Abstract
It has been known for about a century that European eels have a unique life history that includes offshore spawning in the Sargasso Sea about 5000–7000 km away from their juvenile and adult habitats in Europe and northern Africa. Recently hatched eel larvae were historically collected during Danish, German and American surveys in specific areas in the southern Sargasso Sea. During a 31 day period of March and April 2014, Danish and German research ships sampled for European eel larvae along 15 alternating transects of stations across the Sargasso Sea. The collection of recently hatched eel larvae (≤12 mm) from 70° W and eastward to 50° W showed that the European eel had been spawning across a 2000 km wide region of the North Atlantic Ocean. Historical collections made from 1921 to 2007 showed that small larvae had also previously been collected in this wide longitudinal zone, showing that the spatial extent of spawning has not diminished in recent decades, irrespective of the dramatic decline in recruitment. The use of such a wide spawning area may be related to variations in the onset of the silver eel spawning migration, individual differences in their long-term swimming ability, or aspects of larval drift.
Keywords: Anguilla anguilla, spawning area, migration, leptocephali, ocean fronts, Sargasso Sea
1. Introduction
There is increasing evidence that some marine fishes, sea turtles, seabirds and mammals can make remarkably long migrations to very precise areas for feeding or reproduction [1,2]. Marine fishes such as tunas and sharks can make long migrations [2], and diadromous fishes such as anadromous salmon are famous for their long feeding migrations into the ocean before they return to the exact freshwater streams where each fish was born [3]. However, the catadromous reproductive migrations of semelparous anguillid eels out of fresh and coastal waters into offshore oceans for spawning are unique among migratory species. Anguillid eels have fascinated scientists ever since the European eel, Anguilla anguilla, and American eel, A. rostrata, and then the Japanese eel, A. japonica, were found to migrate long distances to spawn offshore [4,5]. This was first discovered about a century ago when small larvae, called leptocephali, of the two Atlantic eel species were collected in the Sargasso Sea [4]. These life histories require both the adults to migrate to their spawning areas and their long-lived leptocephali to drift in ocean currents to their recruitment areas.
The reproductive ecology of anguillid eels has remained mysterious because spawning areas were only known from larval catches [4–7] until Japanese eel eggs and spawning-condition adults were collected recently [8]. Larval catches of the two Atlantic eel species indicate that they spawn in partly overlapping areas of the Sargasso Sea and their small leptocephali are mostly found south of where warm southern surface water meets cold northern water and fronts are formed [6,7,9,10].
The unique oceanic life history of spawning offshore followed by a long larval stage has made it difficult to understand the causes of recent anguillid eel population declines, although the possible contributing factors have been identified [11]. The decline of the European eel is presently reflected in extremely low glass eel recruitment to European coastal waters [12] and in lower larval abundances in the Sargasso Sea [13,14]. Uncertainty about what caused the declines of the Atlantic eels and the Japanese eel has contributed to them being listed as endangered [15]. This has led to new oceanographic surveys to study the European eel spawning area [9,13,16].
We used an unprecedented grid of sampling stations to show that the European eel was spawning across a wide longitudinal zone of the Sargasso Sea during part of their 2014 spawning season. This was achieved by combining the collections of larvae made by two oceanographic research vessels that simultaneously sampled across the entire estimated spawning area. Comparison of our results with an extensive historical collection database shows that small larvae were also confined to this same zone during the same March–April period during past years, which provides the best estimate so far of the width of the spawning area.
