Abstract
Parasitic infection risks in domestic animals may increase as a result of outdoor activities, often leading to transmission events to and from owners, other domestic animals and wildlife. Furthermore, outdoor access has not been quantified in domestic animals as a risk factor with respect to latitude or parasite transmission pathway. Cats are an ideal model to test parasitic infection risk in outdoor animals because there have been many studies analysing this risk factor in this species; and there is a useful dichotomy in cat ownership between indoor-only cats and those with outdoor access. Thus, we used meta-analysis to determine whether outdoor access is a significant risk factor for parasitic infection in domestic pet cats across 19 different pathogens including many relevant to human, domestic animal and wildlife health, such as Toxoplasma gondii and Toxocara cati. Cats with outdoor access were 2.77 times more likely to be infected with parasites than indoor-only cats. Furthermore, absolute latitude trended towards significance such that each degree increase in absolute latitude increased infection likelihood by 4%. Thus, restricting outdoor access can reduce the risk of parasitic infection in cats and reduce the risk of zoonotic parasite transmission, spillover to sympatric wildlife and negative impacts on feline health.
Keywords: felid, latitude, pathogen, pet, transmission, zoonotic
1. Background
Domestic animals, including pets, are responsible for spreading pathogens to humans and sympatric wildlife [1–3]. Notable examples include dogs transmitting rabies to humans [4] or cattle transmitting Cryptosporidium parvum to humans and sympatric wild ruminants [5,6]. However, relatively few domestic animals have such stark dichotomies regarding outdoor access, where environmental contact can, therefore, be evaluated as a means of exposure. Understanding how outdoor access affects infection, and infection by which pathogens are most affected by this risk factor, can have important implications when mitigating parasite transmission among domestic animals, humans and wildlife.
A model organism that is widespread and lives in close proximity to humans is the domestic cat (Felis catus), which has coexisted with humans globally for millennia (ca 9500 years; [7,8]). In fact, pet cats often sit on their owners' laps and sleep in their beds [9]. Furthermore, cats are common as pets around the world, with an estimated 89–90 million in the USA alone [10]. Given that cats are widespread and associated with humans, risk factors for parasitic infections in pet cats are important for zoonotic parasite transmission, with implications for cat health as well as spillover of parasites to sympatric wildlife [11,12].
Domestic pet cats allowed outdoors can also pose health risks to cat owners [13–19]. For instance, Toxoplasma gondii (the causative agent of toxoplasmosis; [15]) and Bartonella henslae (which causes cat-scratch disease; [17]), both infect people worldwide. In addition, there are many infectious diseases that have health consequences for cats themselves. For example, feline immunodeficiency virus (FIV) causes immunosuppression which can increase susceptibility to other infections [20]. Finally, interactions with sympatric wildlife may result in spillover of parasites from domestic cats (table 1). For example, domestic cats have been responsible for the spread of FIV to mountain lions (Puma concolor) and feline panleukopenia to the Florida panther (Puma concolor coryi) [11,12].
Table 1.
Host ranges of pathogens analysed in this study.
| pathogen | hosts | citation(s) |
|---|---|---|
| Aelurostrongylus abstrusus | caracal (Caracal caracal), lion (Panthera leo), serval (Leptailurus serval) | [21] |
| Cystoisospora felis | Felidae (including European wild cat (Felis sylvestris), ocelot (Felis pardalis), serval (Felis serval), tiger (Leo tigris), jaguar (Leo onca), Eurasian lynx (Lynx lynx)), house mouse (Mus musculus), golden hamster (Mesocricetus auratus) | [22] |
| Cystoisospora revolta | Felidae (including European wild cat, jungle cat (Felis chaus), tiger, leopard (Leo pardus)), house mouse, opossum (Didelphis virginiana), Norway rat (Rattus norvegicus), golden hamster | [23] |
| Cytauxzoon spp. | meerkat (Suricata suricatta), bobcat (Lynx rufus), cougar (Puma concolor), Florida panther (Felix concolor coryi), ocelot, puma (Puma yagouaroundi), jaguar (Panthera onca) | [24–27] |
| Dipylidium caninum | crab-eating fox (Cerdocyon thous), red fox (Vulpes vulpes), golden jackal (Canis aureus), wolf (Canis lupus) | [28,29] |
| feline coronavirus | Felidae (including cheetah (Acinonyx jubatus), European wildcat, Canada lynx (Lynx canidensis)) | [30–32] |
| feline leukemia virus | Felidae (including European wildcat), spotted hyena (Crocuta crocuta) | [31–33] |
| feline immunodeficiency virus | Felidae (including European wildcat, sand cat (Felis margarita)), spotted hyena | [31–33] |
| Giardia lamblia | Giardia affects a large number of mammal and bird species, but it appears that the assemblage in domestic cats is not found in other species | [34] |
| Hemoplasma spp. | Iberian lynx, Eurasian lynx, European wildcat, lion, puma, oncilla (Leopardus tigrinis), Geoffroy's cat (Leopardus geoffroyi), margay (Leopardus wiedii), ocelot | [35] |
| Hepatozoon spp. | coyote (Canis latrans), bobcat, ocelot | [36] |
| Mycoplasma spp. | Iberian lynx, Eurasian lynx, lion, European wildcat | [37] |
| Neospora caninum | Canidae (including red fox, grey fox (Urocyon cinereoargeneteus), Australian dingo (Canis familiaris dingo), Chiloé fox (Pseudolapex fulvipes)), cheetah, raccoon (Procyon lotor) | [38,39] |
| Taenia spp. | several Taenia species infect a wide variety of carnivores | [40] |
| Toxocara cati | can infect small mammals (including Guinea pigs (Cavia porcellus) and house mouse but data are lacking | [41] |
| Toxoplasma gondii | wide host range of almost any bird or mammal evaluated | [42] |
| Trichuris spp. | widespread across mammal species depending on species of Trichuris | [43,44] |
| Troglostrongylus brevior | European wild cat | [45] |
Many parasites known to infect cats have life cycles involving transmission from the soil, prey, or other cats [15,46–49]. Here, we hypothesize that cats with outdoor access (free-roaming) will be more likely to be infected with parasites than indoor-only cats. To test our hypothesis, we conducted a meta-analysis of outdoor access as a risk factor for infection across 19 pathogens and 16 countries. Because differences in risk of infection may exist owing to changes in pathogen diversity (i.e. richness and abundance) across transmission type and space [50–52], we considered transmission type and latitude as separate moderators.
