Abstract
Lymphatic malformations and other conditions where lymphatic function is disturbed in the respiratory tract present diagnostic and therapeutic challenges. Advances in lymphatic development, growth regulation, function, and imaging have increased the understanding of lymphatics, but the airways and lungs have not received as much attentions as many other organs. The lung presents challenges for studies of lymphatics because of the complex, densely packed three-dimensional architecture of the airways and vasculature, and because it cannot readily be examined in its entirety. To address this problem, we developed methods for immunohistochemical examination of the lymphatics in mouse lungs, based on approaches we devised for lymphatic vessels and blood vessels in whole mounts of the mouse trachea. This report provides a practical guide for visualizing by fluorescence and confocal microscopy the lymphatics in mouse airways and lungs under normal conditions and in models of disease. Materials and methods are described for immunohistochemical staining of lymphatics in whole mounts of the mouse trachea and 200-μm sections of mouse lung. Also described are mouse models in which lymphatics proliferate in the lung, blocking antibodies for preventing lymphatic growth, methods for fixing mouse lungs by vascular perfusion, and techniques for staining, visualizing, and analyzing lymphatic endothelial cells and other cells in the lung. These methods provide the opportunity to learn as much about lymphatics in the lung as in other organs.
Keywords: Lymphatic vessels, Endothelial cells, Immunohistochemistry, Fluorescence microscopy, Confocal microscopy, Mouse models, Respiratory tract, Lung disease
1. Introduction
The purpose of this chapter is to provide a practical guide for obtaining images of lymphatic vessels in mouse airways and lungs for examination and measurement. The procedures described are derived from methods that we developed to examine lymphatic vessels and blood vessels in whole mounts of the mouse trachea after immunohistochemical staining. The trachea proved to be very informative, because all lymphatics and blood vessels are visible in 3-dimensions when the cylindrical organ is cut longitudinally, stained immunohistochemically as a whole mount, and viewed by fluorescence or confocal microscopy after flat mounting on a microscope slide. By comparison, the lung presents challenges for this type of analysis because of the size, complex, densely packed three-dimensional architecture of the airways and vasculature, and natural tendency to collapse when the thorax is opened. Also, the commonly used lymphatic marker LYVE-1 is not useful because of confounding expression by endothelial cells of lung alveolar capillaries. Other potentially problematic features of mouse lungs are differences from human lungs that include the limited bronchial circulation, presence of cardiac muscle in the wall of large pulmonary veins, and sparseness of lymphatics in the visceral pleura. Nonetheless, many genetic, pharmacologic, and other powerful experimental tools underscore the value of studies of lymphatics in mice. Although the understanding of lymphatics in mouse lungs is still at an early stage [1], reports have described the distribution of lymphatics under normal conditions and changes in lymphatics of mouse airways or lungs after administration of noxious substances [2–4] or infectious organisms [5–11], transgenic overexpression of VEGF-C or interleukin-1beta [12–14], effects of rapamycin [13], and lung transplantation [15]. Here, we describe materials and methods that have been found to be useful for visualizing by fluorescence and confocal microscopy the lymphatics in mouse lungs under normal conditions and in models of disease.
2. Materials
2.1. Transgenic Mice for Studying Lung Lymphatics
Prospero homeobox 1-enhanced green fluorescence protein (Prox1-EGFP) transgenic mice are available from the Mutant Mouse Resource Center at Davis as strain MMRC #036644- UCD and from laboratories that use them [16]. Although the green fluorescence of lymphatics in these mice fades rapidly after fixation with paraformaldehyde, it can be amplified and made permanent by immunohistochemical staining for EGFP. Prox1-EGFP mice can be genotyped by the presence of green fluorescence of Prox1-EGFP in lens cells after a bright light is shone into their eyes.
CCSP-rtTA mice are available from the Jackson Laboratory in FVB/N strain (JAX #006222) and C57BL/6 strain (JAX #006232). TetO-VEGF-C mice have been made by Kari Alitalo at the University of Helsinki, Finland [17]. Breeding these mice yields double transgenic mice [14]. Breeding the double-transgenic mice with Prox1-EGFP mice yields triple transgenic Prox1-EGFP/CCSP/VEGF-C mice. Conventional PCR methods can be used for genotyping.
Like wild-type mice, these transgenic mice should be housed under pathogen-free conditions in filter-top cages to avoid accidental respiratory tract infections, which can have profound effects on lung lymphatics.
2.2. Doxycycline Water to Induce Lymphatic Growth in CCSP/VEGF-C Transgenic Mice
For studies of adult mice, doxycycline (Sigma D8991) is administered in drinking water at a concentration of 1 mg/mL (0.1%) with 4% sucrose (40 mg/mL) added to mask the bitter taste.
2.3. Reagents for Blocking VEGF-C Mediated Lymphatic Growth
Rat monoclonal antibodies DC101 to mouse VEGFR-2 and mF4– 31C1 to mouse VEGFR-3 have been made by ImClone/Eli Lilly. Adeno-associated viral soluble VEGFR-3 (AAV-sVEGFR3; VEGF--C/D Trap) has been made by Kari Alitalo, University of Helsinki, Finland.
2.4. Anesthetics
Ketamine (87 mg/kg) plus xylazine (13 mg/kg) are injected intraperitoneally together in the same syringe (see Note 1).
2.5. Inoculation and Perfusion Apparatus
60° sloping platform made from wood, plastic, or Styrofoam is used for intratracheal administration of substances.
Fiber optic light source (Dolan-Jenner model 180) is used for illumination.
Binocular magnifier (Donegan Optivisor from Fisher Scientific #NC1227372) is used for magnification for inoculation, surgery, and dissection.
100 μL micropipette is used to measure inoculum onto clean Parafilm or other water-repellent surface.
Inoculum is injected intratracheally with a Clay Adams Intramedic PE10 polyethylene tubing (Becton Dickinson #427401) attached to a 28-G needle on a 0.5 mL syringe (Becton Dickinson #329465).
1-liter aspirator bottle with a hose outlet at the bottom (Kimax #14607–1000) is used for perfusion of fixative.
