Abstract
Introduction:
Rotavirus is a leading cause of acute gastroenteritis among Taiwanese children. Two globally licensed rotavirus vaccines recommended for inclusion in routine immunization programs that have been available for private market use in Taiwan since 2006 have been associated with a low risk of intussusception in post-marketing studies conducted in several countries. Our objective was to examine trends and characteristics of intussusception hospitalizations in Taiwan among children aged <12 months before and after rotavirus vaccine licensure to provide updated baseline and early post-licensure data.
Methods:
We extracted data on intussusception-related hospitalizations among children aged <12 months during 2001–2013 from the National Health Insurance Research Database. We examined patient demographics, clinical outcome, and hospitalization trends, focusing on recommended ages for rotavirus vaccination (6–14 weeks, 15–24 weeks, and 25–34 weeks). We compared mean hospitalization rates for pre-vaccine licensure years 2001–2005 with those for post-vaccine licensure years 2007–2013 using Poisson regression analysis.
Results:
During 2001–2013, 1998 intussusceptions hospitalizations were recorded. The mean age of hospitalization was 33 weeks. Almost all children recovered; 3 deaths occurred. The overall intussusception hospitalization rate was 75.1 per 100,000; seasonality was not evident. Hospitalization rates were greatest in children aged ≥25 weeks and occurred more frequently in boys. Pre-vaccine and post-vaccine licensure trends in annual hospitalization rates did not significantly differ. However, mean hospitalization rates were lower during the post-vaccine licensure period for children aged <12 months (RR: 0.84, 95% CI: 0.76–0.92) with the greatest decline among children aged 25–34 weeks (RR: 0.66, 95% CI: 0.55–0.78).
Conclusions:
Infant intussusception in Taiwan occurs at a rate within the range of other Asian countries, is rare among children aged <3 months, has a male predominance, and does not have a clear seasonality pattern. We did not observe a post-licensure increase in intussusception hospitalization rates in children aged 6–14 weeks.
Keywords: intussusception, rotavirus vaccine
INTRODUCTION
Intussusception is a rare clinical condition in which the intestine folds into itself, leading to bowel obstruction that, in turn, can lead to tissue edema, necrosis, and possibly intestinal perforation [1, 2]. Death may occur if the intussusception does not self-resolve or is not managed promptly. Management of intussusception includes reduction by air or hydrostatic enema or surgery [1, 2]. The cause of intussusception in most infant cases is not known. However, some infectious pathogens, such as respiratory adenovirus and various enteric bacteria, have been temporally associated in several studies [3–7]. Additionally, lymphoid hyperplasia, intestinal inflammation, and alterations in gastrointestinal motility have been proposed as mechanisms for intussusception in children [2, 8, 9].
Worldwide, the estimated mean incidence of naturally occurring intussusception among children aged <12 months is 74 cases (range: 9–328) per 100,000 children, with higher rates occurring in certain Asian countries such as Japan, the Republic of Korea, and Vietnam (range: 158–328 cases per 100,000 children) [10, 11]. A previous study conducted in Taiwan for 1998 through 2007 estimated an incidence rate of 77 cases per 100,000 person-years among children ≤12 months of age [12]. Reasons for the higher rates observed in certain Asian countries are not clear, but potential reasons include genetic predisposition, environmental differences including circulating pathogens, different feeding practices, and different diagnostic practices and access to health care [10].
In 1999, the first licensed rotavirus vaccine, Rotashield (Wyeth), was removed from the market due to an association with intussusception on the order of 1 excess case per 10,000 vaccinated infants in the US [13]. Following this, large clinical trials were conducted for the two current globally licensed rotavirus vaccines, Rotarix (GlaxoSmithKline, Rixensart, Belgium) and RotaTeq (Merck & Co., Kenilworth, NJ) [14, 15]. These trials did not detect an increased risk of intussusception. However, post-marketing evaluations in several upper middle to high income countries have detected a risk of vaccine-associated intussusception with both vaccines on the order of ~1–5 excess cases of intussusception per 100,000 vaccinated infants [16–22]. Various national- and global-level expert bodies have reviewed these risks alongside rotavirus vaccination impact data and have concluded that, based on the available evidence, the benefits of rotavirus vaccination outweigh the risks of intussusception [23]. The World Health Organization (WHO) continues to recommend that rotavirus vaccines be included in all national immunization programs [24].