2. Material and methods
Research cruises of the R/V Dana (Denmark) and FR/V Walther Herwig III (Germany) collected anguillid leptocephali along 15 alternating transects of sampling stations occupied moving eastward from 70° W to 49° W from 16 March to 16 April in 2014 (figure 1a). A 9.6 m2 mouth-opening ring net (560 µm mesh; Dana) [9] and a 6.2 m2 mouth-opening Isaacs-Kidd Midwater Trawl (500 µm mesh; Walther Herwig) [13] were used to collect leptocephali during both day and night in the upper 300 m. Thus, different nets and types of flowmeters, and fishing styles were used by the two surveys (double oblique tows to 300 m depth by Walther Herwig, single oblique tows to 200–250 m by Dana; electronic supplementary material), preventing direct abundance comparisons. Therefore, presence/absence of larvae was used as evidence of geographical distribution of spawning. Collected leptocephali were identified using analyses of their DNA sequences, which consisted of the mitochondrial 16sS rRNA gene for species identification and 18S rDNA and restriction fragment length polymorphisms (RFLPs) for detecting hybrids following established protocols [17] (along with some restriction enzyme modifications [18]) for the Walther Herwig specimens, and mitochondrial cytochrome b sequences for the Dana specimens as part of a previous study [19]. This enabled unambiguous species identifications to be made of all European eel larvae, while excluding any American eel larvae that were also collected in the western part of the study area. The spawning area of the American eel extends farther to the west than sampling occurred in 2014 [10], so those data are not included in the present study. Conductivity, temperature, depth (CTD) profiles were made at most stations to examine hydrography. Catch data from a large database [10] for March and April within 75–45° W and 20–32° N were used to show the historical distribution of different sizes of leptocephali across the spawning area.
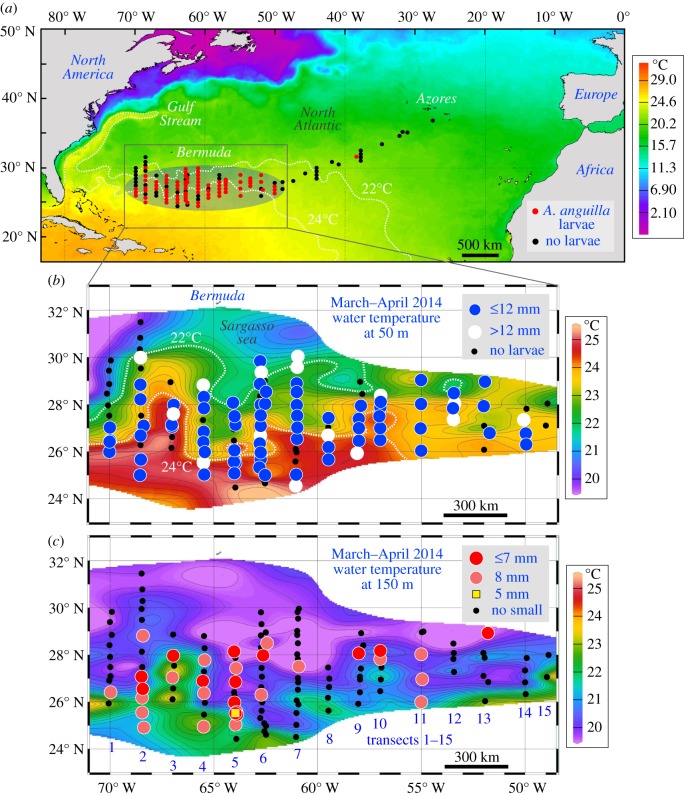
Figure 1.
Maps of the 2014 survey showing catch stations of European eel leptocephali (a), catch locations of leptocephali ≤12 mm (b), and ≤7, 8, 5 mm (c), in relation to ocean temperature at the surface (a), 50 m (b), and 150 m (c) during the survey. The 22 and 24°C isotherms (white lines in (b)) are the temperatures associated with fronts. (Online version in colour.)
3. Results
There were 408 European eel leptocephali collected (29 day capture period: 18 March–15 April 2014) by the Dana (N = 236, 6.3–26.8 mm, mean ± s.d.: 12.7 ± 3.3 mm) and Walther Herwig (N = 172, 5.5–44.0 mm, 12.9 ± 5.5 mm) surveys (electronic supplementary material, figure S1), with no significant differences in larval size between surveys (p = 0.32; t-test). Larvae ≤ 12 mm were distributed across a longitudinal range (50–70° W) of about 2000 km (figures 1b and 2a). Larvae ≤7 and 8 mm were almost as widely distributed from west to east across about 1640 and 1480 km, respectively (figures 1c and 2a). The latitudinal sampling range and minimum larval size in each transect varied considerably, but small larvae were widely distributed (figures 1b,c and 2c). The overall size of larvae increased and the number of small larvae decreased in the eastward direction (electronic supplementary material, figure S2a) in correspondence with the sampling date (electronic supplementary material, figure S2b), but small larvae ≤12 mm were collected in all but the easternmost transect, indicating a wide range of spawning times and locations. Almost all larvae were caught south of the northern front (22°C), but larvae were present on both sides of the southern front (24°C) (figure 1b; electronic supplementary material, figure S3).