Aneroid sphygmomanometer (Medline #MDS9380) is used to generate a fixed pressure for perfusion of fixative from the aspirator bottle.
Silicone tubing and rubber bung are used to connect the sphygmomanometer and perfusion tubing to the aspirator bottle.
Male Luer-to-Luer adapter (Cole Palmer Cadence #6183) attaches the silicone perfusion tubing to an 18-G plastic catheter (Becton Dickinson #511097), 30 mm in length that serves as the perfusion cannula.
Plastic tray similar to a restaurant dinner tray serves as a perfusion table that collects fixative during the perfusion.
The perfusion board is made from cork or a thick sheet of silicone or polystyrene foam (Styrofoam).
Paper tape is used to wrap the limbs of anesthetized mice, and push-pins are used to fix the tape to the perfusion board.
A rubber band attached to front teeth and perfusion board is used to keep the neck and trachea straight during the perfusion.
A shadow-free surgical lamp is used for illumination during surgery, perfusion, and dissection.
70% ethanol is used to moisten mouse fur before surgery and dissection.
2.6. Instruments for Surgery and Dissection
140-mm long coarse scissors are used to open the chest.
90-mm long fine scissors from Fine Science Tools #14060–09 are used to cut the heart and trachea.
Hemostat (locking clamp) with curved smooth tips is used to clamp tubing from perfusion bottle.
Hemostat (locking clamp) with straight serrated tips is used to clamp the rib cage after opening the chest wall.
Dumont #3c or #5 11-cm straight forceps from Fine Science Tools #11231–20 or #11252–20 are used for dissection.
Coarse straight forceps (10 mm long with serrated tips from Fine Science tools #11050–10 are used for surgery and dissection.
Scalpel handle and #10 rounded or #11 straight scalpel blades are used for surgery and dissection.
Surgical silk ligature 3–0 is used to ligate trachea after inflation with fixative.
Binocular dissecting microscope is used for magnification.
2.7. Fixatives, Buffers, Incubation Solutions, Embedding Media
2.7.1. Fixative: 1% Paraformaldehyde in 1× Phosphate-Buffered Saline (PBS)
See Subheading 3.4 Making 1% paraformaldehyde.
2.7.2. Calcium and Magnesium-Free PBS and PBS–Triton
The composition of 1× PBS is 0.2 g/L KH2PO4, 2.16 g/L Na2HPO4∙7H2O, 0.2 g/L KCl, 8.0 g/L NaCl. Store at room temperature. 1× PBS is prepared by diluting 10× PBS with water 1:10. PBS–Triton is made by adding 3 mL of Triton X-100 to 1 L of 1× PBS. Stir with magnetic stirrer until dissolved.
2.7.3. “Immunomix” Antibody Incubation Medium
Immunomix, consisting of 1× PBS containing 0.3% Triton X-100, 0.2% bovine serum albumin BSA, and 0.1% sodium azide, is used for primary antibody incubations for immunohistochemistry.
2.7.4. Cryoprotection Solution
30% sucrose dissolved in PBS is used for cryoprotection of tissues during freezing.
2.7.5. Medium for Embedding and Freezing Tissues
Tissues are embedded in OCT.
2.7.6. “SuperDAPI” Mounting Medium
“SuperDAPI” consists of VECTASHIELD with DAPI mounting with additional DAPI at a final concentration of 50 μg/mL.
2.7.7. Coverslip Sealing Resin
Tissues are mounted on microscope slides with Cytoseal.
2.7.8. Sylgard Silicone Resin for Making Dishes to Pin Whole Mounts
Sylgard 184 silicone elastomer resin is available as a kit (Fisher Scientific # NC9965371).
2.7.9. Stainless Steel Pins for Tracheal Whole Mounts
10-mm long headless stainless steel pins (“Austerlitz Minutien” insect pins: 0.10, 0.15 or 0.20 mm diameter, Fine Science Tools #26002) are used to secure tracheal whole mounts to the layer of Sylgard in dishes.
2.8. Immunological Reagents
Antibodies used for immunohistochemical staining of lymphatics and other types of cells in mouse lungs are listed in Table 1. Combinations of antibodies that stain lymphatics together with other cell types can be very informative. Staining for alpha-smooth muscle actin in smooth muscle cells complements staining for lymphatics because it makes visible the branching architecture of bronchi and large blood vessels. The smooth muscle cell layer is sufficient different in bronchi, pulmonary arteries, and pulmonary veins that it can be used to distinguish each of these structures from the others.
Primary antibodies, including fluorophore-conjugated secondary antibodies (Jackson Immunoresearch), are used at a working concentration of 1 μg/mL (1:100–1:500 dilution) (see Note 2).
Table 1.
