Abstract
Tensan silk, a natural fiber produced by the Japanese oak silk moth (Antherea yamamai, abbreviated to A. yamamai), features superior characteristics, such as compressive elasticity and chemical resistance, when compared to the more common silk produced from the domesticated silkworm, Bombyx mori (B. mori). In this study, the “structure-property” relationships within A. yamamai silk are disclosed from the different structural hierarchies, confirming the outstanding toughness as dominated by the unique mesoscale fibrillar architectures. Inspired by this hierarchical construction, we fabricated A. yamamai silk-like regenerated B. mori silk fibers (RBSFs) with mechanical properties (extensibility and modulus) comparable to natural A. yamamai silk. These RBSFs were further functionalized to form conductive RBSFs that were sensitive to force and temperature stimuli for applications in smart textiles. This study provides a blueprint in exploiting novel designs from one type of silk (A. yamanmai, but rare and expensive as a source material), to an alternative silk (B. mori, common, cost-effective), to empower new material properties.
Keywords: silk fiber, hierarchical structure, spinning, smart textile
1. Introduction
Animal silks have received considerable attention due to their outstanding portfolio of mechanical properties, combining high modulus, strength, and extensibility1–2. Many studies have pursued an understanding of the interplay between the structure and resulting mechanical properties of silks, with a goal toward the transfer of these insights into artificial material designs3. For example, a series of spinning techniques (e.g., wet spinning4, dry spinning5, microfluidic spinning 6, and biomimetic spinning7) have been pursed to spin regenerated fibers with silk-like structures. However, a significant challenge remains to replicate the mechanical properties of animal silks within artificial materials. In particular, there are no regenerated silk fibers are able to combine high strength, modulus and toughness in a fiber like the animal silks display. For example, despite the biomimetic fibers made of recombinant spidroins8 have reached toughness of 189 ± 33 MJ m−3 with the same toughness of natural spider dragline fiber (160 MJ m−3)9, a fiber is produced by the major ampullate gland of spiders as a lifeline and used for the construction of the orb web outer rim and spokes10, their modulus and strength were only half of the spider silk 4±1 GPa and 370±59 MPa, respectively; much lower than that of spider silk fibers (10 GPa in strength and 1.1 GPa in toughness)9.
This mismatch between natural and regenerated fibers is associated with continued limitations in understanding the fundamentals in these unique systems; in particular the contributions of mesostructures (i.e., microfibrils and nanofibrils) on the mechanical properties of the macroscale fibers11. In classical silk structural models, such as the two-phase crosslinking network model12, the mean field theory-based order/disorder fraction model13–14, and the Maxwell model15–16, silks are simplified to a uniform polymer-like fiber, and their mechanical properties are directly related to secondary structures. As a result, most artificial spinning methods inspired by these models attempt to utilize processes that control the content of β-sheets along with molecular orientation5. However, computational modeling has revealed that mesostructures in silks are critical for the toughness of the fibers and lead to mechanical advantages that the synthetic fibers do not display. For instance, silk fibers often have defects (e.g., cavities, cracks, surfaces, tears) that can reach several hundred nanometers in size17. Defects usually are seeds for failure in polymeric materials failure due to the localized stress concentrations18, while silks can protect against these defects through confinement of the diameter of nanofibrils in the 20-80 nm range. In such diameters, the failure stresses and strains of the defective silk fibers (the crack size is 50% the width of the fibers) converges towards defect-free fibers19.
Accordingly, the first aim of this study was to experimentally understand the role of hierarchical structures in the mechanical performance of animal silks. However, different silks show considerable complexity in their construction, mechanical properties and functions due to the diversity of silk types and the species as sources of these proteins20–22. There are more than 30,000 known species of spider and 113,000 species of Lepidoptera insects produce silks23. Thus it is unrealistic to characterize each silk. Here, Anthereayamamai (A. yamamai) silk (refers to tensan silk)24,25 was selected (Figure 1A and B), because such silk could be considered as an bridge (intermedium) to understand the “structure-property-function” relationships of broader silk family. This silk has a primary structure of its protein similar to that of Arachnid spider dragline silk (Nephila clavipes). Both proteins contain large central repetitive regions consisting of poly(alanine) domains alternating with glycine-rich regions20–22. On the other hand, this silk fiber was produced and used by “wild” silkworms to construct cocoons (with the same function as the most of the silks produced by Lepidoptera insects). Moreover, the β-sheet content of A. yamamai silk is intermediate between that of B. mori silk and Nephila spider dragline silk while the mechanical properties of the A. yamamai silk are also intermediate between those of the other two silks24,25. A more critical motive of this study was to construct regenerated functional fibers following fiber design strategies used by the A. yamamai silk moth. Compared with B. mori silk fibers, A. yamamai silk fibers presented more ingenious mesostructures that constructed by highly-organized microfibrils and nanofibrils. The synergistic effects of these mesoscale structures and interfaces made the superior extensibility and toughness of A. yamamai silks. With the above motivations, the advantages in natural designs can be directly transferred into the engineered fibers, providing a blueprint on the design new features and functions, by building on this approach that is emulated from both silkworms and spiders.
Figure 1. Mechanical properties of A. yamamai silkworm cocoon silk fibers.
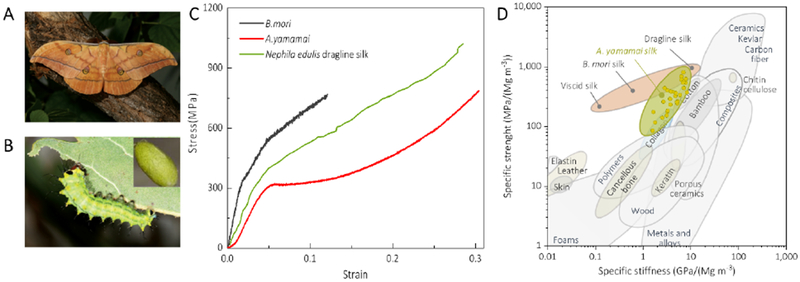
A and B, Alien saturnid orginating from Asia, A. yamamai adult and 2nd instar larva. The insert image is the A. yamamai cocoon. C, Stress-strain curve of A. yamamai silk, B mori silk and Nephila edulis dragline silk. D, Comparison of the specific strength and specific stiffness of A. yamamai silk with other natural and synthetic materials. A and B reproduced with permission from ref (24). Copyright 2014. Pensoft Publishers. The inert picture in B reproduced with permission from ref (25). Copyright 2015. Korean Society of Sericultural Science. The stress-strain curve of Nephila edulis dragline silk use data from ref (26). Ashby plot of natural and synthetic materials are adapted from ref (27).
