Abstract
The origin of Titan’s nitrogen-rich atmosphere is thought to be ammonia ice, but this has not yet been confirmed. Furthermore, it is uncertain whether the building blocks of Titan formed within the Saturnian subnebula or in the colder protosolar nebula (PSN). Recent measurements of the nitrogen isotope ratio in cometary ammonia, combined with evolutionary constraints on the nitrogen isotopes in Titan’s atmosphere provide firm evidence that the nitrogen in Titan’s atmosphere must have originated as ammonia ice formed in the PSN under conditions similar to that of cometary formation. This result has important implications for the projected D/H ratio in cometary methane, nitrogen isotopic fractionation in the PSN and the source of nitrogen for Earth’s atmosphere.
Keywords: comets, general – planets and satellites, atmospheres – planets and satellites, composition – planets and satellites, formation
1. INTRODUCTION
The origin of Titan’s nitrogen-rich atmosphere is still uncertain (Niemann et al. 2010). The ratio of light to heavy nitrogen (14N/15N) in molecular nitrogen, the most abundant constituent in Titan’s atmosphere, has been measured to be (Niemann et al. 2010), a value close to the one recently measured in cometary ammonia (Rousselot et al. 2014; Shinnaka et al. 2014). Here we show that the 14N/15N ratio in Titan’s nitrogen did not significantly evolve over the satellite’s history, irrespective of the atmospheric escape process considered, and remained close to the value in cometary ammonia. This implies that nitrogen was captured in the form of ammonia present in Titan’s building blocks when they formed in the protosolar nebula (PSN). Soon after Titan’s formation, the conversion of ammonia into nitrogen may have been induced by photolysis (Atreya et al. 1978) or shock chemistry (McKay et al. 1988) in the satellite’s atmosphere, or resulted from its thermal decomposition in the interior (Glein et al. 2009). It has already been shown that none of these conversion processes could have significantly changed the 14N/15N ratio (Berezhnoi 2010).
Nitrogen in the PSN was primarily in the form of N2 (Marty et al. 2010) with trace amounts of NH3, HCN and organic material. The reduction of N2 in the warmer subnebula (a gravitationally trapped region of higher density gas) around Saturn is expected to have led to a much greater abundance of NH3 relative to N2 (Prinn & Fegley 1981). If the planetesimals that were the building blocks for Titan formed in the Saturnian subnebula, they would therefore have contained a higher abundance of NH3 than those formed in the PSN. The idea that NH3 might have been more abundant than N2 in Titan’s building blocks was supported by the low non-radiogenic argon (36Ar) abundance measured by the Huygens probe Gas Chromatograph Mass Spectrometer (GCMS; Niemann et al. 2010) which suggested that only NH3 was trapped in Titan’s building blocks because N2 and 36Ar are trapped in ice at much colder temperatures than NH3 (Owen & Bar-Nun 1995). However, laboratory studies have shown that aerosols formed in Titan’s atmosphere can trap sufficient amounts of 36Ar to remove enough of it from the atmosphere over the lifetime of Titan to reduce the 36Ar/N2 ratio from the solar value to the current measurement (Jacovi & Bar-Nun 2008). Hence the low 36Ar abundance is not conclusive in arguing for ammonia as the source of Titan’s nitrogen. Furthermore, an alternative model proposed recently that Titan’s planetesimals formed in the cold PSN and later migrated into the warmer Saturnian subnebula where some of the volatiles in these planetesimals were partly devolatilized (Mousis et al. 2009a). In this model, it was not hot enough in the Saturnian subnebula to vaporize all of the ices or induce chemical conversion. Evaluation of the 14N/15N in Titan’s atmosphere can provide a more definitive constraint on the nitrogen composition of Titan’s building blocks and hence provide a test of these competing models for Titan’s formation.
