Abstract
Contestants use displays to signal their aggressive intent and settle disputes before they escalate. For birds, this is often in the form of song, which can vary in structural complexity. The role of song complexity in signalling aggressive intent has not been fully established, and its efficacy could be influenced by background noise levels. Using playback experiments, we found that in European robins, Erithacus rubecula, song complexity signalled sender aggression and affected receiver response. However, increased noise impacted the ability of contestants to adjust response based on opponent song complexity. These findings provide new evidence regarding the use of acoustic signal complexity for assessing opponent aggression and that noise can influence contest behaviour by interrupting this process, which could impose fitness consequences.
Keywords: contest behaviour, opponent assessment, acoustic communication, agonistic displays, song complexity, noise
1. Background
Across species, contests can be resolved before they escalate to injurious attacks by the exchange and assessment of information on fighting ability and aggressive intent [1,2]. This exchange of information is facilitated by a display phase of a contest where contestants exhibit their morphological features (e.g. weaponry [3] and size [4]) and perform behaviours that signal or demonstrate skill and aggressiveness [5,6]. A particular aspect of behavioural display that has clear effects is intensity, often signalled by the willingness to initiate a display and by its rate or magnitude [1–7]. Display intensity can be transmitted by visual and non-visual signals alike, with the benefit that longer-ranging signals can alert several contenders and reduce future conflicts [3–7]. Yet, some types of long-ranging signals also have the further capacity to harbour information in their structure, such as vocalizations that vary markedly in complexity [8–13].
One such long-range signal used in vocal interactions is bird song. Although the intensity of song displays in birds, such as song rate, has been linked to elevated aggression [6,7], songs also broadly vary in their complexity, which can be used by receivers [11–17]. The term ‘complexity’ has been used for characterizing vocalization structure (e.g. phrases, syllables and notes) in terms of duration, abundance or diversity [11–15]. Notably, these structural components can encode and communicate information [9,16,17]. Therefore, complexity can be used to identify whether information encoded in vocalization structure is assessed when adjusting contest behaviour, for which evidence is limited [7–10].
A candidate for identifying this process is bird song display during territorial disputes [9,18]. Variation in some elements of song structure have been related with aggressive territorial acts [7] and intraspecific variations in song complexity are often suggested to enable the signalling of individual competitive ability [10] and aggressive intent (i.e. their motivation to fight) [18]. Therefore, song complexity may encode information about individual contestants, which may enable them to signal their ability or intent during contests. We hypothesize that song complexity relates to the fight motivation or aggressiveness of the sender and influences the contest behaviour of the receiver [18]. Moreover, the information encoded in song structure is susceptible to background noise, which can mask some of the information [19–22]. Therefore, we also hypothesize that elevated background noise reduces the ability to use song complexity for assessment during contests.
In order to test these hypotheses, we examined the territorial song display of European robins (Erithacus rubecula) in response to playbacks of robin song that varied in structural complexity and background noise level. Measures of robin display intensity, such as their latency to respond and their song rate, provide validated indicators of their aggressive intent [23–25]. Therefore, if vocalization structure also communicates aggressive intent, song complexity is expected to relate to the display intensity of senders and to affect the display intensity of receivers [18]. Under elevated background noise levels, robins decrease their song complexity (syllable diversity) and their display intensity (song rate and approach tendency) [20–22]. However, there is no evidence of noise affecting the perception of information from song complexity. We predict that song complexity from playbacks will have a reduced effect on the display of robins under increased noise levels, because elements of song structure are acoustically masked [19–22]. The aim of this study is to demonstrate whether signal complexity is used in assessment during contests and to quantify the role of noise in modulating this ability.