RESULTS AND DISCUSSION
Figure 1C presents a typical stress-strain curve of A. yamamai silk, which features ductile mechanical behavior within the triphasic region: an elastic phase (from 0 to yield point), a plastic deformation region (after yield point) and a strain hardening region. The ductile failure displayed is also present in spider dragline silk (Figure 1C)26 but in sharp contrast to the brittle fracture of Bombyx mori (B. mori) silk, which shows almost full elastic response until the failure at a stain of 12% (Figure S1). After normalizing the strength and stiffness of all A. yamamai silk fibers by density (Figure 1D), the values are close to that of spider dragline silk instead of B. mori silk, despite all silks present superior strength than most other natural and engineered materials 27 This difference in mechanical behavior between A. yamamai and B. mori silks drove us to further investigate the structure of the A. yamamai silk. We first studied the secondary structure (conformation) of the A. yamamai silk because many studies have confirmed that the conformation of silk fibroin (i.e., β-sheet, random coil and helical structure) plays a vital role in the mechanical properties of the silks. Different characterization techniques, such as X-ray diffraction (XRD) 28–29, nuclear magnetic resonance (NMR)30–33, Raman spectroscopy30–36, and Fourier transform infrared spectroscopy (FTIR)37,38, have been used to evaluate the conformation of animal silks, including spider dragline silk, B. mori silk, A. yamamai silk or the silks produced by other Antherea genus, such as Anthereapernyi (A. pernyi, also known as Chinese tussah silk)38. However, the contents of conformations obtained from the various techniques are different37. Thus, it is difficult to establish “conformation-property” paradigms for A. yamamai silk based on previously reported data.
Herein, synchrotron FTIR microspectroscopy37 was applied to evaluate the silk fibroin conformations in a single fiber of A. yamamai silk. The deconvolution of the amide III band provided an estimation of β-sheet structure in the degummed fiber at 40±10 %, comparable with that of degummed B. mori silk fibers (38±4%). We also compared the orientation of each conformation in A. yamamai silks. S-FTIR microspectra of A. yamamai silk obtained from different infrared polarization angles and their related polar plots are shown in Figure S2 and Figure 2, respectively. Both figures confirmed the high orientation of each conformation in the A. yamamai silk. For example, the coupled absorption β-sheet peaks, such as (1222 cm−1 and 965 cm−1) became progressively smaller from Ω 0° (beam parallel to the fiber axis) to Ω 90° (perpendicular to the fiber axis) and their related polar plot showed considerable order absorbance parallel to the fiber axis direction with a molecular order parameter (Smol) of 0.95. The similar plot patterns and orientation parameters have been detected in a range of animal silks, including B. mori (orientation factor of 0.9239) and A. pernyi silk (orientation factor of 0.9338). These results demonstrate that A. yamamai silk has a similar conformation (both content and orientation) with B. mori silk, opposite to the differences in mechanical performance, where A. yamamai silk displayed ductile failure while B. mori silk was brittle.
Figure 2. Infrared dichroism of single A. yamamai silk fibers.
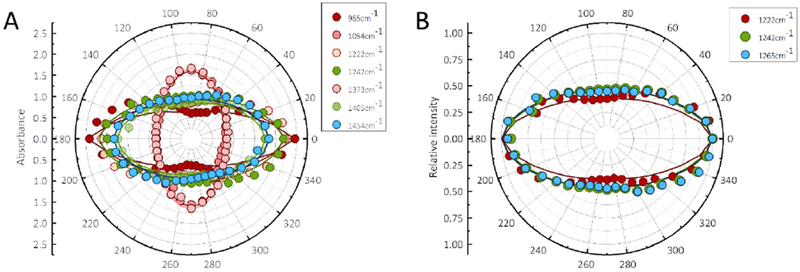
A, Polar plot of the absorbance of the characteristic peaks in SFTIR microspectra in the 1500-800 cm−1 region from single A. yamamai silk fibers. The symbols represent individual experimental data points, while the curves are fitted using Equation 1. The peaks atl454 to 1405 cm−1 are not sensitive to the conformation change. The peak at 1242 cm−1 is attributed to random coil, while the peaks at 1373, 1222, 1054 and 965 cm−1 are assigned to β-sheet. In these β-sheet peaks, 1222 and 965 cm−1 have parallel dichroism, while 1373 and 1054 cm−1 have perpendicular dichroism. B, Polar plot of the relative intensity of different component from the deconvolution of amide III band in S-FTIR microspectra of single A. yamamai silk fibers. The peaks at 1222, 1242, and 1265 cm−1 in amide III band are assigned to β-sheet, random coil and α-helix, respectively. More details of the assignments of these peaks can be found in ref (38).
The reason for the above contradiction can be inferred from the structural difference at the mesoscale because at the macroscale both silks consist of sericin and silk fibroin filaments (Figure 3). Therefore, polarized light microscopy and scanning electron microscopy (SEM) were used to compare the mesostructures between A. yamamai (Figure 3) and B. mori silks (Figure S3). The brilliant color under cross-polarized light confirmed the high-orientation of both kinds of fibers (Figure 3B and Figure S3A). However, comparison of the images in Figure 3B and 3C show that there are highly aligned fibrillar bundles on the A. yamamai silk surface, only a uniform and smooth morphology exists on the surface of the B. mori silk fibers (Figure S3B). To detect the structural details of the A. yamamai microfibrils, we exfoliated the surface layer of the degummed A. yamamai silk fiber. As shown in the surface SEM image (Figure 3D), these microfibrils appeared as ribbon-like structures with a width of 500-1000 nm and were highly oriented along the fiber axis. In addition, the weak adhesion between microfibrils and the numerous pores with a width of ~200 nm were detected from the cross-sectional SEM image (Figure 3E).
Figure 3. Hierarchical structure of A. yamamai silk fiber.
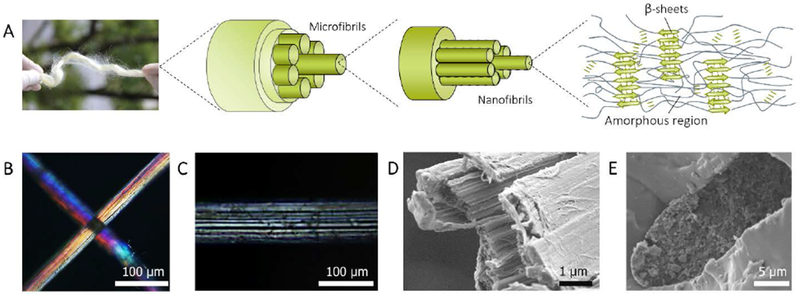
A, Schematic of the hierarchical structure of A. yamamai silk. B and C, The polarized light microscopy image of A. yamamai silk. D, Cross-sectional SEM image of A. yamamai silk after tensile fracture. E, Cross-sectional SEM image of A. yamamai silk fiber. The fiber was embedded in epoxy resin and further broken in liquid nitrogen.
