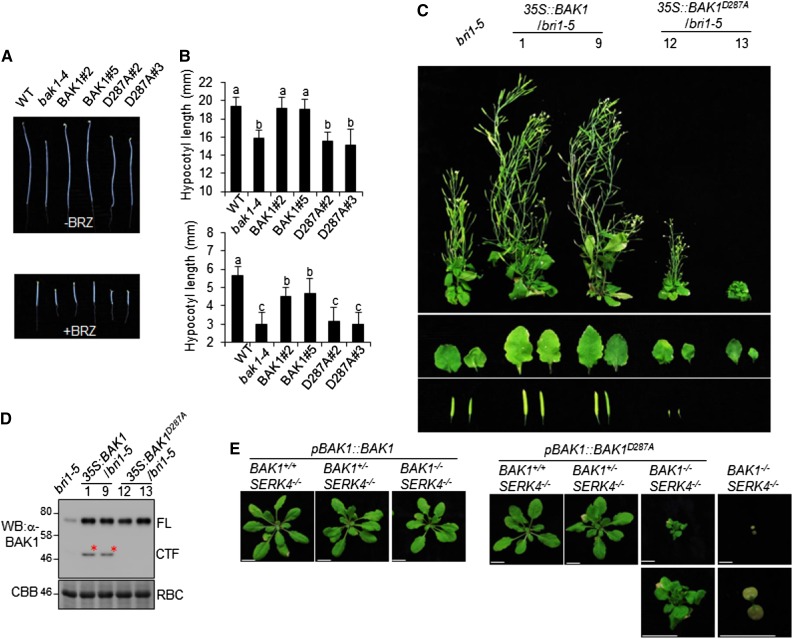
BAK1 plays an important role in BR signaling and the bak1-4 mutant resembles the weak bri1 mutant with compacted rosette leaves and short petioles compared to wild-type Col-0 plants (Li et al., 2002; Nam and Li, 2002). The transgenic plants of pBAK1::BAK1/bak1, but not pBAK1::BAK1D287A/bak1, restored the growth defect of bak1-4 to wild-type Col-0 (Supplemental Fig. S3). When grown in the dark, hypocotyls of pBAK1::BAK1D287A/bak1 transgenic plants and bak1-4 were shorter than those of pBAK1::BAK1/bak1 transgenic plants and wild-type Col-0 in the absence or presence of brassinazole, an inhibitor of BR biosynthesis (Fig. 5, A and B), suggesting an important role of BAK1D287 in mediating BR signaling. BAK1 positively regulates BR signaling by heterodimerization and transphosphorylation with BRI1 (Li et al., 2002; Nam and Li, 2002). Overexpression of functional BAK1 partially alleviates the growth defects of the bri1 mutants. We generated Arabidopsis transgenic plants expressing BAK1 or BAK1D287A under the control of the 35S promoter in the bri1-5 mutant (35S::BAK1/bri1-5 and 35S::BAK1D287A/bri1-5). Multiple transgenic lines were obtained and two lines of each construct with comparable BAK1 protein levels were selected for further study (Fig. 5, C and D). As previously reported, overexpression of BAK1 in bri1-5 partially rescued the dwarf phenotype of bri1-5 with relatively big leaves, elongated stems, and long siliques compared to bri1-5 (Nam and Li, 2002). In contrast, overexpression of BAK1D287A in bri1-5 did not recover the dwarf phenotype of bri1-5, but instead enhanced the growth defects of bri1-5. The 35S::BAK1D287A/bri1-5 transgenic plants were sterile (Fig. 5C). Taken together, the data suggest that BAK1D287 is required for BAK1 function in BR signaling.
Figure 5.
BAK1 D287 is critical for BR signaling and cell death control. A, The reduced hypocotyl length of pBAK1::BAK1D287A/bak1 transgenic plants. The seedlings of wild-type, bak1-4, and transgenic plants were grown in the dark for 5 d on 1/2 MS plates without or with 1 μm of brassinazole. B, Quantification of the hypocotyl length shown in (A). The data are shown as the mean ± sd from 20 biological repeats. The different letters indicate statistically significant difference analyzed with one-way analysis of variance followed by Tukey’s test (P < 0.05). C, BAK1D287A mutant cannot restore bri1-5 dwarf defect. Transgenic plants of 35S::BAK1 and 35S::BAK1D287A in bri1-5 background were photographed at 2 months old. The individual leaves and siliques are shown in the middle and bottom. D, Expression of BAK1 protein in rosette leaves from 4-week-old plants of (C) was detected by western blot (WB) with an α-BAK1 antibody. E, D287 is important for BAK1 function in cell death control. Arabidopsis BAK1+/−SERK4−/− plants were transformed with pBAK1::BAK1 or pBAK1::BAK1D287A. Plants of T1 generation after selection of BAK1 or BAK1D287A transgene were genotyped for endogenous genomic BAK1. Pictures were taken 4 weeks after germination. The above experiments were repeated three times with similar results. BAK1+/+SERK4−/− (BAK1: wild type; SERK4: mutant); BAK1+/−SERK4−/− (BAK1: heterozygous, SERK4: mutant); BAK1−/−SERK4−/− (BAK1: mutant, SERK4: mutant); WT, wild type. Scale bars = 1 cm.
BAK1, together with SERK4 (also called BAK1-LIKE1), play a redundant role in the regulation of cell death, and the bak1/serk4 mutant is seedling-lethal, associated with spontaneous cell death and constitutive H2O2 production (He et al., 2007a). To assess the requirement of BAK1D287 in BAK1/SERK4-regulated cell death, we transformed pBAK1::BAK1 and pBAK1::BAK1D287A into the Arabidopsis BAK1-4+/−SERK4-1−/− mutant and examined the growth phenotypes of T1 generation of transgenic plants. We further differentiated BAK1 wild-type (BAK1+/+), BAK1 heterozygous (BAK1+/−), and BAK1 mutant (BAK1−/−) genotypes by genotyping T-DNA insertions in the transgenic plants. Transgenic plants of pBAK1::BAK1 and pBAK1::BAK1D287A in BAK1+/+SERK4−/− or BAK1+/−SERK4−/− backgrounds displayed normal growth phenotype as compared to the wild-type Col-0 plants (Fig. 5E). However, pBAK1::BAK1D287A transgenic plants in the BAK1−/−SERK4−/− background showed growth defects with much shorter petioles, and small leaves compared to the wild-type Col-0 plants (Fig. 5E). Notably, there are variations of growth defects of pBAK1::BAK1D287A in the BAK1−/−SERK4−/− background, with plants similar to or better than BAK1−/−SERK4−/− (Fig. 5E). Thus, BAK1D287 is partially required for BAK1/SERK4-mediated cell death. In addition, silencing of SERK4 in bak1-4, but not in wild-type Col-0 plants, using the Agrobacterium-mediated virus-induced gene silencing (VIGS) triggers severe growth defects and cell death (de Oliveira et al., 2016; Supplemental Fig. S4A). Silencing of SERK4 in pBAK1::BAK1/bak1 transgenic plants via VIGS did not trigger growth defects, whereas silencing of SERK4 in pBAK1::BAK1D287A/bak1 transgenic plants exhibited severe growth defects with chlorotic leaves and dwarfism (Supplemental Fig. S4A). Trypan blue staining revealed spontaneous cell death in bak1-4 and pBAK1::BAK1D287A/bak1 transgenic plants but not in Col-0 and pBAK1::BAK1/bak1 transgenic plants upon silencing of SERK4 (Supplemental Fig. S4B). Additionally, RT-quantitative PCR (RT-qPCR) analysis indicated that transcripts of pathogenesis-related protein1 (PR1) and PR2 were significantly increased in bak1-4 and pBAK1::BAK1D287A/bak1 plants compared to Col-0 and pBAK1::BAK1/bak1 plants upon silencing of SERK4 (Supplemental Fig. S4C). The data further elaborated that BAK1D287 is required for BAK1 and SERK4-regulated cell death.