In Taiwan, previous studies have estimated that rotavirus accounts for 25–43% of all acute gastroenteritis hospitalizations in children aged <5 years [25, 26]. Both Rotarix and RotaTeq have been available for private market use in Taiwan since 2006. However, costs for these vaccines are not covered by the National Health Insurance Program, so parents must pay out-of-pocket if they would like their children to receive either of these vaccines. Administrative data-based reports from healthcare providers to the Taiwan Centers for Disease Control (TCDC) indicate that rotavirus vaccination coverage of at least one dose of vaccine only reached ~53% by 2014 [27]. As rotavirus remains a leading cause of acute gastroenteritis among children in Taiwan and policy makers consider including rotavirus vaccines in the routine immunization program, updated baseline data and early post-vaccine licensure monitoring of intussusception epidemiology and trends in infants will be useful for post-vaccine introduction safety monitoring. Our objective was to examine trends and describe the epidemiological characteristics of hospitalizations related to intussusception among children aged <12 months before and after licensure of rotavirus vaccines in Taiwan using a robust insurance claims database that represents over 99% of the population of Taiwan.
METHODS
Hospitalization data
We conducted a retrospective analysis of intussusception-related health care visits among children aged <12 months in Taiwan from 2001 through 2013 using data obtained from the National Health Insurance Research Database (NHIRD) [28]. In Taiwan, 99.6% of the population participates in the National Health Insurance (NHI) program, and the NHIRD contains health care claims data for all NHI participants [29].
For this analysis, we defined an intussusception-related hospitalization as one with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code for intussusception (560.0) listed as any diagnosis. Additionally, we identified intussusception hospitalizations requiring surgical intervention as those that had an ICD-9-CM procedure code of 45.0–48.9 or 54.0–54.2 listed as a procedure performed during the hospitalization.
Population data
We calculated intussusception hospitalization rates using mid-year population data for children <12 months of age provided by the Department of Household Registration Affairs, Ministry of the Interior [30]. We assumed births to be evenly distributed throughout the year when calculating intussusception hospitalization rates by age in weeks as data on births by shorter time periods such as weeks or months were not available.
Analysis
We examined patient demographic characteristics, the number of intussusception hospitalizations per child, clinical outcomes, length of hospitalization, and pre-rotavirus vaccine licensure intussusception hospitalization incidence by week of age where incidence was modeled using a spline curve fit to weekly estimates of intussusception incidence. For children who had >1 intussusception hospitalization recorded, hospitalizations that were part of a hospital transfer for the initial course of illness were not counted as a separate intussusception episode. If a child was discharged home and hospitalized again for intussusception at a later date, this was counted as a separate episode. We assessed intussusception seasonality by reviewing monthly hospitalization rates for the entire study period and examined overall trends in the total number and rate of intussusception hospitalizations and rate trends by age groups with a focus on the recommended age windows for rotavirus vaccine administration in Taiwan (i.e., 6–14 weeks, 15–24 weeks, and 25–34 weeks).
To examine intussusception hospitalization rates during pre- and post-rotavirus vaccine licensure time periods by age group, we compared annual rate trends and mean hospitalization rates for pre-vaccine licensure years 2001 through 2005 with those for post-vaccine licensure years 2007 through 2013. We excluded data from 2006 as this was the year of rotavirus vaccine licensure in Taiwan. We calculated rate ratios (RR) and 95% confidence intervals (CI) using Poisson regression analysis.
This study was approved by the Institutional Review Board of the National Health Research Institutes of Taiwan.
RESULTS
Epidemiology of intussusception hospitalizations
During 2001–2013, 1998 intussusceptions hospitalizations were recorded in the NHIRD. The mean age of children at the time of admission was 33 weeks (range: 0 to 51 weeks) (Table 1). During 2001–2005, prior to rotavirus vaccine licensure, intussusception hospitalization rates by week of age showed a steady increase starting at about 8 weeks of age, with a peak in rates between 30 and 44 weeks of age (Figure 1). Of the 1998 recorded hospitalizations, 1887 occurred in children who had one documented intussusception hospitalization over the study period, while the remaining 111 hospitalizations occurred in 52 children who had more than one documented intussusception hospitalization (range: 2 to 5 hospitalizations per child) (Table 1); the median time between hospitalizations for children who had recurrent episodes was 48 days (range: 2 to 280 days). Hospitalization rates were greatest in the older age groups of 25–34 weeks and ≥35 weeks, and there was a male predominance in hospitalizations – 58.1% of hospitalizations occurred in boys, and 41.9% occurred in girls for a ratio of 1.4:1 (Table 1). Overall, 36.7% (annual range: 30.6%−41.5%) of intussusception hospitalizations required surgical treatment, and surgical rates showed no clear trends over time. Most children were discharged home alive (Table 1); only 3 deaths were recorded over the entire study period – one each in 2004, 2009, and 2012 and all in cases that had required surgery. The mean length of hospital stay was 2.7 days (range: 0–40 days) for non-surgical cases and 6.6 days (range: 0–108 days) for surgical cases (Table 1).