2. Results
(a). Overall effects
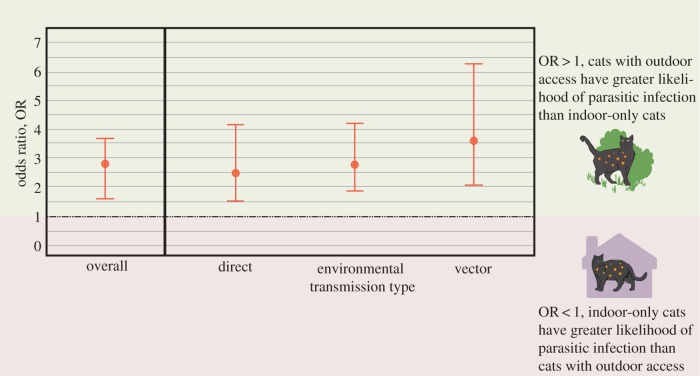
Our synthesis incorporated 21 studies with 31 sets of infection prevalence between indoor-only cats and those with outdoor access (table 2). Among the 21 studies, 19 parasites were analysed (see electronic supplementary material, figure S1 for odds ratios (OR) by parasite and study). According to the overall model, cats with outdoor access are 2.77 (95% confidence limits (95% CL) = 2.10–3.67; p < 0.0001) times as likely to be infected with parasites as indoor-only cats (figure 1). Heterogeneity, or differences in outcomes between studies [70], in the overall model was high (I2 = 84.02%). The publication bias analysis estimated six missing studies on the left side of the funnel plot (figure 2a,b) and incorporation of these randomly created studies using the trim and fill technique still resulted in the effect of outdoor access as a significant risk factor (2.39 OR; p < 0.0001).
Table 2.
Pathogen prevalence in domestic cats (Felis catus) in this study by country.
| pathogen | country | prevalence | citation |
|---|---|---|---|
| Aelurostrongylus abstrusus | Cyprus | 0.02 | [53] |
| Cystoisospora revolta | Cyprus | 0.12 | [53] |
| Cytauxzoon spp. | Spain | 0.01 | [54] |
| Dipylidium caninum | Cyprus | 0.01 | [53] |
| feline coronavirus | Australia | 0.41 | [55] |
| FIV | Australia | 0.10 | [56] |
| 0.31 | [57] | ||
| Canada | 0.63 | [58] | |
| Giardia lamblia | Cyprus | 0.07 | [53] |
| Hemoplasma spp. | Chile | 0.15 | [59] |
| Mycoplasma spp. | Spain | 0.07 | [54] |
| Germany | 0.10 | [60] | |
| Switzerland | 0.09 | [61] | |
| Neospora caninum | Brazil | 0.03 | [62] |
| Taenia spp. | Cyprus | 0.01 | [53] |
| Toxocara spp. | Cyprus | 0.12 | [53] |
| Netherlands | 0.05 | [63] | |
| Toxoplasma gondii | Estonia | 0.62 | [64] |
| Pakistan | 0.26 | [65] | |
| Latvia | 0.53 | [66] | |
| Romania | 0.48 | [67] | |
| Trichuris spp. | St Kitts | 0.22 | [68] |
| Troglostrongylus spp. | Cyprus | 0.05 | [53] |
| Netherlands | 0.20 | [69] |
Figure 1.
Overall effect size and transmission type effect sizes for infection prevalence in cats with outdoor access versus indoor-only cats. Cats with outdoor access are 2.77 (95% CL = 2.10–3.67; p < 0.0001) times as likely to be infected with parasites as indoor-only cats. Transmission types include environmental (soil-borne and intermediate hosts), vector-borne and direct. Transmission type was not a significant moderator (p = 0.62) for outdoor access on infection prevalence in domestic pet cats.
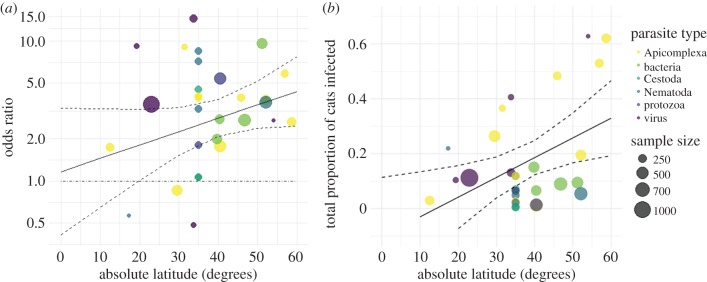
Figure 2.
(a) The relationship between odds ratio for each study/parasite in domestic pet cats across a range of latitudes. For every degree increase in latitude, cats with outdoor access were 1.04 times as likely to be infected with parasites (95% CL = 1.01–1.07). Latitude as a moderator to indoor/outdoor infection risk was trending towards significance (p = 0.081). (b) Total proportions of infected cats for each study/parasite across a range of latitudes where overall proportion of infected cats significantly increased, by 0.7% (95% CL = 0.17–1.3%; p = 0.010) for each degree latitude increase.
