Abstract
Historically, bird song has been regarded as a sex-specific signalling trait; males sing to attract females and females drive the evolution of signal exaggeration by preferring males with ever more complex songs. This view provides no functional role for female song. Historic geographical research biases generalized pronounced sex differences of phylogenetically derived northern temperate zone songbirds to all songbirds. However, we now know that female song is common and that both sexes probably sang in the ancestor of modern songbirds. This calls for research on adaptive explanations and mechanisms regulating female song, and a reassessment of questions and approaches to identify selection pressures driving song elaboration in both sexes and subsequent loss of female song in some clades. In this short review and perspective we highlight newly emerging questions and propose a research framework to investigate female song and song sex differences across species. We encourage experimental tests of mechanism, ontogeny, and function integrated with comparative evolutionary analyses. Moreover, we discuss the wider implications of female bird song research for our understanding of male and female communication roles.
Keywords: female bird song, sex differences, sex-specific communication, female songbirds
1. Female bird song is common and song in both sexes ancestral (the case for studying female bird song)
Bird song serves as a textbook example to illustrate stark sexual dimorphisms shaped by sexual selection: males sing to exclude same-sex competitors and attract females, which select for signal elaboration by preferring quality-linked exaggeration. However, this scenario provides little or no functional explanation for female bird song. Yet with increasing documentation across clades and biogeographic regions, female bird song can no longer be considered a rare exception. On the contrary, when contrasting the previous northern temperate zone-centred view with recent worldwide surveys, it becomes apparent that female song is phylogenetically widespread [1,2]. Moreover, concurrent phylogenetic reconstruction reveals male and female song as the most parsimonious ancestral state [1–3]. This challenges the notion that song dimorphisms resulted from uninterrupted divergent sexual selection on males. Instead, phylogenetic comparisons within and between taxa reveal patterns of sex differences (and absence thereof) suggesting that several evolutionary processes acted on both sexes, including repeated losses and re-gains in females [1,2,4]. A more diverse and dynamic evolutionary landscape underlying trait elaboration and sexual dimorphisms is unfolding that is supported by phylogenetic analyses in other signalling domains (e.g. colours in visual ornaments: [2,5]) and discoveries of single molecular switch mechanisms enabling fast losses and gains of sexual signalling traits in different animal taxa [6]. This new perspective that sexual dimorphisms can arise from both directional selection and repeated secondary trait loss raises new questions about which selection pressures contributed to the initial evolution, maintenance, or elaboration of song in both sexes in some species and the loss of female song in others [1,4].
With this brief review and perspective, we aim to stimulate discussion and coordinated research efforts to address these questions. We highlight the need for: improved documentation and quantification of sex differences in song, studies of the ontogeny and mechanism of female song learning and control, experiments to compare the current function(s) of female and male song, and analyses comparing the evolution of female and male song. Instead of a species-by-species re-run of male bird song research now focusing on females, future approaches should be integrative, simultaneously investigating male and female communication roles and interactions. This requires descriptive and experimental studies to catch up on documenting the natural history of female song, and testing a broader range of hypotheses, since traditional approaches rarely considered the impact of singing social partners on male song function. Sex-inclusive research on bird song also entails parallel testing of hypotheses in both sexes, enabling more consistent interpretation of male and female behaviour than single-sex studies. For example, the use of song for territorial defence is almost by default considered ‘sexually selected’ in males, but socially selected in females (e.g. [7]), whereas we cannot detect obvious contextual differences in many cases (see §2d)—suggesting that either there is more sexually selected song in females or also more instances of socially selected song in males. Our aim is therefore to encourage observations and experimental tests of the function(s) and mechanisms regulating both male and female song and to integrate these findings with phylogenetically-controlled comparative analyses to understand factors driving the evolutionary origins, elaboration, and loss of song across all songbirds (figure 1).
Figure 1.
Investigating function and evolution of female bird song. (a) Phylogenetic comparative studies should (i) quantify song structure, (ii) reconstruct the extent of elaboration in females and males and (iii) compare elaboration with selection pressures. (b) Experimental studies should assess (iv) traditional mate attraction and territory defence functions, (v) potential quality indicators such as song complexity and repertoire size, and (vi) alternative functions, including communication with mates, offspring, or competitors for non-mate resources in both sexes across songbird species. Phylogenetic comparative (a) and experimental studies (b) should complement each other to iteratively refine hypotheses pertaining to female and male song function and evolution.