2. Material and methods
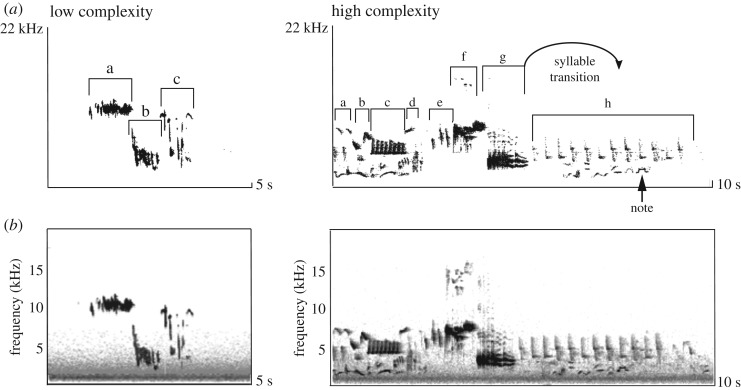
A total of 15 E. rubecula were sampled during the breeding season (March–April 2018; 11.00–16.00) from the resident population of the Lagan valley along the towpath between Belfast and Lisburn, Northern Ireland (54°32′ N, 5°57′ W). Individuals were selected by identifying the territories of birds and mapping their location with GPS. Birds used for the experiments were out of hearing distance of other individuals and thus did not share territory boundaries, and recordings with third-party interference during tests were excluded. Using a within-individual 2 by 2 factorial design, we examined response by robins to four playbacks that varied in song complexity (high or low) and noise level (white noise or ambient control). Songs for playbacks were sourced from an online repository (xenocanto.org) and independent recordings of robin territorial song (as opposed to soft songs, ticks or alarm calls) were selected for being easily classified as having either high or low structural complexity (figure 1a). Robins exhibit no significant geographical variation in daytime singing [26], but can vary individually in their singing and recognize the song of familiar conspecifics [27]. Therefore, the online sourcing provided the opportunity to control for recognition biases. For the noise treatments, the control songs were merged with white noise (low-pass filtered to 100 Hz, 6 dB kHz−1 decrease towards higher frequencies) [20–22,28]. Noise and song exhibited spectral overlap (figure 1b). Using a sound level meter (SL-100; Voltcraft, Hirschau), all playbacks were set to play at a maximum sound level of 85 dBA at 1 m distance and the ambient noise during tests was at levels less than 50 dBA [20–22]. During testing, birds were first visually spotted and then a speaker (SME-AFS loudspeaker; Saul Mineroff Electronics, USA) was directed towards them and placed at ca 5–10 m from their initial location, a distance that varied according to their elevation but in all cases reflected close interaction (less than 10 m) within their territory [27]. Because robins use visual and acoustic signals interchangeably [25], a coloured wooden dummy of a robin was used to reduce effects from the inability of birds to visually locate an opponent near the sound source. The dummy was placed covered in front of the speaker and presented in synchrony to the onset of playbacks, and by using the same dummy across treatments and individuals, we ensured that visual signals were standardized throughout. Following the presentation of the dummy, birds were sequentially exposed to the audio playbacks in a fully randomized order, with each playback running for 1 min and followed by a 1 min no-playback period that served as both an interval and an opportunity to record continuing responses. During tests, both experimenters remained at a 10 m distance from the speaker, at a position that minimized visual interference but limited the opportunity to record visual behavioural measures. Audio was recorded continuously during the sequential playbacks (recorder: Marantz, PMD660; microphone: Sennheiser ME 66/K6).
Figure 1.
Sonograms illustrating variation in the structural complexity of song playbacks and the acoustic make-up of noise treatments. (a) Structural differences between a low and high complexity song playback (xenocanto.org; respective identifiers: XC349141, XC414220), where higher complexity is characterized by longer duration and by greater syllable abundance, diversity (discrete syllable types are denoted by separate letters) and versatility (unique syllable transitions). (b) Noise treatments exhibited spectral overlap with lower frequency elements of songs.
From the audio recordings of tested individuals, we extracted two measures of song display intensity that are regularly used to indicate aggression levels [1,2,6,7], including in robins [23–25]: latency to respond (time between playback start and first song reply; a negative predictor of aggression) and rate of display (number of songs per minute; a positive predictor of aggression) (see data in the electronic supplementary material). We then extracted average values for each of four measures of song complexity (table 1) by examining sonograms of the recordings of each tested individual (44.1 kHz; FFT = 512; 16 bit) generated using the Audacity® software (v. 2.1.3). The measures were highly inter-correlated (Bartlett's , p < 0.001) and fulfilled criteria for using principal components analysis (PCA; table 1) to calculate a composite song complexity score (see the electronic supplementary material), including sampling adequacy (Kaiser–Meyer–Olkin test, KMO = 0.807) and the determinacy of scores (ρ = 0.008). Intra-class correlation (ICC) analysis was used to test repeatability in individual PCA scores across treatments, to examine if song complexity varies as an individual trait. Linear mixed models were used to test song response latency and song rate for fixed effects from noise treatment and playback complexity (to test receiver response), covariate links with song complexity PCA score (to test sender signalling) and interactions. Bird identity was included as a random factor to control for pseudoreplication. Analyses were carried out in Minitab® v. 17 (Minitab Inc., State College, PA, USA).