Table 1.
Demographic characteristics and clinical course of children <12 months of age hospitalized for intussusception − Taiwan, 2001–2013
| Characteristic or clinical course | N (%), Mean (Range) | Rate per 100,000 children |
|---|---|---|
| Total number of intussusception hospitalizations | 1998 | 75.1 |
| Mean age of children with intussusception (range) | 33 weeks (0–51) | |
| Episodes of intussusception per child | ||
| No. of children with 1 episode | 1887 (97%) | |
| No. of children with >1 episode (range: 2–5) | 52 (3%) | |
| Intussusception hospitalizations, by age group | ||
| 0–5 weeks | 14 (0.7%) | 4.6 |
| 6–14 weeks | 108 (5.4%) | 23.5 |
| 15–24 weeks | 369 (18.5%) | 72.4 |
| 25–34 weeks | 532 (26.6%) | 104.4 |
| 35+ weeks | 975 (48.8%) | 112.5 |
| Intussusception hospitalizations, by sex | ||
| Male | 1161 (58.1%) | 83.6 |
| Female | 837 (41.9%) | 65.8 |
| Intussusception hospitalizations, by surgical status | ||
| Non-surgical case | 1264 (63.3%) | 47.5 |
| Surgical case | 734 (36.7%) | 27.6 |
| Outcomes of intussusception hospitalizations | ||
| Discharged home | 1927 (96.4%) | |
| Left hospital against medical advice | 34 (1.7%) | |
| Transferred | 31 (1.5%) | |
| Remained in hospital for other reasons | 3 (0.2%) | |
| Died1 | 3 (0.2%) | |
| Mean length of stay (range), by surgical status | ||
| Non-surgical case | 2.7 days (0–40) | |
| Surgical case | 6.6 days (0–108) |
All deaths occurred in cases that had undergone surgery – 1 death occurred each in 2004, 2009, and 2012
Figure 1.
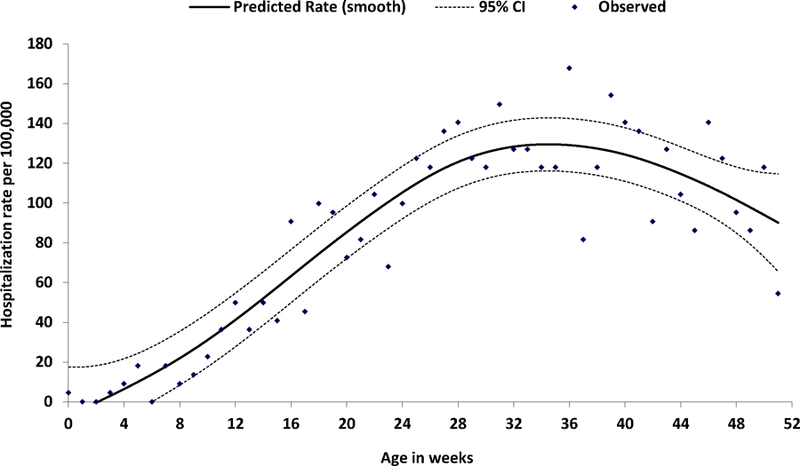
Observed and predicated rates of intussusception hospitalization by week of age — Taiwan, 2001–2005
Trends in intussusception hospitalizations
During 2001–2013, the overall rate of intussusception hospitalizations was 75.1 per 100,000 children aged <12 months. Annual intussusception hospitalizations rates were relatively stable during 2001–2005 (range: 74.2 to 87.5 hospitalizations per 100,000), declined during 2006–2008 (minimum rate of 64.8 per 100,000 in 2008), and subsequently remained relatively stable during 2009–2013 (range: 64.0 to 72.5 per 100,000) (Figure 2). The overall declines seen during 2006–2008 largely reflect declines in hospitalization rates among children in the 25–34 week age group, whereas hospitalizations rates for children in the 6–14 week and 15–24 week age groups did not show similar trends (Figure 3). There was no clear pattern of seasonality in hospitalizations (Figure 4).
Figure 2.
