Abstract
Background
The CRISPR/Cas9 system can effectively introduce site-specific modifications to the genome. The efficiency is high enough to induce targeted genome modifications during embryogenesis, thus increasing the efficiency of producing genetically modified animal models and having potential clinical applications as an assisted reproductive technology. Because most of the CRISPR/Cas9 systems introduce site-specific double-stranded breaks (DSBs) to induce site-specific modifications, a major concern is its potential off-targeting activity, which may hinder the application of the technology in clinics. In this study, we investigated off-targeting events in genome edited pigs/fetuses that were generated through direct injection of the CRISPR/Cas9 system into developing embryos; off-targeting activity of four different sgRNAs targeting RAG2, IL2RG, SCD5, and Ig Heavy chain were examined.
Results
First, bioinformatics analysis was applied to identify 27 potential off-targeting genes from the sgRNAs. Then, PCR amplification followed by sequencing analysis was used to verify the presence of off-targeting events. Off-targeting events were only identified from the sgRNA used to disrupt Ig Heavy chain in pigs; frequency of off-targeting was 80 and 70% on AR and RBFOX1 locus respectively. A potential PAM sequence was present in both of the off-targeting genes adjacent to probable sgRNA binding sites. Mismatches against sgRNA were present only on the 5′ side of AR, suggesting that off-targeting activities are systematic events. However, the mismatches on RBFOX1 were not limited to the 5′ side, indicating unpredictability of the events.
Conclusions
The prevalence of off-targeting is low via direct injection of CRISPR/Cas9 system into developing embryos, but the events cannot be accurately predicted. Off-targeting frequency of each CRISPR/Cas9 system should be deliberately assessed prior to its application in clinics.
Electronic supplementary material
The online version of this article (10.1186/s12896-019-0517-7) contains supplementary material, which is available to authorized users.
Keywords: CRISPR/Cas9, Off-targeting, Genome-editing, Embryo development
Background
Advancements in genome-editing technology permit us to effectively introduce targeted modifications into non-traditional lab animals, such as pigs. Use of Zinc Finger Nucleases (ZFN) and Transcription Activator-Like Effector Nucleases (TALEN) have shown to be successful [1, 2]; however, they are not as affordable or easy to assemble as the CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) and Cas9 system [3]. The CRISPR/Cas9 system is used by bacteria in adaptive immunity and defense mechanisms to prevent invasion of foreign genetic material [4]. An engineered CRISPR/Cas9 system, derived from Streptococcus pyogenes, could customize the use of the CRISPR/Cas9 system as a powerful genome-editing tool [5].
The CRISPR/Cas9 system has contributed to the production of genetically engineered (GE) pigs by increasing efficiency of genetic modifications in somatic cells and allowing genetic modifications during embryogenesis, thus by-passing the need of somatic cell nuclear transfer (SCNT). Numerous GE pigs have been produced by employing the CRISPR/Cas9 technology [6–8] and have shown a lack of off-targeting activities [9–11]. However, the safety of the approach is still being questioned. Specifically, unintended mutations in the genome caused by off-targeting activity of CRISPR/Cas9 system is a leading concern. Because introducing site-specific double-stranded breaks (DSB) on the target locus is the foundation of many genome-editing tools [12], off-targeting events have been reported with the use of CRISPR/Cas9 system [13–15]. Interestingly, the frequency of off-targeting highly depends on the design of single guide RNA (sgRNA) [3], the complimentary RNA sequence that attracts Cas9 to the target genome locus.
Producing GE pigs through direct injection of CRISPR/Cas9 system into developing embryos provides a unique opportunity. Incorporating SCNT during GE pig production often results in developmental defects. However, GE pigs produced via direct injection of the CRISPR/Cas9 system into developing zygotes appear to be normal and the efficiency is as high as 100% [16, 17]. Pigs are similar to humans in size and physiologically, which makes them a good biomedical model for clinical outcomes [18]. In addition, preimplantation embryos originated from the pig and human display similar development trajectory [19], thus ideal to reflect the outcome of applying CRISPR/Cas9 system in clinics.
Recent reports of genome editing in human embryos [20] and the birth of genome-edited babies [21] suggest that the approach may also be expanded to clinical applications; however, safety of the technology has not been fully assessed. Investigating the presence of off-targeting activities in genome edited pigs produced through direct injection of CRISPR/Cas9 system into developing embryos may reflect the frequency of off-targeting events during clinical applications.
In this study, we investigated off-targeting events in RAG2/IL2RG double knockout pigs, SCD5 knockout fetuses, and Ig Heavy chain knockout pigs previously generated via direct injection of the CRISPR/Cas9 system. Frequency of on-target was 100% by the CRISPR/Cas9 systems; however, potential off-targeting activities have not been investigated. The objective of the study was to determine if off-targeting events occurred in knockout pigs by direct injection of CRISPR/Cas9 and examine if the design of sgRNAs affected the occurrence of off-targeting events.