Although several studies have reported that microfibrils in silks are composed of thinner nanofibrils40–48, detailed information about such mesostructures remains unknown due to the lack of an effective experimental approach to isolate or retain these building blocks for characterization. Traditional dissolution processing, such as LiBr/H2O,49 N-methylmorpholine N-oxide (NMMO)/H2O,50–54 and CaCl2/EtOH/H2O,55–58, directly dissolve the silk fibers into silk fibroin molecules or chains. The use of an ultramicrotome allows slicing silk fibers into thin sections for topological characterization12, this method only detects surface morphologies on the sections and is unable to provide detailed 3D structures of the nanofibrils.12 Herein, we modified our recently established “partial dissolution-mechanical isolation” strategy59 to exfoliate A. yamamai silk at the single nanofibril level. Briefly, 0.1 g of degummed A. yamamai silk fibers were immersed in 20 mL 0.075 mM sodium hypochlorite solution and incubated at room temperature to partially dissolve the fibers to microfibrils. After 30 min, ultrasonification processing (120 μm amplitude and 20 KHz frequency, at intervals of 10 sec for 1 hour) was applied to further isolate microfibrils into single nanofibrils. Atomic force microscopy (AFM) identified these isolated nanofibrils with a diameter of 5 nm (Figure S4), similar to the diameter of single B. mori nanofibrils60–61.
Based on the structural characterization, considering structural scales from secondary structure to fiber, we identified significant differences between A. yamamai and B. mori silks at microfibril scale. Previous computational modeling results62–64 suggested that microfibrils may contribute to the toughness of the silks through restricted fibril shearing, controlled slippage, stress transfer, as well as energy dissipation, while direct experimental evidence for the features in silks was lacking. Therefore, we measured the cross-sectional morphologies (Figure 4A) of A. yamamai fibers after tensile failure, where microfibrillar slipping and pulling were indeed found. For comparison, a smooth fracture surface was detected in B. mori silk fibers, which agree well with the brittle fracture observed in mechanical tests (Figure 1C). In addition, mesoscale spring-models19 disclosed that the fibrillar structure could inhibit the transverse growth of cracks through longitudinal splitting when fiber was stretched, so we also tested the mechanical properties of notched silk fibers to compare the response with that of the adjacent un-notched (intact) fibers (Figure 4B–G and Figure S5). As shown in Figure 4B–D, when the notch width was smaller than the half width of the fiber (Figure 4C), the notched fibers exhibited the same stress-strain curves as the un-notched fibers (Figure 4B); only failure to strain was reduced; featuring typical ductile fracture behavior. Cross-sectional SEM images (Figure 4D) of notched A. yamamai fibers after tensile fracture confirmed the ductile failure behavior, where the apparent microfibril splitting and pulling were observed, and the crack was shifted around 90 degrees during tensile processing (the direction indicated by the red arrows). However, the stress-strain curves of notched nylon 66 fibers (Figure 4E), a fiber with similar chemical structure with protein fibers but without the microfibrils (Figure 4F), was significantly different from the unnotched fiber. Linear crack propagation was observed in the notched fiber (Figure 4G) which was fractured before reaching the yield point (strain of 13%).
Figure 4. Comparison of mechanical performance between A. yamamai silk and Nylon 66 fiber.
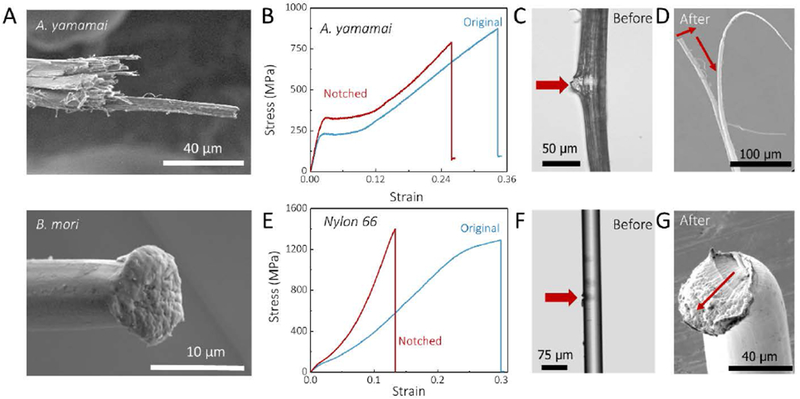
A, SEM images of tensile fracture morphologies of A. yamamai silk and B. mori silk. B, Stress-strain curve of original and notched A. yamamai silk fiber. C and D, The microphotograph of notched A. yamamai silk fiber before (C, optical microscopy image) and after (D, SEM image) the tensile fracture. E, Stress-strain curve of original and notched Nylon 66 fiber. F and G, The microphotograph of notched Nylon 66 fiber before (F, optical microscopy image) and after (G, SEM image) the tensile fracture. The red arrows in C and F indicates the notch of the fibers. These images confirmed the notched width was smaller than the half width of the fiber using in testing. The red arrows in D and G displays the crack propagation direction after tensile failure.
Although the A. yamamai moth has been cultivated in Japan for more than 1,000 years, it is still rare and expensive (more expensive than gold at the same weight). Thus, an alternative approach to the practical use such silk is to mimic the hierarchical structures by using other widely available silk fibers, such as the B. mori silks. However, conventional spinning techniques are challenging to produce regenerated fibers with a hierarchical structure. Inspired by liquid crystal and dry spinning features from the spider and silkworm spinning, we previously reported a dry spinning approach to spin regenerated silk fibers with polymorphic structure5. In this method, degummed B. mori silk fiber was initially dissolved to microfibril features in solution using hexafluoroisopropanol (HFIP) as a solvent and then spun into regenerated fibers by direct extrusion or reeling in the air. With the aim of production of regenerated B. mori silk fibers (RBSF) with A. yamamai silk-like hierarchical structures, we modified this biomimetic spinning route to weaken the degree to which the B. mori silk was dissolved. Thus, this modified approach was suitable for generating regenerated fibers with longer microfibril alignment. In the new spinning protocol, the degum med B. mori silk fiber/HFIP (1/20 wt/wt) system was still incubated at 60°C, but the time was reduced to 3 days. After this process, the single silk fibers were partially dissolved into highly-elongated microfibrils with lengths above centimeters. During spinning in the air, the microfibrils adhered together due to the dissolved silk fibroin, to obtain A. yamamai silk-like structures (Figure 5A–D). These RBSFs exhibited ductile mechanical behavior with a triphasic stress-strain region (the red curve in Figure 5E), very similar to the A. yamamai silk. The average stain to failure of RBSFs reached 23±4%, approximately twice that of B. mori silk (13±4%), and comparable to A. yamamai silk (26±7%). More remarkably, the average modulus of these regenerated fibers was 14±3 GPa, 2 times higher than A. yamamai silks. To confirm that the ductile mechanical behavior of these RBSFs was due to the weak interface and connections between microfibrils, we increased the interface adhesion between microfibrils in RBSFs by adding 4 wt% silk fibroin/HFIP solution into the microfibril pulp before spinning. The tensile stress-strain curves of such RBSFs indeed showed B. mori silk-like brittle failure, with no yield plateau region (the blue curve in Figure 5E).