2. NITROGEN IN THE PSN
The measurements of 14N/15N made throughout the solar system are listed in Table 1 and illustrated in Figure 1. These ratios are either primordial (triangles in Figure 1), or evolved (circles in Figure 1). Primordial ratios are believed to have preserved their value from the early stages of solar system formation. An example of a primordial measurement is 14N/15N in the atmosphere of Jupiter, which is interpreted as primordial because Jupiter’s high-gravity, hydrogen-dense atmosphere maintains a closed chemical system. These ratios are, therefore, presumably a direct record of conditions in the PSN. Evolved ratios are interpreted to have changed in value over the history of the solar system.
Table 1.
Measurements of 14N/15N Throughout the Solar System
| Target | 14N/15N | Primorial or Evolved ratio | Form of nitrogen | Primordial form |
|---|---|---|---|---|
| Solar wind | 442 ± 1001 | Primordial | N | N2 |
| Jupiter | 435 ± 652 | Primordial | NH3 | N2 |
| Several Jupiter family and Oort cloud comets | 157 ± 213 | Primordial | HCN | HCN |
| Summed spectrum of several Jupiter family and Oort cloud comets | 127 ± 324 | Primordial | NH3 | NH3 |
| Comet C/2012 S1 (ISON) | 139 ± 385 | Primordial | NH3 | NH3 |
| Titan’s atmosphere | 167.7 ± 1.76 | Evolved | N2 | NH3 |
| Titan’s building blocks | ⩽ 190 | Primordial | NH3 | |
| Comet 81P/Wild 2 grains | 231 ± 387 | Primordial | Organics with ice | Organics + HCN & NH3 |
| Chondrites | 259 ± 158 | Primordial | Organics | Organics |
| Earth’s atmosphere | 272 ± 0.39 | Evolved | N2 | TBD |
| Earth’s mantle | 270.4 ± 3.39 | Unknown | N2 | |
| Venus’ atmosphere | 273 ± 5610 | Unknown | N2 | |
| Mars meteorite | 280 ± 511 | Unknown | Various N-bearing compounds | |
| Mars’ atmosphere | 168 ± 1712 | Evolved | N2 |
Notes. We identify the form of nitrogen in which the measurement was made and the likely form of nitrogen when it was delivered to the target body. Sources:
Figure 1.
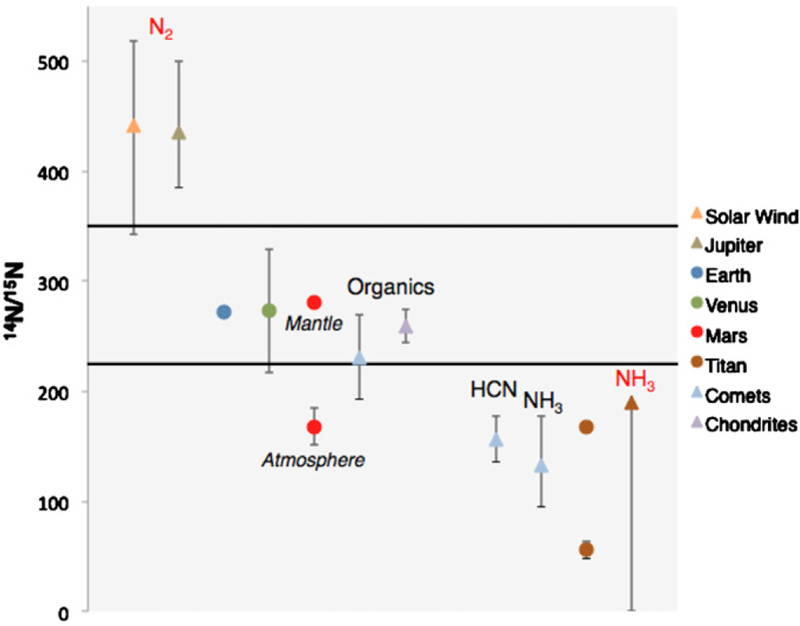
Measurements of nitrogen isotope ratios throughout the solar system. Triangles are primordial values representing 14N/15N in the PSN. Circles are isotope ratios that have evolved over the 4.6 Billion year history of the solar system. The primordial value for Titan is inferred from models of atmospheric evolution. The composition of each primordial 14N/15N is provided as either the constituent that was measured (black) or inferred (red) based on current understanding of the evolution of the solar system.
