Abstract
Background:
Body dysmorphic disorder (BDD) is a psychiatric disorder with specific impairments in social cognition related to excessive concerns about one’s appearance. Individuals with BDD have difficulty identifying emotional expressions and attribute internal factors for others’ emotional expressions in self-referent (but not other-referent) scenarios. Given the role of oxytocin in regulating social approach behavior and social salience, we hypothesized that oxytocin would improve biases in emotion recognition, attributions, and threat interpretations in individuals with BDD, compared to healthy controls (HCs). This is the first study to examine the effects of oxytocin in people with BDD.
Methods:
Eighteen participants with BDD and 16 HCs received a single dose of 24 international units of intranasal oxytocin (Syntocinon®) or matching placebo in a randomized, placebo-controlled, within-subject crossover design. Participants completed the Emotion Recognition Task and Interpretation Questionnaire 45 min after administration.
Results:
Oxytocin, relative to placebo, did not improve emotion recognition accuracy in either self-referent or other-referent contexts for individuals with BDD. However, oxytocin led to greater internal attributions in other-referent contexts for those with BDD compared to HCs. Rather than reducing self-blame, oxytocin led to other-directed blame. Oxytocin did not impact threat interpretations in BDD.
Conclusions:
Oxytocin may only impact specific aspects of higher order social cognition in BDD and may have unwanted effects on emotion attributions. Our results caution its clinical use in BDD.
Keywords: body dysmorphic disorder, emotion recognition, other, oxytocin, self, social cognition
1 |. INTRODUCTION
Individuals with body dysmorphic disorder (BDD) suffer from excessive concerns about perceived flaws in their appearance (American Psychiatric Association, 2013). One of the hallmark features of BDD is social avoidance, which results directly from concerns that others are negatively evaluating their appearance. Experimental research has shown that BDD is associated with specific problems in social cognition. Individuals with BDD are less accurate in identifying facial expressions conveying a neutral or disgust expression (Buhlmann, McNally, Etcoff, Tuschen-Caffier, & Wilhelm, 2004), misidentify neutral expressions as conveying anger or contempt (Buhlmann, Etcoff, & Wilhelm, 2006), and attribute others’ emotional expressions more often to themselves (internal attributions) than to the situation (external attributions) (Buhlmann et al., 2006). People with BDD are less accurate in identifying others’ thoughts and intentions in a video-based test for mind reading difficulties (Buhlmann, Wacker, & Dziobek, 2015). These biases in social cognition extend to interpretive biases for threat in social scenarios (Buhlmann et al., 2002; Clerkin & Teachman, 2008). However, BDD is also associated with intact abilities, such as decoding complex mental states of others from the eye region based on the Reading the Mind in the Eyes Task (Buhlmann, Winter, & Kathmann, 2013). Social cognitive biases in BDD represent an important maintaining factor and treatment target in cognitive behavioral models (Fang & Wilhelm, 2015). Given the limits of the best available pharmacologic and psychological treatments for BDD (Buhlmann, 2011), novel treatment strategies targeting social cognitive factors are needed.
Oxytocin is a promising therapeutic agent, as it plays a major role in mediating socioemotional processing in animals and humans (Liu et al., 2015; MacDonald & Feifel, 2013). Oxytocin is a neuropeptide of nine amino acids that has dual modes of action in the peripheral and central nervous systems, and its receptors are found across diverse brain regions, such as the cortex, limbic areas, basal ganglia, and brainstem (Meyer-Lindenberg, Domes, Kirsch, & Heinrichs, 2011). Oxytocin can also be released dendritically to reach brain areas involved in higher order cognitive processes (Meyer-Lindenberg et al., 2011). As individuals with BDD suffer from emotion recognition deficits, interpretive biases, poor insight, and even delusional beliefs (Fang & Wilhelm, 2015), oxytocin has obvious implications for improving these social cognitive problems in BDD. However, no studies have yet investigated the effects of oxytocin in this population.
Intranasal oxytocin has been shown to improve emotion recognition across many psychiatric populations, from youth with autism (Guastella et al., 2010) to adults with schizophrenia (Averbeck, Bobin, Evans, & Shergill, 2012), however, findings have been inconsistent, possibly due to heterogeneity in participant characteristics (Leppanen, Ng, Tchanturia, & Treasure, 2017) and administration protocols (Guastella et al., 2013). Oxytocin may improve emotion recognition in clinical populations by enhancing eye gaze (Andari et al., 2010; Auyeung et al., 2015; Guastella, Mitchell, & Dadds, 2008). Another possibility is that oxytocin modulates attention toward valence-specific social cues, such as increasing attention toward happy facial expressions (Domes, Steiner, Porges, & Heinrichs, 2013) and shifting attention away from angry and disgust expressions (Domes et al., 2013; Kim et al., 2014; Kim, Eom, Leppanen, Leslie, & Treasure, 2018). Given oxytocin’s effects on socioemotional processes in clinical populations, the goal of this study was to investigate its effects on social cognitive biases in BDD. Our primary hypothesis was that oxytocin would improve emotion recognition accuracy, internal self-blame attributions, and threat interpretations in participants with BDD compared to healthy controls (HCs).