Figure 2.
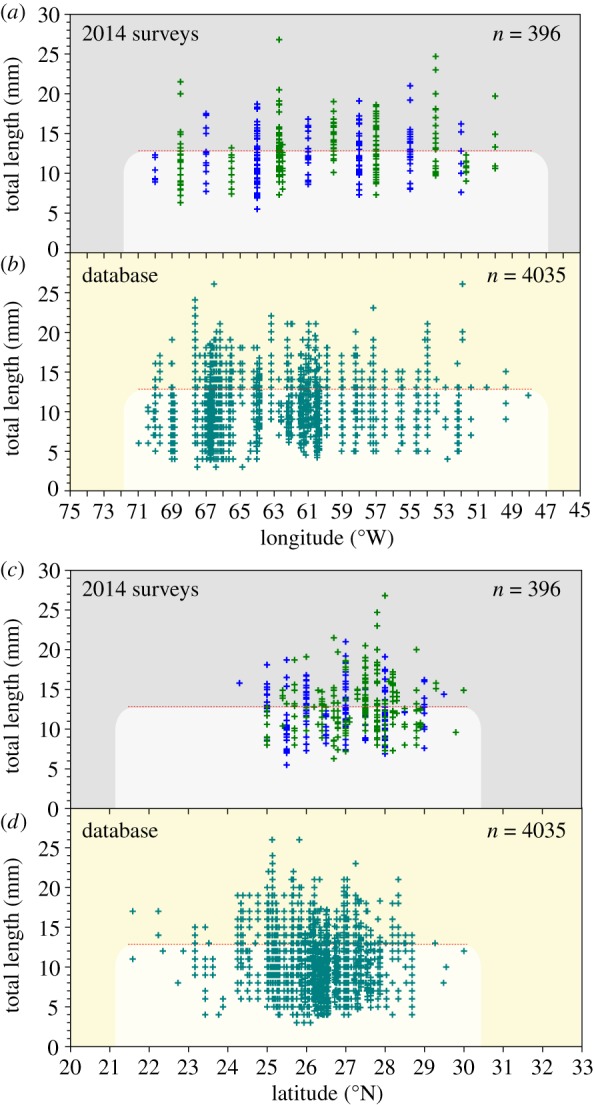
Sizes of individual European eel leptocephali collected during the German (blue crosses) and Danish (green crosses) 2014 surveys (a,c), and in the database containing all larvae collected up to 2007 [10] (b,d) in relation to latitude and longitude, highlighting zones of larvae ≤12 mm (red line and light shading). (Online version in colour.)
Plots of larval sizes in the historical database for March–April in the same region show a strikingly similar pattern in both longitude and latitude (figure 2b,d). The only difference is that larvae were caught farther south than the transects extended in 2014. The longitudinal range of small larvae in the database was almost identical to that in 2014 except a few larvae were collected 1° farther east.
4. Discussion
The collection of ≤12 mm European eel leptocephali across 2000 km of the Sargasso Sea during the two 2014 surveys provides the first clear evidence that spawning can occur across this expansive region within a short time in one spawning season. Larvae ≤12 mm were collected from 70–50° W, indicating widespread spawning several weeks before the surveys, because these larvae may be approximately ≤20 days old [20,21]. More recently spawned ≤7 and 8 mm larvae were almost as widely distributed longitudinally, but were less common, possibly because the main spawning season was ending as the surveys moved eastward. Within this large area of larval distribution, the average growth rates of larvae collected by the Danish 2014 survey showed no distinguishable spatial trends [21], suggesting all areas within the spawning area are equivalent for larval growth. Modelling of drift patterns of the larvae collected by the German 2014 survey suggested that most larvae are not quickly transported very far away from spawning sites [16]. Therefore, catch patterns in the present study may roughly reflect spawning locations.