Results
Bioinformatic analyses identified 9, 8, 6, and 4 potential off-targeting genes carrying 16 bp identity or more with the sgRNA for RAG2, IL2RG, Ig Heavy chain, and SCD5 knockout pigs respectively. Most of the potential off-targeting loci were located in intron regions and only a portion of the potential off-targeting sites possessed –NGG sequences that can act as a PAM sequence (Additional file 1: Table S1-S4). Presence of off-targeting events on the candidate genes were examined as shown in Fig. 1. Sequencing of potential off-target regions revealed the presence of polymorphisms compared to the Sus scrofa reference genome (Sus scrofa 10.2 and 11.1). For instance, mismatches were found in sequencing readings from NTNG1 and 5′ to LRRF1P1 locus. When compared to the known genomic sequence of Sus scrofa breeds and the paternal genomic sequence, the mismatches were identified as polymorphisms due to variations in genomic DNA sequences among breeds. The polymorphism found in the NTNG1 gene was paternally originated since the same polymorphism exists in the genome of the boar used to generate GE pigs. In addition, a polymorphism in PLCXD3 was located in a repetitive element of an intron. All the polymorphisms were detected outside of potential sgRNA binding sites. No off-targeting event was detected from sgRNAs used to introduce modifications on RAG2, IL2RG, or SCD5 (Additional file 1: Table S5, S6, and S7).
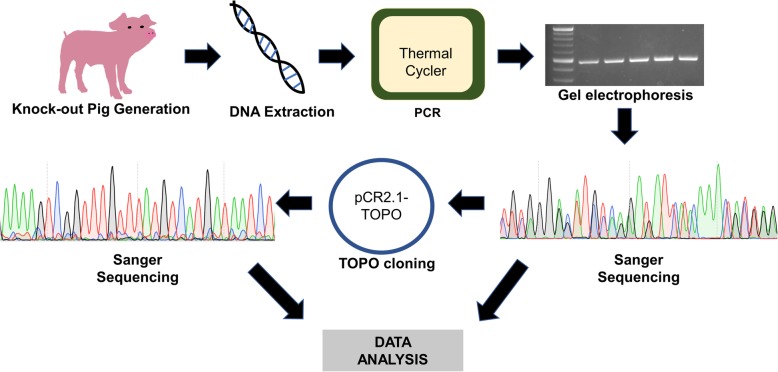
Fig. 1.
Schematic approaches to identify off-targeting events. First, genomic DNAs were isolated from knockout pigs or fetuses, generated using the CRISPR/Cas9 system. Second, a PCR reaction was performed to amplify a fragment of DNA flanking off-target region. Third, PCR products were loaded on a gel and Sanger sequenced. If the results were polymorphic, then TOPO cloning followed by Sanger sequencing was performed to uncover the genotype of each allele
On the contrary, off-targeting events were detected in AR and RBFOX1 genes of Ig Heavy chain knockout pigs. Mismatches were identified where sgRNA could potentially bind and the presence of a potential PAM site was also located adjacent to the sgRNA binding site. The frequency of off-targeting on AR and RBFOX1 was 80 and 70%, respectively (Table 1). Specific types of off-targeting events are summarized in Table 2. It was found that pigs D and E from RBFOX1 gene had a biallelic mutation with no wild-type allele. Pig J carried three different genotypes in the RBFOX1 region with no wild-type sequence present. Genomic DNA of pigs K, L, and M were found to have polymorphisms in the RBFOX1 gene, while still having the wild-type sequence, indicating that the off-targeting affected only one allele. The RBFOX1 gene was modified in a homozygous fashion in the genomic DNA of pig C. Pigs A, B, and N showed no off-targeting or polymorphisms in the RBFOX1 gene. The AR gene was altered in biallelic fashion in the genomic DNA of pig E. Pigs B and M carried the wild-type AR gene, indicating no off-targeting caused by sgRNA targeting Ig Heavy chain. The other pigs examined carried modified AR gene in a heterozygous fashion.
Table 1.
Summary of off-targeting results for Ig Heavy chain. Five pigs were tested for each potential off-target gene. Five additional pigs were tested for the AR and RBFOX1 gene. Eight potential off target events were found in the AR gene and seven potential off-target events were found in the RBFOX1 gene. No off-target events were found in the other potential off-target genes
| Ig Heavy chain | ||
|---|---|---|
| Gene | Number of pigs | Number of off-target events (%) |
| 5′ to LRRF1P1 | 5 | 0 (0%) |
| EDIL3 | 5 | 0 (0%) |
| AR | 10 | 8 (80%) |
| MYLK3 | 5 | 0 (0%) |
| RBFOX1 | 10 | 7 (70%) |
| CCDC60 | 5 | 0 (0%) |
Table 2.