Table 1.
Measures used to describe structural complexity and their contribution to calculating composite song complexity scores using principal components analysis (PCA).
| measure of complexity | description | PCA |
|
|---|---|---|---|
| loadingsa | communalitiesb | ||
| song length | period between first and last note of song. Notes are units of continuous sound traces on the spectrogram [12,13] | 0.836 | 0.699 |
| syllable abundance | number of note complexes (syllables) per song [12,13] | 0.959 | 0.919 |
| syllable diversity | number of different syllable types per song [12,13,18–20] | 0.952 | 0.906 |
| transition versatility | number of unique transitions between two particular syllable types per song [12] | 0.959 | 0.919 |
| eigenvaluec: 3.44 % variance explained: 86.10 |
|||
aCorrelation between component and measure values.
bProportion of measure variance explained by the component.
cOverall variance of transformed data.
3. Results
The song complexity of individual robins (PCA score) was repeatable across treatments (ICC = 0.906; F3,15 = 10.65, p < 0.001) and strongly predicted their latency to respond (R2 = 0.514) and song rate (R2 = 0.450), but the strength of the effect was influenced by interactions with the song complexity of playbacks, low or high (table 2 and figure 2a). Importantly, there was also a significant interaction between playback song complexity and noise (table 2 and figure 2b) such that adjustments in response latency were not evident in noisy conditions.
Table 2.
The effect of song complexity in vocal interactions. Test results from the linear mixed models for all predictors and interactions between them. Statistically significant effects are shown in italics.
| latency to response (s) |
song rate (count min−1) |
|||
|---|---|---|---|---|
| F2,60a | p-valueb | p-valueb | ||
| main predictors | ||||
| own song complexity | 13.75 | <0.001 | 7.49 | 0.006 |
| playback song complexity | 5.18 | 0.028 | 3.49 | 0.062 |
| playback noise | 3.11 | 0.086 | 0.42 | 0.517 |
| interactions | ||||
| own complexity×playback complexity | 9.02 | 0.005 | 4.89 | 0.027 |
| playback noise×playback complexity | 4.32 | 0.044 | 1.08 | 0.298 |
| own complexity×playback noise | 1.35 | 0.252 | 0.51 | 0.476 |
aTest statistic.
bSignificance level.
Figure 2.
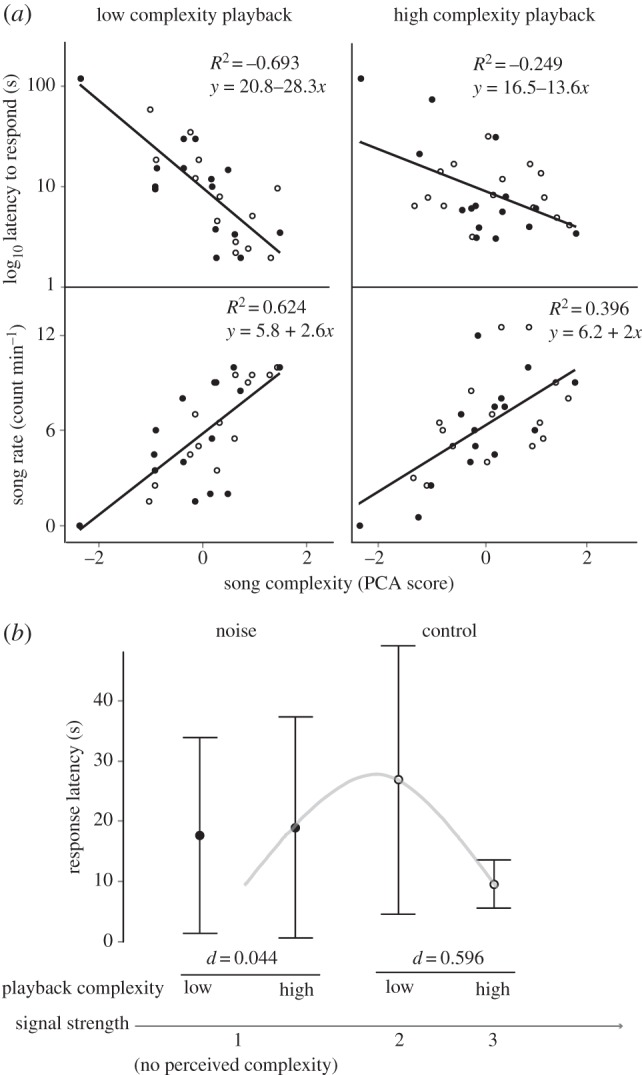
Relationships of sender display-intensity measures with song complexity, and changes in receiver response with playback complexity and noise conditions. (a) Birds that sang more complex songs exhibited shorter response latencies and greater song rates. The relationships were stronger in response to low complexity than high complexity playbacks, but noise had no effects on the relationship (control: open circles; noise treatment: filled circles). (b) Birds responded faster to high complexity playbacks in control conditions, but under the noise treatment, there was no difference in response to low and high complexity playbacks, suggesting a low perception of complexity. Cohen's d denotes the degree of change (0–1) between high and low complexity playbacks, displayed for both noise conditions. The grey curve illustrates how theoretical expectations of response change with perceived signal strength may apply to our results [29].
4. Discussion