The measured 14N/15N can be divided into three categories based on their value.
The solar wind (Marty et al. 2010) and Jupiter (Owen et al. 2001) have the lightest ratios.
Chondrites (Alexander et al. 2012) and grains from comet 81P/Wild 2 (McKeegan et al. 2006), Earth’s atmosphere and mantle (Marty & Zimmermann 1999), Venus (Hoffman et al. 1979) and the mantle of Mars (based on SNC meteorite ratios; Mathew & Marti 2001) have moderately heavy ratios.
Saturn’s moon Titan (Niemann et al. 2010), the atmosphere of Mars (Wong et al. 2013) and HCN (Bockelée-Morvan et al. 2008) and NH3 (Rousselot et al. 2014; Shinnaka et al. 2014) in comets have the heaviest values.
The molecular carrier and the isotopic ratio of nitrogen introduced into Titan during formation was a function of the nebular environment. If Titan’s building blocks formed in an initially warmer and denser Saturnian subnebula, then they would have contained NH3 formed by N2 reduction in the gas phase at high temperatures (Prinn & Fegley 1981). This NH3 would have had a 14N/15N similar to N2 in the PSN because the bulk conversion from N2 to NH3 would produce NH3 with 14N/15N reflecting that of its source material—N2 originating in the PSN. If Titan’s building blocks formed in the colder PSN, then they would have contained either N2, NH3, or a combination of both. The primary form of nitrogen in the PSN was N2 but trace amounts of NH3 and HCN were also present in the PSN and are preserved in primordial ices in bodies such as cometary nuclei (Hily-Blant et al. 2013). The value for 14N/15N in N2 in the PSN can be determined using 14N/15N measured in Jupiter’s atmosphere and the solar wind based on the argument that the Sun and Jupiter derived their nitrogen from N2 in the PSN (Marty et al. 2010; Owen et al. 2001). The agreement between 14N/15N in these two bodies makes this association plausible and gives an estimate for 14N/15N in N2 in the PSN of ~435. If Titan’s nitrogen originated from N2 or from NH3 in the Saturnian subnebula, the primordial ratio of nitrogen in Titan’s building blocks would then have been ~435. If Titan’s building blocks formed in the PSN, any N2 trapped in the ices would have a PSN value of 14N/15N, but any NH3 could have a 14N/15N value that differs from 14N/15N in N2. Two recent measurements of 14N/15N in cometary NH3 demonstrate that ammonia ices formed in the PSN are highly fractionated from 14N/15N in N2: 127 ± 32 (Rousselot et al. 2014) using a summed spectrum of 12 comets, and 139 ± 38 (Shinnaka et al. 2014) from the outburst of comet C/2012 S1 (ISON). Therefore, if Titan’s nitrogen originated from NH3 ice formed in the PSN, its primordial 14N/15N would be ~130.