2 |. MATERIALS AND METHODS
2.1 |. Participants
Participants with BDD according to DSM-IV criteria and HC participants were enrolled through advertisements in the institutional online research recruitment tool and local community. BDD participants were additionally enrolled through a specialty BDD outpatient program. Diagnoses of BDD were confirmed using the Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 2002). The Yale-Brown Obsessive-Compulsive Scale modified for BDD (BDD-YBOCS; Phillips et al., 1997) and Brown Assessment of Beliefs Scale (BABS; Eisen et al., 1998) were administered by a clinically experienced psychologist or doctoral candidate to assess BDD severity and insight, respectively. As (1) estrogen is known to modulate endogenous oxytocin levels, (2) oxytocin levels may differ according to menstrual phase (Salonia et al., 2005), and (3) we were interested in within-subject effects of oxytocin; we included females only if they were taking low-dose oral contraception, that is,<50 mcg daily dose of ethinyl estradiol. Eligible women attended drug administration visits during the active phase of their contraceptive medication (e.g., not during placebo week). Women using multiphasic contraceptives (n = 4, 31% of females) were on the same dose of ethinyl estradiol for drug administration visits. Exclusion criteria for all participants were as follows: history of nasal pathology (recurrent nosebleeds, hypophysectomy, atrophic rhinitis), smoking ≥15 cigarettes per day, serious medical illnesses (e.g., cardiovascular disease and seizures) or endocrine diseases (e.g., untreated thyroid disease and diabetes), taking systemic steroids or hormones except oral contraception, pregnancy, active suicidal or homicidal ideation, and meeting diagnostic criteria for schizophrenia, psychotic disorder, bipolar disorder, or substance abuse/dependence. HC participants did not have any current psychiatric illness. The study protocol was approved by the Partners Human Research Committee and registered through Clinicaltrials.gov (NCT02671266)1. All participants provided written informed consent prior to study procedures.
2.2 |. Procedure
The study consisted of three visits. At visit one, participants were screened for diagnostic and medical eligibility. A study nurse at the Translational Clinical Research Center conducted a history and physical examination to assess medical exclusion criteria. Urine pregnancy tests were given to females at each visit to confirm absence of pregnancy and ensure continued eligibility. Prior to drug administration visits, eligible participants were instructed to avoid alcohol, caffeine, and nicotine for 24 h.
In a randomized, double-blind, placebo-controlled, within-subject, crossover design, two experimental administration visits were conducted, separated by a minimum 1-week washout interval (maximum 28-day interval). Visits began with a study nurse gathering vital signs. Oxytocin (24 IU) and placebo were self-administered in metered dose spray bottles by the participant. Using a standardized protocol, participants inhaled three sprays per nostril (4 IU oxytocin per spray), which represents the most common dose in single-session experimental studies using intranasal oxytocin in humans (Shahrestani, Kemp, & Guastella, 2013). The nasal sprays (Syntocinon® nasal spray; Novartis, Basel, Switzerland) and matched placebo sprays (containing the same ingredients minus the active peptide) were purchased from a pharmacy in Switzerland under an Investigational New Drug (IND#124112) application from the Food and Drug Administration. Approximately 20 min after administration, the nurse assessed adverse events using a symptom checklist, and 45 min after administration, participants began the experimental tasks. Those who endorsed adverse events were monitored to ensure symptoms resolved before the visit ended. After completing the experiment, participants reported their drug expectancies (belief in receiving the active drug, degree of certainty, and reasons why). The MGH Research Pharmacy randomized the order of nasal sprays. All study staff, as well as participants themselves, were blind to drug condition.
2.3 |. Emotion recognition task
The Emotion Recognition Task (ERT; Buhlmann et al., 2006) is a questionnaire designed to assess emotion recognition accuracy and attributions. Participants viewed 24 facial photographs (Ekman & Friesen, 1975), each expressing a different emotion (anger, disgust, neutral, or surprise) with each emotion presented six times. The photographs were drawn from the new facial affect collection (http://www.paulekman.com/). Each photograph was presented within a self-referent scenario (“Imagine this bank teller is looking in your direction”) or other-referent scenario (“Imagine this bank teller is looking in your friend’s direction”). For each scenario, participants were instructed to identify the emotion (with eight possible response options: neutral, anger, contempt, happiness, surprise, sadness, fear, or disgust) and rate whether the person’s emotional expression was caused by the participant or their friend (internal attribution) or by the situation or other reasons (external attribution). Total scores for emotion recognition accuracy ranged from 0 to 24 per condition (self- and other-referent) and 2 to 48 per condition for attribution scores, with higher scores reflecting greater external attributions.
2.4 |. Interpretation questionnaire
The Interpretation Questionnaire (IQ; Buhlmann et al., 2002) is a questionnaire that assesses interpretive biases for threat in BDD in 33 ambiguous scenarios (11 BDD related, 11 social related, and 11 general scenarios). Each scenario included a short description (“Two people are whispering and laughing behind you. You cannot hear them.”) followed by three possible thoughts: “They are making fun of how I look” (threat interpretation), “They must be in a good mood” (non-threat interpretation), and “They are just having a conversation” (non-threat interpretation). Participants were asked to rate the likelihood of each thought coming to mind on a scale from 0 (very unlikely) to 4 (very likely). Ratings for threat-specific interpretations were summed across scenarios making up each subscale to generate three subscales: BDD threat, social threat, and general threat.