2. Towards an integrative framework for studying female and male bird song
(a). Documenting female bird song
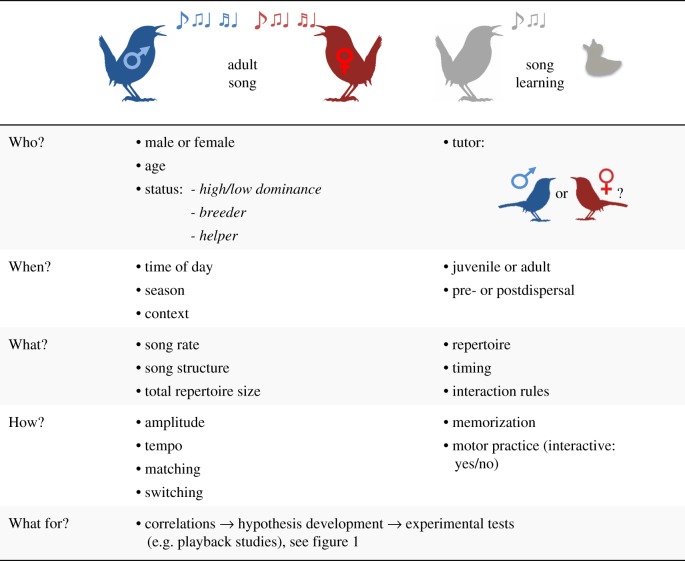
The foundation of integrative research on female and male song is detailed knowledge of its natural history (figure 2). Apart from duetting species [8,9], observational and experimental data on the presence, contexts, and correlates of song are rare for females [10] and females are highly underrepresented in literature and sound collections. For only 27% of all songbird species is there sufficient information to even determine if female song is present or absent [2]. In the two largest collections of bird audio recordings only 0.03% (Macaulay Library) and 0.01% (Xeno-canto) of contributions are labelled ‘female’ [11]. In contrast, the most recent literature-based estimate is that in 64% of songbird species females sing [2]. The current lack of documentation of female song in sound archives is probably partly due to both omission of sex-specific information and mislabelling of some singing females as males in monomorphic species if song is (erroneously) used to infer sex [11]. To avoid new biases, documenting female song ideally should include location, context and season and simultaneous documentation of male and female song [11–13].
Figure 2.
Sex-inclusive song research should collect observational and experimental data in males and females. To stimulate balanced sampling schemes, the left column suggests a list of questions, columns 2 and 3 relevant factors and parameters.
(b). Metrics and analyses to compare female and male song
Studies comparing male and female songs require metrics and analyses that allow accurate assessment of song dimorphism (or lack thereof; see [14]) across the highly variable song structures and singing styles seen between species and the sexes. Past song comparisons often focused on repertoire size or a limited set of parameters only, but analyses should increase the array of metrics in which male and female songs may vary, such as spectral features (e.g. frequency and duration), syllable diversity or repertoires [15], and syntax [16,17]. Analyses that combine multiple structural features over the entire duration of a song or element (e.g. dynamic time warping, [18]) and subsequently capture the relative distances between individuals in trait space i.e. multi-dimensional methods that provide unit-free measures of distances among males and females (as already successfully used for evolutionary analyses of colour dimorphism [5]) seem most promising for multi-species comparisons (e.g. [17,19]). However, more work is needed to develop methods that allow comparison of sex differences in structure and singing styles across species.
(c). Ontogeny and mechanisms regulating song
In songbirds, song development in nature usually involves social learning from conspecifics (generally referred to as song models or tutors), and adult sex differences can result from whom, what and when the sexes learn their songs (see figure 2 and for detailed discussion [20]). Both evolutionary and functional analyses can profit from understanding male and female vocal development and distinguishing between learned versus experience-independent song sex differences. Tutor choice and timing of the song learning process may affect territory establishment, song-based mate choice, and resulting population structure [21–23]. However, compared with male song, we know little about the development of female song. Male and female adult song could differ because during ontogeny the sexes differ in (i) what and how much they learn; (ii) from whom they learn (choosing sex-specific tutors could result in sex-specific song lineages); (iii) the duration of the sensitive phases; (iv) dispersal patterns (thus learning at different times or locations from different tutors); (v) motor practice (timing and amount of motor practice affects song stereotypy); or (vi) how sex-specific vocal tract anatomy affects song learning. Song sex differences are thus not necessarily proof of different learning abilities: some duetting species show pronounced sex-specific repertoires in the wild but learn either repertoire under laboratory conditions [24–26]. Field studies probably best establish from whom males and females learn in their natural social networks (e.g. [27,28]) whereas experimental laboratory studies may provide greater resolution on the what and when of song learning, including lifelong plasticity (examples in [20,26]), which might then help to test functional hypotheses in the field, such as the role of improved song coordination over time in duetting [29,30]. Studies on song production learning should be complemented with studies on song preference learning, not only in females (for examples, see [20]) but also in males (e.g. using operant tests [31]), to ask whether learned preferences may also guide mate choice in males in species where female song functions in mate attraction.