BAK1D287 Is Required for BAK1 Plasma Membrane Localization
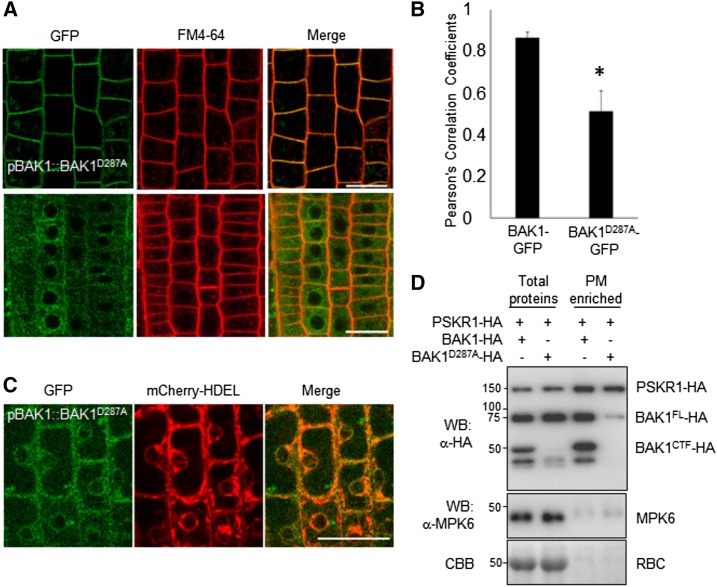
BAK1 is a plasma membrane-localized protein (Li et al., 2002; Nam and Li, 2002), one that is also likely localized to endosomal compartments (Russinova et al., 2004). We tested whether D287A is important for BAK1 localization by generating pBAK1::BAK1-GFP and pBAK1::BAK1D287A-GFP transgenic plants. As reported previously, BAK1-GFP was mainly localized in the plasma membrane, where it colocalized with FM4-64, a lipophilic dye that labels plasma membrane (Fig. 6, A and B). However, the plasma membrane localization of BAK1D287A-GFP was greatly reduced in the pBAK1::BAK1D287A-GFP transgenic plants (Fig. 6, A and B). The BAK1D287A-GFP protein appeared retained in the endoplasmic reticulum (ER) as corroborated by colocalization analyses using the ER marker mCherry-HDEL (Nelson et al., 2007; Fig. 6C) and the ER-Tracker Red (Supplemental Fig. S5). The reduced plasma membrane localization of BAK1D287A was also documented by protein fractionation assay. BAK1-HA and BAK1D287A-HA were transiently expressed in N. benthamiana, and total proteins and plasma membrane-enriched fractions were analyzed with an α-HA immunoblot. The HA-tagged RLK PSKR1 was coexpressed with BAK1-HA or BAK1D287A-HA as a control for the plasma-membrane–localized proteins (Ladwig et al., 2015). Immunoblot for endogenous mitogen‐activated protein kinas 6 (MPK6) with an α-MPK6 antibody was used as a cytoplasmic protein control (Collins et al., 2017). Significantly, compared to wild-type BAK1, the amount of BAK1D287A in the plasma membrane-enriched fraction was greatly reduced, although the amount of PSKR1 in the plasma membrane-enriched fraction stayed the same when it was coexpressed with BAK1 or BAK1D287A (Fig. 6D). Thus, D287 is essential for BAK1 to localize in the plasma membrane, where it functions as a coreceptor to activate diverse signaling pathways.
Figure 6.
D287 is critical for the plasma membrane localization of BAK1. A, Subcellular localization of BAK1-GFP (top) and BAK1D287A-GFP (bottom) in root epidermis of 5-d-old Arabidopsis seedlings. The plasma membrane was stained with FM4-64 (2 μM). B, Pearson’s correlation coefficient (r value) for the colocalization between GFP and FM4-64 fluorescence in the plasma membrane. These values were measured in 15 cells. Error bars = mean ≥ sd. P value (t test), *P < 0.01. C, Colocalization of BAK1D287A-GFP with ER marker, mCherry-HDEL. Scale bars = 20 μm. D, Leaves of 4-week-old N. benthamiana were hand-inoculated with Agrobacteria carrying 35S::BAK1-HA or 35S::BAK1D287A-HA together with 35S::PSKR1-HA. BAK1 from total proteins and enriched plasma membrane proteins were analyzed by western blot (WB) with an α-HA antibody. Cytosolic MPK6 was detected by α-MPK6 WB. The above experiments were repeated three times with similar results.
Overexpression of BAK1D287A Triggers SOBIR1-Dependent and BIK1-Independent Cell Death
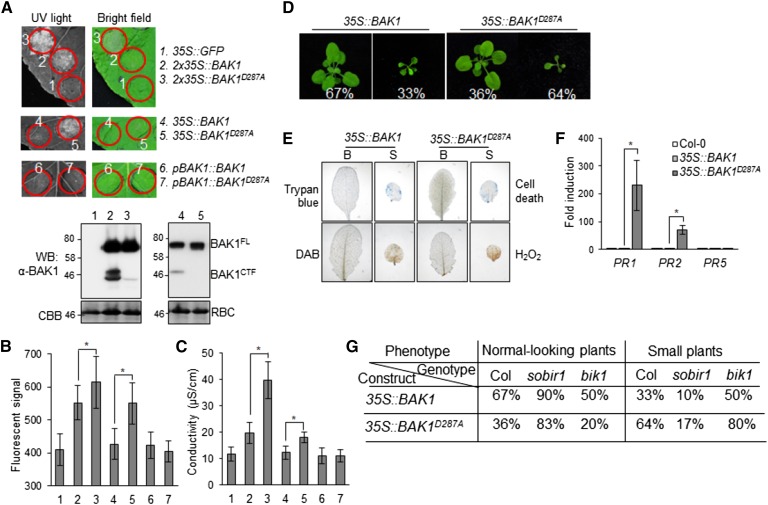
Overexpression of BAK1 under a double 35S (2x35S) promoter in Arabidopsis triggers plant dwarfism accompanied with cell death (Domínguez-Ferreras et al., 2015). Consistently, transient expression of 2x35S::BAK1 triggered cell death in N. benthamiana. Expression of 2x35S::BAK1D287A clearly aggravated the occurrence of cell death in N. benthamiana (Fig. 7A). The elevated cell death by 2x35S::BAK1D287A was also indicated by the autofluorescence signals under the UV light (Fig. 7B) and the electrolyte leakage (Fig. 7C). We did not detect cell death when BAK1 was under the control of a single 35S promoter (35S::BAK1). However, 35S::BAK1D287A triggered cell death when transiently expressed in N. benthamiana (Fig. 7, A–C). No cell death was observed when BAK1 or BAK1D287A was expressed under the control of its native promoter (pBAK1::BAK1 or pBAK1::BAK1D287A; Fig. 7, A–C). The occurrence of cell death by overexpression of BAK1 appeared to be correlated with the level of BAK1 proteins, as 2x35S::BAK1 produced more BAK1 proteins, including both BAK1FL and BAK1CTF, than 35S::BAK1 (Fig. 7A). We further generated Arabidopsis transgenic plants overexpressing BAK1 and BAK1D287A under the control of the 35S promoter in the Col-0 (wild type) background. Among 39 35S::BAK1 plants with positive signals by α-BAK1 immunoblots, 33% (13 out of 39) of transgenic plants were small and showed dwarfism. However, 64% (9 out of 14) of 35S::BAK1D287A transgenic plants were small and dwarfed (Fig. 7D). The small plants were associated with cell death as stained by trypan blue and H2O2 accumulation as stained by DAB (Fig. 7E). RT-qPCR analysis revealed that the expression of PR1 and PR2 was elevated in the 35S::BAK1D287A transgenic plants compared to that in 35S::BAK1 plants (Fig. 7F). Taken together, our data indicate that cleavage of BAK1 might be a way to alleviate cell death when BAK protein level is high in plants.
Figure 7.