Figure 5. Structure and mechanical properties of RBSFs.
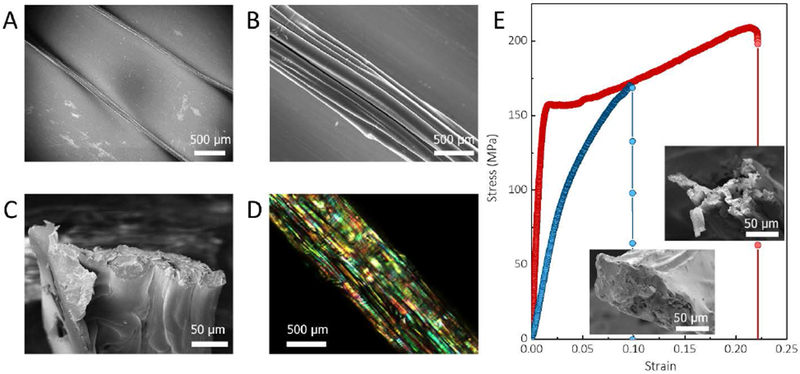
A, SEM image of RBSFs. B and C, the surface and cross-sectional structure of RBSFs. D, Polarized light microscopy image of RBSFs. E, Stress-strain curve of RBSFs with (red curve) and without (blue curve) weak fibrillar interface. The inserts are cross-sectional SEM images of the RBSFs after tensile fracture.
Conductive fibers have received a great deal of interest for electronic, optoelectronic, and energy storage-related fields65. Silks as a robust textile fiber have not been extensively used in this fields because it remains challenging to produce conductive silk fibers while retaining the useful mechanical properties. Wet-spinning66 and dry-spinning techniques67 have been pursued to address this issue, while the content of conductive fillers was insufficient to reach conductive percolation thresholds. Based on the inspiration that toughness of the RBSFs can be enhanced by the organization of high fibrillar orientation, as well as weak fibrillar interfaces, we designed conductive and ductile RBSFs using a yarn-spinning technique. In this route, degummed single B. mori silk fibers were weaved into elongated fiber bundles (consisting of approximate 30 fibers) with a diameter of around 100 μm and length up to 1 m (Figure 6A). Then, 1 gram of the resulting silk fiber bundles was immersed in a 20 mL multi-walled carbon nanotube (MWCNT)-isopropanol-HFIP solution (1:3:17 w/v/v) and sealed for incubation at 60 °C for 3 days to generate conductive RBSFs. In these conductive RBSFs, the MWCNTs bonded with single B. mori silk fibers, and different fibers partially adhered to each other due to the dissolved silk fibroin by the HFIP (Figure S6). Benefiting from these unique hierarchical structures, the conductive RBSFs were sensitive to external tensile elastically. For example, loading-unloading cycles (Figure 6B) showed that neither plastic deformation or loss of strength occurred in the conductive RBSFs at a set strain of 7.5%, and no significant hysteresis loop was found, indicating that the conductive RBSFs exhibited good shape recovery properties after the first cycle. More remarkably, the variation in conductive resistance was synchronized with the tensile strain (Figure 6C). Therefore, such RBSFs can be directly utilized to monitor the deformation of a material. As a prototype (Figure 6D), two conductive RBSFs were weaved into a grid substrate, where the first (fiber i) and the second conductive fiber (fiber ii) were located in the middle and edge of the grid, respectively (Figure 6E). Further, sphere stress was applied and released circularly on the grid. As shown in Figure 6F, the changes in resistance of the two fibers followed the cyclic “press-and-release” process, and fiber i showed more substantial changes than fiber ii, owing to its larger external deformation.
Figure 6. Conductive RBSF-based sensor for monitoring mechanical stimuli.
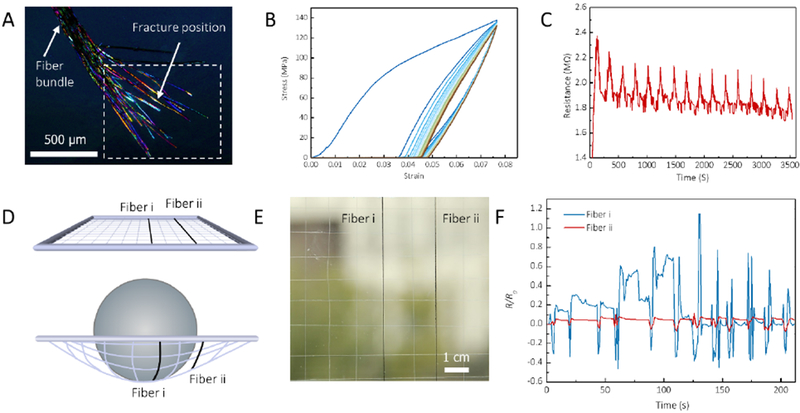
A, Polarized light microscopy image of B. mori silk bundle coated with WMCNTs produced by yarn-spinning technique after the tensile fracture. At the fracture position (indicted by white dashed frame), the silk fibers were spread out while the near adjacent area was still bundled together, presenting similar fracture behaviors with A. yamamai silk fibers. B, The cyclic load-unload stress-strain curve of conductive RBSFs. C, The relationship between strain and electric resistance of conductive RBSFs during cyclic load-unload tensile measurement. D, Schematic of the method to measure the electric resistance response for spherical stress. E, A photograph of the RBSF grid. Two black fibers are conductive RBSFs. The other white fibers are B. mori silk fiber bundles. F, The electric resistance changes of these two fibers with the change of stress. These two fibers followed the cyclic “press and release” process.
In addition, these conductive RBSFs were also sensitive to changes in temperature due to thermal expansion and contraction stress in the CNT-layers. An in-situ experiment was designed to record this temperature response (Figure 7A). A conductive RBSF was first fixed by two tensile clamps, which was then connected by a resistance detector. In this system, a tensile device recorded the force generated by a change in temperature, while the resistance detector collected the resistance changes synchronously. During the testing, a movable heat source was close to (but not touching) the conductive RBSF, and temperature changes of the fiber were monitored in real time by a thermal imager. As shown in Figure 7B, the stress of the conductive RBSF increased immediately when a heat source was close to the fiber and then decrease gradually. More importantly, the stress of the fiber was changed circularly and repeatably with changes of temperature (Figure 7C). Meanwhile, the resistance of the conductive RBSF also changed circularly and repeatably in response to temperature changes (Figure 7D). These stress and temperature sensitive RBSFs may find applications in wearable sensors or as medical implants, considering the biocompatible nature of the composites.
Figure 7. Conductive RBSF-based sensor for monitoring thermal stimuli.
