Abstract
In human cells infected by HIV type 1 (HIV-1), the viral Gag protein directs the assembly of nascent viral particles at the plasma membrane. In murine cells, HIV-1 Gag fails to reach the plasma membrane and instead forms nonfunctional intracellular aggregates. The viral determinants of this species incompatibility are previously undefined. To address this problem, we replaced a region of HIV-1 Gag known to direct its localization, the matrix (MA) domain, with functionally homologous regions from Moloney murine leukemia virus (MLV), a murine retrovirus. An HIV-1 clone carrying such a chimeric Gag protein, designated murine HIV (MHIV), assembled more efficiently than nonchimeric HIV-1 and restored plasma membrane localization of Gag in murine cells. Increased efficiency of viral assembly in murine cells was observed from MHIV constructs carrying MLV MA in place of HIV-1 MA. Efficient processing of the HIV-1 capsid protein from the chimeric Gag polyprotein and subsequent infectivity of MHIV required the presence of MLV p12 in addition to MLV MA. These findings strongly suggest that the HIV-1 MA domain of HIV-1 Gag is responsible for the assembly defect in mouse cells. Although these MHIV do not recruit native HIV-1 Env efficiently, they are capable of single-round infection when produced by high-efficiency transfection of human 293 cells and provided with an HIV-1 Env lacking its cytoplasmic tail. With further adaptation, this chimeric MHIV approach may provide the basis for creating an infectious mouse model for HIV/AIDS.
Understanding the species-specific blocks to HIV-1 replication provides many key insights into HIV–host cell interactions. The CD4 molecule was first demonstrated to be a receptor for HIV-1 when it was observed that expression of this molecule was sufficient to allow HIV-1 infection of human, but not mouse, cells (1). The observation that mouse cells did not support HIV entry led to a coreceptor hypothesis, that an essential, species-specific HIV-1 entry factor was missing from mouse cells. This hypothesis motivated functional cloning experiments in mouse cells that identified a chemokine receptor, CXCR4, as essential for HIV-1 membrane fusion (2).
The addition of CD4 and CXCR4 to mouse cells permitted HIV-1 entry, but subsequent viral gene expression was insufficient to support viral replication. Subsequently, it was found that murine cells did not support Tat, a virally encoded transcriptional activator necessary for efficient HIV gene expression. Tat enhanced viral transcription in human cells, but failed to function in mouse cells. Experiments with human–rodent somatic cell hybrids revealed that a gene on human chromosome 12 restored Tat function (3, 4). Cyclin T1 was identified as a Tat cofactor, located on human chromosome 12, that restored Tat function to mouse cells (5). The addition of human cyclin T1 to mouse cells restored high levels of HIV-1 gene expression, but HIV-1 still did not replicate in these cells (6).
In human cyclin T1-expressing murine cells, HIV-1 Gag was mislocalized, resulting in inefficient viral assembly. Electron micrographs showed electron-dense intracellular structures that seemed to be aggregates of Gag (7). This block to viral assembly was relieved by fusion of infected mouse cells with permissive human cells, strongly suggesting a specific requirement for a human cellular factor(s) in HIV assembly (8, 9).
It is unclear what portion of the HIV-1 genome is incompatible with assembly in mouse cells. The Gag protein alone is sufficient for directing the assembly of virus-like particles (10). HIV-1 Gag is produced as a 55-kDa precursor that is processed by the virally encoded protease into the p15 matrix (MA), p24 capsid (CA), p7 nucleocapsid (NC), and p6 domains. Although Gag processing is not required for virus assembly, it is important for subsequent infectivity of viral particles (11, 12). Plasma membrane localization of the viral Gag protein is critical for viral assembly (13, 14). The N-terminal domain of HIV-1 Gag, MA, is responsible for plasma membrane localization of the viral Gag protein (15, 16). Given that in mouse cells HIV-1 Gag fails to localize to the plasma membrane, it is possible that HIV-1 MA is the primary component of HIV-1 that fails to function in mouse cells. In support of this hypothesis, HIV-1 mutants in MA have been described that mislocalize Gag to intracellular membrane compartments (17–19).
To test our hypothesis, we replaced HIV-1 MA with N-terminal sequences from the Moloney murine leukemia virus (MLV) Gag protein. MLV is a murine retrovirus that replicates in mice. The aim was to make a functional, chimeric HIV-1 Gag protein that allowed virus assembly in mouse cells. We produced a murine HIV (MHIV) expressing a chimeric Gag protein that localized properly and directed assembly more efficiently in murine 3T3 cells than the nonchimeric HIV-1 Gag. Additional modifications of MHIV to develop a virus capable of multiround infection of murine cells are discussed. With further adaptations, MHIV may form the basis for an infectious mouse model for HIV/AIDS.
Materials and Methods
Plasmids and Cell Lines.