(b). Moderators
Transmission type was not a significant moderator (p = 0.62; figure 1), but infection risk in indoor-only pet cats versus those with outdoor access trended towards significance with latitude (figure 2). Specifically, for every degree increase in absolute latitude, cats with outdoor access were 4% more likely to be infected with parasites (95% CL = 1.0–7.0%; p = 0.081; figure 2a). Heterogeneity decreased considerably with the inclusion of this moderator to I2 = 55.7% (from 84.0%), suggesting differences in latitude may account for a significant portion of the variation among studies.
To determine the true effect of increasing latitude (since OR is only a relative comparison of indoor-only and outdoor cats), we also conducted a meta-regression using a raw proportion of the total number of infected cats, with absolute latitude as a moderator. In this model, the overall proportion of infected cats significantly increased, by 0.7% (95% CL = 0.17–1.3%; OR 95% CL = 1.01–1.07; p = 0.010) for each degree latitude increase (figure 2b), indicating that increasing risk of infection in cats with outdoor access with increasing latitude is an important interaction.
3. Discussion
Outdoor access is a significant risk factor for parasitic infection in pet cats, where cats with outdoor access were 2.77 times more likely to be infected with parasites than indoor-only cats, demonstrating support for our hypothesis. Of the 21 studies we included, only three suggested non-significantly higher risk of infection in indoor-only cats. Furthermore, latitude had a marginally significant effect on the likelihood of infection. While there was publication bias indicating positive results for outdoor access as a risk factor, following the trim and fill method the effects were similar and still significant, suggesting publication bias did not influence the significance of the meta-analysis results.
The parasites we analysed have relevance to zoonotic parasite transmission, feline health and wildlife conservation. Given the association between humans and domestic cats [9], habitat and lifestyle risk factors ought to be investigated with respect to zoonotic parasite infection. Furthermore, despite ubiquity of domestic cats, cat–human transmission is likely under-reported [71].
Not only are parasitic infections impactful to feline health, they are also relevant to wildlife. Parasites of domestic cats have already been reported in sympatric wild congeners, such as FIV in cougars (Felis concolor) and Candidatus Mycoplasma haemominutum in wild felids deriving from domestic cats [11,12,37]. Positive associations between feline herpesvirus type 1 (FHV-1) and Bartonella in cougars and urban land-use have also been reported, suggesting interactions with domestic cats [72]. However, further investigation into infection prevalence in wild populations and risk factors for transmission between domestic cats and these species is warranted [12].
Among the transmission types analysed (i.e. direct, vector-borne and environmental), none differed significantly from either of the others with respect to effect of outdoor access on parasitic infection. Two explanations are the small sample size between groups or within studies, and high variability across studies. Additionally, a Bayesian approach using a Markov chain Monte Carlo method may have better accounted for this uncertainty [73]. Directly transmitted parasites (i.e. cat–cat transmission), such as FIV, were not significantly different from other transmission types with respect to outdoor access, which suggests these parasites may be more frequently encountered through contact with feral populations or other pet cats allowed outdoor access rather than from cats in shelters or the household.
Latitude as a moderator on infection risk in cats with outdoor access trended towards a significant positive effect. The trend identified ran contrary to what has been demonstrated for parasite richness and diversity, which typically decrease with increasing latitude [50–52]. Although one might assume that higher parasite diversity results in higher infection risk in hosts, there have been multiple findings demonstrating the opposite—that infection rates decrease with higher parasite diversity [74,75]—which is consistent with our finding that cats with outdoor access in northern regions are at greater risk of infection. Interestingly, these results were also consistent with global patterns of zoonoses in rodents, a common prey of domestic cats, where higher latitudes saw greater numbers of species carrying zoonoses [76]. Higher latitudes also predicted greater risk of helminth parasites from wildlife found in domestic animals [2].
Organizations including the American Bird Conservancy (ABC) and People for the Ethical Treatment of Animals (PETA) have created campaigns that raise awareness about the detrimental impacts of cats with outdoor access in relation to feline health and impacts on wildlife [77,78], though allowing pet cats outdoors is still common occurrence [79,80]. Increased awareness of the risks involved in outdoor access is one facet, but legislation restricting outdoor access in cats would be an ideal outcome [81]. Despite hurdles in enacting new legislation, this issue has a relatively simple solution—keep cats indoors.
Domestic cats act as potent reservoirs for parasites transmissible to wildlife and humans [82–84], and are a unique model for understanding pathogen transmission dynamics given their global ubiquity and contact with humans, other animals and the environment. Our analysis is the first to our knowledge to summarize across many parasites and geographical localities that outdoor access increases the odds of parasitic infection in pet cats as a model for domestic animals. Future research might investigate this risk factor across other domestic species and across factors, such as land use and presence of sympatric congeners. While we do not necessarily advocate that all domestic animals be restricted indoors, determining routes and risk factors of transmission with respect to environmental contact may be useful in mitigating parasitic infection in domestic animals.
4. Methods
(a). Literature search
A literature search using Web of Science was conducted on 11 January 2018, following PRISMA [85] guidelines, with the following keywords: ‘feral cat’ OR ‘feral dog*’ AND ‘infect*’ OR ‘parasit*’ OR ‘disease*’ OR ‘virus*’, excluding reviews. This search returned 500 research articles, which were manually sorted for relevance. Final output was based on the following exclusion criteria: review articles; case studies; sample size less than 20 cats; lack of comparison between indoor-only versus outdoor access pet domestic cats; or outdoor access group included feral or stray cats.
An additional search was performed in Web of Science on 31 May 2018, using the following keywords: ‘domestic cat*’ OR ‘pet cat*’ OR ‘Felis catus’ AND ‘outdoor access’ AND TOPIC: (‘infection*’ OR ‘parasit*’ OR ‘disease*’ OR ‘pathogen*’ OR ‘virus*’ OR ‘sick*’ OR ‘illness*’), which returned 213 additional articles. One search was conducted in Google Scholar using the keywords as follows: domestic OR pet cat OR Felis catus, outdoor access, infection* OR parasit*. This Google Scholar search returned 1190 results. We manually sorted through the first 100 studies using the exclusion criteria described above. After manually sorting the original output of 813 studies, 21 studies fitted the inclusion criteria and were used in the meta-analysis [86] (see https://figshare.com/s/3eebaf42e161c0e7e1ef to access dataset).