In the past it was assumed that Titan’s primordial ratio was the same as the 14N/15N currently measured in Earth’s atmosphere (e.g., Atreya et al. 2009), because comets were suggested to have delivered Earth’s nitrogen in the form of NH3 (Owen et al. 2001). Prior to the measurements referenced above 14N/15N in Earth’s atmosphere was taken to represent 14N/15N in cometary NH3 and was used as the basis for the initial 14N/15N for Titan’s atmosphere (e.g., Lunine et al. 1999). If 14N/15N in NH3 was not homogenous throughout the PSN, then it may be possible that Titan’s building blocks could have contained NH3 with a primordial ratio in the same range as the terrestrial 14N/15N, which is measured at 272. We first explore whether it is possible for Titan’s primordial ratio to fractionate from 272 to its current value of 167.7 and then discuss the implications for the origin of Titan’s nitrogen. It has already been demonstrated that the conversion of NH3 into N2 by photolysis (Atreya et al. 1978) or shock chemistry (McKay et al. 1988) could not have significantly changed the 14N/15N ratio (Berezhnoi 2010) nor could thermal decomposition in the interior (Glein et al. 2009). However, these studies did not consider escape, which is one of the most efficient mechanisms known to substantially fractionate the planetary atmospheres (Pepin 2006). We evaluate this mechanism here to determine if escape can fractionate the 14N/15N ratio in Titan’s atmosphere from 272 to 167.7 within the lifetime of the solar system.
3. FRACTIONATION BY ESCAPE
The evolution of isotope ratios in Titan’s atmosphere depends on processes that fractionate the isotopes. Escape preferentially removes the lighter isotope from the atmosphere thus decreasing 14N/15N. Photochemistry can also fractionate isotopes by preferentially removing either the lighter or the heavier isotope. Photochemistry in Titan’s atmosphere preferentially removes the heavier isotope from N2 through self-shielding processes that incorporate more 15N into HCN relative to 14N (Liang et al. 2007; Mandt et al. 2009). Because we are looking for a limit on how much Titan’s 14N/15N can decrease over the history of the solar system, we will focus only on escape. By doing this, we will define lower limits for the time required to fractionate 14N/15N from the terrestrial value of 272 to Titan’s current value of 167.7. In our previous work, we found that hydrodynamic escape of an atmosphere drags too much of both isotopes out of the atmosphere to effectively change the ratio (Mandt et al. 2009), but the assumption was that the loss rate of the heavy isotope depended on that of the primary constituent, which could either be H, H2 or 14N2. We revisit the question of escape-driven fractionation at Titan based on recent studies of escape processes (Volkov et al. 2011) that allow us to consider mass-dependent fractionation during sputtering, Jeans escape and hydrodynamic escape.
The fractionation of isotopes is described by a fractionation factor, f (Mandt et al. 2009). If f is greater than 1.0 there is preferential loss of the heavy isotope, and if f is less than 1.0 the lighter isotope is preferentially removed. The value of 14N/15N will not change when f is 1.0. The total inventory of a constituent, in terms of the current inventory (i.e., current atmospheric masses of N2), n0/n, required for fractionation from an initial 14N/15N, R0, to the current 14N/15N, R, is given as a function of the fractionation factor:
| (1) |
Currently nitrogen escapes from Titan at a small rate due to sputtering (De la Haye et al. 2007). This process efficiently fractionates the isotopes through diffusion as a function of the temperature in the upper atmosphere, T, and the distance between the exobase and the homopause, or re − rh:
| (2) |
where k is Boltzmann’s constant, G is the gravitational constant, M is the mass of Titan and m is the mass of N2. A reasonable range of f for Titan is 0.720 to 0.850 based on the extent of the atmosphere and possible temperature ranges in the thermosphere as measured by Cassini instruments (Mandt et al. 2012). Under current atmospheric conditions the fractionation factor for sputtering is f = 0.729. Figure 2 shows the inventory that must be lost by sputtering for the ratio to change from 272 to 167.7 (blue shaded region). The escape rate at the current time due to sputtering is ~108 cm−2 s−1 (De la Haye et al. 2007), leading to the time limits shown in Figure 3 (blue line).
Figure 2.