Integrative and comparative studies of male and female song development could be highly informative for research on the neuromolecular bases of sexual differentiation and plasticity of the song system [32–35]. Until recently, the study of the neuroendocrinology of (male) song and sex differences focused on steroid hormones and was predominantly based on species from the most derived clade of the Passerida, which may not necessarily predict sex differences in the quantity and quality of song output in other clades and other biogeographic regions (for review see e.g. [32,34]). In Australian red-backed fairy wrens, Malurus melanocephalus, for example, males and females show clear sex differences in brain morphology and circulating androgen levels but sing equally prolific and complex song [19]. Likewise, the seasonal link between rising plasma testosterone and male song is not necessarily observed in females, or male birds of the tropics and subtropics [32], and physiological levels of testosterone seem poor predictors of variation in singing. Attention has now shifted towards species differences in steroid receptors and associated genomic responses in the song circuitry [33,34], which show surprisingly pronounced species differences [35]. Here, more integrative approaches including early-branching songbird lineages could help to identify the most likely ancestral states and ecological correlates of derived patterns and thus identify target species for physiological and neuromolecular research to update knowledge of the physiology of female song and the neuromolecular underpinning of sex-specific song development.
(d). Song function
Different selection pressures are likely to be at work in species with monomorphic versus dimorphic song. If the sexes sound the same, their songs probably fulfil similar functions, best evidenced where partners can fill in for each other by singing both parts of a duet (so called pseudo-duetting, examples in [36]). In contrast, sex-specific songs suggest a functional advantage for signalling sexual identity (e.g. mate attraction or strong intra-sexual competition) or for sex-specific functions of song. However, since the field of female song function has seen little systematic study, we extrapolate from known male song functions to introduce new questions to further a male–female integrative approach.
Early studies on male song used elegant experiments to demonstrate that song functions primarily to defend territories and to attract females [37]. For example, replacing singing males with speakers delayed territory re-occupation [38] and increased female visitation rates to nest-boxes [39]. Numerous examples have since demonstrated how high song output, complexity, or repertoire size advertises quality and resource holding potential to rivals and prospective mates, but these song functions have been predominantly studied in males [10]. Little is known about females that sing solo songs to defend territories ([10,40,41], but see [42]) and to attract mates in several polygynous and polyandrous species, where females must compete for male attention (e.g. [43,44]). Monogamous females may also use song for mate guarding [45] or for mate attraction [46]. Female song, like male song, can be an indicator trait, e.g. where female song complexity is correlated with age and experience [43,47], and song complexity and quantity predict breeding success [48,49]. However, experiments manipulating state, such as food supplementation [50] or early developmental conditions [51], which demonstrated condition-dependence of male song as an indicator of individual quality, are needed to test causal links for female song.
In many species, males sing year-round and increase song rates in response to (experimentally simulated) territorial intrusion [37]. Likewise, female song is common in species with year-round territories, including during non-breeding periods [52] or when solitary and unpaired [53], which raises the question of whether song also serves other functions in addition to mate attraction and territorial functions. Song in both sexes could mediate social dynamics in dense habitat where vision and low-amplitude calls would be insufficient for partner localization [54,55]. Female, like male song, may mediate competition with hetero-specifics for resources, including nest sites [56]. Both sexes of many species sometimes sing near the nest, potentially to coordinate parental investment and nest defence from predators (reviewed in [57]). Table 1 lists these hypotheses and how new playback experiments should test how males and females respond to the songs of their social partner or group members, whether they approach or match song types [58], and how this response is affected by context, location, season and song complexity. Furthermore, cost–benefit analyses may lead to interesting predictions regarding secondary losses of song, since costs of singing could differ for males and females [59,60].
Table 1.