Overproduction of BAK1 D287A enhances SoBIR1-dependent cell death. A, Expression of BAK1 and BAK1D287A under different promoters in N. benthamiana. Leaves of 4-week-old N. benthamiana were hand-inoculated with Agrobacteria carrying 35S::GFP, 2x35S::BAK1, 2x35S::BAK1D287A, 35S::BAK1, 35S::BAK1D287A, pBAK1::BAK1, or pBAK1::BAK1D287A. Pictures were taken under UV light (left) or visible light (right) 3 d after inoculation. Two d after inoculation and before cell death progressed, protein levels of BAK1 were analyzed by western blot (WB) with an α-BAK1 antibody (bottom). BAK1 proteins from 2x35S::BAK1 (2) and 2x35S::BAK1D287A (3) were detected by WB using SuperSignal West Pico Chemiluminescent Substrate, and BAK1 proteins from 35S::BAK1 (4) and 35S::BAK1D287A (5) were detected using SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific). B, The fluorescent signal from the circled areas in (A) was obtained under the UV light and was quantified as means ± sd from 10 biological repeats. The asterisks indicate statistical significance by using Student’s t test (P < 0.05). C, Measurement of electrolyte leakage of leaf discs from (A). Data are shown as means ± sd from three biological repeats. The asterisks indicate statistical significance by using Student’s t test (P < 0.05). D, Overexpression of BAK1 and BAK1D287A in Arabidopsis. Arabidopsis plants overexpressing 35S::BAK1 or 35S::BAK1D287A were photographed at the 4-week-old stage. Each phenotype of T1 generation plants was calculated as a percentage to the total transgenic plants of that genotype. E, Cell death and H2O2 accumulation in the leaves of 4-week-old plants were examined by trypan blue and DAB staining, respectively. B; normal-looking plants; S; small plants. F, Expression levels of PR genes in 1-week-old transgenic plants were determined by RT-qPCR. The data are shown as means ± sd from three biological repeats. The asterisks indicate statistical significance by using Student’s t test (P < 0.05). G, BAK1 and BAK1D287A cell death is SoBIR1-dependent and BIK1-independent. 35S::BAK1 and 35S::BAK1D287A were transformed into sobir1 and bik1 mutant backgrounds. Each phenotype of T1 generation at the 4-week-old stage was calculated as a percentage as in (D). The above experiments were repeated three times with similar results.
BIK1 is a key component downstream of BAK1 in plant immunity (Lin et al., 2013b; Liang and Zhou, 2018). We tested whether cell death caused by overexpression of BAK1 or BAK1D287A depends on BIK1 by transferring 35S::BAK1 or 35S::BAK1D287A into the bik1 mutant. Compared to wild-type plants, the bik1 mutant did not reduce the portion of small plants of 35S::BAK1 or 35S::BAK1D287A transgenic plants (Fig. 7G), indicating that cell death caused by overexpression of BAK1 or BAK1D287A is BIK1-independent. Thus, it is likely that cleavage of overproduced BAK1 negatively regulates cell death independently of flg22-triggered signaling. It has been reported that overexpression of BAK1-triggered cell death is dependent on Suppressor Of BIR1-1 (SOBIR1), which encodes an LRR-RLK (Gao et al., 2009; Domínguez-Ferreras et al., 2015). We examined whether overexpression of BAK1D287A-triggered cell death also requires SOBIR1 by transferring 35S::BAK1 or 35S::BAK1D287A into the sobir1 mutant. Significantly, the ratio of dwarfed plants in sobir1 was reduced for both 35S::BAK1 (33% to 10%) and 35S::BAK1D287A (64% to 17%) transgenic plants compared to wild-type plants (Fig. 7G). Thus, overexpression of BAK1D287A causes a SOBIR1-dependent, but BIK1-independent, cell death.
DISCUSSION
In animal studies, members of cell surface proteins have been observed to undergo multilayered proteolytic cleavage processes to regulate transmembrane protein functions (Hayashida et al., 2010). For example, proteolytic cleavage of some RTKs upon ligand binding triggers their nuclear translocation to execute their functions in the nucleus (Carpenter and Liao, 2013). Immune receptors TLR7 and TLR9 are ectodomain-cleaved and form the functional receptors in the endolysosome (Ewald et al., 2008; Park et al., 2008; Petes et al., 2017). Similarly, rice LRR–RLK XA21, which confers broad-spectrum immunity to the Gram-negative bacterial pathogens, is cleaved and the intracellular domain is likely translocated to the nucleus, where it interacts with transcription factors for immune activation (Park and Ronald, 2012). The legume RLK SYMBIOSIS RECEPTOR-LIKE KINASE undergoes proteolytic cleavage and the cleaved ectodomain promotes the formation of a complex with NOD-FACTOR RECEPTOR5, which is involved in root symbiosis (Antolín-Llovera et al., 2014). Arabidopsis chitin receptor RLK CERK1 is also likely cleaved, and a mutation that blocks cleavage generates a novel function of CERK1 in chitin-independent cell death control (Petutschnig et al., 2014). An ATP-binding proteomics analysis predicted that two uncharacterized LRR–RLKs (At3g02880 and At5g16590) exist in leaves only as cytoplasmic domains, suggesting that the extracellular domains are cleaved (Villamor et al., 2013). We show here that the shared coreceptor BAK1, and likely other SERK family members, are cleaved by a conserved protease in eukaryotes (Fig. 1). Importantly, this cleavage is enhanced by pathogen or MAMP treatments (Fig. 2), suggesting that the BAK1 cleavage is involved in plant responses to pathogen attacks. Together with previous findings, the proteolytic cleavage of plant RLKs is likely another layer of regulatory mechanism in addition to the well-studied phosphorylation, which controls RLK activation.
We have observed that BAK1 cleavage is Ca2+-dependent (Fig. 1E), and multiple calpain inhibitors blocked BAK1 cleavage in vivo (Fig. 1G). Because the mutation of plant calpain DEK1 is embryonic-lethal (Lid et al., 2005; Johnson et al., 2008), we attempted to silence Arabidopsis DEK1 with VIGS. However, we did not observe the change of BAK1 cleavage in DEK1-silenced plants compared to control plants (Supplemental Fig. S6). Notably, DEK1-silenced plants were phenotypically similar to control plants. It is possible that the reduced DEK1 level in DEK1-silenced plants is sufficient for its normal functions. Despite extensive studies on mammalian calpains, the sequence and structural determinants of cleavage targets are little understood (Johnson et al., 2005), and no substrates of calpain in plants have been reported yet. Plant RLKs are functional counterparts of mammalian RTKs, cleavage of which is stimulated by caspases, metalloproteases, γ-secretase complex, or mRNA splicing (Ancot et al., 2009; Chen and Hung, 2015). The most prevalent mechanism of RTK cleavages occurs within the membrane through the action of a multisubunit γ-secretase complex (Carpenter and Liao, 2013). BAK1 cleavage likely occurs within or immediately after the transmembrane domain (Supplemental Fig. S2B). Arabidopsis also contains genes coding for γ-secretase homologous with the conserved amino acid motifs essential for enzymatic activities (Smolarkiewicz et al., 2014). However, the functions of γ-secretase subunits in plants are largely unknown. In a forward genetic screen for suppressors of the growth phenotype of a weak BRI1 allele bri1-5, BRS1 was identified and demonstrated as a secreted and active Ser carboxypeptidase (Li et al., 2001; Zhou and Li, 2005). Although BRS1’s substrates remain unknown, this work reconciles the importance of proteolytic cleavage processes in BR signaling. The proteolytic cleavage of BAK1 is required for its function in BR signaling and overexpression of BAK1D287A fails to alleviate the growth defects of bri1-5 (Fig. 5, C and D). It remains possible that BRS1 proteolytically processes BAK1 or plays a role in the BAK1 cleavage.