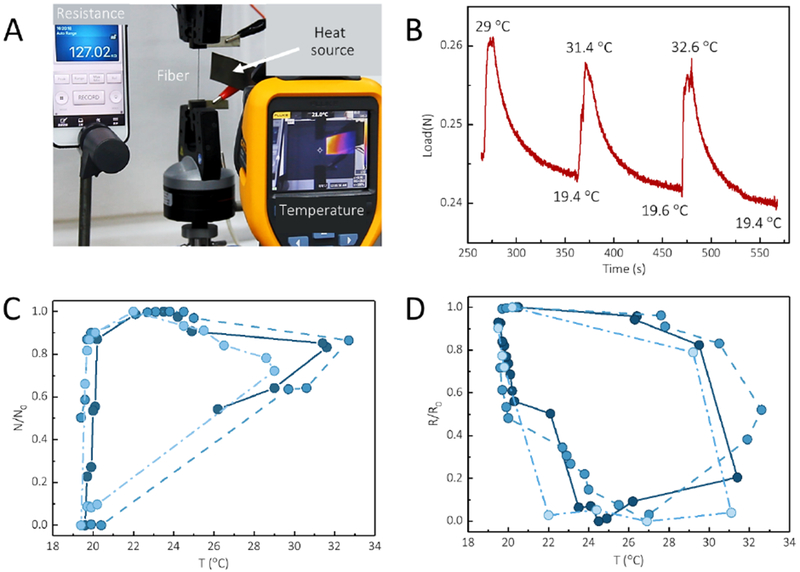
A, Experimental setup for recording the thermal and electric conductivity of conductive RBSFs. B, Time-force load curve of RBSFs with the change of temperature. C and D, The force and resistance of the conductive RBSFs with change of temperature. The force and resistance of the conductive RBSF repeatably response to changes in temperature (3 cycles).
CONCLUSIONS
A. yamamai silk was chosen for study in order to understand the structure-property relationships of natural silks, because of its unique advantages in terms of mechanical extensibility that are superior to B. mori silk. Multiscale structural comparison of A. yamamai and B. mori silks confirmed the unique microfibrillar architectures of A. yamamai silk as dominate in its outstanding extensibility and toughness. Inspired by this fiber construction strategy used by the A. yamamai silk moth, RBSFs were generated experimentally with highly-organized hierarchical structures. These RBSFs had extensibility (23±4%) and modulus (14±3 GPa) that compared to natural A. yamamai silk and were further functionalized to conductive RBSFs by introducing carbon nanotube during the spinning processing. Benefiting from the unique microfibrillar construction and weak microfibrillar interactions, these conductive RBSFs were highly sensitive to force and temperature stimuli and responded as sensors to monitor these changes. These features suggest utility in smart textiles. More importantly, the synergistic integration of multiscale characterization and biomimetic preparation offers a new route to the rational design of functional materials using an optimized “structure-property” relationship.
EXPERIMENTAL SECTION
Preparation of degummed A. yamamai silk fibers
Raw A. yamamai silkworm cocoon silk fibers were degummed by boiling in two 30 min changes of 0.5% (w/w) NaHCO3 solution. The degummed fibers were then washed with distilled water and obtained by drying at room temperature.
Preparation of A. yamamai silk nanofibrils
A. yamamai silk fibers (1 g) was suspended in a beaker containing 100 mL DI water, and then a 30 min chemical oxidation of silk was initiated by adding the desired amount of sodium hypochlorite (NaClO) solution (15 mmol of NaClO per gram of protein). Further, the oxidized A yamamai silk pulp was dialyzed (Pierce, molecular weight cutoff = 10,000) against DI water for 3 days and followed by ultrasonic treatment (QSonica500, USA) at 150 μm amplitude and 20 kHz frequency with interval of 30 min. A. yamamai silk nanofibrils /water dispersion was harvested by centrifugation at 8000 rpm for 5 min.
Preparation of conductive RBSFs
First, B. mori silkworm cocoon silk fibers were degummed by boiling in two 30 min changes of 0.5% (w/w) NaHCO3 solution. The degummed silk fibers were washed with distilled water and allowed to air dry at room temperature. Then, the degummed single B. mori silk fibers were weaved into elongated fiber bundles (consists of 30 fibers) with a diameter of 87 μm and length up to 100 mm. To prepare the MWCNT/isopropanol/HFIP solution, 3 mL isopropanol dispersed MWCNT solution (containing ≈200 mg MWCNT, Chengdu Organic Chemicals Co. Ltd., China) was mixed with 17 mL HFIP in a 20 mL sealed glass bottle with sufficient stirring. Finally, 1 gram of elongated B. mori silk fiber bundles were immersed in MWCNT/isopropanol/HFIP solution. The MWCNT-coated silk fibers were obtained after incubating in airtight containers as mixtures at 60°C for 3 days. The residual solvent was removed thoroughly by drying the fibers at 70°C for 4 hours. All of these steps were conducted in a chemical hood with the necessary precautions as HFIP is a toxic solvent.
Polarized S-FTIR microspectroscopy of single A. yamamai silk fibers
The experiments were performed at BL01B in the Shanghai Synchrotron Radiation Facility (SSRF) with the Nicolet 6700 Fourier transform infrared spectrometer, infrared microscopy, and imaging systems. A 15 × 15 μm square aperture was selected to collect the S-FTIR microspectra of the single A. yamamai silk fiber. This aperture size was smaller than the width of a single fiber, thus avoids the diffraction and the scattering of the infrared light. To study the dichroism of specific absorption bands, a KRS-5 IR polarizer was inserted in the infrared beam. S-FTIR microspectra were collected in the mid-infrared range of 800–3800 cm−1 at a resolution of 4 cm−1 with 256 coadded scans. During the measurement, the background was collected each time before all FTIR spectra of single silk fibers were collected. Deconvolution of amide III band was carried out using PeakFit 4.12. The number of peaks and their positions were obtained from the second derivative spectra and fixed during the subsequent deconvolution process. The orientation of individual moieties can be obtained from the angular dependence of the absorbance A(ν) at wavenumber ν which corresponds to a vibration of the molecular group under investigation. In the general case, the angular dependence of the absorbance can be determined using Equation 1.
| (1) |
Where A(ν, Ω) is the peak intensity of a certain band, Ω is the polarization angle, Ω0 is the angle at maximum absorption, and Amax and Amin are the maximum and minimum absorbance, respectively. The molecular order parameter (Smol) of the corresponding secondary structural component was calculated as Equation 2.
| (2) |
Mechanical testing of natural and regenerated silk fibers
The degummed A. yamamai silk fibers were cut into 40 mm segments for tensile tests. For tensile testing, the 40 mm segments were mounted on a hard-cardboard frame with a base length of 20 mm and fixed with cyanoacrylate. After the cyanoacrylate was dried overnight, the frame was mounted in the testing machine (Instron 5966 machine, Instron, Norwood, USA) and the side support of the frame was cut away so that the force was transmitted through the fibers. Meanwhile, the initial length of the fiber was measured with a caliper at zero load point (the point in which the fibers are tight, but no force exerted on it). To understand the crack propagation mechanism of A yamamai silk fibers, nylon 66 and RBSFs were compared. A fiber was cut into two segments. One segment was further notched by a small incision that operating with the assistance of a microscope, while the other one without a notch was used for the control. All of the tensile measurements were carried out at 25°C and 50% RH with a tensile speed of 2 mm/min. To calibrate the cross-sectional area of the fibers, the fibers were fixed with epoxy resin, and after drying overnight, the samples were sectioned into three segments in liquid nitrogen. The cross-section area (CSA) of the fibers was then measured by SEM observation and the CSA was estimated by ImageJ software (NIH). The average area of three segments was used as the CSA of adjacent RSFs and then used for stress calculations.