Proviral constructs were based on the HIV-1 molecular clone pNL4–3 (20). MLV Gag sequences were derived from packaging vector pCL Eco (21). Chimeric viruses were generated by a two-step PCR-based strategy using primers that joined the exact MLV MA or MA p12 sequence in place of the HIV-1 MA domain. The 5′ primer for MLV MA and MA p12 was 5′-cggaggctagaaggagagagATGGGCCAGACTGTTACCACTC-3′, the 3′ primer for MA was 5′-cctggaggttctgcactataggATAAAGGGAGGATCGAGGCG-3′, and the 3′ primer for p12 was 5′-ccctggaggttctgcactataggGAATGCCTGCGAGGTAGTG-3′. Uppercase letters represent MLV-derived sequence, and lowercase letters represent HIV-1-derived sequence. PCR products were cloned into the unique BssHII and SpeI sites in the proviral clone. All PCR-generated sequences were confirmed by sequence analysis. Luciferase-expressing HIV-1 (HIV Luc) was generated by using plasmid pNL R−E−Luc (22, 23). Luciferase-expressing MLV chimeras were generated by cloning the BssHII and SpeI sites into the identical sites in pNL R−E−Luc. The envelope glycoprotein (Env) expression vector for the wild-type (WT) Env was pSHRS HXB Env, and the cytoplasmic tail-deletion mutant, Δ147 Env, was pSHRS Δ147 Env (24). The 3T3 TXC cell line was generated by infecting 3T3.T4.CXCR4 cells (25) with helper-free retrovirus expressing the human cyclin T1 gene and selection in DMEM with 10% calf serum and 2 μg/ml puromycin. 3T3.T4.CXCR4 cells were obtained through the AIDS Research and Reference Reagent Program, National Institutes of Health (ARRRP-NIH), from Dan Littman. 3T3 TXC cells were confirmed to support HIV-1 Tat function (data not shown). Human embryonic kidney cells (293) were maintained in DMEM containing 10% FCS.
Transfection and Infectivity Assay.
For virus production assays and generation of luciferase-expressing viruses, 293 cells or 3T3 TXC cells were transfected with Lipofectamine Plus reagent (Invitrogen) according to the manufacturer's standard protocol. Transfection efficiencies of the HIV-1 and MHIV plasmids are assumed to be equivalent. Transfection for fluorescence microscopy was performed by standard calcium phosphate methods (Promega). For infectivity assays, the virus-containing supernatants from transfected 293 cells were harvested 2 days after transfection. Supernatants were cleared of cellular debris by centrifugation at 500 × g for 5 min followed by 0.45-μm filtration. Target CD4+ human osteosarcoma cells (HOS CD4, ARRRP-NIH, from Nathaniel Landau) were infected overnight with virus supernatants and incubated for 48 h. Infected cells were lysed with 100 μl of luciferase cell culture lysis buffer (Promega). A 20-μl sample of each lysate was assayed for photon emission after the addition of 100 μl of luciferase assay substrate (Luciferase Assay System, Promega) with a 96-well plate luminometer (Luminoskan RT, Labsystems, Chicago).
ELISA and Western Blot Analyses.
Supernatants were collected from transfected cells and cleared of cellular debris by low-speed centrifugation (500 × g, 5 min), followed by 0.45-μm filtration. Supernatants were inactivated in 0.5% Triton X-100 in PBS before quantitation by commercial ELISA (NEN Life Sciences). Serial 10-fold dilutions of the supernatants were performed in triplicate and concentrations were determined by comparison with dilutions of the kit-based p24 standards. For Western blot analyses, virus particles were purified by centrifugation through a 20% sucrose cushion, 1× TNE buffer (10 mM Tris⋅HCl, pH 7.5/1 mM EDTA/100 mM NaCl). Viral pellets were resuspended in 1× SDS loading buffer and separated by SDS/PAGE, 4–20% polyacrylamide gradient. Proteins were transferred to poly(vinylidene difluoride) membrane (Hybond P, Amersham Pharmacia), blocked with 4% nonfat dried milk/0.1% Tween in PBS. Blots were probed with the following antibodies: human anti-HIV-1 pooled patient antiserum, 1:5,000 dilution (ARRRP-NIH, from Alfred Prince); sheep anti-p24, 1:2,000 dilution (ARRRP-NIH, from Michael Phelan); mouse monoclonal anti-p17, 1:1,000 dilution (Intracel catalog no. 321); and sheep anti-HIV-1 gp120 Env, 1:1,000 dilution (ARRRP-NIH, from Michael Phelan). Western blots were developed with horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch), followed by chemiluminescent detection (ECL, Amersham Pharmacia).
Deconvolution Fluorescence Microscopy.
Transfected 3T3 TXC cells were plated onto treated microscope slides (VWRbrand Superfrost Plus Microslide, VWR Scientific). Cells were fixed with 4% paraformaldehyde in PBS for 10 min, permeablized with 0.15% Triton X-100 for 3 min, and blocked with PBS containing 3% BSA, 0.2% Tween, and 0.2% fish gelatin for 1 h. Primary antibody, a sheep polyclonal anti-p24, 1:50 dilution (ARRRP-NIH, from Michael Phelan), was applied for 1 h at 37°C. Secondary antibody, Texas red-conjugated donkey anti-rabbit, 1:100 dilution (Jackson ImmunoResearch), was applied for 1 h at 37°C. Slides were directly mounted in 4′,6-diamidino-2-phenylindole-containing Vectashield (Vector Laboratories). The cells were imaged on a Nikon Eclipse 800 fluorescence microscope with a 100× oil-immersion lens. Image stacks were recorded at 150-nm intervals (z series) with a Hamamatsu Orca charge-coupled device camera (Hamamatsu Phototonics, Bridgewater, NJ) by using the Metamorph imaging system (Universal Imaging, West Chester, PA) and deconvolved with Huygens software (Bitplane, Switzerland). The images were then reconstructed in three dimensions with IMARIS software (Bitplane) for further analysis.