We have identified D287 as an important site for BAK1 cleavage (Fig. 3). This site is conserved among other SERK family members and likely required for SERK2 and SERK4 cleavage (Fig. 3I). D287 locates at the beginning of BAK1 kinase domain (Fig. 3A). BAK1 is a type-I integral membrane protein with a single transmembrane domain, an extracytoplasmic receptor domain, and a cytoplasmic kinase domain. Based on the molecular weight, BAK1 cleaved products are bigger than the predicted products of cytoplasmic domain (Supplemental Fig. S2, A and B). D287 may not be the cleavage site, but it is essential for the protease-mediated cleavage. Importantly, D287 is essential for BAK1 functions in plant immunity (Fig. 4), BR responses (Fig. 5, A–C), and cell death control (Fig. 5, D and E). Interestingly, the BAK1D287A mutant exhibited an unaltered in vitro kinase activity (Fig. 4G), suggesting that BAK1D287A is still a functional protein. There are several possibilities to explain why BAK1 cleavage is required for its normal functions. Similar with XA21 (Park and Ronald, 2012), the cleaved BAK1 may be released from the plasma membrane and translocated to the nucleus or other subcellular compartments to execute the functions. However, we have not observed a claimable nuclear localization of BAK1. It is also possible that the activated cytoplasmic domain of BAK1 is released to cytoplasm, where it phosphorylates different substrates for bifurcating its different functions. Additionally, the cleaved BAK1 ectodomain may have a higher affinity for the extracellular LRR domains of FLS2 and BRI1 than the FL BAK1, and may promote the ligand-induced receptor complex formation. We have observed that the BAK1 D287A mutant has a reduced plasma membrane localization (Fig. 6). The cleaved BAK1 fragments may facilitate the translocation of BAK1 to the plasma membrane. The BAK1 D287A mutant is likely trapped in the ER during protein maturation process (Fig. 6, C and D). ER-mediated protein quality control is essential for the abundance and signaling of some LRR–RLKs, such as EFR and BRI1 (Liu and Li, 2014; Tintor and Saijo, 2014).
Overexpression of BAK1 or its ectodomain in Arabidopsis triggers growth defects and constitutive activation of defense and cell death (Domínguez-Ferreras et al., 2015). It has been postulated that this cell death might be caused by unbalanced regulatory interactions, such as insufficient amount of the BAK1-INTERACTING RECEPTOR-LIKE KINASE1 (BIR1), which can suppress BAK1 activity (Gao et al., 2009; Domínguez-Ferreras et al., 2015; Liu et al., 2016). We observed that overexpression of the BAK1D287A mutant caused more extensive cell death than wild-type BAK1 in N. benthamiana and Arabidopsis when they were expressed at the same level (Fig. 7, A–F). These data suggest that BAK1 cleavage might provide a means to avoid the detrimental effect of BAK1 overproduction. Both BAK1 and BAK1D287A-induced cell death depends on SOBIR1 (Fig. 7G), which mediates bir1-induced cell death (Gao et al., 2009). SOBIR1 appears to be specifically required for immune responses triggered by certain LRR Receptor–Like Proteins, not LRR–RLKs (Liebrand et al., 2014). Thus, BAK1 overexpression-activated defense is likely mediated by a different mechanism with BAK1-mediated PTI in which BAK1 functions as a coreceptor of multiple LRR–RLKs. Indeed, BAK1 overexpression-activated defense is independent of a key PTI regulatory protein BIK1 (Fig. 7G). Thus, cleavage of BAK1 may have dual roles in plant immunity: mediating BIK1-dependent PTI signaling and attenuating SOBIR1-dependent autoimmunity. Notably, Domínguez-Ferreras et al. (2015) also observed the presence of multiple BAK1 polypeptides in BAK1 overexpression and wild-type plant extracts, and speculated that they were the products of proteolytic cleavage.
Pathogen-effector–mediated proteolytic cleavage is a common mechanism in the activation or attenuation of plant immunity (Dean, 2011; Pogány et al., 2015; Hou et al., 2018). P. syringae effector AvrPphB, a Cys protease, can cleave Arabidopsis receptor-like cytoplasmic kinase AVRPPHB SUSCEPTIBLE1 to initiate cytoplasmic immune receptor RESISTANCE TO P. SYRINGAE5-specified effector-triggered immunity and cleave AVRPPHB SUSCEPTIBLE1-like (PBL) kinases BIK1, PBL1, and PBL2 to inhibit PTI (Shao et al., 2003; Zhang et al., 2010). Interestingly, P. syringae effector HopB1 binds to FLS2 and cleaves immune-activated BAK1 to inhibit plant immunity (Li et al., 2016). In this study, we show that BAK1 proteolytic cleavage is dependent on a host-originated protease(s). In contrast to the HopB1-mediated BAK1 cleavage that occurs within the kinase domain between Arg-297 and Gly-298 (Li et al., 2016), the conserved eukaryotic protease-mediated BAK1 cleavage identified in this study occurs likely within the transmembrane domain, and the cleaved BAK1CTF has a normal kinase activity as the wild-type BAK1 (Fig. 4F). In the case of HopB1-mediated cleavage, the phosphorylation status of BAK1 is critical as two kinase-dead mutants (BAK1K317E and BAK1D416N in the ATP-binding site and catalytic site, respectively) and a phospho-site mutant of BAK1 (BAK1T455A) are resistant to cleavage by HopB1 (Li et al., 2016). In contrast, our studies show that the host-protease–mediated BAK1 cleavage does not require BAK1 kinase activity (Supplemental Fig. S1, D and E). Similarly, the proteolytic cleavage at the ectodomain of mammalian EPIDERMAL GROWTH FACTOR RECEPTOR does not require its kinase activity (Chen et al., 2008; Liao and Carpenter, 2012). These findings show that plants use a different type of protease with bacterial effector HopB1 to cleave BAK1 for a distinct function.
MATERIALS AND METHODS
Plant Materials, Growth Conditions, and Treatments
The following Arabidopsis (Arabidopsis thaliana) mutant lines used here have been described previously: bik1 (Salk_005291), sobir1-12, bak1-4, serk4-1, and bak1/SERK4+/− in Col-0 background (Gao et al., 2009; Lu et al., 2010; de Oliveira et al., 2016) and bri1-5 in Ws-0 background (Lin et al., 2013a). Arabidopsis plants were grown in soil (Metro Mix 366) in a growth room with 23°C, 45% relative humidity, 85 μE m−2 s−1 light, and a photoperiod of 12-h light/12-h dark for 4 weeks before protoplast isolation. To grow Arabidopsis seedlings on medium, the seeds were surface-sterilized with 50% bleach for 15 min, washed with sterilized ddH2O, and then placed on the plates with half-strength Murashige & Skoog medium (1/2 MS) containing 0.5% (w/v) Suc, 0.8% (w/v) agar, and 2.5 mm of 2-(n-morpholino)ethanesulfonic acid (MES) at pH 5.7. The plates were first stored at 4°C for 3 d in the dark for seed stratification, and then moved to the growth room for different periods of time depending on the experiments.
Plasmid Construction and Generation of Transgenic Plants
Protoplasts expression vectors pHBT-35S::BAK1-HA, pHBT-35S::BAK1-FLAG, pHBT-35S::BAK1-GFP, pHBT-35S::SERK1-FLAG, pHBT-35S::SERK2-FLAG, pHBT-35S::SERK4-FLAG, pHBT-35S::BIK1-HA, pHBT-35S::FLS2-HA, protein expression vectors pMAL-BAK1CD, pGST-BIK1KM, and VIGS vector pYL156-SERK4 were used as reported previously (Shan et al., 2008; Lu et al., 2010, 2011; Lin et al., 2013a; Zhou et al., 2014; Meng et al., 2015, 2016; de Oliveira et al., 2016). The yeast expression vector pESC-BAK1-MYC and binary vector pPZP212-pBAK1:BAK1-GFP were obtained from Dr. Jianming Li (Nam and Li, 2002). The binary vector pMDC32-2x35S::BAK1 was obtained from Dr. Chinchilla Delphine (Domínguez-Ferreras et al., 2015). The yeast expression vector pYES2-BAK1-HA was generated by subcloning BAK1-HA from pHBT-35S::BAK1-HA into a modified pYES2 vector using primers listed in Supplemental Table S1. Nontagged FL or truncated BAK1 in pHBT was cloned using pHBT-35S::BAK1-FLAG as the template and primers listed in Supplemental Table S1. To construct pCB302-35S::BAK1 and pCB302-35S::BAK1-HA binary vectors for Agrobacterium tumefaciens–mediated transformation in Arabidopsis, BAK1 was subcloned into a modified plant transformation binary vector pCB302 derivative under the control of the 35S promoter with or without an HA-epitope tag at its C terminus. To construct pHBT-pBAK1::BAK1-GFP and pCB302-pBAK1::BAK1, the 35S promoter in pHBT-35S::BAK1-GFP or pCB302-35S::BAK1 was substituted with the 1.5-kb BAK1 promoter that was amplified by PCR from Col-0 genomic DNA. BAK1/SERK point mutation variants were generated by site-directed mutagenesis with primers listed in Supplemental Table S1 and using respective BAK1 or SERK4 constructs as the templates. FL PSKR1 was amplified by PCR from Col-0 complementary DNA and cloned into a modified plant expression vector pCAMBIA1300 with a HA tag.