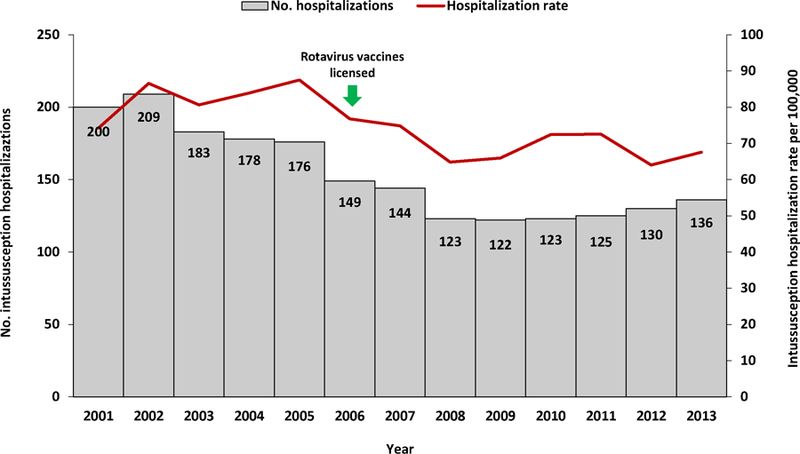
Annual numbers and rates of intussusception hospitalizations among children aged <12 months — Taiwan, 2001–2013
Figure 3.
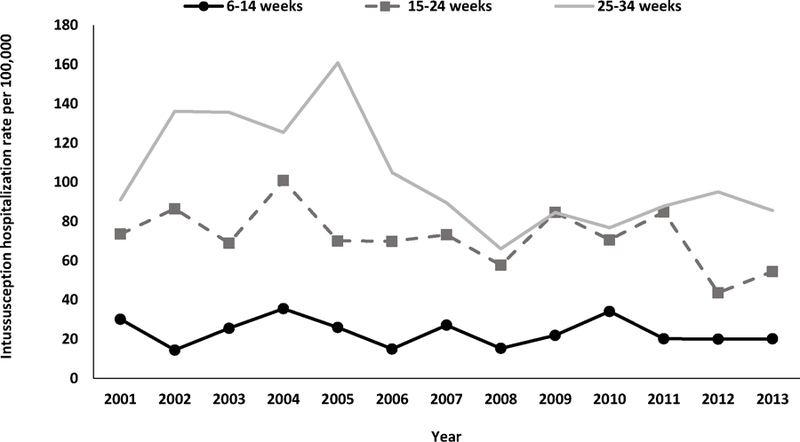
Annual rates of intussusception hospitalizations among children aged <12 months, by age group — Taiwan, 2001–2013
Figure 4.
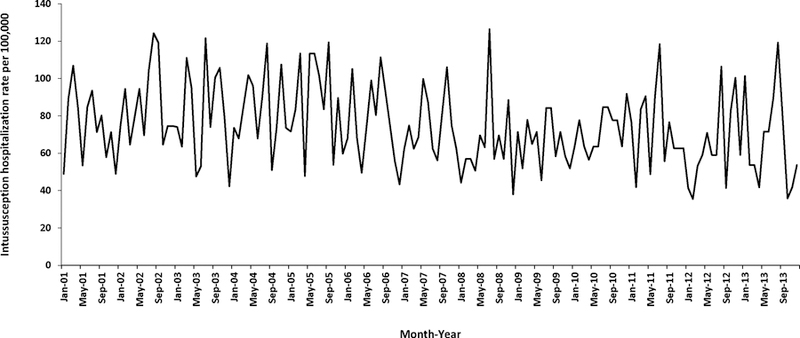
Monthly rates of intussusception hospitalizations among children aged <12 months — Taiwan, 2001–2013
Although annual hospitalization rates varied over time, the overall trend in annual rates during the pre-vaccine licensure period 2001 through 2005 was not significantly different from the overall trend in annual rates for the post-vaccine licensure period 2007 through 2013. However, mean rates of intussusception hospitalization were significantly lower during the post-vaccine licensure period for the overall group of children aged <12 months (RR: 0.84, 95% CI: 0.76–0.92), more specifically for children aged 25–34 weeks (RR: 0.66, 95% CI: 0.55–0.78) and not for children in the other age groups (Table 2).
Table 2.