Results
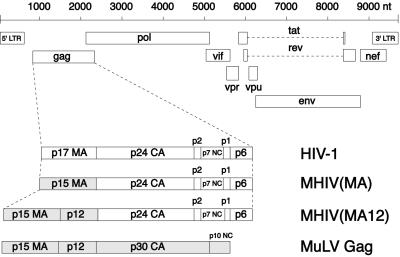
The MA domain of the HIV-1 Gag polyprotein plays a major role in membrane targeting. Because the phenotype of HIV-1 Gag in mouse cells is one of mislocalization and aggregation, we replaced MA from HIV-1 Gag with the MA from MLV Gag (Fig. 1) to determine whether the resulting chimera could function in mouse cells. In a second chimeric construct we replaced HIV-1 MA with a larger piece of MLV Gag that included the MLV MA sequence and the adjacent 3′ sequence referred to as p12. Although the HIV-1 Gag protein has no p12 equivalent, we included it in our construct because p12 plays an essential role in MLV infection—facilitating MLV Gag processing and maintaining viral infectivity (26). Both Gag chimeras were cloned into the context of the full-length HIV-1 molecular clone, pNL4–3 (20), and were designated MHIV(MA) for the MA chimera and MHIV(MA12) for the MA p12 chimera.
Figure 1.
Schematic illustration of the MHIV Gag chimeras. Genomic organization of HIV-1 (Upper). The HIV-1 Gag polyprotein is processed into p17 MA, p24 CA, p2, p7 NC, p1, and p6. Chimeric strategies replace the p17 MA with p15 MA of MLV Gag [MHIV(MA)] or both the p15 MA and p12 domains of MLV Gag [MHIV(MA12)]. The complete MLV Gag consists of p15 MA, p12, p30 CA, and p10 NC (Lower). Scale of viral genome sequence is shown in nucleotides (nt).
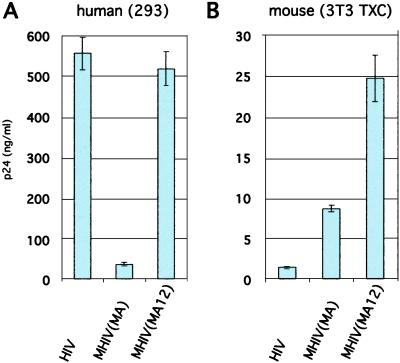
Proviral DNAs for the chimeric viruses were transfected into human embryonic kidney 293 cells, and cell supernatants were tested for HIV-1 Gag CA (p24) by HIV p24 ELISA. The MHIV(MA) chimera produced significantly less p24, but MHIV(MA12) produced levels of HIV-1 p24 comparable with WT HIV-1 (Fig. 2A). When the same viral constructs were transfected into murine cells that stably expressed human CD4, CXCR4, and cyclin T1 (3T3 TXC), the two chimeric viruses produced significantly more supernatant p24 than did WT HIV-1 (Fig. 2B). In particular, the MHIV(MA12) chimera produced 17-fold greater levels of supernatant p24 than did WT HIV-1. These results suggest that the levels of HIV-1 assembly in mouse cells can be significantly improved by replacing only the HIV-1 MA domain with sequences from MLV.
Figure 2.
MHIV(MA12) releases greater levels of supernatant p24 than nonchimeric HIV-1 when transfected into CD4+, CXCR4+, cyclin T1-expressing murine 3T3 cells (3T3 TXC). Graphs are of p24 antigen harvested from supernatants from transfected human embryonic kidney cells 293 (A) and transfected 3T3 TXC cells (B). p24 ELISA was performed in triplicate as described in Materials and Methods. Note different y axis scales. Transfection efficiency and numbers of cells plated per transfection are significantly greater for human 293 cells, making quantitative comparisons between the two cell lines difficult. Results are representative of three independent experiments.
Because the commercial NEN p24 ELISA preferentially recognizes the processed p24 CA protein over unprocessed Gag precursor forms (8), it may be important to determine the efficiency of Gag processing. If Gag processing of the chimeric proteins is poor relative to nonchimeric HIV-1 Gag, the ELISA may underestimate the levels of chimeric Gag protein released. We therefore examined the processing of the p24 antigen of the different viral constructs.