Immunohistochemical markers of lymphatics and other cells in mouse lungs
| Cell type | Marker | Host | Vendor | Catalog # | Comments |
|---|---|---|---|---|---|
| Lymphatic endothelial Cells | EGFP in Prox1-EGFP mice | Chicken | Aves | GFP 1020 | Overall best marker Strong signal. Marks cytoplasm and nucleus |
| Prox1 | Rabbit Goat | AngioBio R&D Systems | 11–002P AF2727 | Nuclei of lymphatic endothelial cells, neuroendocrine cells, and cardiac muscle cells | |
| VEGFR-3 | Goat | R&D Systems | AF743 | Good, but weaker signal and higher background than Prox1 | |
| CCL21 | Goat | R&D Systems | AF469 | In Golgi apparatus of lymphatic endothelial cells | |
| Neuropilin-2 | Goat | R&D Systems | AF564 | Endothelial cells of lymphatics and venules | |
| Podoplanin | Syrian hamster | AngioBio | 11–033 Clone 8.1.1 | Not recommended for the lungs. Expressed by lung and tracheal epithelial cells. | |
| LYVE-1 | Rabbit Rat Goat | AngioBio R&D Systems R&D Systems | 11–034 MAB2125 AF2125 | Not recommended for the lungs. Strongly expressed by blood vessels in lung [18], but not in trachea. | |
| Blood vessel endothelial cells | Pecam1 (CD31) | Armenian hamster Rat | Thermo BioLegend | MA3105 102502 | Clone 2H8 Clone MEC13.3 Weaker expression also on lymphatic EC |
| Endothelial cells | VEGFR-2 | Goat | R&D Systems | AF644 | Weaker expression also on lymphatic EC |
| High endothelial venules (HEV) | Rat (IgM) | BD Bioscience | 553863 | Found in bronchoalveolar lymphoid tissue (BALT) | |
| Smooth muscle cells | α-smooth muscle actin | Mouse | Sigma Sigma | F3777 (FITC) C6198 (Cy3) | Marks large airways and blood vessels |
| Pericytes | NG2 | Rabbit | Millipore | Ab5320 | |
| Epithelial cells (pan) | E-cadherin Pan- Cytokeratin | Rat Rabbit | Thermo Dako | 13–1900 Z0622 | Clone ECCD-2 |
| Clara/Club cells | CCSP | Rabbit | Millipore | 07–623 | |
| Ciliated cells | Acetylated tubulin | Mouse | Sigma | T7451 | Clone 6–11B-1 |
| Alveolar Type 1 cells | Aquaporin-5 | Rabbit | Abcam | Ab78486 | |
| Alveolar Type 2 cells | SPC | Rabbit | Seven Hills | WRAB-09337 | |
| Leukocytes (pan) | CD45 | Rat | BioLegend | 103102 | Clone 30-F11 |
| T-lymphocytes | CD3e | Armenian hamster | BioLegend | 100302 | Clone 145–2C11 |
| B-lymphocytes | B220 | Rat | BioLegend | 103202 | Clone RA3–6B2 |
| Neutrophils | S100A8 A100A9 | Goat Rabbit | R&D Systems Abcam | AF3059 Ab105472 | |
| Macrophages | Iba1 F4/80 | Rabbit Rat | WAKO BioLegend | 019–19741 123102 | Also known as Aif1 Clone BM8 Better in wax sections |
| Dendritic cells | CD11c | Rat | eBioscience | 14–0114-81 | Clone N418 Better in wax sections |
| Fibroblasts | S100A4 | Rabbit | Abcam | Ab27957 | |
| Mesothelial cells | Mesothelin | Goat | Santa Cruz | Sc-27702 |
3. Methods
3.1. Induction of Lymphatic Growth in CCSP/VEGF-C Transgenic Mice by Doxycycline
Adult CCSP/VEGF-C mice are given doxycycline at a concentration of 1 mg/mL (0.1% doxycycline) in drinking water for 7 days after weaning (P28–P35) or later. Newborn CCSP/ VEGF-C mice, which are more sensitive to doxycycline than adults, are given doxycycline at a concentration of 0.001– 0.01 mg/mL, depending on their age [14].
To prepare doxycycline in drinking water, weigh 1 g doxycycline HCl and 40 g sucrose and add to a large screw-cap bottle with magnetic stirrer. Fill bottle to 1000 mL with deionized water and stir to dissolve (see Note 3)
3.2. Procedures for Inducing Lung Injury
Mycoplasma pulmonis bacteria (strain CT7, 106 organisms per mouse [5]) and influenza A virus (H1N1 strain PR8, 100 Units per mouse [19]) can be given to anesthetized mice by intranasal inoculation of 50 μL of inoculum in 0.9% NaCl or PBS in a Class 2 laminar flow hood in the vivarium.
Hold anesthetized mouse vertically by the scruff of the neck and slowly pipette 50 μL of inoculum into the nostrils, by giving 25 μL per nostril. A change in the breathing pattern is evidence that the inoculum entered the lungs.
Intranasal inoculation is easy, but there is a risk that some or all of the inoculum is swallowed, and the amount inhaled is difficult to determine (see Note 4).
Intratracheal administration via a cannula inserted through the mouth [20] is a more reliable route for inoculation when a reproducible dose is important, as for LPS (2.5 mg/kg, Sigma L2880, serotype 055:B5) or bleomycin (0.5–2 Units/kg, Teva Pharmaceuticals).
After giving ketamine–xylazine anesthesia, suspend the mouse by its front teeth with a rubber band attached to the 60° sloping platform.
Use a bright fiber optic light to illuminate the pharynx and larynx through the skin.
A binocular magnifier can be useful to visualize the larynx while leaving both hands free.
Using blunt forceps, open the mouth wide and pull the tongue out and to one side. The diamond-shaped opening of the larynx should be visible, with the vocal cords pulsating at the bottom of the pharynx.
Measure the volume of inoculum with a pipette and expel it as a compact drop onto the Parafilm (see Note 5).
Draw into the syringe 50 μL of air and then all of the drop of inoculum (see Note 6). Inject the inoculum followed by the air into the trachea via the syringe attached to the cannula inserted between the vocal cords.
Remove the mouse from the sloping platform and keep it warm with a heat lamp until it recovers from the anesthetic.
3.3. Pretreatment with Antibodies or Soluble VEGFR-3 to Block Lymphatic Growth
Function-blocking rat monoclonal antibodies can be used to block VEGFR-2 (clone DC101) and/or VEGFR-3 (clone mF4–31C1) (ImClone Systems/Eli Lilly, New York, NY) [7]. Inject antibodies i.p. at a dose of 0.8 mg in a volume of 200 μL of 0.9% sterile NaCl solution per mouse 1 day before infection, then every other day for 14 days. Rat IgG (Jackson ImmunoResearch Labs #012–000-003) is used as a control.
As another approach for blocking VEGFR-3 signaling, treat mice with a recombinant AAV-derived soluble VEGFR-3 receptor that absorbs its ligands VEGF-C and VEGF-D (VEGF-C/D Trap). Inject 150 μL of AAV, at a concentration of approximately 4.5 × 109 virus particles per mL, via a tail vein 1 week before the study day [12].
3.4. Making 1% Paraformaldehyde Fixative
To make 1 L of 1% paraformaldehyde solution, add 10 g of powdered paraformaldehyde to a 1-L Pyrex flask with a magnetic stirring bar (see Note 7).