The BAK1 transgenic plants were generated by Agrobacterium-mediated transformation of Col-0, bri1-5, sobir1-12, bik1, or bak1/SRK4+/−, and screened with the herbicide BASTA (Bayer; resistance conferred by the pCB302 binary vector), antibiotic kanamycin (pPZP212), or hygromycin (pMDC32). BAK1 expression was detected by RT-PCR or western blot with α-HA, α-GFP, or α-BAK1 antibodies. The homozygous lines were selected based on the survival ratio of T2 and T3 generation plants after BASTA spray or antibiotic selection.
pBAK1::BAK1D287A-GFP plants expressing the ER marker were generated by Agrobacterium-mediated transformation with p2x35S::mCherry-HDEL (CD3-959, ER-rk; Nelson et al., 2007). T1 plants were selected on kanamycin and transferred to 1/2 MS without antibiotic for 2 d before imaging.
Arabidopsis Protoplast and Nicotiana benthamiana Transient Assays
For Arabidopsis protoplast transient expression, protoplasts were transfected with genes in the pHBT vector and incubated for 12 h (He et al., 2007b). The cell extracts were added with 2 × sodium dodecyl sulfate (SDS) loading buffer and subjected to immunoblot analysis.
For N. benthamiana transient expression, Agrobacterium strain GV3101 containing binary vector was cultured overnight in Lysogeny broth (LB) medium at 28°C. Bacteria were harvested by centrifugation and resuspended with buffer (10 mm of MES at pH 5.7, 10 mm of MgCl2, and 200 μm of acetosyringone) at A600 = 0.75. Leaves of 4-week–old soil-grown N. benthamiana were hand-infiltrated using a needleless syringe with Agrobacterium cultures. Leaf samples were collected 36 h after infiltration for protein isolation and immunoblot analysis. The cell death phenotype was observed and leaf pictures were taken 3 d after infiltration under UV light with a ChemiDoc system (Bio-Rad).
For electrolyte leakage assays, eight leaf discs (0.5-cm diameter) excised from N. benthamiana 4 d after infiltration were termed “one sample” and was prefloated in 10 mL of ddH2O for 10–15 min to eliminate wounding effect. The ddH2O was then exchanged and electrolyte leakage was measured using a conductivity meter (Traceable Conductivity Meter; VWR) as the average of three samples (n = 3).
In Vitro Phosphorylation, Immunocomplex Kinase, and In Vivo MAP Kinase Assays
For in vitro kinase assay, reactions were performed in 30 μL of kinase buffer (20 mm of Tris-HCl at pH 7.5, 10 mm of MgCl2, 5 mm of EGTA, 100 mm of NaCl, and 1 mm of dithiothreitol [DTT] containing 10 μg of fusion proteins with 0.1 mm of cold ATP and 5 μCi of [32P]γ-ATP) at room temperature for 3 h with gentle shaking. The reactions were stopped by adding 4 × SDS loading buffer. The phosphorylation of fusion proteins was analyzed by autoradiography after separation with 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
For immunocomplex kinase assay, protoplasts were lysed with 0.5 mL of immunoprecipitation (IP) buffer (50 mm of Tris·HCl at pH 7.5, 150 mm of NaCl, 5 mm of EDTA, 1 mm of DTT, 2 mm of NaF, 2 mm of Na3VO3, 1% of Triton, and a protease inhibitor mixture from Roche). After centrifugation at 12,470g for 10 min at 4°C, the supernatant was incubated with an α-FLAG antibody for 2 h and then with protein-G–agarose beads for another 2 h at 4°C with gentle shaking. The beads were collected and washed once with IP buffer and once with kinase buffer (20 mm of Tris·HCl at pH 7.5, 20 mm of MgCl2, 5 mm of EDTA, and 1 mm of DTT). The kinase reactions were performed in 20 μL of kinase buffer with 2 μg of GST fusion proteins, 0.1 mm of cold ATP, and 5 μCi of [32P]γ-ATP at room temperature for 1 h with gentle shaking. The phosphorylation of glutathione s-transferase (GST) fusion proteins was analyzed by 10% SDS-PAGE.
For detecting MAP kinase activity in vivo, 2-week-old seedlings grown on 1/2 MS medium were transferred to water overnight and then treated with 100 nm of flg22 or H2O for the times indicated and frozen in liquid N. The seedlings were homogenized in IP buffer and an equal amount of total protein was electrophoresed on 10% SDS-PAGE. An α-pERK antibody (1:2,000; Cell Signaling) was used to detect phosphorylation status of MPK3 and MPK6 with an immunoblot.
Measurement of ROS Production
Four leaves of each of six 5-week-old Arabidopsis plants were excised into leaf discs of 0.25 cm2, after an overnight incubation in 96-well plate with 100 μL of H2O to eliminate the wounding effect. H2O was replaced by 100 μL of reaction solution containing 50 μm of luminol and 10 μg/mL of horseradish peroxidase (Sigma-Aldrich) supplemented with 100 nm of flg22. The luminescence was measured with a luminometer (2030 Multilabel Reader, Victor X3; Perkin Elmer) immediately after adding the solution, with a 2-min interval reading time for a period of 60 min. The measurement value of ROS production from 24 leaf discs per treatment was indicated as the mean of Relative Light Units.
Callose Deposition
The leaves of 4-week-old plants were incubated with 500 nm of flg22 for 12 h at room temperature. Leaves were immediately cleared in alcoholic lactophenol (95% ethanol/lactophenol [phenol/glycerol/lactic acid/H2O, 1:1:1:1] = 2:1) overnight. Samples were subsequently rinsed with 50% ethanol and H2O. Cleared leaves were stained with 0.01% aniline blue in 0.15 m of P buffer (pH 9.5) and the callose deposits were visualized under a UV filter using a fluorescence microscope. Callose deposits were counted using the software ImageJ 1.43U (http://rsb.info.nih.gov/ij/). The number of deposits was expressed as the mean of six different leaf areas with se.
Pathogen Infection Assays
Pseudomonas syringae pv tomato DC3000 strain was cultured overnight at 28°C in King's B medium with 50 μg/mL of rifampicin. Bacteria were collected, washed, and diluted to the desired density with H2O. Four-week-old Arabidopsis leaves were preinoculated with 200 nm of flg22 or H2O control for 24 h and then infiltrated with bacteria at a concentration of 5 × 105 cfu/mL using a needleless syringe. To measure bacterial growth, two leaf discs were ground in 100 μL of H2O and serial dilutions were plated on King's B medium with appropriate antibiotics. Bacterial-colony–forming units (cfu) were counted 2 d after incubation at 28°C. Each data point is shown as triplicates.
Trypan Blue and 3,3′-Diaminobenzidine Staining
For trypan blue staining, the leaves of 4-week-old plants were excised and subsequently immersed in boiled lactophenol (lactic acid/glycerol/liquid phenol/distilled water, 1:1:1:1) solution with 0.25 mg/mL trypan blue for 1 min. The stained leaves were destained with 95% ethanol/lactophenol solution, and washed with 50% ethanol. For 3,3′-diaminobenzidine (DAB) staining, the leaves of 4-week-old plants were immersed in 1 mg/mL DAB (pH 3.8) solution with low vacuum pressure for 30 min, followed by an overnight incubation at room temperature in the dark. The stained leaves were fixed and cleared in alcoholic lactophenol (95% ethanol/lactic acid/phenol, 2:1:1) at 65°C, and rinsed once with 50% ethanol, and twice with H2O. The destained leaves were subjected to microscopic observation.