(b). Treatment of moderators
Parasite transmission type included direct, vector-borne and environmental pathways (see electronic supplementary material, figure S2 for list of citations for each parasite). Latitude of each study was determined using Google Earth by selecting the middle of the smallest geographical area provided (such as country, state/province or city). Studies that included multiple countries were removed from analysis of this moderator.
(c). Statistical analysis
All analyses were completed in R v.1.1.453 using the metafor package for random effects models to account for between-study heterogeneity using the OR effect size [87,88], where an OR is the probability of an outcome as related to an exposure [89]. Here, the outcome is likelihood of infection as related to outdoor access as the exposure mechanism. OR = 1 means outdoor access does not affect the likelihood of infection; OR < 1 (upper 95% CI is less than 1) means outdoor access is associated with lower odds of infection; and OR > 1 (lower 95% CI is greater than 1) means outdoor access is associated with greater odds of infection. We considered p < 0.05 to indicate the significance of effect size. Two moderators, transmission type and latitude, were evaluated using mixed effects models.
To estimate heterogeneity across studies, we used I2, where a value of 0% indicates no heterogeneity; 25%, low heterogeneity; 50%, moderate; and 75% is considered high heterogeneity [90]. To test for publication bias, we used a trim and fill method to estimate the number of missing studies [91].
Supplementary Material
Acknowledgements
Thanks to the Auburn School of Forestry and Wildlife Science SQUAD (Solving Quantitative, Unusual and Awesome Dilemmas); Todd Steury and Ash Abebe for help with data analysis and interpretation of results; and Patricia Hartman for help conducting the literature search.
Data accessibility
Literature search: Figshare repository figshare.com/s/3eebaf42e161c0e7e1ef [86]. R code in analyses: Figshare repository figshare.com/s/a334c7815b128cb63b98 [87].
Authors' contributions
K.C. designed the study, conducted literature review and analyses, and wrote the manuscript; A.E.W. participated in statistical analyses, study design and manuscript writing; C.A.L. participated in statistical analyses and manuscript writing; S.Z. participated in statistical analyses and manuscript writing. All authors gave approval for the final version of this manuscript, and agree to be accountable for its content.
Competing interests
The authors declare no competing interests.
Funding
K.C. was supported by the Auburn University Cell and Molecular Biology Fellowship Program. Funding for S.Z. was provided by a Young Investigator Award from the USDA National Institute of Food and Agriculture, and CDC-RFA- CK14-1401PPHF. This project was supported by the Alabama Agricultural Experiment Station, the Hatch Program of the National Institute of Food and Agriculture, U.S. Department of Agriculture.
References
- 1.Landaeta-Aqueveque C, Henríquez A, Cattan PE. 2014. Introduced species: domestic mammals are more significant transmitters of parasites to native mammals than are feral mammals. Int. J. Parasitol. 44, 243–249. ( 10.1016/j.ijpara.2013.12.002) [DOI] [PubMed] [Google Scholar]
- 2.Wells K, Gibson DI, Clark NJ, Ribas A, Morand S, McCallum HI. 2018. Global spread of helminth parasites at the human–domestic animal–wildlife interface. Glob. Chang. Biol. 24, 3254–3265. ( 10.1111/gcb.14064) [DOI] [PubMed] [Google Scholar]
- 3.Clark NJ, Seddon JM, Šlapeta J, Wells K. 2018. Parasite spread at the domestic animal - wildlife interface: anthropogenic habitat use, phylogeny and body mass drive risk of cat and dog flea (Ctenocephalides spp.) infestation in wild mammals. Parasites Vectors 11, 8. ( 10.1186/s13071-017-2564-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang X, Luo M, Zhang S, Fooks AR, Hu R, Tu C. 2005. Pivotal role of dogs in rabies transmission, China. Emerg. Infect. Dis. 11, 1970–1972. ( 10.3201/eid1112.050271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alves M, Xiao L, Sulaiman I, Lal AA, Matos O, Antunes F. 2003. Subgenotype analysis of Cryptosporidium isolates from humans, cattle, and zoo ruminants in Portugal. J. Clin. Microbiol. 41, 2744–2747. ( 10.1128/JCM.41.6.2744-2747.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alves M, Xiao L, Antunes F, Matos O. 2006. Distribution of Cryptosporidium subtypes in humans and domestic and wild ruminants in Portugal. Parasitol. Res. 99, 287–292. ( 10.1007/s00436-006-0164-5) [DOI] [PubMed] [Google Scholar]