Add approximately one-third of the total final volume of distilled or de-ionized water. Stir with a magnetic stirrer. The result will be a cloudy white suspension.
Heat the suspension carefully to 55–60 °C on a hot plate with magnetic stirrer in a fume hood. Slowly and carefully add 1 N NaOH drop by drop until the suspension changes from cloudy white to clear. Only a few drops are needed. The suspension will suddenly turn almost clear, but can contain some suspended white precipitate. If the temperature rises above 65 °C, discard the suspension and start over. Allow the suspension to cool and then filter it with coarse filter paper in a funnel to get a clear solution.
Add sufficient 10× PBS to achieve a final PBS concentration of 1× and allow the suspension to cool. When the solution reaches room temperature adjust the pH to approximately 7.4 by adding HCl or NaOH drop by drop. Adjust the volume to the correct final value by adding distilled or de-ionized water.
Store 1% paraformaldehyde at room temperature in a tightly capped bottle for up to 2 weeks. Discard if a precipitate forms (see Note 8).
3.5. Fixation, Pulmonary and Cardiac Perfusion, Dissection, Lung Inflation
After anesthesia, position the mouse ventral side up on the dissection board. Pinch rear paws to ensure adequate anesthesia by lack of reflexes. Secure the mouse to the dissection board with tape around limbs and push pins. Hold the head flat with a rubber band placed between the teeth to keep the trachea straight.
Moisten the fur with 70% ethanol, and cut and remove the skin to expose the ribs and ventral chest wall.
Using coarse scissors, cut the chest open to make a U-shaped flap. The base of the U should be at the level of the eighth rib and across the sternum. Avoid cutting the lungs by gently moving them away from the scissors. Also avoid cutting the diaphragm to prevent the abdominal contents from getting in the way. The vertical portion of the U should end at the level of the fourth rib. Reflect the chest flap and clamp it with the serrated-tipped hemostat.
Hold the beating heart with coarse forceps and, using the fine scissors, make two shallow cuts about 2 mm long, one at the origin of the pulmonary artery (cut 1 in Fig. 1) and the other at the tip of the left ventricle (cut 2 in Fig. 1). Gently tear open the left and right atria with fine forceps to create an exit route for blood and perfused fixative (see Note 9).
Insert the 18-G perfusion catheter into the origin of the pulmonary artery and perfuse fixative at a pressure of 20 mmHg to flush the pulmonary circulation until the lungs turn white. This step removes many blood cells trapped in the pulmonary circulation.
Next, insert the perfusion catheter into the aorta through the opening in the left ventricle and perfuse the systemic vasculature with fixative for 2 min at a pressure of 120 mmHg.
Using the fine scissors, make a shallow 1-mm cut in the trachea at the level of the third cartilage ring (cut 3 in Fig. 1). Do not cut all the way through the trachea. Keep the trachea attached to the esophagus.
To expand the lungs, insert the perfusion catheter through the incision in the trachea and inflate the lungs at a pressure of 20 mmHg. Tie the trachea with a 3–0 surgical silk ligature between the catheter and the tracheal bifurcation to maintain the expansion.
Cut through the entire thickness of the trachea at the rostral end and peel the trachea away from the esophagus. Cut the vena cava and esophagus just above the diaphragm, remove the trachea and lungs as a single unit, and immerse in about 5 mL of fixative for 1 h at room temperature in a 6-well dish.
Wash with several changes of PBS for 1 h at room temperature.
Cut the left lung transversely into three portions with a scalpel, such that the middle portion contains the main bronchus, pulmonary artery, and pulmonary vein cut in cross section. This middle portion should be about 5 mm thick and located approximately 3 mm below the lung hilum. This part of the left lung is particularly useful for systematic analysis, as the main airways and vessels have a reproducible orientation (Fig. 1). The four lobes of the right lung can be kept as one piece.
After the tissues have been fixed and washed, they should be cryoprotected by soaking overnight at 4 °C in 30% sucrose in PBS. Use about ten times the volume of 30% sucrose solution per volume of tissue. Make 1 L of 30% sucrose by dissolving 300 g sucrose in 1 L of 1× PBS using a magnetic stirrer. Store at 4 °C for a few weeks. Discard if cloudy or mold can be seen growing.
Embed the three portions of the left lung flat in OCT medium in three small plastic 5 mL disposable beakers (Fisher # 02–544-30), and freeze them as cryostat blocks using dry ice. Embed and freeze the entire right lung as a single block. Pour just enough OCT medium to cover the bottom of the embedding pot, then place the tissue in the pot in the desired orientation, and freeze the bottom layer of OCT on dry ice. When frozen, add more OCT to cover the frozen specimens. Freezing the tissue in this stepwise manner prevents the lungs from floating to the top of the OCT and losing their orientation. Store the frozen OCT blocks in labeled Ziplockbags at −20 °C. Frozen blocks keep for at least 2 years in a −20 °C freezer.
Fig. 1.