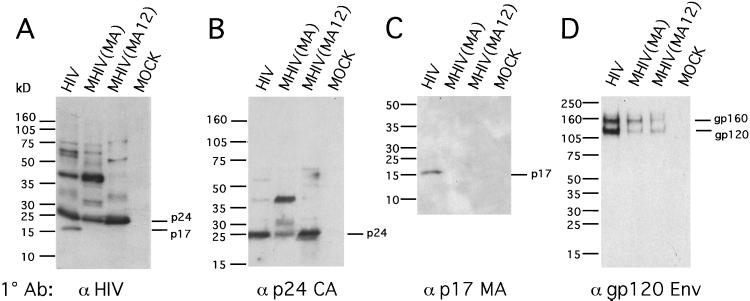
To harvest viral particles for further analysis, equivalent volumes of cell culture supernatants from transfected human 293 cells were centrifuged over a 20% sucrose cushion, and viral pellets were resuspended and separated by SDS/PAGE. Western blot analysis of purified virus particles with two different antibodies revealed that the chimeric MHIV(MA12) virus produced a p24 CA protein that comigrated with WT HIV-1 p24 (Fig. 3 A and B). In contrast, the CA protein from the MHIV(MA) chimera was not processed fully into a 24-kDa species and instead showed a major band at 36 kDa. The level of partially processed MHIV(MA) Gag protein (36-kDa species) seemed similar to the amount of p24 seen in the WT HIV-1 and the MHIV(MA12) constructs, apparently in contrast with the very different levels of p24 Gag predicted from the ELISA (Fig. 2A). Therefore, because the NEN p24 ELISA reacts poorly with unprocessed Gag forms, the ELISA likely underestimates the amount of Gag released by cells transfected with the MHIV(MA) chimera.
Figure 3.
MHIV(MA12) is proteolytically processed in virus particles, but Env incorporation is inefficient. Western blot analysis of purified virus particles performed with human anti-HIV-1 patient serum (A), sheep anti-p24 (B), mouse anti-p17 (C), and sheep anti-gp120 HIV-1 Env (D). Virus construct-transfected or mock-transfected sample (MOCK) are indicated above each lane. Mock samples were transfected with equivalent amount of nonviral plasmid DNA.
The ability of HIV-1 protease to recognize and cleave the MLV p12/HIV p24 CA junction to produce a CA protein of the expected size, and its inability to cleave the MLV MA/HIV p24 junction, may be a function of the HIV protease substrate specificity (27). The two chimeric junctions are modestly different and therefore may be cleaved with differing efficiency. Alternatively, the presence of p12 could be important in promoting a conformational state of the Gag precursor that facilitates processing of the chimeric Gag. In its native context, the p12 domain seems to play a similar role facilitating efficient MLV Gag processing (26).
In a control experiment, Western blotting with an antibody against HIV-1 MA revealed a single band in the HIV-1 lane, but not in the MHIV(MA) or MHIV(MA12) lanes, confirming that the HIV-1 MA protein was present only in WT HIV-1 virus particles and not in either MHIV chimera (Fig. 3C). Because the MA domain of HIV-1 Gag also plays an important role in recruitment of the Env (28, 29), we examined virus particles for the amount of HIV-1 Env they contained. Both MLV chimeras were found to incorporate significantly less HIV-1 Env than WT HIV-1 (Fig. 3D). This result suggests that, although the chimeric viruses are able to assemble more efficiently from mouse cells, they are unable to recruit Env efficiently, a process necessary for viral infectivity.
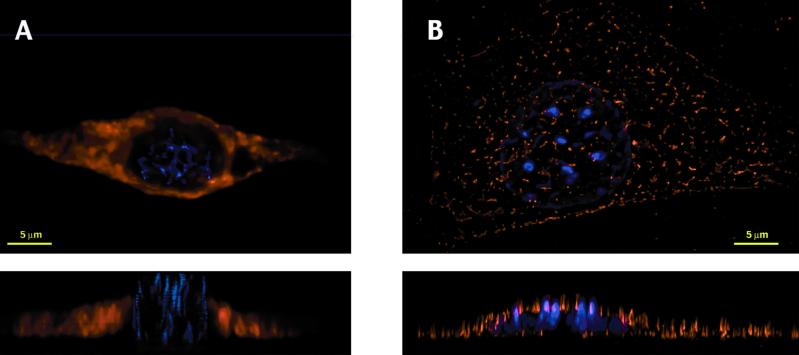
Because WT HIV-1 Gag has been reported to be mislocalized in murine cells, we performed three-dimensional deconvolution immunofluorescence microscopy on transfected 3T3 TXC cells to determine whether a chimeric Gag protein is localized to the plasma membrane. In mouse cells, WT HIV-1 Gag produced a diffuse cytoplasmic signal not localized to the plasma membrane (Fig. 4A). In contrast, the MHIV(MA12) chimera produced a punctate pattern that seems to be associated with the plasma membrane (Fig. 4B). Cross-sectional views illustrate that the staining of the MHIV(MA12) Gag is concentrated at the top and bottom edges of the cell, including the areas of plasma membrane above and below the nucleus. In contrast, WT Gag is found diffusely within the cell and is not found on the membranes above and below the nucleus. These results show that replacement of the HIV-1 MA domain with MLV MA and p12 changes the localization of Gag from a diffusely localized intracellular compartment to the plasma membrane where assembly usually occurs. The punctate pattern observed for the MHIV(MA12) Gag in mouse cells resembles the pattern reported for HIV-1 Gag in human cells (30). It has been proposed that these punctate islands may represent focal points for viral assembly.