- 7.Driscoll CA, et al. 2007. The Near Eastern origin of cat domestication. Science 317, 519–523. ( 10.1126/science.1139518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming PA, Bateman PW. 2018. Novel predation opportunities in anthropogenic landscapes. Anim. Behav. 138, 145–155. ( 10.1016/j.anbehav.2018.02.011) [DOI] [Google Scholar]
- 9.Chomel BB, Sun B. 2011. Zoonoses in the bedroom. Emerg. Infect. Dis. 17, 167–172. ( 10.3201/eid1702.101070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lepczyk CA, Duffy DC. 2018. Feral cats. In Ecology and management of terrestrial vertebrate invasive species in the United States (eds WC Pitt, J Beasley, GW Witmer), pp. 269–310. Boca Raton, FL: CRC Press. [Google Scholar]
- 11.Jessup DA, Pettan KC, Lowenstine LJ, Pedersen NC. 1993. Feline leukemia virus infection and renal spirochetosis in a free-ranging cougar (Felis concolor). J. Zoo Wildl. Med. 24, 73–79. [Google Scholar]
- 12.Roelke ME, Forrester DJ, Jacobson ER, Kollias GV, Scott FW, Barr MC, Evermann JF, Pirtle EC. 1993. Seroprevalence of infectious disease agents in free-ranging Florida panthers (Felis concolor coryi). J. Wildl. Dis. 29, 36–49. ( 10.7589/0090-3558-29.1.36) [DOI] [PubMed] [Google Scholar]
- 13.Lepczyk CA, Lohr CA, Duffy DC. 2015. A review of cat behavior in relation to disease risk and management options. Appl. Anim. Behav. Sci. 173, 29–39. ( 10.1016/j.applanim.2015.07.002) [DOI] [Google Scholar]
- 14.Loyd KAT, Hernandez SM, Abernathy KJ, Shock BC, Marshall GJ. 2013. Risk behaviours exhibited by free-roaming cats in a suburban US town. Vet. Rec. 173, 295 ( 10.1136/vr.101222) [DOI] [PubMed] [Google Scholar]
- 15.Hill D, Dubey JP. 2002. Toxoplasma gondii: transmission, diagnosis and prevention. Clin. Microbiol. Infect. 8, 634–640. ( 10.1046/j.1469-0691.2002.00485.x) [DOI] [PubMed] [Google Scholar]
- 16.Fisher M. 2003. Toxocara cati: an underestimated zoonotic agent. Trends Parasitol. 19, 167–170. ( 10.1016/S1471-4922(03)00027-8) [DOI] [PubMed] [Google Scholar]
- 17.Chomel BB, Boulouis H-J, Maruyama S, Breitschwerdt EB. 2006. Bartonella spp. in pets and effect on human health. Emerg. Infect. Dis. 12, 389–394. ( 10.3201/eid1203.050931) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luft BJ, Remington JS. 1992. Toxoplasmic encephalitis in AIDS. Clin. Infect. Dis. 15, 211–222. ( 10.1093/clinids/15.2.211) [DOI] [PubMed] [Google Scholar]
- 19.Baliu C, Sanclemente G, Cardona M, Castel MA, Perez-Villa F, Moreno A, Cervera C. 2014. Toxoplasmic encephalitis associated with meningitis in a heart transplant recipient. Transpl. Infect. Dis. 16, 631–633. ( 10.1111/tid.12242) [DOI] [PubMed] [Google Scholar]
- 20.Sparkes AH, Hopper CD, Millard WG, Gruffydd-Jones TJ, Harbour DA. 1993. Feline immunodeficiency virus infection clinicopathologic findings in 90 naturally occurring cases. J. Vet. Intern. Med. 7, 85–90. ( 10.1111/j.1939-1676.1993.tb03174.x) [DOI] [PubMed] [Google Scholar]
- 21.Di Cesare A, Laiacona F, Iorio R, Marangi M, Menegotto A.. 2016. Aelurostrongylus abstrusus in wild felids of South Africa. Parasitol. Res. 115, 3731–3735. ( 10.1007/s00436-016-5134-y) [DOI] [PubMed] [Google Scholar]
- 22.Queshi T. 2014. Isospora felis. American Association of Veterinary Parasitologists. See http://www.aavp.org/wiki/catprotozoa/coccidia-apicomplexan/isospora-felis/ (accessed 16 January 2018).
- 23.Bowman A. 2014. Isospora rivolta. American Association of Veterinary Parasitologists. See http://www.aavp.org/wiki/catprotozoa/coccidia-apicomplexan/isospora-rivolta/ (accessed 16 January 2018).
- 24.Leclaire S, Menard S, Berry A. 2015. Molecular characterization of Babesia and Cytauxzoon species in wild South-African meerkats. Parasitology 142, 543–548. ( 10.1017/S0031182014001504) [DOI] [PubMed] [Google Scholar]
- 25.Shock BC, et al. 2011. Distribution and prevalence of Cytauxzoon felis in bobcats (Lynx rufus), the natural reservoir, and other wild felids in thirteen states. Vet. Parasitol. 175, 325–330. ( 10.1016/j.vetpar.2010.10.009) [DOI] [PubMed] [Google Scholar]
- 26.Butt MT, Bowman D, Barr MC, Roelke ME. 1991. Iatrogenic transmission of Cytauxzoon felis from a Florida panther (Felix concolor coryi) to a domestic cat. J. Wildl. Dis. 27, 342–347. ( 10.7589/0090-3558-27.2.342) [DOI] [PubMed] [Google Scholar]
- 27.Bowman A. 2014. Cytauxzoon felis. American Association of Veterinary Parasitologists. See http://www.aavp.org/wiki/catprotozoa/coccidia-apicomplexan/piroplasms-cytauxzoon-babesia/cytauxzoon-felis/ (accessed 16 January 2019).