The mechanical and thermal response of the conductive RBSFs
The ends of the twisted conductive RBSFs were clamped by two pieces of copper sheets and then fixed by tensile devices. Before cyclic tests, the copper sheets were connected with a digital multimeter (CEM, DT-9989). The initial length of the fiber was measured with a caliper at zero load point. The mechanical tests were carried out using an Instron 5966 machine (Instron, Norwood, USA) in tensile and cycle load-unload mode at 25 °C and 50% RH with a tensile speed of 2 mm/min. To test the deformation-electric resistance relationship of the conductive RBSFs, two conductive RBSFs and thirteen weaved B. mori silk fiber bundles were weaved into a grid substrate. These two conductive RBSFs were located in the middle and edge of the grid, respectively. All fibers were fixed with cyanoacrylate. The ends of the conductive RBSFs were clamped by two pieces of copper sheets and further connected with a digital multimeter. During the tests, a 100 mL round-bottom flask, which applied sphere stress on the grid, was used to press the grid intermittently. The resistance values were recorded by a Bluetooth device with a time resolution of 1 s. To detect the thermal response of the conductive RBSFs, a movable copper heater was first moved close to (but not touching) the conductive RBSF for heating and then was removed for cooling. These processes can be iterative. The temperature changes of the fiber were monitored in real time by a thermal imager, and the changes in fiber tension were recorded by using an Instron 5966 machine (Instron, Norwood, USA). The variation of resistance was collected using a digital multimeter (CEM, DT-9989). The resistance and temperature values were extracted from each frame with a time resolution of ≈0.3 s.
Characterization
The surface and cross-sections of all the fibers were observed by SEM (JEOL JSM-7800F) at an acceleration voltage of 5 kV and polarizing optical microscope (Olympus BX51-P, Japan). To prevent electrical charging, all specimens were coated with a 5-nm-thick gold layer before observation. For atomic force microscopy (AFM) measurements, A. yamamai silk nanofibril aqueous dispersion was diluted to ~0.01% (w/v) with DI water. The resultant solution was added dropwise onto a mica substrate for 120 s, followed by purging with nitrogen gas. The topologic structures of nanofibril were characterized by Dimension ICON AFM fast scanning system (Bruker, Germany) with tapping mode. An aluminum reflective coated silicon cantilever with a tip radius 2 nm was used (k = 0.4 N/m).
Supplementary Material
ACKNOWLEDGMENT
We thank the staffs from BL01B beamline of National Center for Protein Science Shanghai (NCPSS) at Shanghai Synchrotron Radiation Facility, for assistance during data collection. Prof. Ling acknowledges the starting grant of ShanghaiTech University.
REFERENCES
- (1).Yarger JL; Cherry BR; van der Vaart A Uncovering the Structure–Function Relationship in Spider Silk. Nature Reviews Materials 2018, 3, 18008–18010. [Google Scholar]
- (2).Omenetto FG; Kaplan DL New Opportunities for An Ancient Material. Science 2010, 329, 528–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Buehler MJ; Keten S; Ackbarow T Theoretical and Computational Hierarchical Nanomechanics of Protein Materials: Deformation and Fracture. Prog. Mater Sci 2008, 53, 1101–1241. [Google Scholar]
- (4).Zhou GQ; Shao ZZ; Knight DP; Yan JP; Chen X Silk Fibers Extruded Artificially from Aqueous Solutions of Regenerated Bombyx mori Silk Fibroin are Tougher than Their Natural Counterparts. Adv. Mater 2009, 21, 366–370. [Google Scholar]
- (5).Ling S; Qin Z; Li C; Huang W; Kaplan DL; Buehler MJ Polymorphic Regenerated Silk Fibers Assembled through Bioinspired Spinning. Nat. Commun 2017, 8, 1387–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Peng Q; Zhang Y; Lu L; Shao H; Qin K; Hu X; Xia X Recombinant Spider Silk from Aqueous Solutions via A Bio-Inspired Microfluidic Chip. Sci. Rep 2016, 6, 36473–36484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Andersson M; Jia Q; Abella A; Lee XY; Landreh M; Purhonen P; Hebert H; Tenje M; Robinson CV; Meng Q; Plaza GR; Johansson J; Rising A Biomimetic Spinning of Artificial Spider Silk from a Chimeric Minispidroin. Nat. Chem. Biol 2017, 13, 262–264. [DOI] [PubMed] [Google Scholar]
- (8).Heidebrecht A; Eisoldt L; Diehl J; Schmidt A; Geffers M; Lang G; Scheibel T Biomimetic Fibers Made of Recombinant Spidroins with the Same Toughness as Natural Spider Silk. Adv. Mater 2015, 27, 2189–2194. [DOI] [PubMed] [Google Scholar]
- (9).Lefèvre T; Auger M Spider Silk as a Blueprint for Greener Materials: A Review. Int. Met. Rev 2016, 61, 127–153. [Google Scholar]
- (10).Vollrath F; Knight DP Liquid Crystalline Spinning of Spider Silk. Nature 2001, 410, 541–548. [DOI] [PubMed] [Google Scholar]
- (11).Keten S; Xu ZP; Ihle B; Buehler MJ Nanoconfinement Controls Stiffness, Strength and Mechanical Toughness of Beta-Sheet Crystals in Silk. Nat. Mater 2010, 9, 359–367. [DOI] [PubMed] [Google Scholar]
- (12).Termonia Y Molecular Modeling of Spider Silk Elasticity. Macromolecules 1994, 27, 7378–7381. [Google Scholar]
- (13).Porter D; Vollrath F; Shao Z Predicting the Mechanical Properties of Spider Silk as A Model Nanostructured Polymer. Eur. Phys. J. E: Soft Matter 2005, 16, 199–206. [DOI] [PubMed] [Google Scholar]
- (14).Vollrath F; Porter D Spider Silk as A Model Biomaterial. Appl. Phys. A 2006, 82, 205–212. [Google Scholar]