Intussusception hospitalizations rates per 100,000 and rate ratios and 95% confidence intervals (CI) by age group in the pre- (2001–2005) and post- (2007–2013) rotavirus vaccine introduction years, by age group
| Age group | 2001–2005 | 2007–2013 | Rate ratio (95% CI) | p-value | ||
|---|---|---|---|---|---|---|
| Average annual no. | Mean rate (min, max) | Average annual no. | Mean rate (min, max) | |||
| <12 months | 189 | 82.2 (77.1, 87.6) | 129 | 68.7 (64.4, 73.3) | 0.84 (0.76, 0.92) | 0.0001 |
| 0–5 weeks | 2 | 6.0 (3.0, 12.1) | 1 | 4.6 (2.2, 9.7) | 0.77 (0.28, 2.11) | 0.6077 |
| 6–14 weeks | 10 | 26.2 (20.0, 34.4) | 7 | 22.5 (17.1, 29.6) | 0.86 (0.58, 1.26) | 0.4418 |
| 15–24 weeks | 35 | 79.8 (68.8, 92.5) | 24 | 66.3 (57.0, 77.2) | 0.83 (0.67, 1.03) | 0.0874 |
| 25–34 weeks | 56 | 127.8 (113.7, 143.6) | 30 | 83.8 (73.2, 95.9) | 0.66 (0.55, 0.78) | <.0001 |
| 35+ weeks | 86 | 114.1 (103.8, 125.4) | 67 | 109.3 (99.8, 119.7) | 0.96 (0.84, 1.09) | 0.5222 |
DISCUSSION
This study provides over a decade of robust, nationally representative data on the epidemiology and trends of intussusception-related hospitalizations among infants in Taiwan. Our findings demonstrate that infant intussusception in Taiwan occurs at a rate that is similar to the global average of 74 per 100,000 children aged <12 months [10, 12] and falls within the range of those reported for other Asian locations with low to very low under-5 mortality rates. The overall rate of 75.1 intussusception hospitalizations per 100,000 children aged <12 months for Taiwan is lower than rates reported for Hong Kong, Japan, the Republic of Korea, and Vietnam (range: 108–328 per 100,000), but higher than those for Malaysia, Singapore, and Thailand (range: 18–51 per 100,000) [10, 31]. One difference in our findings from these other Asian locations is the higher proportion of cases undergoing surgical treatment in Taiwan. In other similar Asian locations, barium or air enema reduction is the predominant treatment for intussusception and is performed in the majority of hospitalized intussusception cases [31–38]. A potential reason for the higher surgical rate observed in Taiwan may be clinician bias to hospitalize only those that will need surgery and discharge those who undergo air or barium enema reduction from the emergency department. Despite this, clinical outcomes of surgical cases were good, and the mortality rate was close to zero.
Similar to other places worldwide, we found that intussusception is very rare among children aged <3 months in Taiwan, is more frequently seen in boys, and occurs without a clear pattern of seasonality [10, 31, 32, 34, 35, 39–46]. The finding of a male predominance in infant intussusception cases is not well understood, but could suggest a genetic predisposition by sex.
For comparisons between pre- and post-vaccine licensure years, we did not observe a post-licensure increase in intussusception hospitalization rates in the 6–14 week age group, the time during which the first dose of rotavirus vaccine that has been associated with an increased risk for intussusception would be given [16–22]. However, given a small birth cohort that ranged from ~170,000 to 200,000 during 2007–2013 and rotavirus vaccination coverage that ranged from 13% in 2007 to 49% in 2013 [27], it would be difficult to see in our data analysis an ecological trend that would point to the vaccine-associated intussusception risk of 1–5 excess cases per 100,000 vaccinated children seen in other countries [16–22]. Even in 2013 when the vaccination coverage was almost 50%, only 1–5 excess intussusception cases would have been expected in a birth cohort of 201,344, should a similar vaccine-associated intussusception risk exist. While the overall decline in hospitalization rates for children aged <12 months is similar to pre-vaccine introduction trends seen in other countries [47–49], it seems to be driven by the significant decline in hospitalizations observed in children aged 25–34 weeks. Reasons for this finding are unknown given that much of the decline occurred in such a specific age group. It is unlikely that earlier hospitalizations rates were affected by something that would have resulted in higher than normal rates of intussusception, such as outbreaks of respiratory adenovirus, as we would have seen the same trend in other age groups, and it is unlikely that later rates were affected by things such as changes in clinical or coding practices as these also would have affected a wider age range.
This study has some limitations. First, intussusception cases were identified by ICD-9-CM code and were not validated by retrospective medical chart review and use of the Brighton Collaboration case definition for a definitive diagnosis of intussusception [50]. This may have resulted in an overestimation of intussusception incidence, which would further support the finding that intussusception is a rare event among infants in Taiwan. Additionally, our findings provide generally representative information on intussusception among infants in Taiwan at the population level, something that would be difficult to obtain without an established active intussusception surveillance network in all hospitals that manage these cases; no such network exists in Taiwan currently. Second, as technology improves and treatment of intussusception shifts toward non-surgical procedures in Taiwan, some cases of intussusception may only be treated in emergency departments and not captured in hospitalization data; this would result in an underestimation of intussusception incidence in later years. Given this possibility, we examined emergency department (ED) visit data included in the NHIRD. However, while an analysis of intussusception-related ED visits for children aged <12 months showed no uniform trends over time, only one of 1828 records with an ICD-9-CM code for intussusception also included a procedure code for intussusception reduction (code 96.29). Therefore, without medical chart confirmation, we could not confidently conclude which cases were confirmed intussusception cases treated in the ED as opposed to cases for which intussusception was ruled out. Third, since rotavirus vaccine is only available on the private market and therefore not included in the NHIRD, we were not able to assess the rotavirus vaccination status of the children hospitalized with intussusception. Should rotavirus vaccine be included in the routine immunization program, future NHIRD analyses will be able to link vaccination data with intussusception records.