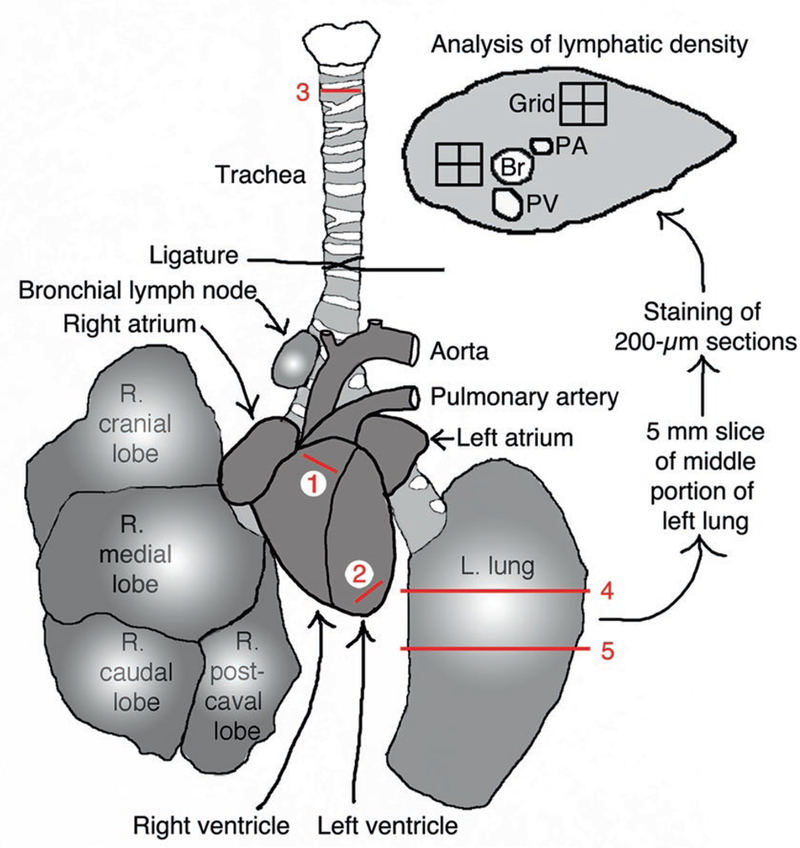
Schematic diagram of mouse heart and lungs showing incision sites 1: in right ventricle at origin of pulmonary artery for pulmonary perfusion; 2: in left ventricle for systemic perfusion; 3: across trachea for lung inflation. An 18-G plastic catheter is inserted at these sites. A thick slice of the middle portion of the left lung is prepared by cutting across sites 4 and 5 with a scalpel when frozen in OCT and is further cut into 200-μm thick cryostat sections. Near the lung hilum the main bronchus and vessels can be readily identified; PA pulmonary artery, Br bronchus, PV pulmonary vein. The lung slice can be analyzed whole or regionally using grids positioned at anatomical regions of interest. Modified from Fig. 1 in [7]
3.6. Cutting Thick Floating Lung Sections
Cut lung sections at 200 μm thickness using the block- trimming function of a Microm or Leica cryostat cooled to −22 °C. Use the anti-roll bar to stabilize and flatten the sections.
Pick up each thick section by the edge with fine forceps and quickly dip it directly in PBS in a 6-well dish or similar. Avoid gripping and damaging the tissue itself in the section. The OCT will dissolve, but the lung sections will float initially.
Wash carefully several times with PBS or PBS–Triton using a plastic pipette to remove the dissolved OCT, taking care not to discard the sections with the wash solution (see Note 10).
3.7. Preparation of Immunomix Antibody Incubation Medium
Add 3 mL of Triton X-100, 2 grams of BSA, and 10 mL of 10% sodium azide solution to a 1 L bottle and fill to 1 L with 1× PBS. Add a magnetic stirring bar and stir slowly to dissolve the BSA. If you attempt to dissolve the BSA quickly, it will clump and take significantly longer to completely dissolve (see Note 11).
3.8. Incubation Procedure for Immunohisto- chemistry
Remove fixative from specimens with several washes of PBS. Time is not critical, provided all of the fixative is rinsed out; more changes, and longer is better. Usually 6 × 10 min is enough.
Immerse lungs in about 5 mL of 30% sucrose in PBS at 4 °C in a 6-well dish. At first, lungs float because they are less dense than the sucrose solution, but when they are fully infiltrated, they sink to the bottom of the tube. Sucrose infiltration helps in the embedding step, as the sucrose has a similar viscosity to OCT. Lungs can be stored in sucrose for several days. Tracheas can be processed as whole mounts immediately without sucrose infiltration.
Incubate lung sections in Immunomix incubation medium containing one or more primary antibodies AND 5–10% blocking serum from the host species in which the secondary antibodies are raised (usually goat or donkey). The blocking step with normal serum reduces nonspecific staining by the primary antibodies. Combinations of primary antibodies must be raised in different species, e.g., rabbit + goat + rat. Incubate tissues at room temperature for at least 6 h (overnight is better) in a 6-well dish on a mechanical rotator.
Wash sections with several changes of PBS–Triton for 5 min each. Wash whole mounts with PBS–Triton for a minimum of 6 h or overnight at room temperature.
Incubate for 1–4 h or longer (overnight is best) for whole mounts in fluorescent secondary antibodies diluted 1:500 in PBS–Triton. Do not use serum in this step. Secondary antibodies conjugated to Cy3 can be used for the red channel, conjugated to Alexa 488 for the green channel, and conjugated to Alexa 647 for the far-red channel of fluorescence or confocal microscopy.
Repeat the wash step with PBS–Triton.
After washing, fix the tissues again briefly for 2 min in 1% PFA. This important step helps to prevent fading and preserves the fluorescence by fixing the labeled antibodies. Wash briefly (3 × 2 min) with PBS to remove the fixative.
Gently fish out individual lung sections from the incubation dish with fine forceps. Hook the forceps under the section and lift up, like picking up spaghetti with a fork. Do not grasp the sections directly to avoid mechanical damage. Transfer the sections into a small volume of SuperDAPI in a watch glass or depression slide (VWR #470005–634). The sections will become clear and almost invisible as the SuperDAPI medium infiltrates them.
To prepare SuperDAPI, make DAPI stock solution by dissolving DAPI dilactate (Sigma; D9564) at 1 mg/mL in deionized water. Store this DAPI stock bottle in the dark at 4 °C.
Next, take a fresh 10 mL dropper bottle of VECTASHIELD mounting medium (H-1000). Pipette 500 μL of DAPI stock solution into an empty glass dropper bottle. Add VECTASHIELD drop by drop and swirl to mix it. Repeat a few times to dilute the DAPI solution with the entire 10 mL volume of VECTASHIELD and mix well. Label the bottle as SuperDAPI and keep it refrigerated in the dark (see Note 12).
Mount the sections in SuperDAPI medium on glass slides, using as little mounting medium as possible. Transfer the lung sections one at a time with fine forceps by hanging them on the tips, as above. Gently spread out the sections flat and orient them in the desired direction by prodding them with the tips of the forceps. Cover the mounted sections with coverslips. If you have used too much mounting medium and the coverslips are floating, absorb the excess by blotting with paper towels.