2.5 |. Analytic approach
Primary outcome variables from the ERT and IQ were analyzed by linear mixed models using maximum likelihood estimation. Group (BDD vs. HC), Drug (oxytocin vs. placebo), and Group by Drug were entered as fixed effects, while Visit was a repeated random effect, which accounted for correlated errors between subjects. Primary hypotheses of interest were tested across seven outcomes: ERT accuracy self, ERT accuracy other, ERT attribution self, ERT attribution other, IQ BDD threat, IQ social threat, IQ general threat. Sex and Order were entered as fixed-effect covariates. Models using different variance–covariance structures were evaluated based on −2 Log Likelihood, Akaike’s Information Criterion (AIC), and Schwarz’s Bayesian Criterion (BIC). Results are reported based on a compound symmetry variance–covariance structure, as it was the simplest model and yielded similar fixed and covariance parameter estimates. We followed-up interaction effects with post hoc single comparisons based on the estimated marginal means. To examine whether participants could correctly guess which days they received oxytocin, we conducted chi-square tests to assess whether participant expectancies of receiving the active drug varied by visit. We also conducted chi-square tests to assess whether frequency of adverse events varied as a function of order for each drug administration visit. All analyses were conducted in SPSS 24.
3 |. RESULTS
3.1 |. Participants
Our final sample included 34 participants who completed at least one drug administration session. Out of all 34 participants, only one HC participant (who was randomized to receive oxytocin first) did not attend the second drug administration session. Excluding this participant from the analyses did not change the overall results of the study. A detailed CONSORT flow diagram of participant enrollment is described in Supporting Information. There were 18 participants with BDD (mean age ± SD: 25.4 ± 4.2 years) and 16 HC participants (mean age ± SD: 27.8 ± 10.5 years). Full descriptive statistics of our sample are reported in Table 1. There was one HC participant who endorsed a history of psychiatric illness (single, 2-week major depressive episode) that had remitted by study entry. No other HC participants endorsed a psychiatric history. Although DSM-IV diagnostic criteria were used during the SCID, all enrolled BDD participants reported multiple past-week repetitive behaviors during the BDD-YBOCS interview (mean number of repetitive behaviors = 6.33, range = 4–10). Thus, all BDD participants would have met DSM-5 criteria for BDD. Four BDD participants (22%) were taking the following psychotropic medications: well-butrin and gabapentin (n = 1), adderall (n = 1), lexapro (n = 1), and trazodone (n = 1). There were no group differences on demographic variables (Table 1).
TABLE 1.
Demographic and clinical data
| Demographic variable | BDD (n = 18) | HC (n = 16) | t/X2 | P |
|---|---|---|---|---|
| Age (in years, M (SD)) | 25.44(4.18) | 27.75(10.52) | -0.86 | 0.40 |
| Sex (n, %) | 0.01 | 0.93 | ||
| Female | 7 (38.89) | 6 (37.50) | ||
| Male | 11(61.11) | 10 (62.50) | ||
| Race(n,%) | 2.55 | 0.64 | ||
| Caucasian | 12 (66.66) | 10 (62.50) | ||
| African American | 0(0) | 1 (6.25) | ||
| Asian | 4 (22.22) | 3 (18.75) | ||
| American Indian/Alaskan Native | 1 (5.56) | 0(0) | ||
| More than one race | 1 (5.56) | 2 (12.50) | ||
| Ethnicity (n, %) | 0.25 | 0.62 | ||
| Hispanic | 2(11.11) | 1 (6.25) | ||
| Non-Hispanic | 16 (88.89) | 15 (93.75) | ||
| Education (n,%) | 0.81 | 0.85 | ||
| Some college | 5 (27.78) | 4(25.00) | ||
| College graduate | 6 (33.33) | 6 (37.50) | ||
| Some postgraduate/professional | 4(22.22) | 2 (12.50) | ||
| Postgraduate/professional | 3(16.67) | 4 (25.00) | ||
| Marital status (n,%) | 2.93 | 0.23 | ||
| Single/never married | 16 (88.89) | 15 (93.50) | ||
| Married | 0(0) | 1 (6.25) | ||
| Living with partner | 2(11.11) | 0(0) | ||
| Clinical variable | ||||
| BDD-YBOCS total score | 25.00 (4.28) | |||
| BABS total score | 12.56 (4.09) | |||
| BDDageof onset (in years) | 13.88 (3.67) | |||
| Any psychotropic medications | 4(22.22) | |||
| Any comorbid disorder | 12 (66.66) | |||
| Social anxiety disorder | 6 (33.33) | |||
| Eating disorder NOS | 4(22.22) | |||
| Major depressive disorder | 2(11.11) | |||
| Dysthymia | 2(11.11) | |||
| Skinpicking disorder | 2(11.11) | |||
| Hypochondriasis | 2(11.11) | |||
| Panic disorder | 1 (5.56) | |||
| Generalized anxiety disorder | 1 (5.56) | |||
| ADHD | 1 (5.56) |
Note. BDD-YBOCS, Yale–Brown Obsessive–Compulsive Scale Modified for BDD; BABS, Brown Assessment of Beliefs Scale; ADHD, attention-deficit hyperactivity disorder.