Our study demonstrates that European robins use song complexity to communicate aggressive intent during territorial contests and that this process is susceptible to background noise (figure 2). The song complexity of individuals was repeatable across treatments and consistently predicted their behaviour, where individuals that sang more complex songs were faster to respond and sang more often (figure 2a). Thus, song complexity is a stable trait encoding information about the individual's motivation or intent to compete (response latency) and fighting ability during contests (rate of display) [1,2,18]. Although the relationship between song complexity and contest behaviour was weaker when birds responded to high complexity song playbacks (figure 2a), this may be due to changes in the perceived ability of their opponent, signalled by the complexity of playbacks.
Overall, response to high song complexity playbacks was faster (table 2), indicating that receivers can associate elevated song complexity with greater aggressive motivation in senders and increase their own motivation in response. The finding is consistent with empirical evidence and theoretical expectations that territorial owners are typically more motivated to fight intruders that they assess as more threatening to their territory [1,2]. This is driven by territory value [30], which in the case of the robins is inflated in the breeding season by prospects of reproductive success [23–25]. However, the elevated aggressive intent towards complex playbacks, as reflected by the shorter latencies to respond, was only expressed during control treatments (figure 2b). Thus, noise affected behavioural adjustments, most likely by disrupting the perception of song complexity because spectral overlap by noise masked information in bird song structure (figure 1b). With noise effects implicating the perception of song complexity alone (table 2), our findings further exclude alternative explanations to masking, such as elevated noise distracting individuals from their opponent's display [31]. The perception of signals often has nonlinear effects on behaviour, e.g. increasing towards medium signal strengths and declining towards higher strengths [29]. In our study, similar changes can be seen across a perceptual gradient that scales from no perception of song complexity under noise, to the perception of low and then high complexity under control conditions (figure 2b; grey line), illustrating how behavioural adjustments rely particularly on the perception of song complexity.
We found that bird song structure can communicate aggressive intent, enabling contestants to assess their opponent, but noise can disrupt this communication by masking the structural complexity of songs. As a result, contestants receive incomplete information on their opponent's aggressive intent and do not appropriately adjust their response. This suggests that under noisy conditions, birds may be limited in their ability to use song complexity to defend or acquire resources, such as territory, and outcomes from vocal interactions can be highly unpredictable and affect subsequent contests [1,2,31]. Any broader fitness consequences from these effects need to be explicitly tested by future studies, which for robins may include attainment of food, attraction of mates (reproductive success) and nest building (parental investment) [23–25]. To our knowledge, this is the first evidence that bird song structure plays a major role in inter-contestant assessment of aggressive intent and that noise can affect contests exclusively by disrupting this process.