Figure 4.
Punctate membrane localization is restored in mouse cells by the MHIV(MA12) chimeric Gag protein. Shown are top and side views of three-dimensional image sets produced by deconvolution immunofluorescence microscopy of murine 3T3 TXC cells transiently transfected with nonchimeric HIV-1 (HIV Luc) (A) or Gag-chimeric HIV [MHIV(MA12) Luc] (B). (Upper) Top views of cells over the entire z stack of images. (Lower) Side view looking at the sum of several cross sections through the middle of the cell. Staining of Gag with polyclonal anti-p24 antibody was developed with Texas red-conjugated secondary antibody (shown in red), and DNA was stained with 4′,6-diamidino-2-phenylindole (shown in blue).
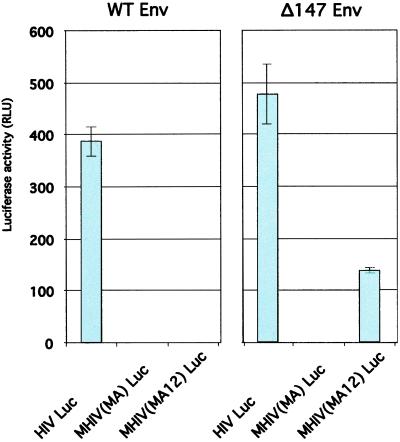
To examine whether the chimeric virus could still produce infectious virus particles, we cloned the chimeric Gag genes into a luciferase-expressing HIV-1 molecular clone, pNL R−E−Luc (22, 23), and produced viruses by high-efficiency transfection of human 293 cells. When luciferase-expressing chimeric viruses, MHIV(MA) Luc or MHIV(MA12) Luc, were produced with WT Env, infectivity was not detected (Fig. 5). As a control, production of the nonchimeric HIV Luc with WT Env produces infectious virus. These results are consistent with our observation that the chimeric viruses do not incorporate HIV-1 Env efficiently.
Figure 5.
MHIV(MA12) Luc virus is able to undergo single-round infection when produced from human cells and provided with an HIV Env with a truncated cytoplasmic tail (Δ147 Env). Virus-containing supernatants were prepared from transfected 293 cells after cotransfection with an Env-deficient, luciferase-encoding provirus [HIV Luc, MHIV(MA) Luc, or MHIV(MA12) Luc] and an Env expression vector (WT Env or Δ147 Env). Infectivity of viruses was measured by luciferase assay of cell lysates prepared from infected CD4+ human osteosarcoma (HOS CD4) cells at 48 h after infection. Luciferase activity was measured in relative light units (RLU).
MLV does not incorporate HIV-1 Env efficiently (31), possibly because steric interference exists between MLV Gag and the large cytoplasmic tail of HIV-1 Env. Indeed, an HIV-1 Env with a truncated cytoplasmic tail (Δ147 Env) is able to pseudotype MLV (31). On that basis, we produced chimeric viruses in which Δ147 Env was provided in trans. When pseudotyped with a Δ147 Env, the MHIV(MA12) chimera is able to produce infectious particles at 25–30% the level of WT HIV-1 (Fig. 5). The MHIV(MA) chimera did not produce infectious particles with WT or Δ147 Env, illustrating that the addition of the p12 domain was important for maintaining infectivity of the chimera. These results suggest that the MHIV(MA12) chimera is compatible with other steps in the HIV-1 life cycle.
Discussion
The MHIV chimeric virus overcomes a major impediment to the development of a murine model for HIV-1 infection, the block to assembly of HIV-1 at the plasma membrane. Grafting MLV MA in place of HIV-1 MA resulted in a modest, but significant, 6-fold increase in p24 antigen release from mouse cells. When one considers that the p24 CA protein from this virus is incompletely processed to a 36-kDa species (Fig. 3), which is poorly recognized by the NEN commercial ELISA (8), the true effect on viral assembly is likely to be significantly larger. In addition, when MHIV chimeras carrying both the MLV MA and p12 domains are examined, in which p24 processing occurs efficiently, we observed 17-fold greater viral antigen production than with WT HIV Gag. The magnitude of this effect correlates roughly with the increase in Gag release when infected murine cells are fused with human cells (8, 9). It is therefore plausible that the assembly defect of HIV-1 in mouse cells can be largely attributed to defects in MA function.
In addition to overcoming the assembly defect, we have shown that an MHIV can be infectious. When MHIV(MA12) is complemented with Δ147 Env and produced by highly efficient transfection of human 293 cells, efficient single-round infection can be observed. This result indicates that MLV MA and p12 are able to complement HIV MA function in the early phase of infection in addition to the late (assembly) phase. Thus, this chimeric virus approach represents an attractive strategy to develop an HIV-based vector that will replicate in a transgenic mouse model. Creation of such a mouse model, however, will require further development of MHIV, so that it can actively replicate in murine cells.