- 28.Dalimi A, Sattari A, Motamedi G. 2006. A study on intestinal helminthes of dogs, foxes and jackals in the western part of Iran. Vet. Parasitol. 142, 129–133. ( 10.1016/j.vetpar.2006.06.024) [DOI] [PubMed] [Google Scholar]
- 29.Segovia JM, Torres J, Miquel J, Llaneza L, Feliu C. 2001. Helminths in the wolf, Canis lupus, from north-western Spain. J. Helminthol. 75, 183–192. ( 10.1079/JOH200152) [DOI] [PubMed] [Google Scholar]
- 30.Heeney JL, Caro T. 1990. Prevalence and implications of feline coronavirus infections of captive and free-ranging cheetahs (Acinonyx jubatus). J. Virol. 64, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daniels MJ, Golder MC, Jarrett O, MacDonald DW. 1999. Feline viruses in wildcats from Scotland. J. Wildl. Dis. 35, 121–124. ( 10.7589/0090-3558-35.1.121) [DOI] [PubMed] [Google Scholar]
- 32.Ostrowski S, Van Vuuren M, Lenain DM, Durand A.. 2003. A serologic survey of wild felids from central west Saudi Arabia. J. Wildl. Dis. 39, 696–701. ( 10.7589/0090-3558-39.3.696) [DOI] [PubMed] [Google Scholar]
- 33.Harrison TM, Mazet JK, Holekamp KE, Dubovi E, Engh AL, Nelson K, Van Horn RC, Munson L.. 2004. Antibodies to canine and feline viruses in spotted hyenas (Crocuta crocuta) in the Masai Mara National Reserve. J. Wildl. Dis. 40, 1–10. ( 10.7589/0090-3558-40.1.1) [DOI] [PubMed] [Google Scholar]
- 34.Feng Y, Xiao L. 2011. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 24, 110–140. ( 10.1128/CMR.00033-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willi B, et al. 2007. Worldwide occurrence of feline Hemoplasma infections in wild felid species. J. Clin. Microbiol. 45, 1159–1166. ( 10.1128/JCM.02005-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mercer SH, Jones LP, Rappole JH, Twedt D, Laack LL, Craig TM. 1988. Hepatozoon sp. in wild carnivores in Texas. J. Wildl. Dis. 24, 574–576. ( 10.7589/0090-3558-24.3.574) [DOI] [PubMed] [Google Scholar]
- 37.Kellner A, Carver S, Scorza V, McKee CD, Lappin M, Crooks KR, VandeWoude S, Antolin MF. 2018. Transmission pathways and spillover of an erythrocytic bacterial pathogen from domestic cats to wild felids. Ecol. Evol. 8, 9779–9792. ( 10.1002/ece3.4451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McAllister MM, Dubey JP, Lindsay DS, Jolley WR, Wills RA, McGuire AM. 1998. Dogs are definitive hosts of Neospora caninum. Int. J. Parasitol. 28, 1473–1478. ( 10.1016/S0020-7519(98)00138-6) [DOI] [PubMed] [Google Scholar]
- 39.Dubey JP. 2003. Review of Neospora caninum and neosporosis in animals. Korean J. Parasitol. 41, 1–16. ( 10.3347/kjp.2003.41.1.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoberg EP. 2006. Phylogeny of Taenia: species definitions and origins of human parasites. Parasitol. Int. 55, S23–S30. ( 10.1016/j.parint.2005.11.049) [DOI] [PubMed] [Google Scholar]
- 41.Nolan T. 2014. Toxocara cati. American Association of Veterinary Parasitologists. See http://www.aavp.org/wiki/nematodes/ascaridida/toxocara-cati/ (accessed 16 January 2019).
- 42.Dubey JP. 2009. Toxoplasmosis of animals and humans. Boca Raton, FL: CRC Press. [Google Scholar]
- 43.Ghai RR, Simons ND, Chapman CA, Omeja PA, Davies TJ, Ting N, Goldberg TL. 2014. Hidden population structure and cross-species transmission of whipworms (Trichuris sp.) in humans and non-human primates in Uganda. PLoS Negl. Trop. Dis. 8, e0003256 ( 10.1371/journal.pntd.0003256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie Y, Zhao B, Hoberg EP, Li M, Zhou X, Gu X, Lai W, Peng X, Yang G. 2018. Genetic characterisation and phylogenetic status of whipworms (Trichuris spp.) from captive non-human primates in China, determined by nuclear and mitochondrial sequencing. Parasites Vectors 11, 5 ( 10.1186/s13071-018-3100-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deak G, Ionică AM, Mihalca AD, Gherman CM. 2017. Troglostrongylus brevior: a new parasite for Romania. Parasites Vectors 10, 599 ( 10.1186/s13071-017-2551-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beaver P. 1975. Biology of soil-transmitted helminths: the massive infection. Health Lab. Sci. 12, 116–125. [PubMed] [Google Scholar]
- 47.Hardy WD, Old LJ, Hess PW, Essex M, Cotter S. 1973. Horizontal transmission of feline leukaemia virus. Nature 244, 266–269. ( 10.1038/244266a0) [DOI] [PubMed] [Google Scholar]
- 48.Allen HA. 2015. Characterizing zoonotic disease detection in the United States: who detects zoonotic disease outbreaks & how fast are they detected? J. Infect. Public Health 8, 194–201. ( 10.1016/j.jiph.2014.09.009) [DOI] [PubMed] [Google Scholar]
- 49.Frenkel JK, Dubey JP. 1972. Rodents as vectors for feline coccidia, Isospora felis and Isospora rivolta. J. Infect. Dis. 125, 69–72. ( 10.1093/infdis/125.1.69) [DOI] [PubMed] [Google Scholar]
- 50.Guernier V, Hochberg ME, Guégan J-F. 2004. Ecology drives the worldwide distribution of human diseases. PLoS Biol. 2, e141 ( 10.1371/journal.pbio.0020141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cashdan E. 2014. Biogeography of human infectious diseases: a global historical analysis. PLoS ONE 9, e0106752 ( 10.1371/journal.pone.0106752) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thieltges DW, Hof C, Dehling DM, Brändle M, Brandl R, Poulin R. 2011. Host diversity and latitude drive trematode diversity patterns in the European freshwater fauna: trematode diversity patterns. Global Ecol. Biogeogr. 20, 675–682. ( 10.1111/j.1466-8238.2010.00631.x) [DOI] [Google Scholar]