Hypothesized functions of bird song and experimental approaches for testing hypotheses in male and female birds simultaneously.
| possible functions of male and female song | suggested experimental approaches |
|---|---|
| Territorial defence | 1. Speaker replacement experiments. Test ‘keep out’ function of song by removing pair and comparing settlement time of male and female immigrants in relation to playback of male and/or female song (e.g. song presence/absence, songs versus calls, repertoires versus single song type). 2. Simulate intrusion (decoy or playback). Test whether female and/or male residents use increases in song rate or song complexity to defend their territory. |
| Distinguishing mate and resource defence | 1. Mate removal experiments. Compare song output of unpaired, monogamous and polygamous individuals; remove mate, document changes in song output. 2. Resource manipulation. Compare song output before and after adding or removing nest sites or supplementing food. |
| Mate attraction | 1. Speaker replacement experiments. Test mate attraction function of song by removing pair and comparing settlement time of male and female immigrants in relation to playback of male and/or female song (test if stimuli as above attract the opposite sex). 2. For cavity-nesting species in which either sex may establish the territory, playback experiments at nest boxes. |
| Mate stimulation | Laboratory playback experiments. Test male preferences for female song, and effects on male reproductive physiology and behaviour. |
| Advertising individual quality (e.g. to attract mate or defend territory) | 1. Food supplementation experiment. Test whether providing food when conditions are harsh leads to increases in song rate. 2. Manipulation of the early rearing environment (food, brood size, parasites). Test whether treatment(s) affects song learning outcomes/repertoire size. |
| Mediating social dynamics | 1. Playback experiments to test the ‘What’ and ‘How’ of adult song (figure 2), particularly interactive playback. 2. Network analyses (microphone arrays in the field, miniature backpack microphones in aviaries). |
To sum up, although experimental studies on the function of female song are rare in comparison with the substantial body of research on male birds of the north-temperate zones, and little is known about song function in the context of inter-sexual or female intra-sexual singing interactions, the studies conducted so far have revealed many different functions of female song. This suggests that song functions and communication roles typical of the highly seasonal northern temperate zones, with predominantly migratory songbird populations, may not extrapolate to other biogeographic regions with year-round residency and pairing, less seasonal breeding and other life-history differences.
(e). Comparative analyses
Across songbirds, there is considerably more variation in female than male song, ranging from species with no female song to species in which female song output or repertoire sizes are equivalent to or more extensive than in males (e.g. [41,61,62]). Phylogenetic comparative studies so far have examined the evolution of female song separately from male song (e.g. [1,4,63]). This was beneficial initially, when knowledge of female bird song was limited and its prevalence was unknown. Now that we understand that female song is widespread, it is important to study female and male song evolution simultaneously. Nevertheless, evolutionary patterns should also be examined for each sex independently within these studies to evaluate how losses and elaboration of song in each sex contribute to overall patterns of dimorphism, as has been done for other elaborate traits [4,5,64]. To this end, phylogenetic comparative studies can correlate ecological and natural history traits to losses and elaborations of song in each sex to identify their associated selection pressures [4]. This requires precisely quantified traits relevant to both sexes; ideally by quantifying and comparing song as a continuous, multi-dimensional trait (see §2b). Past analytical constraints limited phylogenetic comparative studies to correlations among binary character states, with song classified discretely, such as presence/absence or a few ordinal classifications (e.g. [1,63,65]). However, recent advances in phylogenetic comparative tools now allow comparisons of multiple continuous explanatory variables while controlling for phylogeny (e.g. [2,5,66]). Thus, the correlated evolution of complex traits, such as female and male song structure, can now be tested with increased precision. This will require incorporating a broad range of natural history and ecological traits that pertain to both sexes, ideally also measured continuously, representing sexual, social, and natural selection pressures (e.g. [5,7,66]), enabling us to evaluate hypotheses associated with initial gains or elaboration in both sexes, or loss of song in females. Conclusions from these correlational phylogenetic analyses should in turn be tested via experimental studies on the function, costs, and benefits of female and male song. In this way, experimental studies can refine hypotheses supported by comparative studies and, in turn, inform the relevant questions for future phylogenetic comparative research (figure 1).