3.2 |. Emotion recognition accuracy
As demonstrated previously (Buhlmann et al., 2006), in the placebo condition, individuals with BDD performed slightly worse, compared to HC, on emotion recognition accuracy in self-referent contexts, but not in other-referent contexts; however, these differences were not statistically significant (P’s > 0.408) (see Table 2). The mixed model revealed a significant Group by Drug interaction in self-referent accuracy scores, (F(1,33.698) = 7.282, P = 0.011), no effect of Group (F(1,34.364) = 0.059, P = 0.809), and no effect of Drug (F(1,33.698) = 0.001, P = 0.977). Post hoc comparisons revealed no significant differences between BDD and HC within each drug condition, as well as no significant differences between drug conditions within each patient group. In other-referent contexts, there was no Group by Drug interaction (F(1,34.174) = 3.087, P = 0.088), no effect of Group (F(1,34.308) = 3.633, P = 0.065, and no effect of Drug (F(1,34.174) = 0.356, P = 0.554).
TABLE 2.
Raw means and standard deviations of all outcome measures by drug
| Variable | BDD | HC | ||||
|---|---|---|---|---|---|---|
| Accuracy: Self-referent | PBO | OT | Effect size* | PBO | OT | Effect size* |
| Total | 17.61 (3.26) | 18.78 (2.76) | 0.44 | 18.53(3.00) | 17.38 (2.73) | 0.46 |
| Neutral | 3.83 (1.65) | 4.22 (1.67) | 0.29 | 4.67(1.76) | 4.44 (1.26) | 0.21 |
| Surprise | 5.00 (1.19) | 5.17(0.71) | 0.14 | 5.33(1.05) | 5.06 (1.06) | 0.22 |
| Disgust | 4.56(1.54) | 4.72 (1.53)a | 0.12 | 4.07(1.71)b | 3.63 (1.50)a,b | 0.44 |
| Angry | 4.22 (1.70) | 4.67(1.19) | 0.29 | 4.47 (1.36) | 4.25 (1.34) | 0.19 |
| Accuracy: Other-referent | ||||||
| Total | 18.33 (2.57) | 19.17 (2.55)a | 0.28 | 18.07 (2.46) | 16.44 (4.84)a | 0.31 |
| Neutral | 4.39 (2.00) | 4.78 (1.22) | 0.23 | 5.00 (0.93) | 4.00 (1.59) | 0.54 |
| Surprise | 5.06 (0.87) | 5.17(0.79) | 0.16 | 5.20(1.01) | 4.56 (1.63) | 0.37 |
| Disgust | 4.39(1.38) | 4.67(1.19) | 0.20 | 3.60(1.68) | 3.50 (2.13) | 0.06 |
| Angry | 4.50(1.15) | 4.56 (1.38) | 0.05 | 4.27(1.58) | 4.38 (1.75) | 0.06 |
| Attribution: Self-referent | 38.08 (6.06) | 36.15 (4.08)c | 0.42 | 42.46 (4.72) | 42.00 (4.92)c | 0.14 |
| Attribution: Other-referent | 39.73 (5.48)d | 37.20 (5.82)a,d | 0.84 | 40.96 (6.28) | 42.29 (4.97)a | 0.34 |
| Interpretive bias: BDD threat | 24.88 (9.80)c | 25.81 (10.94)c | 0.15 | 7.80 (4.86)c | 9.20 (7.71)c | 0.28 |
| Interpretive bias: SOC threat | 28.31 (7.52)c | 30.25 (11.88)c | 0.21 | 14.27 (6.66)c | 13.41 (7.75)c | 0.16 |
| Interpretive bias: GEN threat | 21.63 (7.92)a | 21.63 (7.32)a | 0.00 | 16.33 (7.39)a | 16.67 (7.56)a | 0.10 |
Note. All pairwise comparisons P’s > .05.
Between group comparisons P < 0.05.
Pairwise comparisons P < 0.05.
Between group comparisons P < 0.01.
Pairwise comparisons P < 0.01.
Effect sizes are based on Cohen’s d for repeated measures and reflect the strength of changes between OT and PBO drug administration sessions (Lakens, 2013).
3.3 |. Attribution
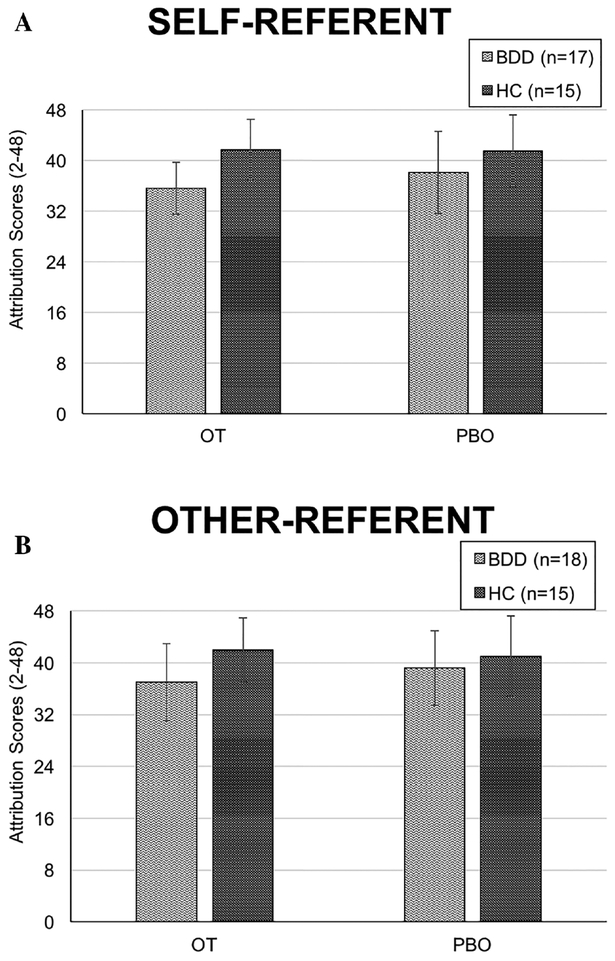
Consistent with previous work (Buhlmann et al., 2006), in the placebo condition, individuals with BDD, compared to HC, displayed slightly worse (more internal) attributions in self-referent versus other-referent contexts; however, these differences were not statistically significant (P’s > 0.05) (see Table 2). The mixed model revealed no Group by Drug interaction (F(1,25.667) = 1.390, P = 0.249), and no effect of Drug (F(1,25.670) = 2.273, P = 0.144) in the self-referent condition (“Why is this person looking in your direction?”). However, there was an effect of Group (F(1,30.110) = 6.400, P = 0.017), as participants with BDD across both drug conditions made more internal attributions than HC. In the other-referent condition (“Why is this person looking in your friend’s direction?”), there was a Group by Drug interaction (F(1,29.388) = 8.984, P = 0.005), but no effect of Group (F(1,32.743) = 3.593, P = 0.067) and no effect of Drug (F(1,29.419) = 1.018, P = 0.321) (see Figure 1). Post hoc comparisons showed that oxytocin led BDD participants to make more internal attributions, compared to HC (F(1,39.332) = 7.709, P = 0.008), but there was no group difference in the placebo condition (F(1,40.908) = 0.681, P = 0.414). Among BDD participants, oxytocin led to more internal attributions, compared to placebo (F(1,29.711) = 8.360, P = 0.007), whereas there was no drug effect within HC participants (F(1,29.123) = 1.902, P = 0.178).