One major obstacle is the poor Env incorporation of MHIV. Because a specific interaction between HIV-1 MA and the cytoplasmic tail of Env is thought to mediate Env recruitment, it is not surprising that MHIV fails to incorporate Env efficiently (Fig. 3). In accord with this observation, the MHIV does not undergo multiple rounds of infection in human or mouse cells (B. K. Chen and P.S.K., unpublished results). The cytoplasmic tail of HIV-1 Env is large and, in the absence of specific recruitment to the viral particle by HIV-1 MA, it may be actively excluded from the MHIV particles. Because elimination of the cytoplasmic tail and overexpression of the Δ147 Env in trans allows enough Env incorporation to make infectious virus, testing the infectivity of proviral constructs carrying truncated Env cytoplasmic domains in cis is warranted. Preliminary efforts to test the ability of a chimeric virus carrying the Δ147 Env on the viral genome have not resulted in a virus that is able to undergo multiple rounds of infection in mouse cells (B. K. Chen and P.S.K., unpublished results). Thus, additional modifications to the cytoplasmic tail or MA itself may be needed to recover the chimeras' ability to incorporate HIV-1 Env.
Prior studies of HIV-1 mutants that delete the Env cytoplasmic tail revealed that the virus is highly attenuated and able to replicate only in a very specific HTLV-I-transformed T cell line, MT-4 (32, 33). Passage of HIV MA/cytoplasmic tail double mutants in MT-4 cells selected for variants with improved replication in other T cell lines (18). Therefore, selection of MHIV cytoplasmic tail-deletion mutants in MT-4 cells may produce viral variants that are better able to replicate in other cell types.
Recent advances in our understanding of viral assembly suggest some other experimental strategies that may allow more efficient Env incorporation onto MHIV. We reported that palmitoylation of HIV-1 Env at two cysteine residues on its cytoplasmic tail was critical for viral incorporation of Env and subsequent infectivity (34). Palmitoylation of Env directs it to lipid microdomains on the plasma membrane, known as lipid rafts, which serve as focal points for viral assembly. It may therefore be helpful to retain a palmitoylation site on truncated Env constructs to promote targeting to lipid rafts. Alternatively, palmitoylated cytoplasmic tails from other viruses (35–38), including MLV itself, could be fused to the HIV-1 Env ectodomain to try to enhance the ability of MHIV to incorporate the resulting chimeric Env.
Although the MHIV(MA12) exhibits many attractive features, alternative chimeric strategies may also be considered to recover Env incorporation. It may be possible to design a chimeric Gag protein that is directed to the plasma membrane in mouse cells by the MLV MA, but at the same time retains MA sequences responsible for HIV-1 Env incorporation. HIV-1 clones engineered to carry tandem copies of HIV-1 MA are still capable of efficient HIV-1 assembly (39). Thus, it may be possible to make a chimeric Gag that keeps the HIV-1 MA and tags the HIV-1 Gag at the N terminus with MLV MA or MA-p12.
The restoration of punctate plasma membrane localization of MLV chimeric Gag in mouse cells further supports that it is the failure of HIV-1 MA to target Gag appropriately in mouse cells that blocks viral assembly. Previous studies suggest that the absence of a human-specific factor in mouse cells limits the ability of HIV-1 to assemble (8, 9). On the basis of our results, it is now likely that one (or more) of these human-specific factors acts as an HIV-1 MA cofactor. Furthermore, because the MA cofactors are human/mouse compatible for MLV MA, but not for HIV-1 MA, our work suggests that the molecular basis of the MA interaction with its cofactor is fundamentally different for these two MA domains. Biochemical or genetic strategies to identify factors that interact with one MA, but not the other, may provide a means for identifying key assembly factors.
The ability of MHIV(MA12) to remain compatible with single-round infection illustrates the remarkable functional conservation of MA in retroviruses of different classes. Because the single-round infection that we observe from MHIV(MA12) produced in highly transfectable 293 cells is severalfold less than WT HIV-1, it is likely that the infectivity per viral particle is somewhat reduced in the chimeras. When transfecting 3T3 TXC cells, it has been technically difficult to produce sufficient virus to determine whether infectivity per unit p24 of the chimeric constructs is reduced relative to virus produced in 293 cells (data not shown), in large part because of the greatly reduced efficiency of transfection of 3T3 TXC cells. Because we have not demonstrated efficient single-round infectivity from mouse cells, it is formally possible that a particle-intrinsic infectivity defect may still occur in virus produced from mouse cells.
Although the MA domain apparently is sufficient to enhance assembly of MHIV, infectivity requires the MLV p12 domain in addition to MLV MA. The p12 domain has no counterpart in HIV-1 Gag, but in MLV it is also essential for maintaining viral infectivity (26). The MLV p12 mutant fails to process its own Gag efficiently and is defective in the MLV protease-mediated Env processing that is unique to MLV. In our experiments, the p12 protein also seems to facilitate processing of chimeric Gag by HIV-1 protease. Previous studies with HIV-1 MA mutants found that proper processing of p24 CA protein is important for infectivity of mutant proviruses (18). It is therefore likely that much of the improved infectivity of the p12-containing virus is due to its improved ability to be processed by HIV-1 protease. MHIV variants designed to improve processing of MHIV MA, or eliminate Gag processing of MHIV MA12, may test directly the role p12 plays in promoting infectivity of MHIV chimeras.