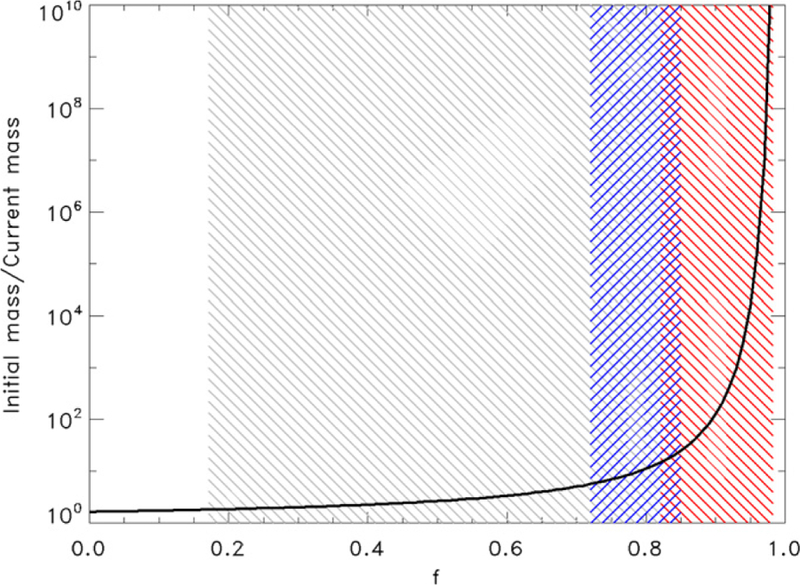
Initial atmospheric mass in terms of the number of current atmospheres required to fractionate 14N/15N from 272 to 167.7, and the fractionation factor, f. Hydrodynamic escape (red range) requires loss of between 13 and 1014 times the current atmosphere, while Sputtering (blue range) only requires loss of 5–24 times the current atmosphere and Jean escape (gray range) requires loss of 0.8–13 times the current atmosphere.
Figure 3.
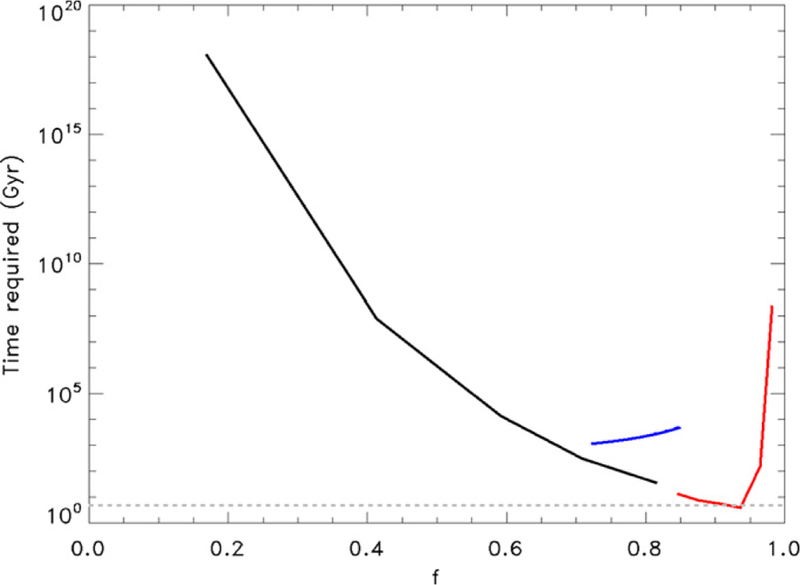
Time required (in billions of years, or Gyr) to change the ratio from 272 to 167.7, shown as a function of f for Hydrodynamic escape (red), Jeans escape (black) and Sputtering (blue). With the exception of one case of Hydrodynamic escape, each type of escape requires longer than the lifetime of the solar system (dashed gray line). The minimum time is ~3.8 Gyr for an escape rate and f that could only have been sustained for 40 Myr.
If we take into account changes in solar activity, sputtering rates early in the history of the solar system could have been up to three orders of magnitude higher for as much as 1 billion years (Gyr) due to the high density and velocity of the solar wind (Penz et al. 2005). These escape rates allow for a total loss of ~0.6 times the current atmospheric mass over the first Gyr of Titan’s lifetime. Although the reasonable range of f for Titan’s current conditions is 0.720–0.850, increased solar extreme ultraviolet (EUV) flux in the early stages of the solar system would increase the temperature and extent of Titan’s atmosphere, increasing f (Equation (2)), and hence reducing fractionation. Thus f of 0.720 is a lower limit, and based on Equation (1) the initial 14N/15N must be lower than 190.