Summary of off-targeting events in AR gene and RBFOX1 gene. Ten pigs were analyzed for off-target events. Eight potential off-targets occurred in AR gene. Seven potential off-targets occurred in RBFOX1 gene
| Ig Heavy chain | ||||
|---|---|---|---|---|
| Pig | AR gene | WT present? | RBFOX1 gene | WT present? |
| WT | TCTCCCCCCAGGTTTTTGTGAGG | N/A | ACACCTCCCAGGCTTTTGTGG | N/A |
| A | TCTCCCCCCAGGTTTTTGTGAGG TCTCCCCCCAGGTTTTTTGTGAGG |
yes | ACACCTCCCAGGCTTTTGTGG | yes |
| B | TCTCCCCCCAGGTTTTTGTGAGG | yes | ACACCTCCCAGGCTTTTGTGG | yes |
| C | TCTCCCCCCAGGTTTTTGTGAGG TCTCCCCCCAGGTTTTTTTGTGA |
yes | ACACCTCCCAGGCTTGCAGGA | no |
| D | TCTCCCCCCAGGTTTTTGTGAGG TCTCCCCCCAGGTGAGGGAAGA |
yes | ACACCTCCCAGGCTTTTGGTG ACACCTCCCAGGCTTGCTGTA |
no |
| E | TCT CCCCCCAGGTTTTTGGTGAGG TCTCCCCCCAGGTTTTTGGGGGG |
no | ACACCTCCCAGGCTTAGCCTG ACACCTCCCAGGCTGGGGCTT |
no |
| J | TCTCCCCCCAGGTTTTTGTGAGG TCTCCCCCCAGGTAATGTAAGTG |
yes | ACACCTCCCAGGCTTAGCCTG ACACCTCCCA-----------GCTTGCAGGA ACACCACTAAAATGGGGC |
no |
| K | TCTCCCCCCAGGTTTTTGTGAGG TCTCCCCCCAGGTGAGGGAAGA |
yes | ACACCTCCCAGGCTTTTGTGG ACACCTCCCACACATCGCCTG |
yes |
| L | TCTCCCCCCAGGTTTTTGTGAGG TCTCCCCCCAGGTTTTTTGTGAAG |
yes | ACACCTCCCAGGCTTTTGTGG ACACCTCCCAGGCTTCCAGGA |
yes |
| M | TCTCCCCCCAGGTTTTTGTGAGG | yes | ACACCTCCCAGGCTTTTGTGG ACACCTCCCAGGCTTGCAGGG |
yes |
| N | TCTCCCCCCAGGTTTTTGTGAGG TCTCCCCCCAGGTTTTGGGAAGGG |
yes | ACACCTCCCAGGCTTTTGTGG | yes |
Following the discovery of off-targeting events in Ig Heavy chain knockout pigs, the frequency of off-targeting from a lower concentration of CRISPR/Cas9 system was examined. Genomic DNA isolated from preimplantation embryos injected with 2.5 ng/μL sgRNA and 5 ng/μL Cas9 mRNA (four times lower than the previous concentration) were used to detect off-targeting events on AR and RBFOX1. On-targeting efficiency to target Ig Heavy chain remained at 100%, although one embryo possessed wild-type allele in a heterozygous fashion. Similarly, although similar off-targeting frequency (6/7 for AR and 5/7 for RBFOX1) was found, there was no biallelic modification on either AR or RBFOX1 and all of the embryos except one (Embryo #3) possessed a wild-type allele (Table 3).
Table 3.
Summary of genotypes in Ig Heavy chain gene, AR gene and RBFOX1 gene in embryos injected with 2.5 ng/μL sgRNA and 5 ng/μL Cas9 mRNA. Seven embryos were analyzed for off-targeting events. Heterozygous: WT and one modified allele; Biallelic: two differently modified alleles; Homozygous: one type of modified alleles; Mosaic: more than two types of alleles and the WT sequence may or may not be present
| Embryo # | Ig Heavy Chain | AR | RBFOX1 |
|---|---|---|---|
| 1 | Biallelic | Heterozygous | WT |
| 2 | Heterozygous | Heterozygous | Heterozygous |
| 3 | Homozygous | Mosaic w/o WT | WT |
| 4 | Homozygous | WT | Mosaic w/ WT |
| 5 | Biallelic | Heterozygous | Heterozygous |
| 6 | Biallelic | Heterozygous | Heterozygous |
| 7 | Mosaic w/o WT | Heterozygous | Mosaic w/ WT |
Our overall off-targeting analyses were based on 4 sgRNAs used to generate GE pigs/fetuses. However, only one sgRNA resulted in off-targeting events. The low prevalence of sgRNA induced mutations indicates that CRISPR/Cas9 system can be applied to produce GE pigs without causing off-target events.
Discussion
The CRISPR/Cas9 system utilizes the crRNA, a 20-nucleotide long sequence that is complimentary to the target DNA sequence, to introduce site-specific modifications by inducing DSB on a PAM sequence [13]. Although effective, the CRISPR/Cas9 system can introduce unintended modifications, i.e. off-targeting, that can be permanent if the modifications are introduced into the germ line. Recent application of CRISPR/Cas9 system to correct genetically inherited diseases in human embryos [20] highlights its potential implication as an assisted reproductive technology. However, safety of the approach should be critically analyzed before being applied in clinics and exploring the genome of GE pigs generated through a similar approach (i.e. direct-injection of CRISPR/Cas9 system [16, 17, 22]) can be used to examine potential off-targeting events.
Among twenty-seven genes screened for off-targeting events, only two genes were found to be modified by the CRISPR/Cas9 system. Previous studies indicated that certain mismatches to sgRNAs can lead to inhibition/prevention of off-targeting events [3, 13, 23]; however, our results demonstrate the logic does not consistently apply. For instance, a previous study reported that cleavage activity of SpCas9 was prohibited by only a single-base mismatch up to 11 bp 5′ of the PAM site; however, mismatches further upstream of the PAM did not affect the nuclease activity [3]. On the contrary, although sgRNA targeting Ig Heavy chain had a mismatch at position 8 bp 5′ to the PAM of RBFOX1, nuclease activity of Cas9 was still active to have caused off-targeting events on the gene. Interestingly, the 5′ to LRRF1P1 locus also possessed similar mismatches (presence of PAM, mismatches on both off-targeting sensitive and insensitive positions as RBFOX1) compared to Ig Heavy chain sgRNA; however, there was no nuclease activity. This discrepancy indicates that it may be more challenging to predict off-target events than previously described. In a different study, it was found that mismatches on the 5′ side of the sgRNA of 1–5 base pairs can be tolerated thus causing off-targeting events, whereas mismatches at the 3′ end are less tolerated [13]. Our results were in agreement with this finding. In the case of AR gene, there were mismatches on the 5′ end only, and the nuclease activity of Cas9 remained and may have caused potential off-targeting. Another gene, EDIL3, also had mismatches only on 5′ end; however no PAM was present, therefore no nuclease activity was observed. These findings suggest that while previous findings are applicable for predicting off-target events, as in the case of AR gene, there was random high incidence of off-targeting found in the case of RBFOX1 gene that did not match previous reports for predicting the likelihood of off-target events.