- (15).Krasnov I; Diddens I; Hauptmann N; Helms G; Ogurreck M; Seydel T; Funari SS; Muller M Mechanical Properties of Silk: Interplay of Deformation on Macroscopic and Molecular Length Scales. Phys. Rev. Lett 2008, 100, 048104–048108. [DOI] [PubMed] [Google Scholar]
- (16).Von Fraunhofer J; Sichina W Characterization of Surgical Suture Materials Using Dynamic Mechanical Analysis. Biomaterials 1992, 13, 715–720. [DOI] [PubMed] [Google Scholar]
- (17).Frische S; Maunsbach A; Vollrath F Elongate Cavities and Skin-Core Structure in Nephila Spider Silk Observed by Electron Microscopy. J. Microsc 1998, 189, 64–70. [Google Scholar]
- (18).Giesa T; Pugno NM; Wong JY; Kaplan DL; Buehler MJ What’s Inside the Box? – Length-Scales that Govern Fracture Processes of Polymer Fibers. Adv. Mater 2014, 26, 412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Giesa T; Arslan M; Pugno NM; Buehler MJ Nanoconfinement of Spider Silk Fibrils Begets Superior Strength, Extensibility, and Toughness. Nano letters 2011, 11, 5038–5046. [DOI] [PubMed] [Google Scholar]
- (20).Lombardi SJ; Kaplan DL The Amino Acid Composition of Major Ampullate Gland Silk (Dragline) of Nephila Clavipes (Araneae, Tetragnathidae). J. Arachnol 1990, 18, 297–306. [Google Scholar]
- (21).Blackledge TA; Cardullo RA; Hayashi CY Polarized Light Microscopy, Variability in Spider Silk Diameters, and the Mechanical Characterization of Spider Silk. Invertebr. biol 2005, 124, 165–173. [Google Scholar]
- (22).Hayashi CY; Lewis RV Molecular Architecture and Evolution of a Modular Spider Silk Protein Gene. Science 2000, 287, 1477–1479. [DOI] [PubMed] [Google Scholar]
- (23).Kaplan D; Adams WW; Farmer B; Viney C Silk: Biology, Structure, Properties, and Genetics. Conf. Magn. Magn. Mater 1994, 24 179–192. [Google Scholar]
- (24).Lopez-Vaamonde C; Agassiz D; Augustin S; De Prins J; De Prins W; Gomboc S; Ivinskis P; Karsholt O; Koutroumpas A; Koutroumpa F Lepidoptera Chapter 11. BioRisk 2018, 4603–668. [Google Scholar]
- (25).Lee K-G; Chung D-E; Kim K-Y; Jo Y-Y; Kim H-B; Kim S-K; Kweon H General Characteristics of Antheraea yamamai Silkworm Cocoon Cultured in Korea. J. Chem. Technol. Biotechnol 2015, 53, 6–11. [Google Scholar]
- (26).Shao Z; Hu XW; Frische S; Vollrath F Heterogeneous Morphology of Nephila edulis Spider Silk and Its Significance for Mechanical Properties. Polymer 1999, 40, 4709–4711. [Google Scholar]
- (27).K. Wegst UG; Ashby MF The Mechanical Efficiency of Natural Materials. Philos. Mag 2004, 84, 2167–2186. [Google Scholar]
- (28).Riekel C; Branden C; Craig C; Ferrero C; Heidelbach F; Muller M Aspects of X-ray Diffraction on Single Spider Fibers. Int. J. Biol. Macromol 1999, 24, 179–186. [DOI] [PubMed] [Google Scholar]
- (29).Riekel C; Madsen B; Knight DP; Vollrath F X-ray Diffraction on Spider Silk during Controlled Extrusion under a Synchrotron Radiation X-Ray Beam. Biomacromolecules 2000, 1, 622–626. [DOI] [PubMed] [Google Scholar]
- (30).Asakura T; Yao JM; Yamane T; Umemura K; Ultrich AS Heterogeneous Structure of Silk Fibers from Bombyx mori Resolved by C-13 Solid-State NMR Spectroscopy. J. Am. Chem. Soc 2002, 124, 8794–8795. [DOI] [PubMed] [Google Scholar]
- (31).Demura M; Minami M; Asakura T; Cross TA Structure of Bombyx mori Silk Fibroin Based on Solid-State NMR Orientational Constraints and Fiber Diffraction Unit Cell Parameters. J. Am. Chem. Soc 1998, 120, 1300–1308. [Google Scholar]
- (32).Asakura T; Yao JM C-13 CP/MAS NMR Study on Structural Heterogeneity in Bombyx mori Silk Fiber and their Generation by Stretching. Protein Sci 2002, 11, 2706–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Gillespie DB; Viney C; Yager P Raman-Spectroscopic Analysis of the Secondary Structure of Spider Silk Fiber. ACS Symp. Ser 1994, 544, 155–167. [Google Scholar]
- (34).Shao Z; Vollrath F; Sirichaisit J; Young RJ Analysis of Spider Silk in Native and Supercontracted States Using Raman Spectroscopy. Polymer 1999, 40, 2493–2500. [Google Scholar]
- (35).Sirichaisit J; Brookes VL; Young RJ; Vollrath F Analysis of Structure/Property Relationships in Silkworm (Bombyx mori) and Spider Dragline (Nephila edulis) Silks Using Raman Spectroscopy. Biomacromolecules 2003, 4, 387–394. [DOI] [PubMed] [Google Scholar]
- (36).Lefevre T; Rousseau ME; Pezolet M Protein Secondary Structure and Orientation in Silk as Revealed by Raman Spectromicroscopy. Biophys. J 2007, 92, 2885–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Ling SJ; Qi ZM; Knight DP; Shao ZZ; Chen X Synchrotron FTIR Microspectroscopy of Single Natural Silk Fibers. Biomacromolecules 2011, 12, 3344–3349. [DOI] [PubMed] [Google Scholar]
- (38).Ling SJ; Qi ZM; Knight DP; Huang YF; Huang L; Zhou H; Shao ZZ; Chen X Insight into the Structure of Single Antheraea pernyi Silkworm Fibers Using Synchrotron FTIR Microspectroscopy. Biomacromolecules 2013, 14, 1885–1892. [DOI] [PubMed] [Google Scholar]
- (39).Fang GQ; Sapru S; Behera S; Yao JR; Shao ZZ; Kundu SC; Chen X Exploration of the Tight Structural-Mechanical Relationship in Mulberry and Non-Mulberry Silkworm Silks. J Mater Chem B 2016, 4, 4337–4347. [DOI] [PubMed] [Google Scholar]
- (40).Du N; Liu XY; Narayanan J; Li L; Lim MLM; Li D Design of Superior Spider Silk: From Nanostructure to Mechanical Properties. Biophys. J 2006, 91, 4528–4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Kenney JM; Knight D; Wise MJ; Vollrath F Amyloidogenic Nature of Spider Silk. Eur. J. Biochem 2002, 269, 4159–4163. [DOI] [PubMed] [Google Scholar]