Thermal escape due to Jeans escape and hydrodynamic escape must also be considered. The current thermal escape rate at Titan is several orders of magnitude less than the sputtering rate for N2, and has no effect on atmospheric evolution. However, thermal escape may have been effective in the distant past when the solar EUV flux was much higher (Penz et al. 2005; Mandt et al. 2009). Thermal escape produces mass-dependent fractionation that varies as a function of the Jeans escape parameter, λ:
| (3) |
The value for λ for N2 in Titan’s atmosphere is presently ~50 (Volkov et al. 2011). Jeans escape is given as:
| (4) |
where ne is the density at the exobase. Fractionation due to Jeans escape for N2 is
| (5) |
The possible values for f due to Jeans escape range between 0.170 when λ = 50 and 0.820 when λ = 6, which is the minimum value for λ before transition to hydrodynamic escape (Volkov et al. 2011). Figure 2 illustrates that 0.8–13 times the current atmosphere (shaded gray area) must be lost through Jeans escape in order to fractionate 14N/15N from 272 to 167.7. Jeans escape only becomes more significant than sputtering when λ< 10. We estimate the time required to lose 13 times the current atmosphere through Jeans escape based on the maximum possible rate, which is found when λ = 6 (Volkov et al. 2011). Based on Equation (3), the temperature at the exobase must be between 650 and 1250 K, depending on the extent of the atmosphere, which could have been as far out as three Titan radii (Penz et al. 2005). The exobase temperature decreases with increasing altitude of the exobase. With these constraints, the range of possible N2 densities at the exobase can be found from the definition that the exobase occurs where the scale height equals the mean free path:
| (6) |
where σ is the collision cross section for N2. This limits the density at the exobase to 1–2 × 106 cm−3, and gives a maximum Jeans escape rate of ~2.8 × 109 cm−2 s−1. Based on this, at least 34.5 Gyr are required to fractionate the isotopes, or more than seven times the lifetime of the solar system. When λ = 10, the time required increases to 305 Gyr because the escape rate is lower, as illustrated in Figure 3 (black line).
In hydrodynamic escape λ gradually becomes smaller while f increases to a value of (Volkov et al. 2011):
| (7) |
as illustrated in Figure 2 (red shaded region). Figure 3 (red line) illustrates estimates for the time required for hydrodynamic escape to fractionate the isotopes. The most effective fractionation occurs when λ = 2, which is the only case where the time required to fractionate the isotopes is within the lifetime of the solar system. In this case we estimate the maximum possible escape rate to be ~4 × 1012 cm−2 s−1 assuming an exobase that could range between its current and three times the current altitude (Penz et al. 2005) and temperatures that could be as high as 75,000 K. Other studies estimated a similar energy-limited loss rate of N2 of 6 × 1012 cm−2 s−1 at the earliest stages of the solar system, but found that this loss rate could not be sustained for more than 40 Myr (Penz et al. 2005). This is much less time than what is required for fractionating the isotopes due to hydrodynamic escape, so the time required for hydrodynamic escape is >100 times too long to fractionate the 14N/15N from 272 to 167.7.