Although the frequency of off-targeting on AR and RBFOX1 was high (above 70%), the efficiency of on-targeting, i.e. disrupting Ig Heavy chain was higher. No wild-type sequence was identified in any of the pigs generated [22], indicating that, although tolerable, mismatches on the 5′ side of sgRNA sequence does reduce the activity of CRISPR/Cas9 system. Similarly, more than two alleles were detected in the RBFOX1 gene of pig J, suggesting that the off-targeting was introduced after cell division, i.e. first cleavage of embryos. Off-targeting events induced by the CRISPR/Cas9 system are presumably through NHEJ, as the changes in nucleotides were random. Interestingly, most of the embryos that received the low concentration of CRISPR/Cas9 retained at least one wild-type allele (13/14), whereas the frequency (14/20) was lower in embryos that received the higher concentration. Due to the low number of observations, it is difficult to draw a statistically significant conclusion; however, the findings suggest that the prevalence of off-targeting may be decreased with the use of lower concentration of CRISPR/Cas9 RNA. On the other hand, the lower concentration impaired on-target efficiency as one embryo (Embryo #2) carried wild-type sequence of Ig Heavy chain, indicating that it is challenging to avoid off-targeting events by lowering concentration of CRISPR/Cas9 system without compromising on-target activity.
Since the oocytes used to generate GE pigs are collected from a local abattoir or purchased from a company, it is impossible to trace genetic background of the maternal side. Therefore, it was difficult to distinguish whether polymorphisms presented through genotyping were due to off-targeting events or naturally occurring polymorphism. Similarly, screening for off-targeting in outbred animals such as humans can be technically challenging because of existing variation in the genome. Traditional inbred animal models such as mice have homozygous genetic backgrounds [24]; however, pigs and humans have diverse genome sequences, which makes it difficult to assess off-targeting events at high accuracy [25, 26]. For instance, conventional surveyor assay and T7E1 analysis [27] may not be able to accurately reflect off-targeting due to the variations in the genome because any allele specific polymorphism may be interpreted as off-targeting events through the assays. Whole genome sequencing can offer a broad map of off-targeting events including unintended locations. However, whole genome sequencing on each genome edited embryo or animal is costly and whole genome sequencing followed by identifying mismatches to the reference genome may not be an absolute solution because any variation from whole genome sequencing needs to be carefully addressed to rule out potential variations from whole genome sequencing [28].
Predicting off-targeting events is technically challenging. Recent developments in computational based web software such as CRISPOR [29] provides a list of potential off-targeting genes that could be affected by designed sgRNA. Interestingly, the website predicts AR and RBFOX1 as potential off-targeting genes linked to the sgRNA used to target Ig Heavy chain, demonstrating its effective predictability. However, the website also suggests multiple off-targeting genes related to other sgRNAs targeting RAG2, IL2RG, or SCD5, although no off-targeting events induced by the sgRNAs were identified. This is not surprising as efficiency of CRISPR/Cas9 system can be dramatically affected by the concentrations of CRISPR/Cas9 system delivered into embryos [16]. Therefore, accurately predicting off-targeting events is a challenging process because of variables associated with utilizing CRISPR/Cas9 system to introduce targeted modifications.
Conclusion
Our study indicates that injecting the CRISPR/Cas9 system into developing embryos results in low incidence of off-targeting events and determining the likelihood of off-target occurrences may be more complex than previous studies have alluded to in outbred animal models. Our findings demonstrate that sequence identities on 5′ side are critical and the presence of a PAM site adjacent to sgRNA is crucial for off-targeting events. However, exceptions to the logic were also identified in our study, indicating that predicting off-targeting can be a challenging process. Off-targeting events reported here were on intron regions, thus unlikely to impact phenotype of resulting GE pigs. However, any unpredicted off-targeting events through clinical application of the technology may result in an irreversible outcome. Unexpected off-targeting events suggest that the technology should be carefully assessed and examined prior to its application.
Methods
Bioinformatic analysis to identify potential off-target sites
Potential off-targeting sites were identified as previously described [30, 31]. Specifically, a single-guide RNA (sgRNA) sequence for each gene was compared against the Sus scrofa reference genome (10.2 and 11.1) using Basic Local Alignment Search Tool (BLAST) through National Center for Biotechnology Information (NCBI). Annotated genes with at least 16 bp identity with the sgRNA sequence were selected regardless of the presence of possible protospacer adjacent motif (PAM) sequences adjacent to the off-target locations. The summary of potential off-target genes is shown in Additional file 1: Table S1-S4. For the RAG2, IL2RG, SCD5, and Ig Heavy chain knockout pigs, 9, 8, 4, and 6 potential off-target genes were manually identified, respectively. Figure 1 provides an overview of the schematic approach.