Supplementary Material
Acknowledgements
We thank Gillian Riddell for technical support.
Data accessibility
All data submitted as electronic supplementary material.
Authors' contributions
K.K. and J.W. collected and analysed data; K.K., H.P.K. and G.A. authored/revised manuscript and designed/managed the project. All authors gave final approval for publication and agree to be accountable for the work.
Competing interests
We declare we have no competing interests.
Funding
The study was funded by Queen's University Belfast as part of the research project of J.W.
References
- 1.Arnott G, Elwood RW. 2009. Assessment of fighting ability in animal contests. Anim. Behav. 77, 991–1004. ( 10.1016/j.anbehav.2009.02.010) [DOI] [Google Scholar]
- 2.Hardy ICW, Briffa M (eds). 2013. Animal contests. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Yasuda CI, Koga T. 2016. Importance of weapon size in all stages of male–male contests in the hermit crab Pagurus minutus . Behav. Ecol. Sociobiol. 70, 2175–2183. ( 10.1007/s00265-016-2221-0) [DOI] [Google Scholar]
- 4.Martín J, Lopez P. 2007. Scent may signal fighting ability in male Iberian rock lizards. Biol. Lett. 3, 125–127. ( 10.1098/rsbl.2006.0589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Payne RJ, Pagel M. 1997. Why do animals repeat displays? Anim. Behav. 54, 109–119. ( 10.1006/anbe.1996.0391) [DOI] [PubMed] [Google Scholar]
- 6.Baker TM, Wilson DR, Mennill DJ. 2012. Vocal signals predict attack during aggressive interactions in black-capped chickadees. Anim. Behav. 84, 965–974. ( 10.1016/j.anbehav.2012.07.022) [DOI] [Google Scholar]
- 7.Searcy WA, Akçay C, Nowicki S, Beecher MD. 2014. Aggressive signaling in song sparrows and other songbirds. Adv. Stud. Behav. 46, 89–125. ( 10.1016/B978-0-12-800286-5.00003-1) [DOI] [Google Scholar]
- 8.Snijders L, Naguib M. 2017. Communication in animal social networks: a missing link. Adv. Stud. Behav. 49, 297–359. ( 10.1016/bs.asb.2017.02.004) [DOI] [Google Scholar]
- 9.Todt D, Naguib M. 2000. Vocal interactions in birds: the use of song as a model in communication. Adv. Stud. Behav. 29, 247–296. ( 10.1016/S0065-3454(08)60107-2) [DOI] [Google Scholar]
- 10.Ten Cate C, Slabbekoorn H, Ballintijn MR. 2002. Birdsong and male–male competition: causes and consequences of vocal variability in the collared dove (Streptopelia decaocto). Adv. Stud. Behav. 31, 31–75. ( 10.1016/S0065-3454(02)80005-5) [DOI] [Google Scholar]
- 11.Hill SD, Amiot C, Ludbrook MR, Ji W. 2015. Seasonal variation in the song structure of tui (Prosthemadera novaeseelandiae). N. Z. J. Ecol. 39, 110–115. [Google Scholar]
- 12.Hill SD, Brunton DH, Anderson MG, Ji W. 2018. Fighting talk: complex song elicits more aggressive responses in a vocally complex songbird. Ibis 160, 257–268. ( 10.1111/ibi.12542) [DOI] [Google Scholar]
- 13.Zeng SJ, Székely T, Zhang XW, Lu K, Liu L, Zuo MX. 2007. Comparative analyses of song complexity and song-control nuclei in fourteen oscine species. Zool. Sci. 24, 1–9. ( 10.2108/zsj.24.1) [DOI] [PubMed] [Google Scholar]
- 14.Briefer E, Osiejuk TS, Rybak F, Aubin T. 2010. Are bird song complexity and song sharing shaped by habitat structure? An information theory and statistical approach. J. Theor. Biol. 262, 151–164. ( 10.1016/j.jtbi.2009.09.020) [DOI] [PubMed] [Google Scholar]