FIGURE 1.
Significant group by drug interaction on emotion attributions. (a) Oxytocin, relative to placebo, did not modulate attributions in BDD versus HC participants in self-referent contexts.(b) Oxytocin led to greater internal attributions in BDD versus HC participants. Post hoc comparisons showed a significant difference between BDD and HC in the oxytocin condition, P = 0.008, 95% CI:[1.441, 9.167], but not in the placebo condition. Within the BDD group, oxytocin also had a significant effect on worsening attributions, relative to placebo, P = 0.007, 95% CI: [−4.229, −.727], and no drug effect within the HC group. Error bars represent ± 1 SD
Given the effect of oxytocin on attributions in the other-referent condition, we examined whether delusionality explained the direction of the effect among those with BDD. With BABS scores added to the mixed model, there was a Drug by Delusionality interaction (F(8,15.250) = 4.627, P = 0.005) and an effect of Drug (F(1,15.245) = 26.310, P = 0.000). Plotting the relationship between drug and delusionality at each level of delusionality revealed no effect of drug at lower levels of delusionality (good and fair insight) (pairwise comparisons, P’s > 0.05). However, at higher levels of delusionality (≥14) reflecting poor and delusional insight, those who received oxytocin reported even greater internal attributions compared to those receiving placebo (pairwise comparisons, P’s ≤ 0.002).
3.4 |. Interpretive bias
Consistent with prior research (Buhlmann et al., 2002), in the placebo condition, those with BDD, compared to HC, displayed greater threat interpretations in BDD threat and social threat scenarios, although those with BDD made more threat interpretations even in the general threat condition (all P’s < 0.05) (see Table 2). The mixed model revealed no Group by Drug interaction (F(1,29.053) = 0.307, P = 0.584) in BDD threat scenarios, and no effect of Drug (F(1,28.925) = 1.674, P = 0.206). However, there was an effect of Group (F(1,32.226) = 35.028, P = 0.000), as individuals with BDD made more threat interpretations in BDD threat scenarios, compared to HC. There was an identical pattern of results for social threat and general threat scenarios, as people with BDD made more threat interpretations overall than HC.
Across all of the mixed models, there was no evidence of carryover effects or sex differences, as indicated by nonsignificant Order and Sex effects, respectively (P’s > 0.05), with one exception. There was a significant effect of Sex in other-referent contexts for Emotion Recognition Accuracy (F(1,34.674) = 5.298, P = 0.027), as males overall performed worse than their female counterparts.
3.5 |. Assessment of blind and adverse events
Participants could not distinguish between placebo and oxytocin, as their beliefs about receiving oxytocin (X2 = 0.130, P = 0.805) and adverse events (P’s > 0.05) were reported as frequently on both drug administration visits. All adverse events were mild (78%) or moderate (22%) in severity. The most common adverse events reported across both drug administrations were as follows: feeling drowsy or sleepy (33%), feeling of calmness or euphoria (17%), difficulty sitting still (17%), and headache (15%).
3.6 |. Medications and comorbidities
We repeated the above analyses while controlling for medication use, as well as having a comorbid disorder, which revealed a similar pattern of results. However, for BDD threat interpretive biases, medication status was significantly associated with reduced threat scores (F(1,30.017) = 7.145, P = 0.012).
4 |. DISCUSSION
This is the first study testing the effects of acute exogenous administration of oxytocin in BDD. Importantly, results indicated that oxytocin, compared to placebo, did not improve emotion recognition accuracy in either self- or other-referent contexts for individuals with BDD. However, we found that BDD participants when given oxytocin, relative to placebo, made greater internal attributions in other-referent, but not self-referent contexts, compared to HC. In other words, oxytocin had the unexpected effect of causing greater other-directed blame, and this effect was related to the degree of delusionality.