Carefully paint the edges of the coverslips with Cytoseal 60 (Fisher Scientific #831016) to seal them to the glass slides. Use a fume hood for this step, as Cytoseal 60 contains the volatile and noxious solvents, toluene and butyl benzyl phthalate. Allow the Cytoseal to dry for about 1 h in a fume hood at room temperature before viewing the slides on a microscope (see Note 13). Store slides in a slide box in the dark in a refrigerator. The fluorescence should last up to 1 year.
3.9. Procedures for Tracheal Whole Mounts
The principles for immunostaining tracheal whole mounts are similar to those for staining lung sections, but since they are thicker, the incubation and washing steps need to be longer, 6 h minimum or overnight if possible.
Cut the trachea along the dorsal midline with fine scissors to make a flat whole-mount. Pin tracheas to a Sylgard layer in a 6-well dish using insect pins.
Instructions for polymerizing Sygard184 silicone resin are included in the kit. Wear gloves. Add one part catalyst by weight to ten parts resin in a plastic beaker and mix gently, trying not to introduce air bubbles into mixture. Use a desiccator bell jar connected to a vacuum source to remove remaining air bubbles. Pour the resin into shallow plastic 6-well dishes or similar containers to a depth of 5–10 mm. Allow to polymerize overnight on a flat surface.
Mouse tracheas can be processed in a small volume (1–2 mL) of incubation medium. Stretch the tracheas and pin them by the corners in the Sylgard dish, with up to eight tracheas per well. Incubate with gentle rotation or agitation at room temperature. Mount the tracheas with a gap between them and the silicone layer so that incubation solutions can circulate and reach both sides of whole mounts and be readily exchanged when washing.
3.10. Microscopy, Photography, and Tiling in Photoshop
Prior to observation with a fluorescence or confocal microscope, take the slides out of the refrigerator and let them come to room temperature for a few minutes so any condensation can evaporate (see Note 14).
Photograph the sections in appropriate color channels with camera exposure adjusted for brightness, contrast, and resolution. For conventional fluorescence microscopy, it is better to photograph red and green channels separately, rather than using dual band red/green fluorescence filters. Some bright fluorescence from one channel can bleed into the other channel. If this occurs, the bleed through image needs to be blackened in Photoshop (Window menu > Channels > select channel with bleed-through image > Edit > Fill > Black 100% > File > Save). Red and green channels can be pasted into R and G channels and separately adjusted and viewed in the Channels window (see Note 15).
Low magnification montages or panoramas of entire lung sections can be made by stitching together multiple photographs taken at a higher magnification and resolution. This “photo tiling” can be done with Photoshop or other applications. For example, 10–20 images at 5× are usually sufficient to make a panorama of an entire section of mouse lung (Fig. 2). Images at 5× have a great depth of field, so almost all lymphatics are in focus (Fig. 3). Photograph individual images so that they have about 30% horizontal and vertical overlap with adjacent images.
In Photoshop, open all of the individual images > File > Auto mate > Photomerge > select ‘Auto Layout’ (if not already clicked) and ‘Blend Images Together’ and click ‘Add Open Files’. Click OK. After processing, Photoshop will assemble the images into a seamless whole. If there are gaps in the image, the original photos have not had enough overlap with their neighbors and need to be rephotographed. Sometimes individual stray images can be dragged manually into the correct position in the panorama. The panorama will be in multiple Photoshop layers and will have a large file size. When you are sure the panorama is correct, flatten the multiple merged images (Layers > Flatten Image > Save as JPG). It is useful to take a photo of a calibrated micrometer scale slide (Ted Pella #2280–13) to calculate the final magnification of the panorama.
Fig. 2.
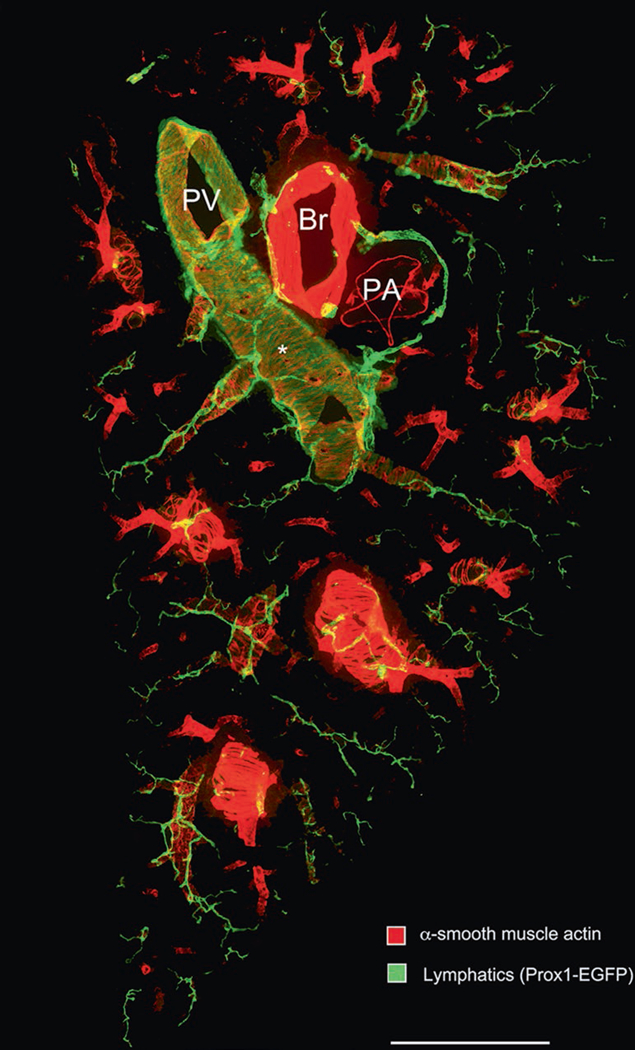
Low magnification panorama of 200-μm thick section of the entire width of the middle portion of left lung of a normal adult C57BL/6 Prox1-EGFP mouse prepared for immunohistochemistry according to the methods described. The section was stained for alpha-smooth muscle actin (red; Cy3- conjugated mouse anti-SMA, Sigma C6198) and Prox1-EGFP (green; Aves #1020 chicken anti-GFP). PV pulmonary vein, Br bronchus, PA pulmonary artery. (Asterisk) Cardiac muscle cells of the pulmonary vein express Prox1-EGFP, but are more weakly stained than the overlying lymphatics. Scale bar: 1 mm
Fig. 3.