Data from Schmidt's collections [4] and more recent surveys in the database [10] showed almost the exact same longitudinal distribution-range of ≤12 mm larvae. The cruise track of the German 19 March–27 April 1979 survey went in various directions over a wider latitudinal zone during a longer period of time, but also found small larvae over a wide area [6]. Therefore, both historical data and our 2014 collections suggest that the European eel can spawn across at least a 2000 km zone of the Sargasso Sea each year, including in recent years after its population decline while its larval abundances appear to be much lower [13,14].
In comparison, American eels seem to spawn in an overlapping, but narrower longitudinal area in the southwest Sargasso Sea [10], and Japanese eels spawn in an even smaller area west of a seamount ridge [8]. This raises the question: Why does the panmictic European eel spawns over such a wide longitudinal zone? Studies have been conducted recently to learn about the spawning migrations of European eels by attaching pop-off satellite-transmitting data storage tags, but so far none of the tracked eels has reached the spawning area [22]. Therefore, where eels from different parts of the European eel continental species range spawn within the wide spawning area is completely unknown.
The possible reasons for spawning over such a wide area may be related to how natural selection has interacted with both the physiological constraints on the long adult migration (approx. 5000–7000 km) and subsequent reproductive success (millions of eggs per female), and the effects of ocean current patterns on larval survival/recruitment success. One factor is that because adult European eels start their spawning migrations out of freshwater during many months of each year, it is questionable if all eels can reach all parts of the spawning area during the main spawning season [22]. Eels that migrate early or from areas closer to the North Atlantic and have adequate time and energy might swim to the western side of the spawning area, while those that migrate later, from farther away, or have low energy reserves could spawn in the east. Western spawning could increase the probability of larvae entering the rapid Gulf Stream transport across the North Atlantic in the traditionally assumed route into the North Atlantic Drift or Azores Current [10]. The implications of eastern spawning closer to Europe is unclear regarding the effect on larval transport, even though some eastward larval transport within frontal-jet countercurrents has been hypothesized [9,10]. These flows and eastern spawning might facilitate retention offshore before the larvae move west, or make it easier to reach the western regions of the Azores Current if late-stage anguillid larvae can swim directionally to overcome drift, as has been discussed [10,23]. While which if any of these possible factors may have been important cannot presently be determined, strong selection against spawning at either margin seems not to have occurred, which has resulted in an unusually wide spawning area.
Regardless of the reasons for such a wide spawning area, the long migrations of European eels to reproduce across an area as wide as 2000 km represent a remarkable life history that will be better understood as new information emerges about the behaviour and reproductive ecology of these mysterious fishes.
Supplementary Material
Acknowledgements
We thank all the scientists, technicians and captains and crews of both research vessels for assistance collecting and sorting the larvae during the two surveys.
Ethics
There are no conflicts with animal-ethics or conservation policies. The net-based sampling described in the methods is a standard approach for the sampling of plankton and larvae of marine animals.
Data accessibility
The DNA sequences used to identify the European eel leptocephali are deposited in the NCBI GenBank under accession nos MK483356-MK483528 for the Walther Herwig III survey, and sequence data from the previous study that identified the larvae from the Dana survey [19] are deposited in GenBank under accession nos KX818059-KX818096, with additional datasets deposited in the Dryad Digital Repository: https://doi.org/10.5061/dryad.v5m24 [24]. Original eel larvae catch data will be used in future publications by the authors, but requests for access will be considered. Historical collection data of all European eel leptocephali collected up to 2007 that were used to construct figure 2b,d are available online (http://www.ices.dk/marine-data/data-portals/Pages/Eggs-and-larvae.aspx).
Authors' contributions
R.H. and P.M. designed and led the research cruises and processing of samples; M.J.M., H.W., P.M. and R.H. analysed catch data and drafted the initial manuscript. All authors participated in the research cruises to collect the European eel larvae, evaluated the manuscript, approved publication, and agree to be accountable for all aspects of the work.
Competing interests
We have no competing interests.
Funding
The FR/V Walther Herwig III survey was funded by the German Federal Ministry of Food and Agriculture, and the R/V Dana survey was funded by the Danish Centre for Marine Research (2013_02) and the Carlsberg Foundation, Denmark (2012_01_0272).