4. DISCUSSION AND CONCLUSIONS
In the previous sections we demonstrated that sputtering would require too much time to fractionate 14N/15N in Titan’s atmosphere from 272 to 167.7, even when taking into account that the early Sun would have allowed higher sputtering rates in the distant past due to a denser solar wind. Fractionation of the isotopes by sputtering would have taken more than 250 times the lifetime of the solar system, as shown in Figure 3. Jeans escape must remove 0.8–13 times the current atmosphere and hydrodynamic escape requires between 13 and 1014 atmospheric masses to be lost. As Figure 3 demonstrates, only one case of hydrodynamic escape provides a timescale that is less than the lifetime of the solar system for an escape process that could have only lasted for ~40 million years (Penz et al. 2005). Therefore, the upper limit for the primordial ratio for Titan is limited to ≤190, which is in agreement with the 14N/15N for NH3 ice in the PSN based on cometary measurements. It is not possible for the primordial ratio of Titan to be the same as the 14N/15N in the Earth’s atmosphere, and unlikely that Titan’s primordial 14N/15N was ~435, thus demonstrating that Titan’s nitrogen must have originated as NH3 ice from the PSN, as was also suggested by Rousselot et al. (2014). A further implication of this result is that cometary NH3 is not likely to be the primary source of Earth’s nitrogen.
Furthermore, a clear difference between 14N/15N in N2 in the PSN and in the NH3 ices formed in the PSN has been demonstrated by our results for Titan and by the measurements of 14N/15N in cometary NH3. This difference can provide some insight into processes at work in the PSN. Observations of prestellar cores suggest that the formation of nitriles (e.g. HCN) and amines (molecules that form by replacing one or more hydrogen atoms in NH3) are decoupled and that processes that enrich nitriles in 15N would not enrich amines in 15N (Hily-Blant et al., 2013). Self-shielding processes in the PSN can explain the enrichment of 15N in nitriles such as HCN (Clayton 2008), because HCN in formed from neutral nitrogen atoms produced by dissociation of N2, while gas–grain chemistry (Charnley & Rodgers 2002; Rodgers & Charnley 2008) provides the best explanation for enrichment of NH3.
Finally, determining that NH3 ices formed in the PSN is the unique source of Titan’s nitrogen allows us to make a prediction for the D/H in methane in comets, which should be measured by the Rosetta mission at comet 67P/Churyumov–Gerasimenko (hereafter CG). The D/H in the water of Enceladus’s plume is 2.9 × 10−4 (Waite et al. 2009), which is in the same range as the enriched values of D/H in water in Oort Cloud comets, suggesting that its building blocks were the same as for this family of comets (Mousis et al. 2009b). Our studies of the evolution of methane in Titan’s atmosphere (Mandt et al. 2012) show that the primordial D/H in Titan’s methane is bracketed between the current value measured by Cassini, 1.6 × 10−4 (Nixon et al. 2012), and the protosolar D/H of 2.1 × 10−5 (Geiss & Gloeckler 1998). The scenario for Titan formation therefore predicts that the D/H in methane in Oort Cloud comets should fall within this range. The D/H in methane in short period comets, such as CG, should be less than that of Oort Cloud comets with a lower limit of the protosolar D/H.
Acknowledgments
Dr. Mandt acknowledges funding of this work through JPL Subcontract 1345493 and NASA grant NNX13AQ99G. The authors thank Christopher Glein for a very helpful review.