In conclusion, this study provides important baseline and early post-rotavirus vaccine licensure data on infant intussusception hospitalizations in Taiwan. In the future, these data will be important for monitoring the safety of rotavirus vaccines as their use becomes more widespread or should they be included in Taiwan’s Expanded Program on Immunization. This study also highlights the utility of databases like NHIRD for monitoring trends in medical conditions and health care use and providing key stakeholders with robust information that can help inform policy decisions.
ACKNOWLEDGEMENTS
This study was sponsored by the Taiwan Centers for Disease Control through grant number MOHW105-CDC-C-114-123302. This study is based in part on data from the National Health Insurance Research Database provided by the National Health Insurance Administration, Ministry of Health and Welfare and managed by National Health Research Institutes. We thank Hsiu-Yun Lo, PhD, from the Taiwan Centers for Disease Control for providing rotavirus vaccination coverage estimates for this manuscript.
Financial support: Grant support provided by the Taiwan Centers for Disease Control through grant number MOHW105-CDC-C-114-123302.
Footnotes
Conflicts of interest: The authors indicate that they have no conflicts of interest relevant to this article to disclose.
Publisher's Disclaimer: Disclaimers: The interpretation and conclusions contained herein do not represent those of the National Health Insurance Administration, Ministry of Health and Welfare or National Health Research Institutes. The finding and conclusions of this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
REFERENCES
- [1].Roeyen G, Jansen M, Hubens G, Vaneerdeweg W, Eyskens E. Intussusception in infants: an emergency in diagnosis and treatment. European journal of emergency medicine : official journal of the European Society for Emergency Medicine 1999;6:73–6. [PubMed] [Google Scholar]
- [2].Stringer MD, Pablot SM, Brereton RJ. Paediatric intussusception. The British journal of surgery 1992;79:867–76. [DOI] [PubMed] [Google Scholar]
- [3].Bines JE, Liem NT, Justice FA, Son TN, Kirkwood CD, de Campo M, et al. Risk factors for intussusception in infants in Vietnam and Australia: adenovirus implicated, but not rotavirus. The Journal of pediatrics 2006;149:452–60. [DOI] [PubMed] [Google Scholar]
- [4].Clarke EJ Jr., Phillips IA, Alexander ER. Adenovirus infection in intussusception in children in Taiwan. Jama 1969;208:1671–4. [PubMed] [Google Scholar]
- [5].May AN, Piper SM, Boutlis CS. Yersinia intussusception: case report and review. Journal of paediatrics and child health 2014;50:91–5. [DOI] [PubMed] [Google Scholar]
- [6].Minney-Smith CA, Levy A, Hodge M, Jacoby P, Williams SH, Carcione D, et al. Intussusception is associated with the detection of adenovirus C, enterovirus B and rotavirus in a rotavirus vaccinated population. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology 2014;61:579–84. [DOI] [PubMed] [Google Scholar]
- [7].Nylund CM, Denson LA, Noel JM. Bacterial enteritis as a risk factor for childhood intussusception: a retrospective cohort study. The Journal of pediatrics 2010;156:761–5. [DOI] [PubMed] [Google Scholar]
- [8].DiFiore JW. Intussusception. Seminars in pediatric surgery 1999;8:214–20. [DOI] [PubMed] [Google Scholar]
- [9].Pang LC. Intussusception revisited: clinicopathologic analysis of 261 cases, with emphasis on pathogenesis. Southern medical journal 1989;82:215–28. [PubMed] [Google Scholar]
- [10].Jiang J, Jiang B, Parashar U, Nguyen T, Bines J, Patel MM. Childhood intussusception: a literature review. PloS one 2013;8:e68482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Noguchi A, Nakagomi T, Kimura S, Takahashi Y, Matsuno K, Koizumi H, et al. Incidence of intussusception as studied from a hospital-based retrospective survey over a 10-year period (2001–2010) in Akita Prefecture, Japan. Jpn J Infect Dis 2012;65:301–5. [PubMed] [Google Scholar]