Three competing hypotheses have been proposed to explain oxytocin’s effects. The prosocial hypothesis predicts that oxytocin promotes positive prosocial behaviors (Kosfeld, Heinrichs, Zak, Fischbacher, & Fehr, 2005); the social salience hypothesis proposes that oxytocin’s effects depend on the salience of the social context (Shamay-Tsoory & Abu-Akel, 2016); and the social approach/withdrawal hypothesis proposes that oxytocin increases social approach emotions and behaviors, such as empathy, and inhibits social withdrawal emotions, such as anxiety (Kemp & Guastella, 2011). Our emotion recognition accuracy finding is inconsistent with these models, all of which predict that oxytocin would improve emotion recognition accuracy. However, our result may be due an underpowered sample size and lack of significant difference within the placebo condition. Compared to the original Buhlmann et al.’s (2006) study, which found group differences in emotion recognition in the self-referent condition, our sample was younger and included more males. Indeed, oxytocin’s effects on emotion recognition may be small at best, as demonstrated by a recent meta-analysis that found a negligible overall effect size of oxytocin on recognition of basic emotions in HC (Leppanen et al., 2017). A study in a related disorder, anorexia nervosa, also showed no effect of oxytocin on emotion recognition (Kim et al., 2015).
Regarding our attribution result, we found that oxytocin worsens attributions in the BDD group in the other-referent condition, which is inconsistent with the prosocial theory and social salience account. The prosocial theory predicts that oxytocin would improve attributions. The social salience account predicts that oxytocin would modulate attributions based on the salience of social behaviors. If social salience were the only mechanism playing a role, we would expect oxytocin to have a similar effect on emotion recognition and attributions within the same context (whether self- or other-referent), which we did not observe. The social approach/withdrawal account best explains oxytocin’s effect on attributions. Oxytocin may have promoted greater empathy (an approach emotion) in BDD participants, which facilitated perspective taking on behalf of a friend in other-referent contexts. However, oxytocin did not correct the attributional bias (possibly linked to delusional beliefs), as BDD participants given oxytocin tended to blame the friend, rather than the situation, for others’ emotional expressions (in the same way our data showed they blamed themselves in self-referent contexts regardless of the drug they received). This is consistent with our finding that oxytocin did not correct threat interpretations in BDD, which may indicate that oxytocin cannot correct strong biases in higher order attributions. The explanation that oxytocin facilitated empathy in BDD participants when making attributions is consistent with a study showing that oxytocin improved emotional empathy in response to both positive and negative stimuli (Hurlemann et al., 2010). Interestingly, our attribution result contrasts with studies demonstrating that intranasal oxytocin reduces positive symptoms in schizophrenia; however, these studies included patients on stable antipsychotic regimes (for a review, see Feifel, Shilling, & MacDonald, 2016).
It is possible that the effect of oxytocin on other-referent attributions is mediated by its effect on self- or other-referential processing. Our results align with one study, which demonstrated that oxytocin helped discriminate between own and other faces in a face morphing paradigm in healthy men (Colonnello, Chen, Panksepp, & Heinrichs, 2013). This contrasts with another study showing that oxytocin weakened the self-reference effect (Zhao et al., 2016). Indeed, self-processing may extend to close others, giving rise to the possibility that oxytocin’s effects on self-processing could actually impact the perception of others as well (Zhao et al., 2017). Further research is needed to closely control for “other” categories, and reconcile differences in design and methodology.
Clinically, individuals with BDD are suffering due to perceived flaws in their appearance, and maladaptive beliefs that others are negatively evaluating their appearance. Our results join a body of research suggesting that oxytocin may impair social cognitive maintaining factors of psychopathology in certain clinical populations (Bartz et al., 2011; Tabak et al., 2016). We found that oxytocin may worsen attributions of others’ emotional expressions, which is particularly troubling for exposure exercises during cognitive behavioral therapy (the first line psychological intervention for BDD). Oxytocin may inadvertently exacerbate maladaptive beliefs about others’ negative intentions and worsen thinking patterns characteristic of personalization and delusions of reference, which are highly predominant in this population (Phillips, 2004).
There are some limitations to the present study. Our sample size was relatively small, with very small cell sizes comparing men and women, and replication is needed. This has been noted as a problem in oxytocin research, as individual studies are often underpowered, which may result in diverse and contradictory findings (Walum, Waldman, & Young, 2016). Despite our small sample size, our within-subject design enhanced statistical power and our findings correspond to large effect sizes. Our study also involved a heterogeneous and relatively young group of individuals with BDD with various comorbidities. A minority of our BDD participants were also taking psychotropic medications. While this enhances generalizability, future research is needed to determine if the effects of oxytocin on emotion attributions are specific to BDD.
5 |. CONCLUSIONS
Consistent with the social approach/withdrawal hypothesis of oxytocin, our study showed that rather than reducing self-blame, oxytocin increased other-directed blame for eliciting emotional expressions in others for people with BDD. We advise against the use of oxytocin in BDD patients and potentially other delusional populations in clinical contexts, which deserves further study.