- 53.Díaz-Regañón D, Villaescusa A, Ayllón T, Rodríguez-Franco F, Baneth G, Calleja-Bueno L, García-Sancho M, Agulla B, Sainz Á. 2017. Molecular detection of Hepatozoon spp. and Cytauxzoon sp. in domestic and stray cats from Madrid, Spain. Parasites Vectors 10, 112 ( 10.1186/s13071-017-2056-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bell ET, Toribio JLML, White JD, Malik R, Norris JM. 2006. Seroprevalence study of feline coronavirus in owned and feral cats in Sydney, Australia. Aust. Vet. J. 84, 74–81. ( 10.1111/j.1751-0813.2006.tb12231.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang-Fung-Martel J, Gummow B, Burgess G, Fenton E, Squires R. 2013. A door-to-door prevalence study of feline immunodeficiency virus in an Australian suburb. J. Feline Med. Surg. 15, 1070–1078. ( 10.1177/1098612X13491959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Norris JM, Bell ET, Hales L, Toribio J-ALML, White JD, Wigney DI, Baral RM, Malik R. 2007. Prevalence of feline immunodeficiency virus infection in domesticated and feral cats in eastern Australia. J. Feline Med. Surg. 9, 300–308. ( 10.1016/j.jfms.2007.01.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ravi M, Wobeser GA, Taylor SM, Jackson ML. 2010. Naturally acquired feline immunodeficiency virus (FIV) infection in cats from western Canada: prevalence, disease associations, and survival analysis. Can. Vet. J. 51, 271–276. [PMC free article] [PubMed] [Google Scholar]
- 58.Walker VR, Morera Galleguillos F, Gómez Jaramillo M, Pereira Almosny NR, Arauna Martínez P, Grob Behne P, Acosta-Jamett G, Müller A. 2016. Prevalence, risk factor analysis, and hematological findings of Hemoplasma infection in domestic cats from Valdivia, southern Chile. Comp. Immunol. Microbiol. Infect. Dis. 46, 20–26. ( 10.1016/j.cimid.2016.03.004) [DOI] [PubMed] [Google Scholar]
- 59.Bergmann M, Englert T, Stuetzer B, Hawley JR, Lappin MR, Hartmann K. 2017. Risk factors of different Hemoplasma species infections in cats. BMC Vet. Res. 13, 52 ( 10.1186/s12917-017-0953-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baneth G, Sheiner A, Eyal O, Hahn S, Beaufils J-P, Anug Y, Talmi-Frank D. 2013. Redescription of Hepatozoon felis (Apicomplexa: Hepatozoidae) based on phylogenetic analysis, tissue and blood form morphology, and possible transplacental transmission. Parasites Vectors 6, 102 ( 10.1186/1756-3305-6-102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meneses IDS, et al. 2014. Frequency of antibodies against Sarcocystis neurona and Neospora caninum in domestic cats in the state of Bahia, Brazil. Rev. Bras. Parasitol. Vet. 23, 526–529. ( 10.1590/S1984-29612014080) [DOI] [PubMed] [Google Scholar]
- 62.Nijsse R, Ploeger HW, Wagenaar JA, Mughini-Gras L. 2016. Prevalence and risk factors for patent Toxocara infections in cats and cat owners' attitude towards deworming. Parasitol. Res. 115, 4519–4525. ( 10.1007/s00436-016-5242-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Must K, Lassen B, Jokelainen P. 2015. Seroprevalence of and risk factors for Toxoplasma gondii infection in cats in Estonia. Vector Borne Zoonotic Dis. 15, 597–601. ( 10.1089/vbz.2015.1809) [DOI] [PubMed] [Google Scholar]
- 64.Ahmad N, Ahmed H, Irum S, Qayyum M. 2014. Seroprevalence of IgG and IgM antibodies and associated risk factors for toxoplasmosis in cats and dogs from sub-tropical arid parts of Pakistan. Trop. Biomed. 31, 777–784. [PubMed] [Google Scholar]
- 65.Deksne G, Petrusēviča A, Kirjušina M. 2013. Seroprevalence and factors associated with Toxoplasma gondii infection in domestic cats from urban areas in Latvia. J. Parasitol. 99, 48–50. ( 10.1645/GE-3254.1) [DOI] [PubMed] [Google Scholar]
- 66.Györke A, Opsteegh M, Mircean V, Iovu A, Cozma V. 2011. Toxoplasma gondii in Romanian household cats: evaluation of serological tests, epidemiology and risk factors. Prev. Vet. Med. 102, 321–328. ( 10.1016/j.prevetmed.2011.07.015) [DOI] [PubMed] [Google Scholar]
- 67.Ketzis JK, Shell L, Chinault S, Pemberton C, Pereira MM. 2015. The prevalence of Trichuris spp. infection in indoor and outdoor cats on St. Kitts. J. Infect. Dev. Ctries 9, 111–113. ( 10.3855/jidc.5778) [DOI] [PubMed] [Google Scholar]
- 68.Opsteegh M, Haveman R, Swart AN, Mensink-Beerepoot ME, Hofhuis A, Langelaar MFM, van der Giessen JWB. 2012. Seroprevalence and risk factors for Toxoplasma gondii infection in domestic cats in The Netherlands. Prev. Vet. Med. 104, 317–326. ( 10.1016/j.prevetmed.2012.01.003) [DOI] [PubMed] [Google Scholar]
- 69.Burling AN, Levy JK, Scott HM, Crandall MM, Tucker SJ, Wood EG, Foster JD. 2017. Seroprevalences of feline leukemia virus and feline immunodeficiency virus infection in cats in the United States and Canada and risk factors for seropositivity. J. Am. Vet. Med. Ass. 251, 187–194. ( 10.2460/javma.251.2.187) [DOI] [PubMed] [Google Scholar]
- 70.Higgins JPT, Thompson SG. 2002. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558. ( 10.1002/sim.1186) [DOI] [PubMed] [Google Scholar]
- 71.Day MJ, et al. 2012. Surveillance of zoonotic infectious disease transmitted by small companion animals. Emerg. Infect. Dis. 18, e1 ( 10.3201/eid1812.120664) [DOI] [Google Scholar]
- 72.Carver S, et al. 2016. Pathogen exposure varies widely among sympatric populations of wild and domestic felids across the United States. Ecol. Appl. 26, 367–381. ( 10.1890/15-0445) [DOI] [PubMed] [Google Scholar]
- 73.Higgins JPT, Thompson SG, Spiegelhalter DJ. 2009. A re-evaluation of random-effects meta-analysis. J. R. Stat. Soc. A 172, 137–159. ( 10.1111/j.1467-985X.2008.00552.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnson PTJ, Hoverman JT. 2012. Parasite diversity and coinfection determine pathogen infection success and host fitness. Proc. Natl Acad. Sci. USA 109, 9006–9011. ( 10.1073/pnas.1201790109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnson PTJ, Preston DL, Hoverman JT, LaFonte BE. 2013. Host and parasite diversity jointly control disease risk in complex communities. Proc. Natl Acad. Sci. USA 110, 16 916–16 921. ( 10.1073/pnas.1310557110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Han BA, Kramer AM, Drake JM. 2016. Global patterns of zoonotic disease in mammals. Trends Parasitol. 32, 565–577. ( 10.1016/j.pt.2016.04.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.American Bird Conservancy. Cats indoors. Better for cats, better for birds, better for people. The Plains, VA: American Bird Conservancy. See https://abcbirds.org/program/cats-indoors/ (accessed 4 April 2019).