- [12].Chen SC, Wang JD, Hsu HY, Leong MM, Tok TS, Chin YY. Epidemiology of childhood intussusception and determinants of recurrence and operation: analysis of national health insurance data between 1998 and 2007 in Taiwan. Pediatr Neonatol 2010;51:285–91. [DOI] [PubMed] [Google Scholar]
- [13].Peter G, Myers MG, National Vaccine Advisory C, National Vaccine Program O. Intussusception, rotavirus, and oral vaccines: summary of a workshop. Pediatrics 2002;110:e67. [DOI] [PubMed] [Google Scholar]
- [14].Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. The New England journal of medicine 2006;354:11–22. [DOI] [PubMed] [Google Scholar]
- [15].Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. The New England journal of medicine 2006;354:23–33. [DOI] [PubMed] [Google Scholar]
- [16].Buttery JP, Danchin MH, Lee KJ, Carlin JB, McIntyre PB, Elliott EJ, et al. Intussusception following rotavirus vaccine administration: post-marketing surveillance in the National Immunization Program in Australia. Vaccine 2011;29:3061–6. [DOI] [PubMed] [Google Scholar]
- [17].Carlin JB, Macartney KK, Lee KJ, Quinn HE, Buttery J, Lopert R, et al. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia’s National Immunization Program. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2013;57:1427–34. [DOI] [PubMed] [Google Scholar]
- [18].Haber P, Patel M, Pan Y, Baggs J, Haber M, Museru O, et al. Intussusception after rotavirus vaccines reported to US VAERS, 2006–2012. Pediatrics 2013;131:1042–9. [DOI] [PubMed] [Google Scholar]
- [19].Patel MM, Lopez-Collada VR, Bulhoes MM, De Oliveira LH, Bautista Marquez A, Flannery B, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. The New England journal of medicine 2011;364:2283–92. [DOI] [PubMed] [Google Scholar]
- [20].Velazquez FR, Colindres RE, Grajales C, Hernandez MT, Mercadillo MG, Torres FJ, et al. Postmarketing surveillance of intussusception following mass introduction of the attenuated human rotavirus vaccine in Mexico. The Pediatric infectious disease journal 2012;31:736–44. [DOI] [PubMed] [Google Scholar]
- [21].Weintraub ES, Baggs J, Duffy J, Vellozzi C, Belongia EA, Irving S, et al. Risk of intussusception after monovalent rotavirus vaccination. The New England journal of medicine 2014;370:513–9. [DOI] [PubMed] [Google Scholar]
- [22].Yih WK, Lieu TA, Kulldorff M, Martin D, McMahill-Walraven CN, Platt R, et al. Intussusception risk after rotavirus vaccination in U.S. infants. The New England journal of medicine 2014;370:503–12. [DOI] [PubMed] [Google Scholar]
- [23].Therapeutic Goods Authority of the Australian Government Department of Health. Rotavirus vaccination and the risk of intussusception 2013. Available from: http://www.tga.ogv.au/alert/rotavirus-vaccination-and-risk-intussusception-0#.U7DWdo2Sx8M. Accessed on 15 April 2016.
- [24].World Health Organization. Rotavirus vaccines. WHO position paper - January 2013. Weekly epidemiological record / Health Section of the Secretariat of the League of Nations 2013;88:49–64. [Google Scholar]
- [25].Chen KT, Chen PY, Tang RB, Huang YF, Lee PI, Yang JY, et al. Sentinel hospital surveillance for rotavirus diarrhea in Taiwan, 2001–2003. The Journal of infectious diseases 2005;192 Suppl 1:S44–8. [DOI] [PubMed] [Google Scholar]
- [26].Wu FT, Liang SY, Tsao KC, Huang CG, Lin CY, Lin JS, et al. Hospital-based surveillance and molecular epidemiology of rotavirus infection in Taiwan, 2005–2007. Vaccine 2009;27 Suppl 5:F50–4. [DOI] [PubMed] [Google Scholar]
- [27].Taiwan Centers for Disease Control. Rotavirus vaccination coverage data, 2006–2014 Unpublished data.
- [28].National Health Research Institutes of Taiwan. National Health Insurance Research Database: Background Available from: http://nhird.nhri.org.tw/en/index.html. Accessed on 15 April 2016.
- [29].Bureau of National Health Insurance, Department of Health of Taiwan. Universal Health Coverage in Taiwan 2012. Available from: http://www.nhi.gov.tw/Resource/webdata/21717_1_20120808UniversalHealthCoverage.pdf. Accessed on 15 April 2016.