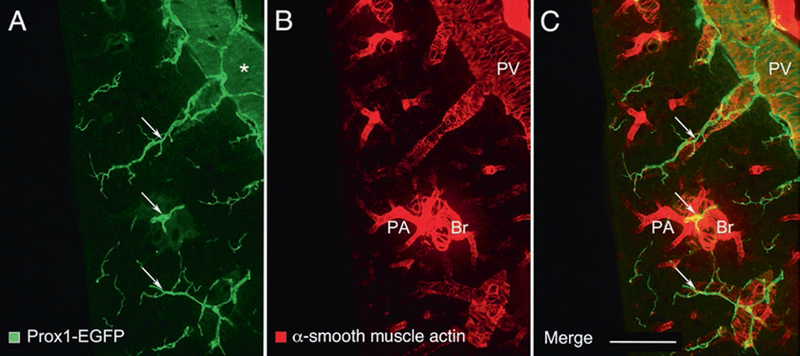
Enlargements of a region near the center of Fig. 2. Left lung of a normal adult C57BL/6 Prox1-EGFP mouse stained for (a) Prox1-EGFP (green) and (b) alpha-smooth muscle actin (red). (c) merged image. Lymphatics (arrows) generally follow the scaffold of smooth muscle cells of airways and blood vessels, especially pulmonary veins (PV): Peripheral branches of the bronchus (Br) and pulmonary artery (PA) are closely associated in pairs, and separate from branches of the pulmonary vein. Each type of vessel has a characteristic coat of smooth muscle cells. The proximal portion of the pulmonary vein has a coat of cardiac muscle cells (asterisk), which express Prox1-EGFP, but are more weakly stained than lymphatics, and also has a thin coat of smooth muscle cells. Scale bar: 500 μm
3.11. Pixel Intensity Analysis or Stereological Point Counting with ImageJ
The area density of immunostained lymphatics in images of lung sections can be measured with ImageJ (https://imagej.nih.gov/ij/ImageJ) after optimization in Photoshop.
Camera settings and Photoshop adjustments must be the same for all images in the batch being analyzed, because the measurement of area density is based on the percentage of pixels above a certain brightness threshold.
In Photoshop adjust the contrast and brightness of the color channel containing lymphatics, such that the background where lymphatics are absent is almost black and the lymphatics are bright.
Launch ImageJ and open the image (File > Open > select the image of interest).
In the “Image” menu of ImageJ scroll down to “Color” then across to “Split Channels.” Keep the color channel containing lymphatics, and discard the others.
Select the region of interest using the “Freehand selection” lasso tool (fourth from the left in the ImageJ menu). Outline the whole lung with the yellow line (see Note 16).
In the “Analyze” menu of ImageJ scroll down to “Histogram” describing the number of pixels in the image with a brightness of 0–225. Click “Copy.”
Paste the data into an Excel spreadsheet, and calculate the total number of pixels with intensity 0–225 in the region of interest bounded by the yellow line.
Also in Excel, calculate the total number of pixels above the chosen brightness threshold, and calculate the percentage they represent of the total number of pixels. The absolute value of the brightness threshold is arbitrary but must be less than the brightness of the structures identified as lymphatics, and must be the same for all images being analyzed.
A plugin called Image-based tool for Counting Nuclei (ICTN) for ImageJ, which can be downloaded from https://imagej.nih.gov/ij/plugins/itcn.html, can be used for counting nuclei of Prox1-stained lymphatic endothelial cells.
Stereological point counting can be used as an alternative to ImageJ for measuring area densities. For this approach, a rectangular test grid is overlaid on the image, and points of intersection are counted manually. A plugin to create such a grid in ImageJ can be accessed by clicking Analyze > scroll down to “Tools” > “Grid.”
Acknowledgments
This work was supported in part by grants R01 HL127402, R01 HL059157, and P01 HL024136 from the NIH National Heart, Lung, and Blood Institute and 11CVD03 from the Leducq Foundation.
4 Notes
Written records must be kept on the use of ketamine, as for other controlled substances.
Secondary antibodies that are preabsorbed for other species are better, but more expensive, than unabsorbed ones.
About 150 mL of doxycycline is needed per water bottle for mouse cages. Make sure the water bottles do not leak. As doxycycline is light-sensitive, the water bottle should be shielded from light. Avoid aluminum foil as shielding as mice will eat it. Doxycycline water should be replaced every 2–3 days to ensure activity.
Appropriate safety precautions should be taken for Mycoplasma pulmonis organisms, which are pathogenic in mice but not in humans, and influenza A virus which is pathogenic in both.
The volume of intratracheal inoculations should be no more than 2 μL per gram of mouse, e.g., 50 μL for a 25-g mouse, to avoid the risk of impairing respiration.
Insert the tubing through the pharynx and larynx into the trachea, and slowly and gently inject the inoculum. The air buffer serves to push the inoculum deep into the lungs. Quickening of breathing is evidence of successful administration.
For safety, wear gloves, mask, and personal protective clothing/equipment. Work in a fume hood to avoid breathing formaldehyde fumes. Also avoid breathing paraformaldehyde powder.
Do not freeze aliquots of paraformaldehyde in solution, as the activity will be lost. Do not use formalin (37% paraformaldehyde + 15% methanol) as the fixative. Local safety regulations may require bottles to have warning labels of “Carcinogen” or similar. Dispose of waste paraformaldehyde in line with local safety regulations.
When creating a route for outflow of blood and fixative during the perfusion, use a toothed forceps and do not cut the heart atria with scissors, as there is a risk of accidental cutting of the aorta or other structures by mistake.