Previous work identified p12 as a protein containing an “L-domain” (26), a proline-rich motif found in many viruses, which plays an important role in the late phase of viral assembly. Although no p12 occurs in HIV-1, an L-domain in HIV-1 is present on p6, which lies at the very C terminus of HIV-1 Gag. The L-domain of HIV-1 is thought to function by recruiting ubiquitin ligation machinery that modifies HIV-1 Gag by covalent addition of ubiquitin on the p6 domain (40, 41). It is therefore possible that p12 may improve particle release of MHIV(MA12) Gag through its function as an L-domain. Mutants in the p12 L-domain may be designed to test this hypothesis directly.
AIDS researchers have long sought a genetically tractable, economical alternative to the costly primate models for HIV-1 infection (42). Transgenic mice expressing human CD4 and CXCR4 or CCR5 on T cells were developed with this goal in mind (43, 44). Although it is possible to detect proviral DNA from the spleen and lymph nodes of infected mice (43), there has been no evidence of ongoing viral replication. Several laboratories are testing the ability of cyclin T1 transgenes to allow HIV-1 to replicate in such transgenic mice. However, these mouse models are still likely to be limited by the inability of HIV-1 to assemble efficiently from mouse cells. Because MHIV provides a solution to the assembly block of HIV-1 in mouse cells, it represents an important step toward a useful transgenic mouse model for HIV-1 infection. Further adaptation of MHIV to undergo multiround infection in mouse cells may permit determination of whether MHIV can cause AIDS-like illness in mice.
Acknowledgments
We thank members of the Kim Laboratory, including John Newman, Sam Sia, Christopher Liu, and Michael Root, for helpful discussions and commentary on the manuscript, Kalle Saksela and George B. Cohen for helpful comments on the manuscript, and Leslie Gaffney for editorial assistance. We thank Katherine Jones for human cyclin T1 cDNAs and Eric Hunter for SRHS Env expression plasmids. Microscopy was conducted by using the W. M. Keck Foundation Biological Imaging Facility at the Whitehead Institute. This research was supported by Grant GM44162 from the National Institutes of Health. B.K.C. was supported by National Institutes of Health National Research Service Award F32 AI10664-01.
Abbreviations
- MA
matrix
- CA
capsid
- NC
nucleocapsid
- MLV
murine leukemia virus
- MHIV
murine HIV
- Env
envelope glycoprotein
- WT
wild type
References
- 1.Maddon P J, Dalgleish A G, McDougal J S, Clapham P R, Weiss R A, Axel R. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 2.Feng Y, Broder C C, Kennedy P E, Berger E A. Science. 1996;272:872–877. [Google Scholar]
- 3.Newstein M, Stanbridge E J, Casey G, Shank P R. J Virol. 1990;64:4565–4567. doi: 10.1128/jvi.64.9.4565-4567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hart C E, Ou C Y, Galphin J C, Moore J, Bacheler L T, Wasmuth J J, Petteway S R, Jr, Schochetman G. Science. 1989;246:488–491. doi: 10.1126/science.2683071. [DOI] [PubMed] [Google Scholar]
- 5.Wei P, Garber M E, Fang S M, Fischer W H, Jones K A. Cell. 1998;92:451–462. doi: 10.1016/s0092-8674(00)80939-3. [DOI] [PubMed] [Google Scholar]
- 6.Garber M E, Wei P, KewalRamani V N, Mayall T P, Herrmann C H, Rice A P, Littman D R, Jones K A. Genes Dev. 1998;12:3512–3527. doi: 10.1101/gad.12.22.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mariani R, Rutter G, Harris M E, Hope T J, Krausslich H G, Landau N R. J Virol. 2000;74:3859–3870. doi: 10.1128/jvi.74.8.3859-3870.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bieniasz P D, Cullen B R. J Virol. 2000;74:9868–9877. doi: 10.1128/jvi.74.21.9868-9877.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mariani R, Rasala B A, Rutter G, Wiegers K, Brandt S M, Krausslich H G, Landau N R. J Virol. 2001;75:3141–3151. doi: 10.1128/JVI.75.7.3141-3151.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freed E O. Virology. 1998;251:1–15. doi: 10.1006/viro.1998.9398. [DOI] [PubMed] [Google Scholar]
- 11.Loeb D D, Swanstrom R, Everitt L, Manchester M, Stamper S E, Hutchison C A., III Nature (London) 1989;340:397–400. doi: 10.1038/340397a0. [DOI] [PubMed] [Google Scholar]