- [30].Ministry of the Interior of Taiwan. Statistical Yearbook of the Interior Taiwan: 2014. Available from: http://sowf.moi.gov.tw/stat/year/elist.htm. Accessed on 15 April 2016. [Google Scholar]
- [31].Van Trang N, Le Nguyen NT, Dao HT, Ho VL, Tran DT, Loewen J, et al. Incidence and Epidemiology of Intussusception among Infants in Ho Chi Minh City, Vietnam. The Journal of pediatrics 2014;164:366–71. [DOI] [PubMed] [Google Scholar]
- [32].Hong Kong Intussusception Study Group. Intussusception trends in Hong Kong children. Hong Kong Med J 2007;13:279–83. [PubMed] [Google Scholar]
- [33].Jo DS, Nyambat B, Kim JS, Jang YT, Ng TL, Bock HL, et al. Population-based incidence and burden of childhood intussusception in Jeonbuk Province, South Korea. Int J Infect Dis 2009;13:e383–8. [DOI] [PubMed] [Google Scholar]
- [34].Nakagomi T, Takahashi Y, Arisawa K, Nakagomi O. A high incidence of intussusception in Japan as studied in a sentinel hospital over a 25-year period (1978–2002). Epidemiol Infect 2006;134:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Phua KB, Lee BW, Quak SH, Jacobsen A, Teo H, Vadivelu-Pechai K, et al. Incidence of intussusception in Singaporean children aged less than 2 years: a hospital-based prospective study. BMC Pediatr 2013;13:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Takeuchi M, Osamura T, Yasunaga H, Horiguchi H, Hashimoto H, Matsuda S. Intussusception among Japanese children: an epidemiologic study using an administrative database. BMC Pediatr 2012;12:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tran LA, Yoshida LM, Nakagomi T, Gauchan P, Ariyoshi K, Anh DD, et al. A High Incidence of Intussusception Revealed by a Retrospective Hospital-Based Study in Nha Trang, Vietnam between 2009 and 2011. Trop Med Health 2013;41:121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wong CW, Chan IH, Chung PH, Lan LC, Lam WW, Wong KK, et al. Childhood intussusception: 17-year experience at a tertiary referral centre in Hong Kong. Hong Kong Med J 2015;21:518–23. [DOI] [PubMed] [Google Scholar]
- [39].Bhowmick K, Kang G, Bose A, Chacko J, Boudville I, Datta SK, et al. Retrospective surveillance for intussusception in children aged less than five years in a South Indian tertiary-care hospital. J Health Popul Nutr 2009;27:660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Buettcher M, Baer G, Bonhoeffer J, Schaad UB, Heininger U. Three-year surveillance of intussusception in children in Switzerland. Pediatrics 2007;120:473–80. [DOI] [PubMed] [Google Scholar]
- [41].Chen YE, Beasley S, Grimwood K, New Zealand Rotavirus Study G. Intussusception and rotavirus associated hospitalisation in New Zealand. Arch Dis Child 2005;90:1077–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Giak CL, Singh HS, Nallusamy R, Leong TY, Ng TL, Bock HL. Epidemiology of intussusception in Malaysia: a three-year review. Southeast Asian J Trop Med Public Health 2008;39:848–55. [PubMed] [Google Scholar]
- [43].Latipov R, Khudoyorov R, Flem E. Childhood intussusception in Uzbekistan: analysis of retrospective surveillance data. BMC Pediatr 2011;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Omore R, Osawa F, Musia J, Rha B, Ismail A, Kiulia NM, et al. Intussusception Cases Among Children Admitted to Referral Hospitals in Kenya, 2002–2013: Implications for Monitoring Postlicensure Safety of Rotavirus Vaccines in Africa. Journal of the Pediatric Infectious Diseases Society 2015. [DOI] [PMC free article] [PubMed]
- [45].Tate JE, Simonsen L, Viboud C, Steiner C, Patel MM, Curns AT, et al. Trends in intussusception hospitalizations among US infants, 1993–2004: implications for monitoring the safety of the new rotavirus vaccination program. Pediatrics 2008;121:e1125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yen C, Tate JE, Steiner CA, Cortese MM, Patel MM, Parashar UD. Trends in intussusception hospitalizations among US infants before and after implementation of the rotavirus vaccination program, 2000–2009. The Journal of infectious diseases 2012;206:41–8. [DOI] [PubMed] [Google Scholar]
- [47].Fischer TK, Bihrmann K, Perch M, Koch A, Wohlfahrt J, Kare M, et al. Intussusception in early childhood: a cohort study of 1.7 million children. Pediatrics 2004;114:782–5. [DOI] [PubMed] [Google Scholar]
- [48].Justice F, Carlin J, Bines J. Changing epidemiology of intussusception in Australia. Journal of paediatrics and child health 2005;41:475–8. [DOI] [PubMed] [Google Scholar]
- [49].Samad L, Cortina-Borja M, Bashir HE, Sutcliffe AG, Marven S, Cameron JC, et al. Intussusception incidence among infants in the UK and Republic of Ireland: a pre-rotavirus vaccine prospective surveillance study. Vaccine 2013;31:4098–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bines JE, Kohl KS, Forster J, Zanardi LR, Davis RL, et al. Acute intussusception in infants and children as an adverse event following immunization: case definition and guidelines of data collection, analysis, and presentation. Vaccine 2004;22:569–574. [DOI] [PubMed] [Google Scholar]