Most air bubbles in the lung will come out during this process or during the incubation steps.
Sodium azide is very toxic and should be handled with care. Please review the accompanying safety sheet before handling. Wear gloves, mask, and protective clothing. Use a large weighing dish to avoid making dust or spilling on the weighing balance. For safety, sodium azide is best handled as a solution. Make a 10% aqueous solution by carefully dissolving 10 g sodium azide powder in 100 mL of deionized water. For safety, sodium azide is best stored and handled as a solution in a break-proof bottle surrounded by a safety container.
VECTASHIELD with DAPI mounting medium (Vector Labs product #H-1200) is too weak to properly stain nuclei in thick tissue sections or tracheal whole mounts. However, it does contain a useful proprietary antifade agent than prolongs fluorescence and minimizes bleaching.
Use of 20× objectives and above will help prevent objective lenses with short working distances coming into contact with Cytoseal or other mounting medium that has not dried.
Keep the microscope stage clean and dry; wipe up any spilled liquid with tissue paper.
When mistakes are made in Photoshop, they can be reversed by using the History window or the Edit > Step Backwards command.
Cardiac muscle cells in the wall of major pulmonary veins stain for Prox1-EGFP, but are less bright than lymphatics. If an image has a large amount of these cells, as near the lung hilum, they can be excluded from measurements by using the Lasso tool in Photoshop or ImageJ.
References
- 1.Stump B, Cui Y, Kidambi P, Lamattina AM, El-Chemaly S (2017) Lymphatic changes in respiratory diseases: more than just remodeling of the lung? Am J Respir Cell Mol Biol 57:272–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E et al. (2007) Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med 204:2349–2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meinecke AK, Nagy N, Lago GD, Kirmse S, Klose R, Schrodter K, Zimmermann A, Helfrich I, Rundqvist H, Theegarten D et al. (2012) Aberrant mural cell recruitment to lymphatic vessels and impaired lymphatic drainage in a murine model of pulmonary fibrosis. Blood 119:5931–5942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kretschmer S, Dethlefsen I, Hagner-Benes S, Marsh LM, Garn H, Konig P (2013) Visualization of intrapulmonary lymph vessels in healthy and inflamed murine lung using CD90/Thy-1 as a marker. PLoS One 8:e55201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker SA et al. (2005) Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest 115:247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baluk P, Yao LC, Feng J, Romano T, Jung SS, Schreiter JL, Yan L, Shealy DJ, McDonald DM (2009) TNF-alpha drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. J Clin Invest 119:2954–2964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baluk P, Adams A, Phillips K, Feng J, Hong YK, Brown MB, McDonald DM (2014) Preferential lymphatic growth in bronchus- associated lymphoid tissue in sustained lung inflammation. Am J Pathol 184:1577–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baluk P, Phillips K, Yao LC, Adams A, Nitschke M, McDonald DM (2014) Neutrophil dependence of vascular remodeling after mycoplasma infection of mouse airways. Am J Pathol 184:1877–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao LC, Baluk P, Feng J, McDonald DM (2010) Steroid-resistant lymphatic remodeling in chronically inflamed mouse airways. Am J Pathol 176:1525–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao LC, Baluk P, Srinivasan RS, Oliver G, McDonald DM (2012) Plasticity of button-like junctions in the endothelium of airway lymphatics in development and inflammation. Am J Pathol 180:2561–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harding J, Ritter A, Rayasam A, Fabry Z, Sandor M (2015) Lymphangiogenesis is induced by mycobacterial granulomas via vascular endothelial growth factor receptor-3 and supports systemic T-cell responses against mycobacterial antigen. Am J Pathol 185:432–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baluk P, Hogmalm A, Bry M, Alitalo K, Bry K, McDonald DM (2013) Transgenic overexpression of interleukin-1beta induces persistent lymphangiogenesis but not angiogenesis in mouse airways. Am J Pathol 182:1434–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baluk P, Yao LC, Flores JC, Choi D, Hong YK, McDonald DM (2017) Rapamycin reversal of VEGF-C-driven lymphatic anomalies in the respiratory tract. JCI Insight 2(16):pii: 90103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao LC, Testini C, Tvorogov D, Anisimov A, Vargas SO, Baluk P, Pytowski B, Claesson-Welsh L, Alitalo K, McDonald DM (2014) Pulmonary lymphangiectasia resulting from vascular endothelial growth factor-C overexpression during a critical period. Circ Res 114:806–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui Y, Liu K, Monzon-Medina ME, Padera RF, Wang H, George G, Toprak D, Abdelnour E, D’Agostino E, Goldberg HJ et al. (2015) Therapeutic lymphangiogenesis ameliorates established acute lung allograft rejection. J Clin Invest 125:4255–4268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi I, Chung HK, Ramu S, Lee HN, Kim KE, Lee S, Yoo J, Choi D, Lee YS, Aguilar B et al. (2011) Visualization of lymphatic vessels by Prox1-promoter directed GFP reporter in a bacterial artificial chromosome-based transgenic mouse. Blood 117:362–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lohela M, Helotera H, Haiko P, Dumont DJ, Alitalo K (2008) Transgenic induction of vascular endothelial growth factor-C is strongly angiogenic in mouse embryos but leads to persistent lymphatic hyperplasia in adult tissues. Am J Pathol 173:1891–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baluk P, McDonald DM (2008) Markers for microscopic imaging of lymphangiogenesis and angiogenesis. Ann N Y Acad Sci 1131:1–12 [DOI] [PubMed] [Google Scholar]
- 19.Gotts JE, Abbott J, Matthay MA (2014) Influenza causes prolonged disruption of the alveolar-capillary barrier in mice unresponsive to mesenchymal stem cell therapy. Am J Physiol Lung Cell Mol Physiol 307:L395–L406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ortiz-Munoz G, Looney MR (2015) Non-invasive intratracheal instillation in mice. Bio Protoc 5(12):pii: e1504. [DOI] [PMC free article] [PubMed] [Google Scholar]