3. Conclusion—towards a sex-inclusive approach to animal communication
Historic and geographical research biases have led to gross underestimation of female bird song. Increasing documentation of female bird song shows it to be widespread, challenging us to revise the traditional male-focused framework and to investigate among-species diversity in sex-specific communication roles. Sex-inclusive research on animal signalling will expand our understanding of the natural history of song in both sexes and variation in the extent of acoustic dimorphism, facilitating experimental and comparative studies to identify factors driving the evolutionary dynamics of female and male bird song. Researching female as well as male song also considerably expands our understanding of social networks in bird song, from a sole focus on male–male networks, to include female–female networks and female–male networks where social dynamics may include both cooperation and conflict (e.g. long-term neighbours and breeding partners). Addressing open questions regarding the different evolutionary scenarios and current selection pressures that led to or maintained so many different forms of sex differences (and their absence) requires concerted research effort into the mechanisms, function, and evolution of female and male bird song. Bird song research has already made many important contributions to the field of animal communication, and improving our knowledge of female bird song not only will rectify our currently incomplete understanding of the evolution and function of bird song, but also offers a tractable model system for improving our understanding of sex-specificity of communication roles, complex social network dynamics, and trait loss and gains.
Data accessibility
This article has no additional data.
Authors' contributions
All authors drafted and wrote the manuscript and approved the final version.
Competing interests
We declare we have no competing interests.
Funding
K.J.O. was funded by the European Union's Horizon 2020 research and innovation programme (Marie Sklodowska-Curie grant no. 703999-YnotSing) and the U.S. National Science Foundation (grant no. 1612861).
References
- 1.Odom KJ, Hall ML, Riebel K, Omland KE, Langmore NE. 2014. Female song is widespread and ancestral in songbirds. Nat. Commun. 5, 3379 ( 10.1038/ncomms4379) [DOI] [PubMed] [Google Scholar]
- 2.Webb W, Brunton D, Aguirre J, Thomas D, Valcu M, Dale J. 2016. Female song occurs in songbirds with more elaborate female coloration and reduced sexual dichromatism. Front. Ecol. Evol. 4, 22 ( 10.3389/fevo.2016.00022) [DOI] [Google Scholar]
- 3.Riebel K, Hall ML, Langmore NE. 2005. Female songbirds still struggling to be heard. Trends Ecol. Evol. 20, 419–420. ( 10.1016/j.tree.2005.04.024) [DOI] [PubMed] [Google Scholar]
- 4.Price JJ. 2015. Rethinking our assumptions about the evolution of bird song and other sexually dimorphic signals. Front. Ecol. Evol. 3, 40 (doi:0.3389/fevo.2015.00040) [Google Scholar]
- 5.Dale J, Dey CJ, Delhey K, Kempenaers B, Valcu M. 2015. The effects of life history and sexual selection on male and female plumage colouration. Nature 527, 367–370. ( 10.1038/nature15509) [DOI] [PubMed] [Google Scholar]
- 6.Kraaijeveld K. 2014. Reversible trait loss: the genetic architecture of female ornaments. Annu. Rev. Ecol. Evol. Syst. 45, 159–177. ( 10.1146/annurev-ecolsys-120213-091550) [DOI] [Google Scholar]
- 7.Tobias JA, Montgomerie R, Lyon BE. 2012. The evolution of female ornaments and weaponry: social selection, sexual selection and ecological competition. Phil. Trans. R. Soc. B 367, 2274–2293. ( 10.1098/rstb.2011.0280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall ML. 2004. A review of hypotheses for the functions of avian duetting. Behav. Ecol. Sociobiol. 55, 415–430. ( 10.1007/s00265-003-0741-x) [DOI] [Google Scholar]
- 9.Hall ML. 2009. A review of vocal duetting in birds. Adv. Study Behav. 40, 67–121. ( 10.1016/s0065-3454(09)40003-2) [DOI] [Google Scholar]
- 10.Langmore NE. 1998. Functions of duet and solo songs of female birds. Trends Ecol. Evol. 13, 136–140. ( 10.1016/S0169-5347(97)01241-X) [DOI] [PubMed] [Google Scholar]
- 11.Odom KJ, Benedict L. 2018. A call to document female bird songs: applications for diverse fields. Auk 135, 314–325. ( 10.1642/auk-17-183.1) [DOI] [Google Scholar]
- 12.Odom KJ, Riebel K. 2017. The Female Bird Song Project: a citizen science project to increase the documentation of female birdsong in biological collections. See http://www.femalebirdsong.org.