ACKNOWLEDGMENTS
This work was supported by an International OCD Foundation Award, National Institute of Mental Health Career Development Award to A.F. (K23 109593–02) and the Neil and Anna Rasmussen Foundation. This work was also supported by the protocol nurses and administrative staff at the MGH Translational Clinical Research Center (TCRC) under grant number 1UL1TR001102. The authors would like to thank the following individuals for their assistance in conducting the study protocol: Dylan Abrams, Aishvarya Arora, Carolyn Davies, Eliza Davidson, Ryan Jacoby, Eric Jenike, Angelina Gomez, Helen Mizrach, Alexandra Shapiro, Alexandra Sullivan, and Charlie Suchin, as well as Samantha Culwell and Cheryl Reilly-Tremblay for their assistance preparing randomization and the study drug at the MGH Research Pharmacy. Finally, thanks to Susanne Hoeppner for statistical consultation.
Funding information
International OCD Foundation, Grant/Award Number: Effect of Intranasal Oxytocin on Social Cognition; National Institute of Mental Health, Grant/Award Number: K23 MH109593–02
CONFLICTS OF INTEREST
A.F. has no financial disclosures to report. E.A.L. is on the scientific advisory board and has a financial interest in OXT Therapeutics, Inc., a company developing an intranasal oxytocin and long-acting analogues of oxytocin to treat obesity and metabolic disease. E.A.L.’s interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflicts of interest policy. S.W. has received research support in the form of free medication and matching placebo from Forest Laboratories for clinical trials funded by the NIH. Dr. Wilhelm is a presenter for the Massachusetts General Hospital Psychiatry Academy in educational programs supported through independent medical education grants from pharmaceutical companies; she has received royalties from Elsevier Publications, Guilford Publications, New Harbinger Publications, and Oxford University Press. Dr. Wilhelm has also received speaking honorarium from various academic institutions and foundations, including the International Obsessive Compulsive Disorder Foundation and the Tourette Association of America. In addition, she received payment from the Association for Behavioral and Cognitive Therapies for her role as Associate Editor for the Behavior Therapy journal, as well as from John Wiley & Sons, Inc. for her role as Associate Editor on the journal Depression & Anxiety. Dr. Wilhelm has also received salary support from Novartis and Telefonica Alpha, Inc.
ENDNOTE
1 Our clinical trials registration reflects our original intention to recruit an obsessive–compulsive disorder clinical comparison group, which was later dropped from the study due to recruitment difficulties.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author. [Google Scholar]
- Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, & Sirigu A (2010). Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proceedings of the National Academy of Sciences of the USA, 107(9), 4389–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auyeung B, Lombardo MV, Heinrichs M, Chakrabarti B, Sule A, Deakin JB, … Baron-Cohen S (2015). Oxytocin increases eye contact during a real-time, naturalistic social interaction in males with and without autism. Translational Psychiatry, 5, e507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbeck BB, Bobin T, Evans S, & Shergill SS (2012). Emotion recognition and oxytocin in patients with schizophrenia. Psychological Medicine, 42(2), 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz J, Simeon D, Hamilton H, Kim S, Crystal S, Braun A, … Hollander E (2011). Oxytocin can hinder trust and cooperation in borderline personality disorder. Social Cognitive and Affective Neuroscience, 6(5), 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhlmann U (2011). Treatment barriers for individuals with body dysmorphic disorder: An internet survey. Journal of Nervous and Mental Disease, 199, 268–271. [DOI] [PubMed] [Google Scholar]
- Buhlmann U, Etcoff NL, & Wilhelm S (2006). Emotion recognition bias for contempt and anger in body dysmorphic disorder. Journal of Psychiatric Research, 40(2), 105–111. [DOI] [PubMed] [Google Scholar]
- Buhlmann U, McNally RJ, Etcoff NL, Tuschen-Caffier B, & Wilhelm S (2004). Emotion recognition deficits in body dysmorphic disorder. Journal of Psychiatric Research, 38(2), 201–206. [DOI] [PubMed] [Google Scholar]
- Buhlmann U, Wacker R, & Dziobek I (2015). Inferring other people’s states of mind: Comparison across social anxiety, body dysmorphic, and obsessive-compulsive disorders. Journal of Anxiety Disorders, 34, 107–113. [DOI] [PubMed] [Google Scholar]
- Buhlmann U, Wilhelm S, McNally RJ, Tuschen-Caffier B, Baer L, & Jenike MA (2002). Interpretive biases for ambiguous information in body dysmorphic disorder. CNS Spectrums, 7(6), 435–443. [DOI] [PubMed] [Google Scholar]
- Buhlmann U, Winter A, & Kathmann N (2013). Emotion recognition in body dysmorphic disorder: Application of the reading the mind in the eyes task. Body Image, 10(2), 247–250. [DOI] [PubMed] [Google Scholar]
- Clerkin EM, & Teachman BA (2008). Perceptual and cognitive biases in individuals with body dysmorphic disorder symptoms. Cognition and Emotion, 22(7), 1327–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnello V, Chen FS, Panksepp J, & Heinrichs M (2013). Oxytocin sharpens self-other perceptual boundary. Psychoneuroendocrinology, 38(12), 2996–3002. [DOI] [PubMed] [Google Scholar]
- Domes G, Steiner A, Porges SW, & Heinrichs M (2013). Oxytocin differentially modulates eye gaze to naturalistic social signals of happiness and anger. Psychoneuroendocrinology, 38(7), 1198–1202. [DOI] [PubMed] [Google Scholar]
- Eisen JL, Phillips KA, Baer L, Beer DA, Atala KD, & Rasmussen SA (1998). The brown assessment of beliefs scale: Reliability and validity. American Journal of Psychiatry, 155(1), 102–108. [DOI] [PubMed] [Google Scholar]