References
- 1.Luschi P. 2013. Long-distance animal migrations in the oceanic environment: orientation and navigation correlates . ISRN Zool. 2013, 631839 ( 10.1155/2013/631839) [DOI] [Google Scholar]
- 2.Block B, et al. 2011. Tracking apex marine predator movements in a dynamic ocean. Nature 475, 86–90. ( 10.1038/nature10082) [DOI] [PubMed] [Google Scholar]
- 3.Quinn TP, Myers KW. 2004. Anadromy and the marine migrations of Pacific salmon and trout: Rounsefell revisited. Rev. Fish Biol. Fish. 14, 421–442. ( 10.1007/s11160-005-0802-5) [DOI] [Google Scholar]
- 4.Schmidt J. 1922. The breeding places of the eel. Phil. Trans. R. Soc. Lond. B 211, 179–208. ( 10.1098/rstb.1923.0004) [DOI] [Google Scholar]
- 5.Tsukamoto K. 1992. Discovery of the spawning area for the Japanese eel. Nature 356, 789–791. ( 10.1038/356789a0) [DOI] [Google Scholar]
- 6.Schoth M, Tesch F-W. 1982. Spatial distribution of 0-group eel larvae (Anguilla sp.) in the Sargasso Sea. Helgoländer Meeresunters. 35, 309–320. ( 10.1007/BF02006139) [DOI] [Google Scholar]
- 7.Kleckner RC, McCleave JD. 1988. The northern limit of spawning by Atlantic eels (Anguilla spp.) in the Sargasso Sea in relation to thermal fronts and surface water masses. J. Mar. Res. 46, 647–667. ( 10.1357/002224088785113469) [DOI] [Google Scholar]
- 8.Tsukamoto K, et al. 2011. Oceanic spawning ecology of freshwater eels in the western North Pacific. Nat. Commun. 2, 179 ( 10.1038/ncomms1174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munk P, et al. 2010. Oceanic fronts in the Sargasso Sea control the early life and drift of Atlantic eels. Proc. R. Soc. B 277, 3593–3599. ( 10.1098/rspb.2010.0900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller MJ, Bonhommeau S, Munk P, Castonguay M, Hanel R, McCleave JD. 2015. A century of research on the larval distributions of the Atlantic eels: a re-examination of the data. Biol. Rev. 90, 1035–1064. ( 10.1111/brv.12144) [DOI] [PubMed] [Google Scholar]
- 11.Drouineau H, Durif C, Castonguay M, Mateo M, Rochard E, Verreault G, Yokouchi K, Lambert P. 2018. Endangered eels: a symbol of the effects of global change. Fish Fish. 19, 903–930. ( 10.1111/faf.12300) [DOI] [Google Scholar]
- 12.ICES. 2018. Report of the Joint EIFAAC/ICES/GFCM Working Group on Eels (WGEEL), 3–10 October 2017, Kavala, Greece ICES CM 2017/ACOM:15. See http://ices.dk/sites/pub/Publication%20Reports/Expert%20Group%20Report/acom/2017/WGEEL/wgeel_2017.pdf.