Production of genome-edited pigs
Genomic DNA of GE pigs are in part derived from our previous reports [16, 22] except for SCD5. To disrupt RAG2, IL2RG, Ig Heavy chain, and SCD5, all sgRNAs were designed using a web-based program (http://crispor.tefor.net/) [29]. Specifically, the region of the target gene was submitted through the CRISPOR software and then potential sgRNA were selected among the numerous sgRNAs suggested by the software. Potential target sites by sgRNAs were then BLASTed against the whole pig genome sequence through NCBI to examine specificity of the designed sgRNA. The sgRNAs and Cas9 mRNA were generated through in vitro transcription as previously described [16, 22, 32].
To generate the knockout pigs, first sow ovaries were obtained from a local abattoir or oocytes were purchased from DeSoto. The cumulus oocyte complexes (COCs) were maturated in IVM medium (TCM-199 based media containing FSH and LH, for 42–44 h at 38.5 °C, 5% CO2). After maturation, cumulus-free oocytes with a visible polar body were collected for in vitro fertilization (IVF). A group of 28–32 maturated oocytes were transferred into IVF media drops and 50 μl sperm (2.5 × 105 sperm/ml) was introduced into IVF media drops that contained maturated oocytes. The gametes were co-incubated for 5 h at 38.5 °C and 5% CO2. After IVF, presumable zygotes were placed in Porcine Zygote Media 3 (PZM-3) and incubated at 38.5 °C, 5% CO2, and 5% O2 for two hours and then the CRISPR/Cas9 system was injected on the heated stage of a Nikon inverted microscope (Nikon Corporation, Tokyo, Japan). Concentration of CRISPR/Cas9 was 5 ng/uL sgRNA and 20 ng/uL Cas9 mRNA for the generation RAG2 knockout and 10 ng/uL sgRNA and 20 ng/uL Cas9 mRNA for Ig Heavy chain, IL2RG, and SCD5 knockout generation. Injected zygotes were washed with PZM-3, then cultured in the PZM-3 until embryo transfer (ET). Day 5–6 after IVF, blastocysts and embryos carrying over 16 cells were transferred into surrogate gilts. The embryos were surgically transferred into the oviduct of the gilts. Pregnancy was determined by ultrasound at day 30–35 of gestation. Two embryo transfers were performed to generate 6 SCD5 fetuses (abortion at embryo day 98) (Additional file 1: Table S8). Genotyping results show that all six fetuses carry modified SCD5 gene without any wild-type sequence (Additional file 1: Table S9).
Screening for off-targeting events
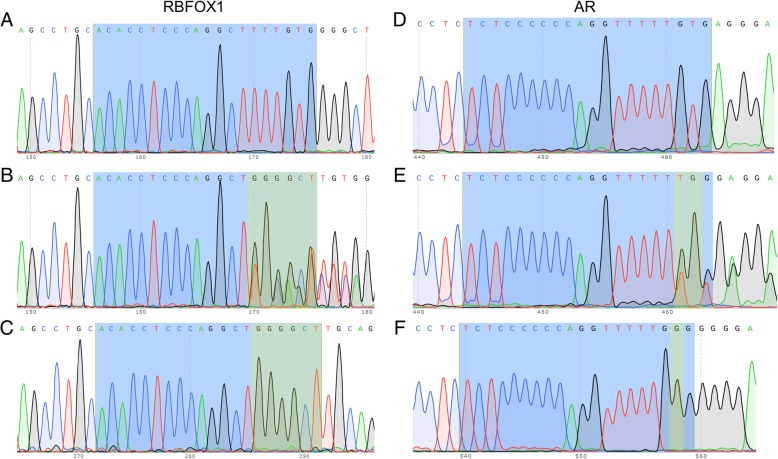
Primers were designed to amplify a fragment of genomic DNA flanking potential sgRNA binding sites (off-target sites). The off-targeting events were screened for five RAG2/IL2RG double knockout pigs, ten Ig Heavy chain knockout pigs, and six 98-day old SCD5 knockout fetuses. Information on the primers are included in Additional file 1: Tables S10-S13. PCR conditions to amplify off-target regions follow; initial denature at 95 °C for 2 min, denature at 95 °C for 30 s, anneal at 58 °C for 30 s, and extension at 72 °C for 30 s for 34 cycles, final extension at 72 °C for 5 min and holding at 4 °C. Then, PCR amplicons were sequenced at the Biocomplexity Institute of Virginia Tech. Genomic DNA from the boar used for IVF was used as a reference to identify potential sequences of paternally derived alleles. Size of the PCR products ranged from approximately 300 bp to 850 bp. In addition to the PCR sequencing, the PCR products were cloned into a sequencing vector to identify possible off-targeting events on each allele. Specifically, PCR products were cloned into the pCR 2.1 TOPO vector following manufacturer’s instructions. Then, plasmids derived from the cloning were sequenced (Fig. 2).
Fig. 2.