- 13.Hall ML, Langmore NE. 2017. Editorial. Fitness costs and benefits of female song. Front. Ecol. Evol. 5, 48 ( 10.3389/fevo.2017.00048) [DOI] [Google Scholar]
- 14.Rose EM, Mathew T, Coss DA, Lohr B, Omland KE. 2018. A new statistical method to test equivalence: an application in male and female eastern bluebird song. Anim. Behav. 145, 77–85. ( 10.1016/j.anbehav.2018.09.004) [DOI] [Google Scholar]
- 15.Greig EI, Price JJ, Pruett-Jones S. 2013. Song evolution in Maluridae: influences of natural and sexual selection on acoustic structure. Emu 113, 270–281. ( 10.1071/mu12078) [DOI] [Google Scholar]
- 16.Kershenbaum A, et al. 2016. Acoustic sequences in non-human animals: a tutorial review and prospectus. Biol. Rev. 91, 13–52. ( 10.1111/brv.12160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lachlan RF, Peters S, Verhagen L, ten Cate C. 2010. Are there species-universal categories in bird song phonology and syntax? A comparative study of chaffinches (Fringilla coelebs), zebra finches (Taeniopygia guttata), and swamp sparrows (Melospiza georgiana). J. Comp. Psychol. 124, 92–108. ( 10.1037/a0016996) [DOI] [PubMed] [Google Scholar]
- 18.Lachlan RF. 2017. Luscinia: a bioacoustics analysis computer program. See https://github.com/rflachlan/Luscinia/wiki.
- 19.Schwabl H, Dowling J, Baldassarre DT, Gahr M, Lindsay WR, Webster MS. 2015. Variation in song system anatomy and androgen levels does not correspond to song characteristics in a tropical songbird. Anim. Behav. 104, 39–50. ( 10.1016/j.anbehav.2015.03.006) [DOI] [Google Scholar]
- 20.Riebel K. 2003. The ‘mute’ sex revisited: vocal production and perception learning in female songbirds. Adv. Study Behav. 33, 49–86. ( 10.1016/s0065-3454(03)33002-5) [DOI] [Google Scholar]
- 21.Riebel K, Lachlan RF, Slater PJB. 2015. Learning and cultural transmission in chaffinch song. Adv. Study Behav. 47, 181–227. ( 10.1016/bs.asb.2015.01.001) [DOI] [Google Scholar]
- 22.Lachlan RF, Anderson RC, Peters S, Searcy WA, Nowicki S. 2014. Typical versions of learned swamp sparrow song types are more effective signals than are less typical versions. Proc. R. Soc. B 281, 20140252 ( 10.1098/rspb.2014.0252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verzijden MN, ten Cate C, Servedio MR, Kozak GM, Boughman JW, Svensson EI. 2012. The impact of learning on sexual selection and speciation. Trends Ecol. Evol. 27, 511–519. ( 10.1016/j.tree.2012.05.007) [DOI] [PubMed] [Google Scholar]
- 24.Gahr M, Sonnenschein E, Wickler W. 1998. Sex differences in the size of the neural song control regions in a duetting songbird with similar song repertoire size of males and females. J. Neurosci. 18, 1124–1131. ( 10.1523/JNEUROSCI.18-03-01124.1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levin RN, Paris TI, Bester JK. 1996. Social versus innate influences on the development of sex-specific song in a tropical duetting wren. Am. Zool. 36, 92A. [Google Scholar]
- 26.Geberzahn N, Gahr M. 2013. Song learning in male and female Uraeginthus cyanocephalus, a tropical songbird species. J. Comp. Psychol. 127, 352–364. ( 10.1037/a0033154) [DOI] [PubMed] [Google Scholar]
- 27.Evans C, Kleindorfer S. 2016. Superb fairy-wren (Malurus cyaneus) sons and daughters acquire song elements of mothers and social fathers. Front. Ecol. Evol. 11, 9 ( 10.3389/fevo.2016.00009) [DOI] [Google Scholar]
- 28.Roper MM, Harmer AMT, Brunton DH. 2018. Developmental changes in song production in free-living male and female New Zealand bellbirds. Anim. Behav. 140, 57–71. ( 10.1016/j.anbehav.2018.04.003) [DOI] [Google Scholar]
- 29.Marshall-Ball L, Slater PJB. 2008. Repertoire sharing by the individual and the pair: insights into duet function and development in the plain wren Thryothorus modestus. J. Avian Biol. 39, 293–299. ( 10.1111/j.0908-8857.2008.04060.x) [DOI] [Google Scholar]