REFERENCES
- Alexander CMO, Bowden R, & Fogel ML 2012, Sci, 337, 721. [DOI] [PubMed] [Google Scholar]
- Atreya SK, Donahue TM, & Kuhn WR 1978, Sci, 201, 611. [DOI] [PubMed] [Google Scholar]
- Atreya SK, Lorenz R, & Waite JH 2009, in Titan from Cassini-Huygens, ed. Brown RH, Lebreton J-P, & Waite JH (Dordrecht: Springer; ), 177 [Google Scholar]
- Berezhnoi AA 2010, SoSyR, 44, 498 [Google Scholar]
- Bockelée-Morvan D, Biver N, Jehin E, et al. 2008, ApJL, 679, L49 [Google Scholar]
- Charnley SB, & Rodgers SD 2002, ApJL, 569, L133 [Google Scholar]
- Clayton RN 2008, in Chemical Evolution Across Space & Time ACS Symp. Ser, Vol. 981, ed. Zaikowski L & Friedrich JM (Washington, D.C.: ACS Publications; ), 141 [Google Scholar]
- De La Haye V, Waite JH, Johnson RE, et al. 2007, JGR, 112, A07309 [Google Scholar]
- Geiss J, & Gloeckler G 1998, SSRv, 84, 239 [Google Scholar]
- Glein CR, Desch S, & Shock E 2009, Icar, 204, 637 [Google Scholar]
- Hily-Blant P, Bonal L, Faure A, & Quirico E 2013, Icar, 223, 582 [Google Scholar]
- Hoffman JH, Hodges RR, McElroy MB, Donahue TM, & Kolpin M 1979, Sci, 205, 49. [DOI] [PubMed] [Google Scholar]
- Jacovi R, & Bar-Nun A 2008, Icar, 196, 302 [Google Scholar]
- Liang M-C, Heays AN, Lewis BR, Gibson ST, & Yung YL 2007, ApJL, 664, L115 [Google Scholar]
- Lunine JI, Yung YL, & Lorenz RD 1999, P&SS, 47, 1291. [DOI] [PubMed] [Google Scholar]
- Mandt KE, Waite JH, Lewis W, et al. 2009, P&SS, 57, 1917 [Google Scholar]
- Mandt KE, Waite JH, Teolis B, et al. 2012, ApJ, 749, 160 [Google Scholar]
- Marty B, & Zimmermann L 1999, GeCoA, 63, 3619 [Google Scholar]
- Marty B, Zimmermann L, Burnard PG, et al. 2010, GeCoA, 74, 340 [Google Scholar]
- Mathew KJ, & Marti K 2001, JGR, 106, 1401 [Google Scholar]
- McKay CP, Scattergood TW, Pollack JB, Borucki WJ, & Van Ghyseghem HT 1988, Natur, 332, 520. [DOI] [PubMed] [Google Scholar]
- McKeegan KD, Aléon J, & Bradley J 2006, Sci, 314, 1724. [DOI] [PubMed] [Google Scholar]
- Mousis O, Lunine JI, Pasek M, et al. 2009a, Icar, 204, 749 [Google Scholar]
- Mousis O, Lunine JI, & Waite JH 2009b, ApJL, 701, L39 [Google Scholar]
- Niemann HB, Atreya SK, Demick JE, et al. 2010, JGR, 115, E12006 [Google Scholar]
- Nixon CA, Temelso B, Vinatier S, et al. 2012, ApJ, 749, 159 [Google Scholar]
- Owen TC, & Bar-Nun A 1995, Icar, 116, 215. [DOI] [PubMed] [Google Scholar]
- Owen TC, Mahaffy PR, Niemann HB, Atreya SK, & Wong MH 2001, ApJL, 553, L77 [Google Scholar]
- Penz T, Lammer H, Kulikov YN, & Biernat HK 2005, AdSpR, 36, 241 [Google Scholar]
- Pepin RO 2006, E&PSL, 252, 1 [Google Scholar]
- Prinn RG, & Fegley B 1981, ApJ, 249, 308 [Google Scholar]
- Rodgers SD, & Charnley SB 2008, MNRAS Lett, 385, L48 [Google Scholar]
- Rousselot P, Pirali O, Jehin E, et al. 2014, ApJL, 780, L17 [Google Scholar]
- Shinnaka Y, Kawakita H, Kobayashi H, Nagashima M, & Boice DC 2014, ApJ, 782, L16 [Google Scholar]
- Volkov AN, Johnson RE, Tucker OJ, & Erwin JT 2011, ApJL, 729, L24 [Google Scholar]
- Waite JH Jr., Lewis WS, Magee BA, et al. 2009, Natur, 460, 487 [Google Scholar]
- Wong MH, Atreya SK, Mahaffy PN, et al. 2013, GRL, 40, 1 [Google Scholar]