Chromatogram showing potential off-target activity on RBFOX1 and AR gene from Ig Heavy chain knockout pig. Similar approach was used to identify any off-target events on RAG2/IL2RG double knockout pigs or SCD5 knockout fetuses. a WT sequence of RBFOX1. b Polymorphic reading from Ig Heavy chain knockout pig E. c Allele specific reading from Ig Heavy chain knockout pig E. d WT sequence of AR. e Polymorphic reading from Ig Heavy chain knockout pig E. f Allele specific reading from Ig Heavy chain knockout pig E. Yellow highlighted portion of sequence indicates deviation from WT sequence reading
Analysis of off-targeting events
Sequencing results identically matching with Sus scrofa reference genome were considered to have no off-targeting events. Any mismatches to the reference genome were considered to be potentially due to off-targeting caused by CRISPR/Cas9 system. Sequence readings carrying the mismatches were BLASTed against all known pig genome sequences to investigate whether the mismatches were from known polymorphism sequences, especially in the intron regions.
Additional file
Table S1. Summary of off-targeting comparison for RAG2. Target gene (RAG2) guide sequence compared to potential off-target sequence. Location on chromosome, intron or exon region and presence of PAM site is indicated. Table S2. Summary of off-targeting comparison for IL2RG. Target gene (IL2RG) guide sequence compared to potential off-target sequence. Location on chromosome, intron or exon region and presence of PAM site is indicated. Table S3. Summary of off-targeting comparison for Ig heavy chain. Target gene (Ig heavy chain) guide sequence compared to potential off-target sequence. Location on chromosome, intron or exon region and presence of PAM site is indicated. Table S4. Summary of off-targeting comparison for SCD5. Target gene (SCD5) guide sequence compared to potential off-target sequence. Location on chromosome, intron or exon region and presence of PAM site is indicated. Table S5. Summary of off-targeting results for RAG2. Five pigs were tested for each potential off-target gene. No off-targeting events were found. Table S6. Summary of off-targeting results for IL2RG. Five pigs were tested for each potential off-target gene. No off-targeting events were found. Table S7. Summary of off-targeting results for SCD5. Six pigs were tested for each potential off-target gene. No off-targeting events were found. Table S8. Embryo transfer to generate SCD5 knockout fetuses. Day 5–6 embryos were transferred into surrogates. A total of 6 fetus were obtained from one surrogate. Table S9. Genotype of SCD5 knockout fetuses. No wild-type allele was identified from the fetuses. Table S10. Summary of primers used to amplify genes tested for potential off-targeting sites as well as original RAG2 target gene. Table S11. Summary of primers used to amplify genes tested for potential off-targeting sites as well as original IL2RG target gene. Table S12. Summary of primers used to amplify genes tested for potential off-targeting sites as well as original Ig heavy chain target gene. Table S13. Summary of primers used to amplify genes tested for potential off-targeting sites as well as original SCD5 gene. (PDF 451 kb)
Acknowledgements
Not applicable.
Funding
This project was supported in part by the Virginia Agricultural Experiment Station through the NIFA, U.S. Department of Agriculture. The funder had no role in the design of this study, data analysis, decision to publish, and preparation of the manuscript.
Availability of data and materials
All data or materials used during this study are available from the corresponding author on reasonable request.
Abbreviations
- BLAST
Basic Local Alignment Search Tool
- COC
Cumulus Oocyte Complex
- CRISPR
Clustered Regularly Interspaced Short Palindromic Repeats
- DSB
Double-Stranded Break
- ET
Embryo Transfer
- GE
Genetically Engineered
- IVF
in vitro Fertilization
- IVM
in vitro Maturation
- NCBI
National Center for Biotechnology Information
- PAM
Protospacer Adjacent Motif
- PZM-3
Porcine Zygote Media 3
- SCNT
Somatic Cell Nuclear Transfer
- sgRNA
Single-Guide RNA
- TALEN
Transcription Activator-Like Effector Nucleases
- WT
Wild-Type
- ZFN
Zinc Finger Nucleases
Authors’ contributions
KC conducted experiments identifying off-targeting events. JR, AJL, BAC, KL designed strategy to disrupt SCD5. JR, KU, SC, KL generated SCD5 knockout fetuses. KL supervised the project. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kayla Carey, Email: kaylac15@vt.edu.
Junghyun Ryu, Email: jhryu@vt.edu.
Kyungjun Uh, Email: joon84@vt.edu.
Andrea J. Lengi, Email: alengi@vt.edu
Sherrie Clark-Deener, Email: sherrie@vt.edu.
Benjamin A. Corl, Email: bcorl@vt.edu
Kiho Lee, Phone: 540-231-1906, Email: kiholee@vt.edu.