- 30.Rivera-Caceres KD, Quiros-Guerrero E, Araya-Salas M, Searcy WA. 2016. Neotropical wrens learn new duet rules as adults. Proc. R. Soc. B 283, 20161819 ( 10.1098/rspb.2016.1819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riebel K, Smallegange IM, Terpstra NJ, Bolhuis JJ. 2002. Sexual equality in zebra finch song preference: evidence for a dissociation between song recognition and production learning. Proc. R. Soc. Lond. B 269, 729–733. ( 10.1098/rspb.2001.1930) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ball GF. 2016. Species variation in the degree of sex differences in brain and behaviour related to birdsong: adaptations and constraints. Phil. Trans. R. Soc. B 371, 20150117 ( 10.1098/rstb.2015.0117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balthazart J, Choleris E, Remage-Healey L. 2018. Steroids and the brain: 50 years of research, conceptual shifts and the ascent of non-classical and membrane-initiated actions. Horm. Behav. 99, 1–8. ( 10.1016/j.yhbeh.2018.01.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gahr M. 2014. How hormone-sensitive are bird songs and what are the underlying mechanisms? Acta Acust. United Acust. 100, 705–718. ( 10.3813/aaa.918749) [DOI] [Google Scholar]
- 35.Frankl-Vilches C, Gahr M. 2018. Androgen and estrogen sensitivity of bird song: a comparative view on gene regulatory levels. J. Comp. Physiol. A 204, 113–126. ( 10.1007/s00359-017-1236-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rek P, Magrath RD. 2017. Deceptive vocal duets and multimodal display in a songbird. Proc. R. Soc. B 284, 20171774 ( 10.1098/rspb.2017.1774) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Catchpole CK, Slater PJB. 2008. Bird song: biological themes and variations, 2nd edn Cambridge, UK: Cambridge University Press. [Google Scholar]
- 38.Krebs JR, Ashcroft R, Webber M. 1978. Song repertoires and territory defence in the great tit. Nature 271, 539–542. ( 10.1038/271539a0) [DOI] [Google Scholar]
- 39.Eriksson D, Wallin L. 1986. Male bird song attracts females—a field experiment. Behav. Ecol. Sociobiol. 19, 297–299. ( 10.1007/BF00300645) [DOI] [Google Scholar]
- 40.Brunton DH, Evans B, Cope T, Ji W. 2008. A test of the dear enemy hypothesis in female New Zealand bellbirds (Anthornis melanura): female neighbors as threats. Behav. Ecol. 19, 791–798. ( 10.1093/beheco/arn027) [DOI] [Google Scholar]
- 41.Illes AE. 2015. Context of female bias in song repertoire size, singing effort, and singing independence in a cooperatively breeding songbird. Behav. Ecol. Sociobiol. 69, 139–150. ( 10.1007/s00265-014-1827-3) [DOI] [Google Scholar]
- 42.Hall ML, Rittenbach MRD, Vehrencamp SL. 2015. Female song and vocal interactions with males in a neotropical wren. Front. Ecol. Evol. 3, 12 ( 10.3389/fevo.2015.00012) [DOI] [Google Scholar]
- 43.Langmore NE, Davies NB, Hatchwell BJ, Hartley IR. 1996. Female song attracts males in the alpine accentor Prunella collaris. Proc. R. Soc. Lond. B 263, 141–146. ( 10.1098/rspb.1996.0022) [DOI] [Google Scholar]
- 44.Yasukawa K, Searcy WA. 1982. Aggression in female red-winged blackbirds—a strategy to ensure male parental investment. Behav. Ecol. Sociobiol. 11, 13–17. ( 10.1007/bf00297660) [DOI] [Google Scholar]
- 45.Rogers AC, Langmore NE, Mulder RA. 2007. Function of pair duets in the eastern whipbird: cooperative defense or sexual conflict? Behav. Ecol. 18, 182–188. ( 10.1093/beheco/arl070) [DOI] [Google Scholar]
- 46.Morton ES, Derrickson KC, Stutchbury BJM. 2000. Territory switching behavior in a sedentary tropical passerine, the dusky antbird (Cercomacra tyrannina). Behav. Ecol. 11, 648–653. ( 10.1093/beheco/11.6.648) [DOI] [Google Scholar]
- 47.Keen S, Meliza CD, Pilowsky J, Rubenstein DR. 2016. Song in a social and sexual context: vocalizations signal identity and rank in both sexes of a cooperative breeder. Front. Ecol. Evol. 4, 46 ( 10.3389/fevo.2016.00046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brunton DH, Roper MM, Harmer AM.T. 2016. Female song rate and structure predict reproductive success in a socially monogamous bird. Front. Ecol. Evol. 4, 13 ( 10.3389/fevo.2016.00013) [DOI] [Google Scholar]