Agrobacterium-Mediated VIGS Assay
Plasmids containing binary Tobacco rattle virus (TRV) vectors pTRV–RNA1 and pTRV–RNA2-derivative pYL156–SERK4 were introduced into A. tumefaciens strain GV3101 by electroporation. Agrobacterium cultures were first grown overnight in LB medium containing 50 μg/mL of kanamycin and 25 μg/mL of gentamicin, and then subcultured in fresh LB medium containing 50 μg/mL of kanamycin and 25 μg/mL of gentamicin supplemented with 10 mm of MES and 20 μm of acetosyringone overnight at 28°C in a roller drum. Cells were pelleted by 4,200 revolutions per min centrifugation, then resuspended in a solution containing 10 mm of MgCl2, 10 mm of MES, and 200 μm of acetosyringone, adjusted to A600 = 1.5 and incubated at 25°C for at least 3 h. Agrobacterium cultures containing pTRV–RNA1 and pYL156–SERK4 were mixed in a 1:1 ratio and inoculated into the first pair of true leaves of 2-week–old soil-grown plants using a needleless syringe.
Plasma Membrane Protein Enrichment
Enrichment of plasma membrane proteins was performed as reported in Lee (2017) with modifications. Briefly, N. benthamiana leaves weighing ∼1 g were ground in liquid N2, and further lysed with 10 mL of ice-cold extraction buffer containing 100 mm of Tris–HCl at pH 8.8, 150 mm of NaCl, 1 mm of EDTA, 20% glycerol, 0.75% polyvinylpolypyrrolidone, 20 mm of NaF, 2 mm of Na3VO4, 1 mm of phenylmethylsulfonyl fluoride, and one complete protease inhibitor cocktail (Roche) per 50 mL. The lysate was centrifuged at 10,000g at 4°C for 10 min to pellet and remove intact cells, cell debris, and intact organelles. Microsomal membranes were then separated by ultracentrifugation at 100,000g for 30 min at 4°C and treated with detergent Brij58 (0.02%; Sigma-Aldrich) to invert plasma membrane vesicles and release contaminating contents (Collins et al., 2017). Pellets were washed with extraction buffer and subjected to a final ultracentrifugation step to collect the enriched plasma membrane fraction.
Confocal Microscopy and Image Analysis
Five- to seven-day-old Arabidopsis seedlings were imaged using a SP8X Inverted Confocal Microscope (Leica) equipped with a HC PL APO CS2 40×/1.10 Water-Corrected Objective (Leica). The excitation wavelength was 488 nm for both GFP and FM4-64, and 561 nm for mCherry by using the white light laser. Emission was detected at 500–530 nm or 495–540 nm for GFP, 570–670 nm for FM4-64, and 600–700 nm for mCherry by using hybrid detectors (Leica). Autofluorescence was removed by adjusting the time gate window between 1 and 4.5 ns or 0.3 and 6 ns. Intensities were manipulated with the LAS-X standard software (Leica) and the software FIJI (National Institutes of Health). The Pearson’s correlation coefficient (r value) was calculated using the “coloc-2 plugin” included in the software FIJI. ER-Tracker Red (BODIPY Glibenclamide; Life Technologies) was used to stain ER in Arabidopsis roots following the manufacturer’s protocol.
Accession Numbers
Sequence data from this article can be found in The Arabidopsis Information Resource database under the following accession numbers: SERK1, AT1G71830; SERK2, AT1G34210; BAK1/SERK3, AT4G33430; SERK4, AT2G13790; FLS2, AT5G46330; BRI1, AT4G39400; BIK1, AT2G39660; SOBIR1, AT2G31880.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Production of C-terminal BAK1 fragments.
Supplemental Figure S2. Identification of BAK1 cleavage site by mutational analyses.
Supplemental Figure S3. Phenotype of BAK1D287A transgenic plants.
Supplemental Figure S4. The D287 site is critical for BAK1/SERK4-mediated cell death.
Supplemental Figure S5. Colocalization of BAK1D287A-GFP with ER marker.
Supplemental Figure S6. The proteolytic cleavage of BAK1 in DEK1-silenced Arabidopsis.
Supplemental Table S1. Primers for point mutations, gene cloning, genotyping, and RT-qPCR.
Acknowledgments
We thank Jianming Li and Delphine Chinchilla for providing materials.
Footnotes
This work was supported by the National Institutes of Health (NIH grant nos. R01GM092893 to P.H. and R01GM097247 to L.S.); the National Science Foundation (NSF grant no. IOS-1252539 to P.H.); the Robert A. Welch Foundation (grant no. A-1795 to L.S.); the Young Eastern Scholar (grant no. QD2016035 to J.Z.); Shanghai Sailing Program (grant no. 17YF1406400 to J.Z.); the China Scholar Council (to P.W. and G.X.); and the Research Foundation-Flanders (grant nos. G022516N and G0E5718N to E.R.).
References
- Ancot F, Foveau B, Lefebvre J, Leroy C, Tulasne D (2009) Proteolytic cleavages give receptor tyrosine kinases the gift of ubiquity. Oncogene 28: 2185–2195 [DOI] [PubMed] [Google Scholar]
- Antolín-Llovera M, Ried MK, Parniske M (2014) Cleavage of the SYMBIOSIS RECEPTOR-LIKE KINASE ectodomain promotes complex formation with Nod factor receptor 5. Curr Biol 24: 422–427 [DOI] [PubMed] [Google Scholar]
- Belkhadir Y, Wang X, Chory J (2006) Arabidopsis brassinosteroid signaling pathway. Sci STKE 2006: cm5. [DOI] [PubMed] [Google Scholar]
- Belkhadir Y, Yang L, Hetzel J, Dangl JL, Chory J (2014) The growth-defense pivot: Crisis management in plants mediated by LRR-RK surface receptors. Trends Biochem Sci 39: 447–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T, Felix G (2009) A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60: 379–406 [DOI] [PubMed] [Google Scholar]
- Carpenter G, Liao HJ (2013) Receptor tyrosine kinases in the nucleus. Cold Spring Harb Perspect Biol 5: a008979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MK, Hung MC (2015) Proteolytic cleavage, trafficking, and functions of nuclear receptor tyrosine kinases. FEBS J 282: 3693–3721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Chen LM, Lin CY, Chai KX (2008) The epidermal growth factor receptor (EGFR) is proteolytically modified by the Matriptase-Prostasin serine protease cascade in cultured epithelial cells. Biochim Biophys Acta 1783: 896–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JD, Felix G, Boller T (2007) A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448: 497–500 [DOI] [PubMed] [Google Scholar]
- Collins CA, Leslie ME, Peck SC, Heese A (2017) Simplified enrichment of plasma membrane proteins from Arabidopsis thaliana seedlings using differential centrifugation and Brij-58 treatment. Methods Mol Biol 1564: 155–168 [DOI] [PubMed] [Google Scholar]
- Dean P. (2011) Functional domains and motifs of bacterial type III effector proteins and their roles in infection. FEMS Microbiol Rev 35: 1100–1125 [DOI] [PubMed] [Google Scholar]
- de Oliveira MV, Xu G, Li B, de Souza Vespoli L, Meng X, Chen X, Yu X, de Souza SA, Intorne AC, de A Manhães AM, et al. (2016) Specific control of Arabidopsis BAK1/SERK4-regulated cell death by protein glycosylation. Nat Plants 2: 15218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez-Ferreras A, Kiss-Papp M, Jehle AK, Felix G, Chinchilla D (2015) An overdose of the Arabidopsis coreceptor BRASSINOSTEROID INSENSITIVE1-ASSOCIATED RECEPTOR KINASE1 or its ectodomain causes autoimmunity in a SUPPRESSOR OF BIR1-1-dependent manner. Plant Physiol 168: 1106–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Gao Y, Zhan Y, Zhang S, Wu Y, Xiao Y, Zou B, He K, Gou X, Li G, Lin H, Li J (2016) Nucleocytoplasmic trafficking is essential for BAK1- and BKK1-mediated cell-death control. Plant J 85: 520–531 [DOI] [PubMed] [Google Scholar]