- Ekman P, & Friesen W (1975). Unmasking the face: A guide to recognizing emotions from facial cues. Englewood Cliffs, NJ: Prentice-Hall. [Google Scholar]
- Fang A, & Wilhelm S (2015). Clinical features, cognitive biases, and treatment of body dysmorphic disorder. Annual Review of Clinical Psychology, 11, 187–212. [DOI] [PubMed] [Google Scholar]
- Feifel D, Shilling PD, & MacDonald K (2016). A review of oxytocin’s effects on the positive, negative and cognitive domains of schizophrenia. Biological Psychiatry, 79, 222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JBW (2002). Structured clinical interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). New York, NY: Biometrics Research. [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, & Hickie IB (2010). Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biological Psychiatry, 67(7), 692–694. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Hickie IB, McGuinness MM, Otis M, Woods EA, Disinger HM, … Banati RB (2013). Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology, 38(5), 612–625. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, & Dadds MR (2008). Oxytocin increases gaze to the eye region of human faces. Biological Psychiatry, 63(1), 3–5. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, … Kendrick KM (2010). Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. Journal of Neuroscience, 30, 4999–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AH, & Guastella AJ (2011). The role of oxytocin in human affect: A novel hypothesis. Current Directions in Psychological Science, 20, 222–231. [Google Scholar]
- Kim YR, Eom JS, Leppanen J, Leslie M, & Treasure J (2018). Effects of intranasal oxytocin on the attentional bias to emotional stimuli in patients with bulimia nervosa. Psychoneuroendocrinology, 91, 75–78. [DOI] [PubMed] [Google Scholar]
- Kim YR, Eom JS, Yang JW, Kang J, & Treasure J (2015). The impact of oxytocin on food intake and emotion recognition in patients with eating disorders: A double blind single dose within-subject cross-over design. PLoS One 10, e0137514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YR, Oh SM, Corfield F, Jeong DW, Jang EY, & Treasure J (2014). Intranasal oxytocin lessens the attentional bias to adult negative faces: A double blind within-subject experiment. Psychiatry Investigation, 11(2), 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, & Fehr E (2005). Oxytocin increases trust in humans. Nature, 435(7042), 673–676. [DOI] [PubMed] [Google Scholar]
- Lakens D (2013). Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Frontiers in Psychology, 4(863), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppanen J, Ng KW, Tchanturia K, & Treasure J (2017). Meta-analysis of the effects of intranasal oxytocin on interpretation and expression of emotions. Neuroscience and Biobehavioral Reviews, 78, 125–144. [DOI] [PubMed] [Google Scholar]
- Liu N, Hadj-Bouziane F, Jones KB, Turchi JN, Averbeck BB, & Unger-leider LG (2015). Oxytocin modulates fMRI responses to facial expression in macaques. Proceedings of the National Academy of Sciences of the USA, 112, E3123–E3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald K, & Feifel D (2013). Helping oxytocin deliver: Considerations in the development of oxytocin-based therapeutics for brain disorders. Frontiers in Neuroscience, 7, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, & Heinrichs M (2011). Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nature Reviews Neuroscience, 12(9), 524–538. [DOI] [PubMed] [Google Scholar]
- Phillips KA (2004). Psychosis in body dysmorphic disorder. Journal of Psychiatric Research, 38, 63–72. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Hollander E, Rasmussen SA, Aronowitz BR, DeCaria C, & Goodman WK (1997). A severity rating scale for body dysmorphic disorder: Development, reliability, and validity of a modified version of the Yale-Brown Obsessive Compulsive Scale. Psychopharmacology Bulletin, 33(1), 17–22. [PubMed] [Google Scholar]
- Salonia A, Nappi RE, Pontillo M, Daverio R, Smeraldi A, Briganti A, … Montorsi F (2005). Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Hormones and Behavior, 47(2), 164–169. [DOI] [PubMed] [Google Scholar]
- Shahrestani S, Kemp AH, & Guastella AJ (2013). The impact of a single administration of intranasal oxytocin on the recognition of basic emotions in humans: A meta-analysis. Neuropsychopharmacology, 38(10), 1929–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, & Abu-Akel A (2016). The social salience hypothesis of oxytocin. Biological Psychiatry, 79(3), 194–202. [DOI] [PubMed] [Google Scholar]
- Tabak BA, Meyer ML, Dutcher JM, Castle E, Irwin MR, Lieber-man MD, & Eisenberger NI (2016). Oxytocin, but not vasopressin, impairs social cognitive ability among individuals with higher levels of social anxiety: A randomized controlled trial. Social Cognitive and Affective Neuroscience, 11(8), 1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H, Waldman ID, & Young LJ (2016). Statistical and methodological considerations for the interpretation of intranasal oxytocin studies. Biological Psychiatry, 79(3), 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Geng Y, Luo L, Zhao Z, Ma X, Xu L, … Kendrick KM (2017). Oxytocin increases the perceived value of both self- and other-owned items and alters medial prefrontal cortex activity in an endowment task. Frontiers in Human Neuroscience, 11, 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Yao S, Li Q, Geng Y, Ma X, Luo L, … Kendrick KM (2016). Oxytocin blurs the self-other distinction during trait judgments and reduces medial prefrontal cortex responses. Human Brain Mapping, 37(7), 2512–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]