References
- 1.Carlson DF, Tan W, Lillico SG, Stverakova D, Proudfoot C, Christian M, Voytas DF, Long CR, Whitelaw CB, Fahrenkrug SC. Efficient TALEN-mediated gene knockout in livestock. Proc Natl Acad Sci U S A. 2012;109(43):17382–17387. doi: 10.1073/pnas.1211446109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang D, Yang H, Li W, Zhao B, Ouyang Z, Liu Z, Zhao Y, Fan N, Song J, Tian J, et al. Generation of PPARgamma mono-allelic knockout pigs via zinc-finger nucleases and nuclear transfer cloning. Cell Res. 2011;21(6):979–982. doi: 10.1038/cr.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science (New York, NY) 2013;339(6121):819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaj Thomas, Gersbach Charles A., Barbas Carlos F. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends in Biotechnology. 2013;31(7):397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu Y, Foden JA, Khayter C, Maeder ML, Reyon D, Joung JK, Sander JD. High frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat Biotechnol. 2013;31(9):822–826. doi: 10.1038/nbt.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitworth KM, Benne JA, Spate LD, Murphy SL, Samuel MS, Murphy CN, Richt JA, Walters E, Prather RS, Wells KD. Zygote injection of CRISPR/Cas9 RNA successfully modifies the target gene without delaying blastocyst development or altering the sex ratio in pigs. Transgenic Res. 2017;26(1):97–107. doi: 10.1007/s11248-016-9989-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanihara F, Hirata M, Nguyen NT, Le QA, Hirano T, Takemoto T, Nakai M, Fuchimoto DI, Otoi T. Generation of a TP53-modified porcine cancer model by CRISPR/Cas9-mediated gene modification in porcine zygotes via electroporation. PLoS One. 2018;13(10):e0206360. doi: 10.1371/journal.pone.0206360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petersen B, Frenzel A, Lucas-Hahn A, Herrmann D, Hassel P, Klein S, Ziegler M, Hadeler KG, Niemann H. Efficient production of biallelic GGTA1 knockout pigs by cytoplasmic microinjection of CRISPR/Cas9 into zygotes. Xenotransplantation. 2016;23(5):338–346. doi: 10.1111/xen.12258. [DOI] [PubMed] [Google Scholar]
- 9.Kang JT, Ryu J, Cho B, Lee EJ, Yun YJ, Ahn S, Lee J, Ji DY, Lee K, Park KW. Generation of RUNX3 knockout pigs using CRISPR/Cas9-mediated gene targeting. Reprod Domest Anim. 2016;51(6):970–978. doi: 10.1111/rda.12775. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Yang HY, Wang Y, Zhang ML, Liu XR, Xiong Q, Zhang LN, Jin Y, Mou LS, Liu Y, et al. Generation of tryptophan hydroxylase 2 gene knockout pigs by CRISPR/Cas9-mediated gene targeting. J Biomed Res. 2017;31(5):445–452. doi: 10.7555/JBR.31.20170026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han K, Liang L, Li L, Ouyang Z, Zhao B, Wang Q, Liu Z, Zhao Y, Ren X, Jiang F, et al. Generation of Hoxc13 knockout pigs recapitulates human ectodermal dysplasia-9. Hum Mol Genet. 2017;26(1):184–191. doi: 10.1093/hmg/ddw378. [DOI] [PubMed] [Google Scholar]
- 12.Yang H, Wu Z. Genome editing of pigs for agriculture and biomedicine. Front Genet. 2018;9:360. doi: 10.3389/fgene.2018.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang XH, Tee LY, Wang XG, Huang QS, Yang SH. Off-target effects in CRISPR/Cas9-mediated genome engineering. Molecular therapy Nucleic acids. 2015;4:e264. doi: 10.1038/mtna.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doench JG, Fusi N, Sullender M, Hegde M, Vaimberg EW, Donovan KF, Smith I, Tothova Z, Wilen C, Orchard R, et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat Biotechnol. 2016;34(2):184–191. doi: 10.1038/nbt.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C, Zhou S, Li Y, Li G, Ding Y, Li L, Liu J, Qu L, Sonstegard T, Huang X, et al. Trio-based deep sequencing reveals a low incidence of off-target mutations in the offspring of genetically edited goats. Front Genet. 2018;9:449. doi: 10.3389/fgene.2018.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lei S, Ryu J, Wen K, Twitchell E, Bui T, Ramesh A, Weiss M, Li G, Samuel H, Clark-Deener S, et al. Increased and prolonged human norovirus infection in RAG2/IL2RG deficient gnotobiotic pigs with severe combined immunodeficiency. Sci Rep. 2016;6:25222. doi: 10.1038/srep25222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitworth KM, Lee K, Benne JA, Beaton BP, Spate LD, Murphy SL, Samuel MS, Mao J, O'Gorman C, Walters EM, et al. Use of the CRISPR/Cas9 system to produce genetically engineered pigs from in vitro-derived oocytes and embryos. Biol Reprod. 2014;91(3):78. doi: 10.1095/biolreprod.114.121723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walters EM, Prather RS. Advancing swine models for human health and diseases. Mo Med. 2013;110(3):212–215. [PMC free article] [PubMed] [Google Scholar]
- 19.Oestrup O, Hall V, Petkov SG, Wolf XA, Hyldig S, Hyttel P. From zygote to implantation: morphological and molecular dynamics during embryo development in the pig. Reprod Domest Anim. 2009;44(Suppl 3):39–49. doi: 10.1111/j.1439-0531.2009.01482.x. [DOI] [PubMed] [Google Scholar]
- 20.Ma H, Marti-Gutierrez N, Park SW, Wu J, Lee Y, Suzuki K, Koski A, Ji D, Hayama T, Ahmed R, et al. Correction of a pathogenic gene mutation in human embryos. Nature. 2017;548(7668):413–419. doi: 10.1038/nature23305. [DOI] [PubMed] [Google Scholar]
- 21.Cyranoski D, Ledford H. Genome-edited baby claim provokes international outcry. Nature. 2018;563(7733):607–608. doi: 10.1038/d41586-018-07545-0. [DOI] [PubMed] [Google Scholar]