- Ewald SE, Lee BL, Lau L, Wickliffe KE, Shi GP, Chapman HA, Barton GM (2008) The ectodomain of Toll-Like Receptor 9 is cleaved to generate a functional receptor. Nature 456: 658–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenteany G, Standaert RF, Lane WS, Choi S, Corey EJ, Schreiber SL (1995) Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science 268: 726–731 [DOI] [PubMed] [Google Scholar]
- Gao M, Wang X, Wang D, Xu F, Ding X, Zhang Z, Bi D, Cheng YT, Chen S, Li X, Zhang Y (2009) Regulation of cell death and innate immunity by two receptor-like kinases in Arabidopsis. Cell Host Microbe 6: 34–44 [DOI] [PubMed] [Google Scholar]
- Gómez-Gómez L, Boller T (2000) FLS2: An LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 5: 1003–1011 [DOI] [PubMed] [Google Scholar]
- Gou X, Yin H, He K, Du J, Yi J, Xu S, Lin H, Clouse SD, Li J (2012) Genetic evidence for an indispensable role of somatic embryogenesis receptor kinases in brassinosteroid signaling. PLoS Genet 8: e1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida K, Bartlett AH, Chen Y, Park PW (2010) Molecular and cellular mechanisms of ectodomain shedding. Anat Rec (Hoboken) 293: 925–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Gou X, Yuan T, Lin H, Asami T, Yoshida S, Russell SD, Li J (2007a) BAK1 and BKK1 regulate brassinosteroid-dependent growth and brassinosteroid-independent cell-death pathways. Curr Biol 17: 1109–1115 [DOI] [PubMed] [Google Scholar]
- He P, Shan L, Sheen J (2007b) The use of protoplasts to study innate immune responses. Methods Mol Biol 354: 1–9 [DOI] [PubMed] [Google Scholar]
- He Y, Zhou J, Shan L, Meng X (2018) Plant cell surface receptor-mediated signaling—a common theme amid diversity. J Cell Sci 131: jcs209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heese A, Hann DR, Gimenez-Ibanez S, Jones AME, He K, Li J, Schroeder JI, Peck SC, Rathjen JP (2007) The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci USA 104: 12217–12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S, Jamieson P, He P (2018) The cloak, dagger, and shield: Proteases in plant-pathogen interactions. Biochem J 475: 2491–2509 [DOI] [PubMed] [Google Scholar]
- Johnson KL, Degnan KA, Ross Walker J, Ingram GC (2005) AtDEK1 is essential for specification of embryonic epidermal cell fate. Plant J 44: 114–127 [DOI] [PubMed] [Google Scholar]
- Johnson KL, Faulkner C, Jeffree CE, Ingram GC (2008) The Phytocalpain Defective Kernel 1 is a novel Arabidopsis growth regulator whose activity is regulated by proteolytic processing. Plant Cell 20: 2619–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlova R, Boeren S, Russinova E, Aker J, Vervoort J, de Vries S (2006) The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE1 protein complex includes BRASSINOSTEROID-INSENSITIVE1. Plant Cell 18: 626–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmerling B, Schwedt A, Rodriguez P, Mazzotta S, Frank M, Qamar SA, Mengiste T, Betsuyaku S, Parker JE, Müssig C, et al. (2007) The BRI1-Associated Kinase 1, BAK1, has a brassinolide-independent role in plant cell-death control. Curr Biol 17: 1116–1122 [DOI] [PubMed] [Google Scholar]
- Ladwig F, Dahlke RI, Stührwohldt N, Hartmann J, Harter K, Sauter M (2015) Phytosulfokine regulates growth in Arabidopsis through a response module at the plasma membrane that includes CYCLIC NUCLEOTIDE-GATED CHANNEL17, H+-ATPase, and BAK1. Plant Cell 27: 1718–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS. (2017) Purification of plant receptor kinases from plant plasma membranes. Methods Mol Biol 1621: 47–56 [DOI] [PubMed] [Google Scholar]
- Li J, Chory J (1997) A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90: 929–938 [DOI] [PubMed] [Google Scholar]
- Li J, Lease KA, Tax FE, Walker JC (2001) BRS1, a serine carboxypeptidase, regulates BRI1 signaling in Arabidopsis thaliana. Proc Natl Acad Sci USA 98: 5916–5921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wen J, Lease KA, Doke JT, Tax FE, Walker JC (2002) BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110: 213–222 [DOI] [PubMed] [Google Scholar]
- Li L, Kim P, Yu L, Cai G, Chen S, Alfano JR, Zhou JM (2016) Activation-dependent destruction of a co-receptor by a Pseudomonas syringae effector dampens plant immunity. Cell Host Microbe 20: 504–514 [DOI] [PubMed] [Google Scholar]
- Li Z, Wang Y, Huang J, Ahsan N, Biener G, Paprocki J, Thelen JJ, Raicu V, Zhao D (2017) Two SERK receptor-like kinases interact with EMS1 to control anther cell fate determination. Plant Physiol 173: 326–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Zhou JM (2018) Receptor-like cytoplasmic kinases: Central players in plant receptor kinase-mediated signaling. Annu Rev Plant Biol 69: 267–299 [DOI] [PubMed] [Google Scholar]
- Liao HJ, Carpenter G (2012) Regulated intramembrane cleavage of the EGF receptor. Traffic 13: 1106–1112 [DOI] [PubMed] [Google Scholar]
- Lid SE, Olsen L, Nestestog R, Aukerman M, Brown RC, Lemmon B, Mucha M, Opsahl-Sorteberg HG, Olsen OA (2005) Mutation in the Arabidopsis thaliana DEK1 calpain gene perturbs endosperm and embryo development while over-expression affects organ development globally. Planta 221: 339–351 [DOI] [PubMed] [Google Scholar]
- Liebrand TW, van den Burg HA, Joosten MH (2014) Two for all: Receptor-associated kinases SOBIR1 and BAK1. Trends Plant Sci 19: 123–132 [DOI] [PubMed] [Google Scholar]
- Lin W, Lu D, Gao X, Jiang S, Ma X, Wang Z, Mengiste T, He P, Shan L (2013a) Inverse modulation of plant immune and brassinosteroid signaling pathways by the receptor-like cytoplasmic kinase BIK1. Proc Natl Acad Sci USA 110: 12114–12119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Ma X, Shan L, He P (2013b) Big roles of small kinases: The complex functions of receptor-like cytoplasmic kinases in plant immunity and development. J Integr Plant Biol 55: 1188–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Li B, Lu D, Chen S, Zhu N, He P, Shan L (2014) Tyrosine phosphorylation of protein kinase complex BAK1/BIK1 mediates Arabidopsis innate immunity. Proc Natl Acad Sci USA 111: 3632–3637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Li J (2014) Endoplasmic reticulum-mediated protein quality control in Arabidopsis. Front Plant Sci 5: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Huang X, Li M, He P, Zhang Y (2016) Loss-of-function of Arabidopsis receptor-like kinase BIR1 activates cell death and defense responses mediated by BAK1 and SOBIR1. New Phytol 212: 637–645 [DOI] [PubMed] [Google Scholar]
- Lu D, Wu S, Gao X, Zhang Y, Shan L, He P (2010) A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc Natl Acad Sci USA 107: 496–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Lin W, Gao X, Wu S, Cheng C, Avila J, Heese A, Devarenne TP, He P, Shan L (2011) Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science 332: 1439–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Xu G, He P, Shan L (2016) SERKing coreceptors for receptors. Trends Plant Sci 21: 1017–1033 [DOI] [PubMed] [Google Scholar]
- Macho AP, Zipfel C (2014) Plant PRRs and the activation of innate immune signaling. Mol Cell 54: 263–272 [DOI] [PubMed] [Google Scholar]
- Meng X, Chen X, Mang H, Liu C, Yu X, Gao X, Torii KU, He P, Shan L (2015) Differential function of Arabidopsis SERK family receptor-like kinases in stomatal patterning. Curr Biol 25: 2361–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Zhou J, Tang J, Li B, de Oliveira MVV, Chai J, He P, Shan L (2016) Ligand-induced receptor-like kinase complex regulates floral organ abscission in Arabidopsis. Cell Reports 14: 1330–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam KH, Li J (2002) BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 110: 203–212 [DOI] [PubMed] [Google Scholar]