- 49.Cain KE, Cockburn A, Langmore NE. 2015. Female song rates in response to simulated intruder are positively related to reproductive success. Front. Ecol. Evol. 3, 119 ( 10.3389/fevo.2015.00119) [DOI] [Google Scholar]
- 50.Thomas RJ. 1999. The effect of variability in the food supply on the daily singing routines of European robins: a test of a stochastic dynamic programming model. Anim. Behav. 57, 365–369. ( 10.1006/anbe.1998.0970) [DOI] [PubMed] [Google Scholar]
- 51.Nowicki S, Peters S, Podos J. 1998. Song learning, early nutrition and sexual selection in songbirds. Am. Zool. 18, 179–190. ( 10.1093/icb/38.1.179) [DOI] [Google Scholar]
- 52.Price JJ. 2009. Evolution and life-history correlates of female song in the New World blackbirds. Behav. Ecol. 20, 967–977. ( 10.1093/beheco/arp085) [DOI] [Google Scholar]
- 53.Schwabl H. 1992. Winter and breeding territorial behavior and levels of reproductive hormones of migratory European robins. Ornis Scandinavica 23, 271–276. ( 10.2307/3676649) [DOI] [Google Scholar]
- 54.Logue DM. 2007. Duetting in space: a radio-telemetry study of the black-bellied wren. Proc. R. Soc. B 274, 3005–3010. ( 10.1098/rspb.2007.1005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mennill DJ, Vehrencamp SL. 2008. Context-dependent functions of avian duets revealed by microphone-array recordings and multispeaker playback. Curr. Biol. 18, 1314–1319. ( 10.1016/j.cub.2008.07.073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tobias JA, Gamarra-Toledo V, Garcia-Olaechea D, Pulgarin PC, Seddon N. 2011. Year-round resource defence and the evolution of male and female song in suboscine birds: social armaments are mutual ornaments. J. Evol. Biol. 24, 2118–2138. ( 10.1111/j.1420-9101.2011.02345.x) [DOI] [PubMed] [Google Scholar]
- 57.Leonard ML. 2008. An overview and comparative analysis of singing on the nest in North American birds. Can. J. Zool. 86, 1101–1110. ( 10.1139/z08-092) [DOI] [Google Scholar]
- 58.Halkin SL. 1997. Nest-vicinity song exchanges may coordinate biparental care of northern cardinals. Anim. Behav. 54, 189–198. ( 10.1006/anbe.1996.0415) [DOI] [PubMed] [Google Scholar]
- 59.Kleindorfer S, Evans C, Mahr K. 2016. Female in-nest chatter song increases predation. Biol. Lett. 12, 20150513 ( 10.1098/rsbl.2015.0513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cain KE, Langmore NE. 2016. Female song and aggression show contrasting relationships to reproductive success when habitat quality differs. Behav. Ecol. Sociobiol. 70, 1867–1877. ( 10.1007/s00265-016-2192-1) [DOI] [Google Scholar]
- 61.Brunton DH, Li XL. 2006. The song structure and seasonal patterns of vocal behavior of male and female bellbirds (Anthornis melanura). J. Ethol. 24, 17–25. ( 10.1007/s10164-005-0155-5) [DOI] [Google Scholar]
- 62.Odom KJ, Omland KE, McCaffrey DR, Monroe MK, Christhilf JL, Roberts NS, Logue DM. 2016. Typical males and unconventional females: songs and singing behaviors of a tropical, duetting oriole in the breeding and non-breeding season. Front. Ecol. Evol. 4, 14 ( 10.3389/fevo.2016.00014) [DOI] [Google Scholar]
- 63.Garamszegi LZ, Pavlova DZ, Eens M, Moller AP. 2007. The evolution of song in female birds in Europe. Behav. Ecol. 18, 86–96. ( 10.1093/beheco/arl047) [DOI] [Google Scholar]
- 64.Price JJ, Eaton MD. 2014. Reconstructing the evolution of sexual dichromatism: current color diversity does not reflect past rates of male and female change. Evolution 68, 2026–2037. ( 10.1111/evo.12417) [DOI] [PubMed] [Google Scholar]
- 65.Price JJ, Lanyon SM, Omland KE. 2009. Losses of female song with changes from tropical to temperate breeding in the New World blackbirds. Proc. R. Soc. B 276, 1971–1980. ( 10.1098/rspb.2008.1626) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tobias JA, Sheard C, Seddon N, Meade A, Cotton AJ, Nakagawa S. 2016. Territoriality, social bonds, and the evolution of communal signaling in birds. Front. Ecol. Evol. 4, 74 ( 10.3389/fevo.2016.00074) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.