- 22.Yugo DM, Heffron CL, Ryu J, Uh K, Subramaniam S, Matzinger SR, Overend C, Cao D, Kenney SP, Sooryanarain H, et al. Infection dynamics of hepatitis E virus in wild-type and immunoglobulin heavy chain knockout JH (−/−) gnotobiotic piglets. J Virol. 2018. [DOI] [PMC free article] [PubMed]
- 23.Zheng T, Hou Y, Zhang P, Zhang Z, Xu Y, Zhang L, Niu L, Yang Y, Liang D, Yi F, et al. Profiling single-guide RNA specificity reveals a mismatch sensitive core sequence. Sci Rep. 2017;7:40638. doi: 10.1038/srep40638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doran AG, Wong K, Flint J, Adams DJ, Hunter KW, Keane TM. Deep genome sequencing and variation analysis of 13 inbred mouse strains defines candidate phenotypic alleles, private variation and homozygous truncating mutations. Genome Biol. 2016;17(1):167. doi: 10.1186/s13059-016-1024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nature ISMWGJ: A map of human genome sequence variation containing 1.42 million single nucleotide polymorphisms. 2001, 409(6822):928. [DOI] [PubMed]
- 26.Archibald Alan L, Bolund Lars, Churcher Carol, Fredholm Merete, Groenen Martien A M, Harlizius Barbara, Lee Kyung-Tai, Milan Denis, Rogers Jane, Rothschild Max F, Uenishi Hirohide, Wang Jun, Schook Lawrence B, Genome Sequencing Consortium Swine. Pig genome sequence - analysis and publication strategy. BMC Genomics. 2010;11(1):438. doi: 10.1186/1471-2164-11-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vouillot L, Thélie A, Pollet N. Comparison of T7E1 and surveyor mismatch cleavage assays to detect mutations triggered by engineered nucleases. G3 (Bethesda, Md) 2015;5(3):407–415. doi: 10.1534/g3.114.015834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ajay SS, Parker SC, Abaan HO, Fajardo KV, Margulies EH. Accurate and comprehensive sequencing of personal genomes. Genome Res. 2011;21(9):1498–1505. doi: 10.1101/gr.123638.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haeussler M, Schonig K, Eckert H, Eschstruth A, Mianne J, Renaud JB, Schneider-Maunoury S, Shkumatava A, Teboul L, Kent J, et al. Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biol. 2016;17(1):148. doi: 10.1186/s13059-016-1012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee K, Kwon DN, Ezashi T, Choi YJ, Park C, Ericsson AC, Brown AN, Samuel MS, Park KW, Walters EM, et al. Engraftment of human iPS cells and allogeneic porcine cells into pigs with inactivated RAG2 and accompanying severe combined immunodeficiency. Proc Natl Acad Sci U S A. 2014;111(20):7260–7265. doi: 10.1073/pnas.1406376111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi YJ, Lee K, Park WJ, Kwon DN, Park C, Do JT, Song H, Cho SK, Park KW, Brown AN, et al. Partial loss of interleukin 2 receptor gamma function in pigs provides mechanistic insights for the study of human immunodeficiency syndrome. Oncotarget. 2016;7(32):50914–50926. doi: 10.18632/oncotarget.10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryu J, Lee K. CRISPR/Cas9-mediated gene targeting during embryogenesis in swine. Methods Mol Biol. 2017;1605:231–244. doi: 10.1007/978-1-4939-6988-3_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of off-targeting comparison for RAG2. Target gene (RAG2) guide sequence compared to potential off-target sequence. Location on chromosome, intron or exon region and presence of PAM site is indicated. Table S2. Summary of off-targeting comparison for IL2RG. Target gene (IL2RG) guide sequence compared to potential off-target sequence. Location on chromosome, intron or exon region and presence of PAM site is indicated. Table S3. Summary of off-targeting comparison for Ig heavy chain. Target gene (Ig heavy chain) guide sequence compared to potential off-target sequence. Location on chromosome, intron or exon region and presence of PAM site is indicated. Table S4. Summary of off-targeting comparison for SCD5. Target gene (SCD5) guide sequence compared to potential off-target sequence. Location on chromosome, intron or exon region and presence of PAM site is indicated. Table S5. Summary of off-targeting results for RAG2. Five pigs were tested for each potential off-target gene. No off-targeting events were found. Table S6. Summary of off-targeting results for IL2RG. Five pigs were tested for each potential off-target gene. No off-targeting events were found. Table S7. Summary of off-targeting results for SCD5. Six pigs were tested for each potential off-target gene. No off-targeting events were found. Table S8. Embryo transfer to generate SCD5 knockout fetuses. Day 5–6 embryos were transferred into surrogates. A total of 6 fetus were obtained from one surrogate. Table S9. Genotype of SCD5 knockout fetuses. No wild-type allele was identified from the fetuses. Table S10. Summary of primers used to amplify genes tested for potential off-targeting sites as well as original RAG2 target gene. Table S11. Summary of primers used to amplify genes tested for potential off-targeting sites as well as original IL2RG target gene. Table S12. Summary of primers used to amplify genes tested for potential off-targeting sites as well as original Ig heavy chain target gene. Table S13. Summary of primers used to amplify genes tested for potential off-targeting sites as well as original SCD5 gene. (PDF 451 kb)
Data Availability Statement
All data or materials used during this study are available from the corresponding author on reasonable request.