- 78.People for the Ethical Treatment of Animals. Animal rights uncompromised: ‘outdoor cats’. Norfolk, VA: PETA. See https://www.peta.org/issues/animal-companion-issues/cruel-practices/outdoor-cats/ (accessed 4 April 2019).
- 79.Lepczyk CA, Mertig AG, Liu J. 2004. Landowners and cat predation across rural-to-urban landscapes. Biol. Conserv. 115, 191–201. ( 10.1016/S0006-3207(03)00107-1) [DOI] [Google Scholar]
- 80.Clancy EA, Moore AS, Bertone ER. 2003. Evaluation of cat and owner characteristics and their relationships to outdoor access of owned cats. J. Am. Vet. Med. Ass. 222, 1541–1545. ( 10.2460/javma.2003.222.1541) [DOI] [PubMed] [Google Scholar]
- 81.Lepczyk CA, et al. 2010. What conservation biologists can do to counter trap-neuter-return: response to Longcore et al. Conserv. Biol. 24, 627–629. ( 10.1111/j.1523-1739.2009.01426.x) [DOI] [PubMed] [Google Scholar]
- 82.Chomel BB. 2014. Emerging and re-emerging zoonoses of dogs and cats. Animals (Basel) 4, 434–445. ( 10.3390/ani4030434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robertson ID, Irwin PJ, Lymbery AJ, Thompson RC. 2000. The role of companion animals in the emergence of parasitic zoonoses. Int. J. Parasitol. 30, 1369–1377. ( 10.1016/S0020-7519(00)00134-X) [DOI] [PubMed] [Google Scholar]
- 84.Hunter PR, Thompson RCA. 2005. The zoonotic transmission of Giardia and Cryptosporidium. Int. J. Parasitol. 35, 1181–1190. ( 10.1016/j.ijpara.2005.07.009) [DOI] [PubMed] [Google Scholar]
- 85.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097 ( 10.1371/journal.pmed.1000097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chalkowski K, Wilson A, Lepczyk C, Zohdy S. 2019. Data from: Who let the cats out: a global meta-analysis on risk of parasitic infection in indoor versus outdoor domestic cats (Felis catus) Figshare Digital Repository (https://figshare.com/s/3eebaf42e161c0e7e1ef). [DOI] [PMC free article] [PubMed]
- 87.Chalkowski K, Wilson A, Lepczyk C, Zohdy S. 2019. Data from: Who let the cats out: a global meta-analysis on risk of parasitic infection in indoor versus outdoor domestic cats (Felis catus). Figshare Digital Repository. See https://figshare.com/s/a334c7815b128cb63b98.
- 88.Viechtbauer W. 2007. Accounting for heterogeneity via random-effects models and moderator analyses in meta-analysis. Z.r Psychol. J. Psychol. 215, 104–121. ( 10.1027/0044-3409.215.2.104) [DOI] [Google Scholar]
- 89.Szumilas M. 2010. Explaining odds ratios. J. Can. Acad. Child Adolesc. Psychiat. 19, 227–229. ( 10.1007/s00787-010-0087-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cuijpers P, van Straten A, Bohlmeijer E, Hollon SD, Andersson G.. 2010. The effects of psychotherapy for adult depression are overestimated: a meta-analysis of study quality and effect size. Psychol. Med. 40, 211 ( 10.1017/S0033291709006114) [DOI] [PubMed] [Google Scholar]
- 91.Duval S, Tweedie R. 2000. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56, 455–463. ( 10.1111/j.0006-341X.2000.00455.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Chalkowski K, Wilson A, Lepczyk C, Zohdy S. 2019. Data from: Who let the cats out: a global meta-analysis on risk of parasitic infection in indoor versus outdoor domestic cats (Felis catus) Figshare Digital Repository (https://figshare.com/s/3eebaf42e161c0e7e1ef). [DOI] [PMC free article] [PubMed]
- Chalkowski K, Wilson A, Lepczyk C, Zohdy S. 2019. Data from: Who let the cats out: a global meta-analysis on risk of parasitic infection in indoor versus outdoor domestic cats (Felis catus). Figshare Digital Repository. See https://figshare.com/s/a334c7815b128cb63b98.
Supplementary Materials
Data Availability Statement
Literature search: Figshare repository figshare.com/s/3eebaf42e161c0e7e1ef [86]. R code in analyses: Figshare repository figshare.com/s/a334c7815b128cb63b98 [87].