- 12.Peng C, Ho B K, Chang T W, Chang N T. J Virol. 1989;63:2550–2556. doi: 10.1128/jvi.63.6.2550-2556.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Göttlinger H G, Sodroski J G, Haseltine W A. Proc Natl Acad Sci USA. 1989;86:5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gheysen D, Jacobs E, de Foresta F, Thiriart C, Francotte M, Thines D, De Wilde M. Cell. 1989;59:103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- 15.Yuan X, Yu X, Lee T H, Essex M. J Virol. 1993;67:6387–6394. doi: 10.1128/jvi.67.11.6387-6394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou W, Parent L J, Wills J W, Resh M D. J Virol. 1994;68:2556–2569. doi: 10.1128/jvi.68.4.2556-2569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Facke M, Janetzko A, Shoeman R L, Krausslich H G. J Virol. 1993;67:4972–4980. doi: 10.1128/jvi.67.8.4972-4980.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reil H, Bukovsky A A, Gelderblom H R, Göttlinger H G. EMBO J. 1998;17:2699–2708. doi: 10.1093/emboj/17.9.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ono A, Orenstein J M, Freed E O. J Virol. 2000;74:2855–2866. doi: 10.1128/jvi.74.6.2855-2866.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naviaux R K, Costanzi E, Haas M, Verma I M. J Virol. 1996;70:5701–5705. doi: 10.1128/jvi.70.8.5701-5705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen B K, Saksela K, Andino R, Baltimore D. J Virol. 1994;68:654–660. doi: 10.1128/jvi.68.2.654-660.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Connor R I, Chen B K, Choe S, Landau N R. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 24.Dubay J W, Roberts S J, Hahn B H, Hunter E. J Virol. 1992;66:6616–6625. doi: 10.1128/jvi.66.11.6616-6625.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng H K, Unutmaz D, KewalRamani V N, Littman D R. Nature (London) 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 26.Yuan B, Li X, Goff S P. EMBO J. 1999;18:4700–4710. doi: 10.1093/emboj/18.17.4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunn B M, Gustchina A, Wlodawer A, Kay J. Methods Enzymol. 1994;241:254–278. doi: 10.1016/0076-6879(94)41068-2. [DOI] [PubMed] [Google Scholar]
- 28.Dorfman T, Mammano F, Haseltine W A, Göttlinger H G. J Virol. 1994;68:1689–1696. doi: 10.1128/jvi.68.3.1689-1696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu X, Yuan X, Matsuda Z, Lee T H, Essex M. J Virol. 1992;66:4966–4971. doi: 10.1128/jvi.66.8.4966-4971.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hermida-Matsumoto L, Resh M D. J Virol. 2000;74:8670–8679. doi: 10.1128/jvi.74.18.8670-8679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schnierle B S, Stitz J, Bosch V, Nocken F, Merget-Millitzer H, Engelstadter M, Kurth R, Groner B, Cichutek K. Proc Natl Acad Sci USA. 1997;94:8640–8645. doi: 10.1073/pnas.94.16.8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akari H, Fukumori T, Adachi A. J Virol. 2000;74:4891–4893. doi: 10.1128/jvi.74.10.4891-4893.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murakami T, Freed E O. Proc Natl Acad Sci USA. 2000;97:343–348. doi: 10.1073/pnas.97.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rousso I, Mixon M B, Chen B K, Kim P S. Proc Natl Acad Sci USA. 2000;97:13523–13525. doi: 10.1073/pnas.240459697. . (First Published November 28, 2000; 10.1073/pnas.240459697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melkonian K A, Ostermeyer A G, Chen J Z, Roth M G, Brown D A. J Biol Chem. 1999;274:3910–3917. doi: 10.1074/jbc.274.6.3910. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Pekosz A, Lamb R A. J Virol. 2000;74:4634–4644. doi: 10.1128/jvi.74.10.4634-4644.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang C, Compans R W. Virology. 1996;221:87–97. doi: 10.1006/viro.1996.0355. [DOI] [PubMed] [Google Scholar]
- 38.Yang C, Spies C P, Compans R W. Proc Natl Acad Sci USA. 1995;92:9871–9875. doi: 10.1073/pnas.92.21.9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang C T, Chen S S, Chiang C C. Virology. 2000;278:289–298. doi: 10.1006/viro.2000.0655. [DOI] [PubMed] [Google Scholar]
- 40.VerPlank L, Bouamr F, LaGrassa T J, Agresta B, Kikonyogo A, Leis J, Carter C A. Proc Natl Acad Sci USA. 2001;98:7724–7729. doi: 10.1073/pnas.131059198. . (First Published June 26, 2001; 10.1073/pnas.131059198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strack B, Calistri A, Accola M A, Palu G, Göttlinger H G. Proc Natl Acad Sci USA. 2000;97:13063–13068. doi: 10.1073/pnas.97.24.13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen J. Science. 2001;293:1034–1036. doi: 10.1126/science.293.5532.1034. [DOI] [PubMed] [Google Scholar]
- 43.Browning J, Horner J W, Pettoello-Mantovani M, Raker C, Yurasov S, DePinho R A, Goldstein H. Proc Natl Acad Sci USA. 1997;94:14637–14641. doi: 10.1073/pnas.94.26.14637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sawada S, Gowrishankar K, Kitamura R, Suzuki M, Suzuki G, Tahara S, Koito A. J Exp Med. 1998;187:1439–1449. doi: 10.1084/jem.187.9.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]