- Nelson BK, Cai X, Nebenführ A (2007) A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J 51: 1126–1136 [DOI] [PubMed] [Google Scholar]
- Ntoukakis V, Schwessinger B, Segonzac C, Zipfel C (2011) Cautionary notes on the use of C-terminal BAK1 fusion proteins for functional studies. Plant Cell 23: 3871–3878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y, Sorimachi H (2012) Calpains: An elaborate proteolytic system. Biochim Biophys Acta 1824: 224–236 [DOI] [PubMed] [Google Scholar]
- Ou Y, Lu X, Zi Q, Xun Q, Zhang J, Wu Y, Shi H, Wei Z, Zhao B, Zhang X, et al. (2016) RGF1 INSENSITIVE 1 to 5, a group of LRR receptor-like kinases, are essential for the perception of ROOT MERISTEM GROWTH FACTOR 1 in Arabidopsis thaliana. Cell Res 26: 686–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CJ, Ronald PC (2012) Cleavage and nuclear localization of the rice XA21 immune receptor. Nat Commun 3: 920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B, Brinkmann MM, Spooner E, Lee CC, Kim YM, Ploegh HL (2008) Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-Like Receptor 9. Nat Immunol 9: 1407–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perraki A, DeFalco TA, Derbyshire P, Avila J, Séré D, Sklenar J, Qi X, Stransfeld L, Schwessinger B, Kadota Y, et al. (2018) Phosphocode-dependent functional dichotomy of a common co-receptor in plant signalling. Nature 561: 248–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petes C, Odoardi N, Gee K (2017) The toll for trafficking: Toll-Like Receptor 7 delivery to the endosome. Front Immunol 8: 1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petutschnig EK, Stolze M, Lipka U, Kopischke M, Horlacher J, Valerius O, Rozhon W, Gust AA, Kemmerling B, Poppenberger B, et al. (2014) A novel Arabidopsis CHITIN ELICITOR RECEPTOR KINASE 1 (CERK1) mutant with enhanced pathogen-induced cell death and altered receptor processing. New Phytol 204: 955–967 [DOI] [PubMed] [Google Scholar]
- Pogány M, Dankó T, Kámán-Tóth E, Schwarczinger I, Bozsó Z (2015) Regulatory proteolysis in Arabidopsis-pathogen interactions. Int J Mol Sci 16: 23177–23194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postel S, Küfner I, Beuter C, Mazzotta S, Schwedt A, Borlotti A, Halter T, Kemmerling B, Nürnberger T (2010) The multifunctional leucine-rich repeat receptor kinase BAK1 is implicated in Arabidopsis development and immunity. Eur J Cell Biol 89: 169–174 [DOI] [PubMed] [Google Scholar]
- Roux M, Schwessinger B, Albrecht C, Chinchilla D, Jones A, Holton N, Malinovsky FG, Tör M, de Vries S, Zipfel C (2011) The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell 23: 2440–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russinova E, Borst JW, Kwaaitaal M, Caño-Delgado A, Yin Y, Chory J, de Vries SC (2004) Heterodimerization and endocytosis of Arabidopsis brassinosteroid receptors BRI1 and AtSERK3 (BAK1). Plant Cell 16: 3216–3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago J, Henzler C, Hothorn M (2013) Molecular mechanism for plant steroid receptor activation by somatic embryogenesis co-receptor kinases. Science 341: 889–892 [DOI] [PubMed] [Google Scholar]
- Santiago J, Brandt B, Wildhagen M, Hohmann U, Hothorn LA, Butenko MA, Hothorn M (2016) Mechanistic insight into a peptide hormone signaling complex mediating floral organ abscission. eLife 5: e15075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan L, He P, Li J, Heese A, Peck SC, Nürnberger T, Martin GB, Sheen J (2008) Bacterial effectors target the common signaling partner BAK1 to disrupt multiple MAMP receptor-signaling complexes and impede plant immunity. Cell Host Microbe 4: 17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao F, Golstein C, Ade J, Stoutemyer M, Dixon JE, Innes RW (2003) Cleavage of Arabidopsis PBS1 by a bacterial type III effector. Science 301: 1230–1233 [DOI] [PubMed] [Google Scholar]
- Shiu SH, Bleecker AB (2003) Expansion of the receptor-like kinase/Pelle gene family and receptor-like proteins in Arabidopsis. Plant Physiol 132: 530–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolarkiewicz M, Skrzypczak T, Michalak M, Leśniewicz K, Walker JR, Ingram G, Wojtaszek P (2014) Gamma-secretase subunits associate in intracellular membrane compartments in Arabidopsis thaliana. J Exp Bot 65: 3015–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Liu L, Wang J, Wu Z, Zhang H, Tang J, Lin G, Wang Y, Wen X, Li W, et al. (2016) Signature motif-guided identification of receptors for peptide hormones essential for root meristem growth. Cell Res 26: 674–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Han Z, Tang J, Hu Z, Chai C, Zhou B, Chai J (2013a) Structure reveals that BAK1 as a co-receptor recognizes the BRI1-bound brassinolide. Cell Res 23: 1326–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Li L, Macho AP, Han Z, Hu Z, Zipfel C, Zhou JM, Chai J (2013b) Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex. Science 342: 624–628 [DOI] [PubMed] [Google Scholar]
- Tintor N, Saijo Y (2014) ER-mediated control for abundance, quality, and signaling of transmembrane immune receptors in plants. Front Plant Sci 5: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubuki S, Saito Y, Tomioka M, Ito H, Kawashima S (1996) Differential inhibition of calpain and proteasome activities by peptidyl aldehydes of di-leucine and tri-leucine. J Biochem 119: 572–576 [DOI] [PubMed] [Google Scholar]
- Villamor JG, Kaschani F, Colby T, Oeljeklaus J, Zhao D, Kaiser M, Patricelli MP, van der Hoorn RA (2013) Profiling protein kinases and other ATP binding proteins in Arabidopsis using Acyl-ATP probes. Mol Cell Proteomics 12: 2481–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Li H, Han Z, Zhang H, Wang T, Lin G, Chang J, Yang W, Chai J (2015) Allosteric receptor activation by the plant peptide hormone phytosulfokine. Nature 525: 265–268 [DOI] [PubMed] [Google Scholar]
- Wang X, Kota U, He K, Blackburn K, Li J, Goshe MB, Huber SC, Clouse SD (2008) Sequential transphosphorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling. Dev Cell 15: 220–235 [DOI] [PubMed] [Google Scholar]
- Yan L, Ma Y, Liu D, Wei X, Sun Y, Chen X, Zhao H, Zhou J, Wang Z, Shui W, Lou Z (2012) Structural basis for the impact of phosphorylation on the activation of plant receptor-like kinase BAK1. Cell Res 22: 1304–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Feng B, He P, Shan L (2017) From chaos to harmony: Responses and signaling upon microbial pattern recognition. Annu Rev Phytopathol 55: 109–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatz M, Starling A (2005) Calpains and disease. N Engl J Med 352: 2413–2423 [DOI] [PubMed] [Google Scholar]
- Zhang J, Li W, Xiang T, Liu Z, Laluk K, Ding X, Zou Y, Gao M, Zhang X, Chen S, et al. (2010) Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe 7: 290–301 [DOI] [PubMed] [Google Scholar]
- Zhou A, Li J (2005) Arabidopsis BRS1 is a secreted and active serine carboxypeptidase. J Biol Chem 280: 35554–35561 [DOI] [PubMed] [Google Scholar]
- Zhou J, Wu S, Chen X, Liu C, Sheen J, Shan L, He P (2014) The Pseudomonas syringae effector HopF2 suppresses Arabidopsis immunity by targeting BAK1. Plant J 77: 235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, Felix G (2006) Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125: 749–760 [DOI] [PubMed] [Google Scholar]