- (42).Shen Y; Johnson MA; Martin DC Microstructural Characterization of Bombyx mori Silk Fibers. Macromolecules 1998, 31, 8857–8864. [Google Scholar]
- (43).Putthanarat S; Stribeck N; Fossey SA; Eby RK; Adams WW Investigation of the Nanofibrils of Silk Fibers. Polymer 2000, 41, 7735–7747. [Google Scholar]
- (44).Miller LD; Putthanarat S; Eby RK; Adams WW Investigation of the Nanofibrillar Morphology in Silk Fibers by Small Angle X-ray Scattering and Atomic Force Microscopy. Int. J. Biol. Macromol 1999, 24, 159–165. [DOI] [PubMed] [Google Scholar]
- (45).Poza P; Pérez-Rigueiro J; Elices M; Llorca J Fractographic Analysis of Silkworm and Spider Silk. Eng. Fract. Mech 2002, 69 , 1035–1048. [Google Scholar]
- (46).Lin T-Y; Masunaga H; Sato R; Malay AD; Toyooka K; Hikima T; Numata K Liquid Crystalline Granules Align in a Hierarchical Structure to Produce Spider Dragline Microfibrils. Biomacromolecules 2017, 18, 1350–1355. [DOI] [PubMed] [Google Scholar]
- (47).Schneider D; Gomopoulos N; Koh CY; Papadopoulos P; Kremer F; Thomas EL; Fytas G Nonlinear Control of High-Frequency Phonons in Spider Silk. Nat. Mater 2016, 15 , 1079–1083. [DOI] [PubMed] [Google Scholar]
- (48).Silva LP; Rech EL Unravelling the Biodiversity of Nanoscale Signatures of Spider Silk Fibres. Nat. Commun 2013, 4, 3014–3022. [DOI] [PubMed] [Google Scholar]
- (49).Rockwood DN; Preda RC; Yucel T; Wang XQ; Lovett ML; Kaplan DL Materials Fabrication from Bombyx mori Silk Fibroin. Nat. Protoc 2011, 6, 1612–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Plaza GR; Corsini P; Marsano E; Pérez-Rigueiro J; Biancotto L; Elices M; Riekel C; Agulló-Rueda F; Gallardo E; Calleja JM; Guinea GV Old Silks Endowed with New Properties. Macromolecules 2009, 42, 8977–8982. [Google Scholar]
- (51).Plaza GR; Corsini P; Marsano E; Pérez-Rigueiro J; Elices M; Riekel C; Vendrely C; Guinea GV Correlation between Processing Conditions, Microstructure and Mechanical Behavior in Regenerated Silkworm Silk Fibers. J. Polym. Sci. Part B: Polym. Phys 2012, 50, 455–465. [Google Scholar]
- (52).Xu Y; Shao HL; Zhang YP; Hu XC Studies on Spinning and Rheological Behaviors of Regenerated Silk Fibroin/N-methylmorpholine-N-oxide Center Dot H2O Solutions. J. Mater. Sci 2005, 40, 5355–5358. [Google Scholar]
- (53).Corsini P; Perez-Rigueiro J; Guinea GV; Plaza GR; Elices M; Marsano E; Carnasciali MM; Freddi G Influence of the Draw Ratio on the Tensile and Fracture Behavior of NMMO Regenerated Silk Fibers. J. Polym. Sci. Part B: Polym. Phys 2007, 45, 2568–2579. [Google Scholar]
- (54).Plaza GR; Corsini P; Perez-Rigueiro J; Marsano E; Guinea GV; Elices M Effect of Water on Bombyx mori Regenerated Silk Fibers and Its Application in Modifying Their Mechanical Properties. J. Appl. Polym. Sci 2008, 109, 1793–1801. [Google Scholar]
- (55).Luo J; Zhang L; Peng Q; Sun M; Zhang Y; Shao H; Hu X Tough Silk Fibers Prepared in Air Using a Biomimetic Microfluidic Chip. Int. J. Biol. Macromol 2014, 66, 319–324. [DOI] [PubMed] [Google Scholar]
- (56).Wei W; Zhang Y; Zhao Y; Shao H; Hu X Studies on the Post-Treatment of the Dry-Spun Fibers from Regenerated Silk Fibroin Solution: Post-Treatment Agent and Method. Mater. Des 2012, 36, 816–822. [Google Scholar]
- (57).Wei W; Zhang Y; Zhao Y; Luo J; Shao H; Hu X Bio-Inspired Capillary Dry Spinning of Regenerated Silk Fibroin Aqueous Solution. Mater. Sci. Eng: C 2011, 31, 1602–1608. [Google Scholar]
- (58).Jin Y; Zhang Y; Hang Y; Shao H; Hu X A Simple Process for Dry Spinning of Regenerated Silk Fibroin Aqueous Solution. J. Mater. Res 2013, 28, 2897–2902. [Google Scholar]
- (59).Boekhoven J; Brizard AM; van Rijn P; Stuart MC; Eelkema R; van Esch JH Programmed Morphological Transitions of Multisegment Assemblies by Molecular Chaperone Analogues. Angew. Chem., Int. Ed 2011, 50, 12285–12289. [DOI] [PubMed] [Google Scholar]
- (60).Ling S; Li C; Adamcik J; Shao Z; Chen X; Mezzenga R Modulating Materials by Orthogonally Oriented β-Strands: Composites of Amyloid and Silk Fibroin Fibrils. Adv. Mater 2014, 26, 4569–4574. [DOI] [PubMed] [Google Scholar]
- (61).Ling S; Qin Z; Huang W; Cao S; Kaplan DL; Buehler MJ Design and Function of Biomimetic Multilayer Water Purification Membranes. Sci. Adv 2017, 3 e1601939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Brown CP; Harnagea C; Gill HS; Price AJ; Traversa E; Licoccia S; Rosei F Rough Fibrils Provide a Toughening Mechanism in Biological Fibers. ACS Nano 2012, 6, 1961–1969. [DOI] [PubMed] [Google Scholar]
- (63).Cranford SW Increasing Silk Fibre Strength through Heterogeneity of Bundled Fibrils. J. R. Soc. Interface 2013, 10 20130148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Xu G; Gong L; Yang Z; Liu X What Makes Spider Silk Fibers So Strong? From Molecular-Crystallite Network to Hierarchical Network Structures. Soft Matter 2014, 10, 2116–2123. [DOI] [PubMed] [Google Scholar]
- (65).Sun H; Zhang Y; Zhang J; Sun X; Peng H Energy Harvesting and Storage in 1D Devices. Nat. Rev. Mater 2017, 2, 17023–17034. [Google Scholar]
- (66).Fang GQ; Zheng ZK; Yao JR; Chen M; Tang YZ; Zhong JJ; Qi ZM; Li Z; Shao ZZ; Chen X Tough Protein-Carbon Nanotube Hybrid Fibers Comparable to Natural Spider Silks. J. Mater. Chem. B 2015, 3, 3940–3947. [DOI] [PubMed] [Google Scholar]
- (67).Zhang C; Zhang Y; Shao H; Hu X Hybrid Silk Fibers Dry-Spun from Regenerated Silk Fibroin/Graphene Oxide Aqueous Solutions. ACS Appl. Mater. Interfaces 2016, 8, 3349–3358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