- 13.Hanel R, Stepputtis D, Bonhommeau S, Castonguay M, Schaber M, Wysujack K, Vobach M, Miller MJ. 2014. Low larval abundance in the Sargasso Sea: new evidence about reduced recruitment of the Atlantic eels. Naturwissenschaften 101, 1041–1052. ( 10.1007/s00114-014-1243-6) [DOI] [PubMed] [Google Scholar]
- 14.Westerberg H, Miller MJ, Wysujack K, Marohn L, Freese M, Pohlmann J-D, Watanabe S, Tsukamoto K, Hanel R. 2018. Larval abundance across the European eel spawning area: an analysis of recent and historic data. Fish Fish. 19, 890–902. ( 10.1111/faf.12298) [DOI] [Google Scholar]
- 15.Jacoby DMP, et al. 2015. Synergistic patterns of threat and the challenges facing global anguillid eel conservation. Glob. Ecol. Conserv. 4, 321–333. ( 10.1016/j.gecco.2015.07.009) [DOI] [Google Scholar]
- 16.Westerberg H, Pacariz S, Marohn L, Fagerström V, Wysujack K, Miller MJ, Freese M, Pohlmann J-D, Hanel R. 2017. Modeling the drift of European (Anguilla anguilla) and American (Anguilla rostrata) eel larvae during the year of spawning. Can. J. Fish. Aquat. Sci. 75, 224–234. ( 10.1139/cjfas-2016-0256) [DOI] [Google Scholar]
- 17.Frankowski J, Bastrop R. 2010. Identification of Anguilla anguilla (L.) and Anguilla rostrata (Le Sueur) and their hybrids based on a diagnostic single nucleotide polymorphism in nuclear 18S rDNA. Mol. Ecol. 10, 173–176. ( 10.1111/j.1755-0998.2009.02698.x) [DOI] [PubMed] [Google Scholar]
- 18.Prigge E, Marohn L, Oeberst R, Hanel R. 2013. Model prediction vs. reality—testing the predictions of a European eel (Anguilla anguilla) stock dynamics model against the in situ observation of silver eel escapement in compliance with the European eel regulation. ICES J. Mar. Sci. 70, 309–318. ( 10.1093/icesjms/fss188) [DOI] [Google Scholar]
- 19.Jacobsen MW, Smedegaard L, Sørensen SR, Pujolar JM, Munk P, Jónsson B, Magnussen E, Hansen MM. 2017. Assessing pre-and post-zygotic barriers between North Atlantic eels (Anguilla anguilla and A. rostrata) Heredity 118, 266–275. ( 10.1038/hdy.2016.96) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuroki M, Marohn L, Wysujack K, Miller MJ, Tsukamoto K, Hanel R. 2017. Hatching time and larval growth of Atlantic eels in the Sargasso Sea. Mar. Biol. 164, 118 ( 10.1007/s00227-017-3150-9) [DOI] [Google Scholar]
- 21.Ayala DJ, Munk P. 2018. Growth rate variability of larval European eels (Anguilla anguilla) across the extensive eel spawning area in the southern Sargasso Sea. Fish. Oceanogr. 27, 525–535. ( 10.1111/fog.12273) [DOI] [Google Scholar]
- 22.Righton D, et al. 2016. Empirical observations of the spawning migration of European eels: the long and dangerous road to the Sargasso Sea. Sci. Adv. 2, e1501694 ( 10.1126/sciadv.1501694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller MJ, Tsukamoto K. 2017. The ecology of oceanic dispersal and survival of anguillid leptocephali. Can. J. Fish. Aquat. Sci. 74, 958–971. ( 10.1139/cjfas-2016-0281) [DOI] [Google Scholar]
- 24.Jacobsen MW, Smedegaard L, Sørensen SR, Pujolar JM, Munk P, Jónsson B, Magnussen E, Hansen MM. 2016. Data from: Assessing pre- and post-zygotic barriers between North Atlantic eels (Anguilla anguilla and A. rostrata) Dryad Digital Repository. ( 10.5061/dryad.v5m24) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Jacobsen MW, Smedegaard L, Sørensen SR, Pujolar JM, Munk P, Jónsson B, Magnussen E, Hansen MM. 2016. Data from: Assessing pre- and post-zygotic barriers between North Atlantic eels (Anguilla anguilla and A. rostrata) Dryad Digital Repository. ( 10.5061/dryad.v5m24) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The DNA sequences used to identify the European eel leptocephali are deposited in the NCBI GenBank under accession nos MK483356-MK483528 for the Walther Herwig III survey, and sequence data from the previous study that identified the larvae from the Dana survey [19] are deposited in GenBank under accession nos KX818059-KX818096, with additional datasets deposited in the Dryad Digital Repository: https://doi.org/10.5061/dryad.v5m24 [24]. Original eel larvae catch data will be used in future publications by the authors, but requests for access will be considered. Historical collection data of all European eel leptocephali collected up to 2007 that were used to construct figure 2b,d are available online (http://www.ices.dk/marine-data/data-portals/Pages/Eggs-and-larvae.aspx).