- 15.Boogert NJ, Giraldeau LA, Lefebvre L. 2008. Song complexity correlates with learning ability in zebra finch males. Anim. Behav. 76, 1735–1741. ( 10.1016/j.anbehav.2008.08.009) [DOI] [Google Scholar]
- 16.Abe K, Watanabe D. 2011. Songbirds possess the spontaneous ability to discriminate syntactic rules. Nat. Neurosci. 14, 1067–1074. ( 10.1038/nn.2869) [DOI] [PubMed] [Google Scholar]
- 17.Bolhuis JJ, Okanoya K, Scharff C. 2010. Twitter evolution: converging mechanisms in birdsong and human speech. Nat. Rev. Neurosci. 11, 747–759. ( 10.1038/nrn2931) [DOI] [PubMed] [Google Scholar]
- 18.Searcy WA, Beecher MD. 2009. Song as an aggressive signal in songbirds. Anim. Behav. 78, 1281–1292. ( 10.1016/j.anbehav.2009.08.011) [DOI] [Google Scholar]
- 19.Brumm H, Slabbekoorn H. 2005. Acoustic communication in noise. Adv. Stud. Behav. 35, 151–209. ( 10.1016/S0065-3454(05)35004-2) [DOI] [Google Scholar]
- 20.McLaughlin KE, Kunc HP. 2013. Experimentally increased noise levels change spatial and singing behaviour. Biol. Lett. 9, 20120771 ( 10.1098/rsbl.2012.0771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montague MJ, Danek-Gontard M, Kunc HP. 2012. Phenotypic plasticity affects the response of a sexually selected trait to anthropogenic noise. Behav. Ecol. 24, 343–348. ( 10.1093/beheco/ars169) [DOI] [Google Scholar]
- 22.McMullen H, Schmidt R, Kunc HP. 2014. Anthropogenic noise affects vocal interactions. Behav. Proc. 103, 125–128. ( 10.1016/j.beproc.2013.12.001) [DOI] [PubMed] [Google Scholar]
- 23.Hoelzel AR. 1986. Song characteristics and response to playback of male and female robins Erithacus rubecula. Ibis 128, 115–127. ( 10.1111/j.1474-919X.1986.tb02098.x) [DOI] [Google Scholar]
- 24.Tobias J, Seddon N. 2000. Territoriality as a paternity guard in the European robin, Erithacus rubecula. Anim. Behav. 60, 165–173. ( 10.1006/anbe.2000.1442) [DOI] [PubMed] [Google Scholar]
- 25.Chantrey DF, Workman L. 1984. Song and plumage effects on aggressive display by the European robin Erithacus rubecula. Ibis 126, 366–371. ( 10.1111/j.1474-919X.1984.tb00257.x) [DOI] [Google Scholar]
- 26.Thomas R, Goldsmith A, Cuthill I, Lidgate H, Proctor SB, Cosgrove D. 2003. The trade-off between singing and mass gain in a daytime-singing bird, the European robin. Behaviour 140, 387–404. ( 10.1163/156853903321826693) [DOI] [Google Scholar]
- 27.Brindley EL. 1991. Response of European robins to playback of song: neighbour recognition and overlapping. Anim. Behav. 41, 503–512. ( 10.1016/S0003-3472(05)80853-X) [DOI] [Google Scholar]
- 28.Halfwerk W, Slabbekoorn H. 2009. A behavioural mechanism explaining noise-dependent frequency use in urban birdsong. Anim. Behav. 78, 1301–1307. ( 10.1016/j.anbehav.2009.09.015) [DOI] [Google Scholar]
- 29.de Kort SR, Eldermire ER, Cramer ER, Vehrencamp SL. 2008. The deterrent effect of bird song in territory defense. Behav. Ecol. 20, 200–206. ( 10.1093/beheco/arn135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnott G, Elwood RW. 2008. Information gathering and decision making about resource value in animal contests. Anim. Behav. 76, 529–542. ( 10.1016/j.anbehav.2008.04.019) [DOI] [Google Scholar]
- 31.Francis CD, Barber JR. 2013. A framework for understanding noise impacts on wildlife: an urgent conservation priority. Front. Ecol. Environ. 11, 305–313. ( 10.1890/120183) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data submitted as electronic supplementary material.